Assessment of Live Lactobacilli Recovery from Probiotic Products for Vaginal Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Probiotic Formulations
2.2. Assessment of Live Lactobacilli Cells from Probiotic Products
2.3. Analysis of the Results
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Petrova, M.I.; Lievens, E.; Malik, S.; Imholz, N.; Lebeer, S. Lactobacillus species as biomarkers and agents that can promote various aspects of vaginal health. Front. Physiol. 2015, 6, 81. [Google Scholar] [CrossRef] [PubMed]
- Fettweis, J.M.; Serrano, M.G.; Girerd, P.H.; Jefferson, K.K.; Buck, G.A. A new era of the vaginal microbiome: Advances using next-generation sequencing. Chem. Biodivers. 2012, 9, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.A.; Beasley, D.E.; Dunn, R.R.; Archie, E.A. Lactobacilli Dominance and Vaginal pH: Why Is the Human Vaginal Microbiome Unique? Front. Microbiol. 2016, 7, 1936. [Google Scholar] [CrossRef] [PubMed]
- Borges, S.; Silva, J.; Teixeira, P. The role of lactobacilli and probiotics in maintaining vaginal health. Arch. Gynecol. Obstet. 2014, 289, 479–489. [Google Scholar] [CrossRef]
- Boris, S.; Barbés, C. Role played by lactobacilli in controlling the population of vaginal pathogens. Microbes Infect. 2000, 2, 543–546. [Google Scholar] [CrossRef]
- Mastromarino, P.; Vitali, B.; Mosca, L. Bacterial vaginosis: A review on clinical trials with probiotics. N. Microbiol. 2013, 36, 229–238. [Google Scholar]
- Nader-Macías, M.E.; Tomás, M.S.J. Profiles and technological requirements of urogenital probiotics. Adv. Drug Deliv. Rev. 2015, 92, 84–104. [Google Scholar] [CrossRef]
- McFarland, L.V.; Elmer, G.W. Pharmaceutical probiotics for the treatment of anaerobic and other infections. Anaerobe 1997, 3, 73–78. [Google Scholar] [CrossRef]
- Reid, G.; Jass, J.; Sebulsky, M.T.; McCormick, J.K. Potential uses of probiotics in clinical practice. Clin. Microbiol. Rev. 2003, 16, 658–672. [Google Scholar] [CrossRef]
- European Commission. Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on Medical Devices, Amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and Repealing Council Directives 90/385/EEC and 93/42/EEC. 2017. Online Resource. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32017R0745 (accessed on 31 July 2023).
- European Pharmacopeia, Live Biotherapeutic Products. Monograph 2017, 3054. Online Resource. Available online: https://www.gmp-compliance.org/gmp-news/revisions-of-ep-chapter-5-1-8-2-6-36-2-6-38-and-monograph-3053 (accessed on 31 July 2023).
- Yunes, R.A.; Poluektova, E.U.; Belkina, T.V.; Danilenko, V.N. Lactobacilli: Legal Regulation and Prospects for New Generation Drugs. Appl. Biochem. Microbiol. 2022, 58, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Pendharkar, S.; Skafte-Holm, A.; Simsek, G.; Haahr, T. Lactobacilli and Their Probiotic Effects in the Vagina of Reproductive Age Women. Microorganisms 2023, 11, 636. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Probiotics in Food: Health and Nutritional Properties and Guidelines for Evaluation. 2006. Online Resource. Available online: http://www.fao.org/3/a-a0512e.pdf (accessed on 31 July 2023).
- Hughes, V.L.; Hillier, S.L. Microbiologic characteristics of Lactobacillus products used for colonization of the vagina. Obstet. Gynecol. 1990, 75, 244–248. [Google Scholar] [PubMed]
- Goldstein, E.J.; Citron, D.M.; Claros, M.C.; Tyrrell, K.L. Bacterial counts from five over-the-counter probiotics: Are you getting what you paid for? Anaerobe 2014, 25, 1–4. [Google Scholar] [CrossRef]
- Patrone, V.; Molinari, P.; Morelli, L. Microbiological and molecular characterization of commercially available probiotics containing Bacillus clausii from India and Pakistan. Int. J. Food Microbiol. 2016, 237, 92–97. [Google Scholar] [CrossRef]
- Mugambi, M.N.; Musekiwa, A.; Lombard, M.; Young, T.; Blaauw, R. Synbiotics, probiotics or prebiotics in infant formula for full term infants: A systematic review. Nutr. J. 2012, 11, 81. [Google Scholar] [CrossRef]
- Al-Ghazzewi, F.H.; Tester, R.F. Biotherapeutic agents and vaginal health. J. Appl. Microbiol. 2016, 121, 18–27. [Google Scholar] [CrossRef]
- Collins, S.L.; McMillan, A.; Seney, S.; van der Veer, C.; Kort, R.; Sumarah, M.W.; Reid, G. Promising Prebiotic Candidate Established by Evaluation of Lactitol, Lactulose, Raffinose, and Oligofructose for Maintenance of a Lactobacillus-Dominated Vaginal Microbiota. Appl. Environ. Microbiol. 2018, 84, e02200-17. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Shen, X.; Xu, L.; Zhang, Z.; Yang, Y.; Li, P.; Ma, T.; Guo, S.; Kwok, L.-Y.; Sun, Z. Postbiotic gel relieves clinical symptoms of bacterial vaginitis by regulating the vaginal microbiota. Front. Cell Infect. Microbiol. 2023, 13, 1114364. [Google Scholar] [CrossRef]
- Migliore, A. On the new regulation of medical devices in Europe. Expert. Rev. Med. Devices 2017, 14, 921–923. [Google Scholar] [CrossRef] [PubMed]
- Owen, D.H.; Katz, D.F. A vaginal fluid simulant. Contraception 1999, 59, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Tomás, M.S.J.; Nader-Macías, M.E. Effect of a medium simulating vaginal fluid on the growth and expression of beneficial characteristics of potentially probiotic lactobacilli. Commun. Curr. Res. Educ. Top. Trends Appl. Microbiol. 2007, 2, 732–739. [Google Scholar]
- Valenta, C. The use of mucoadhesive polymers in vaginal delivery. Adv. Drug Deliv. Rev. 2005, 57, 1692–1712. [Google Scholar] [CrossRef] [PubMed]
- Vermani, K.; Garg, S. The scope and potential of vaginal drug delivery. Pharm. Sci. Technol. Today 2000, 3, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Del Gaudio, G.; Lombardi, L.; Maisetta, G.; Esin, S.; Batoni, G.; Sanguinetti, M.; Senesi, S.; Tavanti, A. Antifungal activity of the non cytotoxic human peptide hepcidin 20 against fluconazole resistant Candida glabrata in human vaginal fluid. Antimicrob. Agents Chemother. 2013, 57, 4314–4321. [Google Scholar] [CrossRef]
- Juárez Tomás, M.S.; De Gregorio, P.R.; Terraf, M.C.L.; Nader-Macías, M.E.F. Encapsulation and subsequent freeze-drying of Lactobacillus reuteri CRL 1324 for its potential inclusion in vaginal probiotic formulations. Eur. J. Pharm. Sci. 2015, 79, 87–95. [Google Scholar] [CrossRef]
- Borges, S.; Costa, P.; Silva, J.; Teixeira, P. Effects of processing and storage on Pediococcus pentosaceus SB83 in vaginal formulations: Lyophilized powder and tablets. Biomed. Res. Int. 2013, 2013, 680767. [Google Scholar] [CrossRef]
- Ventura, M.; Turroni, F.; van Sinderen, D. Probiogenomics as a tool to obtain genetic insights into adaptation of probiotic bacteria to the human gut. Bioeng. Bugs. 2012, 3, 73–79. [Google Scholar] [CrossRef]
- Palmeira-de-Oliveira, R.; Palmeira-de-Oliveira, A.; Martinez-de-Oliveira, J. New strategies for local treatment of vaginal infections. Adv. Drug Deliv. Rev. 2015, 92, 105–122. [Google Scholar] [CrossRef]
- Falagas, M.; Betsi, G.I.; Athanasiou, S. Probiotics for the treatment of women with bacterial vaginosis. Clin. Microbiol. Infect. 2007, 13, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Song, L.; Zhao, W. Effects of probiotics for the treatment of bacterial vaginosis in adult women: A meta-analysis of randomized clinical trials. Arch. Gynecol. Obstet. 2014, 289, 1225–1234. [Google Scholar] [CrossRef] [PubMed]
- Homayouni, A.; Bastani, P.; Ziyadi, S.; Mohammad-Alizadeh-Charandabi, S.; Ghalibaf, M.; Mortazavian, A.M.; Mehrabany, E.V. Effects of probiotics on the recurrence of bacterial vaginosis: A review. J. Low. Genit. Tract Dis. 2014, 18, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.F.; Sobel, J.D.; Akins, R.A.; Hassan, S.S.; Chaiworapongsa, T.; Kusanovic, J.P.; Romero, R. The vaginal microbiome: New information about genital tract flora using molecular based techniques. BJOG 2011, 118, 533–549. [Google Scholar] [CrossRef] [PubMed]
- Harzallah, D.; Belhadj, H. Lactic Acid Bacteria as Probiotics: Characteristics, Selection Criteria and Role in Immunomodulation of Human GI Muccosal Barrier. In Lactic Acid Bacteria–R & D for Food, Health and Livestock Purposes; InTech Open: London, UK, 2013; pp. 197–217. [Google Scholar]
- Klaenhammer, T.R.; Altermann, E.; Pfeiler, E.; Buck, B.L.; Goh, Y.-J.; O’Flaherty, S.; Barrangou, R.; Duong, T. Functional genomics of probiotic Lactobacilli. J. Clin. Gastroenterol. 2008, 42 Pt 2, S160–S162. [Google Scholar] [CrossRef]
- de Vrese, M.; Schrezenmeir, J. Probiotics, prebiotics, and synbiotics. Adv. Biochem. Eng. Biotechnol. 2008, 111, 1–66. [Google Scholar]
- Bharwani, A.; Mian, M.F.; Surette, M.G.; Bienenstock, J.; Forsythe, P. Oral treatment with Lactobacillus rhamnosus attenuates behavioural deficits and immune changes in chronic social stress. BMC Med. 2017, 15, 7. [Google Scholar] [CrossRef]
- Grenov, B.; Namusoke, H.; Lanyero, B.; Nabukeera-Barungi, N.; Ritz, C.; Mølgaard, C.; Friis, H.; Michaelsen, K.F. Effect of Probiotics on Diarrhea in Children With Severe Acute Malnutrition: A Randomized Controlled Study in Uganda. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 396–403. [Google Scholar] [CrossRef]
- Chandel, D.S.; Perez-Munoz, M.E.; Yu, F.; Boissy, R.; Satpathy, R.; Misra, P.R.; Sharma, N.; Chaudhry, R.; Parida, S.; Peterson, D.A.; et al. Changes in the Gut Microbiota After Early Administration of Oral Synbiotics to Young Infants in India. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 218–224. [Google Scholar] [CrossRef]
- Andersson, H.; Tullberg, C.; Ahrné, S.; Hamberg, K.; Ahrén, I.L.; Molin, G.; Sonesson, M.; Håkansson, Å. Oral Administration of Lactobacillus plantarum 299v Reduces Cortisol Levels in Human Saliva during Examination Induced Stress: A Randomized, Double-Blind Controlled Trial. Int. J. Microbiol. 2016, 2016, 8469018. [Google Scholar] [CrossRef]
- De Man, J.; Rogosa, M.; Sharpe, M.E. A medium for the cultivation of lactobacilli. J. Appl. Microbiol. 1960, 23, 130–135. [Google Scholar] [CrossRef]
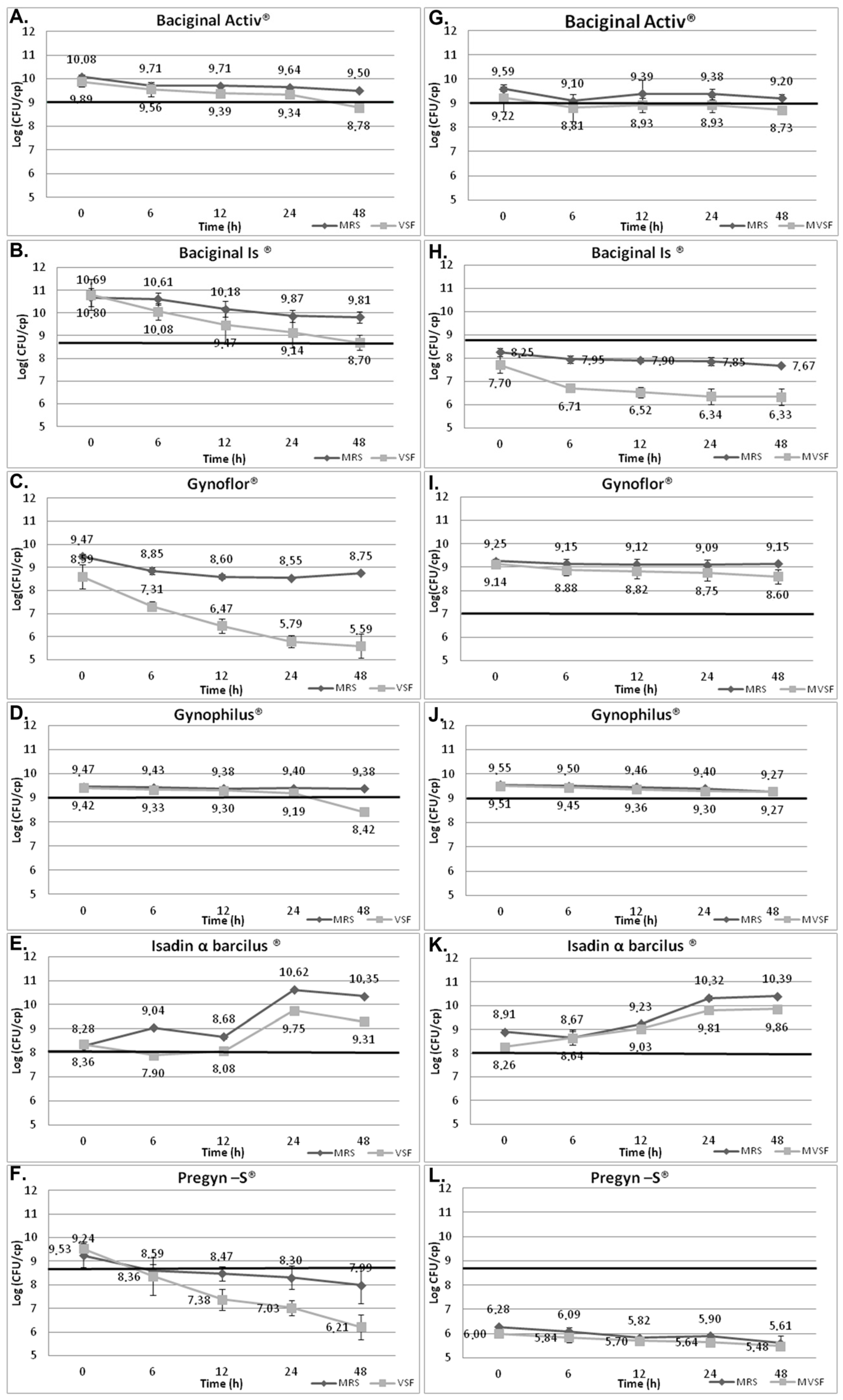
| Product | Claimed Viable Cells per Unit (log) | MRS (t0 h) | MRS (t48 h) | VFS-OK (t0 h) | VFS-OK (t48 h) | MVFS (t0 h) | MVFS (t48 h) | Species |
|---|---|---|---|---|---|---|---|---|
| Baciginal Activ® | 9 | 9.80 | 9.35 | 9.89 | 8.78 | 9.22 | 8.73 | L. acidophilus |
| Baciginal Is® | 8 | 9.47 | 8.74 | 10.80 | 8.70 | 7.70 | 6.33 | L. acidophilus |
| Gynoflor® | 7 | 9.36 | 8.95 | 8.59 | 5.59 | 9.14 | 8.60 | L. acidophilus |
| Gynophilus® | 9 | 9.51 | 9.33 | 9.42 | 8.42 | 9.51 | 9.27 | L. rhamnosus |
| Isadin α barcilus® | 8 | 8.59 | 10.37 | 8.36 | 9.31 | 8.26 | 9.86 | L. plantarum |
| Pregyn–S® | 8.5 | 7.7 | 6.80 | 9.53 | 6.21 | 6.00 | 5.48 | L. acidophilus |
| Product | Recovery (% MRS) 0 h VFS-OK | Recovery (% MRS) 0 h MVFS | p-Value | Recovery (% MRS) 48 h VFS-OK | Recovery (% MRS) 48 h MVFS | p-Value |
|---|---|---|---|---|---|---|
| Baciginal Activ® | 63.3 | 61.7 | p > 0.05 | 22.9 | 14.3 | p > 0.05 |
| Baciginal Is® | 77 | 31.95 | p < 0.05 | 8.49 | 3.93 | p > 0.05 |
| Gynoflor® | 13.4 | 76.15 | p < 0.05 | 0.07 | 28.5 | p < 0.05 |
| Gynophilus® | 89.4 | 92.3 | p > 0.05 | 11.6 | 100 | p < 0.05 |
| Isadin α barcilus® | 120 | 22.3 | p < 0.05 | 9 | 29.4 | p < 0.05 |
| Pregyn–S® | 137.9 | 53.8 | p < 0.05 | 0.97 | 63.4 | p < 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, D.N.; Gaspar, C.; Rolo, J.; Donders, G.G.G.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, R.; Palmeira-de-Oliveira, A. Assessment of Live Lactobacilli Recovery from Probiotic Products for Vaginal Application. Appl. Microbiol. 2023, 3, 1195-1203. https://doi.org/10.3390/applmicrobiol3040082
Sousa DN, Gaspar C, Rolo J, Donders GGG, Martinez-de-Oliveira J, Palmeira-de-Oliveira R, Palmeira-de-Oliveira A. Assessment of Live Lactobacilli Recovery from Probiotic Products for Vaginal Application. Applied Microbiology. 2023; 3(4):1195-1203. https://doi.org/10.3390/applmicrobiol3040082
Chicago/Turabian StyleSousa, Diana Neves, Carlos Gaspar, Joana Rolo, Gilbert G. G. Donders, José Martinez-de-Oliveira, Rita Palmeira-de-Oliveira, and Ana Palmeira-de-Oliveira. 2023. "Assessment of Live Lactobacilli Recovery from Probiotic Products for Vaginal Application" Applied Microbiology 3, no. 4: 1195-1203. https://doi.org/10.3390/applmicrobiol3040082
APA StyleSousa, D. N., Gaspar, C., Rolo, J., Donders, G. G. G., Martinez-de-Oliveira, J., Palmeira-de-Oliveira, R., & Palmeira-de-Oliveira, A. (2023). Assessment of Live Lactobacilli Recovery from Probiotic Products for Vaginal Application. Applied Microbiology, 3(4), 1195-1203. https://doi.org/10.3390/applmicrobiol3040082









