The Development of Novel Edible Films from Single-Cell Protein Produced by the Biotechnological Valorization of Cheese Whey
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials, Microorganisms, and Growth Media
2.2. Fed-Batch Experiment
2.3. Biomass, SCP, Ethanol Determination, and Substrate Consumption
2.4. Preparation of Edible Films
2.5. Characterization of Edible Films
2.5.1. Film Thickness
2.5.2. Color Analysis
2.5.3. Film Opacity
2.5.4. Moisture Content, Solubility, and Swelling Index
2.5.5. Water Vapor Permeability
2.5.6. Mechanical Properties
2.5.7. Wettability
2.6. Statistical Analysis
3. Results and Discussion
3.1. K. marxianus Strain EXF-5288 Growth in Fed-Batch Condition
3.2. The Development and Characterization of SCP-Based Edible Films
3.2.1. Color Film Opacity
3.2.2. Moisture Content, Solubility, and Swelling Index
3.2.3. Water Vapor Permeability
3.2.4. Mechanical Properties
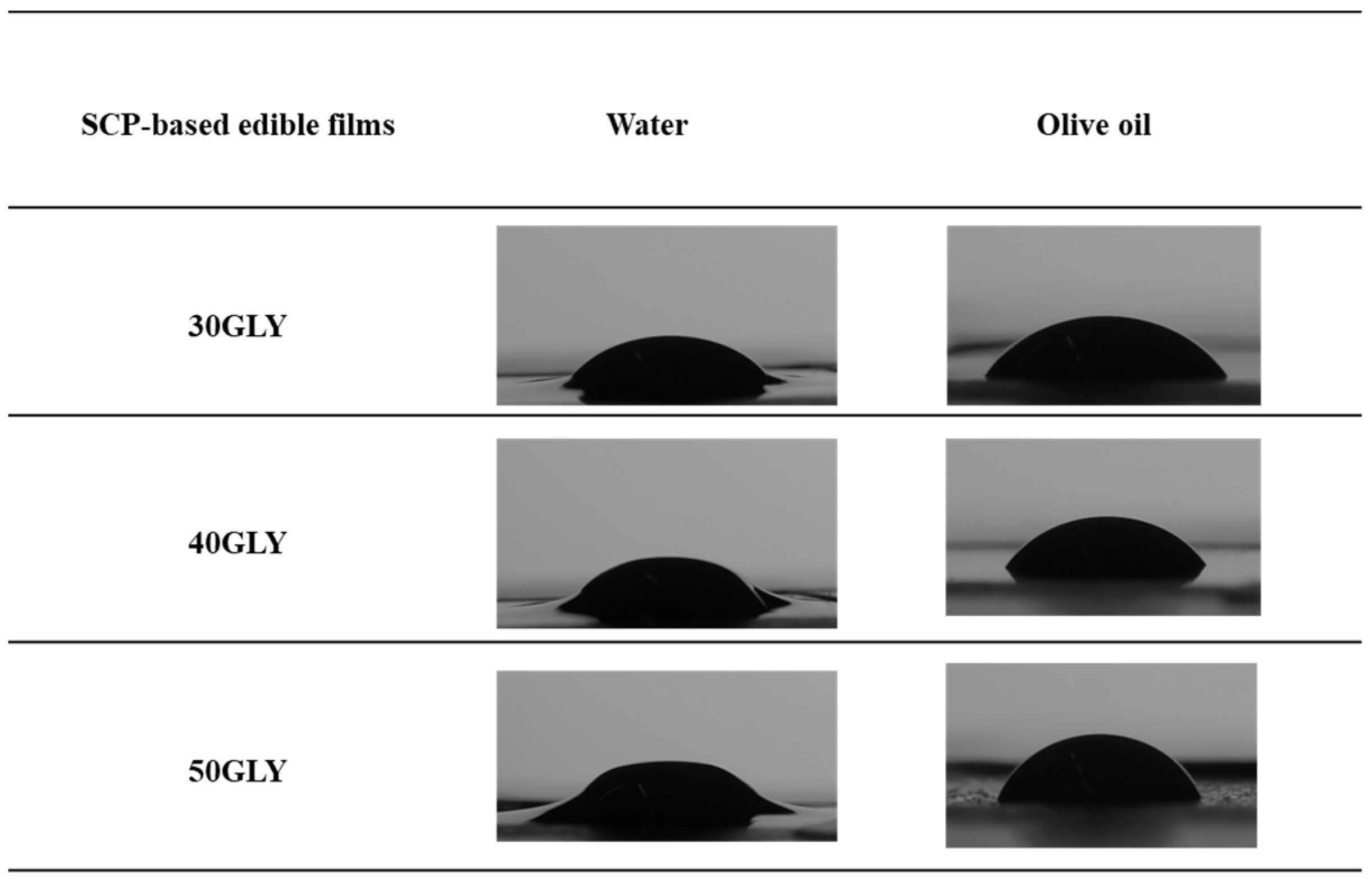
3.2.5. Wettability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Production Forecast of Thermoplastics Worldwide from 2025 to 2050, Statista. 2023. Available online: https://www.statista.com/statistics/664906/plastics-production-volume-forecast-worldwide/ (accessed on 3 June 2024).
- Proposal for a Directive of the European Parliament and of the Council on the Reduction of the Impact of Certain Plastic Products on the Environment, European Commission. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A52018PC0340 (accessed on 3 June 2024).
- Kasznik, D.; Łapniewska, Z. The end of plastic? The EU’s directive on single-use plastics and its implementation in Poland. Environ. Sci. Policy 2023, 145, 151–163. [Google Scholar] [CrossRef]
- Chen, W.; Ma, S.; Wang, Q.; McClements, D.J.; Liu, X.; Ngai, T.; Liu, F. Fortification of edible films with bioactive agents: A review of their formation, properties, and application in food preservation. Crit. Rev. Food Sci. Nutr. 2022, 62, 5029–5055. [Google Scholar] [CrossRef] [PubMed]
- Ramos, Ó.L.; Reinas, I.; Silva, S.I.; Fernandes, J.C.; Cerqueira, M.A.; Pereira, R.N.; Vicente, A.A.; Poças, M.F.; Pintado, M.E.; Malcata, F.X. Effect of whey protein purity and glycerol content upon physical properties of edible films manufactured therefrom. Food Hydrocoll. 2013, 30, 110–122. [Google Scholar] [CrossRef]
- Jiang, L.; Ye, R.; Xie, C.; Wang, F.; Zhang, R.; Tang, H.; He, Z.; Han, J.; Liu, Y. Development of zein edible films containing different catechin/cyclodextrin metal-organic frameworks: Physicochemical characterization, antioxidant stability and release behavior. LWT 2023, 173, 114306. [Google Scholar] [CrossRef]
- Sun, H.; Huang, Y.; Chen, Y.; Liu, X.; Leng, X. Effects of curcumin, phycocyanin, or modified lycopene colorants on the physicochemical and sensory properties of whey protein–cellulose nanocrystal packaging films. Food Chem. 2023, 412, 135541. [Google Scholar] [CrossRef] [PubMed]
- Kyriakidou, A.; Makris, D.P.; Lazaridou, A.; Biliaderis, C.G.; Mourtzinos, I. Physical properties of Chitosan films containing pomegranate peel extracts obtained by deep eutectic solvents. Foods 2021, 10, 1262. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Peng, S.; Shi, C.; Li, C.; Hua, Z.; Cui, H. Preparation and characterization of cassava starch/sodium carboxymethyl cellulose edible film incorporating apple polyphenols. Int. J. Biol. Macromol. 2022, 212, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Guan, W.; Zhou, X.; Lao, M.; Cai, L. The physiochemical and preservation properties of anthocyanidin/chitosan nanocomposite-based edible films containing cinnamon-perilla essential oil pickering nanoemulsions. LWT 2022, 153, 112506. [Google Scholar] [CrossRef]
- Papadaki, A.; Kachrimanidou, V.; Lappa, I.K.; Andriotis, H.; Eriotou, E.; Mandala, I.; Kopsahelis, N. Tuning the physical and functional properties of whey protein edible films: Effect of pH and inclusion of antioxidants from spent coffee grounds. Sustain. Chem. Pharm. 2022, 27, 100700. [Google Scholar] [CrossRef]
- García-Bramasco, C.A.; Blancas-Benitez, F.J.; Montaño-Leyva, B.; Medrano-Castellón, L.M.; Gutierrez-Martinez, P.; González-Estrada, R.R. Influence of Marine Yeast Debaryomyces hansenii on Antifungal and Physicochemical Properties of Chitosan-Based Films. J. Fungi 2022, 8, 369. [Google Scholar] [CrossRef]
- Sáez-Orviz, S.; Marcet, I.; Rendueles, M.; Díaz, M. Preparation of Edible Films with Lactobacillus plantarum and Lactobionic Acid Produced by Sweet Whey Fermentation. Membranes 2022, 12, 115. [Google Scholar] [CrossRef] [PubMed]
- Singha, S.; Mahmutovic, M.; Zamalloa, C.; Stragier, L.; Verstraete, W.; Svagan, A.J.; Das, O.; Hedenqvist, M.S. Novel bioplastic from single cell protein as a potential packaging material. ACS Sustain Chem Eng. 2021, 10, 6337–6346. [Google Scholar] [CrossRef]
- Fradinho, P.; Niccolai, A.; Soares, R.; Rodolfi, L.; Biondi, N.; Tredici, M.R.; Sousa, I.; Raymundo, A. Effect of Arthrospira platensis (spirulina) incorporation on the rheological and bioactive properties of gluten-free fresh pasta. Algal Res. 2020, 45, 101743. [Google Scholar] [CrossRef]
- Khan, M.K.I.; Asif, M.; Razzaq, Z.U.; Nazir, A.; Maan, A.A. Sustainable food industrial waste management through single cell protein production and characterization of protein enriched bread. Food Biosci. 2022, 46, 101406. [Google Scholar] [CrossRef]
- Agboola, J.O.; Lapeña, D.; Øverland, M.; Arntzen, M.Ø.; Mydland, L.T.; Hansen, J.Ø. Yeast as a novel protein source-Effect of species and autolysis on protein and amino acid digestibility in Atlantic salmon (Salmo salar). Aquaculture 2022, 546, 737312. [Google Scholar] [CrossRef]
- Koukoumaki, D.I.; Tsouko, E.; Papanikolaou, S.; Ioannou, Z.; Diamantopoulou, P.; Sarris, D. Recent advances in the production of single cell protein from renewable resources and applications. Carbon Resour. Convers. 2024, 7, 100195. [Google Scholar] [CrossRef]
- Karim, A.; Aider, M. Bioconversion of electro-activated lactose, whey and whey permeate to produce single cell protein, ethanol, aroma volatiles, organic acids and fat by Kluyveromyces marxianus. Int. Dairy J. 2022, 129, 105334. [Google Scholar] [CrossRef]
- Yadav, J.S.S.; Bezawada, J.; Elharche, S.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Simultaneous single-cell protein production and COD removal with characterization of residual protein and intermediate metabolites during whey fermentation by K. marxianus. Bioprocess Biosyst Eng. 2014, 37, 1017–1029. [Google Scholar] [CrossRef]
- Lappa, I.K.; Papadaki, A.; Kachrimanidou, V.; Terpou, A.; Koulougliotis, D.; Eriotou, E.; Kopsahelis, N. Cheese whey processing: Integrated biorefinery concepts and emerging food applications. Foods 2019, 8, 347. [Google Scholar] [CrossRef]
- Dourou, M.; Mizerakis, P.; Papanikolaou, S.; Aggelis, G. Storage lipid and polysaccharide metabolism in Yarrowia lipolytica and Umbelopsis isabellina. Appl. Microbiol. Biotechnol. 2017, 101, 7213–7226. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, D.; Kordowska-Wiater, M.; Sołowiej, B.; Baraniak, B. Physicochemical and Antimicrobial Properties of Biopolymer-Candelilla Wax Emulsion Films Containing Potassium Sorbate—A Comparative Study. Food Bioprocess Technol. 2015, 8, 567–579. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł.; Drozłowska, E.; Trocer, P.; Kostek, M.; Śliwiński, M.; Henriques, M.H.; Bartkowiak, A.; Sobolewski, P. Whey protein concentrate/isolate biofunctional films modified with melanin from watermelon (Citrullus lanatus) seeds. Materials 2020, 13, 3876. [Google Scholar] [CrossRef]
- ASTM. Standard test methods for water vapor transmission of materials. In Annual Book of ASTM Standards; ASTM: Philadelphia, PA, USA, 1995; ASTM E96-95; pp. 697–704. [Google Scholar]
- ASTM. Standard test method for tensile properties of thin plastic sheeting. In Annual Book of ASTM Standards; ASTM: Philadelphia, PA, USA, 2002; ASTM D 882-02; pp. 1–9. [Google Scholar]
- Piccirilli, G.N.; Soazo, M.; Pérez, L.M.; Delorenzi, N.J.; Verdini, R.A. Effect of storage conditions on the physicochemical characteristics of edible films based on whey protein concentrate and liquid smoke. Food Hydrocoll. 2019, 87, 221–228. [Google Scholar] [CrossRef]
- Fonseca, G.G.; Heinzle, E.; Wittmann, C.; Gombert, A.K. The yeast Kluyveromyces marxianus and its biotechnological potential. Appl. Microbiol. Biotechnol. 2008, 79, 339–354. [Google Scholar] [CrossRef]
- Sakihama, Y.; Hidese, R.; Hasunuma, T.; Kondo, A. Increased flux in acetyl-CoA synthetic pathway and TCA cycle of Kluyveromyces marxianus under respiratory conditions. Sci. Rep. 2019, 9, 5319. [Google Scholar] [CrossRef] [PubMed]
- Gabardo, S.; Rech, R.; Rosa, C.A.; Ayub, M.A. Dynamics of ethanol production from whey and whey permeate byimmobilized strains of Kluyveromyces marxianus in batch andcontinuous bioreactors. Renew. Energy 2014, 69, 89–96. [Google Scholar] [CrossRef]
- Tesfaw, A.; Oner, E.T.; Assefa, F. Evaluating crude whey for bioethanol production using non-Saccharomyces yeast, Kluyveromyces marxianus. SN Appl. Sci. 2021, 3, 42. [Google Scholar] [CrossRef]
- Koukoumaki, D.I.; Papanikolaou, S.; Ioannou, Z.; Mourtzinos, I.; Sarris, D. Single-Cell Protein and Ethanol Production of a Newly Isolated Kluyveromyces marxianus Strain through Cheese Whey Valorization. Foods 2024, 13, 1892. [Google Scholar] [CrossRef]
- Ozmihci, S.; Kargi, F. Ethanol fermentation of cheese whey powder solution by repeated fed-batch operation. Enzyme Microb. Technol. 2007, 41, 169–174. [Google Scholar] [CrossRef]
- Cho, S.Y.; Rhee, C. Sorption characteristics of soy protein films and their relation to mechanical properties. LWT 2002, 35, 151–157. [Google Scholar] [CrossRef]
- Galus, S.; Lenart, A. Optical, mechanical, and moisture sorption properties of whey protein edible films. J. Food Process Eng. 2019, 42, e13245. [Google Scholar] [CrossRef]
- Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Gonou-Zagou, Z.; Kopsahelis, N. Trametes versicolor as a Natural Source of Bioactive Compounds for the Production of Whey Protein Films with Functional Properties: A Holistic Approach to Valorize Cheese Whey. Waste Biomass Valorization 2022, 13, 3989–3998. [Google Scholar] [CrossRef]
- Ballesteros-Mártinez, L.; Pérez-Cervera, C.; Andrade-Pizarro, R. Effect of glycerol and sorbitol concentrations on mechanical, optical, and barrier properties of sweet potato starch film. NFS J. 2020, 20, 1–9. [Google Scholar] [CrossRef]
- Wason, S.; Verma, T.; Subbiah, J. Validation of process technologies for enhancing the safety of low-moisture foods: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4950–4992. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Puyol, S.; Benítez, J.J.; Heredia-Guerrero, J.A. Transparency of polymeric food packaging materials. Food Res. Int. 2022, 161, 111792. [Google Scholar] [CrossRef] [PubMed]
- Papadaki, A.; Manikas, A.C.; Papazoglou, E.; Kachrimanidou, V.; Lappa, I.; Galiotis, C.; Mandala, I.; Kopsahelis, N. Whey protein films reinforced with bacterial cellulose nanowhiskers: Improving edible film properties via a circular economy approach. Food Chem. 2022, 385, 132604. [Google Scholar] [CrossRef] [PubMed]
- Khedri, S.; Sadeghi, E.; Rouhi, M.; Delshadian, Z.; Mortazavian, A.M.; de Toledo Guimarães, J.; Mohammadi, R. Bioactive edible films: Development and characterization of gelatin edible films incorporated with casein phosphopeptides. LWT 2021, 138, 110649. [Google Scholar] [CrossRef]
- Pavlath, A.E.; Orts, W. Edible films and coatings: Why, what, and how? In Edible Films and Coatings for Food Applications; Springer: New York, NY, USA, 2009; pp. 1–23. [Google Scholar]
- Pak, E.S.; Ghaghelestani, S.N.; Najafi, M.A. Preparation and characterization of a new edible film based on Persian gum with glycerol plasticizer. J. Food Sci. Technol. 2020, 57, 3284–3294. [Google Scholar] [CrossRef]
- Kokoszka, S.; Debeaufort, F.; Lenart, A.; Voilley, A. Water vapour permeability, thermal and wetting properties of whey protein isolate based edible films. Int. Dairy J. 2010, 20, 53–60. [Google Scholar] [CrossRef]
- Razzaq, Z.U.; Khan, M.K.I.; Maan, A.A.; Rahman, S.U. Characterization of single cell protein from Saccharomyces cerevisiae for nutritional, functional and antioxidant properties. J. Food Meas. Charact. 2020, 14, 2520–2528. [Google Scholar] [CrossRef]
- Sobral, P.J.D.A.; Dos Santos, J.S.; García, F.T. Effect of protein and plasticizer concentrations in film forming solutions on physical properties of edible films based on muscle proteins of a Thai Tilapia. J. Food Eng. 2005, 70, 93–100. [Google Scholar] [CrossRef]
- Shah, Y.A.; Bhatia, S.; Al-Harrasi, A.; Afzaal, M.; Saeed, F.; Anwer, M.K.; Khan, M.R.; Jawad, M.; Akram, N.; Faisal, Z. Mechanical Properties of Protein-Based Food Packaging Materials. Polymers 2023, 15, 1724. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, V.M.; Dias, M.V.; Borges, S.V.; Costa, A.L.R.; Silva, E.K.; Medeiros, A.A.; Nilda de Fátima, F.S. Development of whey protein isolate bio-nanocomposites: Effect of montmorillonite and citric acid on structural, thermal, morphological and mechanical properties. Food Hydrocoll. 2015, 48, 179–188. [Google Scholar] [CrossRef]
- Ellinas, K.; Tserepi, A.; Gogolides, E. From superamphiphobic to amphiphilic polymeric surfaces with ordered hierarchical roughness fabricated with colloidal lithography and plasma nanotexturing. Langmuir 2011, 27, 3960–3969. [Google Scholar] [CrossRef] [PubMed]
- Steele, A.; Bayer, I.; Loth, E. Inherently superoleophobic nanocomposite coatings by Spray Atomization. Nano Lett. 2009, 9, 501–505. [Google Scholar] [CrossRef]
- Pulla-Huillca, P.V.; Gomes, A.; Bittante, A.M.Q.B.; Lourenço, R.V.; do Amaral Sobral, P.J. Wettability of gelatin-based films: The effects of hydrophilic or hydrophobic plasticizers and nanoparticle loads. J. Food Eng. 2021, 297, 110480. [Google Scholar] [CrossRef]
- Athanasopoulou, E.; Bigi, F.; Maurizzi, E.; Karellou, E.I.E.; Pappas, C.S.; Quartieri, A.; Tsironi, T. Synthesis and characterization of polysaccharide- and protein-based edible films and application as packaging materials for fresh fish fillets. Sci. Rep. 2024, 14, 517. [Google Scholar] [CrossRef]
- Kang, H.; Wang, Z.; Zhang, W.; Li, J.; Zhang, S. Physico-chemical properties improvement of soy protein isolate films through caffeic acid incorporation and tri-functional aziridine hybridization. Food Hydrocoll. 2016, 61, 923–932. [Google Scholar] [CrossRef]
 ), SCP (g/L) (
), SCP (g/L) ( ), ethanol (g/L) (
), ethanol (g/L) ( ), and DCW lactose (g/L) (
), and DCW lactose (g/L) ( ).
).
 ), SCP (g/L) (
), SCP (g/L) ( ), ethanol (g/L) (
), ethanol (g/L) ( ), and DCW lactose (g/L) (
), and DCW lactose (g/L) ( ).
).

| Feed | Hours | Biomass (g/L) | SCP (g/L) | Lactosecons (g/L) | Ethanol (g/L) | YSCP/X (g/g) | YX/Laccons (g/g) | YEth/Laccons (g/g) |
|---|---|---|---|---|---|---|---|---|
| Lac = 9.9 ± 0.2 (g/L) | 0.0 | 0.00 | 0.00 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 |
| 4.0 | 0.90 ± 0.05 | 0.41 ± 0.02 | 0.1 ± 0.0 | 0.0 | 0.46 ± 0.00 | 8.18 ± 1.00 | 0.00 | |
| 8.0 | 1.41 ± 0.05 | 0.70 ± 0.10 | 1.6 ± 0.1 | 0.1 ± 0.0 | 0.50 ± 0.05 | 0.87 ± 0.01 | 0.05 ± 0.00 | |
| 10.5 | 2.13 ± 0.04 | 0.89 ± 0.04 | 4.6 ± 0.2 | 0.6 ± 0.0 | 0.42 ± 0.01 | 0.46 ± 0.01 | 0.14 ± 0.00 | |
| 13.0 | 2.58 ± 0.04 | 1.12 ± 0.04 | 8.0 ± 0.2 | 1.9 ± 0.0 | 0.44 ± 0.00 | 0.32 ± 0.00 | 0.23 ± 0.00 | |
| 1st Lac = 0.0 ± 0.0 (g/L) | 14.0 | 2.58 ± 0.04 | 1.12 ± 0.04 | 8.0 ± 0.2 | 2.9 ± 0.1 | 0.44 ± 0.00 | 0.32 ± 0.00 | 0.36 ± 0.01 |
| 22.5 | 3.97 ± 0.06 | 1.89 ± 0.04 | 15.2 ± 0.3 | 5.5 ± 0.3 | 0.48 ± 0.00 | 0.26 ± 0.00 | 0.36 ± 0.02 | |
| 24.0 | 3.97 ± 0.06 | 1.89 ± 0.04 | 15.2 ± 0.3 | 5.9 ± 0.2 | 0.48 ± 0.00 | 0.26 ± 0.00 | 0.39 ± 0.02 | |
| 2nd Lac = 3.7 ± 0.3 (g/L) | 26.0 | 4.95 ± 0.09 | 2.15 ± 0.05 | 19.7 ± 0.1 | 7.6 ± 0.3 | 0.43 ± 0.00 | 0.25 ± 0.00 | 0.39 ± 0.02 |
| 27.0 | 4.95 ± 0.09 | 2.15 ± 0.05 | 21.1 ± 0.5 | 8.7 ± 0.3 | 0.43 ± 0.00 | 0.23 ± 0.00 | 0.41 ± 0.02 | |
| 3rd Lac = 0.0 ± 0.0 (g/L) | 28.0 | 5.36 ± 0.02 | 2.63 ± 0.04 | 23.5 ± 0.1 | 9.8 ± 0.2 | 0.50 ± 0.01 | 0.23 ± 0.00 | 0.42 ± 0.01 |
| SCP-Based Edible Films | L* | a* | b* | C* | h* | Film Opacity |
|---|---|---|---|---|---|---|
| 30GLY | 57.8 ± 1.7 a | 17.8 ± 2.6 a | 49.6 ± 4.7 a | 52.8 ± 5.3 a | 70.5 ± 1.3 a | 3.5 ± 0.7 a |
| 40GLY | 58.3 ± 2.8 a | 17.0 ± 0.3 a | 52.3 ± 3.7 a | 55.2 ± 3.4 a | 72.0 ± 1.5 a | 6.0 ± 2.2 a |
| 50GLY | 58.0 ± 0.7 a | 17.2 ± 1.4 a | 48.6 ± 2.4 a | 51.6 ± 2.4 a | 70.5 ± 1.6 a | 5.1 ± 0.6 a |
| SCP-Based Edible Films | Moisture Content (%) | Solubility (%) | S.I (%) | WVP (g·mm/m2·d·kPa) |
|---|---|---|---|---|
| 30GLY | 11.2 ± 0.7 a | 48.5 ± 2.5 a | 33.2 ± 2.5 a | 20.5 ± 4.1 a |
| 40GLY | 15.0 ± 2.7 a | 75.0 ± 1.3 b | 34.4 ± 1.8 a | 19.2 ± 3.8 a |
| 50GLY | 25.9 ± 0.3 b | 58.2 ± 2.8 c | 38.8 ± 1.1 a | 21.4 ± 1.2 a |
| SCP-Based Edible Films | TS (Mpa) | E (%) | OCA° |
|---|---|---|---|
| 30GLY | 1.3 ± 0.6 a | 6.9 ± 2.0 a | 47.1°±0.5 a |
| 40GLY | 0.3 ± 0.1 a | 5.6 ± 0.8 a | 46.7° ± 1.3 a |
| 50GLY | 0.4 ± 0.1 a | 4.7 ± 0.5 a | 54.0° ± 0.5 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koukoumaki, D.I.; Papanikolaou, S.; Ioannou, Z.; Gkatzionis, K.; Sarris, D. The Development of Novel Edible Films from Single-Cell Protein Produced by the Biotechnological Valorization of Cheese Whey. Appl. Microbiol. 2024, 4, 1030-1041. https://doi.org/10.3390/applmicrobiol4030070
Koukoumaki DI, Papanikolaou S, Ioannou Z, Gkatzionis K, Sarris D. The Development of Novel Edible Films from Single-Cell Protein Produced by the Biotechnological Valorization of Cheese Whey. Applied Microbiology. 2024; 4(3):1030-1041. https://doi.org/10.3390/applmicrobiol4030070
Chicago/Turabian StyleKoukoumaki, Danai Ioanna, Seraphim Papanikolaou, Zacharias Ioannou, Konstantinos Gkatzionis, and Dimitris Sarris. 2024. "The Development of Novel Edible Films from Single-Cell Protein Produced by the Biotechnological Valorization of Cheese Whey" Applied Microbiology 4, no. 3: 1030-1041. https://doi.org/10.3390/applmicrobiol4030070
APA StyleKoukoumaki, D. I., Papanikolaou, S., Ioannou, Z., Gkatzionis, K., & Sarris, D. (2024). The Development of Novel Edible Films from Single-Cell Protein Produced by the Biotechnological Valorization of Cheese Whey. Applied Microbiology, 4(3), 1030-1041. https://doi.org/10.3390/applmicrobiol4030070








