Nourishing the Human Holobiont to Reduce the Risk of Non-Communicable Diseases: A Cow’s Milk Evidence Map Example
Abstract
:1. Introduction
2. Advancing Knowledge of Milk and Milk Microbiota
3. Historical Influences of Milk Production Practices on Health and Safety
4. Regulating the Microimmunosome
5. Balancing the Immune System via the Microimmunosome
6. Effects of Raw Cow’s Milk on the Microimmunosome and Risk of Allergies and Asthma: Proof of Concept
- Immunologically active whey IgG increases the attachment/colonization of Bifidobacterium longum ssp. infantis (B. infantis) and increases colonization resistance against Campylobacter jejuni [60].
- Raw cow’s milk administered in a mouse ovalbumin-sensitized mouse model increases the prevalence of Lachnospiraceae UCG-001, Lachnospiraceae UCG-008, and Ruminiclostridium 5 (Clostridial clusters XIVa and IV) and increases butyrate producers while decreasing inflammation. In contrast, pasteurized cow’s milk produces the opposite effect, resulting in microbiome dysbiosis and elevated pro-inflammatory Proteobacteria. The protective effect of raw cow’s milk against ovalbumin-associated food allergy is heat sensitive. It is associated with immunological changes including a reduction in allergen specific Th2 cells responsiveness and an increase in Treg activity [56].
- Cow’s milk oligosaccharrides in combination with B. infantis, can correct diet-associated microbial dysbiosis, reduce gut permeability, and reduce inflammation [61].
- Raw cow’s milk prevents airway inflammation from developing in a mouse model of house dust mite-induced asthma [62].
- Using a mouse animal model, raw cow’s milk but not processed milk appears to have epigenetic effects on FoxP3+ T regulatory cells resulting in reduced allergic symptoms [63].
- A biomarker, Neu5Gc and antibodies produced against it, are associated with consumption of raw cow’s milk. This is a useful biomarker for predicting protection against allergies and asthma [59].
7. Approach for Creating the Evidence Map for Cow’s Milk
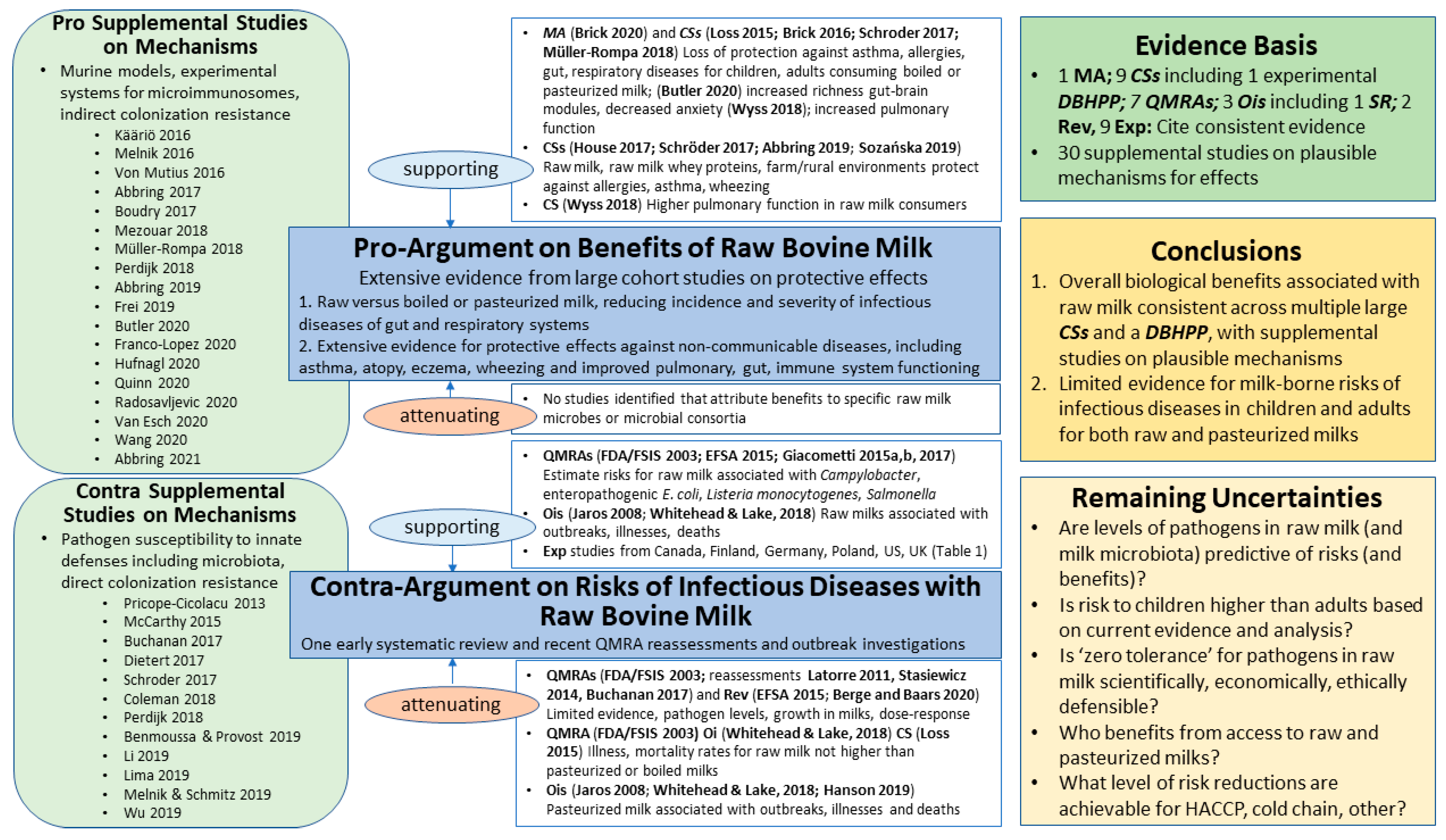
8. Results for Cow’s Milk Evidence Mapping
8.1. Benefits: Pro-Argument
8.1.1. Supporting
8.1.2. Attenuating
8.2. Risks: Contra-Argument
8.2.1. Supporting
8.2.2. Attenuating
8.3. Risk–Benefit Conclusions
8.4. Remaining Uncertainties
- Are the presence and level of potential pathogens in raw milk predictive of risks (illness)? Are the presence and levels of the natural milk microbiota (or smaller consortia) predictive of benefits (protection against infectious illness and NCDs)? Do we need metrics from monitoring of both potential pathogens and the core consortia of the milk microbiota to assess the balance of benefits and risks?
- Is the assumption that risk (likelihood and/or severity of infectious illness and NCDs) to children drinking raw milk is higher compared to adults supported by current evidence and analysis?
- Is ‘zero tolerance’ for pathogens (or their toxins) in raw milks scientifically, economically, and ethically defensible, given current evidence and analysis?
- Who benefits from access to raw, pasteurized and dry milks?
- What level of risk reduction can be achieved by HACCP programs, cold chain, and other farm management practices that maximize herd health and minimize: (i) frequency and duration of mastitis; and (ii) frequency and level of contamination by potential pathogens in raw milk from farm to table?
9. Opening Dialogue and Future Directions
9.1. Updating Preconceived Notions from 20th-Century Decision Science
9.2. Updating Preconceived Notions from 20th-Century Microbial Ecology
9.3. Updating Preconceived Notions from 20th-Century Immunology
9.4. Future Directions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dietert, R.R. Microbiome First Approaches to Rescue Public Health and Reduce Human Suffering. Biomedicines 2021, 9, 1581. [Google Scholar] [CrossRef]
- World Health Organization Preventing Noncommunicable Diseases. Available online: https://www.who.int/activities/improving-treatment-for-snakebite-patients (accessed on 27 August 2021).
- Yang, X.; Xie, L.; Li, Y.; Wei, C. More than 9,000,000 Unique Genes in Human Gut Bacterial Community: Estimating Gene Numbers inside a Human Body. PLoS ONE 2009, 4, e6074. [Google Scholar] [CrossRef] [Green Version]
- Henrick, B.M.; Rodriguez, L.; Lakshmikanth, T.; Pou, C.; Henckel, E.; Arzoomand, A.; Olin, A.; Wang, J.; Mikes, J.; Tan, Z.; et al. Bifidobacteria-Mediated Immune System Imprinting Early in Life. Cell 2021, 184, 3884–3898.e11. [Google Scholar] [CrossRef]
- Dietert, R.R. Microbiome First Medicine in Health and Safety. Biomedicines 2021, 9, 1099. [Google Scholar] [CrossRef]
- Dietert, R.R.; Dietert, J.M. Microbiome First Approaches in Pain Prevention and Management. Amer. J. Biomed. Sci. Res. 2021, 14, 184–192. [Google Scholar] [CrossRef]
- Dietert, R.R. The Microbiome-Immune-Host Defense Barrier Complex (Microimmunosome) and Developmental Programming of Noncommunicable Diseases. Reprod. Toxicol. Elmsford N 2017, 68, 49–58. [Google Scholar] [CrossRef]
- Vighi, G.; Marcucci, F.; Sensi, L.; Di Cara, G.; Frati, F. Allergy and the Gastrointestinal System. Clin. Exp. Immunol. 2008, 153 (Suppl. 1), 3–6. [Google Scholar] [CrossRef]
- Witkowska-Zimny, M.; Kaminska-El-Hassan, E. Cells of Human Breast Milk. Cell. Mol. Biol. Lett. 2017, 22, 11. [Google Scholar] [CrossRef]
- Oikonomou, G.; Addis, M.F.; Chassard, C.; Nader-Macias, M.E.F.; Grant, I.; Delbès, C.; Bogni, C.I.; Le Loir, Y.; Even, S. Milk Microbiota: What Are We Exactly Talking About? Front. Microbiol. 2020, 11, 60. [Google Scholar] [CrossRef] [Green Version]
- Parente, E.; Ricciardi, A.; Zotta, T. The Microbiota of Dairy Milk: A Review. Int. Dairy J. 2020, 107, 104714. [Google Scholar] [CrossRef]
- Zimmermann, P.; Curtis, N. Breast Milk Microbiota: A Review of the Factors That Influence Composition. J. Infect. 2020, 81, 17–47. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, Y.; Jay-Russell, M.; Lemay, D.G.; Mills, D.A. Reservoirs of Antimicrobial Resistance Genes in Retail Raw Milk. Microbiome 2020, 8, 99. [Google Scholar] [CrossRef] [PubMed]
- Urbaniak, C.; Cummins, J.; Brackstone, M.; Macklaim, J.M.; Gloor, G.B.; Baban, C.K.; Scott, L.; O’Hanlon, D.M.; Burton, J.P.; Francis, K.P.; et al. Microbiota of Human Breast Tissue. Appl. Environ. Microbiol. 2014, 80, 3007–3014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiménez, E.; de Andrés, J.; Manrique, M.; Pareja-Tobes, P.; Tobes, R.; Martínez-Blanch, J.F.; Codoñer, F.M.; Ramón, D.; Fernández, L.; Rodríguez, J.M. Metagenomic Analysis of Milk of Healthy and Mastitis-Suffering Women. J. Hum. Lact. Off. J. Int. Lact. Consult. Assoc. 2015, 31, 406–415. [Google Scholar] [CrossRef]
- Derakhshani, H.; Plaizier, J.C.; De Buck, J.; Barkema, H.W.; Khafipour, E. Composition of the Teat Canal and Intramammary Microbiota of Dairy Cows Subjected to Antimicrobial Dry Cow Therapy and Internal Teat Sealant. J. Dairy Sci. 2018, 101, 10191–10205. [Google Scholar] [CrossRef] [Green Version]
- Ruegg, P.L. A 100-Year Review: Mastitis Detection, Management, and Prevention. J. Dairy Sci. 2017, 100, 10381–10397. [Google Scholar] [CrossRef] [Green Version]
- USDA. Dairy 2014 Milk Quality, Milking Procedures, and Mastitis on US Dairies, 2014; USDA Animal Plant Health Inspection Service: Fort Collins, CO, USA, 2016.
- Patel, S.H.; Vaidya, Y.H.; Patel, R.J.; Pandit, R.J.; Joshi, C.G.; Kunjadiya, A.P. Culture Independent Assessment of Human Milk Microbial Community in Lactational Mastitis. Sci. Rep. 2017, 7, 7804. [Google Scholar] [CrossRef] [Green Version]
- Fitzstevens, J.L.; Smith, K.C.; Hagadorn, J.I.; Caimano, M.J.; Matson, A.P.; Brownell, E.A. Systematic Review of the Human Milk Microbiota. Nutr. Clin. Pract. Off. Publ. Am. Soc. Parenter. Enter. Nutr. 2017, 32, 354–364. [Google Scholar] [CrossRef]
- Scheu, A.; Powell, A.; Bollongino, R.; Vigne, J.-D.; Tresset, A.; Çakırlar, C.; Benecke, N.; Burger, J. The Genetic Prehistory of Domesticated Cattle from Their Origin to the Spread across Europe. BMC Genet. 2015, 16, 54. [Google Scholar] [CrossRef] [Green Version]
- Egan, M. Organizing Protest in the Changing City: Swill Milk and Social Activism in New York City, 1842–1864. N. Y. Hist. 2005, 86, 205–225. [Google Scholar]
- Obladen, M. From Swill Milk to Certified Milk: Progress in Cow’s Milk Quality in the 19th Century. Ann. Nutr. Metab. 2014, 64, 80–87. [Google Scholar] [CrossRef]
- Condran, G.A.; Crimmins, E. Mortality Differentials between Rural and Urban Areas of States in the Northeastern United States 1890–1900. J. Hist. Geogr. 1980, 6, 179–202. [Google Scholar] [CrossRef]
- Alsan, M.; Goldin, C. Watersheds in Child Mortality: The Role of Effective Water and Sewerage Infrastructure, 1880 to 1920. J. Polit. Econ. 2019, 127, 586–638. [Google Scholar] [CrossRef]
- Feigenbaum, J.J.; Muller, C.; Wrigley-Field, E. Regional and Racial Inequality in Infectious Disease Mortality in u.s. Cities, 1900–1948. Demography 2019, 56, 1371–1388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crimmins, E.M.; Condran, G.A. Mortality Variation in U.S. Cities in 1900: A Two-Level Explanation by Cause of Death and Underlying Factors. Soc. Sci. Hist. 1983, 7, 31–60. [Google Scholar] [CrossRef] [PubMed]
- Heckman, J.R. Securing Fresh Food from Fertile Soil, Challenges to the Organic and Raw Milk Movements. Renew. Agric. Food Syst. 2019, 34, 472–485. [Google Scholar] [CrossRef] [Green Version]
- Whitehead, J.; Lake, B. Recent Trends in Unpasteurized Fluid Milk Outbreaks, Legalization, and Consumption in the United States. PLoS Curr. 2018, 10, currents.outbreaks.bae5a0fd685616839c9cf857792730d1. [Google Scholar] [CrossRef]
- Brucker, R.M.; Bordenstein, S.R. The Hologenomic Basis of Speciation: Gut Bacteria Cause Hybrid Lethality in the Genus Nasonia. Science 2013, 341, 667–669. [Google Scholar] [CrossRef]
- Lim, S.J.; Bordenstein, S.R. An Introduction to Phylosymbiosis. Proc. Biol. Sci. 2020, 287, 20192900. [Google Scholar] [CrossRef] [Green Version]
- Dietert, R.R. Safety and Risk Assessment for the Human Superorganism. Hum. Ecol. Risk Assess. Int. J. 2017, 23, 1819–1829. [Google Scholar] [CrossRef] [Green Version]
- Dietert, R.R. A Focus on Microbiome Completeness and Optimized Colonization Resistance in Neonatology. NeoReviews 2018, 19, e78–e88. [Google Scholar] [CrossRef]
- Hill, C. RDA for Microbes—Are You Getting Your Daily Dose? Biochemist 2018, 40, 22–25. [Google Scholar] [CrossRef]
- Marco, M.L.; Hill, C.; Hutkins, R.; Slavin, J.; Tancredi, D.J.; Merenstein, D.; Sanders, M.E. Should There Be a Recommended Daily Intake of Microbes? J. Nutr. 2020, 150, 3061–3067. [Google Scholar] [CrossRef] [PubMed]
- Stein, R.R.; Bucci, V.; Toussaint, N.C.; Buffie, C.G.; Rätsch, G.; Pamer, E.G.; Sander, C.; Xavier, J.B. Ecological Modeling from Time-Series Inference: Insight into Dynamics and Stability of Intestinal Microbiota. PLoS Comput. Biol. 2013, 9, e1003388. [Google Scholar] [CrossRef] [PubMed]
- Buffie, C.G.; Bucci, V.; Stein, R.R.; McKenney, P.T.; Ling, L.; Gobourne, A.; No, D.; Liu, H.; Kinnebrew, M.; Viale, A.; et al. Precision Microbiome Reconstitution Restores Bile Acid Mediated Resistance to Clostridium Difficile. Nature 2015, 517, 205–208. [Google Scholar] [CrossRef] [Green Version]
- Becattini, S.; Pamer, E.G. Multifaceted Defense against Listeria Monocytogenes in the Gastro-Intestinal Lumen. Pathog. Basel Switz. 2017, 7, 1. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Covington, A.; Pamer, E.G. The Intestinal Microbiota: Antibiotics, Colonization Resistance, and Enteric Pathogens. Immunol. Rev. 2017, 279, 90–105. [Google Scholar] [CrossRef]
- Toscano, M.; De Grandi, R.; Peroni, D.G.; Grossi, E.; Facchin, V.; Comberiati, P.; Drago, L. Impact of Delivery Mode on the Colostrum Microbiota Composition. BMC Microbiol. 2017, 17, 205. [Google Scholar] [CrossRef]
- Phillips-Farfán, B.; Gómez-Chávez, F.; Medina-Torres, E.A.; Vargas-Villavicencio, J.A.; Carvajal-Aguilera, K.; Camacho, L. Microbiota Signals during the Neonatal Period Forge Life-Long Immune Responses. Int. J. Mol. Sci. 2021, 22, 8162. [Google Scholar] [CrossRef]
- McFadden, J.P.; Thyssen, J.P.; Basketter, D.A.; Puangpet, P.; Kimber, I. T Helper Cell 2 Immune Skewing in Pregnancy/Early Life: Chemical Exposure and the Development of Atopic Disease and Allergy. Br. J. Dermatol. 2015, 172, 584–591. [Google Scholar] [CrossRef]
- Uebelhoer, L.S.; Lancioni, C.L. CD4+ T Cell Activation during the Newborn Period: Barriers against and Pathways toward Th1 Immunity. Crit. Rev. Immunol. 2018, 38, 1–15. [Google Scholar] [CrossRef]
- Dietert, R.R.; Piepenbrink, M.S. The Managed Immune System: Protecting the Womb to Delay the Tomb. Hum. Exp. Toxicol. 2008, 27, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Walker, W.A.; Iyengar, R.S. Breast Milk, Microbiota, and Intestinal Immune Homeostasis. Pediatr. Res. 2015, 77, 220–228. [Google Scholar] [CrossRef] [Green Version]
- Ojo-Okunola, A.; Nicol, M.; du Toit, E. Human Breast Milk Bacteriome in Health and Disease. Nutrients 2018, 10, 1643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oddy, W.H. Breastfeeding, Childhood Asthma, and Allergic Disease. Ann. Nutr. Metab. 2017, 70 (Suppl. 2), 26–36. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, M.; Dimova, T.; Shey, M.; Briel, L.; Veldtsman, H.; Khomba, N.; Africa, H.; Steyn, M.; Hanekom, W.A.; Scriba, T.J.; et al. Fetal Public Vγ9Vδ2 T Cells Expand and Gain Potent Cytotoxic Functions Early after Birth. Proc. Natl. Acad. Sci. USA 2020, 117, 18638–18648. [Google Scholar] [CrossRef] [PubMed]
- Ravens, S.; Fichtner, A.S.; Willers, M.; Torkornoo, D.; Pirr, S.; Schöning, J.; Deseke, M.; Sandrock, I.; Bubke, A.; Wilharm, A.; et al. Microbial Exposure Drives Polyclonal Expansion of Innate Γδ T Cells Immediately after Birth. Proc. Natl. Acad. Sci. USA 2020, 117, 18649–18660. [Google Scholar] [CrossRef]
- Willers, M.; Ulas, T.; Völlger, L.; Vogl, T.; Heinemann, A.S.; Pirr, S.; Pagel, J.; Fehlhaber, B.; Halle, O.; Schöning, J.; et al. S100A8 and S100A9 Are Important for Postnatal Development of Gut Microbiota and Immune System in Mice and Infants. Gastroenterology 2020, 159, 2130–2145. [Google Scholar] [CrossRef]
- Zimmermann, J.; Macpherson, A.J. Breast Milk Modulates Transgenerational Immune Inheritance. Cell 2020, 181, 1202–1204. [Google Scholar] [CrossRef]
- Dharmage, S.C.; Perret, J.L.; Custovic, A. Epidemiology of Asthma in Children and Adults. Front. Pediatr. 2019, 7, 246. [Google Scholar] [CrossRef]
- American College of Allergy, Asthma, & Immunology Facts and Stats-50 Million Americans Have Allergies | ACAAI Patient. Available online: https://acaai.org/allergies/allergies-101/facts-stats/ (accessed on 4 November 2021).
- Seppo, A.E.; Bu, K.; Jumabaeva, M.; Thakar, J.; Choudhury, R.A.; Yonemitsu, C.; Bode, L.; Martina, C.A.; Allen, M.; Tamburini, S.; et al. Infant Gut Microbiome Is Enriched with Bifidobacterium Longum Ssp. Infantis in Old Order Mennonites with Traditional Farming Lifestyle. Allergy 2021, 76, 3489–3503. [Google Scholar] [CrossRef]
- Nguyen, M.; Holdbrooks, H.; Mishra, P.; Abrantes, M.A.; Eskew, S.; Garma, M.; Oca, C.-G.; McGuckin, C.; Hein, C.B.; Mitchell, R.D.; et al. Impact of Probiotic B. Infantis EVC001 Feeding in Premature Infants on the Gut Microbiome, Nosocomially Acquired Antibiotic Resistance, and Enteric Inflammation. Front. Pediatr. 2021, 9, 618009. [Google Scholar] [CrossRef] [PubMed]
- Abbring, S.; Engen, P.A.; Naqib, A.; Green, S.J.; Garssen, J.; Keshavarzian, A.; van Esch, B.C.A.M. Raw Milk-Induced Protection against Food Allergic Symptoms in Mice Is Accompanied by Shifts in Microbial Community Structure. Int. J. Mol. Sci. 2021, 22, 3417. [Google Scholar] [CrossRef] [PubMed]
- Abbring, S.; Ryan, J.T.; Diks, M.A.P.; Hols, G.; Garssen, J.; van Esch, B.C.A.M. Suppression of Food Allergic Symptoms by Raw Cow’s Milk in Mice Is Retained after Skimming but Abolished after Heating the Milk-a Promising Contribution of Alkaline Phosphatase. Nutrients 2019, 11, 1499. [Google Scholar] [CrossRef] [Green Version]
- Abbring, S.; Xiong, L.; Diks, M.A.P.; Baars, T.; Garssen, J.; Hettinga, K.; van Esch, B.C.A.M. Loss of Allergy-Protective Capacity of Raw Cow’s Milk after Heat Treatment Coincides with Loss of Immunologically Active Whey Proteins. Food Funct. 2020, 11, 4982–4993. [Google Scholar] [CrossRef] [PubMed]
- Frei, R.; Roduit, C.; Ferstl, R.; O’Mahony, L.; Lauener, R.P. Exposure of Children to Rural Lifestyle Factors Associated with Protection against Allergies Induces an Anti-Neu5Gc Antibody Response. Front. Immunol. 2019, 10, 1628. [Google Scholar] [CrossRef] [Green Version]
- Quinn, E.M.; Kilcoyne, M.; Walsh, D.; Joshi, L.; Hickey, R.M. A Whey Fraction Rich in Immunoglobulin G Combined with Bifidobacterium Longum Subsp. Infantis ATCC15697 Exhibits Synergistic Effects against Campylobacter Jejuni. Int. J. Mol. Sci. 2020, 21, 4632. [Google Scholar] [CrossRef]
- Boudry, G.; Hamilton, M.K.; Chichlowski, M.; Wickramasinghe, S.; Barile, D.; Kalanetra, K.M.; Mills, D.A.; Raybould, H.E. Bovine Milk Oligosaccharides Decrease Gut Permeability and Improve Inflammation and Microbial Dysbiosis in Diet-Induced Obese Mice. J. Dairy Sci. 2017, 100, 2471–2481. [Google Scholar] [CrossRef] [Green Version]
- Abbring, S.; Verheijden, K.A.T.; Diks, M.A.P.; Leusink-Muis, A.; Hols, G.; Baars, T.; Garssen, J.; van Esch, B.C.A.M. Raw Cow’s Milk Prevents the Development of Airway Inflammation in a Murine House Dust Mite-Induced Asthma Model. Front. Immunol. 2017, 8, 1045. [Google Scholar] [CrossRef] [Green Version]
- Abbring, S.; Wolf, J.; Ayechu-Muruzabal, V.; Diks, M.A.P.; Alhamwe, B.A.; Alhamdan, F.; Harb, H.; Renz, H.; Garn, H.; Garssen, J.; et al. Raw Cow’s Milk Reduces Allergic Symptoms in a Murine Model for Food Allergy-A Potential Role for Epigenetic Modifications. Nutrients 2019, 11, 1721. [Google Scholar] [CrossRef] [Green Version]
- Coleman, M.E.; North, D.W.; Dietert, R.R.; Stephenson, M.M. Examining Evidence of Benefits and Risks for Pasteurizing Donor Breastmilk. Appl. Microbiol. 2021, 1, 408–425. [Google Scholar] [CrossRef]
- Wiedemann, P.; Schütz, H.; Spangenberg, A.; Krug, H.F. Evidence Maps: Communicating Risk Assessments in Societal Controversies: The Case of Engineered Nanoparticles. Risk Anal. Off. Publ. Soc. Risk Anal. 2011, 31, 1770–1783. [Google Scholar] [CrossRef]
- Dietert, R.R.; Dietert, J.M. Twentieth Century Dogmas Prevent Sustainable Healthcare. Am. J. Biomed. Sci. Res. 2021, 13, 409–417. [Google Scholar] [CrossRef]
- Food and Drug Administration; Center for Food Safety and Applied Nutrition; US Department of Health and Human Services; Food Safety and Inspection Service & US Department of Agriculture (FDA/FSIS). Quantitative Assessment of Relative Risk to Public Health from Foodborne Listeria Monocytogenes among Selected Categories of Ready-to-Eat Foods; FDA: Silver Spring, MA, USA, 2003.
- Jaros, P.; Cogger, N.; French, N. A Systematic Review of the Human Disease Evidence Associated with the Consumption of Raw Milk and Raw Milk Cheeses. A Report Prepared for the New Zealand Food Safety Authority (NZFSA); Massey University: Palmerston North, New Zealand, 2008; p. 92. [Google Scholar]
- Latorre, A.A.; Pradhan, A.K.; Van Kessel, J.A.S.; Karns, J.S.; Boor, K.J.; Rice, D.H.; Mangione, K.J.; Gröhn, Y.T.; Schukken, Y.H. Quantitative Risk Assessment of Listeriosis Due to Consumption of Raw Milk. J. Food Prot. 2011, 74, 1268–1281. [Google Scholar] [CrossRef]
- Stasiewicz, M.J.; Martin, N.; Laue, S.; Gröhn, Y.T.; Boor, K.J.; Wiedmann, M. Responding to Bioterror Concerns by Increasing Milk Pasteurization Temperature Would Increase Estimated Annual Deaths from Listeriosis. J. Food Prot. 2014, 77, 696–712. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards (BIOHAZ). Scientific Opinion on the Public Health Risks Related to the Consumption of Raw Drinking Milk. EFSA J. 2015, 13, 3940. [Google Scholar] [CrossRef] [Green Version]
- Giacometti, F.; Bonilauri, P.; Amatiste, S.; Arrigoni, N.; Bianchi, M.; Losio, M.N.; Bilei, S.; Cascone, G.; Comin, D.; Daminelli, P.; et al. Human Campylobacteriosis Related to the Consumption of Raw Milk Sold by Vending Machines in Italy: Quantitative Risk Assessment Based on Official Controls over Four Years. Prev. Vet. Med. 2015, 121, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Giacometti, F.; Bonilauri, P.; Albonetti, S.; Amatiste, S.; Arrigoni, N.; Bianchi, M.; Bertasi, B.; Bilei, S.; Bolzoni, G.; Cascone, G.; et al. Quantitative Risk Assessment of Human Salmonellosis and Listeriosis Related to the Consumption of Raw Milk in Italy. J. Food Prot. 2015, 78, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Loss, G.; Depner, M.; Ulfman, L.H.; van Neerven, R.J.J.; Hose, A.J.; Genuneit, J.; Karvonen, A.M.; Hyvärinen, A.; Kaulek, V.; Roduit, C.; et al. Consumption of Unprocessed Cow’s Milk Protects Infants from Common Respiratory Infections. J. Allergy Clin. Immunol. 2015, 135, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Brick, T.; Schober, Y.; Böcking, C.; Pekkanen, J.; Genuneit, J.; Loss, G.; Dalphin, J.-C.; Riedler, J.; Lauener, R.; Nockher, W.A.; et al. ω-3 Fatty Acids Contribute to the Asthma-Protective Effect of Unprocessed Cow’s Milk. J. Allergy Clin. Immunol. 2016, 137, 1699–1706.e13. [Google Scholar] [CrossRef] [Green Version]
- Giacometti, F.; Bonilauri, P.; Piva, S.; Scavia, G.; Amatiste, S.; Bianchi, D.M.; Losio, M.N.; Bilei, S.; Cascone, G.; Comin, D.; et al. Paediatric HUS Cases Related to the Consumption of Raw Milk Sold by Vending Machine in Italy: Quantitative Risk Assessment Based on Escherichia Coli O157 Official Controls over 7 Years. Zoonoses Public Health 2017, 64, 505–516. [Google Scholar] [CrossRef]
- House, J.S.; Wyss, A.B.; Hoppin, J.A.; Richards, M.; Long, S.; Umbach, D.M.; Henneberger, P.K.; Beane Freeman, L.E.; Sandler, D.P.; Long O’Connell, E.; et al. Early-Life Farm Exposures and Adult Asthma and Atopy in the Agricultural Lung Health Study. J. Allergy Clin. Immunol. 2017, 140, 249–256.e14. [Google Scholar] [CrossRef] [Green Version]
- Schröder, P.C.; Illi, S.; Casaca, V.I.; Lluis, A.; Böck, A.; Roduit, C.; Depner, M.; Frei, R.; Genuneit, J.; Pfefferle, P.I.; et al. A Switch in Regulatory T Cells through Farm Exposure during Immune Maturation in Childhood. Allergy 2017, 72, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Müller-Rompa, S.E.K.; Markevych, I.; Hose, A.J.; Loss, G.; Wouters, I.M.; Genuneit, J.; Braun-Fahrländer, C.; Horak, E.; Boznanski, A.; Heederik, D.; et al. An Approach to the Asthma-Protective Farm Effect by Geocoding: Good Farms and Better Farms. Pediatr. Allergy Immunol. Off. Publ. Eur. Soc. Pediatr. Allergy Immunol. 2018, 29, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Wyss, A.B.; House, J.S.; Hoppin, J.A.; Richards, M.; Hankinson, J.L.; Long, S.; Henneberger, P.K.; Beane Freeman, L.E.; Sandler, D.P.; O’Connell, E.L.; et al. Raw Milk Consumption and Other Early-Life Farm Exposures and Adult Pulmonary Function in the Agricultural Lung Health Study. Thorax 2018, 73, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Abbring, S.; Kusche, D.; Roos, T.C.; Diks, M.A.P.; Hols, G.; Garssen, J.; Baars, T.; van Esch, B.C.A.M. Milk Processing Increases the Allergenicity of Cow’s Milk-Preclinical Evidence Supported by a Human Proof-of-Concept Provocation Pilot. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2019, 49, 1013–1025. [Google Scholar] [CrossRef] [Green Version]
- Hanson, H.; Whitfield, Y.; Lee, C.; Badiani, T.; Minielly, C.; Fenik, J.; Makrostergios, T.; Kopko, C.; Majury, A.; Hillyer, E.; et al. Listeria Monocytogenes Associated with Pasteurized Chocolate Milk, Ontario, Canada. Emerg. Infect. Dis. 2019, 25, 581–584. [Google Scholar] [CrossRef] [Green Version]
- Sozańska, B. Raw Cow’s Milk and Its Protective Effect on Allergies and Asthma. Nutrients 2019, 11, 469. [Google Scholar] [CrossRef] [Green Version]
- Berge, A.C.; Baars, T. Raw Milk Producers with High Levels of Hygiene and Safety. Epidemiol. Infect. 2020, 148, e14. [Google Scholar] [CrossRef] [Green Version]
- Brick, T.; Hettinga, K.; Kirchner, B.; Pfaffl, M.W.; Ege, M.J. The Beneficial Effect of Farm Milk Consumption on Asthma, Allergies, and Infections: From Meta-Analysis of Evidence to Clinical Trial. J. Allergy Clin. Immunol. Pract. 2020, 8, 878–889.e3. [Google Scholar] [CrossRef]
- Butler, M.I.; Bastiaanssen, T.F.S.; Long-Smith, C.; Berding, K.; Morkl, S.; Cusack, A.-M.; Strain, C.; Busca, K.; Porteous-Allen, P.; Claesson, M.J.; et al. Recipe for a Healthy Gut: Intake of Unpasteurised Milk Is Associated with Increased Lactobacillus Abundance in the Human Gut Microbiome. Nutrients 2020, 12, 1468. [Google Scholar] [CrossRef]
- Andrzejewska, M.; Szczepańska, B.; Śpica, D.; Klawe, J.J. Prevalence, Virulence, and Antimicrobial Resistance of Campylobacter Spp. in Raw Milk, Beef, and Pork Meat in Northern Poland. Foods 2019, 8, 420. [Google Scholar] [CrossRef] [Green Version]
- McLauchlin, J.; Aird, H.; Elliott, A.; Forester, E.; Jørgensen, F.; Willis, C. Microbiological Quality of Raw Drinking Milk and Unpasteurised Dairy Products: Results from England 2013-2019. Epidemiol. Infect. 2020, 148, e135. [Google Scholar] [CrossRef]
- Willis, C.; Jørgensen, F.; Aird, H.; Elviss, N.; Fox, A.; Jenkins, C.; Fenelon, D.; Sadler-Reeves, L.; McLauchlin, J. An Assessment of the Microbiological Quality and Safety of Raw Drinking Milk on Retail Sale in England. J. Appl. Microbiol. 2018, 124, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, M.M.; Coleman, M.E. Database of Primary Microbial Testing Program Data for Raw Milk Stored in Microsoft Access®. Final Report Submitted to Weston A. Price Foundation on 27 August, 2021. Available online: https://www.realmilk.com/safety/ (accessed on 8 March 2021).
- Castro, H.; Ruusunen, M.; Lindström, M. Occurrence and Growth of Listeria Monocytogenes in Packaged Raw Milk. Int. J. Food Microbiol. 2017, 261, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jaakkonen, A.; Castro, H.; Hallanvuo, S.; Ranta, J.; Rossi, M.; Isidro, J.; Lindström, M.; Hakkinen, M. Longitudinal Study of Shiga Toxin-Producing Escherichia Coli and Campylobacter Jejuni on Finnish Dairy Farms and in Raw Milk. Appl. Environ. Microbiol. 2019, 85, e02910-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Collo, L.P.; Karns, J.S.; Biswas, D.; Lombard, J.E.; Haley, B.J.; Kristensen, R.C.; Kopral, C.A.; Fossler, C.P.; Van Kessel, J.A.S. Prevalence, Antimicrobial Resistance, and Molecular Characterization of Campylobacter Spp. in Bulk Tank Milk and Milk Filters from US Dairies. J. Dairy Sci. 2017, 100, 3470–3479. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Nguyen, Q.D.; Tran, T.T.M.; Tang, M.T.; Tsuruta, T.; Nishino, N. Rumen Fluid, Feces, Milk, Water, Feed, Airborne Dust, and Bedding Microbiota in Dairy Farms Managed by Automatic Milking Systems. Anim. Sci. J. Nihon Chikusan Gakkaiho 2019, 90, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Marks, H.M.; Coleman, M.E.; Lin, C.T.J.; Roberts, T. Topics in Microbial Risk Assessment: Dynamic Flow Tree Process. Risk Anal. 1998, 18, 309–328. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission (CAC). Principles and Guidelines for the Conduct of Microbiological Risk Assessment. Available online: http://www.fao.org/3/y1579e/y1579e05.htm (accessed on 8 March 2021).
- Coleman, M.E.; Dietert, R.R.; North, D.W.; Stephenson, M.M. Enhancing Human Superorganism Ecosystem Resilience by Holistically ‘Managing Our Microbes’. Appl. Microbiol. 2021, 1, 471–497. [Google Scholar] [CrossRef]
- Coleman, M.E.; Sandberg, S.; Anderson, S.A. Impact of Microbial Ecology of Meat and Poultry Products on Predictions from Exposure Assessment Scenarios for Refrigerated Storage. Risk Anal. Off. Publ. Soc. Risk Anal. 2003, 23, 215–228. [Google Scholar] [CrossRef]
- Buchanan, R.L.; Gorris, L.G.M.; Hayman, M.M.; Jackson, T.C.; Whiting, R.C. A Review of Listeria Monocytogenes: An Update on Outbreaks, Virulence, Dose-Response, Ecology, and Risk Assessments. Food Control 2017, 75, 1–13. [Google Scholar] [CrossRef]
- Robertson, R.C.; Manges, A.R.; Finlay, B.B.; Prendergast, A.J. The Human Microbiome and Child Growth–First 1000 Days and Beyond. Trends Microbiol. 2019, 27, 131–147. [Google Scholar] [CrossRef] [Green Version]
- North, D.W. Uncertainties, Precaution, and Science: Focus on the State of Knowledge and How It May Change. Risk Anal. Int. J. 2011, 31, 1526–1529. [Google Scholar] [CrossRef] [PubMed]
- Blaser, M.J. The Microbiome Revolution. J. Clin. Invest. 2014, 124, 4162–4165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, B.J.K.; Li, C.X.; Nachman, K.E. A Literature Review of the Risks and Benefits of Consuming Raw and Pasteurized Cow’s Milk 2014. Available online: https://clf.jhsph.edu/sites/default/files/2019-05/a-literature-revie-of-the-risks-and-benefits-of-consuming-raw-milk.pdf (accessed on 15 January 2021).
- Lucey, J.A. Raw Milk Consumption: Risks and Benefits. Nutr. Today 2015, 50, 189–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verhagen, H.; Alonso-Andicoberry, C.; Assunção, R.; Cavaliere, F.; Eneroth, H.; Hoekstra, J.; Koulouris, S.; Kouroumalis, A.; Lorenzetti, S.; Mantovani, A.; et al. Risk-Benefit in Food Safety and Nutrition-Outcome of the 2019 Parma Summer School. Food Res. Int. Ott. Ont. 2021, 141, 110073. [Google Scholar] [CrossRef] [PubMed]
- Coleman, M.; Elkins, C.; Gutting, B.; Mongodin, E.; Solano-Aguilar, G.; Walls, I. Microbiota and Dose Response: Evolving Paradigm of Health Triangle. Risk Anal. 2018, 38, 2013–2028. [Google Scholar] [CrossRef] [PubMed]
- Collineau, L.; Boerlin, P.; Carson, C.A.; Chapman, B.; Fazil, A.; Hetman, B.; Smith, B.A. Integrating Whole-Genome Sequencing Data into Quantitative Risk Assessment of Foodborne Antimicrobial Resistance: A Review of Opportunities and Challenges. Front. Microbiol. 2019, 10, 1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischhoff, B.; Brewer, N.T.; Downs, J.S. Communicating Risks and Benefits: An Evidence-Based User’s Guide 2011; FDA: Silver Spring, MA, USA, 2011.
- Nauta, M.J.; Andersen, R.; Pilegaard, K.; Pires, S.M.; Ravn-Haren, G.; Tetens, I.; Poulsen, M. Meeting the Challenges in the Development of Risk-Benefit Assessment of Foods. Trends Food Sci. Technol. 2018, 76, 90–100. [Google Scholar] [CrossRef]
- Burns, C.J.; LaKind, J.S.; Mattison, D.R.; Alcala, C.S.; Branch, F.; Castillo, J.; Clark, A.; Clougherty, J.E.; Darney, S.P.; Erickson, H.; et al. A Matrix for Bridging the Epidemiology and Risk Assessment Gap. Glob. Epidemiol. 2019, 1, 100005. [Google Scholar] [CrossRef]
- National Research Council. Understanding Risk: Informing Decisions in a Democratic Society 1996. The National Academies Press. Available online: https://www.nap.edu/catalog/5138/understanding-risk-informing-decisions-in-a-democratic-society (accessed on 15 January 2021).
- North, D.W. Risk Analysis, Decision Analysis, Causal Analysis, and Economics: A Personal Perspective from More than 40 Years Experience. Risk Anal. 2020, 40, 2178–2190. [Google Scholar] [CrossRef]
- Dietert, R.R. The Human Superorganism: How the Microbiome Is Revolutionizing the Pursuit of a Healthy Life; Dutton: New York, NY, USA, 2016. [Google Scholar]
- Wang, S.; Ryan, C.A.; Boyaval, P.; Dempsey, E.M.; Ross, R.P.; Stanton, C. Maternal Vertical Transmission Affecting Early-Life Microbiota Development. Trends Microbiol. 2020, 28, 28–45. [Google Scholar] [CrossRef]
- Van Daele, E.; Knol, J.; Belzer, C. Microbial Transmission from Mother to Child: Improving Infant Intestinal Microbiota Development by Identifying the Obstacles. Crit. Rev. Microbiol. 2019, 45, 613–648. [Google Scholar] [CrossRef]
- Wang, J.; Kalyan, S.; Steck, N.; Turner, L.M.; Harr, B.; Künzel, S.; Ibrahim, S.M. Analysis of Intestinal Microbiota in Hybrid House Mice Reveals Evolutionary Divergence in a Vertebrate Hologenome. Nat. Commun. 2015, 6, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, W.; Hine, B.C.; Wallace, O.A.M.; Callaghan, M.; Bibiloni, R. Transfer of Intestinal Bacterial Components to Mammary Secretions in the Cow. PeerJ 2015, 3, e888. [Google Scholar] [CrossRef]
- Vuitton, D.A.; Dalphin, J.-C. From Farming to Engineering: The Microbiota and Allergic Diseases. Engineering 2017, 3, 98–109. [Google Scholar] [CrossRef]
- Curone, G.; Filipe, J.; Cremonesi, P.; Trevisi, E.; Amadori, M.; Pollera, C.; Castiglioni, B.; Turin, L.; Tedde, V.; Vigo, D.; et al. What We Have Lost: Mastitis Resistance in Holstein Friesians and in a Local Cattle Breed. Res. Vet. Sci. 2018, 116, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Carnes, M.U.; Butz, N.; Azcarate-Peril, M.A.; Richards, M.; Umbach, D.M.; Thorne, P.S.; Beane Freeman, L.E.; Peddada, S.D.; London, S.J. Exposures Related to House Dust Microbiota in a U.S. Farming Population. Environ. Health Perspect. 2018, 126, 067001. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, G.T.; Lynch, S.V.; Bloomberg, G.R.; Kattan, M.; Wood, R.A.; Gergen, P.J.; Jaffee, K.F.; Calatroni, A.; Bacharier, L.B.; Beigelman, A.; et al. Early-Life Home Environment and Risk of Asthma among Inner-City Children. J. Allergy Clin. Immunol. 2018, 141, 1468–1475. [Google Scholar] [CrossRef] [Green Version]
- Parajuli, A.; Grönroos, M.; Siter, N.; Puhakka, R.; Vari, H.K.; Roslund, M.I.; Jumpponen, A.; Nurminen, N.; Laitinen, O.H.; Hyöty, H.; et al. Urbanization Reduces Transfer of Diverse Environmental Microbiota Indoors. Front. Microbiol. 2018, 9, 84. [Google Scholar] [CrossRef] [Green Version]
- Andrews, T.; Neher, D.A.; Weicht, T.R.; Barlow, J.W. Mammary Microbiome of Lactating Organic Dairy Cows Varies by Time, Tissue Site, and Infection Status. PLoS ONE 2019, 14, e0225001. [Google Scholar] [CrossRef] [Green Version]
- Dhakal, S.; Wang, L.; Antony, L.; Rank, J.; Bernardo, P.; Ghimire, S.; Bondra, K.; Siems, C.; Lakshmanappa, Y.S.; Renu, S.; et al. Amish (Rural) vs. Non-Amish (Urban) Infant Fecal Microbiotas Are Highly Diverse and Their Transplantation Lead to Differences in Mucosal Immune Maturation in a Humanized Germfree Piglet Model. Front. Immunol. 2019, 10, 1509. [Google Scholar] [CrossRef] [Green Version]
- Haahtela, T. A Biodiversity Hypothesis. Allergy 2019, 74, 1445–1456. [Google Scholar] [CrossRef] [Green Version]
- Melnik, B.C.; Schmitz, G. Exosomes of Pasteurized Milk: Potential Pathogens of Western Diseases. J. Transl. Med. 2019, 17, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nero, L.A.; de Carvalho, A.F. Challenges for Production and Consumption of Raw Milk and Raw Milk Products. In Raw Milk; Elsevier: Amsterdam, The Netherlands, 2019; pp. 351–362. ISBN 978-0-12-810530-6. [Google Scholar]
- Ohtsuka, H.; Hirose, H.; Murakami, K.; Murata, R.; Kato, T.; Tajima, M. Relationship between Mrna of Immune Factors Expressed by Milk Somatic Cells and Bacteria Present in Healthy Lactating Holstein Cows. J. Vet. Res. 2019, 63, 369–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savin, K.W.; Zawadzki, J.; Auldist, M.J.; Wang, J.; Ram, D.; Rochfort, S.; Cocks, B.G. Faecalibacterium Diversity in Dairy Cow Milk. PLoS ONE 2019, 14, e0221055. [Google Scholar] [CrossRef] [Green Version]
- Franco-Lopez, J.; Duplessis, M.; Bui, A.; Reymond, C.; Poisson, W.; Blais, L.; Chong, J.; Gervais, R.; Rico, D.E.; Cue, R.I.; et al. Correlations between the Composition of the Bovine Microbiota and Vitamin B12 Abundance. mSystems 2020, 5, e00107-20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hettinga, K.; van Valenberg, H.; de Vries, S.; Boeren, S.; van Hooijdonk, T.; van Arendonk, J.; Vervoort, J. The Host Defense Proteome of Human and Bovine Milk. PLoS ONE 2011, 6, e19433. [Google Scholar] [CrossRef]
- Ward, T.L.; Hosid, S.; Ioshikhes, I.; Altosaar, I. Human Milk Metagenome: A Functional Capacity Analysis. BMC Microbiol. 2013, 13, 116. [Google Scholar] [CrossRef] [Green Version]
- Kuhn, T.S. The Structure of Scientific Revolutions; Fiftieth Anniversary; University of Chicago Press: Chicago, IL, USA, 2012. [Google Scholar]
- Pearl, J.; Mackenzie, D. The Book of Why: The New Science of Cause and Effect, 1st ed.; Basic Books: New York, NY, USA, 2018; ISBN 978-0-465-09760-9. [Google Scholar]
- North, D.W. Commentary on “Should Health Risks of Air Pollution Be Studied Scientifically? By Louis Anthony Cox, Jr.”. Glob. Epidemiol. 2020, 2, 100021. [Google Scholar] [CrossRef]

| Country (Reference) | Dates (State if US) | Campylobacter | E. coli O157:H7 or EHECs | L. monocytogenes | Salmonella |
|---|---|---|---|---|---|
| Canada (BCHA, 2021; website listed above) | 2015–2021 | 0/192 | 0/192 | 0/192 | 0/192 |
| Poland (Andrzejewska et al., 2019 [87]) | 2014–2018 | 0/113 vending machines; 26/221 (12%) C. jejuni, directly from farmers | Not Tested | Not Tested | Not Tested |
| UK (McLauchlin et al., 2020 [88]) | 2017–2019 | 18/635 (2.8%) | 0/58 O157; 3/304 EHEC (0%, 1%) | 1/642 (0.2%) | 3/622 (0.5%) |
| UK (Willis et al., 2018 [89]) | 2014–2016 (routine monitoring) | 2/770 (<0.01%) | 2/770 (<0.01%) | 2/770 >100 cfu/mL (<0.01%) | 0/770 |
| US State Monitoring (database of FOIA source data from licensed farms; Stephenson and Coleman, 2021 [90]) | 2009–2014 (CA) | 0/61 | 0/61 | 0/61 | 0/61 |
| 2009–2014 (NY) | 6/783 (0.7%) | 0/782 | 1/781 (0.1%) | 0/780 | |
| 2009–2014 (TX) | 4/601 (0.7%) | 0/596 | 4/596 (0.7%) | 11/606 (1.8%) | |
| 2012–2015 (WA) | 0/497 | 0/502 2/501 (0.4%) | 0/502 | 0/494 | |
| Germany (Berge & Baars, 2020 [84]) | 2001–2015 (VZM) | 7/2352 (0.3%) | 17/2737 (0.7%) | 30/2999 (1%) | 0/3367 |
| Germany (Berge & Baars, 2020 [84]) | 2001–2015 (not for direct consumption raw, pre-pasteurized) | 17/2258 (0.8%) | 82/5433 (1.5%) | 52/2355 (2.2%) | 0/1084 |
| Finland (Castro et al., 2017 [91]) | 2013–2015 | Not Tested | Not Tested | 5/105 retail bottles (4.8%) 2/115 bulk tanks (1.7%) | Not Tested |
| Finland (Jaakkonen et al., 2019 [92]) | 2014–2015 | 0/789 | 0/789 O157:H7; 2/789 O121:H19 (<1%) | Not Tested | Not Tested |
| US (Del Collo et al., 2017, [93]) | 2014 (17 states) | 13/234 culture; 27/234 PCR (6%; 12%) | Not Tested | Not Tested | Not Tested |
| OVERALL PERCENTAGE POSITIVE | 93/9740 (0.01%) | 26/10,934 (<0.01%) | 40/9118 (<0.01%) | 14/7976 (<0.01%) | |
|
|
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dietert, R.R.; Coleman, M.E.; North, D.W.; Stephenson, M.M. Nourishing the Human Holobiont to Reduce the Risk of Non-Communicable Diseases: A Cow’s Milk Evidence Map Example. Appl. Microbiol. 2022, 2, 25-52. https://doi.org/10.3390/applmicrobiol2010003
Dietert RR, Coleman ME, North DW, Stephenson MM. Nourishing the Human Holobiont to Reduce the Risk of Non-Communicable Diseases: A Cow’s Milk Evidence Map Example. Applied Microbiology. 2022; 2(1):25-52. https://doi.org/10.3390/applmicrobiol2010003
Chicago/Turabian StyleDietert, Rodney R., Margaret E. Coleman, D. Warner North, and Michele M. Stephenson. 2022. "Nourishing the Human Holobiont to Reduce the Risk of Non-Communicable Diseases: A Cow’s Milk Evidence Map Example" Applied Microbiology 2, no. 1: 25-52. https://doi.org/10.3390/applmicrobiol2010003






