Stimulation of Heme-Dependent Catalase Enhanced the Cytoprotective Effect of Lactobacillus plantarum against Oxidative Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains, Cells and Culture Conditions
2.2. Preparation of Bacterial Cells and Cell Lysates
2.3. Analysis of Catalase Activity
2.4. Scavenging Free Radical Abilities of L. plantarum CGMCC 6888
2.5. The Cytoprotective Effects of L. plantarum CGMCC 6888 against Oxidative Stress
2.5.1. Intestinal Epithelial Cell Viability Assay
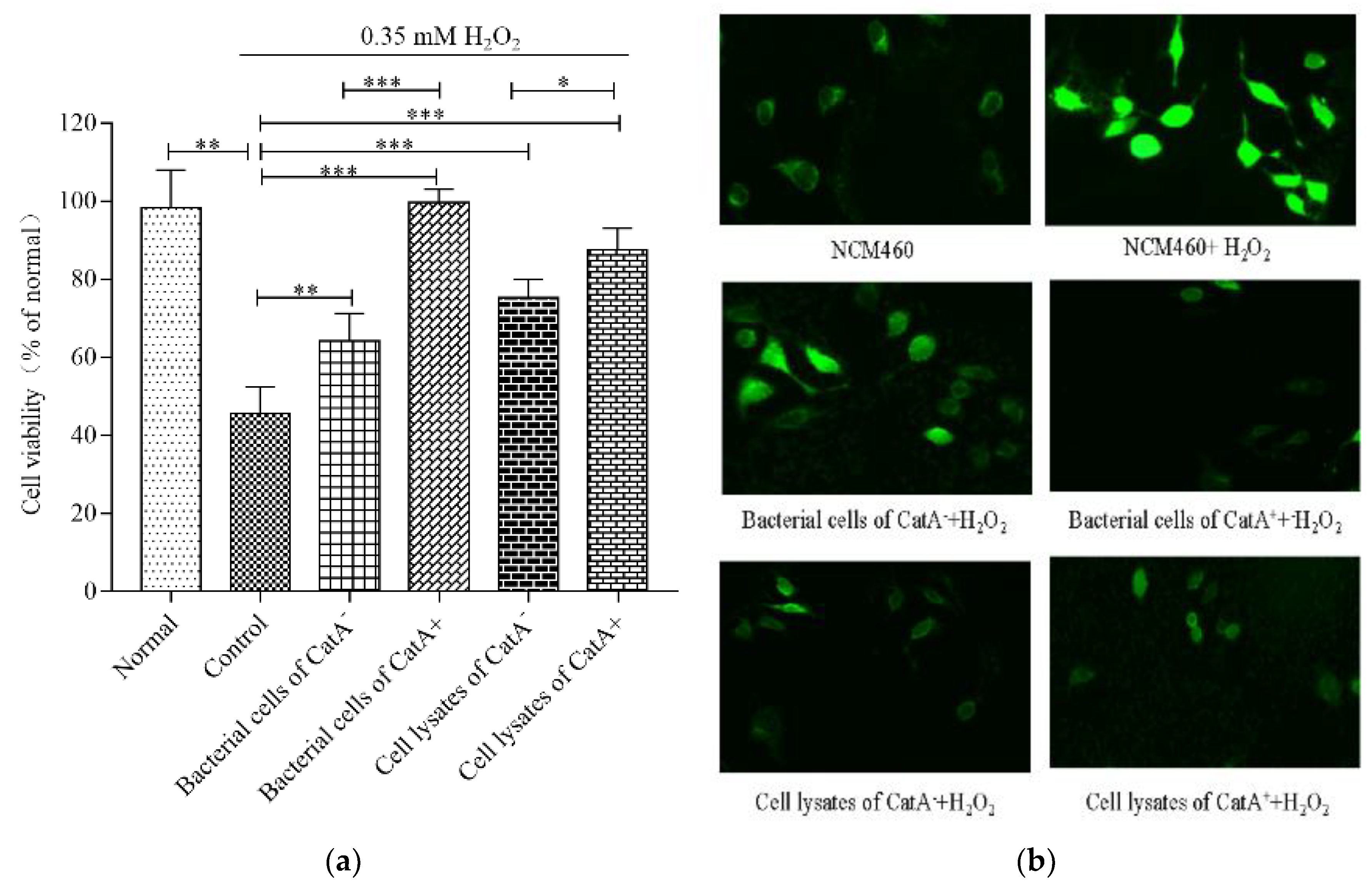
2.5.2. Intracellular ROS Detection in NCM460 Cells
2.5.3. Determination of CAT, GSH-px and SOD Activities in NCM460 Cells
2.5.4. Analysis of the Gene Transcription Levels of the Antioxidant Enzyme Genes and Tight Junction Protein Genes
2.5.5. Statistical Analysis
3. Results
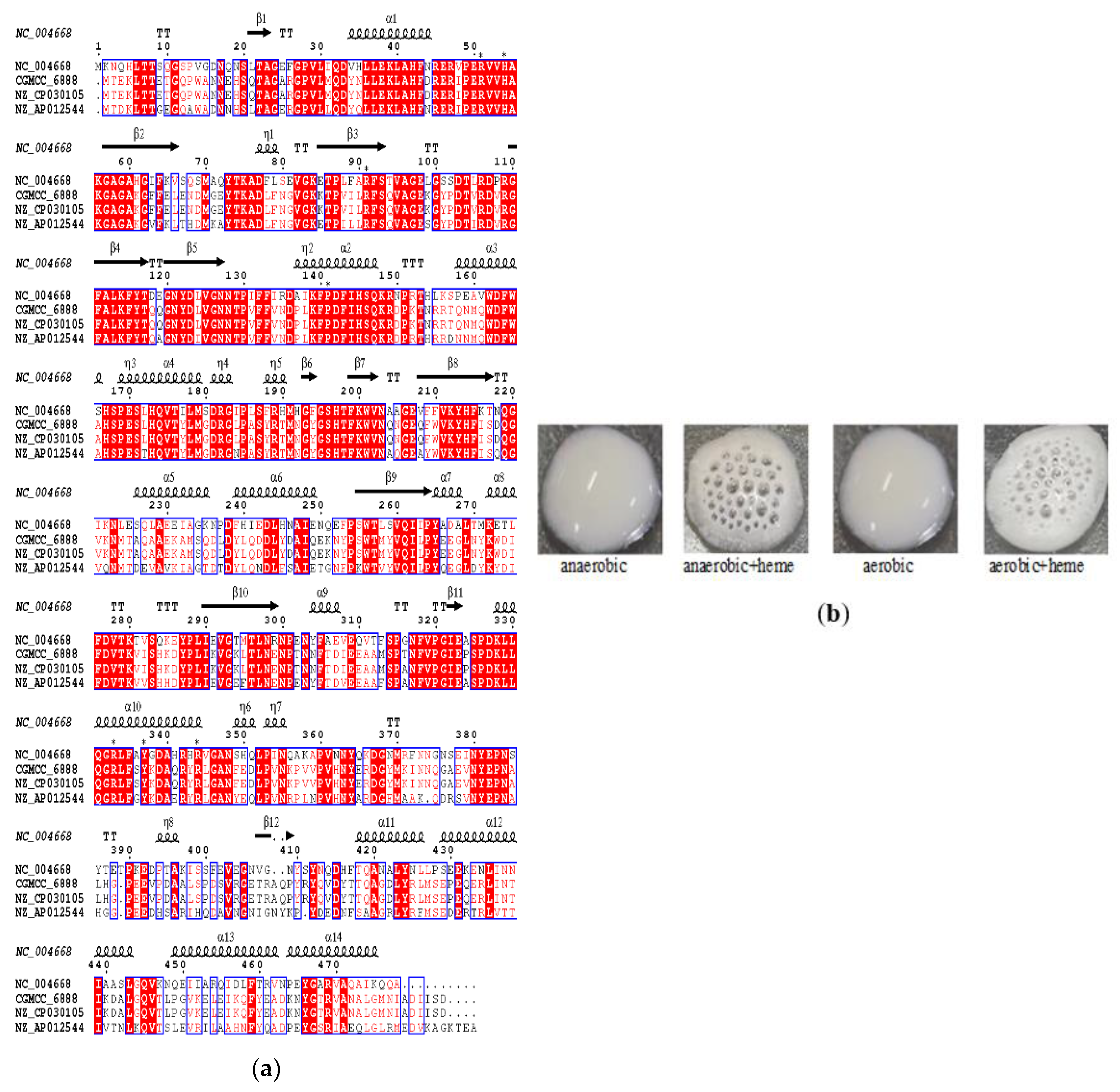
3.1. Identification and Activity Analysis of Catalase A in L. plantarum CGMCC 6888
3.2. Scavenging Free Radical Activities of L. plantarum CGMCC 6888
3.3. Effects of L. plantarum CGMCC 6888 on Oxidative Stress in NCM460 Cells
3.4. Antioxidant Enzyme Activities in NCM460 Cells Promoted by L. plantarum CGMCC 6888
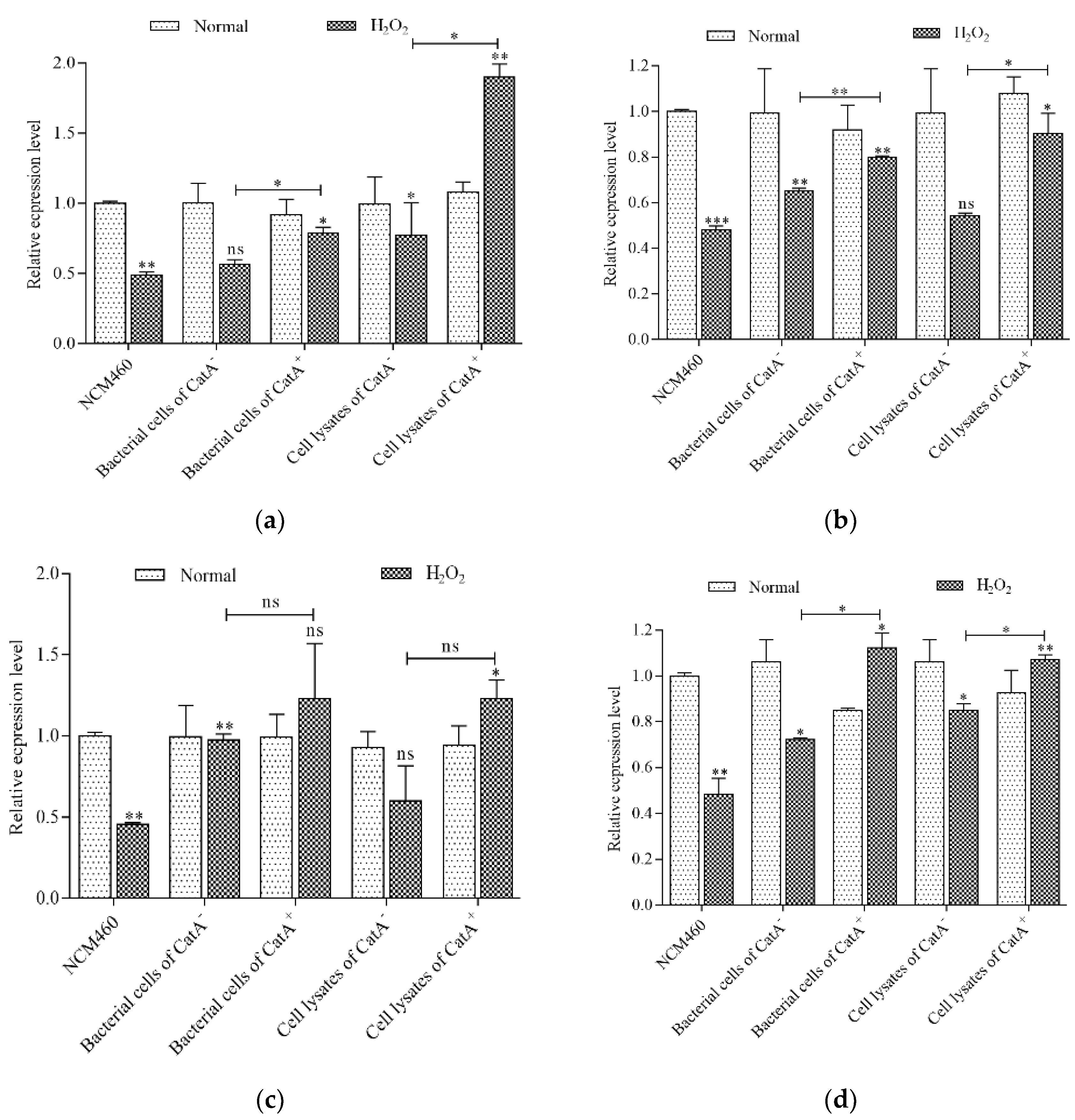
3.5. L. plantarum CGMCC 6888 Enhanced the Transcription Level of Antioxidant Enzyme Genes
3.6. CatA-Mediated Protection of L. plantarum CGMCC 6888 on the Intestinal Barrier
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lykkesfeldt, J.; Svendsen, O. Oxidants and antioxidants in disease: Oxidative stress in farm animals. Vet. J. 2007, 173, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Shah, C.; Mokashe, N.; Chavan, R.; Yadav, H.; Prajapati, J. Probiotics as Potential Antioxidants: A Systematic Review. J. Agric. Food Chem. 2015, 63, 3615–3626. [Google Scholar] [CrossRef] [PubMed]
- D’Autréaux, B.; Toledano, M.B. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007, 8, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Pulford, D.J. The glutathione S-transferase supergene family: Regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit. Rev. Biochem. Mol. Biol. 1995, 30, 445–600. [Google Scholar] [CrossRef] [PubMed]
- Townsend, D.M.; Tew, K.D. The role of glutathione-S-transferase in anti-cancer drug resistance. Oncogene 2003, 22, 7369–7375. [Google Scholar] [CrossRef]
- Barrera, G.; Cucci, M.; Grattarola, M.; Dianzani, C.; Muzio, G.; Pizzimenti, S. Control of Oxidative Stress in Cancer Chemoresistance: Spotlight on Nrf2 Role. Antioxidants 2021, 10, 510. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
- Nowak, A.; Paliwoda, A.; Błasiak, J. Anti-proliferative, pro-apoptotic and anti-oxidative activity of Lactobacillus and Bifidobacterium strains: A review of mechanisms and therapeutic perspectives. Crit. Rev. Food Sci. Nutr. 2019, 59, 3456–3467. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Zerrouki, M.; Benkaci-Ali, F. DFT study of the mechanisms of nonenzymatic DNA repair by phytophenolic antioxidants. J. Mol. Model. 2018, 24, 78. [Google Scholar] [CrossRef]
- De Boeck, I.; Spacova, I.; Vanderveken, O.M.; Lebeer, S. Lactic acid bacteria as probiotics for the nose? Microb. Biotechnol. 2021, 14, 859–869. [Google Scholar] [CrossRef]
- De Filippis, F.; Pasolli, E.; Ercolini, D. The food-gut axis: Lactic acid bacteria and their link to food, the gut microbiome and human health. FEMS Microbiol. Rev. 2020, 44, 454–489. [Google Scholar] [CrossRef]
- Amanatidou, A.; Smid, E.J.; Bennik, M.H.; Gorris, L.G. Antioxidative properties of Lactobacillus sake upon exposure to elevated oxygen concentrations. FEMS Microbiol. Lett. 2001, 203, 87–94. [Google Scholar] [CrossRef]
- Hernández-Delgado, N.C.; Torres-Maravilla, E.; Mayorga-Reyes, L.; Martín, R.; Langella, P.; Pérez-Pastén-Borja, R.; Bermúdez-Humarán, L.G. Antioxidant and Anti-Inflammatory Properties of Probiotic Candidate Strains Isolated during Fermentation of Agave (Agave angustifolia Haw). Microorganisms 2021, 9, 1063. [Google Scholar] [CrossRef]
- Pan, Y.; Ning, Y.; Hu, J.; Wang, Z.; Chen, X.; Zhao, X. The Preventive Effect of Lactobacillus plantarum ZS62 on DSS-Induced IBD by Regulating Oxidative Stress and the Immune Response. Oxidative Med. Cell. Longev. 2021, 2021, 9416794. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Y.; Wang, X.; Wang, S.; Bi, D. The Impact of Lactobacillus plantarum on the Gut Microbiota of Mice with DSS-Induced Colitis. Biomed. Res. Int. 2019, 2019, 3921315. [Google Scholar]
- Ge, Q.; Yang, B.; Liu, R.; Jiang, D.; Yu, H.; Wu, M.; Zhang, W. Antioxidant activity of Lactobacillus plantarum NJAU-01 in an animal model of aging. BMC Microbiol. 2021, 21, 182. [Google Scholar] [CrossRef]
- Mu, G.; Li, H.; Tuo, Y.; Gao, Y.; Zhang, Y. Antioxidative effect of Lactobacillus plantarum Y44 on 2,2’-azobis(2-methylpropionamidine) dihydrochloride (ABAP)-damaged Caco-2 cells. J. Dairy Sci. 2019, 102, 6863–6875. [Google Scholar] [CrossRef]
- Scillato, M.; Spitale, A.; Mongelli, G.; Privitera, G.F.; Mangano, K.; Cianci, A.; Stefani, S.; Santagati, M. Antimicrobial properties of Lactobacillus cell-free supernatants against multidrug-resistant urogenital pathogens. Microbiologyopen 2021, 10, e1173. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, Y.; Zhou, H.; Tian, F.; Ni, Y. Antimicrobial activities and in vitro properties of cold-adapted Lactobacillus strains isolated from the intestinal tract of cold water fishes of high latitude water areas in Xinjiang, China. BMC Microbiol. 2019, 19, 247. [Google Scholar] [CrossRef]
- Qiao, Y.; Qiu, Z.; Tian, F.; Yu, L.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Pediococcus acidilactici Strains Improve Constipation Symptoms and Regulate Intestinal Flora in Mice. Front. Cell. Infect. Microbiol. 2021, 11, 655258. [Google Scholar] [CrossRef] [PubMed]
- Song, M.W.; Jang, H.J.; Kim, K.-T.; Paik, H.-D. Probiotic and Antioxidant Properties of Novel Lactobacillus brevis KCCM 12203P Isolated from Kimchi and Evaluation of Immune-Stimulating Activities of Its Heat-Killed Cells in RAW 264.7 Cells. J. Microbiol. Biotechnol. 2019, 29, 1894–1903. [Google Scholar] [CrossRef] [PubMed]
- Bayat, E.; Moosavi-Nasab, M.; Fazaeli, M.; Majdinasab, M.; Mirzapour-Kouhdasht, A.; Garcia-Vaquero, M. Wheat Germ Fermentation with Saccharomyces cerevisiae and Lactobacillus plantarum: Process Optimization for Enhanced Composition and Antioxidant Properties In Vitro. Foods 2022, 11, 1125. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, N.; Li, D.; Wang, M.; Li, C.; Tian, H. Mechanism of gastrointestinal adaptability and antioxidant function of infant-derived Lactobacillus plantarum BF_15 through genomics. Food Sci. Biotechnol. 2022, 31, 1451–1462. [Google Scholar] [CrossRef]
- Wang, Z.; Feng, Y.; Yang, N.; Jiang, T.; Xu, H.; Lei, H. Fermentation of kiwifruit juice from two cultivars by probiotic bacteria: Bioactive phenolics, antioxidant activities and flavor volatiles. Food Chem. 2022, 373, 131455. [Google Scholar] [CrossRef]
- Kostelac, D.; Gerić, M.; Gajski, G.; Frece, J. Probiotic and paraprobiotic derivates exhibit anti-inflammatory and genoprotective effects during induced stress. J. Appl. Microbiol. 2022, 133, 819–829. [Google Scholar] [CrossRef]
- Lechardeur, D.; Cesselin, B.; Fernandez, A.; Lamberet, G.; Garrigues, C.; Pedersen, M.; Gaudu, P.; Gruss, A. Using heme as an energy boost for lactic acid bacteria. Curr. Opin. Biotechnol. 2011, 22, 143–149. [Google Scholar] [CrossRef]
- Pedersen, M.B.; Gaudu, P.; Lechardeur, D.; Petit, M.-A.; Gruss, A. Aerobic Respiration Metabolism in Lactic Acid Bacteria and Uses in Biotechnology. Annu. Rev. Food Sci. Technol. 2012, 3, 37–58. [Google Scholar] [CrossRef]
- Tang, W.; Xing, Z.; Li, C.; Wang, J.; Wang, Y. Molecular mechanisms and in vitro antioxidant effects of Lactobacillus plantarum MA2. Food Chem. 2017, 221, 1642–1649. [Google Scholar] [CrossRef]
- Quatravaux, S.; Remize, F.; Bryckaert, E.; Colavizza, D.; Guzzo, J. Examination of Lactobacillus plantarum lactate metabolism side effects in relation to the modulation of aeration parameters. J. Appl. Microbiol. 2006, 101, 903–912. [Google Scholar] [CrossRef]
- Zhang, R.; Li, Z.; Gu, X.; Zhao, J.; Guo, T.; Kong, J. Probiotic Bacillus subtilis LF11 Protects Intestinal Epithelium Against Salmonella Infection. Front. Cell. Infect. Microbiol. 2022, 12, 837886. [Google Scholar] [CrossRef]
- Sun, Z.; Huang, L.; Kong, J.; Hu, S.; Zhang, X.; Kong, W. In vitro evaluation of Lactobacillus crispatus K313 and K243: High-adhesion activity and anti-inflammatory effect on Salmonella braenderup infected intestinal epithelial cell. Vet. Microbiol. 2012, 159, 212–220. [Google Scholar] [CrossRef]
- Håkansson, K.O.; Brugna, M.; Tasse, L. The three-dimensional structure of catalase from Enterococcus faecalis. Acta Crystallogr. Sect. D Biol. Crystallogr. 2004, 60, 1374–1380. [Google Scholar] [CrossRef]
- Igarashi, T.; Kono, Y.; Tanaka, K. Molecular Cloning of Manganese Catalase from Lactobacillus plantarum. J. Biol. Chem. 1996, 271, 29521–29524. [Google Scholar] [CrossRef]
- Zotta, T.; Parente, E.; Ricciardi, A. Aerobic metabolism in the genus Lactobacillus: Impact on stress response and potential applications in the food industry. J. Appl. Microbiol. 2017, 122, 857–869. [Google Scholar] [CrossRef]
- Barynin, V.V.; Whittaker, M.M.; Antonyuk, S.V.; Lamzin, V.S.; Harrison, P.M.; Artymiuk, P.J.; Whittaker, J.W. Crystal Structure of Manganese Catalase from Lactobacillus plantarum. Structure 2001, 9, 725–738. [Google Scholar] [CrossRef]
- Fu, L.; Kong, J.; Sun, Z.; Zhang, L.; Zhang, X.; Guo, T. Enhancing the oxidative resistance of yoghurt starter bacteria with heterologous catalase expression in Streptococcus thermophilus. Int. Dairy J. 2013, 30, 68–72. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Wang, Y.; Xu, H.; Mei, X.; Yu, D.; Li, W. Antioxidant Properties of Probiotic Bacteria. Nutrients 2017, 9, 521. [Google Scholar] [CrossRef]
- Finamore, A.; Ambra, R.; Nobili, F.; Garaguso, I.; Raguzzini, A.; Serafini, M. Redox Role of Lactobacillus casei Shirota Against the Cellular Damage Induced by 2,2’-Azobis (2-Amidinopropane) Dihydrochloride-Induced Oxidative and Inflammatory Stress in Enterocytes-Like Epithelial Cells. Front Immunol. 2018, 9, 1131. [Google Scholar] [CrossRef]
- Min, W.-H.; Fang, X.-B.; Wu, T.; Fang, L.; Liu, C.-L.; Wang, J. Characterization and antioxidant activity of an acidic exopolysaccharide from Lactobacillus plantarum JLAU103. J. Biosci. Bioeng. 2019, 127, 758–766. [Google Scholar] [CrossRef]
- Hou, Y.; Li, X.; Liu, X.; Zhang, Y.; Zhang, W.; Man, C.; Jiang, Y. Transcriptomic responses of Caco-2 cells to Lactobacillus rhamnosus GG and Lactobacillus plantarum J26 against oxidative stress. J. Dairy Sci. 2019, 102, 7684–7696. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T. Regulation of the intestinal barrier by nutrients: The role of tight junctions. Anim. Sci. J. 2020, 91, e13357. [Google Scholar] [CrossRef] [PubMed]
- Adora, W.; Pinaki, P. Effects of Lactobacillus plantarum on Caco-2 cell tight junction proteins after infection with enteric bacteria. Gastroenterology 2003, 124, A479. [Google Scholar]
- Yu, Q.; Yuan, L.; Deng, J.; Yang, Q. Lactobacillus protects the integrity of intestinal epithelial barrier damaged by pathogenic bacteria. Front. Cell. Infect. Microbiol. 2015, 5, 26. [Google Scholar] [CrossRef]
- Cuevas-González, P.; Aguilar-Toalá, J.; García, H.; González-Córdova, A.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Protective Effect of the Intracellular Content from Potential Probiotic Bacteria against Oxidative Damage Induced by Acrylamide in Human Erythrocytes. Probiotics Antimicrob. Proteins 2020, 12, 1459–1470. [Google Scholar] [CrossRef]
- Lin, M.-Y.; Chang, F.-J. Antioxidative Effect of Intestinal Bacteria Bifidobacterium longum ATCC 15708 and Lactobacillus acidophilus ATCC 4356. Dig. Dis. Sci. 2000, 45, 1617–1622. [Google Scholar] [CrossRef]
- Saide, J.; Gilliland, S. Antioxidative Activity of Lactobacilli Measured by Oxygen Radical Absorbance Capacity. J. Dairy Sci. 2005, 88, 1352–1357. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.E.; Hall, F.G.; Urbizo-Reyes, U.C.; Garcia, H.S.; Vallejo-Cordoba, B.; González-Córdova, A.F.; Hernández-Mendoza, A.; Liceaga, A.M. In Silico Prediction and In Vitro Assessment of Multifunctional Properties of Postbiotics Obtained from Two Probiotic Bacteria. Probiotics Antimicrob. Proteins 2020, 12, 608–622. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.E.; Estrada-Montoya, M.C.; Liceaga, A.M.; Garcia, H.S.; González-Aguilar, G.A.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. An insight on antioxidant properties of the intracellular content of Lactobacillus casei CRL-431. LWT 2018, 102, 58–63. [Google Scholar] [CrossRef]
- Li, S.; Zhao, Y.; Zhang, L.; Zhang, X.; Huang, L.; Li, D.; Wang, Q. Antioxidant activity of Lactobacillus plantarum strains isolated from traditional Chinese fermented foods. Food Chem. 2012, 135, 1914–1919. [Google Scholar] [CrossRef]
- Ahire, J.J.; Mokashe, N.U.; Patil, H.J.; Chaudhari, B.L. Antioxidative potential of folate producing probiotic Lactobacillus helveticus CD6. J. Food Sci. Technol. 2013, 50, 26–34. [Google Scholar] [CrossRef]
- Endo, H.; Niioka, M.; Kobayashi, N.; Tanaka, M.; Watanabe, T. Butyrate-Producing Probiotics Reduce Nonalcoholic Fatty Liver Disease Progression in Rats: New Insight into the Probiotics for the Gut-Liver Axis. PLoS ONE 2013, 8, e63388. [Google Scholar] [CrossRef]
- Kullisaar, T.; Zilmer, M.; Mikelsaar, M.; Vihalemm, T.; Annuk, H.; Kairane, C.; Kilk, A. Two antioxidative lactobacilli strains as promising probiotics. Int. J. Food Microbiol. 2002, 72, 215–224. [Google Scholar] [CrossRef]

| Gene | Forward (from 5′ to 3′) | Reverse (from 5′ to 3′) |
|---|---|---|
| β-actin | AGTTGCGTTACACCCTTTCTTG | TCACCTTCACCGTTCCAGTTT |
| Nrf2 1 | ACACGGTCCACAGCTCATC | TGTCAATCAAATCCATGTCCTGH |
| HO-1 | CTTCTTCACCTTCCCCAACA | AGCTCCTGCAACTCCTCAAA |
| GCLC | GGAGACCAGAGTATGGGAGTT | CCGGCGTTTTCGCATGTTG |
| NQO-1 | ATGTATGACAAAGGACCCTTCC | TCCCTTGCAGAGAGTACATTGG |
| TXNRD1 | ATATGGCAAGAAGGTGATGGTCC | GGGCTTGTCCTAACAAAGCTG |
| CLDN-1 | GTGCCTTGATGGTGGTTG | TGTTGGGTAAGAGGTTGT |
| OCLN | GCAGCTACTGGACTCTACG | ATGGGACTGTCAACTCTTTC |
| ZO-1 | AAGAGTGAACCACGAGAC | TCCGTGCTATACATTGAG |
| JAM-1 | GATGTGCCTGTGGTGCTG | GCTCTGCCTTGAGATAAGAA |
| Sample | Scavenging Rate (%) | ||
|---|---|---|---|
| DPPH | O2− | OH. | |
| Bacterial cells of CatA− | 16.74 ± 1.35 | 5.51 ± 0.31 | 10.76 ± 0.67 |
| Bacterial cells of CatA+ | 18.54 ± 5.51 | 8.99 ± 0.97 * | 13.93 ± 0.01 * |
| Cell lysates of CatA− | 10.00 ± 2.34 | 2.90 ± 0.44 | 5.82 ± 0.50 |
| Cell lysates of CatA+ | 12.90 ± 1.81 | 6.01 ± 0.60 ** | 8.00 ± 0.47 ** |
| Sample | CAT (U/mg Prot) | GSH-px (U/mg Prot) | SOD (U/mg Prot) |
|---|---|---|---|
| Normal | 0.55 ± 0.07 | 39.42 ± 0.51 | 1.12 ± 0.05 |
| Control | 0.11 ± 0.01 | 28.91 ± 0.56 | 0.37 ± 0.03 |
| Bacterial cells of CatA− | 0.73 ± 0.13 ** | 40.55 ± 0.58 *** | 0.45 ± 0.05 * |
| Bacterial cells of CatA+ | 0.81 ± 0.15 ** | 42.98 ± 0.12 *** | 0.44 ± 0.03 * |
| Cell lysates of CatA− | 1.41 ± 0.13 *** | 41.62 ± 2.26 *** | 1.21 ± 0.08 *** |
| Cell lysates of CatA+ | 1.43 ± 0.02 *** | 44.43 ± 1.07 *** | 1.11 ± 0.13 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, X.; Zhu, X.; Wang, M.; Guo, T.; Kong, J. Stimulation of Heme-Dependent Catalase Enhanced the Cytoprotective Effect of Lactobacillus plantarum against Oxidative Stress. Appl. Microbiol. 2023, 3, 131-144. https://doi.org/10.3390/applmicrobiol3010011
Tian X, Zhu X, Wang M, Guo T, Kong J. Stimulation of Heme-Dependent Catalase Enhanced the Cytoprotective Effect of Lactobacillus plantarum against Oxidative Stress. Applied Microbiology. 2023; 3(1):131-144. https://doi.org/10.3390/applmicrobiol3010011
Chicago/Turabian StyleTian, Xingfang, Xiaoce Zhu, Meng Wang, Tingting Guo, and Jian Kong. 2023. "Stimulation of Heme-Dependent Catalase Enhanced the Cytoprotective Effect of Lactobacillus plantarum against Oxidative Stress" Applied Microbiology 3, no. 1: 131-144. https://doi.org/10.3390/applmicrobiol3010011
APA StyleTian, X., Zhu, X., Wang, M., Guo, T., & Kong, J. (2023). Stimulation of Heme-Dependent Catalase Enhanced the Cytoprotective Effect of Lactobacillus plantarum against Oxidative Stress. Applied Microbiology, 3(1), 131-144. https://doi.org/10.3390/applmicrobiol3010011






