Assessment of the Spoilage Microbiota and the Growth Potential of Listeria monocytogenes in Minced Free-Range Chicken Meat Stored at 4 °C in Vacuum: Comparison with the Spoilage Community of Resultant Retail Modified Atmosphere Packaged Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chicken Meat Samples and Their Inoculation, Storage, and Sampling Conditions
2.2. Microbiological Sampling and Analyses
2.3. Isolation and Biochemical Characterization of the Dominant Chicken Meat Spoilage Microbiota
2.4. Physicochemical Analyses
2.5. Comparison of Minced Free-Range Chicken Meat VP Batch Samples with Resultant Retail MAP Products
2.6. Statistical Analysis
3. Results
3.1. Changes in pH and Organic Acid Contents in Minced VP Free-Range Chicken Meat Stored at 4 °C
3.2. Evolution of the Spoilage Microbiota in Minced VP Free-Range Chicken Meat during Storage at 4 °C
3.3. Behavior of Listeria monocytogenes in the Minced Free-Range Chicken Meat Batch Samples during Storage at 4 °C in Vacuum Packages
3.4. Predominance of Latilactobacillus sakei in Terminally Spoiled, Minced, Free-Range Chicken Meat Stored at 4 °C in Vacuum Packages
3.5. Major Variations in the Spoilage Pattern of the Fresh VP Chicken Meat Minces Compared to the Resultant MAP Products and the High-Acetate-Containing VP + AA Mince during Storage at 4 °C
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rouger, A.; Tresse, O.; Zagorec, M. Bacterial contaminants of poultry meat: Sources, species, and dynamics. Microorganisms 2017, 5, 50. [Google Scholar] [CrossRef] [PubMed]
- Yimenu, S.M.; Koo, J.; Kim, B.S.; Kim, J.H.; Kim, J.Y. Freshness-based real-time shelf-life estimation of packaged chicken meat under dynamic storage conditions. Poult. Sci. 2019, 98, 6921–6930. [Google Scholar] [CrossRef] [PubMed]
- Lytou, A.E.; Renieri, C.T.; Doulgeraki, A.I.; Nychas, G.J.E.; Panagou, E.Z. Assessment of the microbiological quality and safety of marinated chicken products from Greek retail outlets. Int. J. Food Microbiol. 2020, 320, 108506. [Google Scholar] [CrossRef] [PubMed]
- Samapundo, S.; de Baenst, I.; Aerts, M.; Cnockaert, M.; Devlieghere, F.; Van Damme, P. Tracking the sources of psychrotrophic bacteria contaminating chicken cuts during processing. Food Microbiol. 2019, 81, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Hulankova, R.; Borilova, G.; Abdullah, F.A.A.; Buchtova, H. Microbiological quality of organic chicken meat during refrigerated storage in air and modified atmospheres. Brit. Poult. Sci. 2018, 59, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Yang, L.; Zang, H.; Xu, Y.; Chen, X.; Chen, X.; Liu, P.; Geng, Z. Influence of free-range days on growth performance, carcass traits, meat quality, lymphoid organ indices, and blood biochemistry of Wannanyellow chickens. Poult. Sci. 2019, 98, 6602–6610. [Google Scholar] [CrossRef] [PubMed]
- Samelis, J. Managing microbial spoilage in the meat industry. In Food Spoilage Microorganisms; Blackburn, C.W., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2006; pp. 213–286. [Google Scholar]
- Odeyemi, O.A.; Alegbeleye, O.O.; Strateva, M.; Stratev, D. Understanding spoilage microbial community and spoilage mechanisms in foods of animal origin. Comp. Rev. Food Sci. Food Saf. 2020, 19, 311–331. [Google Scholar] [CrossRef]
- Lauritsen, C.V.; Kjeldgaard, J.; Ingmer, H.; Bisgaard, M.; Christensen, H. Microbiota encompassing putative spoilage bacteria in retail packaged broiler meat and commercial broiler abattoir. Int. J. Food Microbiol. 2019, 300, 14–21. [Google Scholar] [CrossRef]
- Rouger, A.; Moriceau, N.; Prévost, H.; Remenant, B.; Zagorec, M. Diversity of bacterial communities in French chicken cuts stored under modified atmosphere packaging. Food Microbiol. 2018, 70, 7–16. [Google Scholar] [CrossRef]
- Franke, C.; Höll, L.; Langowski, H.C.; Petermeier, H.; Vogel, R.F. Sensory evaluation of chicken breast packed in two different modified atmospheres. Food Packag. Shelf Life 2017, 13, 66–75. [Google Scholar] [CrossRef]
- Moutiq, R.; Misra, N.; Mendonça, A.; Keener, K. In-package decontamination of chicken breast using cold plasma technology: Microbial, quality and storage studies. Meat Sci. 2020, 159, 107942. [Google Scholar] [CrossRef] [PubMed]
- Rossaint, S.; Klausmann, S.; Kreyenschmidt, J. Effect of high-oxygen and oxygen-free modified atmosphere packaging on the spoilage process of poultry breast fillets. Poult. Sci. 2015, 94, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Morales, P.A.; Aguirre, J.S.; Troncoso, M.R.; Figueroa, G.O. Phenotypic and genotypic characterization of Pseudomonas spp. present in spoiled poultry fillets sold in retail settings. LWT Food Sci. Technol. 2016, 73, 609–614. [Google Scholar] [CrossRef]
- Mellor, G.E.; Bentley, J.A.; Dykes, G.A. Evidence for a role of biosurfactants produced by Pseudomonas fluorescens in the spoilage of fresh aerobically stored chicken meat. Food Microbiol. 2011, 28, 1101–1104. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.Y.; Wang, H.H.; Han, Y.W.; Xing, T.; Ye, K.P.; Xu, X.L.; Zhou, G.H. Evaluation of the spoilage potential of bacteria isolated from chilled chicken in vitro and in situ. Food Microbiol. 2017, 63, 139–146. [Google Scholar] [CrossRef]
- Labadie, J. Consequences of packaging on bacterial growth. Meat is an ecological niche. Meat Sci. 1999, 52, 299–305. [Google Scholar] [CrossRef]
- Herbert, U.; Albrecht, A.; Kreyenschmidt, J. Definition of predictor variables for MAP poultry fillets stored under different temperature conditions. Poult. Sci. 2015, 94, 424–432. [Google Scholar] [CrossRef]
- Chmiel, M.; Roszko, M.; Hać-Szymańczuk, E.; Adamczak, L.; Florowski, T.; Pietrzak, D.; Cegiełka, A.; Bryła, M. Time evolution of microbiological quality and content of volatile compounds in chicken fillets packed using various techniques and stored under different conditions. Poult. Sci. 2020, 99, 1107–1116. [Google Scholar] [CrossRef]
- Nieminen, T.T.; Nummela, M.; Björkroth, J. Packaging gas selects lactic acid bacterial communities on raw pork. J. Appl. Microbiol. 2015, 119, 1310–1316. [Google Scholar] [CrossRef]
- Dhananjayan, R.; Han, I.Y.; Acton, J.C.; Dawson, P.L. Growth depth effects of bacteria in ground turkey meat patties subjected to high carbon dioxide or high oxygen atmospheres. Poult. Sci. 2006, 85, 1821–1828. [Google Scholar] [CrossRef]
- Klein, D.; Maurer, S.; Herbert, U.; Kreyenschmidt, J.; Kaul, P. Detection of volatile organic compounds arising from chicken breast filets under modified atmosphere packaging using TD-GC/MS. Food Anal. Methods 2018, 11, 88–98. [Google Scholar] [CrossRef]
- Dainty, R.H.; Mackey, B.M. The relationship between the phenotypic properties of bacteria from chill-stored meat and spoilage processes. J. Appl. Microbiol. 1992, 73, 103S–114S. [Google Scholar] [CrossRef] [PubMed]
- Casaburi, A.; Piombino, P.; Nychas, G.J.; Villani, F.; Ercolini, D. Bacterial populations and the volatilome associated to meat spoilage. Food Microbiol. 2015, 45, 83–102. [Google Scholar] [CrossRef] [PubMed]
- Patsias, A.; Badeka, A.V.; Savvaidis, I.N.; Kontominas, M.G. Combined effect of freeze chilling and MAP on quality parameters of raw chicken fillets. Food Microbiol. 2008, 25, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Höll, L.; Behr, J.; Vogel, R.F. Identification and growth dynamics of meat spoilage microorganisms in modified atmosphere packaged poultry meat by MALDI-TOF MS. Food Microbiol. 2016, 60, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Chouliara, E.; Karatapanis, A.; Savvaidis, I.N.; Kontominas, M.G. Combined effect of oregano essential oil and modified atmosphere packaging on shelf-life extension of fresh chicken breast meat, stored at 4 °C. Food Microbiol. 2007, 24, 607–617. [Google Scholar] [CrossRef]
- Luong, N.D.M.; Coroller, L.; Zagorec, M.; Membré, J.M.; Guillou, S. Spoilage of chilled fresh meat products during storage: A quantitative analysis of literature data. Microorganisms 2020, 8, 1198. [Google Scholar] [CrossRef]
- Rothrock, J.M.J.; Micciche, A.C.; Bodie, A.R.; Ricke, S.C. Listeria occurrence and potential control strategies in alternative and conventional poultry processing and retail. Front. Sustain. Food Syst. 2019, 3, 33. [Google Scholar] [CrossRef]
- Roberts, B.N.; Chakravarty, D.; Gardner, J.C., III; Ricke, S.C.; Donaldson, J.R. Listeria monocytogenes response to anaerobic environments. Pathogens 2020, 9, 210. [Google Scholar] [CrossRef]
- Samelis, J.; Metaxopoulos, J. Incidence and principal sources of Listeria spp. and Listeria monocytogenes contamination in processed meats and a meat processing plant. Food Microbiol. 1999, 16, 465–477. [Google Scholar] [CrossRef]
- Tsafrakidou, P.; Sameli, N.; Bosnea, L.; Chorianopoulos, N.; Samelis, J. Assessment of the spoilage microbiota in minced free range chicken meat during storage at 4 °C in retail modified atmosphere packages. Food Microbiol. 2021, 99, 103822. [Google Scholar] [CrossRef] [PubMed]
- Vandera, E.; Lianou, A.; Kakouri, A.; Feng, J.; Koukkou, A.I.; Samelis, J. Enhanced control of Listeria monocytogenes by Enterococcus faecium KE82, a multiple enterocin-producing strain, in different milk environments. J. Food Prot. 2017, 80, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Samelis, J.; Kakouri, A.; Rementzis, J. The spoilage microflora of cured, cooked turkey breasts prepared commercially with or without smoking. Int. J. Food Microbiol. 2000, 56, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Samelis, J.; Kakouri, A. Growth inhibitory and selective pressure effects of sodium diacetate on the spoilage microbiota of frankfurters stored at 4 °C and 12 °C in vacuum. Foods 2021, 10, 74. [Google Scholar] [CrossRef] [PubMed]
- Association of the Official Chemists (AOAC) International. Official Methods of Analysis; Cunniff, P., Ed.; AOAC International: Rockville, MD, USA, 1998. [Google Scholar]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Sakala, R.M.; Kato, Y.; Hayashidani, H.; Murakami, M.; Kaneuchi, C.; Ogawa, M. Lactobacillus fuchuensis sp. nov., isolated from vacuum-packaged refrigerated beef. Int. J. Syst. Evol. Microbiol. 2002, 52, 1151–1154. [Google Scholar]
- Shaw, B.G.; Harding, C.D. Leuconostocgelidum sp. nov. and Leuconostoccarnosum sp. nov. from chill-stored meats. Int. J. Syst. Bacteriol. 1989, 39, 217–223. [Google Scholar] [CrossRef]
- Collins, M.D.; Farrow, J.A.E.; Phillips, B.A.; Ferusu, S.; Jones, D. Classification of Lactobacillus divergens, Lactobacillus piscicola, and some catalase-negative, asporogenous, rod-shaped bacteria from poultry in a new genus, Carnobacterium. Int. J. Syst. Bacteriol. 1987, 37, 310–316. [Google Scholar] [CrossRef]
- Lee, J.S.; Lee, K.C.; Ahn, J.S.; Mheen, T.I.; Pyun, Y.R.; Park, Y.H. Weissellakoreensis sp. nov., isolated from kimchi. Int. J. Syst. Evol. Microbiol. 2002, 52, 1257–1261. [Google Scholar]
- Dal Bosco, A.; Mattioli, S.; Cartoni Mancinell, A.; Cotozzolo, E.; Castellini, C. Extensive rearing systems in poultry production: The right chicken for the right farming system. A review of twenty years of scientific research in Perugia University, Italy. Animals 2021, 11, 1281. [Google Scholar] [CrossRef]
- Castellini, C.; Mugnai, C.; Dal Bosco, A. Effect of organic production system on broiler carcass and meat quality. Meat Sci. 2002, 60, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Fanatico, A.C.; Pillai, P.B.; Emmert, J.L.; Owens, C.M. Meat quality of slow- and fast-growing chicken genotypes fed low-nutrient or standard diets and raised indoors or with outdoor access. Poult. Sci. 2007, 86, 2245–2255. [Google Scholar] [CrossRef] [PubMed]
- Husak, R.L.; Sebranek, J.G.; Bregendahl, K. A survey of commercially available broilers marketed as organic, free-range, and conventional broilers for cooked meat yields, meat composition, and relative value. Poult. Sci. 2008, 87, 2367–2376. [Google Scholar] [CrossRef] [PubMed]
- Dal Bosco, A.; Mugnai, C.; Mattioli, S.; Rosati, A.; Ruggeri, S.; Ranucci, D.; Castellini, C. Transfer of bioactive compounds from pasture to meat in organic free-range chickens. Poult. Sci. 2016, 95, 2464–2471. [Google Scholar] [CrossRef]
- Abdullah, F.A.A.; Buchtová, H. Quantity and quality properties of breast and thigh of chicken broilers from organic and conventional production systems. J. Food Saf. Food Quality—Arch. Für Leb. 2017, 68, 56–62. [Google Scholar]
- Abdullah, F.A.A.; Buchtová, H.; Turek, P. Influence of modified atmosphere packaging on freshness parameters of organic chicken meat—Short communication. Czech J. Food Sci. 2017, 35, 466–468. [Google Scholar] [CrossRef]
- Borch, E.; Kant-Muermans, M.L.; Blixt, Y. Bacterial spoilage of meat and cured meat products. Int. J. Food Microbiol. 1996, 33, 103–120. [Google Scholar] [CrossRef]
- Vihavainen, E.; Lundström, H.S.; Susiluoto, T.; Koort, J.; Paulin, L.; Auvinen, P.; Björkroth, K.J. Role of broiler carcasses and processing plant air in contamination of modified-atmosphere-packaged broiler products with psychrotrophic lactic acid bacteria. Appl. Environ. Microbiol. 2007, 73, 1136–1145. [Google Scholar] [CrossRef]
- Melero, B.; Vinuesa, R.; Diez, A.M.; Jaime, I.; Rovira, J. Application of protective cultures against Listeria monocytogenes and Campylobacter jejuni in chicken products packaged under modified atmosphere. Poult. Sci. 2013, 92, 1108–1116. [Google Scholar] [CrossRef]
- Argyri, A.A.; Papadopoulou, O.S.; Sourri, P.; Chorianopoulos, N.; Tassou, C.C. Quality and safety of fresh chicken fillets after high pressure processing: Survival of indigenous Brochothrixthermosphacta and inoculated Listeria monocytogenes. Microorganisms 2019, 7, 520. [Google Scholar] [CrossRef]
- Zeitoun, A.A.M.; Debevere, J.M. Decontamination with lactic acid/sodium lactate buffer in combination with modified atmosphere packaging effects on the shelf life of fresh poultry. Int. J. Food Microbiol. 1992, 16, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, S.M.; Salsi, M.S.; Tiburzi, M.C.; Rafaghelli, R.C.; Pirovani, M.E. Combined use of acetic acid treatment and modified atmosphere packaging for extending the shelf-life of chilled chicken breast portions. J. Appl. Microbiol. 1999, 87, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Dave, D.; Ghaly, A.E. Meat spoilage mechanisms and preservation techniques: A critical review. Am. J Agric. Biol. Sci. 2011, 6, 486–510. [Google Scholar]
- Kakouri, A.; Nychas, G.J.E. Storage of poultry meat under modified atmospheres or vacuum packs: Possible role of microbial metabolites as indicator of spoilage. J. Appl. Bacteriol. 1994, 76, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Nychas, G.J.E.; Skandamis, P.N.; Tassou, C.C.; Koutsoumanis, K.P. Meat spoilage during distribution. Meat Sci. 2008, 78, 77–89. [Google Scholar] [CrossRef]
- Latou, E.; Mexis, S.F.; Badeka, A.V.; Kontakos, S.; Kontominas, M.G. Combined effect of chitosan and modified atmosphere packaging for shelf-life extension of chicken breast fillets. LWT Food Sci. Technol. 2014, 55, 263–268. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, Y. Non-invasive qualitative and quantitative assessment of spoilage attributes of chilled pork using hyperspectral scattering technique. Appl. Spectrosc. 2016, 70, 1309–1320. [Google Scholar] [CrossRef]
- Doulgeraki, A.I.; Ercolini, D.; Villani, F.; Nychas, G.J.E. Spoilage microbiota associated to the storage of raw meat in different conditions. Int. J. Food Microbiol. 2012, 157, 130–141. [Google Scholar] [CrossRef]
- Dalgaard, P.; Gram, L.; Huss, H.H. Spoilage and shelf life of cod fillets packed in vacuum or modified atmosphere. Int. J. Food Microbiol. 1993, 19, 283–294. [Google Scholar] [CrossRef]
- Gram, L.; Ravn, L.; Rasch, M.; Bruhn, J.B.; Christensen, A.B.; Givskov, M. Food spoilage—Interactions between food spoilage bacteria. Int. J. Food Microbiol. 2002, 78, 79–97. [Google Scholar] [CrossRef]
- Susiluoto, T.; Korkeala, H.; Björkroth, K.J. Leuconostocgasicomitatum is the dominating lactic acid bacterium in retail modified-atmosphere-packaged marinated broiler meat strips on sell-by-day. Int. J. Food Microbiol. 2003, 80, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Laursen, B.G.; Bay, L.; Cleenwerck, I.; Vancanneyt, M.; Swings, J.; Dalgaard, P.; Leisner, J.J. Carnobacteriumdivergens and Carnobacteriummaltaromaticum as spoilers or protective cultures in meat and seafood: Phenotypic and genotypic characterization. Syst. Appl. Microbiol. 2005, 28, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Pothakos, V.; Snauwaert, C.; De Vos, P.; Huys, G.; Devlieghere, F. Psychrotrophic members of Leuconostocgasicomitatum, Leuconostocgelidum and Lactococcus piscium dominate at the end of shelf-life in packaged and chilled-stored food products in Belgium. Food Microbiol. 2014, 39, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Pothakos, V.; Devlieghere, F.; Villani, F.; Björkroth, J.; Ercolini, D. Lactic acid bacteria and their controversial role in fresh meat spoilage. Meat Sci. 2015, 109, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Nychas, G.; Dillon, V.; Board, R. Glucose, the key substrate in the microbiological changes occurring in meat and certain meat products. Biotechnol. Appl. Biochem. 1988, 10, 203–231. [Google Scholar] [CrossRef] [PubMed]
- Borch, E.; Agerhem, H. Chemical, microbial and sensory changes during the anaerobic cold storage of beef inoculated with a homofermentative Lactobacillus sp. or a Leuconostoc sp. Int. J. Food Microbiol. 1992, 15, 99–108. [Google Scholar] [CrossRef]
- Kim, K.H.; Chun, B.H.; Baek, J.H.; Roh, S.W.; Lee, S.H.; Jeon, C.O. Genomic and metabolic features of Lactobacillus sakei as revealed by its pan-genome and the metatranscriptome of kimchi fermentation. Food Microbiol. 2020, 86, 103341. [Google Scholar] [CrossRef]
- Borch, E.; Berg, H.; Holst, O. Heterolactic fermentation by a homofermentative Lactobacillus sp. during glucose limitation in anaerobic continuous culture with complete cell recycle. J. Appl. Bacteriol. 1991, 71, 265–269. [Google Scholar] [CrossRef]
- DeBruyn, I.N.; Holzapfel, W.H.; Visser, L.; Louw, A.I. Glucose-metabolism by Lactobacillus divergens. Microbiology 1988, 134, 2103–2109. [Google Scholar] [CrossRef]
- Papadopoulou, O.S.; Iliopoulos, V.; Mallouchos, A.; Panagou, E.Z.; Chorianopoulos, N.; Tassou, C.C.; Nychas, G.J.E. Spoilage potential of Pseudomonas (P. fragi, P. putida) and LAB (Leuconostocmesenteroides, Lactobacillus sakei) strains and their volatilome profile during storage of sterile pork meat using GC/MS and data analysis. Foods 2020, 9, 633. [Google Scholar] [CrossRef]
- Chen, S.; Liu, S.; Ma, J.; Xu, X.; Wang, H. Evaluation of the spoilage heterogeneity of meat-borne Leuconostocmesenteroides by metabonomics and in-situ analysis. Food Res. Int. 2022, 156, 111365. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, F.; Laghi, L.; Gardini, F.; Montanari, C.; Tabanelli, G. Metabolism of Lactobacillus sakei Chr82 in the presence of different amounts of fermentable sugars. Foods 2020, 9, 720. [Google Scholar] [CrossRef] [PubMed]
- Egan, A.F.; Shay, B.J.; Rogers, P.J. Factors affecting the production of hydrogen sulphide by Lactobacillus sakei L13 growing on vacuum-packaged beef. J. Appl. Bacteriol. 1989, 67, 255–262. [Google Scholar] [CrossRef]
- Leisner, J.J.; Greer, G.G.; Stiles, M.E. Control of beef spoilage by a sulfide-producing Lactobacillus sake strain with bacteriocinogenic Leuconostocgelidum UAL187 during anaerobic storage at 2 °C. Appl. Environ. Microbiol. 1996, 62, 2610–2614. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.K.; David, J.R.D.; Gilbreth, S.E.; Smith, G.; Nietfeldt, J.; Legge, R.; Kim, J.; Sinha, R.; Duncan, C.E.; Ma, J.; et al. Microbial successions are associated with changes in chemical profiles of a model refrigerated fresh pork sausage during an 80-day shelf-life study. Appl. Environ. Microbiol. 2014, 80, 5178–5194. [Google Scholar] [CrossRef] [PubMed]
- Bouju-Albert, A.; Pilet, M.F.; Guillou, S. Influence of lactate and acetate removal on the microbiota of French fresh pork sausages. Food Microbiol. 2018, 76, 328–336. [Google Scholar] [CrossRef]
- Irkin, R.; Kizilirmak Esmer, O. Control of Listeria monocytogenes in ground chicken breast meat under aerobic, vacuum and modified atmosphere packaging conditions with or without the presence of bay essential oil at 4 °C. Food Sci. Technol. Res. 2010, 16, 285–290. [Google Scholar] [CrossRef]
- Gonçalves-Tenório, A.; Nunes Silva, B.; Rodrigues, V.; Cadavez, V.; Gonzales-Barron, U. Prevalence of pathogens in poultry meat: A meta-analysis of European published studies. Foods 2018, 7, 69. [Google Scholar] [CrossRef]
- Doulgeraki, A.I.; Paramithiotis, S.; Kagkli, D.M.; Nychas, G.J.E. Lactic acid bacteria population dynamics during minced beef storage under aerobic or modified atmosphere packaging conditions. Food Microbiol. 2010, 27, 1028–1034. [Google Scholar] [CrossRef]
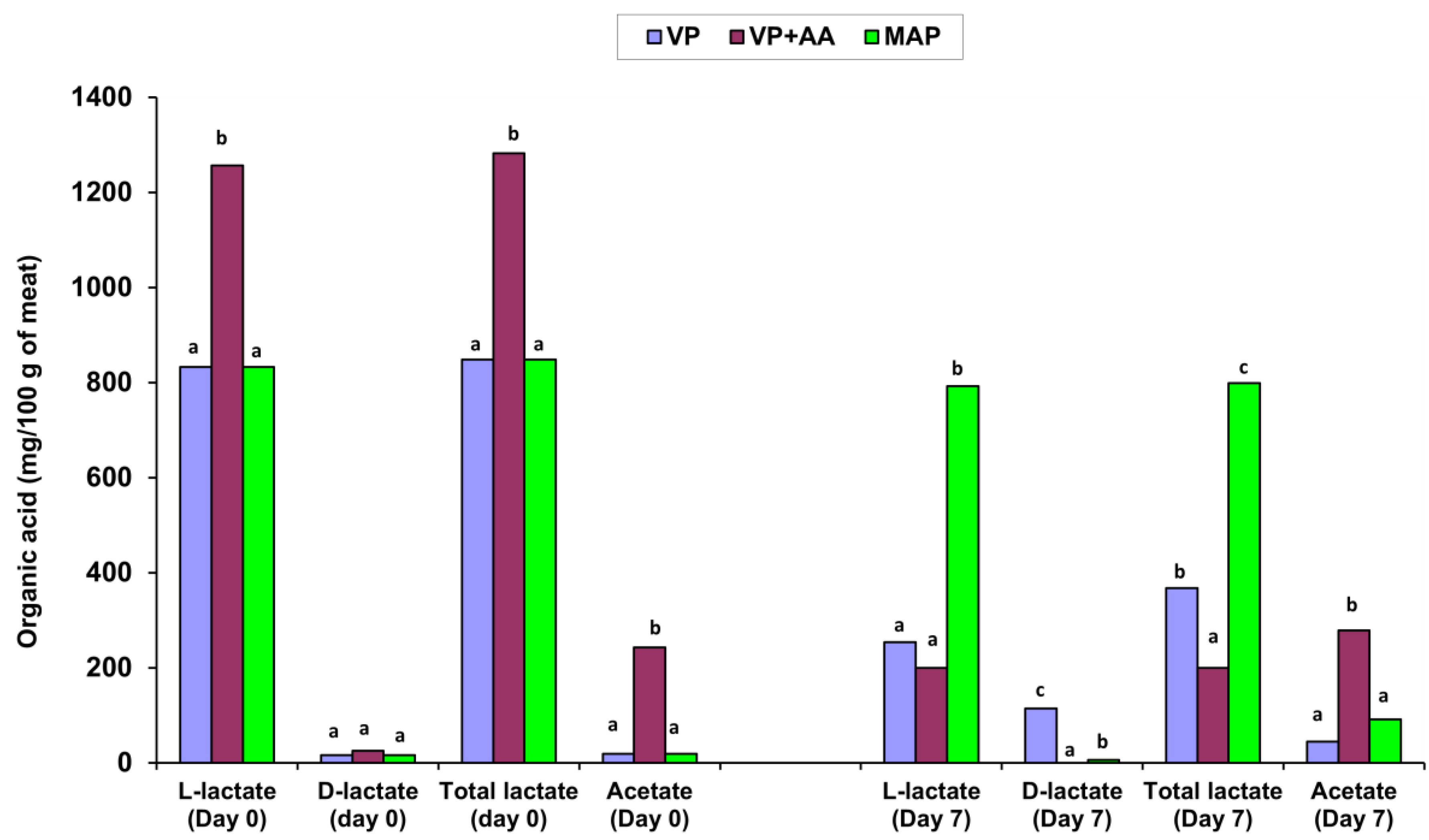

| Biochemical Parameter | Treatment | Storage at 4 °C (Days) | ||||
|---|---|---|---|---|---|---|
| 0 | 3 | 5 | 7 | 10 | ||
| Chicken meat pH | VP | 6.04 ± 0.05 Bb | 5.93 ± 0.08 Bab | 5.88 ± 0.13 Ba | 5.96 ± 0.08 Bb | 5.91 ± 0.01 Bab |
| VP + AA | 5.77 ± 0.03 Aa | 5.80 ± 0.05 Aab | 5.76 ± 0.11 Aa | 5.86 ± 0.01 Ab | 5.73 ± 0.07 Aa | |
| Organic acid 2 | ||||||
| L-lactic acid | VP | 748.3 ± 251.5 Ab | NT | NT | 217.9 ± 92.4 Aa | NT |
| VP + AA | 1256.6 ± 590.3 Bb | NT | NT | 199.8 ± 92.9 Aa | NT | |
| D-lactic acid | VP | 17.1 ± 3.7 Aa | NT | NT | 80.3 ± 100.7 Bb | NT |
| VP + AA | 25.6 ± 0.0 Ab | NT | NT | n.d. Aa | NT | |
| Total lactic acid | VP | 765.4 ± 255.2 Ab | NT | NT | 298.2 ± 193.1 Aa | NT |
| VP + AA | 1282.2 ± 590.3 Bb | NT | NT | 199.8 ± 92.9 Aa | NT | |
| Acetic acid | VP | 17.4 ± 23.3 Aa | NT | NT | 37.5 ± 15.2 Aa | NT |
| VP + AA | 242.9 ± 17.0 Ba | NT | NT | 278.2 ± 37.2 Ba | NT | |
| Microbial Group | Treatment | Storage at 4°C (Days) | ||||
|---|---|---|---|---|---|---|
| 0 | 3 | 5 | 7 | 10 | ||
| Total mesophilic microbiota | VP | 5.33 ± 0.14 Aa | 5.75 ± 0.17 Aab | 6.30 ± 0.10 Bb | 6.93 ± 0.38 Bc | 7.47 ± 0.02 Bd |
| VP + AA | 5.51 ± 0.00 Aa | 5.42 ± 0.03 Aa | 5.30 ± 0.06 Aa | 5.57 ± 0.11 Aa | 6.58 ± 0.39 Ab | |
| Lactic acid bacteria | VP | 4.15 ± 0.59 Aa | 4.81 ± 0.34 Aa | 5.69 ± 0.32 Bb | 6.53 ± 0.51 Bc | 7.19 ± 0.08 Bd |
| VP + AA | 4.36 ± 0.06 Aa | 4.59 ± 0.16 Aa | 4.45 ± 0.23 Aa | 4.89 ± 0.27 Aa | 6.31 ± 0.21 Ab | |
| Pseudomonad-like bacteria | VP | 4.17 ± 0.26 Aa | 4.30 ± 0.30 Aa | 4.59 ± 0.52 Aa | 5.14 ± 0.53 Bab | 6.11 ± 0.22 Bb |
| VP + AA | 4.50 ± 0.01 Aa | 4.52 ± 0.04 Aa | 4.40 ± 0.02 Aa | 4.47 ± 0.16 Aa | 4.38 ± 0.06 Aa | |
| Enterobacteriaceae | VP | 2.25 ± 0.19 Aa | 2.95 ± 0.23 Ab | 3.22 ± 0.58 Bab | 3.50 ± 0.58 Bb | 3.88 ± 0.37 Bb |
| VP + AA | 2.74 ± 0.37 Ac | 2.69 ± 0.13 Ac | 2.15 ± 0.21 Aab | 2.29 ± 0.01 Ab | 1.84 ± 0.20 Aa | |
| Enterococci | VP | 3.14 ± 0.56 Aa | 3.06 ± 0.18 Aa | 3.18 ± 0.30 Aa | 3.18 ± 0.33 Ba | 2.69 ± 0.13 Aa |
| VP + AA | 3.91 ± 0.29 Ab | 3.00 ± 0.00 Aa | 2.63 ± 0.21 Aa | 2.50 ± 0.28 Aa | 2.93 ± 0.04 Aa | |
| Staphylococci | VP | 4.05 ± 0.29 Aa | 4.00 ± 0.17 Aa | 3.95 ± 0.28 Aa | 3.78 ± 0.56 Aa | 3.96 ± 0.14 Aa |
| VP + AA | 4.07 ± 0.01 Aa | 4.13 ± 0.09 Aa | 4.04 ± 0.08 Aa | 4.01 ± 0.06 Aa | 3.71 ± 0.18 Aa | |
| Yeasts | VP | 3.61 ± 0.37 Aa | 3.28 ± 0.84 Aa | 3.60 ± 0.43 Aa | 3.67 ± 0.42 Aa | 3.54 ± 0.08 Aa |
| VP + AA | 4.04 ± 0.02 Aa | 3.89 ± 0.00 Aa | 4.01 ± 0.08 Aa | 4.14 ± 0.20 Aa | 3.83 ± 0.18 Aa | |
| Listeria monocytogenes | VP | 3.24 ± 0.07 Aa | 3.09 ± 0.09 Aa | 3.31 ± 0.13 Aa | 3.12 ± 0.28 Aa | 3.19 ± 0.08 Aa |
| VP + AA | 3.28 ± 0.03 Aa | 3.48 ± 0.00 Βa | 3.30 ± 0.31 Aa | 2.89 ± 0.58 Aa | 3.14 ± 0.08 Aa | |
| LAB Species | Basic Differentiating Phenotypic Characteristics | Basic Differentiating Sugar Fermentation Reactions | Total Isolates | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MA | CO2 | NH3 | 37 °C | 45 °C | 6.5% | 8% | LAra | Gal | Mal | Mel | Suc | Treh | Xyl | ||
| Latilactobacillus sakei (typical isolates) | R | − | + | + | − | + | + | + | + | − | +/+d | + | + | − | 48 |
| Latilactobacillus sakei (atypical isolates) | R | − | + | + | − | + | −/+d | − | + | − | − | + | + | − | 2 |
| Latilactobacillus curvatus | CR | − | − | + | − | + | + | − | + | + | − | 2/4 | − | − | 4 |
| Latilactobacillus fuchuensis | SR | − | − | − | − | − | − | − | + | + | − | − | + | + | 7 |
| Leuconosto ccarnosum | CB | + | − | − | − | + | 3/5 | − | − | + | − | + | + | − | 5 |
| Carnobacterium divergens | SR | (+)d | + | + | − | +d | − | − | 2/5 | + | − | + | + | − | 5 |
| Weissella koreensis | R | (+) | + | − | − | − | − | + | − | − | − | − | − | + | 2 |
| Enterococcus faecalis | C | − | + | + | + | + | + | − | + | + | + | + | + | − | 2 |
| Abiotrophia/Facklamia | C | − | (+) | − | − | − | − | − | − | ((+)) | − | (+) | (+) | − | 1 |
| 76 | |||||||||||||||
| Biochemically Identified LAB Species | Number of Isolates | Corresponding Group/Biotype in Retail MAP Products 1 | Representative 16S rRNA Identified MAP Isolates 2 | Number of Isolates from Each Chicken Meat Batch and Isolation Agar Medium | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Batch 1 (VP1) | Batch 2 (VP + AA) | Batch 3 (VP3) | Batch 4 (VP4) | ||||||||
| TSAYE | MRS | TSAYE | MRS | TSAYE | MRS | TSAYE | MRS | ||||
| Latilactobacillus sakei(typical) | 48 | A (A1 + A2) | MCM3, MCM10, MCM34, MCM46 | 3 | 10 | 6 | 8 | 2 | 9 | 3 | 7 |
| Latilactobacillus sakei (atypical) | 2 | A (A3) | MCM44 | - | - | - | - | - | - | 1 | 1 |
| Latilactobacillus curvatus | 4 | n.d. in MAP 3 | - | 2 | - | 1 | - | - | 1 | - | - |
| Latilactobacillus fuchuensis | 7 | E | MCM40 | 1 | - | 3 | 1 | - | - | 2 | - |
| Leuconostoc carnosum | 5 | B | MCM43 | - | - | - | 1 | - | - | 2 | 2 |
| Carnobacterium divergens | 5 | C | MCM9, MCM31 | 3 | - | - | - | 2 | - | - | - |
| Weissella koreensis | 2 | D | MCM50 | 1 | - | - | - | 1 | - | - | - |
| Enterococcus faecalis | 2 | n.d. in MAP 3 | - | - | - | - | - | - | - | 2 | - |
| Abiotrophia/ Facklamia | 1 | F | MCM37 | - | - | - | - | 1 | - | - | - |
| Total isolates | 76 | 10 | 10 | 10 | 10 | 6 | 10 | 10 | 10 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsafrakidou, P.; Sameli, N.; Kakouri, A.; Bosnea, L.; Samelis, J. Assessment of the Spoilage Microbiota and the Growth Potential of Listeria monocytogenes in Minced Free-Range Chicken Meat Stored at 4 °C in Vacuum: Comparison with the Spoilage Community of Resultant Retail Modified Atmosphere Packaged Products. Appl. Microbiol. 2023, 3, 1277-1301. https://doi.org/10.3390/applmicrobiol3040088
Tsafrakidou P, Sameli N, Kakouri A, Bosnea L, Samelis J. Assessment of the Spoilage Microbiota and the Growth Potential of Listeria monocytogenes in Minced Free-Range Chicken Meat Stored at 4 °C in Vacuum: Comparison with the Spoilage Community of Resultant Retail Modified Atmosphere Packaged Products. Applied Microbiology. 2023; 3(4):1277-1301. https://doi.org/10.3390/applmicrobiol3040088
Chicago/Turabian StyleTsafrakidou, Panagiota, Nikoletta Sameli, Athanasia Kakouri, Loulouda Bosnea, and John Samelis. 2023. "Assessment of the Spoilage Microbiota and the Growth Potential of Listeria monocytogenes in Minced Free-Range Chicken Meat Stored at 4 °C in Vacuum: Comparison with the Spoilage Community of Resultant Retail Modified Atmosphere Packaged Products" Applied Microbiology 3, no. 4: 1277-1301. https://doi.org/10.3390/applmicrobiol3040088
APA StyleTsafrakidou, P., Sameli, N., Kakouri, A., Bosnea, L., & Samelis, J. (2023). Assessment of the Spoilage Microbiota and the Growth Potential of Listeria monocytogenes in Minced Free-Range Chicken Meat Stored at 4 °C in Vacuum: Comparison with the Spoilage Community of Resultant Retail Modified Atmosphere Packaged Products. Applied Microbiology, 3(4), 1277-1301. https://doi.org/10.3390/applmicrobiol3040088







