Advances in Kombucha Tea Fermentation: A Review
Abstract
:1. Introduction
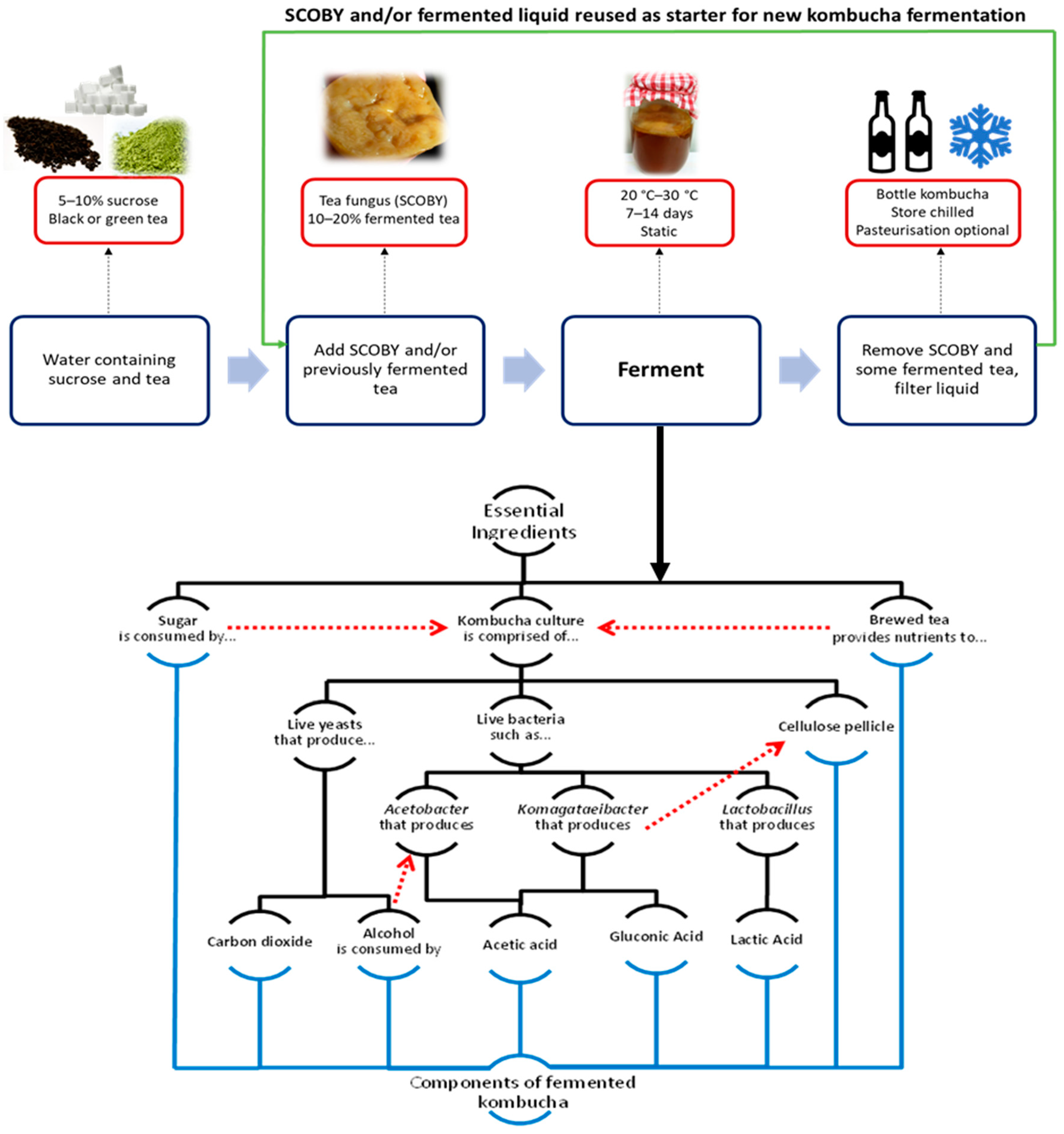
2. Kombucha Tea Production
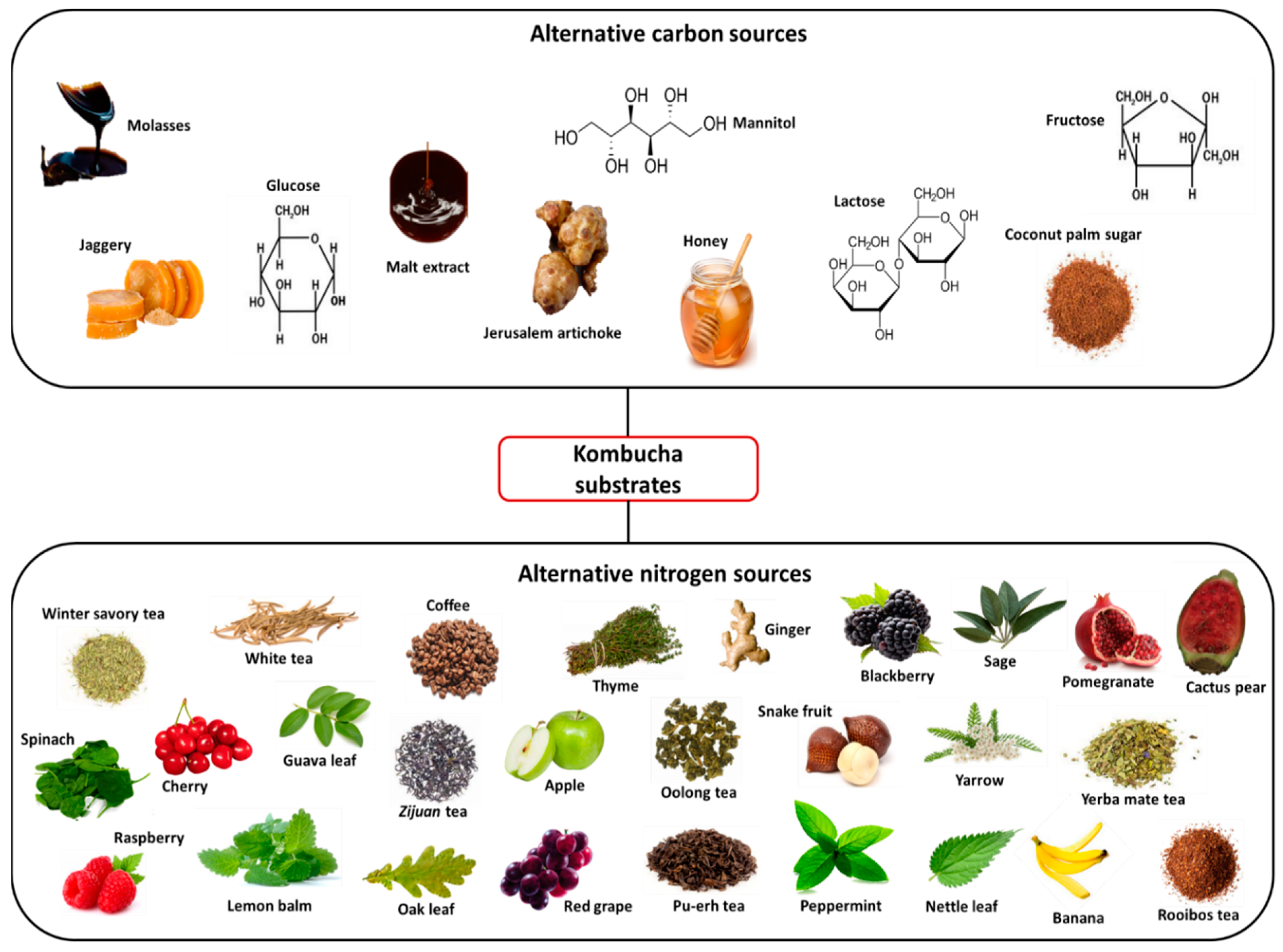
2.1. Alternative Substrates
2.2. Adjustment of Fermentation Conditions
3. Microbial Diversity
Use of Defined Starter Cultures
4. Potential Health Benefits
4.1. Clinical Trials
4.2. In Vitro and In Vivo Studies
5. Safety of Kombucha
5.1. Potential Toxicity
5.2. Risk Analysis of Kombucha Production
6. Kombucha Market
6.1. Market Analysis and Current Trends
6.2. Commercial Kombucha Products
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soares, M.G.; de Lima, M.; Reolon Schmidt, V.C. Technological aspects of kombucha, its applications and the symbiotic culture (SCOBY), and extraction of compounds of interest: A literature review. Trends Food Sci. Technol. 2021, 110, 539–550. [Google Scholar] [CrossRef]
- Greenwalt, C.J.; Steinkraus, K.H.; Ledford, R.A. Kombucha, the fermented tea: Microbiology, composition, and claimed health effects. J. Food Prot. 2000, 63, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Adhikari, K. Current trends in kombucha: Marketing perspectives and the need for improved sensory research. Beverages 2020, 6, 15. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Hsu, W.; Lee, F.; Liao, C. The isolation and identification of microbes from a fermented tea beverage, Haipao and their interactions during Haipao fermentation. Food Microbiol. 1996, 13, 407–415. [Google Scholar] [CrossRef]
- Mayser, P.; Fromme, S.; Leitzmann, G.; Gründer, K. The yeast spectrum of the ‘tea fungus kombucha’. Mycoses 1995, 38, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Marsh, A.J.; Sullivan, O.O.; Hill, C.; Ross, R.P.; Cotter, P.D. Sequence-based analysis of the bacterial and fungal compositions of multiple kombucha (tea fungus) samples. Food Microbiol. 2014, 38, 171–178. [Google Scholar] [CrossRef]
- Chakravorty, S.; Bhattacharya, S.; Chatzinotas, A.; Chakraborty, W.; Bhattacharya, D.; Gachhui, R. Kombucha tea fermentation: Microbial and biochemical dynamics. Int. J. Food Microbiol. 2016, 220, 63–72. [Google Scholar] [CrossRef]
- Sievers, M.; Lanini, C.; Weber, A.; Schuler-Schmid, U.; Teuber, M. Microbiology and fermentation balance in a kombucha beverage obtained from a tea fungus fermentation. Syst. Appl. Microbiol. 1995, 18, 590–594. [Google Scholar] [CrossRef]
- Jayabalan, R.; Malbaša, R.V.; Lončar, E.S.; Vitas, J.S.; Sathishkumar, M. A review on kombucha tea-microbiology, composition, fermentation, beneficial effects, toxicity, and tea fungus. Compr. Rev. Food Sci. Food Saf. 2014, 13, 538–550. [Google Scholar] [CrossRef]
- Villarreal-Soto, S.A.; Beaufort, S.; Bouajila, J.; Souchard, J.P.; Taillandier, P. Understanding kombucha tea fermentation: A review. J. Food Sci. 2018, 83, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Watawana, M.I.; Jayawardena, N.; Gunawardhana, C.B.; Waisundara, V.Y. Health, wellness, and safety aspects of the consumption of kombucha. J. Chem. 2015, 2015, 591869. [Google Scholar] [CrossRef] [Green Version]
- Fortune Business Insights Kombucha Market Size, Share & COVID-19 Impact Analysis, by Type (Natural and Flavored), Distribution Channel (Supermarkets/Hypermarkets, Convenience Stores, Health Stores, and Online Retail), and Regional Forecast, 2020–2027. Available online: https://www.fortunebusinessinsights.com/industry-reports/kombucha-market-100230 (accessed on 29 November 2021).
- Kapp, J.M.; Sumner, W. Kombucha: A systematic review of the empirical evidence of human health benefit. Ann. Epidemiol. 2019, 30, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.; Smolinske, S.; Greenbaum, D. Probable gastrointestinal toxicity of kombucha tea: Is this beverage healthy or harmful? J. Gen. Intern. Med. 1997, 12, 643–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gedela, M.; Potu, K.C.; Gali, V.L.; Alyamany, K.; Jha, L.K. A case of hepatotoxicity related to kombucha tea consumption. SD Med. 2016, 69, 26–28. [Google Scholar]
- Kole, A.; Jones, H.D.; Christensen, R.; Gladtsein, J. A case of kombucha tea toxicity. J. Intensiv. Care Med. 2009, 24, 205–207. [Google Scholar] [CrossRef] [PubMed]
- Perron, A.; Patterson, J.; Yanofsky, N.N. Kombucha “mushroom” hepatotoxicity. Ann. Emerg. Med. 1995, 26, 660–661. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.E.; Walia, K.; Farber, J.M. Safety aspects and guidance for consumers on the safe preparation, handling and storage of kombucha—A fermented tea beverage. Food Prot. Trends 2018, 38, 329–337. [Google Scholar]
- Goh, W.N.; Rosma, A.; Kaur, B.; Fazilah, A.; Karim, A.A.; Bhat, R. Fermentation of black tea broth (kombucha): I. Effects of sucrose concentration and fermentation time on the yield of microbial cellulose. Int. Food Res. J. 2012, 19, 109–117. [Google Scholar]
- Malbaša, R.; Lončar, E.; Djurić, M.; Došenović, I. Effect of sucrose concentration on the products of kombucha fermentation on molasses. Food Chem. 2008, 108, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Chatterjee, J. Efficient extraction strategies of tea (Camellia sinensis) biomolecules. J. Food Sci. Technol. 2015, 52, 3158–3168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harbowy, M.E.; Balentine, D.A.; Davies, A.P.; Cai, Y. Tea chemistry. CRC Crit. Rev. Plant Sci. 1997, 16, 415–480. [Google Scholar] [CrossRef]
- Roussin, M. Analyses of Kombucha Ffrments. Available online: https://research.kombuchabrewers.org/wp-content/uploads/kk-research-files/analyses-of-kombucha-ferments.pdf (accessed on 21 November 2021).
- Reiss, J. Influence of different sugars on the metabolism of the tea fungus. Z. Lebensm. Unters. Forsch. 1994, 198, 258–261. [Google Scholar] [CrossRef]
- Malbaša, R.; Lončar, E.; Djurić, M. Comparison of the products of kombucha fermentation on sucrose and molasses. Food Chem. 2008, 106, 1039–1045. [Google Scholar] [CrossRef]
- Muhialdin, B.J. Effects of sugar sources and fermentation time on the properties of tea fungus (kombucha) beverage. Int. Food Res. J. 2019, 26, 481–487. [Google Scholar]
- Vohra, B.M.; Fazry, S.; Sairi, F.; Babul-airianah, O. Effects of medium variation and fermentation time on the antioxidant and antimicrobial properties of kombucha. Malays. J. Fundam. Appl. Sci. 2019, 15, 298–302. [Google Scholar] [CrossRef]
- Cvetković, D.D.; Markov, S.L. Cultivation of tea fungus on malt extract medium. Acta Period. Technol. 2002, 33, 117–126. [Google Scholar] [CrossRef]
- Malbaša, R.V.; Lončar, E.S.; Kolarov, L.J.A. Sucrose and inulin balance during tea fungus fermentation. Rom. Biotechnol. Lett. 2002, 7, 573–576. [Google Scholar]
- Lončar, E.S.; Malbaša, R.V.; Kolarov, L.A. Kombucha fermentation on raw extracts of different cultivars of Jerusalem artichoke. Acta Period. Technol. 2007, 38, 37–44. [Google Scholar] [CrossRef]
- Francisco, Á.R.; Jose, R.M.; Igor, H. Development of a no added sugar kombucha beverage based on germinated corn. Int. J. Gastron. Food Sci. 2021, 24, 100355. [Google Scholar] [CrossRef]
- Degirmencioglu, N.; Yildiz, E.; Sahan, Y.; Guldas, M.; Gurbuz, O. Impact of tea leaves types on antioxidant properties and bioaccessibility of kombucha. J. Food Sci. Technol. 2021, 58, 2304–2312. [Google Scholar] [CrossRef]
- Tanticharakunsiri, W.; Mangmool, S.; Ochaikul, D. Characteristics and upregulation of antioxidant enzymes of kitchen mint and oolong tea kombucha beverages. J. Food Biochem. 2020, 45, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Tsuru, V.H.; Gomes, R.J.; Silva, J.R.; Prudencio, S.H.; Costa, G.N.; Spinosa, W.A. Physicochemical, antioxidant and sensory properties of kombucha beverages obtained from oolong or yerba mate tea fermentation. Res. Soc. Dev. 2021, 10, e62101118790. [Google Scholar] [CrossRef]
- Gaggìa, F.; Baffoni, L.; Galiano, M.; Nielsen, D.S.; Jakobsen, R.R.; Castro-Mejía, J.L.; Bosi, S.; Truzzi, F.; Musumeci, F.; Dinelli, G.; et al. Kombucha beverage from green, black and rooibos teas: A comparative study looking at microbiology, chemistry and antioxidant activity. Nutrients 2019, 11, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, C.; Li, R.; Chen, J.; Wang, F.; Gao, Y.; Fu, Y.; Xu, Y.; Yin, J. Zijuan tea-based kombucha: Physicochemical, sensorial, and antioxidant profile. Food Chem. 2021, 363, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Reiss, J. Der Teepilz und seine Stoffwechselproducte. Dtsch. Leb. 1987, 83, 286–290. [Google Scholar]
- Velićanskí, A.S.; Cvetković, D.D.; Markov, S. Characteristics of kombucha fermentation on medicinal herbs from Lamiaceae family. Rom. Biotechnol. Lett. 2013, 18, 8034–8042. [Google Scholar]
- Zhang, J.; Van Mullem, J.; Dias, D.R.; Schwan, R.F. The chemistry and sensory characteristics of new herbal tea-based kombuchas. J. Food Sci. 2021, 86, 740–748. [Google Scholar] [CrossRef]
- Vitas, J.S.; Cvetanović, A.D.; Mašković, P.Z.; Švarc-Gajić, J.V.; Malbaša, R.V. Chemical composition and biological activity of novel types of kombucha beverages with yarrow. J. Funct. Foods 2018, 44, 95–102. [Google Scholar] [CrossRef]
- Četojević-Simin, D.D.; Velićanski, A.S.; Cvetković, D.D.; Markov, S.L.; Mrdanović, J.Ž.; Bogdanović, V.V.; Šolajić, S.V. Bioactivity of lemon balm kombucha. Food Bioprocess Technol. 2012, 5, 1756–1765. [Google Scholar] [CrossRef]
- Velićanski, A.S.; Cvetkovic, D.D.; Markov, S.L.; Tumbas Šaponjac, V.T.; Vulić, J.J. Antioxidant and antibacterial activity of the beverage obtained by fermentation of sweetened lemon balm (Melissa officinalis L.) tea with symbiotic consortium of bacteria and yeasts. Food Technol. Biotechnol. 2014, 52, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Cabral, B.D.; Rocha-Guzmán, N.E.; Gallegos-Infante, J.A.; González-Herrera, S.M.; González-Laredo, R.F.; Moreno-Jiménez, M.R.; Córdova-Moreno, I.T.S. Chemical and sensory evaluation of a functional beverage obtained from infusions of oak leaves (Quercus resinosa) inoculated with the kombucha consortium under different processing conditions. Nutrafoods 2014, 13, 169–178. [Google Scholar] [CrossRef]
- Vázquez-Cabral, B.; Larrosa-Perez, M.; Gallegos-Infante, J.A.; Moreno-Jimenez, M.R.; Gonzalez-Laredo, R.; Rutiago-Quinones, J.G.; Gamboa-Gomez, C.I.; Rocha-Guzman, N.E. Oak kombucha protects against oxidative stress and inflammatory processes. Chem. Biol. Interact. 2017, 272, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pure, A.E.; Pure, M.E. Antioxidant and antibacterial activity of kombucha beverages prepared using banana peel, common nettles and black tea infusions. Appl. Food Biotechnol. 2016, 3, 125–130. [Google Scholar] [CrossRef]
- Salafzoon, S.; Mahmoodzadeh Hosseini, H.; Halabian, R. Evaluation of the antioxidant impact of ginger-based kombucha on the murine breast cancer model. J. Complement. Integr. Med. 2018, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ayed, L.; Hamdi, M. Manufacture of a beverage from cactus pear juice using “tea fungus” fermentation. Ann. Microbiol. 2015, 65, 2293–2299. [Google Scholar] [CrossRef]
- Ayed, L.; Abid, S.B.; Hamdi, M. Development of a beverage from red grape juice fermented with the kombucha consortium. Ann. Microbiol. 2017, 67, 111–121. [Google Scholar] [CrossRef]
- Aspiyanto, A.S.; Iskandar, J.M.; Melanie, H.; Maryati, Y.; Lotulung, P.D. Characteristic of fermented spinach (Amaranthus spp.) polyphenol by kombucha culture for antioxidant compound. AIP Conf. Proc. 2017, 1803, 20018. [Google Scholar]
- Yavari, N.; Mazaheri-Assadi, M.; Mazhari, Z.H.; Moghadam, M.B.; Larijani, K. Glucuronic acid rich kombucha-fermented pomegranate juice. J. Food Res. 2017, 7, 61. [Google Scholar] [CrossRef] [Green Version]
- Yavari, N.; Assadi, M.M.; Larijani, K.; Moghadam, M.B. Response surface methodology for optimization of glucuronic acid production using kombucha layer on sour cherry juice. Aust. J. Basic Appl. Sci. 2010, 4, 3250–3256. [Google Scholar]
- Yavari, N.; Assadi, M.M.; Moghadam, M.B.; Larijani, K.; Researchers, Y. Optimizing glucuronic acid production using tea fungus on grape juice by response surface methodology. Aust. J. Basic Appl. Sci. 2011, 5, 1788–1794. [Google Scholar]
- Zofia, N.-L.; Aleksandra, Z.; Tomasz, B.; Martyna, Z.; Tomasz, W. Effect of fermentation time on antioxidant and anti-ageing properties of green coffee. Molecules 2020, 25, 5394. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.; Tang, S.; Azi, F.; Hu, W.; Dong, M. Use of kombucha consortium to transform soy whey into a novel functional beverage. J. Funct. Foods 2019, 52, 81–89. [Google Scholar] [CrossRef]
- Loňcar, E.; Djurić, M.; Malbaša, R.; Kolarov, L.; Klašnja, A. Influence of working conditions upon kombucha conducted fermentation of black tea. Food Bioprod. Process. 2006, 84, 186–192. [Google Scholar] [CrossRef]
- Lončar, E.S.; Kanurić, K.; Malbaša, R.; Đjuric, M.; Milanović, S.D. Kinetics of saccharose fermentation by kombucha. Chem. Ind. Chem. Eng. Q. 2014, 20, 345–352. [Google Scholar] [CrossRef] [Green Version]
- Vitas, J.S.; Malbaša, R.V.; Grahovac, J.A.; Lončar, E.S. The antioxidant activity of kombucha fermented milk products with stinging nettle and winter savory. Chem. Ind. Chem. Eng. Q. 2013, 19, 129–139. [Google Scholar] [CrossRef]
- Neffe-Skocińska, K.; Sionek, B.; Ścibisz, I.; Kołożyn-Krajewska, D. Acid contents and the effect of fermentation condition of kombucha tea beverages on physicochemical, microbiological and sensory properties. CyTA—J. Food 2017, 15, 601–607. [Google Scholar] [CrossRef] [Green Version]
- De Filippis, F.; Dario, A.; Vitaglione, P.; Ercolini, D. Different temperatures select distinctive acetic acid bacteria species and promotes organic acids production during kombucha tea fermentation. Food Microbiol. 2018, 73, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Hur, J.; Lee, S.; Kim, Y.-C.; Choi, I.; Kim, G.-B. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Cvetković, D.D.; Markov, S.L. Preparation of kombucha from winter savory (Satureja montana L.) in the laboratory bioreactor. Acta Period. Technol. 2005, 266, 187–196. [Google Scholar] [CrossRef]
- Malbaša, R.; Lončar, E.S.; Vitas, J.; Čanadanovi-Brunet, J.M. Influence of starter cultures on the antioxidant activity of kombucha beverage. Food Chem. 2011, 127, 1727–1731. [Google Scholar] [CrossRef]
- Teoh, A.; Heard, G.; Cox, J. Yeast ecology of kombucha fermentation. Int. J. Food Microbiol. 2004, 95, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, X.; Qi, X.; Ren, L. Isolation and identification of a bacterial cellulose synthesizing strain from kombucha in different conditions: Gluconacetobacter xylinus ZHCJ618. Food Sci. Biotechnol. 2018, 27, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.T.; Flanagan, B.; Gidley, M.J.; Dykes, G.A. Characterization of cellulose production by a Gluconacetobacter xylinus strain from kombucha. Curr. Microbiol. 2008, 57, 449–453. [Google Scholar] [CrossRef]
- Mikkelsen, D.; Flanagan, B.M.; Dykes, G.A.; Gidley, M.J. Influence of different carbon sources on bacterial cellulose production by Gluconacetobacter xylinus strain ATCC 53524. J. Appl. Microbiol. 2009, 107, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Coton, M.; Pawtowski, A.; Taminiau, B.; Burgaud, G.; Deniel, F.; Coulloumme-Labarthe, L.; Fall, A.; Daube, G.; Coton, E. Unraveling microbial ecology of industrial-scale kombucha fermentations by metabarcoding and culture-based methods. FEMS Microbiol. Ecol. 2017, 93, fix048. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Z.; Xin, X. Isolation and identification of microorganisms from kombucha fungus culture. J. Beijing Union Univ. 2011, 25, 42–46. [Google Scholar]
- Wu, W.; Gai, B.-C.; Ji, B.-P. Study on the isolation and identification of microbes of kombucha. J. Food Sci. 2004, 25, 55–58. [Google Scholar]
- Arikan, M.; Mitchell, A.; Finn, R.; Gurel, F. Microbial composition of kombucha determined using amplicon sequencing and shotgun metagenomics. J. Food Sci. 2020, 85, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Harrison, K.; Curtin, C. Microbial composition of SCOBY starter cultures used by commercial kombucha brewers in North America. Microorganisms 2021, 9, 1060. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Yan, F.; Cao, Z.; Xie, F.; Lin, J. Antioxidant activities of kombucha prepared from three different substrates and changes in content of probiotics during storage. Food Sci. Technol. 2014, 34, 123–126. [Google Scholar] [CrossRef] [Green Version]
- Mukadam, T.A.; Punjabi, K.; Deshpande, S.D. Isolation and characterization of bacteria and yeast from kombucha tea. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 32–41. [Google Scholar] [CrossRef]
- Watawana, M.I.; Jayawardena, N.; Ranasinghe, S.J.; Waisundara, V.Y. Evaluation of the effect of different sweetening agents on the polyphenol contents and antioxidant and starch hydrolase inhibitory properties of kombucha. J. Food Process. Preserv. 2017, 41, e12752. [Google Scholar] [CrossRef] [Green Version]
- Angela, C.; Young, J.; Kordayanti, S.; Devanthi, P.V.P.; Katherine. Isolation and screening of microbial isolates from kombucha culture for bacterial cellulose production in sugarcane molasses medium. In Proceedings of the 2019 International Conference on Biotechnology and Life Sciences, Kolkata, India, 8–10 August 2019; CRC Press: Boca Raton, FL, USA, 2020; Volume 2020, pp. 111–127. [Google Scholar]
- Villarreal-Soto, S.A.; Bouajila, J.; Pace, M.; Leech, J.; Cotter, P.D.; Souchard, J.; Taillandier, P.; Beaufort, S. Metabolome-microbiome signatures in the fermented beverage, kombucha. Int. J. Food Microbiol. 2020, 333, 108778. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, L.; Qi, L.; Liang, H.; Lin, X.; Li, S.; Yu, C.; Ji, C. Effect of synthetic microbial community on nutraceutical and sensory qualities of kombucha. Int. J. Food Sci. Technol. 2020, 55, 3327–3333. [Google Scholar] [CrossRef]
- Leonarski, E.; Cesca, K.; Borges, O.M.A.; de Oliveira, D.; Poletto, P. Typical kombucha fermentation: Kinetic evaluation of beverage and morphological characterization of bacterial cellulose. J. Food Process. Preserv. 2021, 45, e16100. [Google Scholar] [CrossRef]
- Savary, O.; Mounier, J.; Thierry, A.; Poirier, E.; Jourdren, J.; Maillard, M.; Penland, M.; Decamps, C.; Coton, E.; Coton, M. Tailor-made microbial consortium for kombucha fermentation: Microbiota-induced biochemical changes and biofilm formation. Food Res. Int. 2021, 147, 110549. [Google Scholar] [CrossRef]
- Tran, T.; Grandvalet, C.; Winckler, P.; Verdier, F.; Martin, A.; Alexandre, H.; Tourdot-Maréchal, R. Shedding light on the formation and structure of kombucha biofilm using two-photon fluorescence microscopy. Front. Microbiol. 2021, 12, 1–13. [Google Scholar] [CrossRef]
- Ma, C.; Lin, J.; Yang, M.; Liu, Z. Optimization of technical conditions in the fermentation of kombucha. Food Res. Dev. 2008, 29, 36–38. [Google Scholar]
- Edwards, C.G.; Collins, M.D.; Lawson, P.A.; Rodriguez, A.V. Lactobacillus nagelii sp. nov., an organism isolated from a partially fermented wine. Int. J. Syst. Evol. Microbiol. 2000, 50, 699–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furukawa, S.; Yoshida, K.; Ogihara, H.; Yamasaki, M.; Morinaga, Y. Mixed-species biofilm formation by direct cell-cell contact between brewing yeasts and lactic acid bacteria. Biosci. Biotechnol. Biochem. 2010, 74, 2316–2319. [Google Scholar] [CrossRef] [PubMed]
- May, A.; Narayanan, S.; Alcock, J.; Varsani, A.; Maley, C.; Aktipis, A. Kombucha: A novel model system for cooperation and conflict in a complex multi-species microbial ecosystem. PeerJ 2019, 2019, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Bergström, H. The Effect of the Fermented Tea Beverage Kombucha on the Gut Microflora. Master’s Thesis, Lund University, Lund, Sweden, 2018. [Google Scholar]
- U.S. National Library of Medicine. The Effect of the Fermented Tea Beverage Kombucha on the Oral and Gut Microflora (NCT03873350). Available online: https://clinicaltrials.gov/ct2/show/NCT03873350?term=Kombucha&draw=2&rank=1 (accessed on 8 December 2021).
- U.S. National Library of Medicine. Evaluating the Effects of Kombucha as a Hyperglycaemic Therapeutic Agent within Diabetic Human Subjects (NCT04107207). Available online: https://clinicaltrials.gov/ct2/show/NCT04107207?term=Kombucha&draw=2&rank=2 (accessed on 8 December 2021).
- U.S. National Library of Medicine. The Effect of Kombucha on Blood Sugar Levels in Humans (NCT04051294). Available online: https://clinicaltrials.gov/ct2/show/NCT04051294?term=Kombucha&draw=2&rank=3 (accessed on 8 December 2021).
- Shahbazi, H.; Hashemi Gahruie, H.; Golmakani, M.T.; Eskandari, M.H.; Movahedi, M. Effect of medicinal plant type and concentration on physicochemical, antioxidant, antimicrobial, and sensorial properties of kombucha. Food Sci. Nutr. 2018, 6, 2568–2577. [Google Scholar] [CrossRef] [PubMed]
- Sknepnek, A.; Pantić, M.; Matijašević, D.; Miletić, D.; Lević, S. Novel kombucha beverage from Lingzhi or Reishi medicinal mushroom Ganoderma lucidum with antibacterial and antioxidant affects. Int. J. Med. Mushrooms 2018, 20, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.C.; Muhialdin, B.J.; Meor Hussin, A.S. Influence of storage conditions on the quality, metabolites, and biological activity of Soursop (Annona muricata. L.) kombucha. Front. Microbiol. 2020, 11, 2982. [Google Scholar] [CrossRef] [PubMed]
- Zubaidah, E.; Dewantari, F.J.; Novitasari, F.R.; Srianta, I.; Blanc, P.J. Potential of snake fruit (Salacca zalacca (Gaerth.) Voss) for the development of a beverage through fermentation with the kombucha consortium. Biocatal. Agric. Biotechnol. 2018, 13, 198–203. [Google Scholar] [CrossRef] [Green Version]
- Al-Mohammadi, A.R.; Ismaiel, A.A.; Ibrahim, R.A.; Moustafa, A.H.; Zeid, A.A.; Enan, G. Chemical constitution and antimicrobial activity of kombucha fermented beverage. Molecules 2021, 26, 5026. [Google Scholar] [CrossRef] [PubMed]
- Ivanisova, E.; Menhartova, K.; Terentjeva, M.; Harangozo, L.; Kantor, A.; Kacaniova, M. The evaluation of chemical, antioxidant, antimicrobial and sensory properties of kombucha tea beverage. J. Food Sci. Technol. 2020, 57, 1840–1846. [Google Scholar] [CrossRef]
- Nazemi, L.; Hashemi, S.J.; Ghazvini, R.D.; Saeedi, M.; Khodavaisy, S.; Barac, A.; Modiri, M.; Dana, M.A.; Shahrabadi, Z.Z.; Rezaie, S. Investigation of cgrA and cyp51A gene alternations in Aspergillus fumigatus strains exposed to kombucha fermented tea. Curr. Med. Mycol. 2019, 5, 36–42. [Google Scholar] [CrossRef]
- Fu, N.; Wu, J.; Lv, L.; He, J.; Jiang, S. Anti-foot-and-mouth disease virus effects of Chinese herbal kombucha in vivo. Braz. J. Microbiol. 2015, 46, 1245–1255. [Google Scholar] [CrossRef] [PubMed]
- Cobbina, E.; Akhlaghi, F. Non-alcoholic fatty liver disease (NAFLD)—Pathogenesis, classification, and effect on drug metabolizing enzymes and transporters. Drug Metab. Rev. 2018, 49, 197–211. [Google Scholar] [CrossRef]
- Cardoso, R.; Moreira, L.P.; de Campos Costa, M.; Toledo, R.C.; Grancieri, M.; Nascimento, T.P.; Ferreira, M.S.; da Matta, S.L.; Eller, M.R.; Duarte Martine, H.; et al. Kombucha from green and black teas reduce oxidative stress, liver steatosis and inflammation, and improve glucose metabolism in Wistar rats fed a high-fat high-fructose diet. Food Funct. 2021, 12, 10813–10827. [Google Scholar] [CrossRef]
- Lee, C.; Kim, J.; Wang, S.; Sung, S.; Kim, N.; Lee, H.; Seo, Y.; Jung, Y. Hepatoprotective effect of kombucha tea in rodent model of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Int. J. Mol. Sci. 2019, 20, 2369. [Google Scholar] [CrossRef] [Green Version]
- Urrutia, M.A.; Ramos, A.; Menegusso, R.B.; Lenz, R.; Ramos, M.; Tarone, A.; Cazarin, C.B.; Cottica, S.; da Silva, S.A.; Bernardi, D. Effects of supplementation with kombucha and green banana flour on Wistar rats fed with a cafeteria diet. Heliyon 2021, 7, e07081. [Google Scholar] [CrossRef]
- Zubaidah, E.; Apriyadi, T.E.; Kalsum, U.; Widyastuti, E.; Estiasih, T.; Srianta, I.; Blanc, P.J. In vivo evaluation of snake fruit kombucha as hyperglycemia therapeutic agent. Int. Food Res. J. 2018, 25, 453–457. [Google Scholar]
- Zubaidah, E.; Afgani, C.A.; Kalsum, U.; Srianta, I.; Blanc, P.J. Comparison of in vivo antidiabetes activity of snake fruit kombucha, black tea kombucha and metformin. Biocatal. Agric. Biotechnol. 2019, 17, 465–469. [Google Scholar] [CrossRef]
- Constantinescu, C.S.; Farooqi, N.; Brien, K.O.; Gran, B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br. J. Pharmacol. 2011, 164, 1079–1106. [Google Scholar] [CrossRef]
- Haghmorad, D.; Yazdanpanah, E.; Sadighimoghaddam, B.; Yousefi, B.; Sahafi, P. Kombucha ameliorates experimental autoimmune encephalomyelitis through activation of Treg and Th2 cells. Acta Neurol. Belg. 2021, 121, 1685–1692. [Google Scholar] [CrossRef]
- Sknepnek, A.; Tomić, S.; Miletić, D.; Lević, S.; Čolić, M. Kombucha beverages and immunomodulatory potential of their polysaccharide extracts. Food Chem. 2021, 342, 128344. [Google Scholar] [CrossRef] [PubMed]
- Kovacevic, Z.; Davidovic, G.; Vuckovic-Filipovic, J.; Janicijevic-Petrovic, M.A.; Janicijevic, K.; Popovic, A. A toxic hepatitis caused the kombucha tea—Case report. Open Access Maced. J. Med. Sci. 2014, 2, 128–131. [Google Scholar] [CrossRef] [Green Version]
- Derk, C.T.; Sandorfi, N.; Curtis, M.T. A case of anti-Jo1 myositis with pleural effusions and pericardial tamponade developing after exposure to a fermented kombucha beverage. Clin. Rheumatol. 2004, 23, 355–357. [Google Scholar] [CrossRef]
- Wood, B.; Rademaker, M.; Oakley, A.; Wallace, J. Pellagra in a woman using alternative remedies. Australas. J. Dermatol. 1998, 39, 42–44. [Google Scholar] [CrossRef]
- Moreira, P.L.; Lourencao, T.B.; Pinto, J.P.A.N.; Rall, V.L.M. Microbiological quality of spices marketed in the city of Botucatu, Sao Paulo, Brazil. J. Food Prot. 2009, 72, 421–424. [Google Scholar] [CrossRef]
- Sagoo, S.K.; Little, C.L.; Greenwood, M.; Mithani, V.; Grant, K.A.; McLauchlin, J.; de Pinna, E.; Threlfall, E.J. Assessment of the microbiological safety of dried spices and herbs from production and retail premises in the United Kingdom. Food Microbiol. 2009, 26, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Sospedra, I.; Soriano, J.M.; Mañes, J. Assessment of the microbiological safety of dried spices and herbs commercialized in Spain. Plant Foods Hum. Nutr. 2010, 65, 364–368. [Google Scholar] [CrossRef]
- Ossowski, M.; Nowakowicz-Dębek, B.; Wlazło, L.; Król, J.; Kasela, M.; Maksym, P.; Malm, A. Evaluation of microbiological contamination of black and green teas. Adv. J. Food Sci. Technol. 2019, 17, 65–71. [Google Scholar] [CrossRef]
- Carraturo, F.; De Castro, O.; Troisi, J.; De Luca, A.; Masucci, A.; Cennamo, P.; Trifuoggi, M.; Aliberti, F.; Guida, M. Comparative assessment of the quality of commercial black and green tea using microbiology analyses. BMC Microbiol. 2018, 18, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centers for Disease Control and Prevention. Unexplained severe illness possibly associated with consumption of kombucha tea—Iowa, 1995. Morb. Mortal. Wkly. Rep. 1995, 44, 892–893. [Google Scholar]
- Sadjadi, J. Cutaneous anthrax associated with the kombucha “mushroom” in Iran. J. Am. Med. Assoc. 1998, 280, 1567–1568. [Google Scholar] [CrossRef]
- Brewer, S.S.; Lowe, C.A.; Beuchat, L.R.; Ortega, Y.R. Survival of Salmonella and Shiga toxin-producing Escherichia coli and changes in indigenous microbiota during fermentation of home-brewed kombucha. J. Food Prot. 2021, 84, 1366–1373. [Google Scholar]
- Nummer, B.A. Kombucha brewing under the Food and Drug Administration model Food Code: Risk analysis and processing guidance. J. Environ. Health 2013, 76, 8–11. [Google Scholar]
- Sundermann, A.; Zhao, S.; Young, C.; Lam, L.; Jones, S. Alcohol use in pregnancy and miscarriage: A systematic review and meta-analysis. Alcohol. Clin. Exp. Res. 2019, 43, 1606–1616. [Google Scholar] [CrossRef]
- Hon, K.L.; Leung, A.K.C.; Cheung, E.; Lee, B.; Tsang, M.M.C.; Torres, A.R. An overview of exposure to ethanol-containing substances and ethanol intoxication in children based on three illustrated cases. Drugs Context 2018, 7, 5–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phan, T.G.; Estell, J.; Duggin, G.; Beer, I.; Smith, D.; Ferson, M.J. Lead poisoning from drinking kombucha tea brewed in a ceramic pot. Med. J. Aust. 1998, 169, 644–646. [Google Scholar] [CrossRef]
- PepsiCo. PepsiCo Announces Definitive Agreement to Acquire KeVita, a Leader in Fermented Probiotic Beverages. Available online: https://www.pepsico.com/news/press-release/pepsico-announces-definitive-agreement-to-acquire-kevita-a-leader-in-fermented-p11222016 (accessed on 1 December 2021).
- Frost, P. Molson Coors acquires Clearly Kombucha. Available online: https://www.molsoncoorsblog.com/news/molson-coors-acquires-clearly-kombucha (accessed on 1 December 2021).
- Coca-Cola. The Coca-Cola Company Adds Its First Line of Kombucha through Acquisition of Australian-Based Organic & Raw Trading Co. Available online: https://www.coca-colacompany.com/au/media-centre/media-releases/coca-cola-company-adds-kombucha-acquisition-australian-organic-raw-trading-co (accessed on 1 December 2021).
- Caballero, M. Health-Ade Lands $20M Coke Equity Investment. Available online: https://www.bevnet.com/news/2019/report-coke-veb-reups-in-health-ade/ (accessed on 1 December 2021).
- Peel, A.G. First Bev Buys Controlling Stake in Kombucha Brand Health-Ade. Available online: https://www.foodbev.com/news/first-bev-buys-controlling-stake-in-kombucha-brand-health-ade/ (accessed on 1 December 2019).
- Caballero, M. Peet’s Acquire Majority Stake in Revive Kombucha. Available online: https://www.bevnet.com/news/2018/peets-acquires-majority-stake-in-revive-kombucha/ (accessed on 1 December 2021).
- World Health Organisation. Global Status Report on Alcohol and Health 2018; World Health Organisation: Geneva, Switzerland, 2018. [Google Scholar]
- Treatt. Supplier of Flavour, Fragrance Ingredients. Available online: https://www.treatt.com/ (accessed on 1 December 2021).
- International Food Information Council. 2021 Food & Health Survey; International Food Information Council: Washington, DC, USA, 2021. [Google Scholar]
- Mellentin, J. 10 Key Trends in Food, Nutrition & Health 2022; New Nutrition Business: London, UK, 2022. [Google Scholar]
- Maathuis, A.J.H.; Keller, D.; Farmer, S. Survival and metabolic activity of the GanedenBC30 strain of Bacillus coagulans in a dynamic in vitro model of the stomach and small intestine. Benef. Microbes 2010, 1, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Majeed, M.; Nagabhushanam, K.; Natarajan, S.; Sivakumar, A.; Eshuis-de Ruiter, T.; Booij-Veurink, J.; de Vries, Y.P.; Ali, F. Evaluation of genetic and phenotypic consistency of Bacillus coagulans MTCC 5856: A commercial probiotic strain. World J. Microbiol. Biotechnol. 2016, 32, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavrentev, F.V.; Ashikhmina, M.S.; Ulasevich, S.A.; Morozova, O.V.; Orlova, O.Y.; Skorb, E.V.; Iakovchenko, N.V. Perspectives of Bacillus coagulans MTCC 5856 in the production of fermented dairy products. LWT 2021, 148, 111623. [Google Scholar] [CrossRef]
- Majeed, M.; Majeed, S.; Nagabhushanam, K.; Arumugam, S.; Beede, K.; Ali, F. Evaluation of probiotic Bacillus coagulans MTCC 5856 viability after tea and coffee brewing and its growth in GIT hostile environment. Food Res. Int. 2019, 121, 497–505. [Google Scholar] [CrossRef]
- Jang, S.S.; McIntyre, L.; Chan, M.; Brown, P.; Finley, J.; Chen, S.X. Ethanol concentration of kombucha teas in British Columbia, Canada. J. Food Prot. 2021, 84, 1878–1883. [Google Scholar] [CrossRef]
- Food Safety Authority of Ireland Food Safety Guidance Published for Unpasteurised Fermented Plant-Based Products. Available online: https://www.fsai.ie/news_centre/press_releases/fermented_products_guidance_23092021.html (accessed on 2 December 2021).
- Food Standards Australia New Zealand Food Recall-Lychee Rose Kombucha 330 mL. Available online: https://www.foodstandards.gov.au/industry/foodrecalls/recalls/Pages/Lychee-Rose-Kombucha-330ml-.aspx (accessed on 2 December 2021).
- LeBlanc, C.S. The Kombucha Tea Recall. Available online: https://www.law.uh.edu/healthlaw/perspectives/2010/leblanc_kombucha.pdf (accessed on 2 December 2021).
- Kombucha Brewers International. The Kombucha Act (H.R. 2124/S. 892) Is Changing Our Industry. Available online: https://kombuchabrewers.org/lobbying/ (accessed on 2 December 2021).

| Yeast | Lactic Acid Bacteria | Acetic Acid Bacteria | Culture | Inoculum Type | Fermentation Substrates | Fermentation Temperature | Fermentation Time | Reference |
|---|---|---|---|---|---|---|---|---|
| Brettanomyces, Zygosaccharomyces, Saccharomyces, Candida | n.d. | n.d. | Dependent | Pellicle and liquid (n = 34) | n/a | n/a | n/a | [5] |
| Zygosaccharomyces sp. | n.d. | Acetobacter (A.) xylinum | Dependent | Pellicle (3–4 layers) and liquid (10% v/v) | 67.5 g/L sucrose, 1.5 g/L tea | 20 °C–22 °C | 60 d | [8] |
| Zygosaccharomyces (Z.) bailii, Saccharomyces (S.) cerevisiae, Brettanomyces (B.) bruxellensis | n.d. | A. aceti, A. pasteurianus | Dependent | Pellicle and liquid (n = 3) | n/a | n/a | n/a | [4] |
| Z. bailii, Schizosaccharomyces (Sc.) pombe, Torulospora delbreuckii, Rhodotorula mucilaginosa, B. bruxellensis, Candida (C.) stellata | n.d. | n.d. | Dependent | Pellicle and liquid (10% v/v) | 100 g/L sucrose, 5.4 g/L black tea | 20 °C–22 °C | 14 d | [63] |
| Isolate 5/3, isolate 7/2, isolate 2/1 | n.d. | Isolate 5, isolate 9 | Dependent | Defined starter culture of yeasts and AAB (1 × 107–4 × 108 CFU/mL) | 70 g/L sucrose, 7.5 g/L winter savory tea | 28 °C | 11 d | [61] |
| Zygosaccharomyces sp., S. cerevisiae | n.d. | Acetobacter | Dependent | Defined starter culture of yeasts (5.8 × 104 CFU/mL) and AAB (105 CFU/mL) | 7% sucrose, 1.5 g/L black or green tea | 28 °C | 10 d | [62] |
| S. cerevisiae | Lactobacillus (Lb.) plantarum | Gluconacetobacter sp. | Dependent | Defined starter culture of yeasts, LAB and AAB (1:1:1) | 5% sucrose, 7.5 g/L black tea, green tea or tea powder | 30 °C | 90 h | [72] |
| Dekkera (D.) bruxellensis, D. anomala, Z. bisporus, Z. lentus, Pichia (P.) kudriavzevii, Davidiella tassiana, Wallemia sebi, Lachancea (L.) fermentati, Leucosporidiella fragaria, Kazachstania unispora, Kluyveromyces marxianus, Naumovozyma castelli, Hanseniaspora (H.) vineae | Lactobacillus, Lactococcus, Leuconostoc, Bifidobacterium | Acetobacter, Gluconacetobacter | Independent | Pellicle (10% w/v) and liquid (10% v/v) | 10% sucrose, 0.49% black tea | 23 °C | 10 d | [6] |
| Candida, Lachancea, Kluyveromyces, Debaryomyces, Pichia, Waitea, Eromothecium, Meyerozyma, Zygowilliopsis, Saccharomyces, Saccharomycopsis, Hanseniaspora, Kazachstania, Starmera, Merimbla, Sporopachydermia, Sugiyamaella | Lactobacillus, Weissella, Bifidobacterium | Komagataeibacter, Gluconobacter | Independent | Pellicle and liquid (10% v/v) | 10% sucrose, 5 g/L black tea | 28 °C | 21 d | [7] |
| Z. bailii | n.d. | Komagataeibacter (K.) saccharivorans | Dependent | Pellicle (3% w/v) and liquid (10% v/v) | 10% sucrose, 2% tea | Room temp | 28 d | [73] |
| C. boidinii, D. anomala, D. bruxellensis, H. valbyensis, Wickerhamomyces anomalus, P. membranifaciens, S. cerevisiae, S. uvarum, Torulaspora microellipsoides, Z. bailii, Z. florentina | Oenococcus (O.) oeni, Lb. nagelii, L. satsumensis | A. lovaniensis, A. okinawensis, A. peroxydans, A syzgii, A. tropicalis, Gluconacetobacter (Ga.) eurapaeus, Ga. hansenii, Ga. intermedius, Ga. liquefaciens, Ga. xylinus, Gluconobacter (G.) cerinus, G. oxydans, Tanticharoemia sakaeratensis | Dependent and independent | n/a | Sweetened black and green tea | n/a | 8 d | [67] |
| Dekkera, Pichia, Zygosaccharomyces | Lactobacillus, Leuconostoc, Lactococcus, Bifidobacterium | Gluconacetobacter, Acetobacter | Independent | Pellicle (3% w/v) | 10% sugar source (Aspartame, bees’ honey, glucose, Caryota urens honey or Palmyrah jaggery), 10 g/L black tea | 24 °C | 7 d | [74] |
| n.d. | Lactobacillus, Lactococcus, Streptococcus | Acetobacter, Ga. xylinus, Ga. saccharivorans | Dependent and independent | Pellicle (20 g/L) and liquid (3% v/v) | 100 g/L sucrose, 10 g/L black or green tea | 20 °C or 30 °C | 21 d | [59] |
| B. bruxellensis, Z. parabailii | Lactobacillaceae, Leuconostocaceae, Streptococcaceae | K. intermedius, K. rhaeticus, Ga. entanii | Dependent and independent | Pellicle (3% w/v) and liquid (10% v/v) | 80 g/L sucrose, 8 g/L black, green or rooibos tea | 27 °C | 14 d | [35] |
| B. bruxellensis | n.d. | Komagataeibacter sp. DS1MA.62A, K. xylinus, K. saccharivorans, Ga. saccharivorans | Dependent | Liquid (10% v/v) | 10% molasses in acetate buffer (200 mM, pH 4.75) | Room temp | 6 d | [75] |
| Zygosaccharomyces, Z. bailii | n.d. | Komagataeibacter, K. rhaeticus | Independent | Pellicle (100 mm2) and liquid (10% v/v) | 10 g/L sucrose, 6 g/L tea | 28 °C | 15 d | [70] |
| C. arabinofermentans, B. bruxellensis, Sc. pombe, Z. bailii | n.d. | A. maolroum, A. pasteurianus, A. pomorum, A. tropicalis, K. rhaeticus, K. xylinus, K. europaeus, K. intermedius, G. oxydans, Gluconacetobacter sp. SXCC-1 | Independent | Pellicle (2% w/v) and liquid (2% v/v) | 70 g/L sucrose, 10 g/L black tea | 25 °C | 14 d | [76] |
| Z. bailii | n.d. | A. pasteurianus, Ga. xylinus | Dependent | Defined starter culture of Z. bailii (105 CFU/mL), A. pasteurianus (105 CFU/mL) and Ga. xylinus (106 CFU/mL) | 100 g/L sucrose, 8 g/L black tea | 29 °C | 10 d | [77] |
| Brettanomyces, Zygosaccharomyces, Starmerella, Lachancea, Saccharomycetales, Candida, Hanseniaspora, Pichia, Ascomycota, Kregervanrija | Lactobacillus, Oenococcus | Komagataeibacter, Acetobacter, Gluconobacter, Gluconacetobacter | Independent | Pellicle (n = 103) | n/a | n/a | n/a | [71] |
| B. bruxellensis, Z. bisporus | n.d. | Acetobacteraceae, K. rhaeticus, K. hansenii, K. xylinus | Independent | Pellicle (4% w/v) and liquid (10% v/v) | 70 g/L glucose and fructose mixture (1:1), 0.5% green tea | 30 °C | 15 d | [78] |
| D. bruxellensis, H. uvarum, Z. bailii | Lb. nagelii, O. oeni | A. tropicalis, A. okinawensis, K. hansenii, G. oxydans | Independent | Defined starter culture of yeasts, LAB and AAB (105 CFU/mL of each) | 55 g/L blond sugar, green tea | 25 °C | 27 d | [79] |
| B. bruxellensis, H. valbyensis, S. cerevisiae | n.d. | A. papayae, Ga. takamatsuzukensis | Dependent | Liquid (12% v/v) | 60 g/L sucrose, 1% black tea | 26 °C | 3 d | [80] |
| Study Title | Study Status | Participants | Study Type | Intervention Treatment | Location | Source |
|---|---|---|---|---|---|---|
| The effect of the fermented tea beverage kombucha on the oral and gut microflora | Completed (2018) | 42 | Interventional; randomised allocation; double-blind | Participants will be divided into three groups, in which one is given living kombucha (intervention), one heat-sterilised kombucha (placebo) and one tap water (control). Dosage: one bottle (330 mL) daily for 3 weeks | Lund University, Sweden | [85,86] |
| Evaluating the effects of kombucha as a hyperglycaemic therapeutic agent within diabetic human subjects (completed) | Completed (2020) | 12 | Interventional; randomised allocation; double-blind | Either ginger kombucha (intervention) or ginger water (placebo) will be given to subjects for weeks 1–4 followed by the reciprocal beverage for weeks 6–10 | Georgetown University, USA | [87] |
| The effect of kombucha on blood sugar levels in humans (recruiting) | Recruiting | 20 (planned) | Interventional; randomised allocation; single-blind (participant) | At first visit, participants will be divided into four groups, in which one is given commercial kombucha (intervention 1), one brewed kombucha (intervention 2), one tea (control 1) and one tap water (control 2). At each subsequent visit, subjects will be randomly allocated into one of the remaining groups until they have completed each intervention treatment Dosage: 8 oz (237 mL) | University of Missouri, USA | [88] |
| Step | Procedure | Hazard Type | Potential Hazard | Preventative Measure |
|---|---|---|---|---|
| 1 | Boil water | Biological | Presence of pathogens in water | Boil water to kill pathogens |
| 2 | Dissolve sugar and steep tea in boiled water. Cool heated tea. | Biological | Introduction of contaminants prior to fermentation | Use clean and sanitised utensils and vessels. Ensure fermentation and preparation areas are clean and sanitary. Cover fermentation vessel with a porous cloth. Cool tea to 20 °C within 2 h and start fermentation as soon as possible. |
| 3 | Inoculate kombucha with SCOBY and liquid kombucha | Biological | Use of contaminated SCOBY or liquid kombucha inoculum | Use a commercial culture for first fermentation. Do not reuse a culture with signs of mould contamination. Do not reuse a culture which did not reach a pH of ≤4.2 in a previous fermentation. |
| 4 | Ferment for 7–10 days | Biological | Introduction or growth of contaminants during fermentation | Ensure fermentation areas are clean and sanitary. Cover fermentation vessel with a porous cloth. Ferment aerobically to ensure acetic acid production and ferment until a pH ≤ 4.2 is reached |
| Chemical | Leaching of chemical contaminants from fermentation vessel due to low pH of kombucha | Use food-grade fermentation vessels such as glass, stainless steel, high-density polyethylene (HDPE) or propylene (PP) | ||
| Chemical | Excessive production of organic acids due to prolonged fermentation | Stop fermentation before excess acid is produced. The pH of the final product should be ≥2.5 | ||
| Chemical | 1 Excessive production of ethanol due to prolonged fermentation | Stop fermentation before excess ethanol is produced OR allow fermentation to proceed and include an additional manufacturing step to reduce ethanol (e.g., distillation) | ||
| 5 | Remove SCOBY. Filter kombucha (optional) | Biological | Contamination of kombucha. Contamination of SCOBY if not stored/re-used correctly | Use clean and sanitised utensils and vessels. Ensure fermentation and preparation areas are clean and sanitary. Use SCOBY in new kombucha fermentation or store refrigerated in fresh sweetened tea until use. |
| 6 | Refrigerate | Biological | Continued fermentation and production of excess alcohol and acetic acid | Refrigerate at 4 °C to prevent continued fermentation |
| 7A | Consume at home | Chemical | Excess consumption could result in toxicity or acidosis | Ensure pH of final product is ≥2.5. Do not consume more than the daily recommended amount. |
| 7B | Package for retail sale | Chemical | Leaching of chemical contaminants from fermentation vessel due to low pH of kombucha | Use suitable food-grade packaging materials |
| Biological | Introduction of contaminants from packaging materials | Ensure packaging materials are clean and sanitised |
| Brand Name | Country of Production | No. of Products/Flavours Available | Base Ingredients | Calories (kcal/100 mL) | Sugar (g/100 mL) | Alcohol (% v/v) | Product Claims |
|---|---|---|---|---|---|---|---|
| GT’s Living Foods Kombucha | USA | 50 (12 Classic; 31 Synergy; 7 Hard) | Black tea, green tea, cane sugar, kiwi juice, live cultures (S. boulardii: 4 billion CFU, Lactobacillus bacterium: 4 billion CFU, Bacillus coagulans GBI-306086: 1 billion CFU) | ≤31 | 2.5–4.2 | Classic: 0.5–1%; Hard: 3%; Synergy: <0.5% | Organic; Vegan; Non-GMO; Gluten-Free; Raw |
| Wonder Drink Kombucha | USA | 5 | Filtered water, green tea, black tea, kombucha culture (yeast and bacteria), cane sugar, prebiotic corn fibre (xylo-oligosaccharides), natural flavour, stevia leaf extract | ≤14 | 2.3–2.8 | <0.5% | Organic; Vegan; Non-GMO; Gluten-free; BPA free; Low-sugar; Non-alcoholic; Raw |
| Humm Kombucha | USA | 24 (13 Original; 6 Zero; 5 Whole30 Approved) | Filtered water, green tea, black tea, white grape juice, cane sugar, live kombucha cultures, natural flavours, Bacillus subtilis (2 billion CFU), vitamin B12 | ≤18 | 2.4–3.9 | <0.5% | Organic; Vegan; Non-GMO; Gluten-free; Keto-friendly; Non-alcoholic; Raw |
| KÖE Kombucha | USA | 7 | Purified sparkling water, black tea, sugar, active culture, erythritol, natural flavour, lime juice concentrate, citric acid, ascorbic acid, B. coagulans, fruit and vegetable juice, stevia extract, green tea extract, green coffee extract | ≤9 | 2.3 | <0.5% | Organic; Vegan; Non-GMO; Gluten-free; Non-alcoholic; Raw |
| Rowdy Mermaid Kombucha | USA | 9 | Filtered water, green tea, black tea, cane sugar, live cultures (Lactobacillus plantarum) | ≤14 | 2.5–2.8 | <0.5% | Vegan; Non-GMO; Gluten-free; Non-alcoholic; Raw |
| Kevita Master Brew Kombucha | USA | 15 | Filtered water, kombucha culture, black tea, green tea, natural flavour, cane sugar, B. coagulans LactoSpore MTCC 5856, black tea essence, caffeine (green coffee bean extract), stevia leaf extract | ≤17 | 3.3–3.6 | <0.5% | Organic; Non-GMO; Kosher; Non-alcoholic; Raw |
| Brew Dr. Kombucha | USA | 13 (10 Classic; 3 Tranquil (CBD)) | Filtered water, green tea, cane sugar, live kombucha culture (yeast, bacteria) | ≤19 | 2.9–3.6 | <0.5% | Organic; Non-GMO; Gluten-free; Kosher; Alcohol-extracted; Raw |
| Clearly Kombucha | USA | 4 | Filtered water, active culture, cane sugar, black tea, green tea, ginger root, natural flavours, fruit and vegetable juice, live probiotic (B. coagulans, I billion CFU) | ≤5 | 0.0–1.2 | <0.5% | Organic; Non-GMO; Gluten-free; Kosher; Non-alcoholic; Raw |
| Health-Ade Kombucha | USA | 22 (15 Original; 7 Health-Ade Plus) | Filtered water, green tea, black tea, cane sugar, live kombucha cultures (yeast and bacteria) | ≤17 | 2.7–3.6 | Trace amounts | Organic; Vegan; non-GMO; Gluten-free; Raw |
| Aqua ViTea Kombucha | USA | 11 (9 Original; 2 CBD) | Filtered water, black tea, green tea, cane sugar, kombucha culture | ≤15 | 1.3–2.1 | <0.5% | Organic; Vegan; Non-GMO; Gluten-free; Paleo; Alcohol-extracted; Raw |
| Dominga Kombucha | Mexico | 4 | Filtered water, green tea, cane sugar, SCOBY | n/a | n/a | n/a | Raw |
| Gutsy Kombucha | Canada | 8 (6 Classic; 2 No Sugar) | Filtered water, black tea, cane sugar, living cultures (bacteria and yeast) | ≤14 | 0.0–3.7 | <0.5% | Organic; Vegan; Non-GMO; Gluten-free; Raw |
| Hoochy Booch Kombucha | Canada | 10 | Filtered water, green tea, cane sugar, kombucha culture | 8 | 2.1 | Trace amounts | Organic; Vegan; Gluten free; Raw |
| Vitae Kombucha | Spain | 9 | Volcanic mineral water, green tea, cane sugar, kombucha culture | ≤16 | 1.6–3.7 | <0.5% | Organic; Vegan; Gluten free; Lactose-free; Raw |
| The GUTsy Captain Kombucha | Portugal | 15 (9 Traditional; 6 Zero) | Filtered water, cane sugar, green tea (0.4%), stevia leaves, kombucha cultures, Bacillus coagulans | ≤18 | 0.0–4.4 | <0.5% | Organic; Vegan; Gluten free; Low-calorie; Non-alcoholic; Raw |
| SynerChi Kombucha | Ireland | 11 (6 Classic Kombucha Bottles; 3 Kombucha Cans; 2 Kombucha Shots) | Filtered water, sencha green tea, golden cane sugar, kombucha cultures | ≤38 | 0.0–4.5 | <0.5% | Organic; Vegan; Gluten free; Dairy free; Soy-free; Non-alcoholic; Raw |
| Holo Kombucha | Ireland | 2 | Filtered purified water, green tea, black tea, sugar, kombucha cultures | ≤7 | 3.1 | <1% | Organic; Vegan; Non-GMO; Gluten free; Dairy free; Raw |
| Leave Your Sword Kombucha | Netherlands | 7 | Filtered water, tea, beetroot sugar, kombucha culture | n/a | 4.5 | 2.5% | Raw |
| Equinox Kombucha | UK | 12 | Pure spring water, chun-mee green tea, raw cane sugar, kombucha cultures | ≤18 | 2.5–4.3 | <0.5% | Organic; Vegan; Gluten free; Plant-based; Non-alcoholic; Raw |
| Biona Kombucha | UK | 3 | Tea herbal extract (natural mineral water, green tea, black tea, mate leaves, lime blossom, lemon verbena, cornflower blossom, lemon balm, mint, nettle, woodruff, elder blossom, raspberry leaves, marigold blossom, blackberry leaves, liquorice root), raw cane sugar, kombucha cultures, natural carbon dioxide | ≤18 | 4.7 | <0.5% | Organic; Vegan; Non-alcoholic; Pasteurised |
| MOJO Kombucha | Australia | 15 (6 Activated Kombucha; 4 Gut Shot; 3 Kombucha Soda; 2 Superbooch) | Water, sugar, tea, kombucha culture, B. coagulans GBI-30 6086 (1 billion CFU) | ≤22 | 1.7–2.4 | <0.5% | Organic; Vegan; Low-sugar; Non-alcoholic; Raw |
| Remedy Kombucha | Australia | 13 | Sparkling water, sugar, black tea, green tea, wild kombucha culture, erythritol, steviol glycosides | 4 | 0.0 | <0.5% | Organic; Vegan; Gluten free; Non-alcoholic; Raw; Halal |
| Naughty Booch Kombucha | Australia | 2 | Kombucha apple cider blend, lime juice, monk fruit, natural flavours | ≤36 | 1.4–2.1 | 4.6% | Vegan; Low-sugar |
| Swig Kombucha | New Zealand | 4 | Purified Wanaka alpine water, sugar, green tea, black tea, kombucha culture | n/a | n/a | n/a | Organic; Raw |
| BomBooch Kombucha | China | 6 | Filtered water, green tea, black Ceylon tea, raw unrefined cane sugar, SCOBY culture | ≤19 | 1.7 | <0.5% | Raw |
| Wild Kombucha | Malaysia | 17 | Filtered water, tea leaves, cane sugar, kombucha culture | 11 | 1.5 | <1% | Organic; Raw |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nyhan, L.M.; Lynch, K.M.; Sahin, A.W.; Arendt, E.K. Advances in Kombucha Tea Fermentation: A Review. Appl. Microbiol. 2022, 2, 73-103. https://doi.org/10.3390/applmicrobiol2010005
Nyhan LM, Lynch KM, Sahin AW, Arendt EK. Advances in Kombucha Tea Fermentation: A Review. Applied Microbiology. 2022; 2(1):73-103. https://doi.org/10.3390/applmicrobiol2010005
Chicago/Turabian StyleNyhan, Laura M., Kieran M. Lynch, Aylin W. Sahin, and Elke K. Arendt. 2022. "Advances in Kombucha Tea Fermentation: A Review" Applied Microbiology 2, no. 1: 73-103. https://doi.org/10.3390/applmicrobiol2010005
APA StyleNyhan, L. M., Lynch, K. M., Sahin, A. W., & Arendt, E. K. (2022). Advances in Kombucha Tea Fermentation: A Review. Applied Microbiology, 2(1), 73-103. https://doi.org/10.3390/applmicrobiol2010005






