Development of a Standardised International Protocol for Evaluation of the Disinfection Efficacy of Healthcare Laundry Wash Processes
Abstract
:1. Introduction
2. Methods
2.1. Chemicals
2.2. Microorganisms
2.3. Phase 1: To Determine the Efficacy of Current Methods for Measuring Disinfection within Healthcare Laundry Wash Processes
Sample Preparation
2.4. Suspension Methods
2.4.1. Recovery Media
2.4.2. Agitation Method
2.4.3. Recovery Agar
2.5. Surface Sampling Methods
2.6. Bioindicators
2.6.1. Recovery of Microorganisms from DES Controller Bioindicators
2.6.2. E. faecium DES Controller Bioindicator Retention Efficacy
2.6.3. E. faecium DES Controller Bioindicator Permeability to Disinfectants and Detergents
2.7. Comparison of Semi-Quantitative and Quantitative Enumeration Methods
2.8. Field Test
2.9. Phase 2: Development of Alternative E. faecium Strains and a Bioindicator Membrane for Low-Temperature Laundering
2.10. Disinfectant, Detergent and Thermal Tolerance on Cotton
2.11. Bioindicator Membrane Assessment for Low-Temperature Laundering
Flow Rate of the Solution through PES
2.12. Detergent and Disinfectant Membrane Permeability
2.12.1. Preparation of Samples for 1H NMR Analysis
2.12.2. Acquisition of 1H NMR Spectra
2.12.3. Spectrophotometric Determinations of Hypochlorite, Hypochlorous acid and Hydrogen Peroxide
2.12.4. PES Bioindicator Permeability to Disinfectants and Detergents
2.12.5. Comparison of PES Bioindicators and Swatch Methodologies within the Wash Process
2.13. Statistical Analysis
3. Results
3.1. Phase 1: The Efficacy of Current Methods for Measuring Disinfection within Healthcare Laundry Wash Processes
Methods for the Recovery of Microorganisms from Textiles
3.2. Recovery of Microorganisms from DES Controller Bioindicators
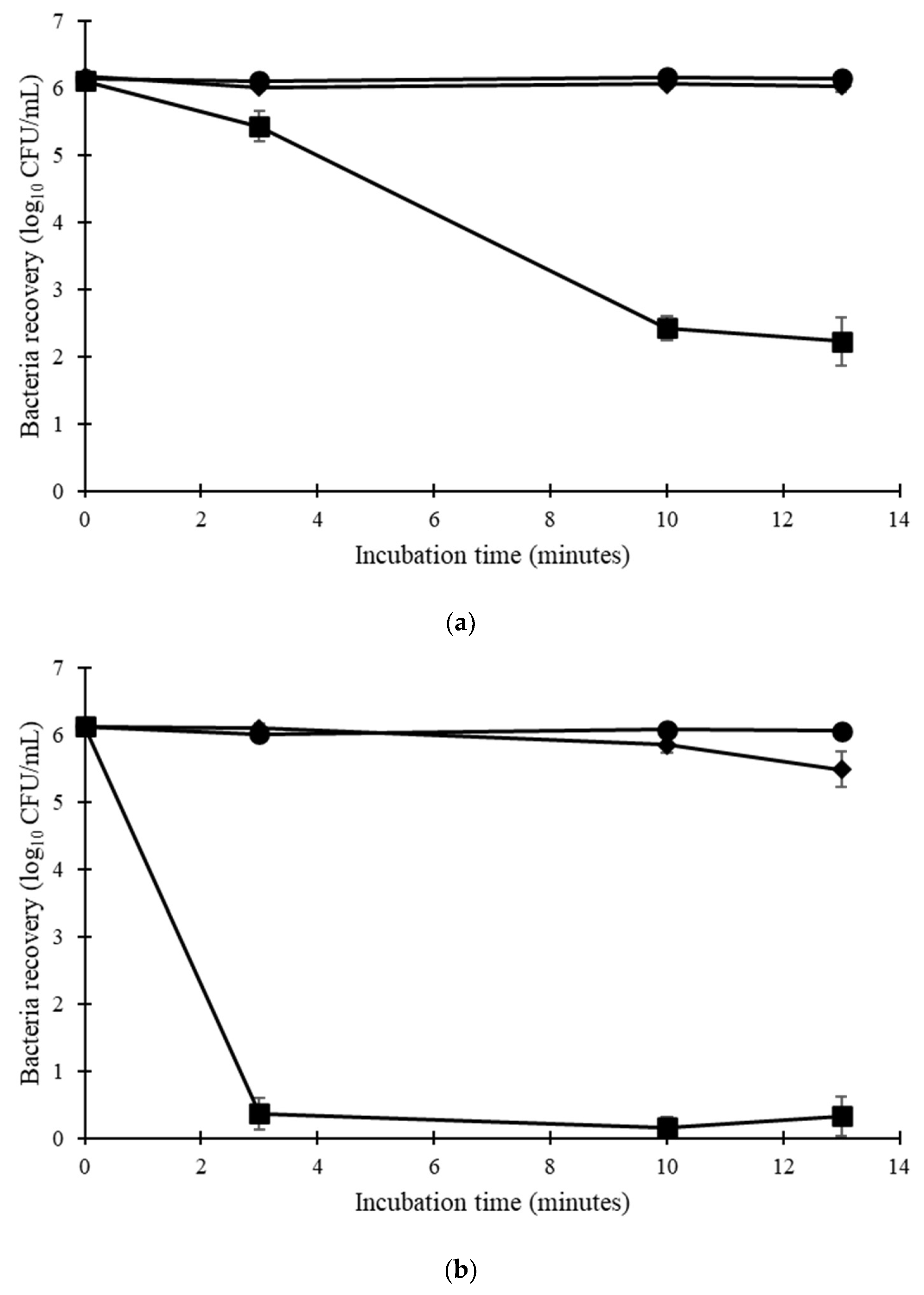
3.3. E. faecium DES Controller Bioindicator Retention Efficacy
3.4. E. faecium DES Controller Bioindicator Permeability to Disinfectants and Detergents
3.5. Comparison of Semi-Quantitative and Quantitative Enumeration Methods for Commercially Available DES Controller Bioindicators
3.6. Phase 2: Development of Alternative E. faecium Strains and a Bioindicator Membrane for Low-Temperature Laundering
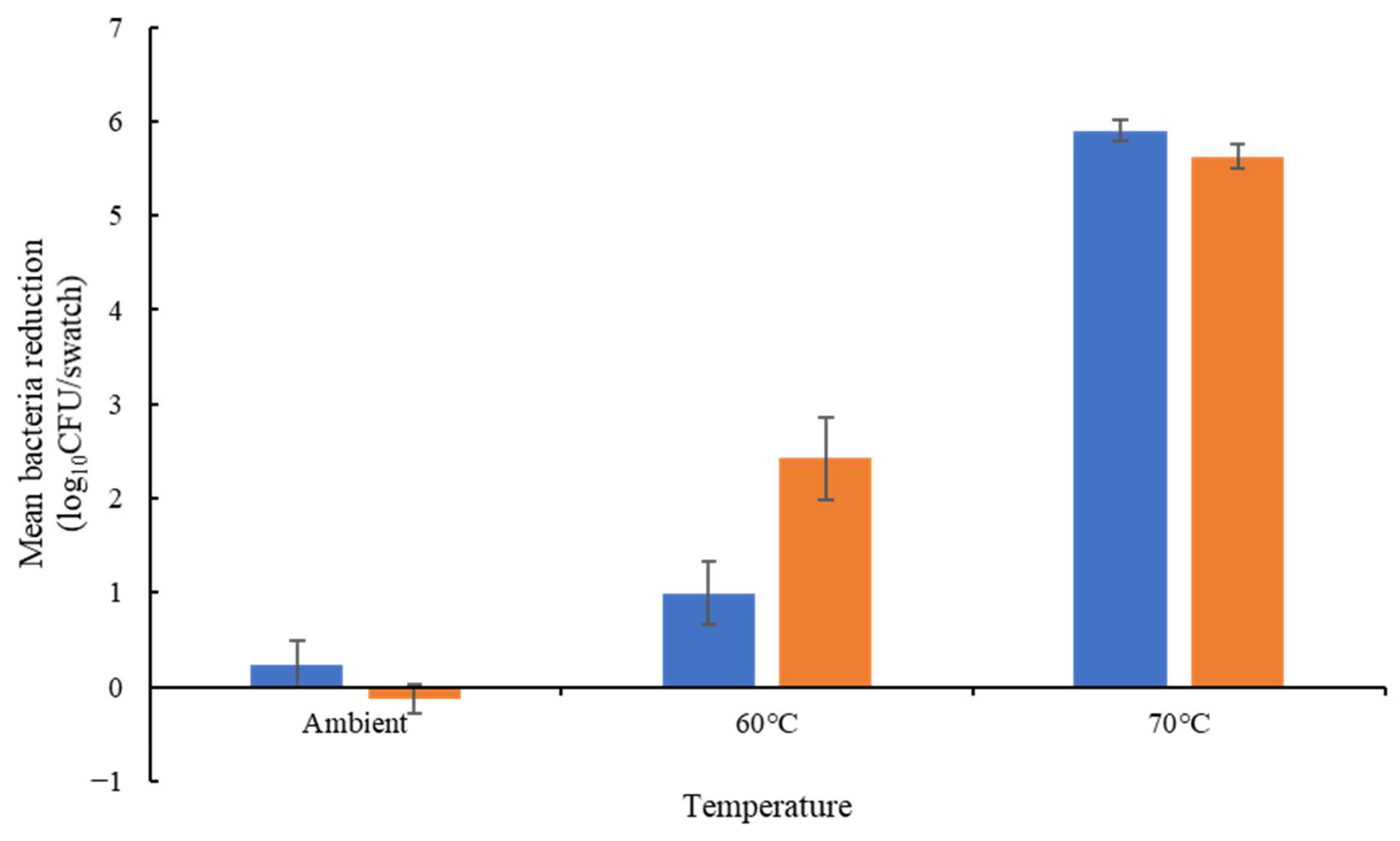
Disinfectant, Detergent and Thermal Tolerance on Cotton
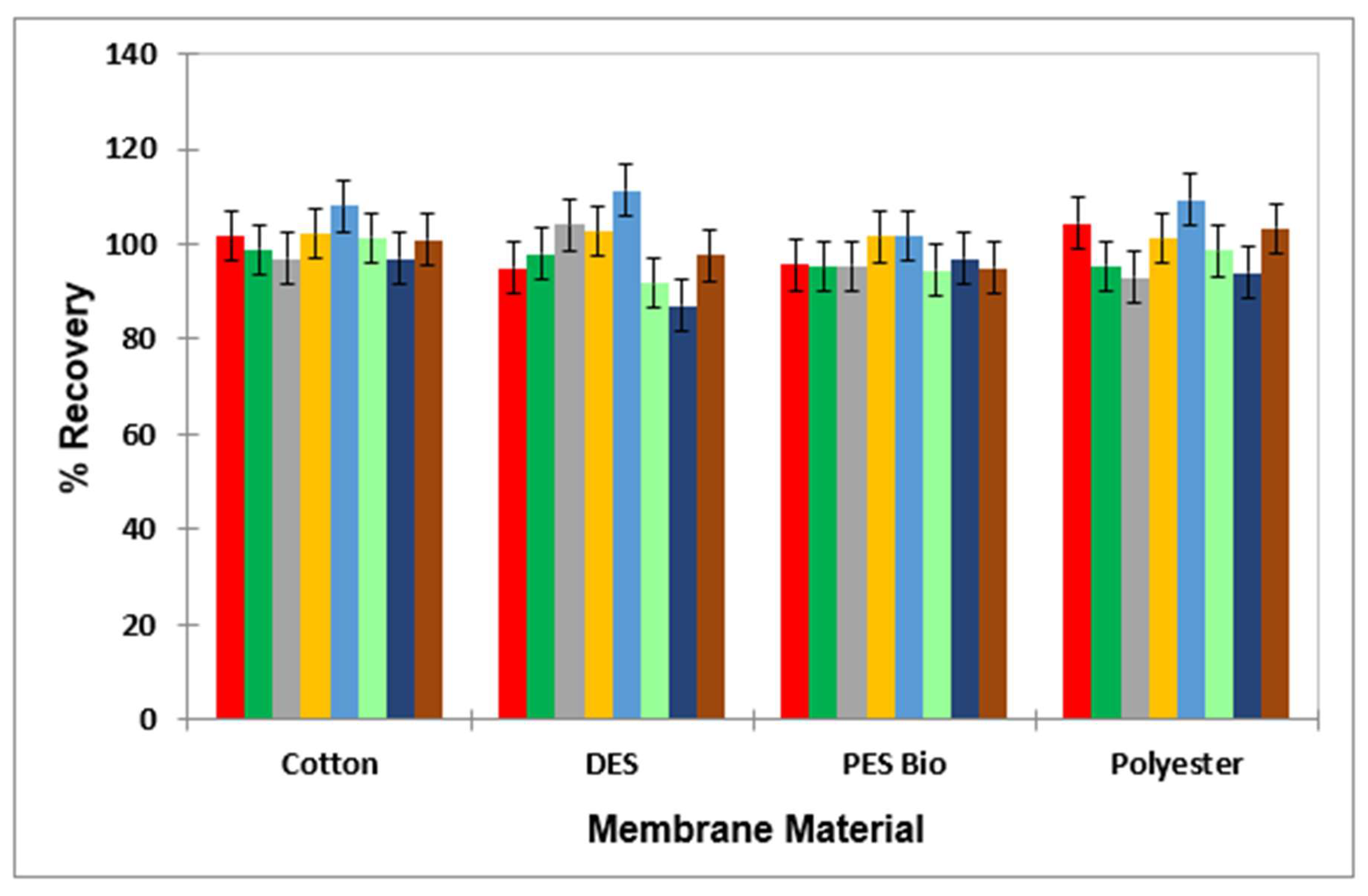
3.7. Assessment of Stability of the PES Membrane
3.8. Flow Rate of Solution through the PES Membrane
3.9. Detergent and Disinfectant Membrane Passage Assessment
3.10. PES Bioindicator Permeability to Disinfectants and Detergents during the Wash Process
3.11. Comparison of PES Bioindicators and Swatch Methodologies within the Wash Process
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Glowicz, J.; Benowitz, I.; Arduino, M.J.; Li, R.; Wu, K.; Jordan, A.; Toda, M.; Garner, K.; Gold, J.A. Keeping Healthcare Linens Clean: Underrecognized Hazards and Critical Control Points to Avoid Contamination of Laundered Healthcare Textiles. Am. J. Infect. Control, 2022; in press. [Google Scholar]
- Bockmühl, D.P. Laundry hygiene—How to get more than clean. J. Appl. Microbiol. 2017, 122, 1124–1133. [Google Scholar] [CrossRef] [PubMed]
- CDC. Guidelines for Environmental Infection Control in Health-Care Facilities: G. Laundry and Bedding. 2003. Available online: https://www.cdc.gov/infectioncontrol/guidelines/environmental/background/laundry.html (accessed on 25 July 2022).
- Department of Health. Health Technical Memorandum 01-04: Decontamination of Linen for Health and Social Care: Management and Provision. 2016. Available online: https://www.england.nhs.uk/wp-content/uploads/2021/05/Mgmt_and_provision.pdf (accessed on 25 July 2022).
- Kampf, G. How long can nosocomial pathogens survive on textiles? A systematic review. GMS Hyg. Infect. Control 2020, 15, Doc10. [Google Scholar] [PubMed]
- Mallick, D.; Gupta, D.; Sharma, S. Transfer of bacteria between fabric and surrogate skin. Am. J. Infect. Control 2022, 50, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Handorean, A.; Robertson, C.E.; Harris, J.K.; Frank, D.; Hull, N.; Kotter, C.; Stevens, M.J.; Baumgardner, D.; Pace, N.R.; Hernandez, M. Microbial aerosol liberation online soiled textiles isolated during routine residuals handling in a modern health care setting. Microbiome 2015, 3, 72. [Google Scholar] [CrossRef] [PubMed]
- Loveday, H.P.; Wilson, J.A.; Hoffman, P.N.; Pratt, R.J. Public perception and the social and microbiological significance of uniforms in the prevention and control of healthcare-associated infections: An evidence review. Br. J. Infect. Control 2007, 8, 10–21. [Google Scholar]
- Mitchell, A.; Spencer, M.; Edmiston Jr, C. Role of healthcare apparel and other healthcare textiles in the transmission of pathogens: A review of the literature. J. Hosp. Infect. 2015, 90, 285–292. [Google Scholar] [PubMed]
- Overcash, M.R.; Sehulster, L.M. Estimated incidence rate of healthcare-associated infections (HAIs) linked to laundered reusable healthcare textiles (HCTs) in the United States and United Kingdom over a 50-year period: Do the data support the efficacy of approved laundry practices? Infect. Control Hosp. Epidemiol. 2021, 43, 1510–1512. [Google Scholar] [PubMed]
- Sehulster, L.M. Healthcare laundry and textiles in the United States: Review and commentary on contemporary infection prevention issues. Infect. Control Hosp. Epidemiol. 2015, 36, 1073–1088. [Google Scholar] [PubMed]
- EN 14065; Textiles-Laundry Processed Textiles-Biocontamination Control System. CEN: Brussels, Belgium, 2016.
- BS EN 14065:2016; Textiles-Laundry Processed Textiles-Biocontamination Control System. British Standards Institute (BSI): London, UK, 2016.
- TRSA. Standard for Producing Hygienically Clean Reusable Textiles for Use in the Healthcare Industry. 2021. Available online: https://hygienicallyclean.org/wp-content/uploads/2021/01/HCH_Standard_011421.pdf (accessed on 25 July 2022).
- Fijan, S.; Cencic, A.; Turk, S.Š. Hygiene monitoring of textiles used in the food industry. Braz. J. Microbiol. 2006, 37, 356–361. [Google Scholar]
- Department of Health. Health Technical Memorandum 01-04: Decontamination of Linen for Health and Social Care: Engineering, Equipment and Validation. 2016. Available online: https://www.england.nhs.uk/wp-content/uploads/2021/05/Engineering.pdf (accessed on 25 July 2022).
- NHS Wales Shared Services Partnership–Specialist Estates Services. WHTM 01-04–Decontamination of Linen for Health and Social Care -Engineering, Equipment and Validation. 2017. Available online: https://nwssp.nhs.wales/a-wp/all-wales-laundry-service-review/all-wales-laundry-service-documents/useful-documents/whtm-01-04-2017-linen-engineering/ (accessed on 7 July 2023).
- Owen, L.; Laird, K. The role of textiles as fomites in the healthcare environment: A review of the infection control risk. PeerJ 2020, 8, e9790. [Google Scholar] [PubMed]
- Riley, K.; Williams, J.; Owen, L.; Shen, J.; Davies, A.; Laird, K. The effect of low-temperature laundering and detergents on the survival of Escherichia coli and Staphylococcus aureus on textiles used in healthcare uniforms. J. Appl. Microbiol. 2017, 123, 280–286. [Google Scholar] [PubMed]
- BS EN ISO 6330:2012; Textiles. Domestic Washing and Drying Procedures for Textile Testing. British Standards Institute (BSI): London, UK, 2012.
- BS EN 1040:2005; Chemical Disinfectants and Antiseptics. Quantitative Suspension Test for the Evaluation of Basic Bactericidal Activity of Chemical Disinfectants and Antiseptics. Test Method and Requirements (Phase 1). British Standards Institute (BSI): London, UK, 2005.
- Horváth, A.K.; Nagypál, I. Kinetics and mechanism of the reaction between hypochlorous acid and tetrathionate ion. Int. J. Chem. Kinet. 2000, 32, 395–402. [Google Scholar] [CrossRef]
- Beers, R.F.; Sizer, I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar] [PubMed]
- BS EN 16616:2022; Chemical Disinfectants and Antiseptics. Chemical-Thermal Textile Disinfection. Test Method and Requirements (Phase 2, Step 2). British Standards Institute (BSI): London, UK, 2022; 2.
- Rabuza, U.; Šostar Turk, S.; Fijan, S. Efficiency of four sampling methods used to detect two common nosocomial pathogens on textiles. Text. Res. J. 2012, 82, 2099–2105. [Google Scholar] [CrossRef]
- Tarrant, J.; Jenkins, R.O.; Laird, K.T. Online ward to washer: The survival of Clostridium difficile spores on hospital bed sheets through a commercial UK NHS healthcare laundry process. Infect. Control Hosp. Epidemiol. 2018, 39, 1406–1411. [Google Scholar] [CrossRef] [PubMed]
- Kagemann, G.; Hilgenberg, B.; Rech, J.; Heintz, M.; Vossebein, L. Use of Biomonitors for the Validation of Chemo-thermal Disinfecting Washing Procedures. Tenside Surfactants Deterg. 2008, 45, 334–339. [Google Scholar] [CrossRef]
- Kopit, L.M.; Kim, E.B.; Siezen, R.J.; Harris, L.J.; Marco, M.L. Safety of the Surrogate Microorganism Enterococcus faecium NRRL B-2354 for Use in Thermal Process Validation. Appl. Environ. Microbiol. 2014, 80, 1899–1909. [Google Scholar] [PubMed]
- Cheng, V.C.; Chen, J.H.; Leung, S.S.; So, S.Y.; Wong, S.C.; Wong, S.C.; Tse, H.; Yuen, K.Y. Seasonal outbreak of Bacillus bacteremia associated with contaminated linen in Hong Kong. Clin. Infect. Dis. 2017, 64, S91–S97. [Google Scholar] [PubMed]

| Condition | Log10 CFU (% Recovery) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S. aureus | E. faecium | E. coli | B. cereus Spores | ||||||||||
| 8 | 2 | 1 | 8 | 2 | 1 | 8 | 2 | 1 | 8 | 2 | 1 | ||
| Recovery Media | PBS | 7.01 ± 0.37 (1.14) | - | - | 7.61 ± 0.04 (21.48) | - | - | 7.66 ± 0.10 (38.04) | - | - | 7.09 ± 0.40 (4.38) | - | - |
| PBS-T | 7.18 ± 0.30 (1.69) | - | - | 7.62 ± 0.04 (21.84) | - | - | 7.76 ± 0.11 (48.14) | - | - | 6.96 ± 0.27 (3.21) | - | - | |
| MRD | 6.95 ± 0.42 (0.98) | - | - | 7.65 ± 0.06 (23.25) | - | - | 7.68 ± 0.03 (48.14) | - | - | 7.14 ± 0.36 (4.89) | - | - | |
| MRD-T | 7.04 ± 0.40 (1.22) | - | - | 7.62 ± 0.06 (23.25) | - | - | 7.65 ± 0.11 (37.05) | - | - | 7.02 ± 0.37) | - | - | |
| Agitation Method | Vortexing | 7.18 ± 0.30 (1.69) | 0.00 ± 0.00 (0.17) | - | 7.62 ± 0.04 (21.84) | 1.70 ± 0.04 (54.23) | - | 7.76 ± 0.11 (48.14) | 1.79 ± 0.48 (57.75) | - | 6.96 ± 0.27 (0.03) | 2.02 ± 0.02 (77.24) | - |
| Shaking by Hand | 7.17 ± 0.09 (4.42) | 0.54 ± 0.27 (2.40) | 0.60 ± 0.30 (13.94) | 7.63 ± 0.03 (49.99) | 1.22 ± 0.24 (17.72) | 0.99 ± 0.34 (76.47) | 7.59 ± 0.09 (26.11) | 2.31 ± 0.27 (>100) | 0.00 ± 0.00 (0.00) | 7.74 ± 0.14 (22.44) | 2.28 ± 0.13 (>100) | 1.06 ± 0.39 (2.34) | |
| Rotary Shaking | 6.85 ± 0.34 (2.08) | 0.44 ± 0.13 (1.89) | - | 7.32 ± 0.46 (24.32) | 1.92 ± 0.19 (90.20) | - | 7.53 ± 0.10 (22.90) | 2.36 ± 0.24 (>100) | - | 7.58 ± 0.20 (15.53) | 2.19 ± 0.15 (>100) | - | |
| Stomaching (30 s) | 7.39 ± 0.06 (10.56) | 1.21 ± 0.27 (0.91) | - | 7.60 ± 0.05 (15.79) | 1.24 ± 0.12 (6.91) | - | 7.67 ± 0.03 (24.08) | 2.42 ± 0.19 (>100) | - | 7.32 ± 0.38 (7.92) | 2.99 ± 0.61 (>100) | - | |
| Stomaching (1 min) | 6.67 ± 0.03 (1.40) | 0.40 ± 0.13 (1.72) | - | 7.59 ± 0.52 (45.88) | 1.65 ± 0.40 (48.32) | - | 7.84 ± 0.10 (46.46) | 2.26 ± 0.61 (>100) | - | 7.65 ± 0.15 (18.33) | 2.39 ± 0.12 (182.67) | - | |
| Surface Method | Swabbing | 4.48 ± 0.39 (0.01) | 0.00 ± 0.00 (0.00) | - | 4.50 ± 0.23 (0.02) | 0.00 ± 0.00 (0.00) | - | 4.42 ± 0.28 (0.02) | 1.23 ± 0.44 (9.85) | - | 4.75 ± 1.46 (0.00) | 0.00 ± 0.00 (0.00) | - |
| Dip Slide | TNTC | 0.90 ± 0.34 (2.79) | - | TNTC | 0.44 ± 0.15 (2.16) | - | TNTC | 0.63 ± 0.24 (1.83) | - | TNTC | 0.74 ± 0.30 (0.11) | - | |
| RODAC | TNTC | 0.00 ± 0.00 (0.00) | - | TNTC | 0.00 ± 0.12 (0.38) | - | TNTC | 0.48 ± 0.24 (1.83) | - | TNTC | 0.00 ± 0.12 (0.17) | - | |
| Recovery Agar | Nutrient Agar | 7.61 ± 0.66 (19.86) | 0.48 ± 0.22 (1.45) | - | 8.26 ± 0.05 (>100) | 2.23 ± 0.14 (>100) | - | 8.14 ± 0.07 (94.66) | 3.67 ± 0.18 (>100) | - | 7.18 ± 0.36 (35.44) | 0.TN00 ± 0.00 (0.00) | - |
| Selective Agar | 8.32 ± 0.05 (17.77) | 0.00 ± 0.12 (0.36) | - | 8.06 ± 0.07 (75.44) | 2.28 ± 0.12 (>100) | - | 8.09 ± 0.08 (83.64) | 3.69 ± 0.12 (>100) | - | 6.81 ± 0.02 (15.18) | 0.00 ± 0.00 (0.00) | - | |
| Microorganism | Condition | Expected Count (Log10 CFU) | Actual Count |
|---|---|---|---|
| E. faecium | Bioindicator | 6 | 5.60 ± 0.06 |
| 5 | 4.47 ± 0.17 | ||
| 4 | 5.12 ± 0.13 | ||
| 3 | 4.75 ± 0.15 | ||
| Inoculated Swatch | 6 | 5.44 ± 0.05 | |
| 5 | 5.47 ± 0.28 | ||
| 4 | 4.90 ± 0.34 | ||
| 3 | 3.63 ± 0.26 | ||
| E. coli | Bioindicator | 6 | 4.55 ± 0.12 |
| 5 | 3.07 ± 0.58 | ||
| 4 | 3.82 ± 0.58 | ||
| 3 | 2.50 ± 0.33 | ||
| B. subtilis spores | Bioindicator | 6 | 5.96 ± 0.06 |
| Bioindicator 70 °C, 30 min | 6 | 5.96 ± 0.08 |
| Condition | Enclosure | Log10 CFU Recovery | Log10 Reduction from Untreated Swatch | Difference in Log10 Reduction from Loose Swatch | |
|---|---|---|---|---|---|
| Pre-Treatment | Post-Treatment | ||||
| Water Only | Loose Inoculated Swatch | 5.46 ± 0.13 | 3.63 ± 0.08 | 1.83 | - |
| Cotton Bag, Hemming Web | 5.85 ± 0.03 | 4.81 ± 0.12 | 1.04 | −0.79 | |
| Cotton Bag, Sewn | 5.85 ± 0.033 | 4.35 ± 0.16 | 1.50 | −0.32 | |
| Laundry Bag | 5.85 ± 0.03 | 4.39 ± 0.20 | 1.46 | −0.36 | |
| Nylon Membrane Filter | 5.82 ± 0.07 | 5.48 ± 0.10 | 0.34 | −1.48 | |
| Reusable Autoclave Bag | 5.89 ± 0.04 | 4.64 ± 0.31 | 1.25 | −0.57 | |
| Bioindicator (no membrane) | 6.48 ± 0.12 | 4.82 ± 0.30 | 1.66 | - | |
| Bioindicator (with membrane) | 6.48 ± 0.12 | 6.03 ± 0.03 | 0.45 | −1.21 | |
| Detergent | Loose Swatch | 6.13 ± 0.31 | 1.27 ± 0.35 | 4.86 | |
| Cotton Bag, Hemming Web | 5.85 ± 0.07 | 3.57 ± 0.29 | 2.28 | −2.58 | |
| Nylon Membrane Filter | 5.82 ± 0.07 | 5.74 ± 0.03 | 0.08 | −4.78 | |
| Reusable Autoclave Bag | 5.77 ± 0.17 | 4.11 ± 0.13 | 1.66 | −3.20 | |
| Bioindicator (no membrane) | 6.48 ± 0.12 | 3.73 ± 0.10 | 2.75 | - | |
| Bioindicator (with membrane) | 6.48 ± 0.12 | 6.32 ± 0.19 | 0.16 | −2.59 | |
| Temperature | Disinfectant | Treatment | Log10 CFU Recovery | Log10 Reduction | Log10 Reduction Difference with Membrane |
|---|---|---|---|---|---|
| Ambient | Water-Only Control | No membrane | 5.84 ± 0.11 | - | −0.31 |
| With membrane | 6.15 ± 0.04 | −0.31 | |||
| Peracetic Acid (0.64 mL/L) | No membrane | 0.88 ± 0.37 * | 4.96 | +0.43 | |
| With membrane | 0.76 ± 0.36 * | 5.39 | |||
| Sodium Hypochlorite (0.80 mL/L) | No membrane | 3.88 ± 0.32 | 1.96 | +0.56 | |
| With membrane | 3.63 ± 0.15 * | 2.52 | |||
| Hypochlorous Acid (3.13 mL/L) | No membrane | 4.84 ± 0.10 | 1.00 | −0.66 | |
| With membrane | 5.81 ± 0.07 | 0.34 | |||
| Hydrogen Peroxide (4.69 mL/L) | No membrane | 5.00 ± 0.12 | 0.84 | −0.58 | |
| With membrane | 5.89 ± 0.03 | 0.26 | |||
| BAC (0.093 mL/L) | No membrane | 4.73 ± 0.08 | 1.11 | −1.49 § | |
| With membrane | 6.53 ± 0.05 | −0.38 | |||
| DDAC (0.15 mL/L) | No membrane | 5.20 ± 0.14 | 0.64 | −0.94 | |
| With membrane | 6.45 ± 0.08 | −0.30 | |||
| SDS (0.06% w/v) | No membrane | 1.12 ± 0.28 * | 4.72 | −5.36 § | |
| With membrane | 6.49 ± 0.13 | −0.64 | |||
| 60 °C | Water | No Membrane | 1.80 ± 0.43 | - | - |
| With Membrane | 6.01 ± 0.07 | - | |||
| SDS (0.06% w/v) | No Membrane | 0.00 ± 0.00 | ≥1.80 | +2.47 | |
| With Membrane | 1.74 ± 0.32 | 4.27 | |||
| 50 °C | Water | No Membrane | 5.57 ± 0.51 | - | - |
| With Membrane | 6.85 ± 0.03 | - | |||
| SDS (0.06% w/v) | No Membrane | 0.69 ± 0.40 | 4.88 | −4.83 | |
| With Membrane | 6.79 ± 0.05 | 0.05 |
| Microorganism | Wash | Temperature (°C) | Detergent | Quantitative Method | Semi-Quantitative Method | ||||
|---|---|---|---|---|---|---|---|---|---|
| Log10 CFU | Log10 Reduction (Unlaundered) | Log10 Reduction (Water Only) | Pass/Fail (5 log10 Reduction) | Log10 Reduction | Pass/Fail (5 log10 Reduction) | ||||
| E. faecium | Unlaundered | N/A | N/A | 6.34 ± 0.03 | - | - | - | - | - |
| Domestic | Ambient | None | 7.02 ± 0.05 | - | - | Fail | 0 | Fail | |
| 60 | None | 6.76 ± 0.08 | - | 0.26 | Fail | 0 * | Fail | ||
| Non-Biological | 2.22 ± 0.22 | 4.12 | 4.80 | Fail | 4 | Fail | |||
| Biological | 0.67 ± 0.43 | 5.67 | 6.35 | Pass | 4 | Fail | |||
| Industrial | Ambient | None | 6.03 ± 0.03 | 0.34 | - | Fail | 0 | Fail | |
| 67 | None | 0.78 ± 0.51 | 5.56 | 5.25 | Pass | 5 | Pass | ||
| Industrial Detergent | 0.00 ± 0.00 | 6.34 | 6.03 | Pass | 6 | Pass | |||
| 75 | None | 0.00 ± 0.00 | 6.34 | 6.03 | Pass | 6 | Pass | ||
| E. coli | Unlaundered | N/A | N/A | 4.02 ± 0.29 | - | - | - | 0 | - |
| Domestic | Ambient | None | 4.60 ± 0.02 | - | - | § | - | § | |
| 60 | None | 0.00 ± 0.00 | 4.02 | 4.60 | § | 6 | * | ||
| Non-Biological | 0.00 ± 0.00 | 4.02 | 4.60 | § | 6 | Pass | |||
| Biological | 0.00 ± 0.00 | 4.02 | 4.60 | § | 6 | Pass | |||
| Industrial | Ambient | None | 3.93 ± 0.31 | 0.09 | - | § | 0 | Fail | |
| 67 | None | 0.08 ± 0.08 | 3.94 | 3.86 | § | 6 | Pass | ||
| Industrial Detergent | 0.00 ± 0.00 | 4.02 | 3.93 | § | 6 | Pass | |||
| 75 | None | 0.00 ± 0.00 | 4.02 | 3.93 | § | 6 | Pass | ||
| B. subtilis spores | Unlaundered | N/A | N/A | 5.93 ± 0.02 | - | - | - | - | - |
| Domestic | Ambient | None | 6.20 ± 0.03 | - | - | Fail | 0 | Fail | |
| 60 | None | 6.15 ± 0.08 | - | 0.05 | Fail | 0 | Fail | ||
| Non-Biological | 5.98 ± 0.03 | - | 0.23 | Fail | 0 | Fail | |||
| Biological | 6.10 ± 0.05 | - | 0.10 | Fail | 0 | Fail | |||
| Industrial | Ambient | None | 6.20 ± 0.03 | - | - | Fail | 0 | Fail | |
| 67 | None | 5.99 ± 0.01 | - | 0.21 | Fail | 0 | Fail | ||
| Industrial Detergent | 4.52 ± 0.11 | 1.41 | 1.69 | Fail | 0 | Fail | |||
| 75 | None | 5.95 ± 0.04 | - | 0.25 | Fail | 0 | Fail | ||
| Microorganism | Condition | Quantitative Method | Semi-Quantitative Method | |
|---|---|---|---|---|
| Log10 CFU | Log10 Reduction | Log10 Reduction | ||
| B. subtilis spores | Unlaundered | 5.83 ± 0.13 | ≥4.51 | ≤6 |
| Laundered | ≥1.32 ± 0.94 * | |||
| E. coli | Unlaundered | ≥3.05 ± 0.39 * | ≥3.03 | 6 |
| Laundered | 0.02 ± 0.01 | |||
| E. faecium | Unlaundered | 6.35 ± 0.12 | 6.35 | 6 |
| Laundered | 0.00 ± 0.00 | |||
| Treatment Type * | Interfering Substance (Defibrinated Sheep Blood) | Mean log10 CFU Recovery/Swatch | Mean log10 CFU Reduction/Swatch ** | |
|---|---|---|---|---|
| Water | In solution | - | 6.36 ± 0.05 | NA |
| In solution | + | 6.32 ± 0.06 | 0.04 ± 0.1 | |
| In wash | - | 5.95 ± 0.08 | 0.41 ± 0.07 | |
| In wash | + | 5.82 ± 0.06 | 0.54 ± 0.11 | |
| Chlorine (80 ppm) | In solution | - | 0 | 6.54 ± 0.11 |
| In solution | + | 6.25 ± 0.12 | 0 ± 0.11 | |
| In wash | - | 0.62 ± 0.32 | 5.92 ± 0.29 | |
| In wash | + | 0 | 6.54 ± 0.11 | |
| SDS (0.06% w/v) | In solution | - | 4.47 ± 0.22 | 1.93 ± 0.24 |
| In solution | + | 6.25 ± 0.04 | 0.15 ± 0.05 | |
| In wash | - | 4.68 ± 0.18 | 1.72 ± 0.22 | |
| In wash | + | 5.60 ± 0.17 | 0.80 ± 0.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Owen, L.; Cayrou, C.; Page, G.; Grootveld, M.; Laird, K. Development of a Standardised International Protocol for Evaluation of the Disinfection Efficacy of Healthcare Laundry Wash Processes. Appl. Microbiol. 2024, 4, 194-214. https://doi.org/10.3390/applmicrobiol4010014
Owen L, Cayrou C, Page G, Grootveld M, Laird K. Development of a Standardised International Protocol for Evaluation of the Disinfection Efficacy of Healthcare Laundry Wash Processes. Applied Microbiology. 2024; 4(1):194-214. https://doi.org/10.3390/applmicrobiol4010014
Chicago/Turabian StyleOwen, Lucy, Caroline Cayrou, Georgina Page, Martin Grootveld, and Katie Laird. 2024. "Development of a Standardised International Protocol for Evaluation of the Disinfection Efficacy of Healthcare Laundry Wash Processes" Applied Microbiology 4, no. 1: 194-214. https://doi.org/10.3390/applmicrobiol4010014
APA StyleOwen, L., Cayrou, C., Page, G., Grootveld, M., & Laird, K. (2024). Development of a Standardised International Protocol for Evaluation of the Disinfection Efficacy of Healthcare Laundry Wash Processes. Applied Microbiology, 4(1), 194-214. https://doi.org/10.3390/applmicrobiol4010014







