Abstract
The selection of probiotic bacteria based on their beneficial characteristics does not necessarily mean they can be later scaled up and used for technological applications and formula design. Three probiotic strains—Lactobacillus acidophilus CRL2074, Limosilactobacillus fermentum CRL2085, and Limosolactobacillus mucosae CRL2069, originally isolated from feedlot cattle feces—have demonstrated beneficial characteristics when used as in-feed probiotics. Therefore, the current study was conducted to develop a low-cost culture medium to optimize growth conditions to enhance biomass production. The study also sought to identify appropriate cryoprotective agents to sustain high functional cell numbers after freeze drying. A central composite design was applied to determine the optimal medium composition. This yielded a simplified, low-cost effective medium containing 3% molasses and industrial yeast extracts (0.5 to 2.5%) as carbon and nitrogen sources, which were added to a basal medium for each strain. Established production conditions at 37 °C, without agitation, and pH-controlled for the CRL2085 and CRL2069 strains, and free pH for the CRL2074 strain, allowed us to obtain biomass yields of 12.95, 18.20, and 12.25 g, respectively, at 24-h incubation, compared with the MRS medium. In addition, the cryoprotective effect of the selected agents was demonstrated to be strain-dependent. Thus, the highest viability (109–1010 CFU/g), stability during 30-d storage, and survival rate (88–99%) were achieved when 10% MSG (monosodium glutamate), sucrose + fructose + trehalose + WPC (whey protein concentrate) + 10% MSG, and 1.2% WPC + 10% trehalose, were used for freeze drying CRL2074, CRL2085, and CRL2069, respectively. Moreover, the probiotic strains retained their probiotic functionality when hydrophobic characteristics were evaluated. These results highlight the need to perform strain-specific evaluation of the critical factors involved in the large-scale production of probiotic lactobacilli to sustain viability and stability after the freeze drying and storage processes.
1. Introduction
There is growing research and commercial interest in the use of probiotics for in-feed supplementation of meat animals to prevent disease and improve productivity [1,2]. The in-feed administration of probiotics during farm animals’ production has emerged as an alternative to the use of antibiotics as growth promoters, whose overuse resulted in the appearance of drug resistant pathogenic bacteria in veterinary practice and human medicine [2,3]. According to the definition used by the International Scientific Association for Probiotics and Prebiotics (ISAPP), a probiotic is a live, non-pathogenic microorganism with a positive physiological effect on the host [4]. The administration of probiotics contributes to the establishment of a beneficial intestinal population for the host and serves as an antagonist for disease-causing bacteria, improving the efficiency of intensive animal production systems [1,5,6]. Many studies have been reported on the administration of probiotics involving yeasts and bacteria species. Among the bacterial probiotics applied to farm animals, lactic acid bacteria (LAB) as lactobacilli, enterococci, and pediococci, were the most commonly used [3,7,8,9].
Because their effects are niche- and host-specific, selected strains must be properly identified by genotypic methods, and their functional and safety aspects must be characterized in order to improve the design of probiotic formulas. The potential benefits of probiotics must also be assayed in the animals to which they are to be administered. However, given the huge/large scale of animal production systems, it is necessary to deliver high numbers of probiotic live bacteria biomass produced in low-cost media, using appropriate fermentation procedures. LAB are exigent microorganisms requiring carbohydrates, amino acids, peptides, vitamins, and Mg/Mn salts for adequate growth. In commercial media, MRS [10] is optimal; however, due to its high cost, it is inappropriate for large-scale biomass production. In previous studies, the presence of yeast extract, starch hydrolysate, vitamins, and ammonium sulfate/ammonium phosphate salts enhanced lactic acid and cell biomass production [11,12,13]; however, this also proved to be expensive. In order to obtain high biomass yield for lactobacilli on a large production scale, the use and/or supplementation with agro-industrial residues, such as cheese whey, whey protein concentrate, molasses, soybean protein, corn-derived products, and pectin, among others, resulted in improved production [11,13,14,15]. In addition to medium formulation, LAB probiotic activity is affected by culture conditions, such as temperature, pH, and oxygen/redox level, as well as the industrial process. As a rule, an optimal temperature is set for growth [16,17]. Since probiotic strains for farm animals were isolated from their feces/GIT, 37 °C is deemed appropriate. Concerning optimal pH, the medium acidification by LAB growth is challenging for industrial production; lactic acid accumulation may affect cell physiology, and growth interruption or reduction may occur. Thus, pH control close to neutrality can support a higher growth rate. In addition, the growth of LAB is rarely enhanced by the presence of oxygen given the microaerophyllical nature of LAB probiotics; therefore, high agitation of the medium must be avoided [17]. The industrial application of LAB probiotics depends on biomass concentration and preservation technologies to ensure long-term stability in terms of viability and functional activity [18]. For long-term storage, water must be eliminated; therefore, cell harvesting and concentration after fermentation is commonly carried out by centrifugation or membrane filtration. Freeze drying is by far the most conventional process used in the industrial production of dried bacterial cultures. Consequently, the use of cryoprotectants is essential for cell survival [17]. Hence, this study evaluates the optimal conditions for the growth and preservation of LAB probiotic strains isolated from feedlot cattle, with the benefits of these strains being confirmed by in-feed supplementation to animals by our research group [9,19,20].
2. Material and Methods
2.1. Probiotic Bacteria Used in This Study
The probiotic bacteria used in this study were LAB that were previously isolated from feedlot cattle and selected for their beneficial characteristics [21,22], namely, Lactobacillus acidophilus CRL2074, Limosilactobacillus fermentum CRL2085, and Limosilactobacillus mucosae CRL2069. The inoculum of strains was prepared by transferring milk–yeast extract (13% non-fat milk, 0.5% yeast extract, and 1% glucose) and glycerol 20% stock cultures to MRS broth (Merck, Darmstadt, Germany) and sub-cultured twice in the same medium at 37 °C for 16 h.
2.2. Optimization of Culture Media
To optimize the culture medium for each probiotic strain, the previously formulated Mgl medium containing (g/L): glucose, 10; yeast extract, 20; ammonium citrate, 2; sodium acetate 5; MgSO4.7H2O, 0.1; MnSO4.H2O, 0.05; Tween 80, 1 mL; and pH 6.5 [21] was used as the basal medium. In this medium, glucose and yeast extract were replaced by the assayed carbon and yeast extract sources.
2.3. Carbon Sources
Different agro-industrial products and residues were evaluated as carbon sources at different concentrations to select the optimal culture medium for each probiotic strain. High-fructose corn syrup (HFCS 55%, Fructo AR55, Arcor, Arroyito, Argentina), lactose (Cicarelli, Iriondo, Argentina), molasses (Arcor, Arroyito, Argentina), pectin (PuraQuimica, Argentina), IMO corn syrup (Isomaltooligosaccharides fiber, Arcor, Arroyito, Argentina), and glucose (Britania, Buenos Aires, Argentina) as a control, each at concentrations ranging from 0.25 to 1.5%, were evaluated. The assessment of different carbon sources was carried out using a simplex–centroid mixture statistical model [23], in which a center-point run with equal amounts of all the ingredients was included. In this study, agro-industrial residues were used as mixtures of carbon sources in different combinations and in concentrations ranging from 0.25 to 1.5%. Thus, concentrated solutions (10%) of each carbon source, and the Mgl medium without glucose, and a pH of 6.5 ± 0.2, were prepared. All ingredients and culture media were sterilized (121 °C; 20 min). Active probiotic strains were inoculated (1% v/v) in Mgl (5 mL), to which the evaluated carbon source/s were previously added at a volume to reach the concentration given by the mixture design experiment (Table 1). MRS broth was also included as a control. Most of the growth protocols were conducted using sterile polystyrene microplates (Merck, Darmstadt, Germany). Growth kinetics were evaluated in 200 μL of previously inoculated Mgl medium and the OD560 of each well was measured using a microplate reader (VersaMax Molecular Devices, San Josè, CA, USA) over 14 h at 37 °C. By applying the simplex—centroid mixture model, 60 samples (broth culture media) were generated for each probiotic lactobacillus. The samples contained only one carbon source (6), combinations of two carbon sources (45), and combinations of the six components (9). The results of the effect of carbon sources on probiotic growth were analyzed using Minitab® 17.1 software. This was done by selecting the optimal carbon source in the culture medium for each probiotic strain by the maximal OD560 at 14 h. The growth rate μ (h−1) for each culture medium was also calculated. Statistical analysis was performed considering the maximal OD560 values obtained up to 14 h of growth.

Table 1.
Statistical model applied (simplex–centroid mixture matrix) for the selection of carbon sources to optimize culture media ingredients for probiotic strains.
2.4. Nitrogen Sources
To optimize nitrogen sources for the growth of the probiotic strains, we evaluated laboratory yeast extracts (Britania, Buenos Aires, Argentina and Neogen, Buenos Aires, Argentina) and industrial dehydrated yeast Tulev (Lince SA, Córdoba, Argentina), FM503, FM902, FM985, and LM (lactobacilli medium) (Angel Yeast Co., Ltd., Yichang, China) at concentrations ranging from 0.5% to 2.5%; urea (MP Biomedicals, Irvine, CA, USA) 1% and (NH4)2SO4, (Cicarelli, Iriondo, Argentina) 0.05%. Mgl basal medium (5 mL, from which yeast extract was omitted) was used along with each nitrogen source at different concentrations, as previously specified, and molasses (3%) as a carbon source. After media sterilization (121 °C; 20 min), active probiotic strains were inoculated (1% v/v). Growth kinetics were evaluated using polystyrene microplates (Merck, Darmstadt, Germany) in 200 μL of previously inoculated Mgl medium, and the OD560 of each well was measured using a microplate reader (VersaMax Molecular Devices, San Josè, CA, USA) over 14 h at 37 °C. The results were then analyzed using Minitab v17.1 Statistics Software to select the optimal nitrogen source for the culture medium, which would allow the highest growth of the probiotic strains. Statistical analysis was performed considering the maximal OD560 values obtained over up to 14 h of growth.
2.5. Optimization of Growth Conditions for Biomass Production
2.5.1. Evaluation of Temperature and pH in MRS Medium
The influence of temperature (30 and 37 °C) and pH (free and controlled) on biomass production was evaluated for each probiotic strain. Flasks containing 250 mL of MRS broth adjusted to pH 6.5 ± 0.2 were inoculated (1% v/v) with the active cultures of probiotic strains. Incubation was carried out at both temperatures over 24 h, with free and controlled pH, and with/without agitation. Growth with agitation and controlled pH was conducted using flask incubation in a temperature-controlled shaker operated at 100 rpm (PsycroTherm, New Brunswick Scientific, Enfield, CT, USA). Samples were aseptically collected at 0, 3, 6, 9, and 24 h, and growth was determined by OD560 (Spectronic 20, Bausch & Lomb, Rochester, NY, USA). The pH values were measured by a PT-10 pH meter with penetration electrodes (Sartorius AG, Göttingen, Germany). In addition, biomass production at 24 h was determined after centrifugation (Beckman Coulter Avanti J-E, Brea, CA, USA) by weight difference (Flaskpellet—Flaskempty). The obtained data were used to calculate μmax and ODmax. All the protocols were carried out in duplicate and the results were expressed as mean ± SD.
2.5.2. Biomass Production in a Laboratory Bioreactor
To evaluate the influence of controlled or free pH on the probiotic strains, batch mode fermentation was used. A 1.5 L bioreactor (Benchtop Bioreactor Infors HT Minifors, Minneapolis, MI, USA) containing 1 L of optimized culture medium was used, with in situ sterilization (121 °C; 20 min). The growth temperature was maintained at 37 °C and a broth formulated with Mgl (without glucose and with Britania laboratory yeast extract) + 3% Mol was used. For the free pH trials, they were initially adjusted to 6.5, while controlled pH was achieved through automatic adjustment with 1N NaOH. The culture medium without aeration was stirred at a speed of 75 rpm to maintain the homogeneity of the ingredients during growth. A speed of 200 rpm was used for the controlled pH experiments. After inoculation (1% v/v) with the active cultures of probiotic strains, fermentation was carried out over 24 h. Samples were taken aseptically from the bioreactor at 0, 3, 6, 9, and 24 h. LAB growth was determined by viable cell numbers, quantified after seriated dilutions and plating on MRS agar (incubated at 37 °C for 16 h). For pH measurements (free pH), a PT-10 pH meter with a penetration electrode (Sartorius AG, Göttingen, Germany) was used, and probiotic biomass (g/L) production at 24 h was determined by weighting the cellular pellet after centrifugation (Beckman Coulter Avanti J-E, Brea, CA, USA).
2.6. Preservation of the Probiotic Biomass
To preserve the probiotic biomass, freeze drying (lyophilization) was used. Probiotic strains were grown at 37 °C for 16 h in the optimized medium for each strain. Biomass from each optimal medium culture was obtained by centrifugation (10,000× g, 15 min at 4 °C). The cell pellets were washed twice with sterile distilled water, centrifuged (7000× g; 10 min at 4 °C) (Beckman Coulter Avanti J-E, Brea, CA, USA), and re-suspended in solutions with the following protectors at a concentration of 10%: fructose (Sigma, St. louis, MI, USA), sucrose (Cicarelli, Iriondo, Argentina), trehalose (Anedra, Buenos Aires, Argentina), industrial monosodium glutamate (MSG, Fufeng, Qingdao, China), and whey protein concentrate (WPC, Sancor, Sunchales, Argentina). Additionally, the following combinations were used: GMS (1.2%) + trehalose (10%) and fructose + sucrose + MSG + WPC (10% each). Each cryoprotectant was suspended in water, and aliquots (0.3 mL) of each bacterial suspension were dispensed in sterile ampoules, frozen at −20 °C and dried in a chamber-type freeze-drier (Lyovac GT2; Leybold, Köln, Cologne, Germany) for 16 h at 0.3 mbar to obtain <1% residual moisture. Samples were collected post freezing at −20 °C; after freeze drying and storage at 4 °C over 30 and 90 days serially diluted, plated in MRS agar, and incubated at 37 °C for 48 h and CFU/mL were counted. The survival rate was expressed as N/Ni, in which N: log CFU/mL after treatment and Ni: log CFU/mL before treatment.
2.7. Maintenance of the Probiotic Properties of the Lactobacilli
After producing dry-concentrated probiotic biomass through freeze drying, the evaluation of probiotic properties, including hydrophobicity and auto-aggregation of the lactobacillus strains, was performed using the methodology described by Maldonado et al. [22].
2.8. Statistics
The results were expressed as the mean value ± standard deviation of the data. All the assays were performed in duplicate or triplicate. A value of p < 0.05 was considered statistically significant by applying an analysis of variance (ANOVA). The results were analyzed using Minitab® 17 software (manufacture, city, if any state, country). The statistical analysis was carried out considering the maximal values of OD560nm up to 14 h of growth and the log CFU/mL in each one of the growth media assayed and selected as optimal.
3. Results
3.1. Selection of Carbon and Nitrogen Sources
In a previous study, three probiotic lactobacilli were selected from those that were isolated, characterized, and evaluated as feed supplementation for feedlot cattle [9,19,20,22]. In the search for low-cost medium formulation to produce a large-scale probiotic biomass as an alternative to commercial culture media, Mgl was designed as a basal medium by omitting peptone, beef extract, and H2PO4 (ingredients in MRS broth). Glucose (10 g/L) and yeast extract (20 g/L) were decreased and increased, respectively, compared with MRS. These ingredients resulted in a medium with a final pH of 5.9, allowing the highest cell number production (>109 CFU/mL), as previously reported by Aristimuño Ficoseco et al. [21]. Therefore, the Mgl medium was formulated using only yeast extract and glucose as nitrogen and carbon sources, respectively.
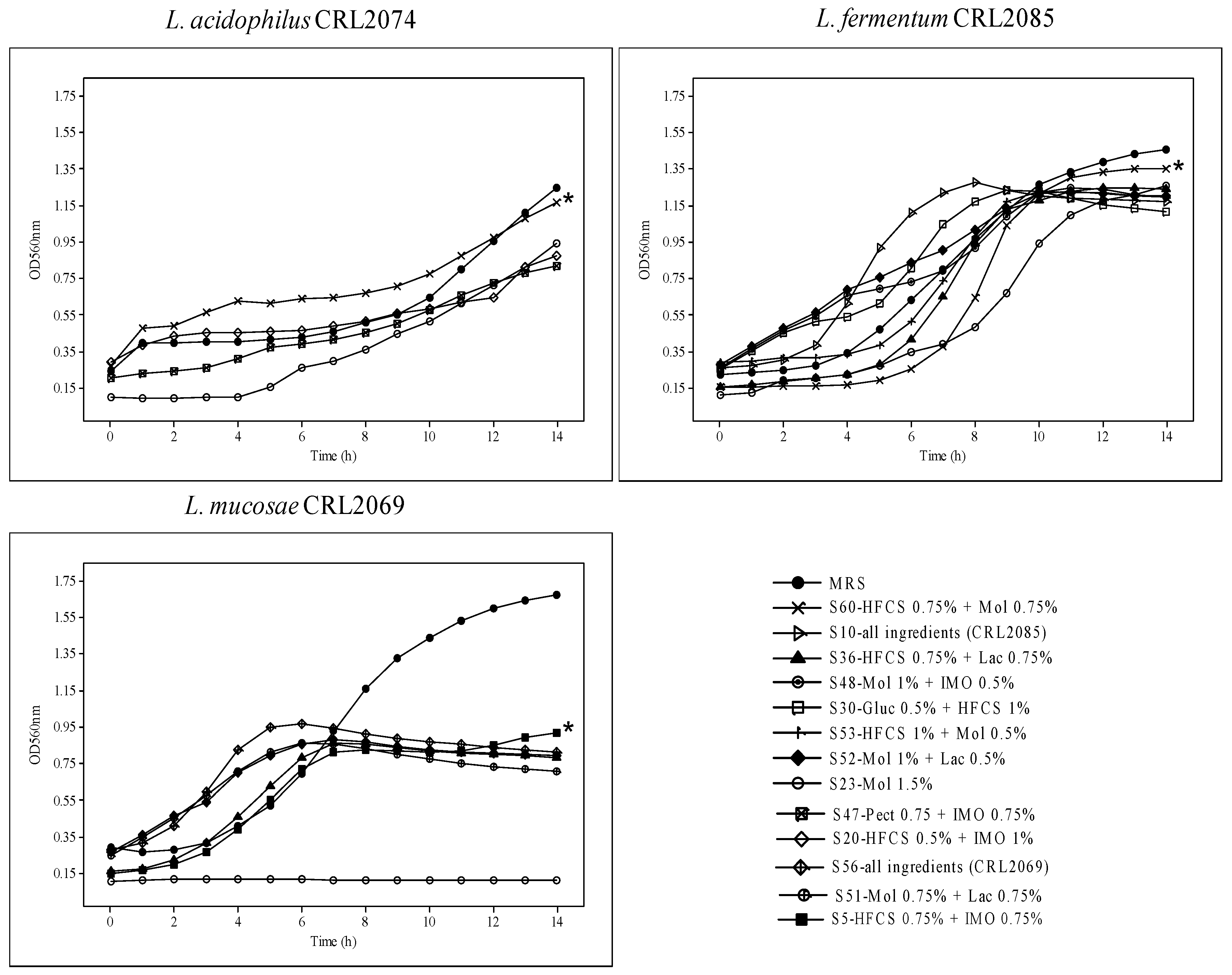
To optimize a large-scale medium for probiotic biomass production, in this study, we used the Mgl basal medium with added agro-industrial residues/products as sources of carbon and nitrogen. For the selection of carbon sources, we used the simplex–centroid mixture statistical model to establish the relationships between the proportions of different variables when either all of the components (mixtures of glucose, lactose, molasses, pectin, sucrose, and corn syrup) or the individual components were added, at the same range (0.25–1.5%). Using this statistical design, 60 different carbon source mixes were generated (Table 1) and inoculated with each of the probiotic strains (L. acidophilus CRL2074, L. fermentum CRL2085, and L. mucosae CRL2069). The different growth responses at OD560nm showed that the growth kinetics of probiotic lactobacilli were dependent both on the strain and the culture medium composition (Figure 1; Table 2).
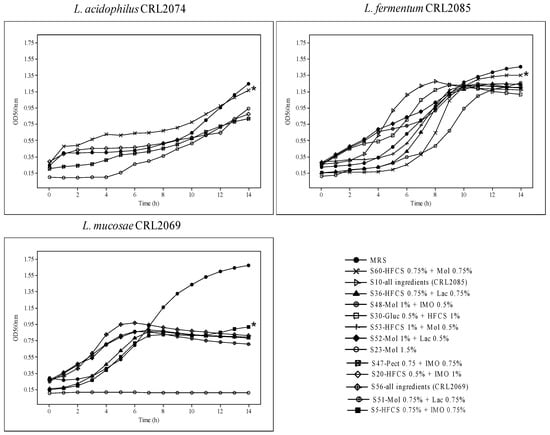
Figure 1.
Viable cell numbers of probiotic strains grown in different carbon sources. * Significant differences (p < 0.05) are indicated after evaluating growth in different culture media, tested using the Tukey tests.

Table 2.
Growth parameters of probiotic lactobacilli strains on selected culture media.
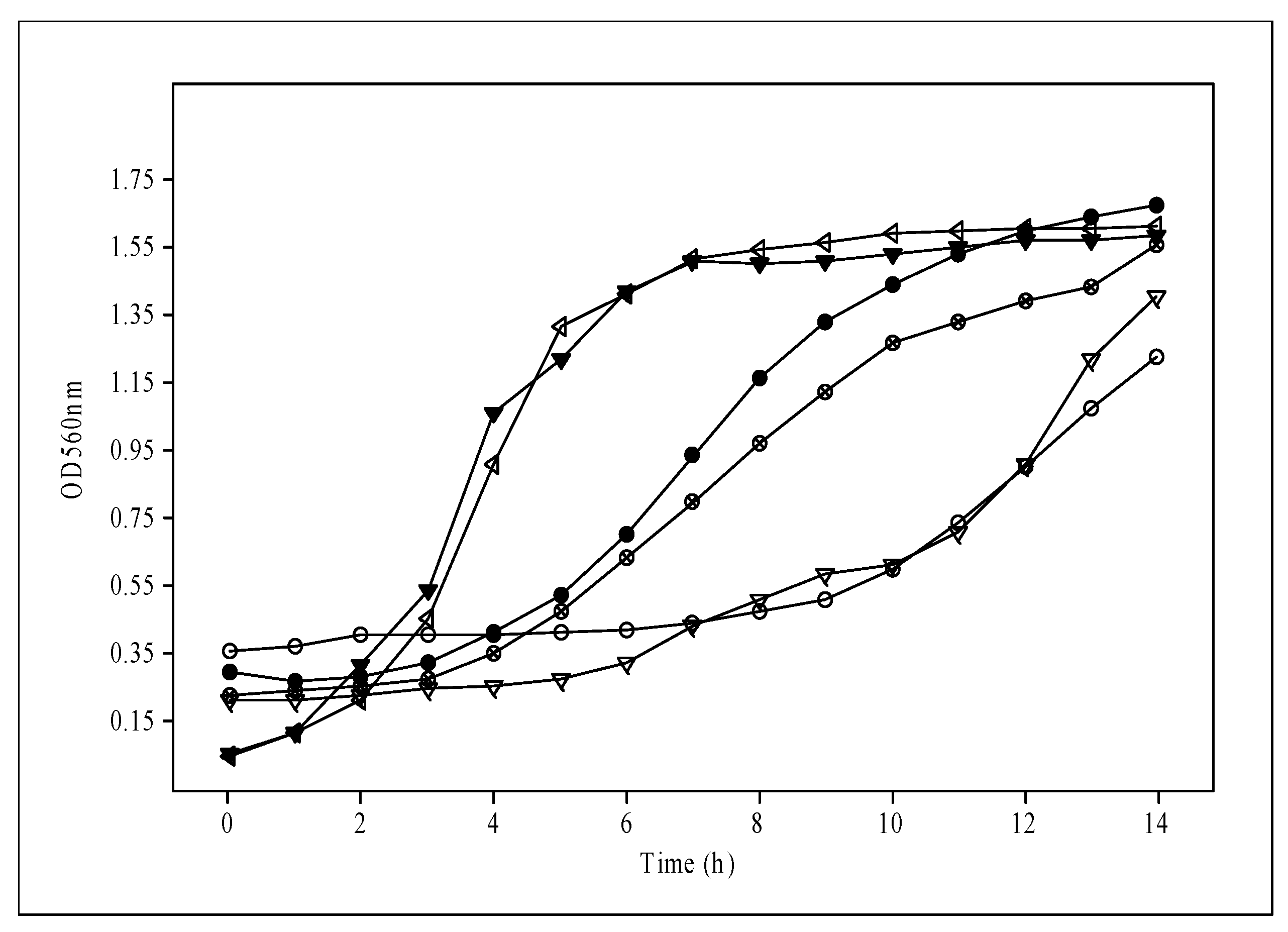
The results for the MRS broth used as a control showed max OD560 with values of 1.24, 1.46, and 1.68 for CRL2074, CRL2085, and CRL2069, respectively, at 14 h. However, lower maximal OD560 values were reached for the formulated media of probiotic lactobacilli. When we analyzed the time (in hours) taken by the bacteria to reach max OD560, the L. fermentum CRL2085 and L. mucosae CRL2069 probiotic strains showed 8 to 10 h for S10, S30, S52, and S53 media, and 4 to 7 h for S36, S51, S52, and S56 media, respectively. L. acidophilus CRL2074 showed 14 h for all the assayed media to reach max OD560, indicating great dependence on the culture medium ingredients and the strain. However, the highest maximum growth rate (µmax) reached by the probiotic lactobacilli ranged from 0.334 ± 0.02 (1.5% Mol) to 0.480 ± 0.01 h−1 (0.75% HFCS + 0.75% Mol) for CRL2085, and from 0.157 ± 0.02 (1% Mol + 05% Lac) to 0.346 ± 0.05 h−1 (0.75% HFCS + 0.75% Lac) for CRL2069. However, a lower µmax was found for probiotics grown in MRS (<0.260 h−1) after 14 h of incubation (Table 2). In contrast, none of the assayed culture media inoculated with L. acidophilus CRL2074 were able to reach µmax values higher than those obtained with the MRS medium (0.196 ± 0.02 h−1). Given that the highest growth rates and/or the shortest time to reach max OD were recorded for probiotic lactobacilli in media containing fructose (HFCS) or molasses (S5, S10, S23, S36, S48, S51, S52, S53, S56, S60), or both (S53, S60), it is suggested that these should be the preferred carbon sources used for the probiotic strains. Indeed, L. acidophilus CRL2074 and L. fermentum CRL2085 reached the highest max OD560nm growing in the Mgl + 0.75% Fruct + 0.75% Mol (S60) medium, while a medium containing all the ingredients (S56) allowed L. mucosae CRL2069 to reach max OD560. These results provide evidence that HFCS and molasses containing sucrose, fructose, and glucose as major saccharides may be used as cost-efficient carbohydrate sources for the industrial production of probiotic bacteria. Consequently, and in order to simplify the assays by using a single carbon source, molasses at a concentration of 3% added to the Mgl medium was also evaluated. L. fermentum CRL2085 and L. mucosae CRL2069 exhibited higher growth rates (μmax: 0.425 h−1 and 0.495 h−1, respectively), initiating the stationary phase after 8 h, compared with MRS (Figure 2; Table 2). L. acidophilus CRL2074 showed a similar growth rate in 3% Mol and MRS broth up to 12 h, reaching higher max OD560 at 14 h in the presence of molasses. It is known that the shorter the time to reach the stationary phase, the greater the impact on production economics.
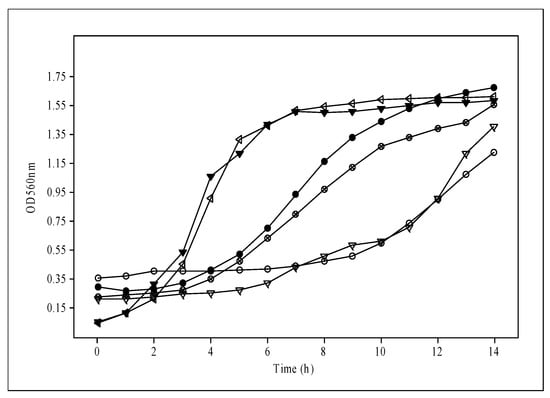
Figure 2.
Growth of probiotic strains in media with added 3% molasses compared with the MRS medium.
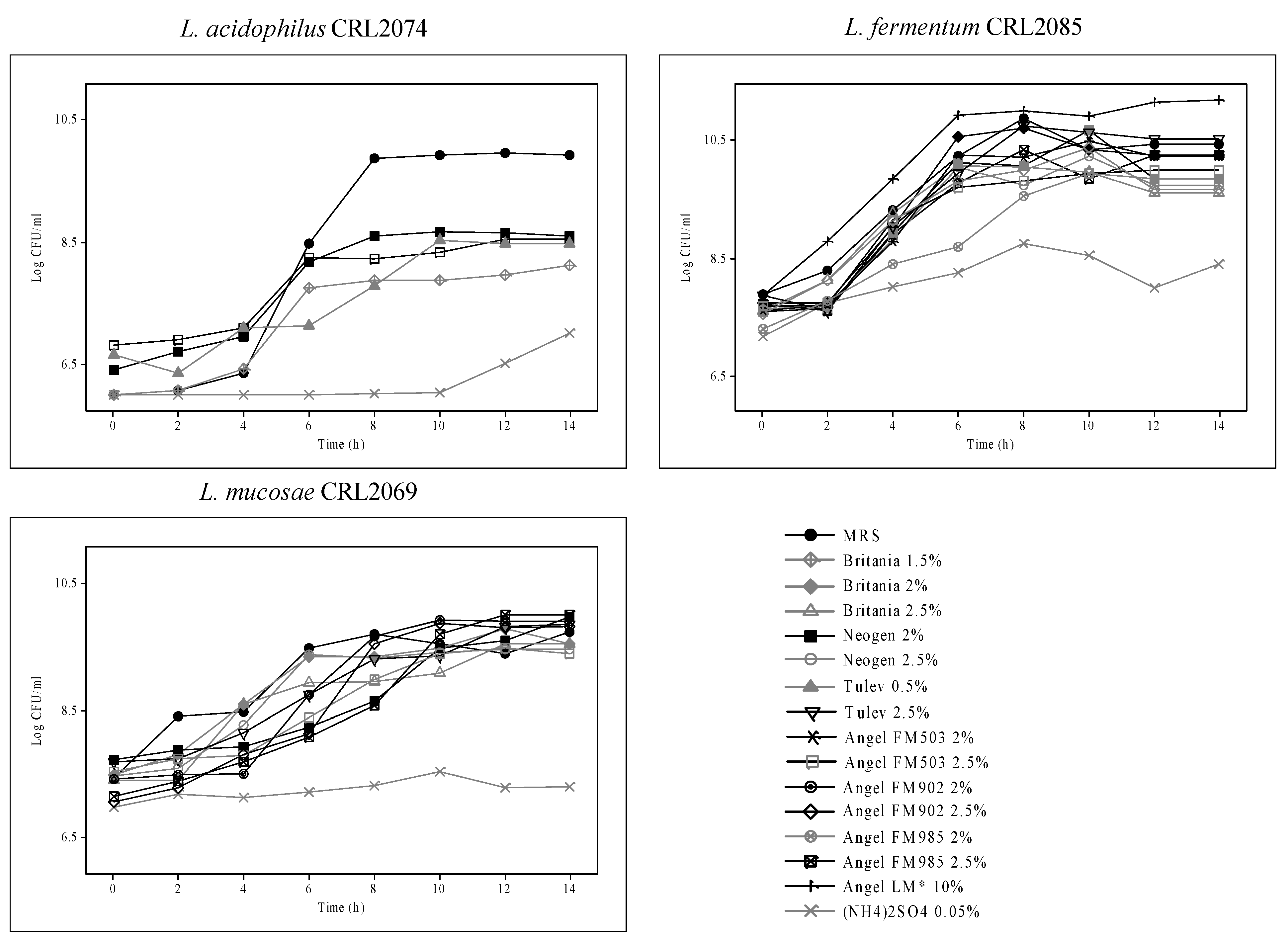
To optimize the nitrogen source for the production of probiotic strains, different yeast extracts (laboratory and industrial products), ammonium sulfate (0.05%), and urea (1%) were assayed, with MRS used as a control (Figure 3). Different yeast extracts (YEs) as nitrogen compounds were added to basal medium Mgl + 3% Mol, and the log CFU/mL of probiotic strains during 14 h of growth was evaluated. A high dependence of YEs as a nitrogen source used for probiotic lactobacilli growth was observed. Indeed, compared with MRS broth, industrial 2.0% FM503 and 2.5% Tulev yielded the highest L. fermentum CRL2085 growth, with cell numbers >10.5 log CFU/mL after 14 h. Similarly, the highest growth with final cell counts of around 10 log CFU/mL was observed for L. mucosae CRL2069, compared with MRS, in the presence of laboratory 2.0% Neogen YE and industrial dehydrated 2.5% Tulev, 2.5% FM985, and 2.0% FM902 YEs added to the basal medium. Conversely, no growth or lower than MRS growth was exhibited by the L. acidophilus CRL2074 strain; maximal cell counts of 8 log CFU/mL were attained at 14 h when 2% Neogen, 0.5% Tulev, and 2.5% FM503 YEs were assayed (Figure 3). Moreover, none of probiotic strains were able to grow in the presence of urea, and very low growth was observed when (NH4)2SO4 was used as a nitrogen source. Of the yeast extracts evaluated, both industrial dehydrated products, Tulev and Angel Yeast (FM503, FM985, and FM902), as well as the laboratory Neogen YE enhanced CRL2085 and CRL2069 probiotic growth. In contrast, MRS was optimal for CRL2074. Based on these results, 2% Neogen, 0.5% Tulev, 2% FM503, and 2% FM985 were selected for further production condition assays.
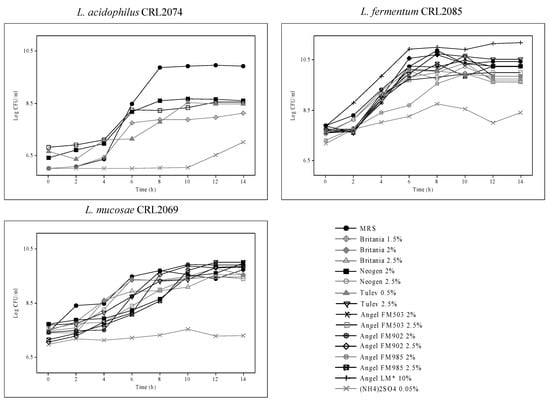
Figure 3.
Viable cell numbers of probiotic strains grown in different commercial nitrogen sources.
3.2. pH and Incubation Temperature Impact on Biomass Production for Probiotic Lactobacilli
To further optimize the growth rate, the effect of physicochemical operating conditions, medium pH, and incubation temperature were evaluated. Since the probiotic lactobacilli were isolated from bovine feces, it may be assumed that a temperature between 30 and 37 °C would be optimal for growth. An initial pH of 6.5, free or controlled, under static or agitated conditions during 24 h was assayed. The results showed biomass production as being higher for L. acidophilus CRL2074, L. fermentum CRL2085, and L. mucosae CRL2069 at 37 °C under static conditions and free pH, with a production of 12.9, 9.4, and 13.0 g/L of cell mass, respectively, during 24 h (Table 3). When bacterial fermentation was conducted with agitation under free and controlled pH at both temperatures, biomass yield was much lower compared with static conditions. All three strains reduced pH below 5.0; maximal pH reduction (4.10) was reached by L. acidophilus CRL2074 at 24 h. In addition, the production of probiotic lactobacilli was scaled up in a 1.5 L laboratory bioreactor with a work volume of 1 L. A previously optimized culture medium of Mgl + 3% Mol + selected yeast extract, gentle agitation for nutrient homogenization, and 37 °C under free and controlled pH conditions were used for growth and biomass production during 24 h, which was monitored by CFU/mL increase.

Table 3.
Probiotic lactobacillus growth conditions and cell biomass production in MRS medium.
The results in Table 4 show that the L. fermentum CRL2085 and L. mucosae CRL2069 probiotic strains reached maximal cell counts of 8.65 × 1010 and 2.3 × 1010 CFU/mL, respectively, under controlled pH, while wet biomass production in the bioreactor resulted in 12.95 and 18.20 g/L, respectively. L. acidophilus CRL2074 growing under free pH showed maximal cell numbers of 1.0 × 109 CFU/mL, while a biomass of 12.25 g/L was produced at 24 h when pH 3.6 was reached. Biomass production scaled up in a lab bioreactor using optimized medium and conditions showed that CRL2085 and CRL2069 exhibited significantly higher biomass production of 27 and 30%, respectively, compared with that in the MRS broth (as a control), while CRL2074 produced a similar biomass to that of MRS. None of the probiotic strains was able to grow in the presence of urea as a nitrogen source, while ammonium sulfate enabled very low cell mass for all three probiotic strains.

Table 4.
Probiotic lactobacillus growth and biomass production in bioreactor with optimized media at free and controlled pH (6.5) at 37 °C.
3.3. Biomass Preservation
Probiotics need to have high viability when they are administered; therefore, it is necessary to ensure the preservation of their high numbers in the obtained biomass. Methods to maintain the high survival of probiotic cells include spray drying and freeze drying or lyophilization. In this study, freeze drying was used because to the advantages of this method due to the enhanced stability reached by probiotic microorganisms during storage by the use of cryoprotectants. Thus, different cryoprotective additives were evaluated (Table 5). The probiotic cells were differently affected by the cryoprotectants used; survival between 88 and 99% after 30 d of storage at 4 °C was found. When suspended in MSG, L. acidophilus CRL2074 showed the highest viability (8.74 log CFU/g), with an estimated survival rate of 96% after 30 d. In contrast, L. fermentum CRL2085 and L. mucosae CRL2069 reached maximal viability when suspended in MIX (fructose + sucrose + trehalose + MSG + WPC; 10% each) and 1.2% WPC + 10% Treh, respectively, increasing their viability after freeze drying to 9.96 and 9.99 log CFU/g. Moreover, after 30 d of storage, CRL2085 and CRL2069 showed survival rates of 88 and 99%, respectively (Table 5), and 70% at 90 d. The results showed mono- and di-saccharides as exhibiting a low cryoprotective effect when used alone. MSG and the WPC + Treh and MIX combinations served as the best suspending media to preserve the probiotics’ high viability.

Table 5.
Effect of cryoprotective additives on the viability of probiotic lactobacilli after freeze drying, survival rate and stability.
3.4. Maintenance of Probiotic Properties after Freeze Drying
To evaluate the effect of freeze drying on the probiotic properties of lactobacilli, bacterial surface properties were evaluated after 30 days and a comparison was conducted with those that were previously obtained. The results in Table 6 show higher auto-aggregation values in MRS and Mgl + 3% Mol (optimal selected medium) for L. acidophilus CRL2074 and L. fermentum CRL2085. The results were somewhat lower for L. mucosae CRL2069 when compared with the original values. However, although the affinity of the probiotic strains to both solvents was lower in both assayed media after freeze drying than than originally detected affinity [22], their hydrophobic character was maintained with higher values for xylene than toluene.

Table 6.
Surface properties of probiotic lactobacilli at 30 days after freeze drying.
4. Discussion
Large-scale and low-cost probiotic production to deliver high numbers of bacteria to intensive animal systems is becoming an important issue. The formulation of industrial culture media must ensure the necessary nutritional requirements to support the growth of each lactobacillus selected for the probiotic formula. Because the cost of culture media has a significant impact on the mass production of probiotics, substitution with low-cost nutrient ingredients, simplification of the culture media, and optimization of growth conditions is crucial for production economics. This study sought to sustainability optimizes the culture media and conditions for large-scale production of probiotic lactobacilli as well as controlling their properties. Lactic acid bacteria are strain-dependent, fastidious microorganisms with respect to nutrient and growth condition requirements, and these factors are key environmental parameters for their growth. Even when the MRS medium represents rich and suitable conditions supporting optimal lactobacillus growth, its high formulation cost and potential environmental hazards make it unviable for large-scale commercial applications. In a previous study, Mgl containing yeast extract (20 g/L), glucose (10 g/L), ammonium citrate (2 g/L), sodium acetate (5 g/L), Mg and Mn salts (0.1 and 0.05 g/L), Tween 80 (1 mL/L), and pH 5.9–6.0 was preliminarily selected for probiotic lactobacillus growth [21]. Casein/beef peptones as nitrogenous sources in the Mgl medium were omitted because of environmental concerns due to the high amount of waste generated. Moreover, after bovine spongiform encephalopathy, it is necessary to avoid using ingredients of animal origin in culture media for probiotics intended for cattle [24].
At an industrial scale, the optimization of culture medium ingredients to obtain high cell biomass yields is closely related to the availability of components that are low cost and mainly regionally available. These factors will influence the probiotics’ efficacy, functional attributes, survival, and stability during the production stages and storage of the designed formula. Among industrial products/residues that are locally available for use as low-cost carbon sources, molasses, pectin, high-fructose, and IMO corn syrup (derived from sugarcane, citrus, and corn processing) as well as the disaccharide lactose (selected due to its high availability) were used to replace glucose in the Mgl basal medium. From the different carbon source combinations generated by the applied/designed statistical model, L. acidophilus CRL2074, L. fermentum CRL2085, and L. mucosae CRL2069 showed maximal growth when glucose was replaced by single molasses, HFCS, or both, although these combinations were unable to reach higher OD560 than that those found for MRS broth. However, when the molasses concentration was increased to 3% and used as the sole carbon source, maximal OD560 values were higher than those obtained from MRS.
Molasses and HFCS are the main byproducts of the sugarcane and corn industry. Molasses contain (average on dry matter base) sucrose (48.8%), fructose (8.1%), and glucose (5.3%), amino acids, vitamins, minerals, and lactic acid as essential nutrients [25]. Sucrose as a disaccharide containing glucose and fructose represents a major substrate for LAB growth. Acidification was reported to promote sucrose hydrolysis in molasses into the inverted sugar fructose and glucose; this process is produced by LAB growth, in addition to the spontaneous acidification of molasses [26]. HFCS (fructose 55% and glucose 37–40%, according to the manufacturer) is produced from corn starch, which is broken down by enzymes. Some of its glucose is then further isomerized into fructose [27]. The higher growth of the CRL2074 and CRL2085 probiotic strains when molasses and/or fructose were used as carbon sources, agrees with their ability to utilize sucrose, fructose, and glucose for growth [28,29]. However, lower growth was observed for CRL2069 when molasses/fructose was present at low concentrations (≤1.5%) in the formulated media. This result being correlated with the inability of some L. mucosae strains to utilize fructose, but their ability to use sucrose, as reported when comparative genomics analysis was applied [30]. However, the higher growth of L. mucosae CRL2069 when 3% Mol was added as the sole carbon source showed a shorter lag phase than MRS, which correlates with this strain’s ability to use sucrose, the main component of molasses. The use of molasses as a low-cost component and undesired waste from the sugar industry to optimize large-scale probiotic biomass production has also been reported; moreover, soybean meal or wheat stillage as carbon and nitrogen sources (to replace costly yeast extract) for the intensive growth of L. mucosae, L. johnsonii, and L. plantarum probiotics, as well as for the production of mannitol by L. intermedius and protease by the Bacillus strains has also been reported [11,31,32,33]. Similarly, molasses and corn steep liquor (nitrogen source) as components of a low-cost medium enable high cell mass and lactic acid production by L. salivarius [34]. Although the use of pectin (waste from the citrus industry) in the formulation of culture media has been reported to enhance the growth of lactobacilli and bifidobacteria [14], no increase in the growth of probiotic strains was found in this study. However, the addition of isomaltooligosaccharide (IMO) corn syrup (a high maltose prebiotic) showed much lower growth of probiotic strains; maximal OD560 was exhibited when IMO combined with molasses was added to the Mgl base medium. Consequently, a lower number of live cells of the L. acidophilus probiotic strain at 24 h was reported when the medium was supplemented with fructooligosaccharide commercial prebiotic compared with mono- and di-saccharides [35]. Lactobacilli conversion of α-glycosides involves the widely distributed dextranase DexB for IMO hydrolysis; although metabolic enzymes for oligosaccharide metabolism are intracellular, their transport is limited [36].
When nitrogen sources were assayed, even when laboratory YEs allowed probiotics to reach growth similar or higher to MRS at 14 h, these are not considered low-cost nitrogen sources for industrial biomass production. However, when industrial Angel YEs (FM503, FM983, and FM902) were used as nitrogen sources, higher maximal growth of heterofermentative probiotics (CRL2085 and CRL2069) was promoted, while Tulev YE enhanced the growth of the CRL2074 strain. Despite efforts to find a suitable partial/total replacement for yeast extract owing to its high cost, it has proven difficult to replace its effectiveness in the cultivation of lactobacilli [37]. Yeast extracts as industrial nitrogen sources were used in this study; these are claimed by the manufacturer to contain balanced free amino acids, peptides, vitamins, nucleotides, and trace elements, and as they are bulk produced and commercialized, they would be less expensive. Industrial waste products, soybean meal (45% protein) and corn steep licquor, were also used to replace YE as low-cost nitrogen sources for lactobacillus biomass production [11,13].
The effects of the initial pH of the media and culturing temperature on probiotics growth and biomass production were investigated using an optimized medium. This is because not only medium formulation, but also physicochemical parameters are critical factors. The results showed that both initial and maintained pH (during growth) and incubation temperature affected the biomass production of the probiotic lactobacilli. The preliminary growth of the CRL2074, CRL2085, and CRL2069 probiotic strains in MRS at 37 °C under static conditions showed maximal biomass production. Lower or similar biomass was produced under controlled pH at the same temperature, while agitation reduced biomass production at both temperatures. It is known that pH can influence probiotics’ functionality, such as tolerance to gastric conditions, adhesion to intestinal epithelia, or immunomodulatory activity. Indeed, the higher resistance of L. rhamnosus E800 to bile salts and gastric acidity was reported when biomass production was conducted at controlled pH 5.0 and not at pH 5.8 [38]. Moreover, although a similar biomass was obtained at pH between 6.0 and 4.0, IL-12 production was higher at pH 4.0 [39]. The self-imposed acid stress by lactobacilli during growth on different carbon sources make them relatively acid-tolerant; this feature is important for probiotic lactobacilli whose growth/survival in the presence of metabolizable sugars may be enhanced by the acidic environment [40]. In this study, compared with the MRS medium, biomass production in the lab bioreactor using optimized media and culture conditions increased by 37 and 46%, reaching cell numbers of 8.65 × 1010 and 2.3 × 1010 CFU/mL at 24 h for CRL2085 and CRL2069 probiotics, respectively. A cell mass increase of 22% was observed for the CRL2074 strain. Because the probiotic strains were isolated from bovine feces, higher biomass production was obtained at the optimal growth temperature of 37 °C. Consequently, biomass production by L. plantarum 200655 was enhanced by using a lab-scale bioreactor containing maltose, yeast extract, and soytone as critical components to obtain yields higher than 50% compared with the MRS medium [41]. Similarly, biotherapeutic L. reuteri DSM 20016T and L. plantarum AS-14 biomass was reported to increase by 55% and 57% in optimized media compared with un-optimized or MRS media [13,42]. In this study, heterofermentative CRL2085 and CRL2069 probiotics registered similar viable counts (1010 CFU/g) with controlled pH, producing higher biomass when compared with free pH cultures. Similarly, higher cell counts were achieved by L. rhamnosus 64 grown in a dairy-based medium and batch fermentation with controlled pH than in under free pH conditions [43]. The significant difference in biomass amount produced by the CRL2085 and CRL2069 strains when pH-controlled batch fermentation conditions were used may be attributed to the different cell morphology developed by each strain. This has been previously reported for the largely different dry biomass amounts produced by the L. acidophilus (La-5) and L. paracasei (Lp-01) probiotics, even when exhibiting similar viable counts [24]. Nevertheless, since probiotics must be administered in high numbers, with high viability, the amount of biomass may not be as relevant as the final viable counts obtained after technological production; cells can be of different weights depending on the production culture medium, and can generate more biomass with fewer viable cells [13]. In addition, bacterial biomass does not take into account the viability of cells with sub-lethal damage or dead cell weight.
The industrial application of LAB depends on the concentration and preservation technologies to ensure the stability of cultures in terms of viability and functional activity. Freeze drying is by far the most conventional process used for the industrial production of dried bacterial cultures [17]. It is the most suitable and widely used method of preserving probiotic bacteria, yeasts, and sporulating fungi by reducing water activity on water removal; long-term storage is achieved by reducing water activity to values lower than 0.2 [18]. The stability of probiotic bacteria during freeze drying and storage can be enhanced by adding cryoprotectants; these agents are adsorbed on bacterial cell membranes to form a viscous layer, inhibiting the intracellular formation of ice [44]. In this study, seven cryoprotectants were assayed, both individually and combined. The results indicate that MSG was the only agent to protect CRL2074, while combinations of all the ingredients assayed and WPC + Treh were shown to protect CRL2085 and CRL2069, respectively, during freeze drying and storage. MSG was reported as the better agent to increase the survival of lactobacilli, either alone or combined with sucrose and skim milk; it provided significantly high viable counts and increased survivability after freeze drying of L. acidophilus TTC4962 and L. salivarius W13 [37,45]. However, L. mucosae CRL2069 exhibited maximal protection after freeze drying when the cells were suspended in MSG and WPC + Treh mix. WPC consists of about 80% protein, while the disaccharide trehalose, protects cell membranes by replacing water molecules and stabilizing them in the dry state [46]. Trehalose showed a high protective effect for probiotic lactobacilli; a high survival rate after lyophilization was observed with media containing skim milk and trehalose [47]. When the effect of cryoprotectants was evaluated after freeze drying and storage of L. fermentum CRL2085, a maximal survival rate was observed with a medium containing a mixture of five components involving Treh. Consequently, adding trehalose prior to freezing had a cryoprotective effect on the survival of L. fermentum present in wheat sourdough [48]. The disaccharides, fructose and sucrose, as well as WPC, have also been described as lactobacillus protectants upon freeze drying [44,49]. It was reported that a combination of protein and sugar can have synergistic effect on cell survival, rather than acting individually [50]. However, the viability of the three probiotic strains was found to be non-significant when fructose or sucrose were added individually or in combination with other agents; this loss in viability may be due to the use of these monosaccharaides by bacterial cells.
As lyophilization and storage are strain-specific, this may interfere with the probiotics’ features and functionality, and consequently, this must be taken into consideration in product development. In this study, cell surface hydrophobicity related to the adhesion ability to the intestinal epithelium was used as a control of freeze drying and storage. When the superficial features of probiotic cells were determined and compared with those previously exhibited [21], the results indicated that their functionality was retained after freeze drying when using previously selected cryoprotectants and 30-d of storage at 4 °C. Similarly, the degree of cell surface hydrophobicity of freeze-dried L. plantarum strains in skim milk + sucrose and a combination of rice protein–fructooligosaccharides was not different from the original cells, as previously demonstrated for LAB from different ecosystems [50,51,52,53].
5. Conclusions
To support the large-scale production of the L. acidophilus CRL2074, L. fermentum CRL2085, and L. mucosae CRL2069 probiotic strains, in this study, we optimized a low-cost culture medium and cultivation conditions, which significantly enhanced biomass production for the three probiotics strains. The use of molasses (3%) as undesired waste from the sugar industry as well as low-cost industrial yeast extracts showed maximal, large-scale probiotic biomass production. Selected and effective cryoprotectants were established in a targeted, strain-specific way for high probiotic biomass production, with increased cell viability and survivability after freeze drying and storage without a decrease in functionality. Bacterial mass production by probiotic lactobacilli was enhanced to obtain higher yields than in the MRS medium. Thus, these results can be successfully used as a basis for the industrial cultivation of these probiotics.
Author Contributions
Conceptualization, G.M.V. and M.E.F.N.-M.; methodology, C.A.F. and F.I.M.; validation, C.A.F. and F.I.M.; formal analysis, M.E.F.N.-M.; investigation, C.A.F. and F.I.M.; writing original draft preparation, G.M.V.; writing—review and editing, G.M.V. and M.E.F.N.-M.; funding acquisition, G.M.V. and M.E.F.N.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Consejo Nacional de Investigaciones Científicas y Técnicas (PIP 744) and Agencia Nacional de Promoción Científica y Tecnológica (PICT 2018-664-PICT 2017-4032).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Adjei-Fremah, S.; Ekwemalor, K.; Worku, M.; Ibrahim, S. Probiotics and Ruminant Health. In Probiotics. Current Knowledge and Future Prospects; Enany, S., Ed.; IntechOpen: London, UK, 2018; Chapter 8; pp. 133–150. [Google Scholar] [CrossRef]
- Alayande, K.A.; Aiyegoro, O.A.; Ateba, C.N. Probiotics in animal husbandry: Applicability and associated risk factors. Sustainability 2020, 12, 1087. [Google Scholar] [CrossRef]
- Zamojska, D.; Nowak, A.; Nowak, I.; Macierzyňska-Piotrowska, E. Probiotics and Postbiotics as substitutes of antibiotics in farm animals: A Review. Animals 2021, 11, 3431. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Anee, I.J.; Alam, S.; Begum, R.A.; Md Shahjahan, R.; Khandaker, A.M. The role of probiotics on animal health and nutrition. J. Basic Appl. Zool. 2021, 82, 52. [Google Scholar] [CrossRef]
- Kober, A.K.M.H.; Riaz Rajoka, M.S.; Mehwish, H.M.; Villena, J.; Kitazawa, H. Immunomodulation potential of probiotics: A novel strategy for improving livestock health, immunity, and productivity. Microorganisms 2022, 10, 388. [Google Scholar] [CrossRef] [PubMed]
- Bajagay, Y.S.; Klieve, A.V.; Dart, P.J.; Bryden, W.L. Probiotics in Animal Nutrition–Production, Impact and Regulation. In FAO Animal Production and Health Paper; FAO: Rome, Italy, 2016. [Google Scholar]
- Cangiano, L.R.; Yohe, T.T.; Steele, M.A.; Renaud, D.L. Strategic use of microbial-based probiotics and prebiotics in dairy calf rearing. Appl. Anim. Sci. 2020, 36, 630–651. [Google Scholar] [CrossRef]
- Mansilla, F.I.; Aristimuño Ficoseco, C.; Miranda, M.H.; Puglisi, E.; Nader-Macías, M.E.F.; Vignolo, G.M.; Fontana, C.A. Administration of probiotic lactic acid bacteria to modulate fecal microbiome in feedlot cattle, Sci. Rep. 2022, 12, 12957. [Google Scholar] [CrossRef]
- De Mann, J.C.; Rogosa, M.; Sharpe, E.M. A medium for the cultivation of lactobacilli. J. Appl. Bacteriol. 1960, 23, 130–135. [Google Scholar] [CrossRef]
- Chiang, M.L.; Chen, H.C.; Chen, K.N.; Lin, Y.C.; Lin, Y.T.; Chen, M.J. Optimizing production of two potential probiotic lactobacilli strains isolated from piglet feces as feed additives for weaned piglets. Asian-Australas. J. Anim. Sci. 2015, 28, 1163–1170. [Google Scholar] [CrossRef]
- Hwang, C.F.; Chang, J.H.; Houng, J.Y.; Tsai, C.C.; Lin, C.K.; Tsen, H.Y. Optimization of medium composition for improving biomass production of Lactobacillus plantarum Pi06 using the Taguchi Array design and the Box-Behnken method. Biotechnol. Bioprocess Eng. 2012, 17, 827–834. [Google Scholar] [CrossRef]
- Manzoor, A.; Qazi, J.I.; Haq, I.; Mukhtar, H.; Rasool, A. Significantly enhanced biomass production of a novel bio-therapeutic strain Lactobacillus plantarum (AS-14) by developing low cost media cultivation strategy. J. Biol. Eng. 2017, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, E.; Manuel, S.G.A.; Hassan, S.H. Effect of fruit pectin on growth of lactic acid bacteria. J. Prob. Health 2016, 4, 2. [Google Scholar] [CrossRef]
- Coghetto, C.C.; Bettker Vasconcelos, C.; Brusch Brinques, G.; Záchia Ayubet, M.A. Lactobacillus plantarum BL011 cultivation in industrial isolated soybean protein acid residue. Braz. J. Microbiol. 2016, 47, 941–948. [Google Scholar] [CrossRef]
- Śliżewska, K.; Chlebicz-Wójcik, A. Growth kinetics of probiotic Lactobacillus strains in the alternative, cost-efficient semi-solid fermentation medium. Biology 2020, 9, 423. [Google Scholar] [CrossRef] [PubMed]
- Vinderola, G.; Champagne, C.P.; Desfossès-Foucailt, E. The Production of Lactic Acid Bacteria Starters and Probiotics. An Industrial Perspective. In Lactic Acid Bacteria. Microbioloical and Functional Aspects; Vinderola, G., Ouwehand, A.C., Salminen, S., von Wright, A., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2019; Chapter 20; pp. 317–336. [Google Scholar]
- Carvalho, A.S.; Silva, J.; Ho, P.; Teixeira, P.; Malcata, F.X.; Gibbs, P. Relevant factors for the preparation of freeze-dried lactic acid bacteria. Int. Dairy J. 2004, 14, 835–847. [Google Scholar] [CrossRef]
- Mansilla, F.I.; Miranda, M.H.; Uezen, J.D.; Maldonado, N.C.; D’Urso Villar, M.A.; Merino, L.A.; Vignolo, G.M.; Nader-Macias, M.E.F. Effect of probiotic lactobacilli supplementation on growth parameters, blood profile, productive performance, and fecal microbiology in feedlot cattle. Res. Vet. Sci. 2023, 155, 76–87. [Google Scholar] [CrossRef]
- Mansilla, F.I.; Miranda, M.H.; Aristimuño Ficoseco, C.; Obregozo, M.; D’Urso Villar, M.; Nader-Macias, M.E.F.; Vignolo, G.M. Use of probiotic lactobacilli as alternative to monensin in beef feedlot cattle. Res. Vet. Sci. 2023; in press. [Google Scholar]
- Aristimuño Ficoseco, C.; Mansilla, F.I.; Maldonado, N.C.; Miranda, H.; Nader-Macias, M.E.F.; Vignolo, G.M. Safety and growth optimization of lactic acid bacteria isolated from feedlot cattle for probiotic formula design. Front. Microbiol. 2018, 9, 2220. [Google Scholar] [CrossRef]
- Maldonado, N.C.; Aristimuño Ficoseco, C.; Mansilla, F.I.; Melián, C.; Hébert, E.M.; Vignolo, G.M.; Nader-Macías, M.E.F. Identification, characterization and selection of autochthonous lactic acid bacteria as probiotic for feedlot cattle. Livest. Sci. 2018, 212, 99–110. [Google Scholar] [CrossRef]
- Buruk Şahin, Y.; Aktar Demirtaş, E.; Burnak, N. Mixture design: A review of recent applications in the food industry. Pamukkale Univ. J. Eng. Sci. 2016, 22, 297–304. [Google Scholar] [CrossRef]
- Heenan, C.N.; Adams, M.C.; Hosken, R.W.; Fleet, G.H. Growth medium for culturing probiotic bacteria for applications in vegetarian food products. LWT-Food Sci. Technol. 2002, 35, 171–176. [Google Scholar] [CrossRef]
- Palmonari, A.; Cavallini, D.; Sniffen, C.J.; Fernandes, L.; Holder, P.; Fagioli, L.; Fusaro, I.; Biagi, G.; Formigoni, A.; Mammi, L. Characterization of molasses chemical composition. J. Dairy Sci. 2020, 103, 6244–6249. [Google Scholar] [CrossRef]
- Sen, K.Y.; Hazwan Hussin, M.; Baidurah, S. Biosynthesis of poly(3-hydroxybutyrate) (PHB) by Cupriavidus necator from various pretreated molasses as carbon source. Biocatal. Agric. Biotechnol. 2019, 17, 51–59. [Google Scholar] [CrossRef]
- Parker, K.; Salas, M.; Nwosu, V.C. High fructose corn syrup: Production, uses and public health concerns. Biotechnol. Mol. Biol. Rev. 2010, 5, 71–78. [Google Scholar]
- Srinivas, D.; Mital, B.K.; Garg, K. Utilization of sugars by Lactobacillus acidophilus strains. Int. J. Food Microbiol. 1990, 10, 51–57. [Google Scholar] [CrossRef]
- Verce, M.; De Vuyst, L.; Weckx, S. Comparative genomics of Lactobacillus fermentum suggests a free-living lifestyle of this lactic acid bacterial species. Food Microbiol. 2020, 89, 103448. [Google Scholar] [CrossRef]
- Jia, Y.; Yang, B.; Ross, P.; Stanton, C.; Zhang, H.; Zhao, J.; Chenet, W. Comparative genomics analysis of Lactobacillus mucosae from different niches. Genes 2020, 11, 95. [Google Scholar] [CrossRef]
- Krzywonos, M.; Eberhard, T. High density process to cultivate Lactobacillus plantarum biomass using wheat stillage and sugar beet molasses. Electron. J. Biotechnol. 2011, 14, 6. [Google Scholar] [CrossRef]
- Saha, B.C. A low-cost medium for mannitol production by Lactobacillus intermedius NRRL B-3693. Appl. Microbiol. Biotechnol. 2006, 72, 676–680. [Google Scholar] [CrossRef]
- Tari, C.; Genckal, H.; Tokatlı, F. Optimization of a growth medium using a statistical approach for the production of an alkaline protease from a newly isolated Bacillus sp. L21. Process Biochem. 2006, 41, 659–665. [Google Scholar] [CrossRef]
- KiBeom, L.; Kee, K.S.; Jun, J.C. A low-cost Lactobacillus salivarius L29 growth medium containing molasses and corn steep liquor allows the attainment of high levels of cell mass and lactic acid production. Afr. J. Biotechnol. 2013, 12, 2013–2018. [Google Scholar] [CrossRef]
- Goderska, K.; Nowak, J.; Czarnecki, Z. Comparison of the growth of Lactobacillus acidophilus and Bifidobacterium bifidum species in media supplemented with selected saccharides including prebiotics. Acta Sci. Pol. Technol. Aliment. 2008, 7, 5–20. [Google Scholar]
- Gänzle, M.; Follador, R. Metabolism of oligosaccharides and starch in lactobacilli: A Review. Front. Microbiol. 2012, 3, 340. [Google Scholar] [CrossRef]
- Yeo, S.; Shin, H.; Lee, H.W.; Hong, D.; Park, H.; Holzapfel, W.; Kim, E.B.; Huh, C.S. Determination of optimized growth medium and cryoprotective additives to enhance the growth and survival of Lactobacillus salivarius. J. Microbiol. Biotechnol. 2018, 28, 718–731. [Google Scholar] [CrossRef] [PubMed]
- Saarela, M.H.; Alakomi, H.L.; Puhakka, A.; Mättö, J. Effect of the fermentation pH on the storage stability of Lactobacillus rhamnosus preparations and suitability of in vitro analyses of cell physiological functions to predict it. J. Appl. Microbiol. 2009, 106, 1204–1212. [Google Scholar] [CrossRef]
- Sashihara, T.; Sueki, N.; Furuichi, K.; Ikegami, S. Effect of growth conditions of Lactobacillus gasseri OLL2809 on the immunostimulatory activity for production of interleukin-12 (p70) by murine splenocytes. Int. J. Food Microbiol. 2007, 120, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, B.M.; Stanton, C.; Fitzgerald, G.F.; Ross, R.P. Survival of probiotic lactobacilli in acidic environments is enhanced in the presence of metabolizable sugars. Appl. Environ. Microbiol. 2005, 71, 3060–3067. [Google Scholar] [CrossRef]
- Choi, G.H.; Lee, N.K.; Paik, H.D. Optimization of medium composition for biomass production of Lactobacillus plantarum 200655 using response surface methodology. J. Microbiol. Biotechnol. 2021, 31, 717–725. [Google Scholar] [CrossRef]
- Selvamani, S.; Dailin, D.J.; Rostom, M.; Malek, R.A.; Gupta, V.K.; El-Enshasy, H.A. Optimizing medium components to enhance high cell mass production of biotherapeutic strain Lactobacillus reuteri DSM 20016T by statistical method. J. Sci. Ind. Res. 2020, 79, 798–803. [Google Scholar]
- Lavari, L.; Ianniello, R.; Paez, R.; Zotta, T.; Cuatrin, A.L.; Reinheimer, J.A.; Parente, E.; Vinderola, C.G. Growth of Lactobacillus rhamnosus 64 in whey permeate and study of the effect of mild stresses on survival to spray drying. LWT-Food Sci. Technol. 2015, 63, 322–330. [Google Scholar] [CrossRef]
- Bagad, M.; Pande, R.; Dubey, V.; Ghosh, A.S. Survivability of freeze-dried probiotic Pediococcus pentosaceus strains GS4, GS17 and Lactobacillus gasseri (ATCC 19992) during storage with commonly used pharmaceutical excipients within a period of 120 days. Asian Pac. J. Trop. Biomed. 2017, 7, 921–929. [Google Scholar] [CrossRef]
- Pyar, H.; Peh, K.K. Cost effectiveness of cryoprotective agents and modified De Man Rogosa Sharpe medium on growth of Lactobacillus acidophilus. Pak. J. Biol. Sci. 2014, 17, 462–471. [Google Scholar] [CrossRef]
- Duong, T.; Barrangou, R.; Russell, W.M.; Klaenhammer, T.R. Characterization of the tre locus and analysis of trehalose cryoprotection in Lactobacillus acidophilus NCFM. Appl. Env. Microbiol. 2006, 72, 1218–1225. [Google Scholar] [CrossRef] [PubMed]
- Jalali, M.; Abedi, D.; Varshosaz, J.; Najjarzadeh, M.; Mirlohi, M.; Tavakoli, N. Stability evaluation of freeze-dried Lactobacillus paracasei subsp. tolerance and Lactobacillus delbrueckii subsp. bulgaricus in oral capsules. Res. Pharm. Sci. 2012, 7, 31–36. [Google Scholar] [PubMed]
- Stefanello, R.F.; Nabeshima, E.H.; Iamanaka, T.; Ludwig, A.; Martins Fries, L.L.; Olivier Bernardi, A.; Venturini Copetti, M. Survival and stability of Lactobacillus fermentum and Wickerhamomyces anomalus strains upon lyophilisation with different cryoprotectant agents. Food Res. Int. 2019, 115, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Tian, F.; Liu, X.; Zhao, J.; Zhang, H.; Chen, W. Effects of cryoprotectants on viability of Lactobacillus reuteri CICC6226. Appl. Microbiol. Biotechnol. 2011, 92, 609–616. [Google Scholar] [CrossRef]
- Savedboworn, W.; Teawsomboonkit, K.; Surichay, S.; Riansa-ngawong, W.; Rittisak, S.; Charoen, R.; Phattayakorn, K. Impact of protectants on the storage stability of freeze-dried probiotic Lactobacillus plantarum. Food Sci. Biotechnol. 2019, 28, 795–805. [Google Scholar] [CrossRef]
- Montel Mendoza, G.; Pasteris, S.; Otero, M.C.; Nader-Macías, M.E.F. Survival and beneficial properties of lactic acid bacteria from raniculture subjected to freeze-drying and storage. J. Appl. Microbiol. 2014, 116, 157–166. [Google Scholar] [CrossRef]
- JuárezTomás, M.S.; De Gregorio, P.R.; LecceseTerraf, M.C.; Nader-Macías, M.E. Encapsulation and subsequent freeze-drying of Lactobacillus reuteri CRL 1324 for its potential inclusion in vaginal probiotic formulations. Eur J Pharm Sci. 2015, 15, 87–95. [Google Scholar] [CrossRef]
- Miranda, M.H.; AristimuñoFicoseco, M.E.; Nader-Macias, M.E.F. Safety, environmental and technological characterization of beneficial autochthonous lactic bacteria, and their vaginal administration to pregnant cows for the design of homologous multi-strain probiotic formulas. Braz. J. Microbiol. 2021, 52, 2455–2473. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).