Isolation, Identification and Characterization of Bioflocculant-Producing Bacteria from Activated Sludge of Vulindlela Wastewater Treatment Plant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation of Bioflocculant-Producing Microorganisms
2.2. Screening of Bacteria for Bioflocculant Production
2.3. Determination of Bioflocculant Activity
2.4. Identification of a Bioflocculant-Producing Bacterium
2.4.1. Morphological Identification of the Bioflocculant-Producing Bacterium
2.4.2. Molecular Identification of Bioflocculant–Producing Bacterium
2.5. Activation of the Isolate for Fermentation
2.6. Optimisation of Bioflocculant Production Conditions
2.6.1. Determination of the Inoculation Volume
2.6.2. Effect of Carbon and Nitrogen Sources on Bioflocculating Activity
2.6.3. Effect of Metal Ions on Bioflocculating Activity
2.6.4. Effect of Initial pH of the Production Media
2.6.5. Effect of Shaking Speed on Bioflocculant Production
2.6.6. Effect of Cultivation Temperature on Bioflocculating Activity
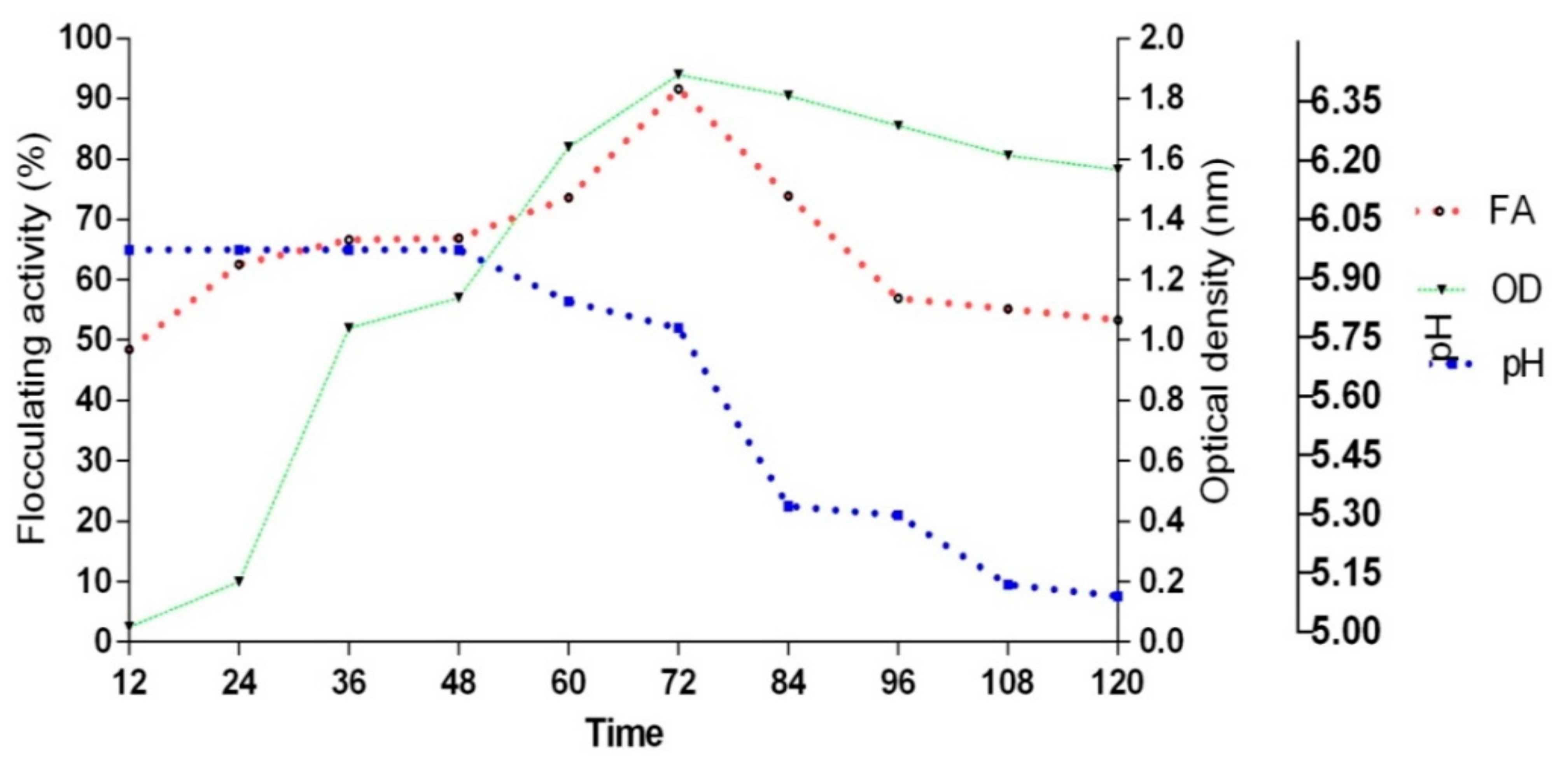
2.6.7. Time Course Assay
2.7. Extraction and Purification of Bioflocculant
2.8. Chemical Analysis of the Purified Bioflocculant
2.8.1. Composition Analysis of the Purified Bioflocculant
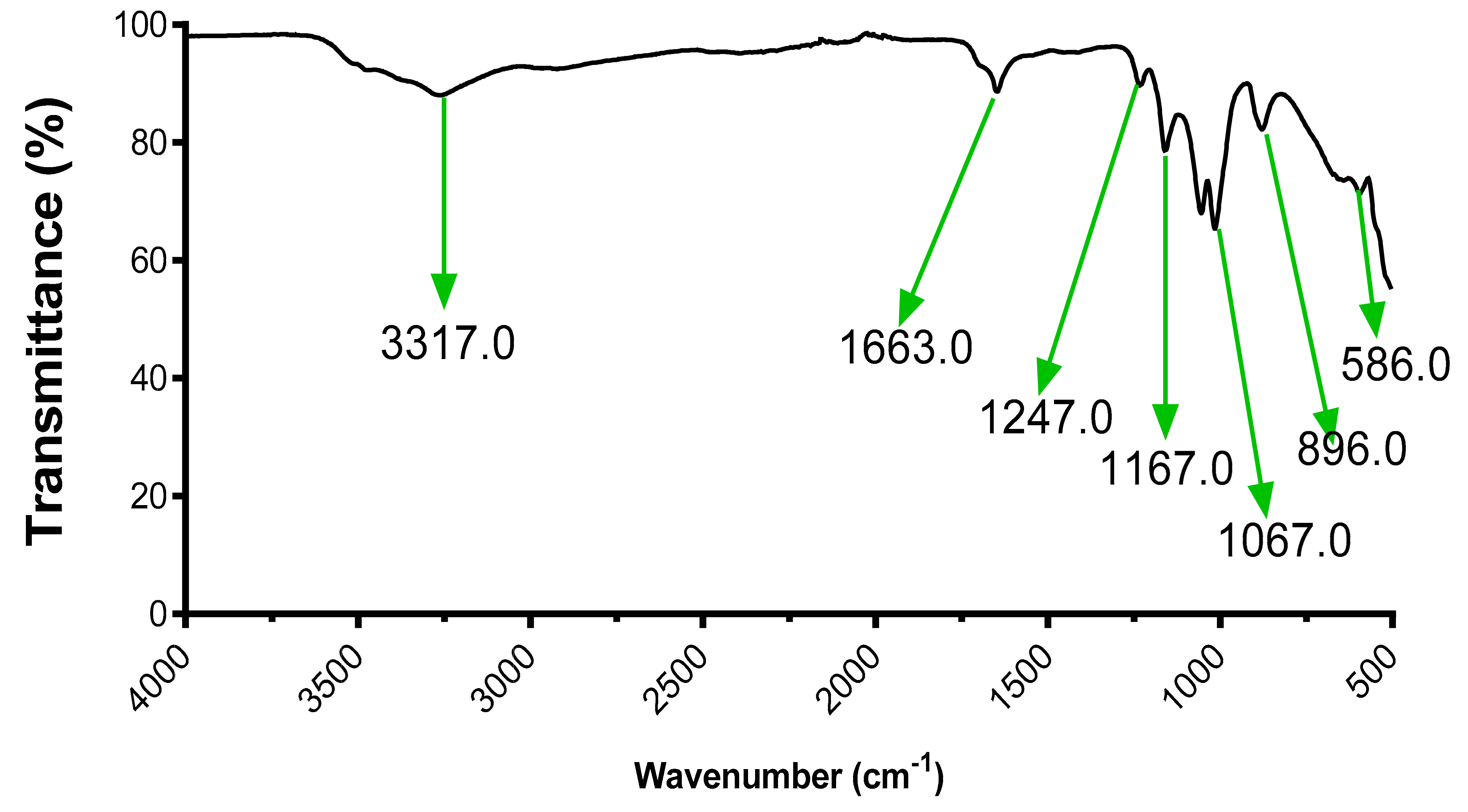
2.8.2. FTIR Analysis
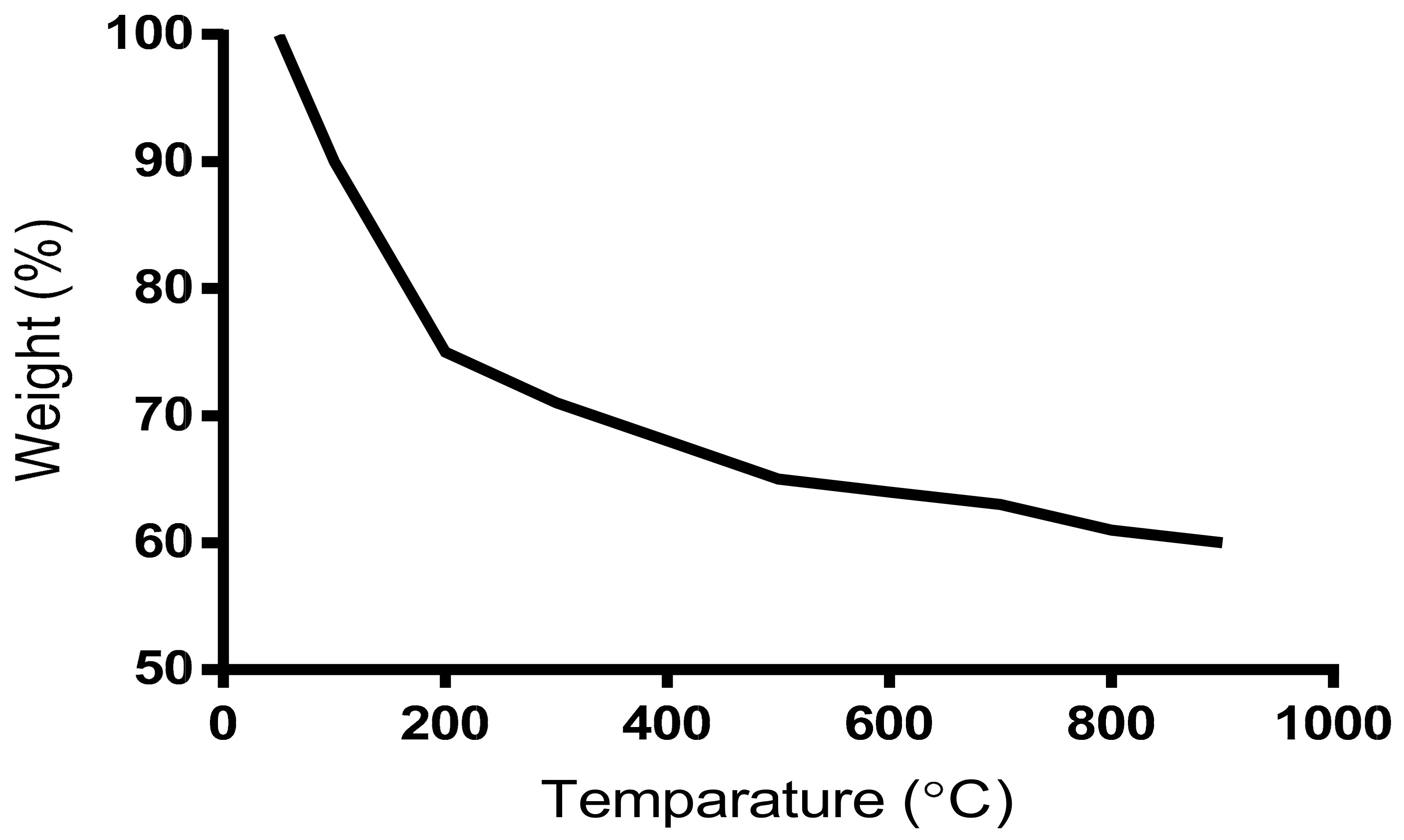
2.8.3. Thermo-Gravimetric Analysis (TGA) of a Bioflocculant
2.8.4. Elementary Analysis
2.9. Flocculation Characteristics of a Purified Bioflocculant
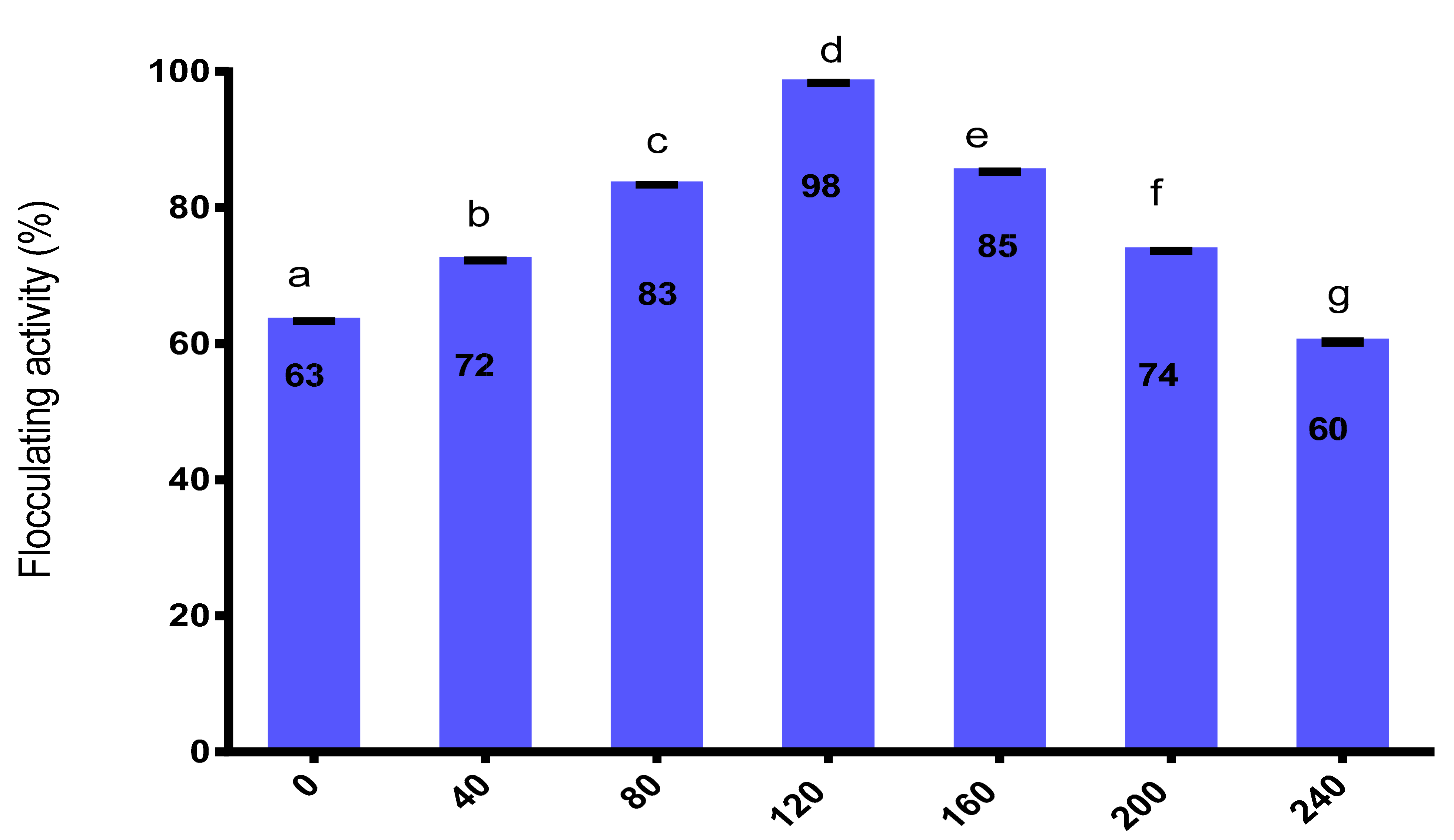
2.9.1. Effect of Dosage Concentration on Flocculating Activity
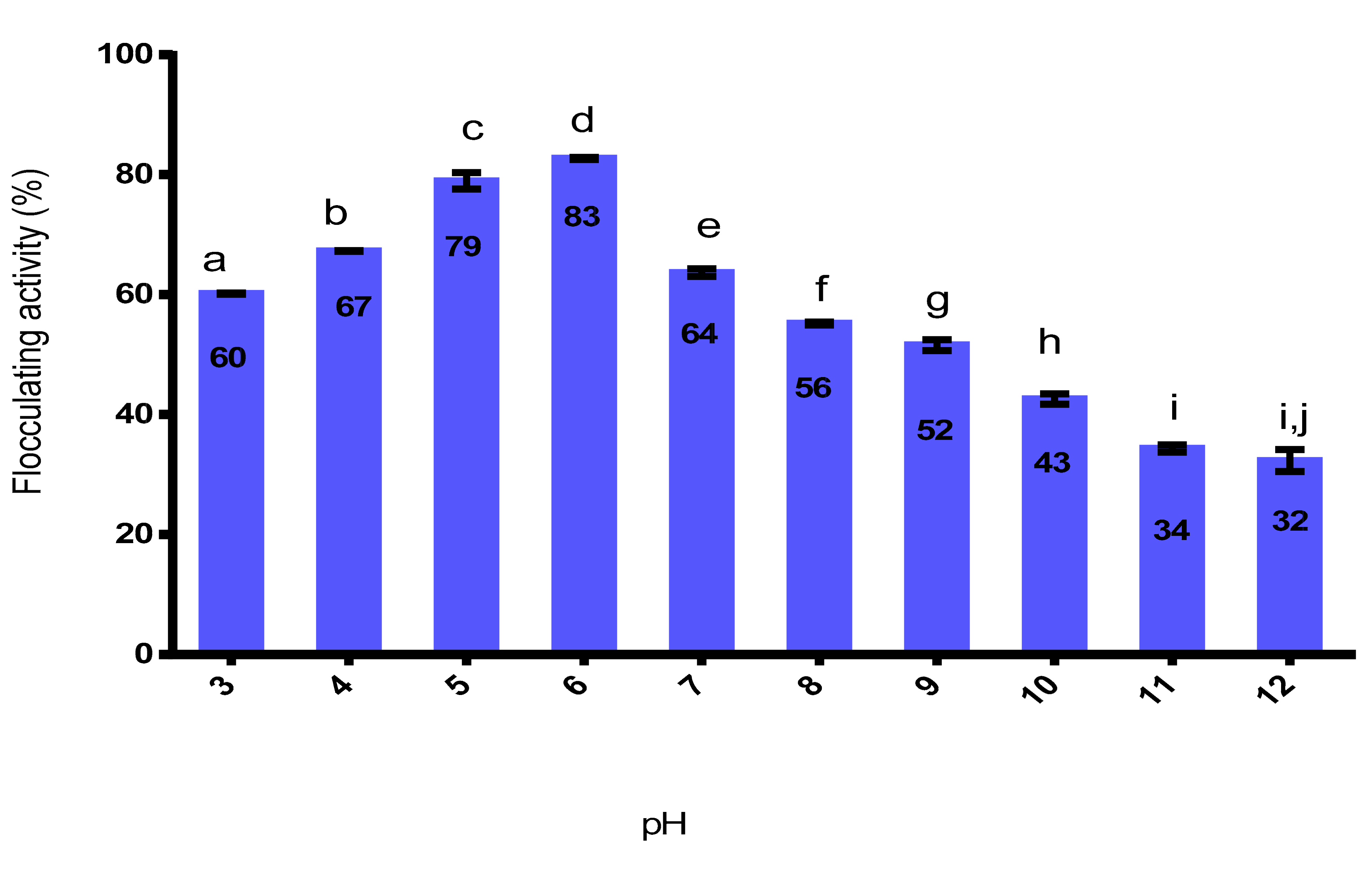
2.9.2. Effect of pH on the Flocculating Activity of Purified Bioflocculant
2.9.3. Effect of Metal Ions on Flocculating Activity
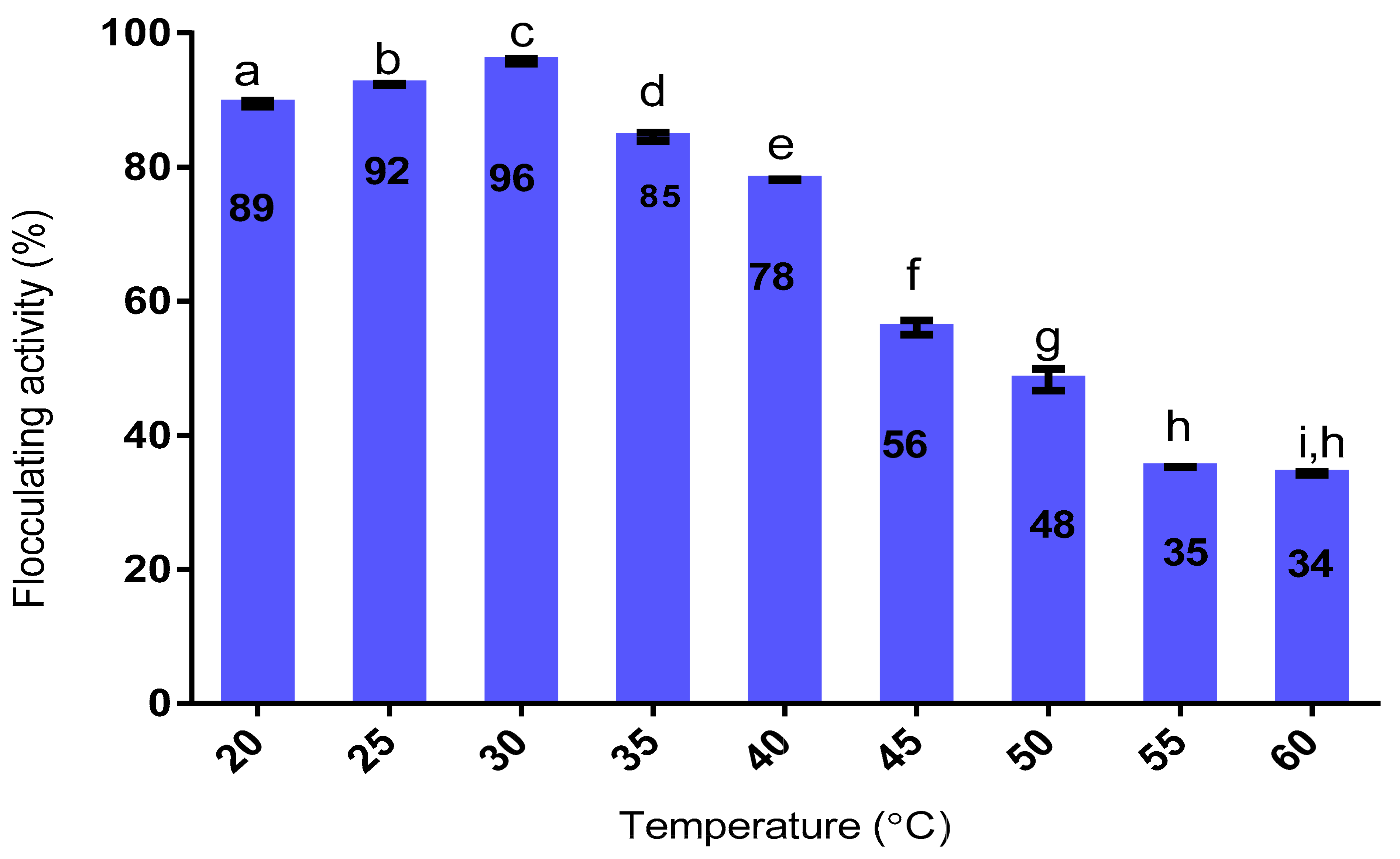
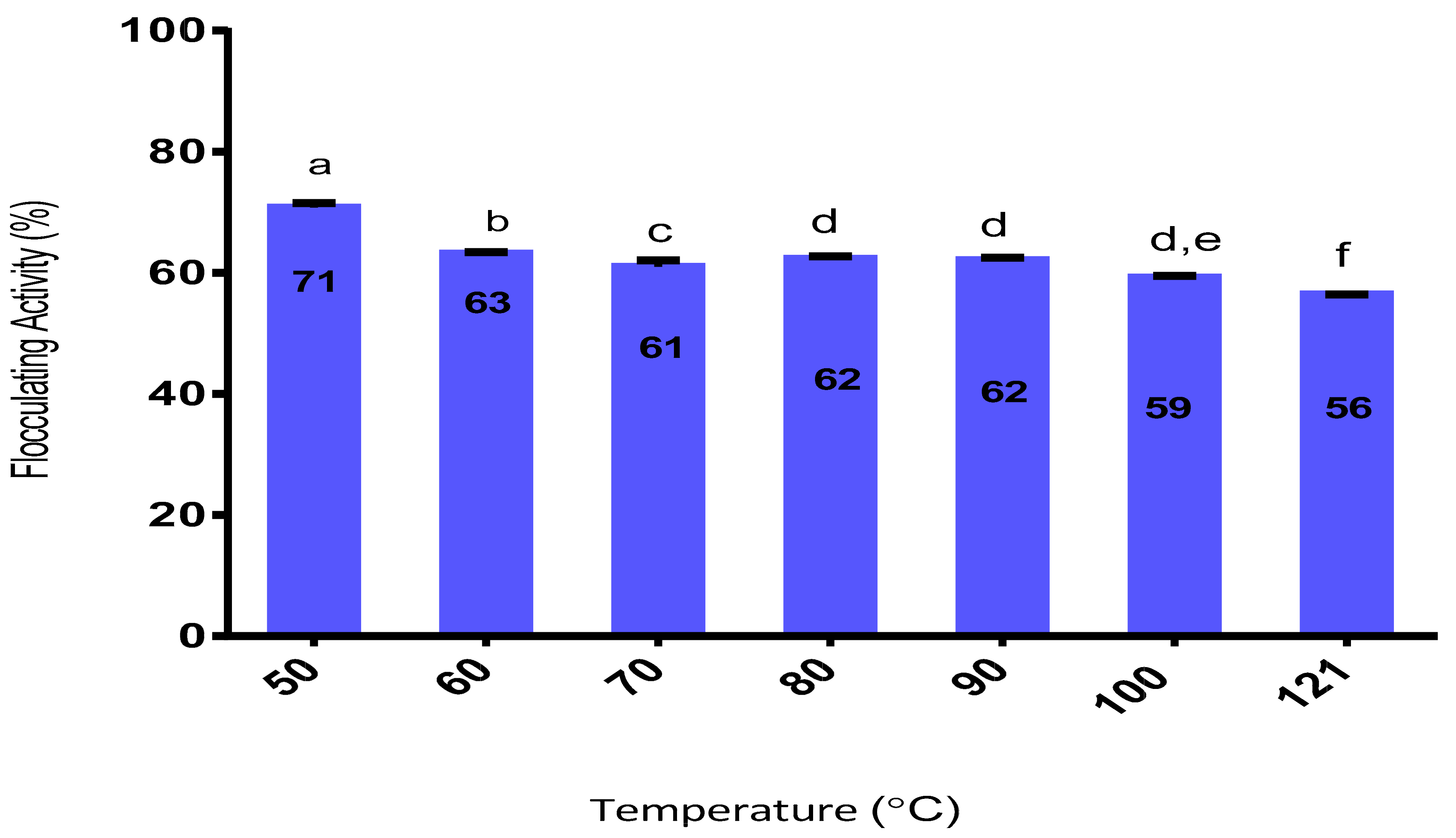
2.9.4. Effect of Heat on Flocculating Activity
2.10. Statistical Analysis
3. Results and Discussion
3.1. Isolation, Screening and Identification of Bioflocculant-Producing Bacterium
3.2. Optimisation of Culture Conditions
3.2.1. Effect of Inoculation Volume on Bioflocculant Production
3.2.2. Effect of Carbon Source on Biofloccculant Production
3.2.3. Effect of Nitrogen Sources on Bioflocculant Production
3.2.4. Effect of Metal Ions on Bioflocculant Production
3.2.5. Effect of pH on Bioflocculant Production
3.2.6. Effect of Shaking Speed on Bioflocculant Production
3.2.7. Effect of Temperature on Bioflocculant Production
3.2.8. Time Course on Bioflocculant Production
3.3. Extraction and Purification of a Bioflocculant
3.4. Chemical composition of the Purified Bioflocculant
3.5. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
3.6. Thermogravimetric TGA Analysis of the Purified Bioflocculant
3.7. Elemental Analysis of the Purified Bioflocculant
3.8. SEM Analysis of the Purified Bioflocculant
3.9. Conditions for Flocculating Activity of the Purified Bioflocculant
3.9.1. Effect of Dosage Concentration on the Purified Bioflocculant
3.9.2. Effect of pH on the Purified Bioflocculant
3.9.3. The Effective Use of Metal Ions on the Purified Bioflocculant
3.9.4. Effect of Heat on the Purified Bioflocculant
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Okaiyeto, K.; Nwodo, U.U.; Okoli, S.A.; Mabinya, L.V.; Okoh, A.I. Implications for public health demands alternatives to inorganic and synthetic flocculants: Bioflocculants as important candidates. MicrobiologyOpen 2016, 5, 177–211. [Google Scholar] [CrossRef]
- Quina, M.J.; Soares, M.A.R.; Quinta-Ferreira, R. Applications of industrial eggshell as a valuable anthropogenic resource. Resour. Conserv. Recycl. 2017, 123, 176–186. [Google Scholar] [CrossRef]
- Pathak, S.; Sneha, C.; Mathew, B.B. Bioplastics: Its timeline based scenario & challenges. J. Polym. Biopolym. Phys. Chem. 2014, 2, 84–90. [Google Scholar]
- Bruno, P.; Campo, R.; Giustra, M.; De Marchis, M.; Di Bella, G. Bench scale continuous coagulation-flocculation of saline industrial wastewater contaminated by hydrocarbons. J. Water Process Eng. 2020, 34, 101156. [Google Scholar] [CrossRef]
- Nwodo, U.U.; Agunbiade, M.O.; Green, E.; Mabinya, L.V.; Okoh, A.I. A freshwater Streptomyces, isolated from Tyume river, produces a predominantly extracellular glycoprotein bioflocculant. Int. J. Mol. Sci. 2012, 13, 8679–8695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nwodo, U.U.; Okoh, A.I. Mixed culture fermentation and media optimization by response surface model: Streptomyces and Brachybacterium species in bioflocculant production. Molecules 2014, 19, 11131–11144. [Google Scholar] [PubMed] [Green Version]
- Aljuboori, A.H.R.; Idris, A.; Abdullah, N.; Mohamad, R. Production and characterization of a bioflocculant produced by Aspergillus flavus. Bioresour. Technol. 2013, 127, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Mabinya, L.V.; Cosa, S.; Nwodo, U.; Okoh, A.I. Studies on bioflocculant production by Arthrobacter sp. Raats, a freshwater bacteria isolated from Tyume River, South Africa. Int. J. Mol. Sci. 2012, 13, 1054–1065. [Google Scholar] [CrossRef] [PubMed]
- Salehizadeh, H.; Yan, N. Recent advances in extracellular biopolymer flocculants. Biotechnol. Adv. 2014, 32, 1506–1522. [Google Scholar] [CrossRef] [PubMed]
- Ntozonke, N.; Okaiyeto, K.; Okoli, A.S.; Olaniran, A.O.; Nwodo, U.U.; Okoh, A.I. A Marine Bacterium, Bacillus sp. Isolated from the Sediment Samples of Algoa Bay in South Africa Produces a Polysaccharide-Bioflocculant. Int. J. Environ. Res. Public Health 2017, 14, 1149. [Google Scholar] [CrossRef] [Green Version]
- Okaiyeto, K.; Nwodo, U.U.; Mabinya, L.V.; Okoh, A.I. Characterization of a bioflocculant produced by a consortium of Halomonas sp. Okoh and Micrococcus sp. Leo. Int. J. Environ. Res. Public Health 2013, 10, 5097–5110. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Y.; Wang, Y.; Yu, Y.; Li, Q.; Wang, H.; Chen, R.; He, N. Production and characterization of a novel bioflocculant from Bacillus licheniformis. Appl. Environ. Microbiol. 2010, 76, 2778–2782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maliehe, T.S. Production, Characterisation and Application of Bioflocculants from Pure Bacterial Strains and their Consortia Isolated from Sodwana Bay in Kwa-Zulu Natal, South Africa. Ph.D. Thesis, University of Zululand, Richards Bay, South Africa, 2018. [Google Scholar]
- Oladele, A.M. Studies on Bioflocculants Produced by Three Freshwater Actinomycetes (Streptomyces Sp. Gansen, Cellulomonas Sp, Bola and Brachybacterium Sp, UFH) Isolated from Tyume River. Available online: http://hdl.handle.net/10353/6550 (accessed on 27 April 2011).
- Zhong, C.; Cao, G.; Rong, K.; Xia, Z.; Peng, T.; Chen, H.; Zhou, J. Characterization of a microbial polysaccharide-based bioflocculant and its anti-inflammatory and pro-coagulant activity. Colloids Surf. B Biointerfaces 2018, 161, 636–644. [Google Scholar] [CrossRef]
- Akapo, C.S.O. Production and Characterisation of Bioflocculant Produced by Bacterial Isolates from Richards Bay Harbour, Kwazulu Natal. Ph.D. Thesis, University of Zululand, Richards Bay, South Africa, 2019. [Google Scholar]
- Sepehri, A.; Sarrafzadeh, M.-H.; Avateffazeli, M. Interaction between Chlorella vulgaris and nitrifying-enriched activated sludge in the treatment of wastewater with low C/N ratio. J. Clean. Prod. 2020, 247, 119164. [Google Scholar] [CrossRef]
- Giri, S.S.; Harshiny, M.; Sen, S.S.; Sukumaran, V.; Park, S.C. Production and characterization of a thermostable bioflocculant from Bacillus subtilis F9, isolated from wastewater sludge. Ecotoxicol. Environ. Saf. 2015, 121, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Pu, S.; Ma, H.; Deng, D.; Xue, S.; Zhu, R.; Zhou, Y.; Xiong, X. Isolation, identification, and characterization of an Aspergillus niger bioflocculant-producing strain using potato starch wastewater as nutrilite and its application. PLoS ONE 2018, 13, e0190236. [Google Scholar] [CrossRef] [PubMed]
- Mathias, D.; Hammantola, S.D.; Ishaku, G.A. Isolation and characterization of bioflocculant-producing bacteria from wastewater at Jimeta, Adamawa State. J. Adv. Biol. Biotechnol. 2017, 15, 1–7. [Google Scholar] [CrossRef]
- Jiang, J.; Liu, L.; Nie, W.; Chen, Y.; Wang, Z. Screening of a high bioflocculant-producing bacterial strain from an intensive fish pond and comparison of the bioflocculation effects with Rhodococcus erythropolis. Aquac. Res. 2019, 50, 1047–1056. [Google Scholar] [CrossRef]
- Ugbenyen, A.M.; Simonis, J.J.; Basson, A.K. Screening for Bioflocculant-Producing Bacteria from the Marine Environment of Sodwana Bay, South Africa. Ann. Sci. Technol. 2018, 3, 16–20. [Google Scholar] [CrossRef] [Green Version]
- Cupples, A.M.; Sims, G.K. Identification of in situ 2, 4-dichlorophenoxyacetic acid-degrading soil microorganisms using DNA-stable isotope probing. Soil Biol. Biochem. 2007, 39, 232–238. [Google Scholar] [CrossRef]
- Abu Tawila, Z.M.; Ismail, S.; Dadrasnia, A.; Usman, M.M. Production and characterization of a bioflocculant produced by Bacillus salmalaya 139SI-7 and its applications in wastewater treatment. Molecules 2018, 23, 2689. [Google Scholar]
- Xia, S.; Zhang, Z.; Wang, X.; Yang, A.; Chen, L.; Zhao, J.; Leonard, D.; Jaffrezic-Renault, N. Production and characterization of a bioflocculant by Proteus mirabilis TJ-1. Bioresour. Technol. 2008, 99, 6520–6527. [Google Scholar] [CrossRef]
- Liu, W.; Wang, K.; Li, B.; Yuan, H.; Yang, J. Production and characterization of an intracellular bioflocculant by Chryseobacterium daeguense W6 cultured in low nutrition medium. Bioresour. Technol. 2010, 101, 1044–1048. [Google Scholar] [CrossRef]
- Adebami, G.; Adebayo-Tayo, B. Comparative effect of medium composition on bioflocculant production by microorganisms isolated from wastewater samples. Rep. Opin. 2013, 5, 46–53. [Google Scholar]
- Maliehe, T.S.; Basson, A.K.; Dlamini, N.G. Removal of pollutants in mine wastewater by a non-cytotoxic polymeric bioflocculant from Alcaligenes faecalis HCB2. Int. J. Environ. Res. Public Health 2019, 16, 4001. [Google Scholar]
- Dlamini, N.G.; Basson, A.K.; Pullabhotla, V. Biosynthesis and characterization of copper nanoparticles using a bioflocculant extracted from Alcaligenis faecalis HCB2. Adv. Sci. Eng. Med. 2019, 11, 1064–1070. [Google Scholar] [CrossRef]
- Mu, J.; Zhou, H.; Chen, Y.; Yang, G.; Cui, X. Revealing a novel natural bioflocculant resource from Ruditapes philippinarum: Effective polysaccharides and synergistic flocculation. Carbohydr. Polym. 2018, 186, 17–24. [Google Scholar] [PubMed]
- Devi, K.K.; Natarajan, K. Isolation and characterization of a bioflocculant from Bacillus megaterium for turbidity and arsenic removal. Min. Metall. Explor. 2015, 32, 222–229. [Google Scholar]
- Jing, Y.; Cui, X.; Chen, Z.; Huang, L.; Song, L.; Liu, T.; Lv, W.; Yu, R. Elucidation and biological activities of a new polysaccharide from cultured Cordyceps militaris. Carbohydr. Polym. 2014, 102, 288–296. [Google Scholar] [CrossRef]
- Luo, Z.; Chen, L.; Chen, C.; Zhang, W.; Liu, M.; Han, Y.; Zhou, J. Production and characteristics of a bioflocculant by Klebsiella pneumoniae YZ-6 isolated from human saliva. Appl. Biochem. Biotechnol. 2014, 172, 1282–1292. [Google Scholar] [CrossRef]
- Dlamini, N.G.; Basson, A.K.; Pullabhotla, R.V. Green Synthesis of Iron Nanoparticles by a Polysaccharide Bioflocculant from Marine Alcaligenes faecalis HCB2 and Characterization. Adv. Sci. Eng. Med. 2020, 12, 1034–1039. [Google Scholar]
- Rasulov, B.A.; Li, L.; Liu, Y.-H.; Mohamad, O.A.; Xiao, M.; Ma, J.-B.; Li, W.-J. Production, characterization and structural modification of exopolysaccharide-based bioflocculant by Rhizobium radiobacter SZ4S7S14 and media optimization. 3 Biotech 2017, 7, 179. [Google Scholar] [CrossRef]
- Cosa, S.; Okoh, A. Bioflocculant Production by a Consortium of Two Bacterial Species and Its Potential Application in Industrial Wastewater and River Water Treatment. Pol. J. Environ. Stud. 2014, 23, 689–696. [Google Scholar]
- Gomaa, E.Z. Production and characteristics of a heavy metals removing bioflocculant produced by Pseudomonas aeruginosa. Pol. J. Microbiol. 2012, 61, 281–289. [Google Scholar]
- Masuku, S.K. Synthesis and Application of a Grafted Flocculant Produced from a Chemical Combination of a Bioflocculant TKT and Acrylamide (AM). Ph.D. Thesis, University of Zululand, Richards Bay, South Africa, 2019. [Google Scholar]
- Makapela, B.; Okaiyeto, K.; Ntozonke, N.; Nwodo, U.U.; Green, E.; Mabinya, L.V.; Okoh, A.I. Assessment of Bacillus pumilus isolated from fresh water milieu for bioflocculant production. Appl. Sci. 2016, 6, 211. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, J.N.; Wan Dagang, W.R.Z. Implications for industrial application of bioflocculant demand alternatives to conventional media: Waste as a substitute. Water Sci. Technol. 2019, 80, 1807–1822. [Google Scholar] [PubMed]
- Li, Z.; Zhong, S.; Lei, H.-y.; Chen, R.-w.; Yu, Q.; Li, H.-L. Production of a novel bioflocculant by Bacillus licheniformis X14 and its application to low temperature drinking water treatment. Bioresour. Technol. 2009, 100, 3650–3656. [Google Scholar] [PubMed]
- Ugbenyen, A.; Cosa, S.; Mabinya, L.; Babalola, O.O.; Aghdasi, F.; Okoh, A. Thermostable bacterial bioflocculant produced by Cobetia spp. isolated from Algoa Bay (South Africa). Int. J. Environ. Res. Public Health 2012, 9, 2108–2120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahadat, M.; Teng, T.T.; Rafatullah, M.; Shaikh, Z.; Sreekrishnan, T.; Ali, S.W. Bacterial bioflocculants: A review of recent advances and perspectives. Chem. Eng. J. 2017, 328, 1139–1152. [Google Scholar]
- Zheng, Y.; Ye, Z.-L.; Fang, X.-L.; Li, Y.-H.; Cai, W.-M. Production and characteristics of a bioflocculant produced by Bacillus sp. F19. Bioresour. Technol. 2008, 99, 7686–7691. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Z.; Wang, S.; Jiang, P. Characterization of a bioflocculant produced by the marine myxobacterium Nannocystis sp. NU-2. Appl. Microbiol. Biotechnol. 2002, 59, 517–522. [Google Scholar] [PubMed]
- Sheng, Y.; Zhang, Q.; Sheng, Y.; Li, C.; Wang, H. Screening and flocculating properties of bioflocculant-producing microorganisms. J. Univ. Sci. Technol. Beijing Miner. Metall. Mater. 2006, 13, 289–292. [Google Scholar] [CrossRef]
- Vimala, R.; Escaline, J.L.; Sivaramakrishnan, S. Characterization of self-assembled bioflocculant from the microbial consortium and its applications. J. Environ. Manag. 2020, 258, 110000. [Google Scholar] [CrossRef] [PubMed]
- Cosa, S.; Mabinya, L.V.; Olaniran, A.O.; Okoh, O.O.; Bernard, K.; Deyzel, S.; Okoh, A.I. Bioflocculant production by Virgibacillus sp. Rob isolated from the bottom sediment of Algoa Bay in the Eastern Cape, South Africa. Molecules 2011, 16, 2431–2442. [Google Scholar] [CrossRef] [PubMed]
- Li-Fan, L.; Cheng, W. Characteristics and culture conditions of a bioflocculant produced by Penicillium sp. Biomed. Environ. Sci. 2010, 23, 213–218. [Google Scholar]
- Ntsangani, N.; Okaiyeto, K.; Uchechukwu, N.U.; Olaniran, A.O.; Mabinya, L.V.; Okoh, A.I. Bioflocculation potentials of a uronic acid-containing glycoprotein produced by Bacillus sp. AEMREG4 isolated from Tyhume River, South Africa. 3 Biotech 2017, 7, 78. [Google Scholar] [CrossRef] [Green Version]
- Sekelwa, C.; Anthony, U.M.; Vuyani, M.L.; Anthony, O.I. Characterization of a thermostable polysaccharide bioflocculant produced by Virgibacillus species isolated from Algoa bay. Afr. J. Microbiol. Res. 2013, 7, 2925–2938. [Google Scholar]
- Chen, Z.; Liu, P.; Li, Z.; Yu, W.; Wang, Z.; Yao, H.; Wang, Y.; Li, Q.; Deng, X.; He, N. Identification of key genes involved in polysaccharide bioflocculant synthesis in Bacillus licheniformis. Biotechnol. Bioeng. 2017, 114, 645–655. [Google Scholar] [CrossRef]
- Agunbiade, M.O.; Van Heerden, E.; Pohl, C.H.; Ashafa, A.T. Flocculating performance of a bioflocculant produced by Arthrobacter humicola in sewage waste water treatment. BMC Biotechnol. 2017, 17, 51. [Google Scholar] [CrossRef]
- Yim, J.H.; Kim, S.J.; Ahn, S.H.; Lee, H.K. Characterization of a novel bioflocculant, p-KG03, from a marine dinoflagellate, Gyrodinium impudicum KG03. Bioresour. Technol. 2007, 98, 361–367. [Google Scholar] [CrossRef]
- Buthelezi, S.P.; Olaniran, A.O.; Pillay, B. Textile dye removal from wastewater effluents using bioflocculants produced by indigenous bacterial isolates. Molecules 2012, 17, 14260–14274. [Google Scholar] [CrossRef] [Green Version]
- Nie, M.; Yin, X.; Jia, J.; Wang, Y.; Liu, S.; Shen, Q.; Li, P.; Wang, Z. Production of a novel bioflocculant MNXY1 by Klebsiella pneumoniae strain NY1 and application in precipitation of cyanobacteria and municipal wastewater treatment. J. Appl. Microbiol. 2011, 111, 547–558. [Google Scholar]
- Zulkeflee, Z.; Shamsuddin, Z.H.; Aris, A.Z.; Yusoff, M.K.; Komilis, D.; Sánchez, A. Glutamic acid independent production of bioflocculants by Bacillus subtilis UPMB13. Environ. Process. 2016, 3, 353–367. [Google Scholar] [CrossRef] [Green Version]
- Adebayo-Tayo, B.C.; Adebami, G.E. Production, characterization and effect of cultural condition on bioflocculant produced by Alcaligenes aquatilis AP4. J. Appl. Life Sci. Int. 2017, 14, 1–12. [Google Scholar] [CrossRef]
- Luvuyo, N.; Nwodo, U.U.; Mabinya, L.V.; Okoh, A.I. Studies on bioflocculant production by a mixed culture of Methylobacterium sp. Obi and Actinobacterium sp. Mayor. BMC Biotechnol. 2013, 13, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayangbenro, A.S.; Babalola, O.O.; Aremu, O.S. Bioflocculant production and heavy metal sorption by metal resistant bacterial isolates from gold mining soil. Chemosphere 2019, 231, 113–120. [Google Scholar] [CrossRef]
- AMBARSARI, L.; ARTIKA, I.M.; SUSANTI, H.E. Characterization of bioflocculant producing-bacteria isolated from tapioca waste water. HAYATI J. Biosci. 2011, 18, 193–196. [Google Scholar]
- Ntsaluba, L.; Agunbiade, M.; Mabinya, L.; Okoh, A. Studies on bioflocculant production by Methylobacterium sp. Obi isolated from a freshwater environment in South Africa. Afr. J. Microbiol. Res. 2011, 5, 4533–4540. [Google Scholar] [CrossRef]
- Lin, J.; Harichund, C. Isolation and characterization of heavy metal removing bacterial bioflocculants. Afr. J. Microbiol. Res. 2011, 5, 599–607. [Google Scholar]
- Okaiyeto, K.; Nwodo, U.U.; Okoli, A.S.; Leonard, L.V.L.; Okoh, A.I. Studies on bioflocculant production by Bacillus sp. AEMREG7. Pol. J. Environ. Stud. 2016, 25, 241–250. [Google Scholar] [CrossRef]
- Ma, L.; Liang, J.; Liu, Y.; Zhang, Y.; Ma, P.; Pan, Z.; Jiang, W. Production of a bioflocculant from Enterobacter sp. P3 using brewery wastewater as substrate and its application in fracturing flowback water treatment. Environ. Sci. Pollut. Res. 2020, 27, 18242–18253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-Q.; Bo, L.; Xia, S.-Q.; Wang, X.-J.; YANG, A.-M. Production and application of a novel bioflocculant by multiple-microorganism consortia using brewery wastewater as carbon source. J. Environ. Sci. 2007, 19, 667–673. [Google Scholar] [CrossRef]
- Tang, W.; Song, L.; Li, D.; Qiao, J.; Zhao, T.; Zhao, H. Production, characterization, and flocculation mechanism of cation independent, pH tolerant, and thermally stable bioflocculant from Enterobacter sp. ETH-2. PLoS ONE 2014, 9, e114591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.; Liu, H.; Zhou, J. Characterization of a bioflocculant MBF-5 by Klebsiella pneumoniae and its application in Acanthamoeba cysts removal. Bioresour. Technol. 2013, 137, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Salam, A.E.; Abd-El-Haleem, D.; Youssef, A.S.; Zaki, S.; Abu-Elreesh, G.; El-Assar, S.A. Isolation, characterization, optimization, immobilization and batch fermentation of bioflocculant produced by Bacillus aryabhattai strain PSK1. J. Genet. Eng. Biotechnol. 2017, 15, 335–344. [Google Scholar] [CrossRef]
- Dermlim, W.; Prasertsan, P.; Doelle, H. Screening and characterization of bioflocculant produced by isolated Klebsiella sp. Appl. Microbiol. Biotechnol. 1999, 52, 698–703. [Google Scholar]
- Guo, H.; Hong, C.; Zheng, B.; Lu, F.; Jiang, D.; Qin, W. Bioflocculants’ production in a biomass-degrading bacterium using untreated corn stover as carbon source and use of bioflocculants for microalgae harvest. Biotechnol. Biofuels 2017, 10, 306. [Google Scholar] [CrossRef] [Green Version]
- Bisht, V.; Lal, B. Exploration of performance kinetics and mechanism of action of a potential novel bioflocculant BF-VB2 on clay and dye wastewater flocculation. Front. Microbiol. 2019, 10, 1288. [Google Scholar] [CrossRef] [Green Version]
- Okaiyeto, K.; Nwodo, U.U.; Mabinya, L.V.; Okoh, A.I. Bacillus toyonensis strain AEMREG6, a bacterium isolated from South African marine environment sediment samples produces a glycoprotein bioflocculant. Molecules 2015, 20, 5239–5259. [Google Scholar] [CrossRef]
- Tlou, N.S. Characterization of Selected Microbial Species for Bioflocculant Producing Potential and Comparison with Traditional Flocculants in Industrial Waste Water Treatment. Ph.D. Thesis, University of Zululand, Richards Bay, South Africa, 2017. [Google Scholar]
- Pathak, M.; Devi, A.; Bhattacharyya, K.; Sarma, H.; Subudhi, S.; Lal, B. Production of a non-cytotoxic bioflocculant by a bacterium utilizing a petroleum hydrocarbon source and its application in heavy metal removal. RSC Adv. 2015, 5, 66037–66046. [Google Scholar] [CrossRef]
- He, J.; Zou, J.; Shao, Z.; Zhang, J.; Liu, Z.; Yu, Z. Characteristics and flocculating mechanism of a novel bioflocculant HBF-3 produced by deep-sea bacterium mutant Halomonas sp. V3a’. World J. Microbiol. Biotechnol. 2010, 26, 1135–1141. [Google Scholar] [CrossRef]
- Zaki, S.A.; Elkady, M.F.; Farag, S.; Abd-El-Haleem, D. Characterization and flocculation properties of a carbohydrate bioflocculant from a newly isolated Bacillus velezensis 40B. J. Environ. Biol. 2013, 34, 51. [Google Scholar] [PubMed]


| Ingredients | Amount (g) |
|---|---|
| Glucose | 20.0 |
| K2HPO4 | 5.0 |
| K2PO4 | 2.0 |
| Urea | 0.5 |
| Yeast extract | 0.5 |
| MgSO4·7H2O | 0.2 |
| (NH4)2SO4 | 0.2 |
| NaCl | 0.1 |
| Activated sludge water (filtered) | 1 Litre |
| Sample | Un-Diluted (CFU/mL) | 10−1 (CFU/mL) | 10−2 (CFU/mL) |
|---|---|---|---|
| Wastewater | TN | TN | 8 × 103 |
| Inoculation Volume (%) | FA (%) ± SD | Carbon Sources | FA (%) ± SD | Nitrogen Source | FA (%) ± SD | Cations | FA (%) ± SD |
|---|---|---|---|---|---|---|---|
| 1 | 80.5 ± 0.41 a | Lactose | 52.7 ± 0.92 a | Casein | 52.4 ± 2.48 a | Control | 46.2 ± 0.13 a |
| 2 | 89.2 ± 0.14 b | Galactose | 56.1 ± 0.98 a | Ammonium sulphate | 64.3 ± 1.32 b | Li+ | 65.7 ± 1.15 b |
| 3 | 90.2 ± 0.14 c | Xylose | 63.9 ± 1.34 b | Ammonium acetate | 66.9 ± 0.17 b,c | K+ | 66.7 ± 0.05 b |
| 4 | 63.3 ± 0.15 d | Maltose | 71.7 ± 4.45 c | Ammonium chloride | 77.2 ± 0.57 d | Na+ | 86.1 ± 1.86 c |
| 5 | 49.4 ± 0.04 e | Fructose | 83.4 ± 2.59 d | Urea | 90.1 ± 2.39 e | Ba2+ | 90.4 ± 0.08 d |
| - | - | Starch | 83.8 ± 0.99 d,e | Yeast extract | 91.4 ± 0.41 e,f | Ca2+ | 90.8 ± 0.02 d,e |
| - | - | Glucose | 89.3 ± 1.68 d,f | Peptone | 92.1 ± 0.11 e,g | Mg2+ | 93.1 ± 0.02 e,f |
| - | - | Sucrose | 94.7 ± 3.10 e | Mixture of nitrogen | 63.1 ± 0.44 b,h | Mn2+ | 95.1 ± 0.25 f,g |
| - | - | Mixture (CHO) | 68.9 ± 1. 3 b,c,h | - | - | Fe3+ | 21.1 ± 1.01 h |
| Sample | Concentration (%) |
|---|---|
| Total sugar | 64.25% |
| Total protein | 10.42% |
| Uronic acid | 23.51% |
| Dosage (mg/mL) | FA (%) ± SD | pH | FA (%) ± SD | FA (%) ± SD | Metal Ions |
|---|---|---|---|---|---|
| 0.2 | 57.1 ± 5.78 a | 3 | 81.1 ± 0.07 a | Na+ | 63.3 ± 0.15 a |
| 0.4 | 81.9 ±1.96 b | 4 | 86.4 ± 1.35 b | Li+ | 65.3 ± 0.13 a |
| 0.6 | 80.8 ± 6.31 b,c | 5 | 88.9 ± 0.48 c | K+ | 64.0 ± 0.21 a |
| 0.8 | 65.5 ± 1.75 a | 6 | 97.4 ± 0.05 d | Ca2+ | 70.2 ± 0.11 b |
| 1.0 | 49.4 ± 1.62 a,d | 7 | 93.3 ± 0.32 e | Ba2+ | 77.2 ± 0.42 c |
| - | - | 8 | 80.2 ± 0.23 a | Mn2+ | 98.4 ± 0.13 d |
| - | - | 9 | 78.2 ± 0.14 a,f | Mg2+ | 98.3 ± 0.14 d |
| - | - | 10 | 65.6 ± 1.86 g | Fe3+ | 90.2 ± 0.16 e |
| - | - | 11 | 60.4 ± 0.58 h | Control | 43.3 ± 0.10 f |
| - | - | 12 | 63.7 ± 0.45 g,i | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nkosi, N.C.; Basson, A.K.; Ntombela, Z.G.; Maliehe, T.S.; Pullabhotla, R.V.S.R. Isolation, Identification and Characterization of Bioflocculant-Producing Bacteria from Activated Sludge of Vulindlela Wastewater Treatment Plant. Appl. Microbiol. 2021, 1, 586-606. https://doi.org/10.3390/applmicrobiol1030038
Nkosi NC, Basson AK, Ntombela ZG, Maliehe TS, Pullabhotla RVSR. Isolation, Identification and Characterization of Bioflocculant-Producing Bacteria from Activated Sludge of Vulindlela Wastewater Treatment Plant. Applied Microbiology. 2021; 1(3):586-606. https://doi.org/10.3390/applmicrobiol1030038
Chicago/Turabian StyleNkosi, Nkanyiso Celukuthula, Albertus K. Basson, Zuzingcebo G. Ntombela, Tsolanku S. Maliehe, and Rajasekhar V. S. R. Pullabhotla. 2021. "Isolation, Identification and Characterization of Bioflocculant-Producing Bacteria from Activated Sludge of Vulindlela Wastewater Treatment Plant" Applied Microbiology 1, no. 3: 586-606. https://doi.org/10.3390/applmicrobiol1030038
APA StyleNkosi, N. C., Basson, A. K., Ntombela, Z. G., Maliehe, T. S., & Pullabhotla, R. V. S. R. (2021). Isolation, Identification and Characterization of Bioflocculant-Producing Bacteria from Activated Sludge of Vulindlela Wastewater Treatment Plant. Applied Microbiology, 1(3), 586-606. https://doi.org/10.3390/applmicrobiol1030038







