Abstract
The oral microbiome, a complex ecosystem of microbes, is crucial for oral health. Imbalances in this ecosystem can lead to various oral diseases. Probiotics, live beneficial bacteria, offer a potential solution by strengthening oral defences. This study aimed to develop and evaluate a novel toothpaste containing Streptococcus salivarius M18, a probiotic strain. After ensuring compatibility with toothpaste ingredients, a stable formulation with desirable properties was created. The toothpaste demonstrated cleaning efficacy and antimicrobial activity against oral pathogens in vitro. A clinical trial involving healthy adults showed that all doses of the probiotic toothpaste significantly increased S. salivarius M18 levels in saliva, with the effect persisting even after discontinuation. These findings suggest that the toothpaste effectively delivers the probiotic to the oral cavity and promotes colonisation. Further research is needed to optimise the formulation and assess its long-term impact on oral health.
1. Introduction
The human oral cavity is a teeming microcosm, harbouring a complex and dynamic ecosystem of over 700 bacterial species [1]. This diverse community, known as the oral microbiome, plays a critical role in maintaining our oral health [2]. A delicate balance exists within this microbiome, and disruptions can lead to various oral diseases, including dental caries, periodontal disease, and halitosis [3]. Traditionally, oral hygiene practices focus on mechanical plaque removal through brushing and flossing, along with antiseptic mouthwashes. While effective at removing debris and reducing bacteria, these methods often fail to address the underlying microbial imbalances that contribute to oral health issues [4]. A paradigm shift is underway, moving from simply eliminating all bacteria to promoting a healthy microbiome. This has led to the exploration of novel therapeutic strategies, including probiotics. Defined by the World Health Organization (WHO) as “live microorganisms which when administered in adequate amounts confer a health benefit on the host” [5], probiotics like Streptococcus salivarius M18 offer promise in oral health [6,7].
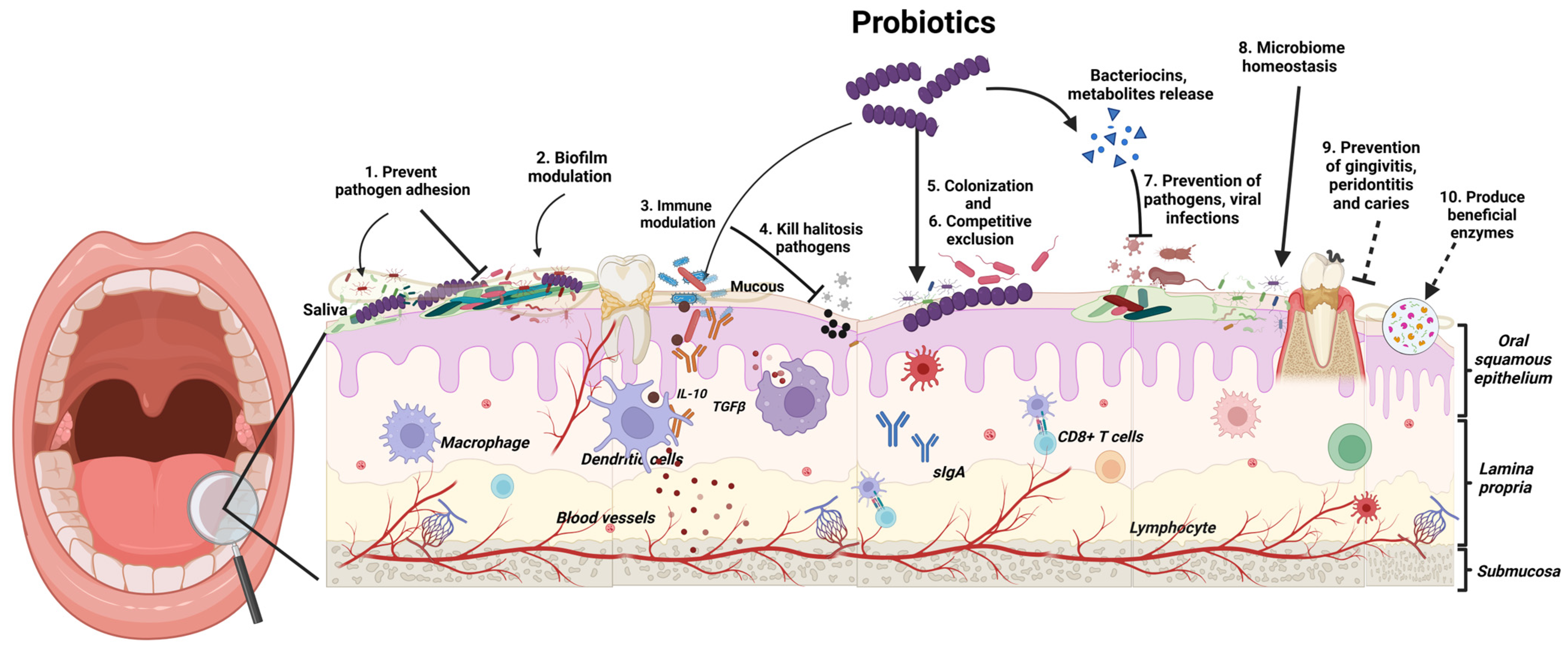
Probiotics exert their beneficial effects in the oral cavity through several mechanisms (Figure 1). These mechanisms include enhanced epithelial barrier function: Probiotics can act as a first line of defence against invading pathogens by strengthening the barrier formed by epithelial cells in the mouth [8]. Competitive exclusion: Probiotics compete with pathogenic bacteria for adhesion sites on oral surfaces. This limits the ability of harmful bacteria to colonise and form biofilms, which are sticky films that contribute to oral diseases [8]. Antimicrobial production: Probiotics can produce substances like bacteriocins, which directly inhibit the growth of pathogenic bacteria [8]. Modulation of immune response: Probiotics can stimulate the production of anti-inflammatory cytokines and suppress pro-inflammatory mediators. This promotes a balanced immune response within the oral cavity, further aiding in oral health [8].
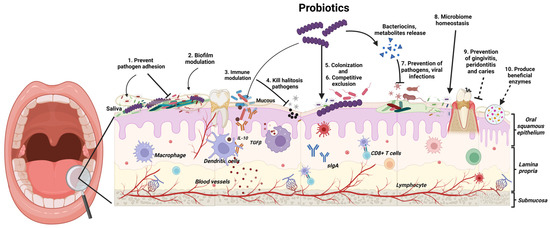
Figure 1.
Probiotics exert their beneficial effects in the oral cavity through several mechanisms. (Created in https://BioRender.com/g66c495, accessed on 21 January 2025).
Clinical research is increasingly backing the use of probiotics for preventing and managing various oral diseases. Studies show that probiotic supplements can reduce dental cavities in both children and adults [6,9]. These beneficial bacteria have also been effective in tackling plaque and gingivitis, the early stages of gum disease [10]. More recent research suggests probiotics may even help with halitosis (bad breath) by reducing levels of bad-smelling sulphur compounds produced by certain oral bacteria [11].
Targeted Delivery: Toothpaste as a Novel Platform
The delivery of probiotics to the oral cavity is crucial for their efficacy. Various delivery systems have been explored, including lozenges and powders containing probiotic strains. However, these methods often have limitations, such as short residence time in the oral cavity. A probiotic toothpaste presents a novel and promising platform for delivering probiotics directly to the oral biofilm. Toothpaste offers several advantages, including its widespread use in daily oral hygiene routines, prolonged contact time with oral tissues, and the ability to target specific areas within the oral cavity [4,9,10]. While research on probiotic toothpaste is still emerging, limited initial clinical trials have yielded promising results. Studies have shown that probiotic toothpaste can be as effective as conventional fluoride toothpaste in reducing plaque and gingivitis, the early stages of gum disease [11]. Additionally, user safety and tolerability of probiotic toothpaste have been established [12].
The probiotic Streptococcus salivarius is a naturally dominant bacterial species in the human oral cavity, particularly on the tongue [13,14,15]. In 2010, a specific strain, S. salivarius M18 (BLIS M18™), was identified for its potential benefits in oral health. This strain produces four unique bacteriocins (salivaricin A, 9, MPS, and M) [16] that inhibit the growth of dental pathogens like Streptococcus mutans and Actinomyces viscosus, which cause caries (cavities) and periodontal disease [17].This sets S. salivarius M18 apart from other probiotics and targets its activity specifically for dental applications. In addition to in vitro studies [6,18], several clinical trials have shown the effectiveness of daily lozenges containing Blis M18 in promoting oral health by reducing dental infections [17,19,20,21,22,23]. However, no published reports have yet evaluated the efficacy of S. salivarius M18 delivered in a toothpaste format.
This study employed a systematic approach to evaluate the in vivo efficacy of a probiotic toothpaste containing live Streptococcus salivarius M18. The evaluation encompassed the antimicrobial activity of the probiotic strain against common dental pathogens, the compatibility of all ingredients within the toothpaste formulation, the cleansing ability of the toothpaste itself, and finally, microbiological analysis of saliva samples collected from healthy adult volunteers following a clinical trial.
2. Materials and Methods
2.1. Materials
S. salivarius M18 (formerly denoted as Mia) has been deposited in the internationally recognised culture collections Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH—German Collection of Microorganisms and Cell Cultures DSMZ 14685 and American Type Culture Collections ATCC BAA2593).
S. salivarius M18 freeze-dried raw ingredient powder was supplied by Blis Technologies Ltd., Dunedin, New Zealand. The (USP grade) vehicle Medium Chain Triglyceride (MCT—Radia 7104) oil was purchased from Oleon, Malaysia. A colloidal hydrophobic silica oleogelator/viscosity modifier (>99.8%, USP grade) was a gift from Chemiplas, Auckland, New Zealand. The dispersing and wetting agent polysorbate 80 (USP grade) was purchased from Sigma Aldrich, Auckland, New Zealand, and abrasive calcium carbonate and flavouring agents, spearmint oil and peppermint (organic) oil, were purchased from Pure Ingredients, Auckland, New Zealand. Bubble gum flavour oil and grape flavour oil were purchased from Lorann Oils, Auckland, New Zealand. Fluoride (sodium monofluorophosphate) was purchased from Alfa Aesar, Thermo Fisher Scientific, Auckland, New Zealand. Xylitol (fine grain—Xylisorb-90) was a gift from Roquette, Frankfurt am Main, Germany. Synergy Flavour (PF 7513) was a gift from Pacific Flavours, Auckland, New Zealand. Xanthan gum was purchased from Lotus, Auckland, New Zealand. Smoothenol was a gift from Sensient, Auckland, New Zealand. Low-foaming agents Sodium Cocyl Isethionate, Coco Glucoside, Decyl Glucoside, Lauryl glucoside, and Caprylyl glucoside were purchased from Pure Nature, Auckland, New Zealand. Teeth-whitening agent hydrogen peroxide was purchased from Ecostore, Auckland, New Zealand. Dilutions for cell counts were prepared using analytical-grade phosphate buffered saline (PBS) (Oxoid, Dulbeco A) purchased from Thermofisher Scientific, Auckland, New Zealand. The sample dispersions were prepared using filter stomacher bags (BagPage+ full-page filter bag, microperforated filter, 400 mL, Interscience, Auckland, New Zealand).
Bacterial cultures Streptococcus mutans 10449 (ATCC 25175), Streptococcus pyogenes 71–698, Streptococcus pyogenes FF22, Streptococcus pyogenes W-1, Streptococcus pyogenes 71–679, Streptococcus mutans ATCC 10449, Streptococcus mutans 31c, and Actinomyces viscosus T14 were supplied by BLIS Technologies Ltd., Dunedin, New Zealand, on request. Bacterial cultures were grown on human blood agar supplemented with 0.1% (w/v) calcium carbonate (hBaCa), and Columbia blood agar plates (CABK12) were purchased from Fort Richard Labs, Auckland, New Zealand. Sodium surfactin was received as a gift sample from Kaneka Corporation, Tokyo, Japan. Distilled water was used for preparing buffers and agar medium.
2.2. Methods
2.2.1. Probiotic Excipient Compatibility Study
This study investigated whether commonly used toothpaste ingredients (abrasives, flavours, foaming agents) affected the viability of S. salivarius M18 by employing a modified minimum inhibitory concentration assay on an agar plate. Briefly, a suspension was prepared by mixing 1.1 g of freeze-dried powder of S. salivarius M18 in 9.9 g of PBS in a stomacher bag and mixed for 5 min in a stomacher (Masticator Basic, IUL Instruments, Barcelona, Spain). The suspension was then serially diluted (1 in 10 dilution), and 10−5 dilution was spread on CABK12 and hBaCa agar plates to prepare a lawn of S. salivarius M18. Each test excipient was also serially diluted with PBS or MCT oil to prepare a range of concentrations. Then, 10 µL of each test excipient concentration was spot plated (up to 8 spots per plate, highest to lowest concentration in a clockwise direction) on the lawn of S. salivarius M18, and the plates were incubated at 37 °C, 5% CO2 for 24 h. After incubation, the zones of inhibition were recorded.
2.2.2. Formulation of S. salivarius M18 Toothpaste
The composition of the probiotic toothpaste is shown in Table 1. Briefly, in a 250 mL beaker, the required quantities (Table 1) of MCT oil and polysorbate 80 were thoroughly mixed to form a uniform mixture. S. salivarius M18 freeze-dried powder, hydrophobic silica, xylitol, calcium carbonate, xanthan gum, sweetener, and flavours were added, and the mixture was homogenised at 8000–15,000 rpm for 3–5 min (intermittently to avoid heat build-up) using a high shear homogeniser (D-160, DLAB, Auckland, New Zealand). This approach ensures all ingredients are thoroughly combined while minimising potential damage to the probiotic strain caused by excessive heat.

Table 1.
S. salivarius M18 toothpaste composition.
2.2.3. Enumeration and Recovery of S. salivarius M18 in Toothpaste Formulations
A method for the enumeration of S. salivarius M18 in toothpaste formulation was developed to determine the cell count in samples prepared for in vitro testing and samples for a colonisation efficacy trial. In a filter stomacher bag, 1.1 g of S. salivarius M18 toothpaste formulation was weighed and diluted with 9.9 g of pre-warmed (37 °C) sterile PBS supplemented with 0.005% v/v sodium surfactin. The mixture was homogenised by mixing using a stomacher for 5 min at room temperature (20 °C ± 2 °C) to obtain a homogeneous dispersion. Samples (100 µL) were ten-fold serially diluted in sterile PBS, spread-plated onto CABK12 agar, and incubated at 37 °C in an atmosphere of 5% CO2 in the air for 24 h. The S. salivarius M18 colonies were counted and recorded as colony-forming units (CFU)/g using a Q-Count Automatic Colony Counter (Spiral Biotech, Auckland, New Zealand).
2.2.4. Physicochemical Properties of Probiotic Toothpaste
Determination of the pH: The pH of the 10% toothpaste formulations was determined by using a digital pH metre (Mettler Toledo, Greifensee, Switzerland). Briefly, in a 50 mL beaker, 1 g of the toothpaste formulations was mixed with 9 g of PBS (10% w/v), and the pH of the mixture was measured at room temperature (~22 °C) in triplicate.
Determination of the viscosity: The viscosity of the toothpaste formulation was measured using a Brookfield viscometer (Brookfield LVDVI—Prime using Brookfield Helipath Spindle, Middleboro, MA, USA). Briefly, in a 100 mL beaker, 80 g of toothpaste formulation was taken, the viscometer spindle S94 was immersed into it, and the viscosity was measured in triplicate at 0.5 RPM (rotations per minute) and 25 °C.
2.2.5. Determination of the Cleaning Ability of the Probiotic Toothpaste to Remove Stained Pellicle
Briefly, the cleaning ability of the probiotic toothpaste and a standard reference material (silica) was tested by an independent laboratory based in the USA, following the protocol described here. Bovine, permanent, central incisors were cut to obtain labial enamel specimens approximately 10 × 10 mm. The enamel specimens were embedded in an autopolymerising methacrylate resin so that only the enamel surfaces were exposed. The enamel surfaces were then smoothed and polished on a lapidary wheel and lightly etched to expedite stain accumulation and adherence. They were placed on a rotating rod (~37 °C incubator), which alternately exposed them to air and a solution consisting of PGY broth, tea, coffee, mucin, FeCl3, and Micrococcus luteus. The staining broth was changed, and specimens were rinsed daily until a uniform stain had accumulated. After approximately seven days, a darkly stained pellicle film was apparent on the enamel surfaces. Specimens were rinsed, allowed to air dry, and refrigerated until used. All products were tested using specimens prepared at the same time.
Scoring and Set-Up: The amount of in vitro stain was photometrically graded using only the L value of the L*a*b* scale using a spectrophotometer (Konica Minolta CM2600d, Konica Minolta Sensing Americas, Ramsey, NJ, USA). The area of the specimens scored was a 1/4-inch-diameter circle in the centre of the 10 × 10 mm enamel. Specimens with scores between 30 and 42 (30 being more darkly stained) were used. Based on these scores, the specimens were divided into groups of 16 specimens each, with each group having approximately the same average baseline score.
Procedure: The specimens were mounted on a mechanical V-8 cross-brushing machine equipped with soft nylon filament (Oral-B 40) toothbrushes. Tension on the enamel surface was adjusted to 150 g. The dentifrices were tested as slurries prepared by mixing 25 g of dentifrice with 40 mL of deionised water. The standard reference material was tested as a slurry by mixing 10 g of reference silica with 50 mL of 0.5% CMC solution. The specimens were brushed for 800 strokes (4.5 min). To minimise mechanical variables, two specimens per group were brushed on each of the eight brushing heads. Different test products were used on each run, with one tube of slurry made up for each product. The fresh slurry was made after being used to brush four specimens. Following brushing, specimens were rinsed, blotted dry, and scored again for stain, as previously described.
Calculations: The mean decrement between the pre- and post-brushing stain scores was determined for the standard reference material group and assigned a pellicle cleaning ratio (PCR) value of 100. A constant value was calculated by dividing the mean decrement (corrected for the use of silica instead of Ca2P2O7) of the standard reference material into 100. The individual PCR value for each specimen was calculated by multiplying its decrement by the calculated constant. The mean, standard deviation, and SEM (standard error of the mean) for each test group (N = 16) were then calculated using the individual PCR values. The larger the PCR value, the greater the amount of stained pellicle removed from the enamel surface in this test.
2.2.6. Determination of the Relative Dentin Abrasion Level of Probiotic Toothpaste
This study was conducted by an independent laboratory based in the USA. The procedure used in this study was the Heffernan abrasivity test [6] recommended by the ADA and ISO 11609 [24] for the determination of dentifrice abrasiveness in dentin. The abrasivity limit specified by ISO 11609 at 2.5× that of the standard reference material (ISO reference silica) may be considered in the interpretation of the results of this test. Therefore, since the current protocol has assigned an arbitrary value of 100 to the standard reference material, the RDA abrasivity limit is 250.
Specimen preparation: Eight human dentin specimens were subjected to neutron bombardments resulting in the formation of radioactive phosphorus (32P) within the specimens under the controlled conditions outlined by the ADA. The specimens were mounted in methyl methacrylate, so they fit in a V-8 cross-brushing machine. The specimens were brushed for a 1500-stroke precondition run using a slurry consisting of 10 g of standard reference material (Evonik, Essen, Germany) in 50 mL of a 0.5% CMC glycerine solution. The brushes used were those specified by the ADA with a brush tension of 150 g.
Procedure: Following the precondition run, the test was performed using the above parameters (150 g and 1500 strokes) in a “sandwich design”. Before and after brushing with the test product (25 g product/40 mL water), each tooth set was brushed with the standard reference material (10 g of ISO Reference Silica/50 mL 0.5% CMC). The procedure was repeated additional times so that each product was assayed on each tooth set. The treatment design was the modified Latin square design so that no treatment followed another treatment consistently.
Calculations: One ml samples were taken, each weighed (~1 g), and added to 5 mL of the “Ultima Gold” scintillation cocktail. The samples were mixed well and immediately put on a liquid scintillation counter for radiation detection. Following counting, the net counts per minute (CPM) values were divided by the weight of the sample to calculate a net CPM/gram of slurry. The net CPM/g of the pre- and post-standard reference materials was multiplied by a correction factor (calculated by the difference between the calcium pyrophosphate and ISO reference silica materials in in-house testing) and then averaged to use in the calculation of RDA (relative dentin abrasion) for the test material. The standard reference material was assigned a value of 100, and its ratio to the test material was calculated.
2.2.7. Antimicrobial Activity of Probiotic Toothpaste Against the Indicator Microorganisms
Bacteriocin production was assessed using the deferred antagonism test [25], where the test strain secretes bacteriocin(s) into the culture medium, and following this, various bacteriocin-susceptible (indicator) strains were applied to the bacteriocin-containing agar. If the bacteriocin inhibits the indicator strain, there is a corresponding absence of its growth on the bacteriocin-impregnated agar (i.e., an inhibition zone). S. salivarius M18 freeze-dried raw ingredient powder and S. salivarius M18-containing toothpaste formulation were assessed for their in vitro inhibitory activity against a wide range of oral bacteria, in particular S. mutans.
2.2.8. Preliminary Safety, Tolerability, and Colonisation Efficacy Trial in Healthy Human Adults
A randomised, baseline-controlled, parallel-group trial was conducted in healthy human adult participants in August 2022 to assess safety, colonisation efficacy, and colonisation towards the use of the S. salivarius M18 toothpaste formulation. The toothpaste was first tested for the absence of microbial contaminants by an independent laboratory (Cawthron Institute, Nelson, New Zealand). All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki and approved by the Health and Disability Ethics Committee, Ministry of Health, Wellington, New Zealand (2022 EXP 13043) and registered with ClinicalTrials.gov (NCT05480020).
2.2.9. Pilot Cosmetic Trial Design
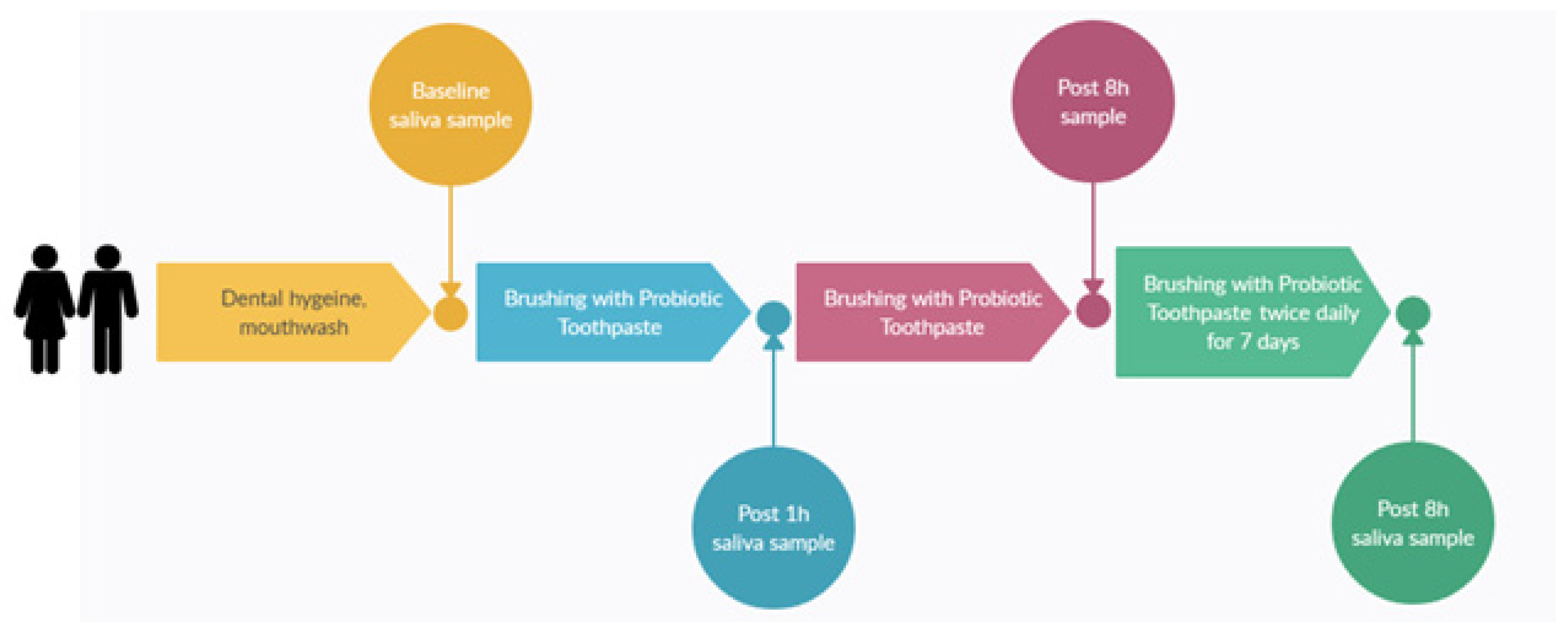
The primary aim of the pilot trial was to evaluate the microbial colonisation efficacy, that is, levels in the saliva samples of S. salivarius M18 when delivered in a toothpaste format. The toothpaste composition remained similar as per Table 1 except for the amount of S. salivarius M18, which was varied to achieve different cfu/dose. Thirty subjects participated in a single-site, parallel-group, double-blind, randomised, baseline-controlled trial. Potential participants were enrolled if they met the following inclusion criteria: healthy adults 18−75 y of age having generally good health and practicing good oral hygiene. Participants were excluded if they had a history of autoimmune disease or were currently being treated with either antibiotics or anti-inflammatories (e.g., steroids, non-steroidal anti-inflammatory drugs). The study design and dosing regimens are shown in Figure 2. Briefly, participants were randomly assigned to one of the three groups to brush their teeth twice daily for 7 days with a probiotic toothpaste containing different doses of S. salivarius M18. Saliva samples were collected and stored frozen (−20 °C) until they were analysed.
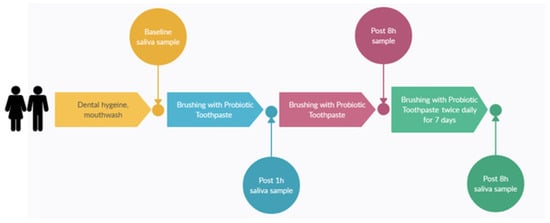
Figure 2.
Study design.
The participants were randomly assigned to three groups (n = 10 per group) receiving toothpastes with varying S. salivarius M18 concentrations (CFU/dose): Study Group A: 1 million Cfu/dose (n = 10, 8M, 2F); Study Group B: 10 million Cfu/dose (n = 10, 5M, 5F); and Study Group C: 100 million Cfu/dose (n = 10, 4M, 6F). Participants were also asked to report any adverse event, fill out a compliance diary, and complete a survey indicating their experience of using the probiotic toothpaste.
Sample size justification: A sample size calculation was employed and was specifically tailored to detect a statistically significant difference in our primary outcome measure: colonisation of viable S. salivarius M18 cells in saliva. An online sample size calculator (link to clincalc.com/stats/samplesize.aspx, accessed on 18 October 2021) was utilised, considering the following parameters:
Power: 80%—this ensures a high probability of detecting a true colonisation effect if it exists. Significance level: 0.05—this represents the acceptable chance of obtaining a statistically significant result by random chance. The margin of error: 20%—this reflects the expected variability in measuring viable S. salivarius M18 cells, a common value for microbial analysis. Based on these parameters, the initial target sample size was eight participants per group. To account for potential participant dropout, a frequent occurrence in clinical trials, we aimed to recruit 10 participants per group. This approach provides a buffer and ensures an adequate final sample size for robust statistical analysis of the colonisation data. Additionally, a sample size of 10 participants per group allows for the detection of any potential adverse events associated with the intervention within a reasonable timeframe, thus addressing safety considerations throughout this study.
2.2.10. Microbial Analysis
The frozen saliva samples were defrosted at room temperature and then vortex-mixed for 1 min at 2600 rpm in a Class II biological safety cabinet (Labguard (ES), NUAIRE, Plymouth, MN, USA) before 10-fold serially diluting in PBS. Samples (50 µL) of the initial suspension and each serial dilution were spiral-plated (Whitley Automated Spiral Plater, Don Whitley Scientific, Auckland, New Zealand) on Mitis–Salivarius agar plates (an S. salivarius selective growth media) and incubated at 37 °C in air supplemented with 5% CO2 for 24 h. After incubation, S. salivarius M18 and a closely related oral strain were analysed.
S. salivarius K12 colonies were further differentiated based on their inhibition activity against the specific indicator strains I1 (Micrococcus luteus T18) and I3 (Streptococcus cosntellatus T29). The indicator strains were pre-plated on sheep blood agar plates (sBaCa) (using a 10−1 dilution of a liquid suspension of I3 made by swabbing from an area of I3 culture (on sBaCa) approximately 1 cm2; for I1 only one colony was picked from the agar plate using a sterile cotton swab and suspended in 900 µL PBS). With the help of a sterile toothpick, the S. salivarius isolates grown from the saliva samples on the Mitis–Salivarius agar were transferred to the sBaCa plates containing the pre-seeded indicator lawns and incubated for 24 h at 37˚C in an atmosphere of 5% CO2 in the air. The inhibition capacity of each isolate was then compared to the S. salivarius M18 controls, which were added to each plate, enabling the identification of each isolate as either S. salivarius M18 or other.
2.2.11. Consumer Experience Survey
To assess participants’ experience with the probiotic toothpaste, a survey was conducted. Participants rated their overall liking of the toothpaste on a 9-point scale (1 = dislike extremely, 9 = like extremely) and indicated their preference for it over their usual toothpaste on a 5-point scale (1 = dislike much more, 5 = like much more). The researchers felt the minimal amount of S. salivarius M18 in the toothpaste would not significantly impact taste, so the taste was not evaluated. Instead, the survey focused on overall experience and preference.
2.2.12. Statistical Analysis
Statistical analysis and data visualisation for the saliva samples were performed using Prism 9.4.0 software (GraphPad Software). A repeated-measures one-way ANOVA (analysis of variance) to compare S. salivarius M18 colonisation levels across the different dosage groups (1 million CFU/dose, 10 million CFU/dose, and 100 million CFU/dose) was carried out. Following the ANOVA, Tukey’s multiple comparisons test was used to identify statistically significant differences in colonisation between specific dose groups. The level of significance was set at p ≤ 0.05. Additionally, the overall colonisation rate (the percentage of participants with detectable S. salivarius M18) within each dose group was reported. Ratings from the participant experience survey were reported as means ± SDs.
3. Results
3.1. Probiotic Excipient Compatibility Study
An assessment of S. salivarius M18’s compatibility with common toothpaste ingredients (Table 2) revealed that its viability was significantly inhibited by several components. These included the teeth-whitening agent hydrogen peroxide, both low- and high-foaming agents, and, surprisingly, even a commercially available probiotic toothpaste.

Table 2.
Compatibility of S. salivarius M18 with common toothpaste components.
3.2. Preparation and Enumeration of S. salivarius M18 Toothpaste
The non-aqueous formulation was a smooth, viscous paste with a toothpaste-like consistency. The enumeration method was suitable for the enumeration with 100% recovery of probiotics from the toothpaste matrix.
pH: the pH of the 10% solution was 6.96.
Viscosity: The toothpaste prototype had a viscosity of >192,000 cp at 25 °C.
3.3. Relative Dentin Abrasion Level of Probiotic Toothpaste
The Table 3 below summarises the results of the relative dentin abrasivity (RDA) testing. It presents the mean RDA values for the tested groups (±standard error of the mean).

Table 3.
Relative dentin abrasion score for probiotic toothpaste.
3.4. Cleaning Ability of the Probiotic Toothpaste to Remove Stained Pellicle
The Table 4 below summarises the results of the stained pellicle removal test, listing PCR values (mean ± SEM) in descending order (higher values indicate greater efficacy). Based on statistical analysis and the ranking in the table, the standard reference material displayed greater effectiveness in removing stained pellicles compared to the probiotic toothpaste. It is important to note, however, that commercially available toothpastes exhibit a wide range of PCR values, with silica-containing products typically scoring lower (around 25) and silica/alumina combinations reaching higher values (around 138). The cleaning efficiency index (CEI) should be considered for a more comprehensive assessment of stain cleaning efficacy.

Table 4.
Pellicle cleaning ratio for the probiotic toothpaste.
3.5. Cleaning Efficiency Index (CEI)
A cleaning efficiency index (CEI) was calculated to assess both stain removal and abrasive effects. This index considers both the relative dentin abrasivity (RDA) and pellicle cleaning ratio (PCR) values using the following formula:
CEI = (RDA + PCR − 50)/RDA
A higher CEI value indicates greater cleaning efficacy with a balance between stain removal and reduced abrasiveness. In this study, the Blis M18 toothpaste achieved a CEI of 1.32.
3.6. Antimicrobial Activity of Probiotic Toothpaste Against the Indicator Microorganisms
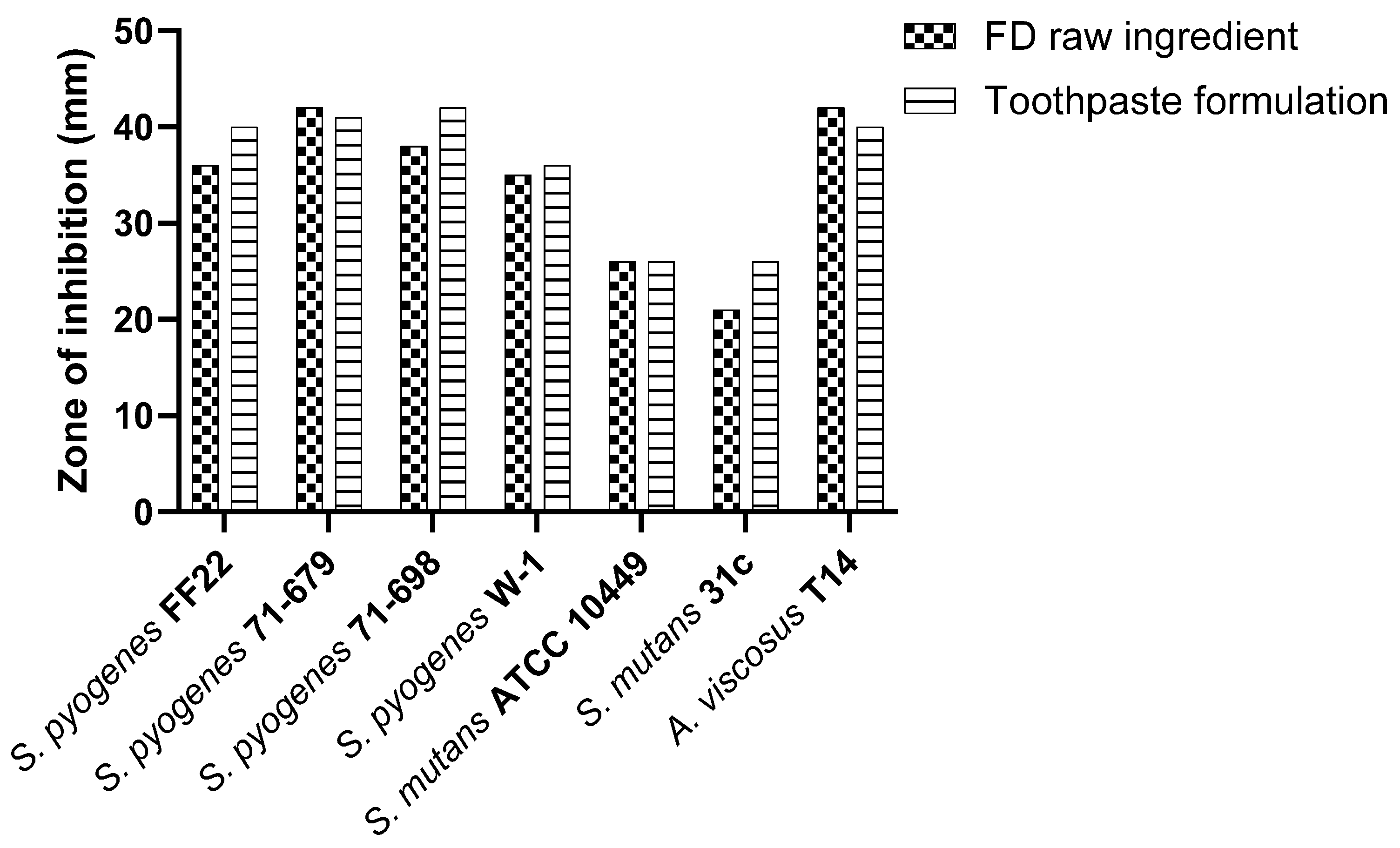
S. salivarius M18 in the toothpaste formulation exhibited inhibitory activity against oral pathogens, comparable to or slightly exceeding the control (Figure 3).
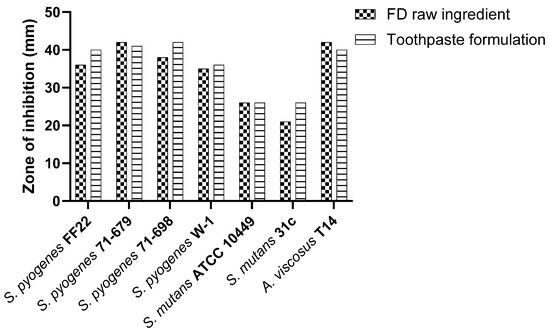
Figure 3.
Comparative in vitro inhibitory efficacy of S. salivarius M18 in toothpaste formulation.
The activity remains comparable to the freeze-dried raw ingredient, suggesting that the toothpaste matrix does not interfere with the antimicrobial efficacy of the probiotic.
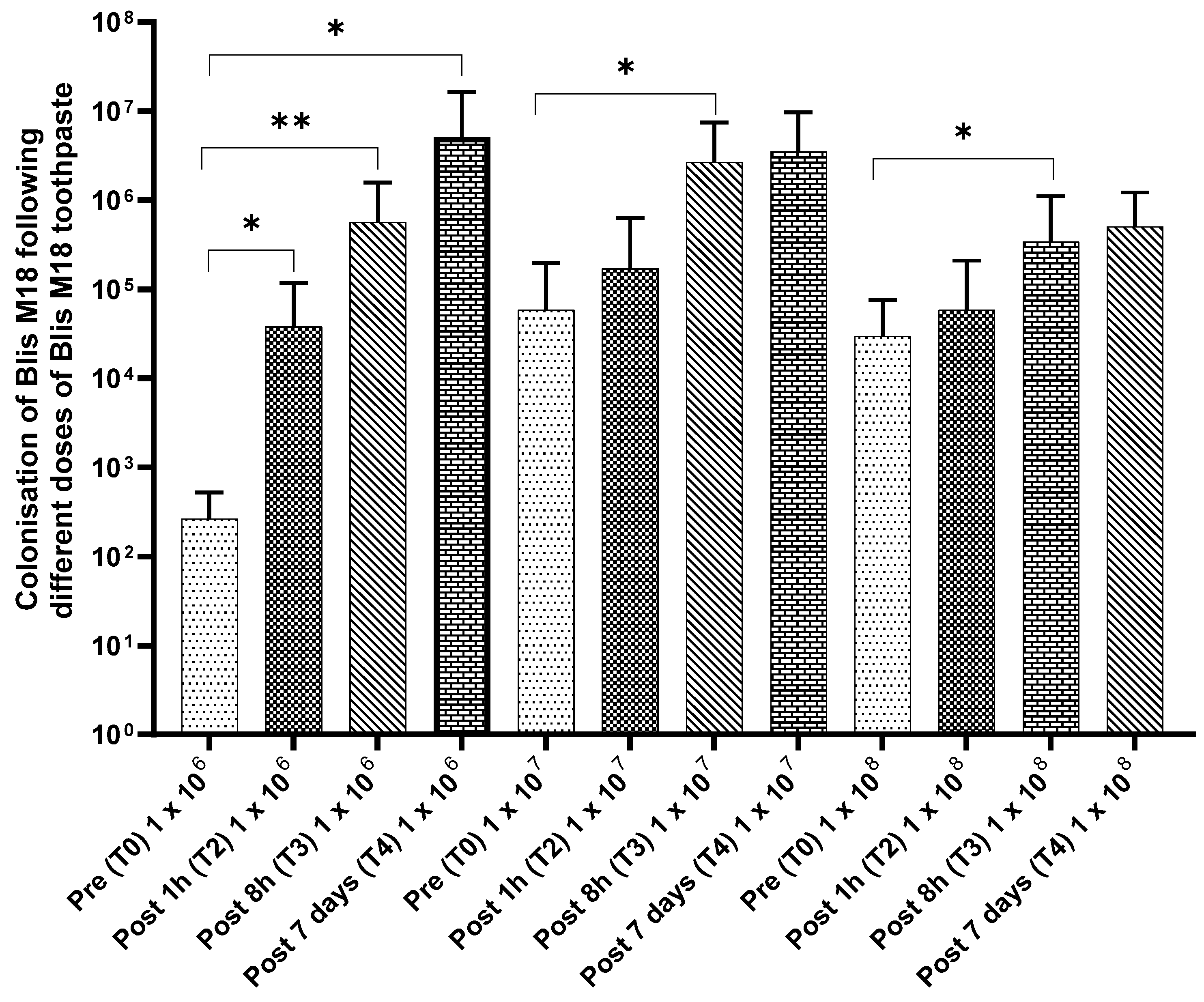
3.7. Colonisation Trials
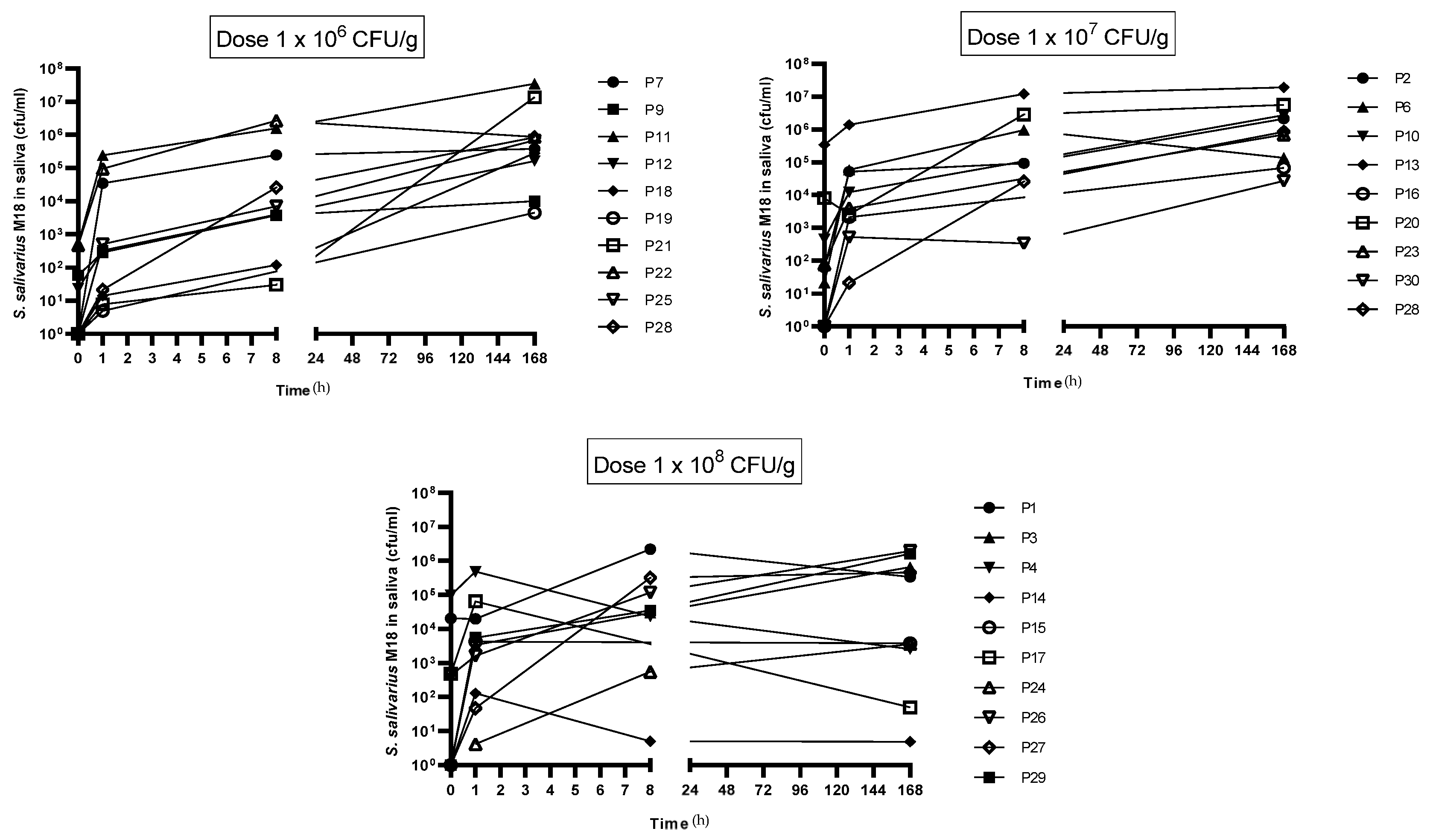
A July/August 2022 colonisation trial assessed if a toothpaste containing S. salivarius M18 could establish the probiotic in participants’ saliva. Encouragingly, all 30 participants finished with no adverse effects, and saliva analysis (Figure 4) showed a significant rise in S. salivarius M18 across all doses. This increase began as early as 1 h after the first use and persisted even 7 days after participants stopped using the toothpaste, indicating potential colonisation.
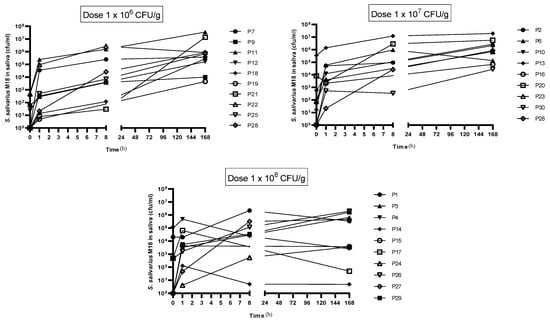
Figure 4.
Change in S. salivarius M18 cell count compared to baseline in participants saliva. Top left: 1 × 106 cfu/dose; top right: 1 × 107 cfu/dose; and bottom left: 1 × 108 cfu/dose. N = 10 per group.
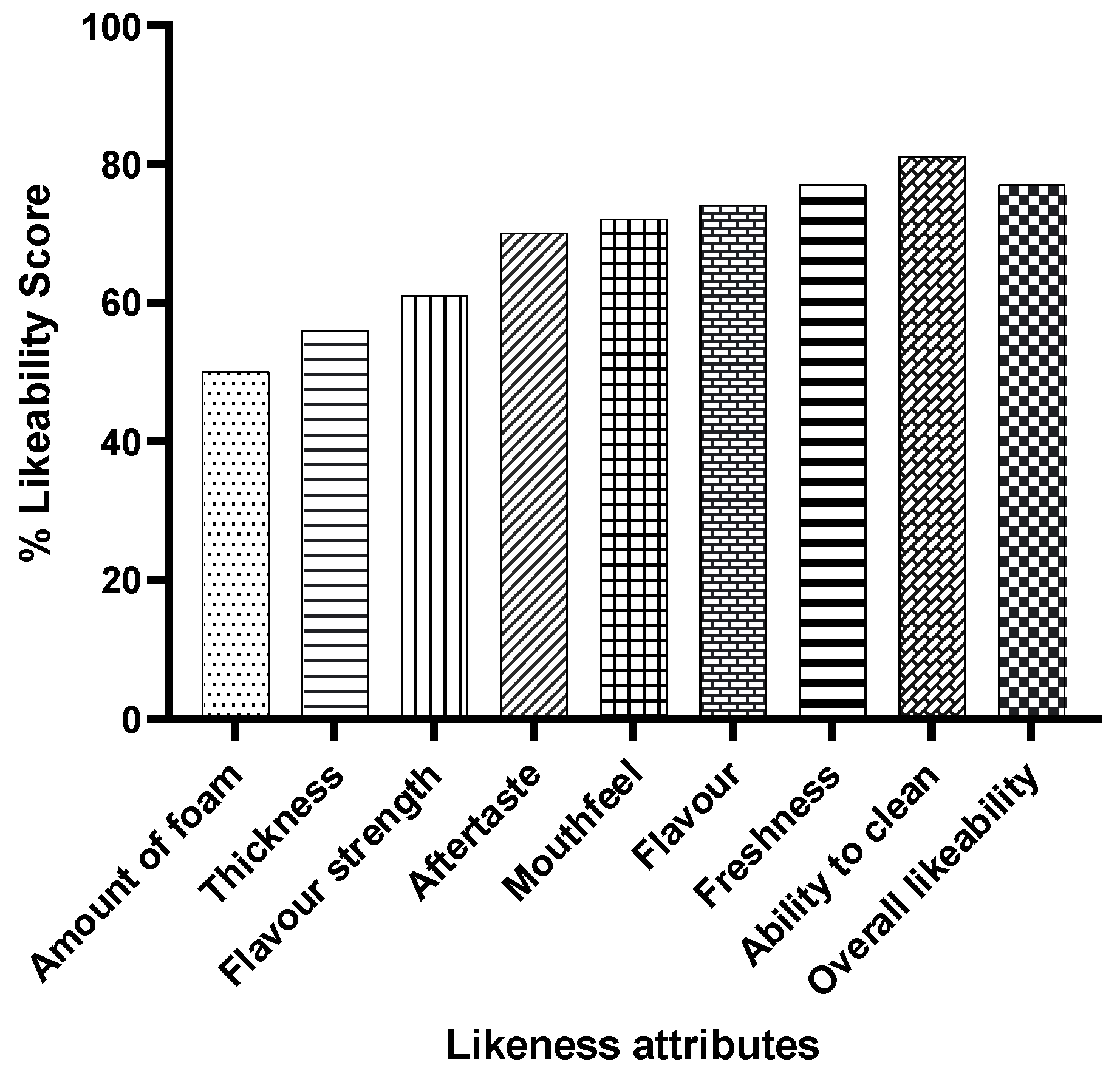
The toothpaste received overwhelmingly positive feedback on its sensory properties (Figure 5). Over 80% of participants reported a clean feeling after use, and more than 70% enjoyed the flavour strength, mouthfeel, and overall experience.
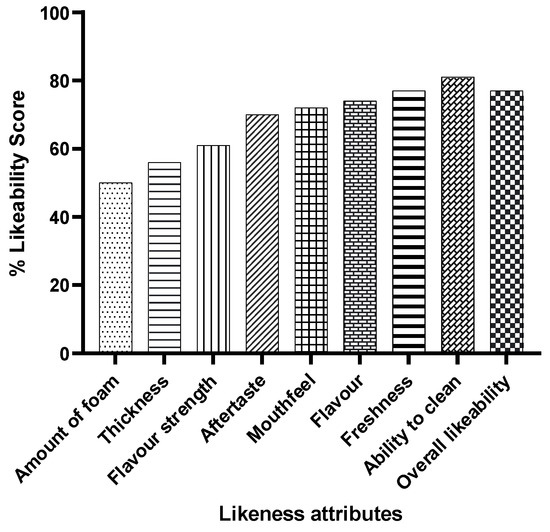
Figure 5.
Feedback on the sensory properties of probiotic S. salivarius M18 toothpaste. Data show the combined feedback of all 30 participants (all three doses).
Compared to the baseline, a significant increase in the cell counts for all time points was observed for 1 × 106 cfu/dose. In comparison, a similar increase was observed for the 1 × 107 cfu/dose (significant at post 7 days) and 1 × 108 cfu/dose (significant at post 8 h) (Figure 6).
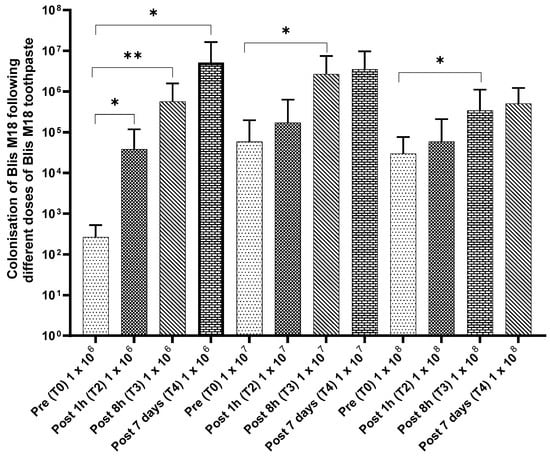
Figure 6.
Colonisation of S. salivarius M18 following different doses of S. salivarius M18 toothpaste. Statistical significance is indicated by the p-value, with lower p-values reflecting stronger evidence. p ≤ 0.05 (Statistically significant *) and p ≤ 0.01 (Highly significant **).
4. Discussions
The rise of probiotics in oral care marks a significant shift in how we approach dental health. Probiotics offer a novel and promising strategy for preventing and managing oral diseases by fostering a balanced oral microbiome. Probiotic toothpaste, with its convenience and targeted delivery, has the potential to revolutionise daily hygiene routines. However, further research is crucial to identify optimal probiotic strains, combinations, and dosages for specific conditions. Additionally, long-term clinical trials are needed to ensure sustained efficacy and safety. Despite these considerations, the oral microbiome’s role in oral health is undeniable [1,3,4]. Probiotics offer a promising approach to promoting balance and preventing disease, with probiotic toothpaste acting as a novel and convenient delivery system. As research progresses, probiotics have the potential to become a cornerstone of future oral healthcare strategies.
The human oral cavity harbours a diverse microbial community known as the oral microbiome, which includes both beneficial and harmful bacteria, fungi, and viruses [1]. A balanced oral microbiome is essential for oral health, as imbalances (dysbiosis) can lead to problems like bad breath, cavities, and gum disease. This microbial landscape is established at birth, influenced by delivery type (vaginal vs. C-section) [25,26], and continues to evolve throughout life. Individual variations arise due to internal factors like genetics and diet, as well as external factors like oral hygiene practices and environmental exposures. These influences create unique microbiomes that differ between people (interpersonal) and even within a single person over time (intrapersonal).
The diverse oral microbiome, teeming with both beneficial commensal and harmful pathogenic bacteria in various niche cavities [3,4], plays a crucial role in maintaining oral health through symbiosis [3,17]. However, a shift from a balanced state to disease can be triggered by a complex interplay of factors. While the specifics remain unclear, it is likely due to a combination of microbial changes, environmental factors, and even physical damage to the oral lining (mucosa) caused by, for example, antibiotics or specific pathogens [3]. Recent research suggests that the specific types of bacteria present and their interactions within the oral cavity can significantly influence the progression of oral disease [3,27,28].
S. salivarius M18 is a strain of probiotic bacteria that resides in the oral cavity of healthy human adults and has been identified to produce a unique antimicrobial spectrum that is inhibitory towards pathogenic bacteria associated with many oral and dental diseases [17]. S. salivarius M18 exerts its antimicrobial and immunomodulatory effects through multiple mechanisms. Primarily, it produces bacteriocins [16,17,29,30,31,32,33], which inhibit the growth of other bacteria, notably Streptococcus mutans, a key cariogenic bacterium [34]. Furthermore, S. salivarius M18 competes with pathogens for nutrients and attachment sites on oral surfaces, limiting their colonisation. Additionally, it may modulate the host’s immune response by influencing cytokine production and potentially enhancing innate and adaptive immunity [28,35,36,37,38].
Blis M18 has established a safety profile and is marketed as a lozenge to prevent teeth and gum-related issues [17]. The efficacy of S. salivarius M18 has been studied concerning oral health benefits and shown to help promote oral and dental health, supporting a healthy balance of oral microbiota and potentially reducing the risk of dental caries [7,18,22,29,38]. So far, the efficacy of this probiotic is seen when it is delivered in lozenge form via Blis M18 ToothGuard lozenges (www.blis.co.nz accessed on 22 November 2022) [7,18,22,29,38]. Currently, there has been no toothpaste containing S. salivarius M18 due to the poor shelf life of this probiotic strain in standard toothpaste preparations. This is because a common component of toothpaste is water, which is detrimental to the probiotic stability. Further other ingredients in the toothpaste, such as foaming agents and certain flavours, result in lysis of the probiotic, rendering it both microbiologically and commercially non-viable. A recently published systematic review suggests that probiotics, in the form of lozenges taken twice daily, may offer additional clinical benefits when used as an adjunct to non-surgical periodontal therapy [39]. This study investigates the potential of toothpaste as a delivery vehicle for S. salivarius M18. This approach offers a convenient and potentially more effective means of administration, as toothpaste provides consistent and widespread contact with oral surfaces, facilitating colonisation and enhancing the probiotic’s beneficial effects.
Initial experiments tested the compatibility of S. salivarius M18 with common toothpaste ingredients. Mixing it with a commercial toothpaste resulted in a significant loss of viability (>1 log in 1 h and >4 logs in 7 days). This highlighted the need for a more in-depth compatibility assessment. Further studies using a modified minimum inhibitory concentration method revealed that even low-foaming agents (Table 1) and teeth-whitening agents like hydrogen peroxide negatively impact the viability of S. salivarius M18.
In the in vitro assessments against the reference standards, the S. salivarius M18 toothpaste stands out for its gentle yet effective cleaning. Its low abrasivity (RDA of 50 ± 5) makes it safe for long-term use, especially for sensitive teeth, and falls well within regulatory limits set by the ADA (<250) and FDA (<200) [40,41] . Unlike some harsher toothpastes (RDA value between 70–150) that require dentist supervision, S. salivarius M18 probiotic toothpaste offers a gentler approach [40] . Despite its low abrasiveness, it removes stains effectively, achieving a PCR value (66) comparable to commercial brands (ranging from 25 for silica-based pastes to 138 for silica/alumina ones). This stain removal efficacy is further confirmed by its cleaning efficiency index (CEI) of 1.32, which matches popular options like Colgate Total Whitening, Simply White, and Cavity Protection [40]. In summary, the S. salivarius M18 probiotic toothpaste offers a low-abrasive, enamel-friendly formula for safe long-term use, effectively removing surface films and stains without compromising tooth sensitivity.
Even when incorporated into the toothpaste matrix, S. salivarius M18 maintained its antimicrobial activity, comparable to the control strain. This confirms the format’s suitability for delivering the probiotic’s benefits. Additionally, the toothpaste formulation offers a two-year shelf life for the probiotic strain. In the clinical trial, we assessed the impact of S. salivarius M18 probiotic toothpaste on the oral microbiome. Traditional agar plate techniques identified S. salivarius M18 in saliva samples, confirming its presence alongside other oral bacteria. Interestingly, all dosage groups showed colonisation of S. salivarius M18, with increasing probiotic concentration observed over time compared to baseline. This colonisation mirrors the effects seen with S. salivarius M18 lozenges [6] despite the toothpaste’s limited contact with tooth surfaces. This suggests that the toothpaste’s “oily matrix” plays a role, potentially adhering to teeth and gums and slowly releasing S. salivarius M18 to aid colonisation, as previously reported in our research [42]. While the lack of a placebo control and the relatively small sample size limit definitive conclusions, these preliminary findings provide a strong foundation for future research. Larger, well-designed, placebo-controlled trials with longer follow-up periods and more diverse populations are warranted to fully explore the potential of this novel approach for improving oral health. Given the preliminary nature of this study, future studies incorporating WGS could offer valuable insights into the complex interactions within the oral microbiome and the precise impact of the probiotic toothpaste on its composition.
Despite limitations like the absence of a placebo group and reliance on conventional analysis techniques (rather than whole genome sequencing for increased specificity), the clinical trial yielded promising results. The findings suggest that this toothpaste format offers a viable method for delivering the beneficial S. salivarius M18 probiotic and potentially promoting oral and dental health.
5. Conclusions
This study developed a promising probiotic toothpaste containing S. salivarius M18. The formulation safeguards the probiotic while offering potential oral health benefits. By significantly increasing S. salivarius M18 levels in saliva, the toothpaste suggests it could promote a healthy oral microbiome and potentially reduce dental problems. While further research is needed to optimise cleaning and confirm long-term effects, this study demonstrates the potential of this probiotic toothpaste for promoting oral health.
6. Patents
Some of the work included in this paper has been part of the provisional patent [42].
Author Contributions
Conceptualisation, S.S.S., R.J. and J.D.F.H.; methodology, S.S.S., R.J. and J.D.F.H.; formal analysis, S.S.S., R.J. and J.D.F.H.; investigation, S.S.S. and R.J.; writing—original draft preparation, R.J.; writing—review and editing, S.S.S., R.J. and J.D.F.H.; project administration, R.J. and J.D.F.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Health and Disability Ethics Committee, Ministry of Health, Wellington, New Zealand (2022 EXP 13043 approved on 27 July 2022) and registered with ClinicalTrials.gov (NCT05480020 registered on 29 July 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study, and written informed consent was obtained from the participants(s) to publish this paper.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to restrictions, e.g., information that could compromise the privacy of research participants.
Conflicts of Interest
All authors are past or current employees of Blis Technologies Limited, the manufacturer of S. salivarius M18 probiotics. The authors were responsible for the design and execution of this study, but the results were analysed independently by blinded third parties.
References
- Deo, P.; Deshmukh, R. Oral microbiome: Unveiling the fundamentals. J. Oral Maxillofac. Pathol. 2019, 23, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Willis, J.R.; Gabaldón, T. The Human Oral Microbiome in Health and Disease: From Sequences to Ecosystems. Microorganisms 2020, 8, 308. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, R.; Badran, Z.; Boghossian, A.; Alharbi, A.M.; Alfahemi, H.; Khan, N.A. The increasing importance of the oral microbiome in periodontal health and disease. Future Sci. OA 2023, 9, FSO856. [Google Scholar] [CrossRef]
- Kilian, M.; Chapple, I.L.C.; Hannig, M.; Marsh, P.D.; Meuric, V.; Pedersen, A.M.L.; Tonetti, M.S.; Wade, W.G.; Zaura, E. The oral microbiome—An update for oral healthcare professionals. Br. Dent. J. 2016, 221, 657–666. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Burton, J.P.; Wescombe, P.A.; Macklaim, J.M.; Chai, M.H.C.; MacDonald, K.; Hale, J.D.F.; Tagg, J.; Reid, G.; Gloor, G.B.; Cadieux, P.A. Persistence of the Oral Probiotic Streptococcus salivarius M18 Is Dose Dependent and Megaplasmid Transfer Can Augment Their Bacteriocin Production and Adhesion Characteristics. PLoS ONE 2013, 8, e65991. [Google Scholar] [CrossRef]
- Scariya, L.; Nagarathna, D.V.; Varghese, M. Probiotics in periodontal therapy. J. Marmara Univ. Inst. Health Sci. 2015. [Google Scholar] [CrossRef]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of Action of Probiotics. Adv. Nutr. Int. Rev. J. 2019, 10, S49–S66. [Google Scholar] [CrossRef]
- Sheen, S.; Pontefract, H.; Moran, J. The benefits of toothpaste—Real or imagined? The effectiveness of toothpaste in the control of plaque, gingivitis, periodontitis, calculus and oral malodour. Dent. Updat. 2001, 28, 144–147. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, L.; Zhao, Y. Awareness of the benefits and risks related to using fluoridated toothpaste among doctors: A population-based study. Med. Sci. Monit. 2019, 25, 6397–6404. [Google Scholar] [CrossRef]
- Bolya, P.; Mutha, A.; Nagora, A.; Sharma, R.; Choudhary, S. A comparison of the efficacy of a probiotic toothpaste, a fluoridated toothpaste in management of Streptococci mutans in plaque around orthodontic brackets—An in vivo study. J. Contemp. Orthod. 2024, 8, 1–5. [Google Scholar] [CrossRef]
- Bharath, N.; Selvaraj, K.; Natarajan, R.; Dinesh, S.; Murugesan, S.; Selvaraj, S. Comparative evaluation of antimicrobial efficacy of toothpastes containing probiotic and neem as primary ingredient on salivary Streptococcus mutans in Melmaruvathur population: An in vivo study. J. Pharm. Bioallied Sci. 2020, 12, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, J.; Grahnén, H.; Jonsson, G.; Wikner, S. Establishment of Streptococcus sanguis in the mouths of infants. Arch. Oral Biol. 1970, 15, 1143–1148. [Google Scholar] [CrossRef]
- Favier, C.F.; Vaughan, E.E.; De Vos, W.M.; Akkermans, A.D.L. Molecular monitoring of succession of bacterial communities in human neonates. Appl. Environ. Microbiol. 2002, 68, 219–226. [Google Scholar] [CrossRef]
- Park, H.K.; Shim, S.S.; Kim, S.Y.; Park, J.H.; Park, S.E.; Kim, H.J.; Kang, B.C.; Kim, C.M. Molecular analysis of colonized bacteria in a human newborn infant gut. J. Microbiol. 2005, 43, 345–353. [Google Scholar]
- Heng, N.C.K.; Haji-Ishak, N.S.; Kalyan, A.; Wong, A.Y.C.; Lovrić, M.; Bridson, J.M.; Artamonova, J.; Stanton, J.-A.L.; Wescombe, P.A.; Burton, J.P.; et al. Genome sequence of the bacteriocin-producing oral probiotic Streptococcus salivarius strain M18. J. Bacteriol. 2011, 193, 6402–6403. [Google Scholar] [CrossRef]
- Hale, J.; Jain, R.; Wescombe, P.; Burton, J.; Simon, R.; Tagg, J. Safety assessment of Streptococcus salivarius M18 a probiotic for oral health. Benef. Microbes 2022, 13, 47–60. [Google Scholar] [CrossRef]
- Burton, J.P.; Drummond, B.K.; Chilcott, C.N.; Tagg, J.R.; Thomson, W.M.; Hale, J.D.F.; Wescombe, P.A. Influence of the probiotic Streptococcus salivarius strain M18 on indices of dental health in children: A randomized double-blind, placebo-controlled trial. J. Med. Microbiol. 2013, 62, 875–884. [Google Scholar] [CrossRef]
- Burton, J.; Wescombe, P.; Cadieux, P.; Tagg, J. Beneficial microbes for the oral cavity: Time to harness the oral streptococci? Benef. Microbes 2011, 2, 93–101. [Google Scholar] [CrossRef]
- Burton, J.; Chilcott, C.; Moore, C.; Speiser, G.; Tagg, J. A preliminary study of the effect of probiotic Streptococcus salivarius K12 on oral malodour parameters. J. Appl. Microbiol. 2006, 100, 754–764. [Google Scholar] [CrossRef]
- Di Pierro, F.; Zanvit, A.; Nobili, P.; Risso, P.; Fornaini, C. Cariogram outcome after 90 days of oral treatment with Streptococcus salivarius M18 in children at high risk for dental caries: Results of a randomized, controlled study. Clin. Cosmet. Investig. Dent. 2015, 7, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Poorni, S.; Nivedhitha, M.S.; Srinivasan, M.; Balasubramaniam, A. Effect of Probiotic Streptococcus salivarius K12 and M18 Lozenges on the Cariogram Parameters of Patients With High Caries Risk: A Randomised Control Trial. Cureus 2022, 14, e23282. [Google Scholar] [CrossRef]
- Srinivasan, M.; Poorni, S. Comparing the Effect of Probiotic Streptococcus salivarius K12 and M18 on the Streptococcus Mutans Count, Salivary Ph and Buffer Capacity: A Randomized Double Blinded Clinical Trial. Cumhur. Dent. J. 2022, 24, 346–354. [Google Scholar] [CrossRef]
- ISO 11609:2017; Dentistry—Dentifrices—Requirements, Test Methods and Marking. Available online: https://www.iso.org/standard/70956.html (accessed on 21 January 2025).
- Tagg, J.R.; Bannister, L.V. “Fingerprinting”-haemolytic streptococci by their production of and sensitivity to bacteriocine-like inhibitors. J. Med. Microbiol. 1979, 12, 397–411. [Google Scholar] [CrossRef]
- Hefferren, J.J. A Laboratory Method for Assessment of Dentrifrice Abrasivity. J. Dent. Res. 1976, 55, 563–573. [Google Scholar] [CrossRef]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Delgado Palacio, S.; Arboleya Montes, S.; Mancabelli, L.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, e00036-17. [Google Scholar] [CrossRef]
- Huttenhower, C.; Gevers, D.; Knight, R. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef]
- Babina, K.; Salikhova, D.; Doroshina, V.; Makeeva, I.; Zaytsev, A.; Uvarichev, M.; Polyakova, M.; Novozhilova, N. Antigingivitis and Antiplaque Effects of Oral Probiotic Containing the Streptococcus salivarius M18 Strain: A Randomized Clinical Trial. Nutrients 2023, 15, 3882. [Google Scholar] [CrossRef]
- Park, J.-A.; Lee, G.R.; Lee, J.-Y.; Jin, B.-H. Oral Probiotics, Streptococcus salivarius K12 and M18, Suppress the Release of Volatile Sulfur Compounds and a Virulent Protease from Oral Bacteria: An In-Vitro Study. Oral Health Prev. Dent. 2023, 21, 259–269. [Google Scholar] [CrossRef]
- Yoo, H.; Jwa, S.; Kim, D.; Ji, Y. Inhibitory effect of Streptococcus salivarius K12 and M18 on halitosis in vitro. Clin. Exp. Dent. Res. 2020, 6, 207–214. [Google Scholar] [CrossRef]
- Koelemaji, M.E.; Hale, J.D.F.; Jain, R. Oral Probiotics Containing Streptococcus salivarius M18 for the Prevention of Dental Plaque: A Systematic Review. Int. J. Pharma Bio Sci. 2021, 12, 43–49. [Google Scholar] [CrossRef]
- Stowik, T. Contribution of Probiotics Streptococcus salivarius Strains K12 and M18 to Oral Health in Humans: A Review. 2016. Available online: https://digitalcommons.lib.uconn.edu/srhonors_theses/488/ (accessed on 21 January 2025).
- Reichardt, E.; Shyp, V.; Alig, L.; Verna, C.; Kulik, E.M.; Bornstein, M.M. Antimicrobial effect of probiotic bacteriocins on Streptococcus mutans biofilm in a dynamic oral flow chamber model—An in vitro study. J. Oral Microbiol. 2024, 16, 2304971. [Google Scholar] [CrossRef]
- Mallikarjun, S.B.; Salim, H.P.; Raju, S.; Surendranath, A.R. Randomized Clinical Trial of Oral Probiotic Streptococcus salivarius M18 on Salivary Streptococcus mutans in Preprimary Children. Int. J. Clin. Pediatr. Dent. 2023, 16, 259–263. [Google Scholar] [CrossRef]
- Harold, L.K.; Jones, N.C.; Barber, S.L.; Voss, A.L.; Jain, R.; Tagg, J.R.; Hale, J.D.F. Specific Synbiotic Sugars Stimulate Streptococcus salivarius BLIS K12 and BLIS M18 Lantibiotic Production to Expand Bacterial Inhibition Range and Potency. Appl. Microbiol. 2024, 4, 1320–1334. [Google Scholar] [CrossRef]
- Kaci, G.; Goudercourt, D.; Dennin, V.; Pot, B.; Doré, J.; Ehrlich, S.D.; Renault, P.; Blottière, H.M.; Daniel, C.; Delorme, C. Anti-Inflammatory properties of streptococcus salivarius, a commensal bacterium of the oral cavity and digestive tract. Appl. Environ. Microbiol. 2014, 80, 928–934. [Google Scholar] [CrossRef]
- Bardellini, E.; Amadori, F.; Gobbi, E.; Ferri, A.; Conti, G.; Majorana, A. Does Streptococcus salivarius Strain M18 Assumption Make Black Stains Disappear in Children? Oral Health Prev. Dent. 2020, 18, 161–164. [Google Scholar] [CrossRef]
- Ausenda, F.; Barbera, E.; Cotti, E.; Romeo, E.; Natto, Z.S.; Valente, N.A. Clinical, microbiological and immunological short, medium and long-term effects of different strains of probiotics as an adjunct to non-surgical periodontal therapy in patients with periodontitis. Systematic review with meta-analysis. Jpn. Dent. Sci. Rev. 2023, 59, 62–103. [Google Scholar] [CrossRef]
- Schemehorn, B.R.; Moore, M.H.; Putt, M.S. Abrasion, polishing, and stain removal characteristics of various commercial dentifrices in vitro. J. Clin. Dent. 2011, 22, 11–18. [Google Scholar]
- Sharma, V.; Rath, S.K.; Pratap, C.; Chaturvedi, T. Abrasivity of dentrifices: An update. SRM J. Res. Dent. Sci. 2016, 7, 96–100. [Google Scholar] [CrossRef]
- Hale, J.D.F.; Jain, R. Oral Composition. Patent WO2022172230, 2022. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2022172230&_cid=P12-M4J5QQ-89193-1 (accessed on 21 January 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).