Abstract
Improving the quality of oil-contaminated soils remains a critical challenge, and bioaugmentation using allochthonous bacteria offers promising perspectives. This study proposes a framework for exogenous bioaugmentation using a bacterial consortium, composed of strains from diverse climates, immobilized in alginate beads and combined with calcium peroxide as an oxygen-releasing compound. Two conditions were tested: freshly prepared beads (BA) and lyophilized beads (LA). Their performance was compared to natural attenuation (NA) and to landfarming coupled with bioaugmentation using a free autochthonous consortium. Hydrocarbon degradation was assessed through total petroleum hydrocarbon (TPH) and alkane depletion (GC-MS), microbial community dynamics (amplicon sequencing), and abundance of the alkB gene (qPCR). In three months, the BA treatment achieved a 44% TPH reduction, outperforming LA (34%) and NA (10% less than BA). However, LA induced a marked increase in alkB gene copies and microbial biomass at the end of the experiment, suggesting greater long-term potential. Dominant genera varied across treatments: Rhodococcus in NA, Gordonia in BA, and Pseudomonas in LA. In parallel, the autochthonous consortium achieved up to 80% oil degradation. This study demonstrates the viability of lyophilized microbial consortia in scalable, ready-to-use formulations and provides an operational methodology for exogenous bioaugmentation as a tool for the remediation of hydrocarbon-contaminated soils.
1. Introduction
Soil pollution is a critical environmental issue driven by both natural processes and human activities [1,2]. Among the most widespread environmental contaminants, petroleum hydrocarbons pose a serious threat to soil ecosystems globally. Weathering processes modulate their distribution over time, allowing bioavailable fractions, particularly saturated and low-molecular-weight hydrocarbons, to persist and exert toxic effects on living organisms. In contrast, high-molecular-weight hydrocarbons progressively bind to soil organic matter and mineral components, reducing their mobility and long-term bioavailability [1,3,4,5].
In this regard, given the recurrence of oil spills, rapid and effective response strategies are essential to mitigate environmental damage, including ecosystem disruption, groundwater contamination, and bioaccumulation within the food web [6,7,8,9]. In recent years, increasing awareness of this issue has led to extensive research on natural and sustainable methods to restore soils contaminated with petroleum hydrocarbons. A consensus has emerged on utilizing the inherent purifying abilities of microorganisms, particularly bacteria, for efficient and eco-friendly oil recovery [6,9,10,11,12]. Over 200 microbial species capable of degrading hydrocarbons have been identified. Among them, various bacterial species adapted to contaminated environments produce catabolic enzymes that facilitate the breakdown and mineralization of petroleum hydrocarbons [6,9]. A notable example is the transmembrane non-heme iron alkane monooxygenase AlkB, encoded by the alkB gene (E.C. 1.14.15.3; KEGG Orthology K00496), which is widely distributed among soil bacteria and oxidizes compounds with five or more carbon atoms [13].
Bioaugmentation has emerged in recent years as one of the most promising bioremediation techniques, drawing growing interest within the scientific community due to its encouraging results [14,15,16,17]. This approach involves the inoculation of indigenous (native or autochthonous) or exogenous (foreign to the environment or allochthonous) microbial strains in the contaminated environment. Microbial consortia generally outperform individual strains owing to the synergistic and complementary action of their catabolic enzymes [2,9,18,19,20]. However, the use of exogenous species in bioaugmentation remains contentious. Some authors claim that this approach is less effective compared to autochthonous consortia [21,22,23], which tend to be better adapted to site-specific conditions and more resilient to environmental stress [22,24]. Moreover, competition between indigenous and introduced microorganisms may hinder the latter’s survival, activity, and reproduction [11,25].
This assertion has been tested in several studies. For example, [23] observed a decrease in bioremediation efficiency when a loamy German sunflower field soil characterized by a moderate temperature (21 °C), a slightly alkaline pH (7.5), high moisture content (41.7%), high total organic carbon (13.1%) and total nitrogen (1.5%) levels, was inoculated with strains from an Egyptian garden soil. The latter exhibited substantially different physicochemical properties, including a higher temperature (33 °C), an alkaline pH (8.2), a low moisture content (13.3%), as well as reduced total organic carbon (1.3%) and nitrogen (0.3%) contents. Similarly, an attempt by [24] to enhance remediation using consortia derived from soils across three continents failed. Nonetheless, successful applications of exogenous bioaugmentation have also been documented [25]. Despite this, large-scale implementation of exogenous consortia for oil recovery remains uncommon. There is, therefore, a significant gap in advancing robust, adaptable formulations that can perform across diverse environmental conditions [23]. Such a solution would facilitate timely and effective responses to recurring hydrocarbon pollution incidents across various regions.
To overcome the aforementioned limitations, researchers have explored complementary strategies to improve microbial survival and functionality post-inoculation. One of the most effective involves immobilizing microbial strains on biodegradable carriers that function as physical shields against environmental stress. Indeed, as seen in the study by [23], contrasting physicochemical parameters considerably contribute to functional mismatch between the host environment and the introduced microbial consortia, ultimately impairing the bioaugmentation outcome. The support for immobilization not only protect the strains but also allow nutrient exchange with the surrounding environment, fostering optimal conditions for microbial activity. Within this confined microenvironment, dense biomass formation enhances metabolic activity and minimizes cell loss, ultimately improving degradation efficiency [10,26,27]. A commonly used immobilization technique is the entrapment of bacterial strains in alginate beads, which has yielded promising results in various studies [11,26,27,28,29,30]. In addition, several authors have engaged in lyophilization or freeze-drying to extend the shelf life of microbial consortia, up to 50 years in some cases, while preserving viability and functionality. Freeze-drying offers significant operational advantages, including volume reduction, ease of storage, and simplified transport and field application [25,31]. Furthermore, lyophilized products can be rehydrated and reused without restarting the formulation process, offering enhanced operational flexibility and cost-efficiency [25].
In parallel, oxygen availability often represents a major limiting factor in hydrocarbon degradation, especially for in situ bioremediation strategies [32,33]. To address this challenge, the use of oxygen-releasing compounds (ORCs) has been increasingly integrated into bioremediation frameworks. Among available ORCs, calcium peroxide (CaO2) is one of the most studied, due to its low cost, chemical stability, ease of application, and sustained oxygen-release profile, which aligns well with microbial oxygen demand. Its effectiveness has been demonstrated in various contexts, including the complete removal of benzene from contaminated groundwater using encapsulated CaO2 formulations [32,33,34].
In this context, the present study aims to address the gap in developing a bioremediation model employing a microbial culture cocktail suitable for diverse environmental settings. Indeed, while some commercial formulations have been proposed [24,35], no documented study has demonstrated their successful simultaneous application across different foreign environments. Hence, the goal is to establish a reproducible methodology for producing a ready-to-use microbial mixture, which can subsequently be improved and adapted to diverse climatic conditions. The research does not claim to provide a definitive solution to this complex issue. Rather, it aims to contribute relevant proposals to support the development of more reliable and effective exogenous bioaugmentation strategies for the remediation of oil-contaminated soils.
A methodological framework is therefore proposed and tested on a soil sample that has been contaminated for three months. A bacterial consortium composed of strains isolated from oil-contaminated soils located in both Equatorial and Mediterranean regions was built and entrapped in alginate beads. The resulting entrapped microbial blend, named COBIONA, was immobilized in alginate beads and subsequently lyophilized.
To the best of our knowledge, this is the first study reporting the bioaugmentation application of an entrapped microbial consortium comprising strains from diverse climates and lyophilized for oil recovery purposes.
2. Experimental Design
The methodological framework developed in this study included a tailored toolbox to address the limitations associated with site-specific conditions and microbial competition in exogenous bioaugmentation. Key components of the strategy included:
- The integration of indigenous and exogenous bacterial strains selected for relevant phenotypic and genotypic traits, particularly biosurfactant production, which enhances the solubilization and bioavailability of hydrophobic pollutants [12,18,19,36,37].
- The choice of a molecular marker linked to the type of contamination to be tackled.
- The application of a biodegradable carrier to immobilize the bacterial consortium and ensure sustained activity.
- The addition of a solid oxygen-releasing compound (ORC) to address oxygen limitations in the soil. The ORC offers a practical alternative to mechanical soil aeration, which is typically labor-intensive and disruptive, especially in large-scale applications [38].
- Here, the bacterial strains were isolated from oil-contaminated soils in Equatorial and Mediterranean climates. Their selection was guided by criteria focused on the degradation of aliphatic hydrocarbons typical of recent contamination events (3 months in this study). To this end, candidate strains were chosen based on the presence of the alkB gene, the chosen molecular marker, and the ability to produce biosurfactants. The strains were subsequently entrapped in alginate beads, and calcium peroxide, the chosen ORC, was added to ensure sustained aerobic conditions. To assess field-readiness, the entrapped bacterial consortium was also tested as a lyophilized formulation.
For comparative evaluation, this exogenous bioaugmentation strategy was tested against indigenous approaches. The latter involved landfarming on one hand, and landfarming combined with bioaugmentation employing a native bacterial consortium, on the other hand.
The three approaches were monitored using amplicon sequencing and real-time polymerase chain reaction (qPCR). These molecular tools allow the characterization of microbial community dynamics and the quantification of functional gene expression throughout the remediation process [17,39]. The data obtained are critical to establishing links between microbial dynamics and contaminant degradation, and identifying the main microbial players involved in the process [11].
3. Materials and Methods
3.1. Entrapment and Lyophilization
In this study, the consortium COBIONA was used for the bioaugmentation experiments. The consortium was composed of the Mediterranean bacterial strains Achromobacter pestifer S-BM4 (Genbank accession number OM568680.1), Pseudomonas benzenivorans S-AR5 (Genbank accession number OM568662.1), Rhodococcus qingshengii C-M4 (Genbank accession number OM850318.1), and the Equatorial strain Shinella zoogloeoides LFG9 (Genbank accession number MT672424.1), previously isolated by an enrichment technique [30], a major step in biodegradation studies [40,41]. These strains exhibited the presence of the alkB gene and/or the synthesis of biosurfactant-like molecules with an emulsification index against crude oil ranging between 34.9% and 68.1%. Bacterial strains were grown individually in LB broth for 48–72 h at 30 °C or 37 °C, respectively, for Mediterranean and Equatorial strains. The cultures were standardized to an optical density of 1.6 < OD > 1.7 at 600 nm before centrifugation (Eppendorf® Centrifuge 5427 R, Framingham, MA, USA) at 12,327× g for 10 min and 4 °C. Afterward, the pellets were washed (centrifugation at 5547× g for 15 min) twice with the mineral salt medium (MSM: KH2PO4 15 g.L−1; Na2HPO4 5 g.L−1; NH4Cl 10 g.L−1; NaCl 1 g.L−1, Sigma-Aldrich, Burlington, MA, USA).
The alginate beads were prepared according to a previous methodology with some modifications [30]. Equal volumes of the bacterial suspensions were mixed with a 2% sodium alginate solution in a ratio of 1:1. The mixture was stirred and extruded into a calcium chloride solution. The obtained alginate beads were washed thrice with sterile MSM, left to strengthen at room temperature, and stored at 4 °C when not used.
A lyophilization process was also performed. The alginate beads were suspended in a sterile cryopreservation medium (1% sodium glutamate and 7% sucrose) in microcentrifuge tubes before freeze-drying. The tubes were agitated by mild inversion and left to settle for 10 min to impregnate the beads with the solution. The mixture was then centrifuged at 17,258× g for 10 min, and the supernatant was discarded. The alginate beads were subsequently stored at −80 °C overnight. Finally, the frozen beads contained in the Eppendorf tubes were transferred to a freeze-dryer. Drying was performed for 10 h with a final temperature of 45 °C and a pressure of 0.51 bars. The lyophilized beads containing the bacterial consortium were stored at 4 °C when not used.
3.2. Soil Treatment: Mesocosm Study of Natural Attenuation, Bioaugmentation, and Landfarming
Plot Experiment
Bioaugmentation has been set up to understand the biodegradation efficiency of the COBIONA consortium at the mesocosm scale.
For each remediation strategy, the experiments were conducted in duplicate on short-term contaminated soil C. The soil was collected from a previously surveyed contaminated site using a mini-excavator, with nine samples taken from both superficial and deep layers in correspondence with historical sampling points. To ensure representativeness, the collected samples were homogenized and quartered in the field. At the laboratory, stones and debris were removed by sieving through a 2 mm mesh. The soil showed a slightly alkaline pH of 8 and was characterized by a sandy texture, with 88% sand, 4.6% clay, and 7.4% silt at 0–1 m depth, and 90.4% sand, 2.9% clay, and 6.7% silt at 1–2 m depth. Based on these properties and following the World Reference Base for Soil Resources (WRB), the soil can be classified as an arenosol. Hydrocarbon analysis indicated the presence of all the typical aliphatic hydrocarbons belonging to diesel (n-C16-n-C25). The total organic carbon content was evaluated at 5513.25 mg.kg−1. When not used, soil samples were kept at 4 °C for further analysis and at −20 °C before molecular analysis.
Six tanks (38 × 28 × 20 cm) were filled each with 1 kg of contaminated soil. Bioaugmentation was carried out with fresh (BA treatment) and lyophilized beads (LA treatment).
The BA treatment was performed using 50 g of fresh beads. Quantification of colony-forming units (CFU) on agar plates 24 h after entrapment confirmed the viability of the bacterial consortium within the beads, with a mean concentration of 3.29 × 105 CFU.mL−1. In each tank, the beads were disposed in a layer on top of 500 g of soil and covered by the remaining 500 g of soil. The beads were distributed into 5 points of 10 g of beads each.
In the LA treatment, 3.8 g of lyophilized beads were used. The 3.8 g of lyophilized beads were obtained by freeze-drying 50 g of freshly prepared beads. Both formulations originated from the same batch, using bacterial suspensions standardized to similar optical densities for each strain. This approach ensured a uniform initial inoculum composition across both formulations. The lyophilized beads were evenly spread over 500 g of soil, and the remaining 500 g of soil was used to cover them.
To maintain oxygen supply without the need for soil mixing, 5 g of calcium peroxide (Sigma-Aldrich, Burlington, MA, USA) was spread on the soil top layer in each tank, for BA and LA treatments.
For comparison, two tanks with identical soil quantities (1 kg) but without any amendments were prepared to create the Natural Attenuation (NA) process. It is important to note that in this context, natural attenuation has been carried out without the addition of bacteria.
To evaluate the efficiency of the developed methodology, a comparison was drawn between the outcomes of the exogenous bioaugmentation within the current experimental configuration and the results previously attained using the same soil subjected to landfarming combined with indigenous bioaugmentation. In the latter approach, landfarming facilitates oxygen supply through soil mixing, while bioaugmentation involves introducing autochthonous strains into the soil.
Within this experimental setup, two scenarios were explored: landfarming alone without bacterial inoculation (L treatment) and landfarming combined with bioaugmentation, wherein an autochthonous bacterial consortium was introduced (LB treatment). These specific conditions, along with Natural Attenuation (NA), were examined in duplicate, involving weekly moistening of the soil and periodic soil mixing to enhance oxygenation. The inoculum used in LB conditions was composed of strains Kocuria arsenatis and Bacillus wiedmannii. The consortium was added once at a concentration of about 1011 colony-forming units (CFU) in 1 kg of soil.
All the experiments, including landfarming and exogenous bioaugmentation, were monitored for 90 days. In all tanks, the soil was periodically moistened to field capacity every five days on average by adding 50 mL of sterile distilled water. This procedure aimed to maintain adequate moisture conditions (neither dry nor saturated), thereby preventing dryness while avoiding the introduction of exogenous microorganisms, particularly in the bioaugmented treatments. Humidity was determined periodically by determining fluctuations in the soil weight before and after watering. Soil samples were also collected at different time intervals, i.e., 30 (T1), 60 (T2), and 90 (T3) days, for chemical and molecular analysis.
The different treatments are described in Table 1.

Table 1.
Description of the different treatments.
3.3. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
Ten grams of soil samples were dried and used to extract total petroleum hydrocarbons using dichloromethane as solvent. For each sample, the soil mixed with an equal weight of inert quartz, was inserted in extraction cells of a pressurized liquid extractor (Buchi Speed Extractor E916, BÜCHI Labortechnik AG, Flawil, Switzerland) set at 100 °C and 100 bar. The extracting cells contained about 10 g of inert material in order to avoid contact with the filters and tube clogging. In addition, the transportation of aqueous residues into the extracts was prevented by introducing 2 g of hygroscopic diatomaceous earth between the inert material and soil sample. The extracts were then up to 10 mL via a concentrator turbovap at 37 °C in a nitrogen stream and filtered by standard filter vials 0.45 µm PTFE.
Subsequently, each extract was diluted to a final concentration of 5 mg.mL−1 in dichloromethane supplemented with a known amount of n-hexadecane as an internal standard. Up to 2 µL of each sample was injected on a single quadrupole GC-MS (Trace-DSQ, Thermo Scientific, Ann Arbor, MI, USA) Finnigan Trace DSQ (Thermo Scientific, Ann Arbor, MI, USA) interfaced to a Finnigan TraceGC Ultra (Thermo Scientific, Milan, Italy) equipped with a capillary column coated with MDN-5S (30 m × 0.25 mm i.d., 0.25 µm film thickness) using helium as the carrier gas (1 mL.min−1). The elution protocol was as following: initial temperature of 40 °C for 4 min and a temperature ramp of 40° to 320 °C at a rate of 10 °C min−1. The mass spectra were recorded in electron impact mode at 70 eV and processed using XcaliburTM version 4.2. The chromatogram peaks were identified by comparison with the NIST libraries.
3.4. Next-Generation Sequencing (NGS) to Determine Soil Community Structures
The dynamics of the bacterial community following each treatment were evaluated by Ion torrent sequencing of the 16S rRNA gene. Briefly, for each aliquoted sample, the genomic DNA was extracted from 500 mg of soil using the Fast DNATM Spin Kit (MP Biomedicals, CA, USA) according to the manufacturer’s instructions and then quantified with Qubit® 2.0 fluorometer (Invitrogen, Waltham, MA, USA). Three ng DNA were used to amplify the 16S rRNA gene using the primers 331F (5′-TCCTACGGGAGGCAGCAGT-3′) and 797R (5′--GGACTACCAGGGTATCTAATCCTGTT-3′) [42]. The amplicons were then purified and linked to specific barcodes. The libraries were diluted to 33 pM, pooled to a final volume of 25 µL and then the preparation of the sequencing of the libraries followed the standard protocols for the Ion GeneStudio S5 Systems (i.e., Ion Chef ™ System and Ion GeneStudio S5 Sequencer) provided by the manufacturer (Thermo Fisher Scientific, Ann Arbor, MI, USA). The run is based on the workflow Metagenomics 16S w1.1 handling the Database Curated microSEQ® 16 S and the reference Library 2013.1. The 250 bp sequences were aligned with the Torrent Suite™ Software (version 5.8) using the Torrent Mapping Alignment Program (TMAP). The resulting BAM file gives the percentage of reads which pass all filters (i.e., enrichment, no template, clonal and polyclonal discrimination, % of test fragments, % of adapter dimer, and % of low quality). The obtained Operational Taxonomic Units (OTU) are then processed in order to determine their relative abundances (%) in the entire sample.
The data have been deposited in Genbank under the accession numbers PRJNA1000427 (exogenous bioaugmentation) and PRJNA1000434 (landfarming).
3.5. Diversity Assessment
Alpha diversity analyses were conducted using Past 4.03 software. Richness and diversity were estimated by calculating Chao1, Abundance-based, Shannon’s Index, Simpsons’ Diversity Index, and Pielou’s Evenness.
3.6. Quantification of Microbial Community and Alkanes Degradation Gene alkB by qPCR
Quantitative PCR (qPCR) using CFX96TM Real-Time System (Bio-Rad, Hercules, CA, USA) was applied to estimate the bacterial community size in soil during the different treatments. The 16S rRNA gene was amplified with primers 331F (5′-TCCTACGGGAGGCAGCAGT-3′) and 797R (5′-GGACTACCAGGGTATCTAATCCTGTT-3′) [42] in 20-μL reaction volume containing 0.2 µL of each primer (100 µM), 1 µL of DNA, 10 µL of FluoCycle II SYBR 2X (Euroclone, Pero, Italy) and 8.6 µL of nuclease-free water. The amplification reaction consisted of polymerase activation at 95 °C for 4 min followed by 40 cycles of denaturation at 95 °C for 15 s, annealing at 60 °C for 30 s and final extension at 72 °C for 30 s. The quantification of the 16S rRNA gene copies was performed in comparison to the amplification curve obtained from serial dilutions of a standard DNA. The standard DNA used was the 466 bp fragment of E. coli strain DH10B amplified with the same primers. The abundance of alkB copies was also determined by qPCR amplification, using the primers alkH-1F (5′ -CIGIICACGAIITIGGICACAAGAAGG-3′) and alkH-3R (5′- IGCITGITGATCIIIGTGICGCTGIAG-3′) amplifying a fragment of approximately 544 bp [13]. In this case, the standard DNA was extracted from Rhodococcus sp.
3.7. Statistical Analysis
The experiments were conducted in duplicate, and the data are expressed as mean values. Beta diversity of the bacterial community and the Principal Coordinates Analysis (PCoA) were performed by the EMPeror v0.9.60 software, integrated into QIIME 2. This software is a useful tool for the visualization of high-throughput microbial community data [43].
4. Results
4.1. NGS Results
4.1.1. Microbial Community Changes
To gain a better understanding of the efficiency and effects of each bioremediation approach, changes in the bacterial community were closely monitored through 16S rRNA amplicon sequencing. A total of 1,774,300 reads were obtained from the run, with more than 140,000 reads per sample and a mean length of 233 bp. The obtention of rarefaction curves validated the community assessment (Supplementary Figure S1).
A total of 13 phyla were detected in the untreated soil. Bacteria were also grouped into 18 classes, 30 orders, 63 families and 74 classified genera. The presence of hydrocarbon-degrading orders and genera was used to characterize the bacterial community in both treated and untreated soils. These ranks presented less unclassified taxa and with relative abundances lower than 1%. It should be noted that the untreated soil refers to the soil at the initial sampling time T0.
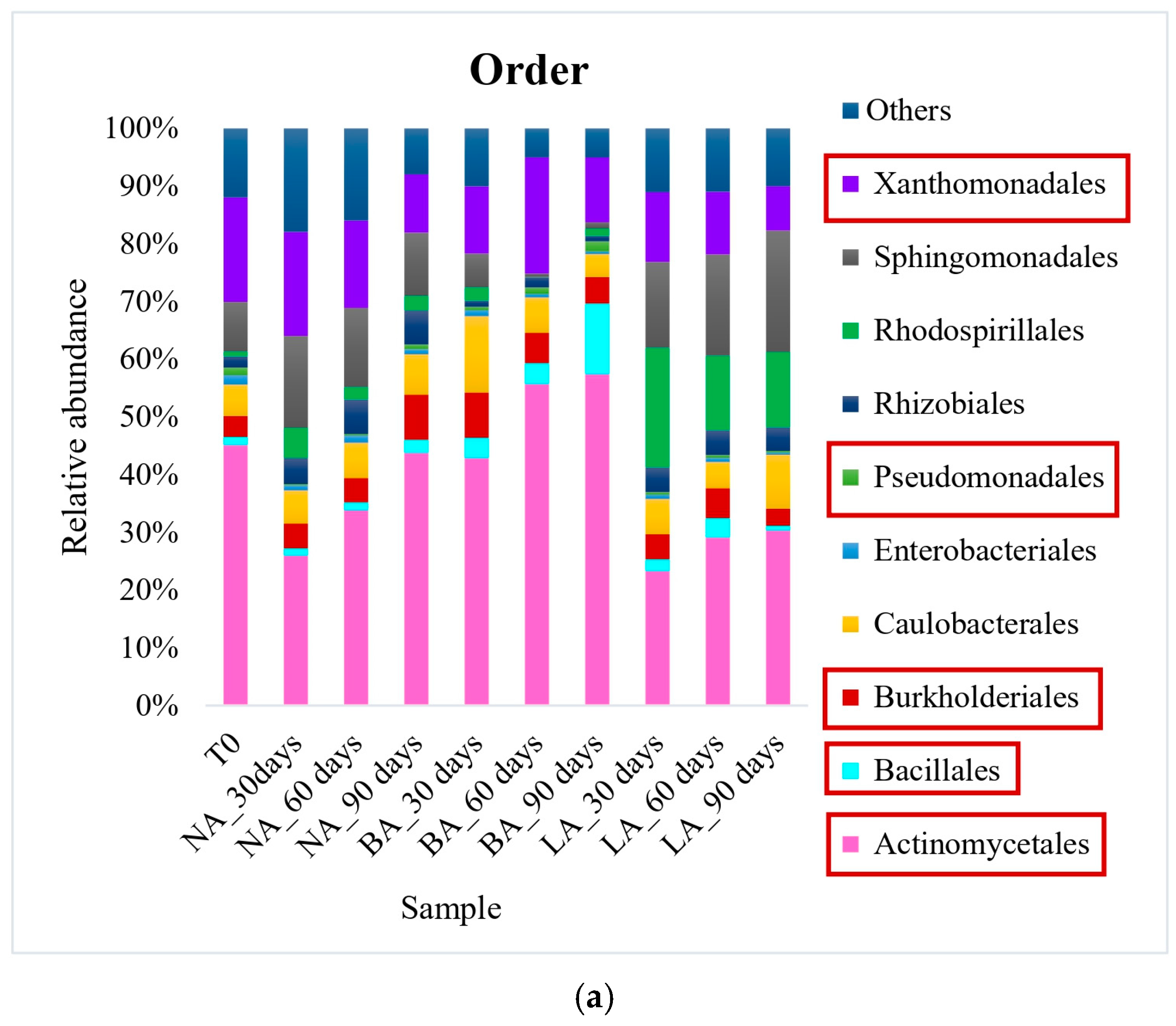
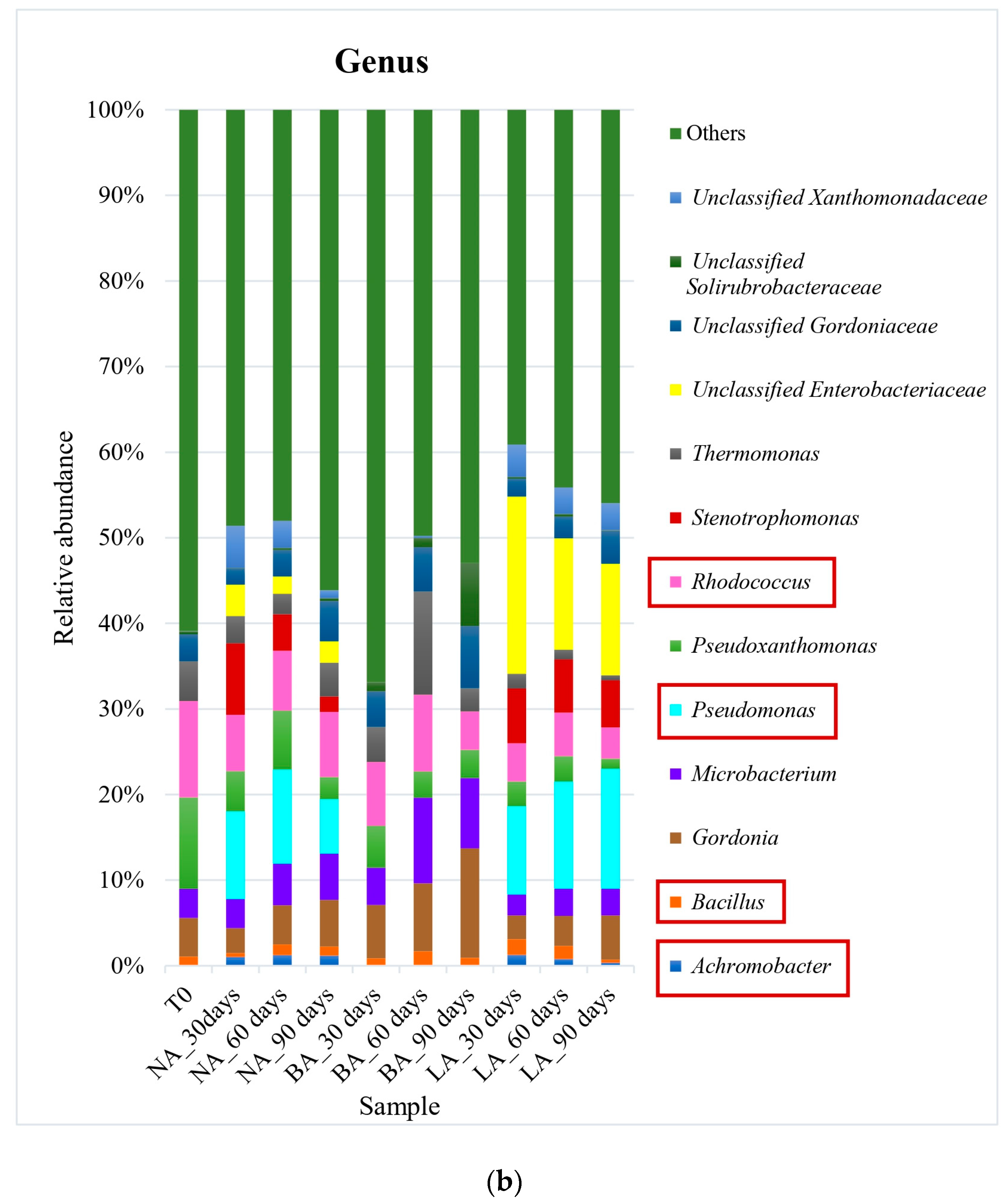
Actinomycetales was the most prevalent order in all treatments at the end of the experiment when using exogenous bioaugmentation. The representative genera included Rhodococcus (8% relative abundance) in NA, as well as Gordonia and Microbacterium in BA (13% and 8% of relative abundance, respectively) (Figure 1). In the LA treatment on the other hand, Pseudomonas (14% relative abundance) from the Pseudomonadales order was the most abundant at the genus level. Furthermore, while the relative abundance of Actinomycetales after 90 days (44% of relative abundance) was similar to the untreated soil T0 (45% of relative abundance) in the natural attenuation experiment, a decrease was observed in the LA treatment (30% of relative abundance) after the same period (Figure 1). On the other hand, a small rise in this pattern was noticed during BA treatment, with the Actinomycetales order detected at 57% of relative abundance after 90 days. This was caused by an increase in the Gordoniaceae family, which saw a relative abundance increase, from 8% to 20% in BA, in contrast to T0 (the initial untreated soil). Xanthomonadales was the second most prevalent order in T0, with a relative abundance of 18%, which experienced a decline across all treatments, ranging from 8% to 11%. This correlated with the Xanthomonadaceae family’s relative abundance, also from this order, which fell from 17% in T0 (untreated soil) to an average of 10% of relative abundance in the treated soils. Other main hydrocarbon-degrading orders were detected at lower levels. Sphingomonadales increased in NA and LA, with a relative abundance of 11% and 21% compared to 9% in T0. However, the order was significantly reduced to a relative abundance of 1% in BA.
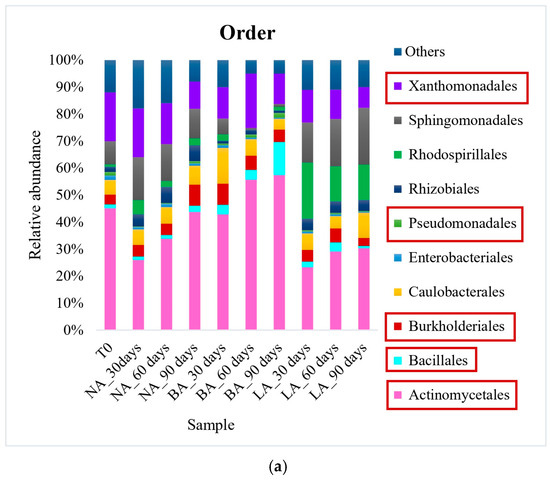
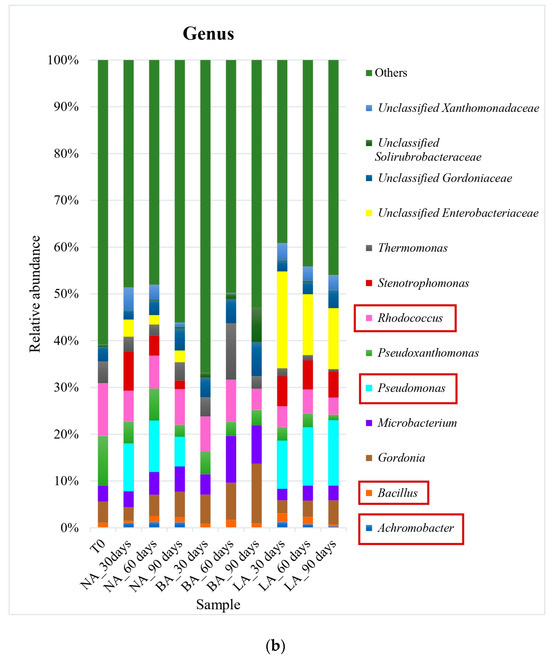
Figure 1.
Bacterial community at order (a) and genus (b) levels during exogenous bioaugmentation. The most representative taxa as well as those included in the exogenous consortium used are represented while the rest are grouped in the category Others. The consortium used comprised Achromobacter, Rhodococcus, and Pseudomonas genera. The most interesting orders and genera in this study have been marked in square to highlight their representation within the entire microbial community.
During the experiment, a noticeable reverse trend was observed in the Bacillales order. While its relative abundance increased from 1% in T0 to 12% in the BA treatment at the end of the experiment (90 days), the NA and LA treatments remained consistent with similar abundances as T0 (untreated soil). Similarly, the Burkholderiales order doubled in relative abundance in NA compared to T0, reaching 8%, whereas BA and LA had only 5% and 3% relative abundance, which was comparable to T0 (4%). The increase in the Burkholderiales order in NA was mainly attributed to the augmented Alcaligenaceae family, which was not detectable in T0 but represented 3% of relative abundance in NA. It is also worth mentioning that the Enterobacteriales order was found in very low abundances, with only 2% relative abundance in T0, and 1%, 0.5%, 0.4% in NA, BA, and LA, respectively. Nonetheless, the only family belonging to this order in this study, i.e., Enterobacteriaceae, was undetectable in T0 and the BA treatment but represented 3% and 14% of relative abundance in NA and LA, correspondingly. Similarly, the Pseudomonadales order was detected in both the untreated (initial sampling time T0) and the treated soils at a similar average relative abundance of 1%. However, the Pseudomonadaceae family belonging to this order, showed a significant increase in relative abundance from 1% in T0 to 8% and 21% in NA and LA, respectively, after 90 days. This increase was not observed in BA, where the relative abundance remained at 1% in the course of the experiment.
Regarding the landfarming approach, the hydrocarbon-degrading bacterial community included the orders Acidimicrobiales, Actinomycetales, Bacillales, Pseudomonadales, Rhizobiales, Sphingobacteriales, Sphingomonadales, and Xanthomonadales. Actinomycetales was the most abundant order with relative abundances of 32%, 23%, and 28% recorded in the NA, L, and LB treatments, respectively, after 90 days. Initially, the Acidimicrobiales order had a low average relative abundance of 0.3% across all treatments. However, it increased significantly, reaching 8%, 12%, and 13% in NA, L, and LB treatments, respectively, by the end of the experiment. In addition, there was a notable increase in the order Sphingobacteriales in L (17% relative abundance) and LB (14% relative abundance) treatments, unlike in NA (1% relative abundance). This increase was primarily due to a rise in the Chitinophagaceae family, which had relative abundance values of 2.4% in L and 7% in LB. Inversely, the Xanthomonadales order decreased in all soil samples, with final abundances of 13%, 5% and 7%, respectively, in NA, L and LB treatments at 90 days.
4.1.2. Alpha Diversity
The diversity index and richness calculated at the phylum level indicated an uneven distribution of taxa in the exogenous bioaugmentation treatment. The Chao-1 index revealed that taxonomic composition varied according to the treatments applied and the duration of exposure. Both the Simpson and Shannon indices demonstrated low microbial diversity across all treatments (Table 2). A similar trend was observed in the landfarming experiment (Table 3).

Table 2.
Alpha diversity in exogenous bioaugmentation soil samples.

Table 3.
Alpha diversity in landfarming soil samples.
4.1.3. Ordination and Clustering Analysis
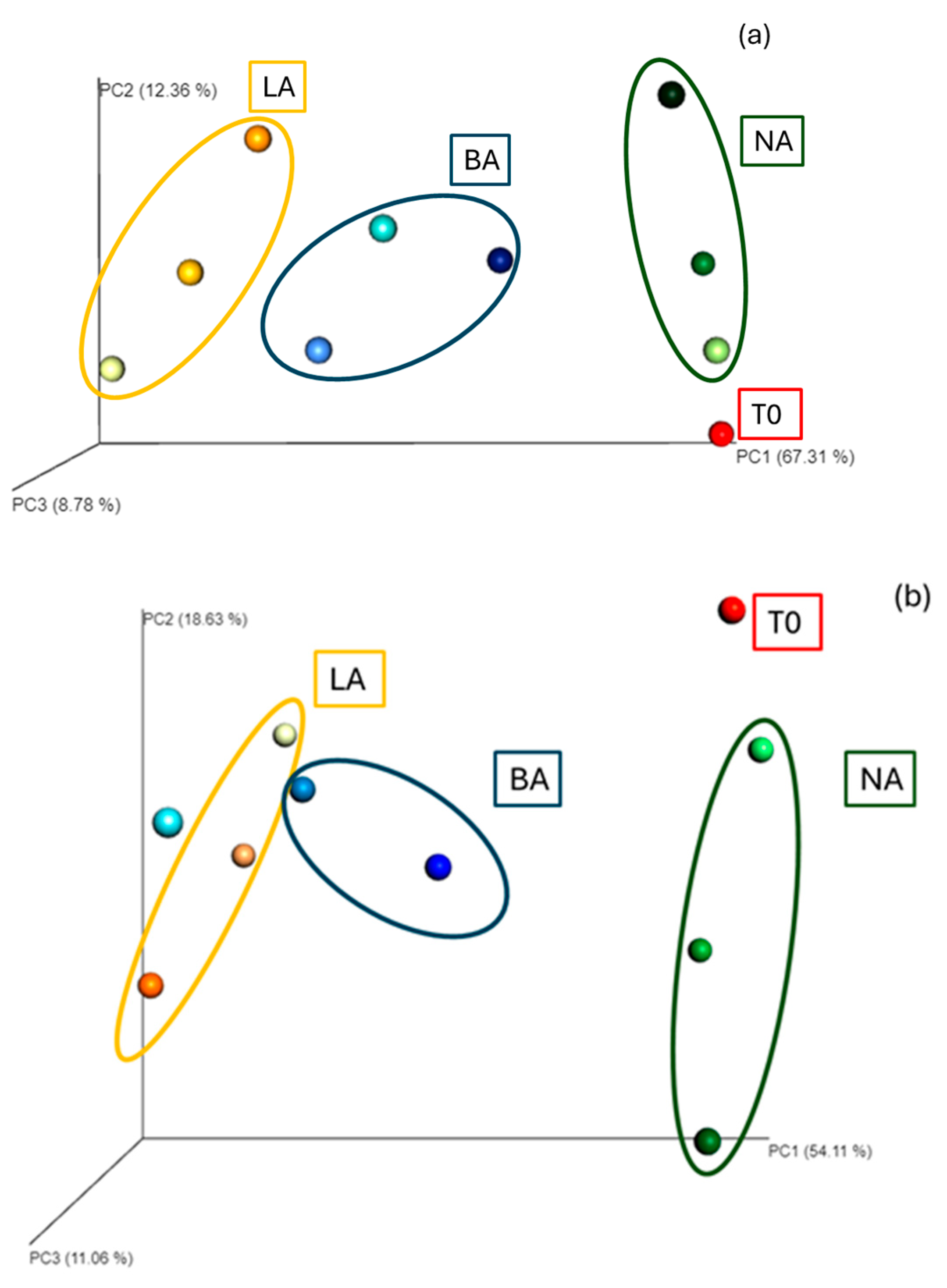
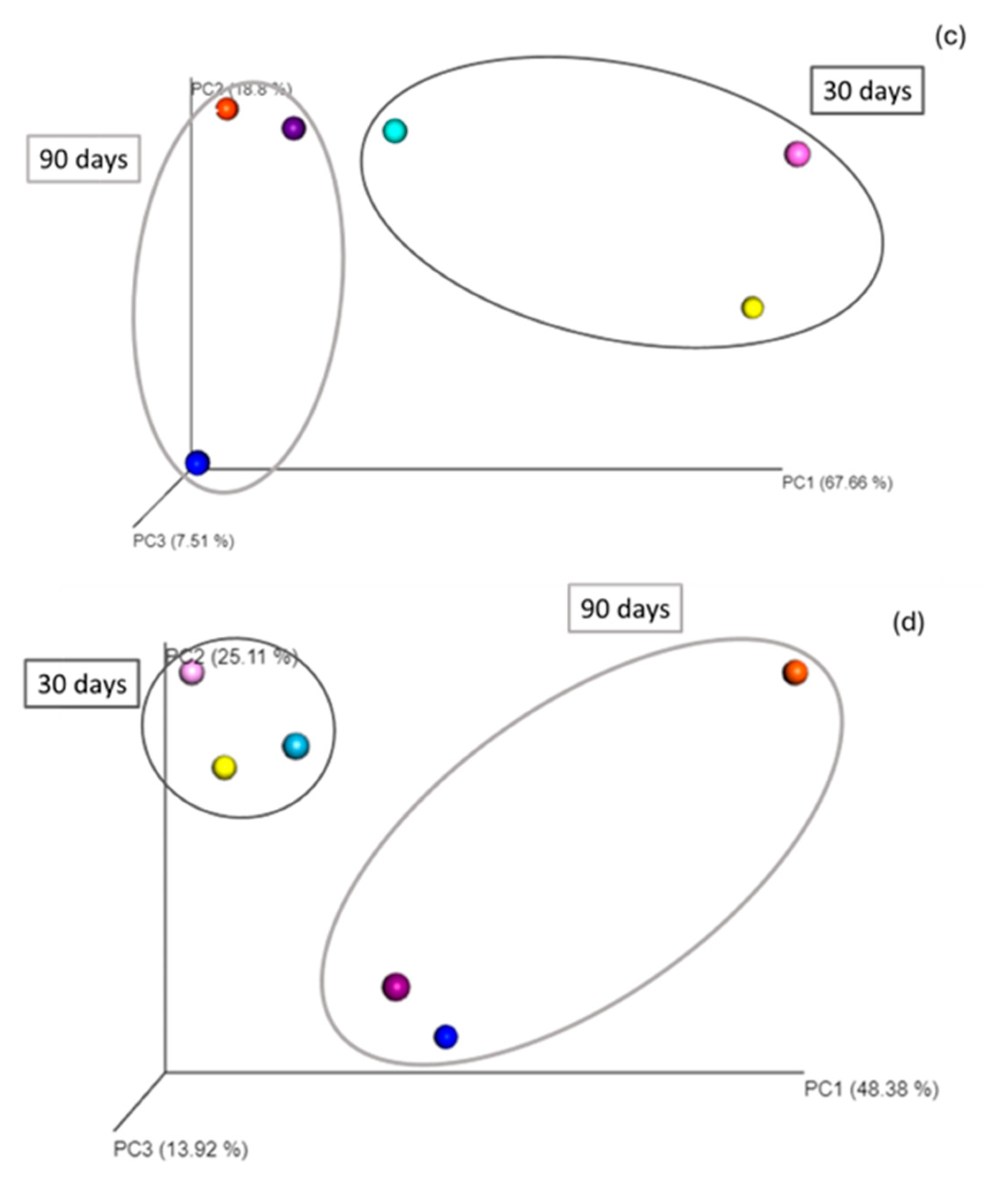
The Euclidean distance analysis showed that the communities in the three treatments (NA, BA, LA) clustered, respectively, at 30 and 90 days, indicating alterations of the bacterial community composition at the genus level between the end of the beginning of the experiment. In addition, clusters were formed, grouping the community diversity at the different sampling points, i.e., 30, 60, and 90 days for each treatment and indicating strong differences with the initial community (T0) at the genus level and throughout the remediation process (Figure 2).
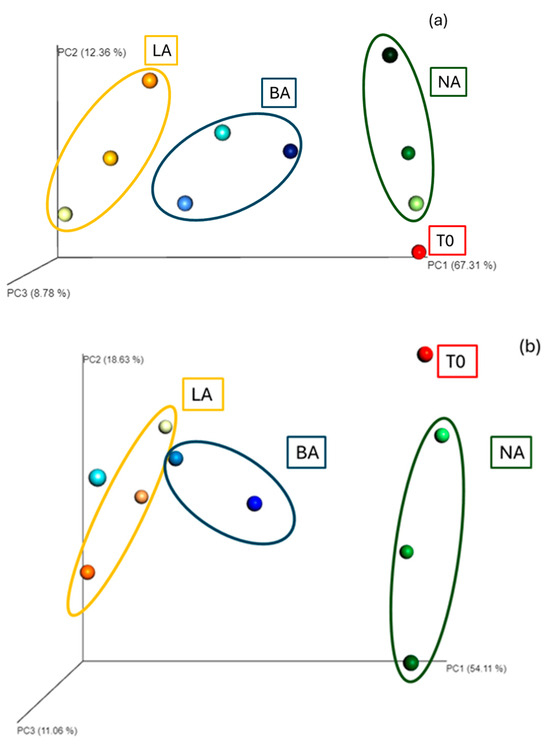
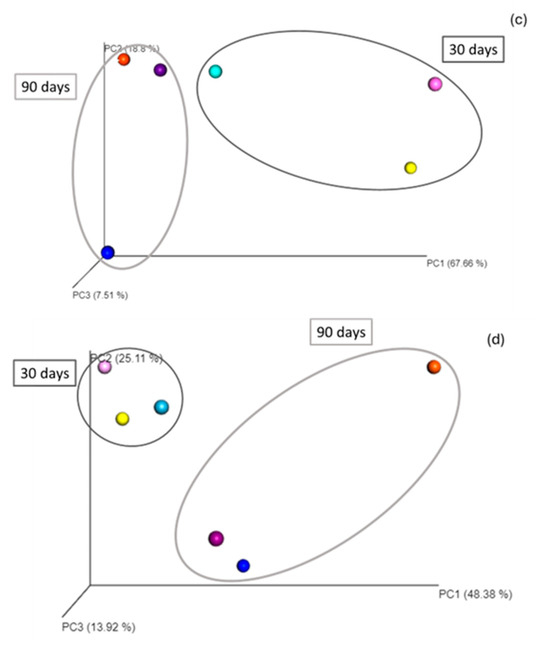
Figure 2.
T0: initial time; T1: 30 days; T2: 60 days; T3: 90 days; NA: natural attenuation; BA: bioaugmentation with fresh beads; LA: bioaugmentation with lyophilized beads; (a) Euclidean distance representation of beta diversity at the genus level of the NA, BA and LA treatments; (b) Bray–Curtis distance representation of beta diversity at the genus level of the NA, BA and LA treatments; (c) Euclidean distance representation of beta diversity at the genus level of the NA, L and LB treatments; (d) Bray–Curtis distance representation of beta diversity at the genus level of the NA, L and LB treatments. Light, middle and darker intensity colors represent the diversity at 30 days, 60 days and 90 days, respectively. In this case the Principal Coordinate Analysis shows significant clustering for the different treatments.
4.1.4. Bacterial Community Size and alkB Gene Copies
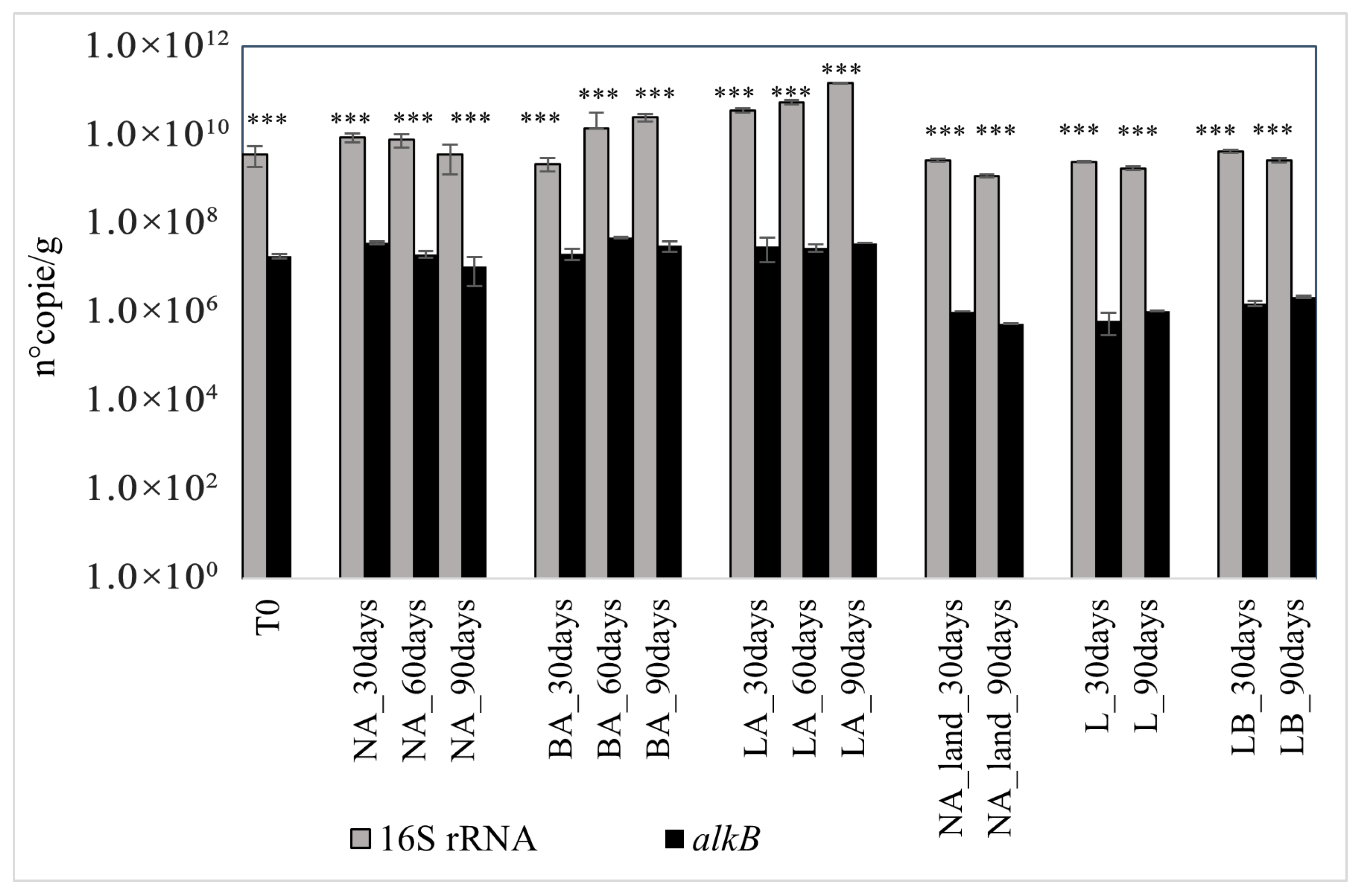
The qPCR results showed that the average bacterial 16S rRNA gene copy count in the original soil was 2.63 × 109 g−1.
Regarding the bioaugmentation approach, the community size increased in BA and LA treatments, reaching 1.77 × 1010 and 1.05 × 1011 of 16S rRNA gene copies after 90 days. On the contrary, the community size slightly decreased in NA treatment with 2.58 × 109 gene copies recorded at the end of the experimentation. Different behaviors were also observed between the treatments. Bioaugmentation with lyophilized beads (LA) was the only treatment presenting a continuous increase of 16S rRNA gene copies from the beginning of the experiment, with recorded values of 2.53 × 1010 and 3.93 × 1010, respectively, after 30 and 60 days. Instead, treatment with fresh beads (BA) primarily induced a reduction in the gene copy number after 30 days (1.58 × 109) before an augmentation was registered from day 60 (1 × 1010) until the end of the experiment. On the contrary, in natural attenuation (NA), 16S RNA gene copies rose during the first 30 days (6.25 × 109) before decreasing until the end of the experiment.
A similar trend was observed in NA within the landfarming experiment. The 16S rRNA gene copy number increased during the first 30 days, reaching a count of 2.71 × 109, and declined to reach 1.22 × 109 copies recorded on day 90. In the L treatment, the number of gene copies decreased from day 30 (2.53 × 109) until the end of the experiment, with a count of 1.80 × 109 copies on day 90. Inversely, the 16S rRNA gene copy number increased in the LB treatment in the first 30 days (4.37 × 109) but also slightly decreased, reaching 2.77 × 109 on day 90.
The count of alkB indicated that the untreated soil T0 harbored 1.30 × 107 copies of the gene per gram. A slight increase in this value was observed in all treatments after 30 days. While at this time the gene copies in BA were almost the same as in T0, with a recorded value of 1.51 × 107, this increase doubled the copies of the gene in NA and LA, respectively, with 2.63 × 107 and 2.20 × 107 values recorded. However, variations in the alkB copies differed between the treatments. Natural attenuation presented a gene copy reduction profile that indicated the decline of the number under the initial T0 value after 90 days, with the recorded count of 7.61 × 106. Instead, bioaugmentation with fresh and lyophilized beads induced an increase in alkB copies after 90 days of treatment. The number of copies doubled in the LA treatment compared to T0, reaching a count of 2.60 × 107 copies of the gene. A similar increase was observed in the BA treatment with 2.23 × 107 copies counted on day 90. However, the increase in gene copy number was not continuous in the two treatments. A peak of the gene copies was registered on day 60 in BA (3.44 × 107) before slightly decreasing to the last value recorded on day 90. Instead, in LA treatment, alkB copies decreased by 1.56 × 106 at day 60 compared to day 30, before increasing to the last value recorded on day 90 (Figure 3).
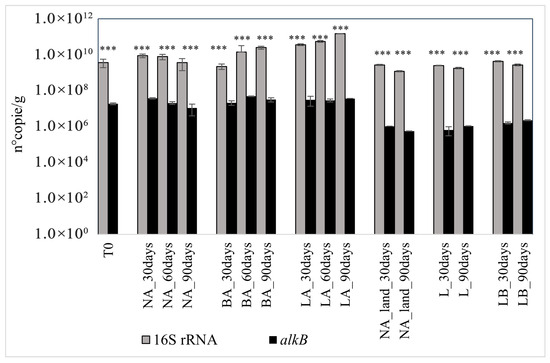
Figure 3.
16S rRNA and alkB gene copies in the different treatments over time. NA: natural attenuation; BA: fresh alginate beads; LA: lyophilized beads; NA_land: natural attenuation of landfarming; L: landfarming; LB: landfarming + bioaugmentation. The *** mark indicates significant differences observed in the ratio alkB/16S rRNA (p < 0.001 ***).
For the landfarming approach, a reduction in alkB gene copies was also registered in natural attenuation from day 30 (1.05 × 106 copies) and at the end of the experiment, with 5.55 × 105 copies counted on day 90. Inversely, in L and LB treatments, the gene copies increased from 6.42 × 105 and 1.61 × 106 on day 30 to 1.08 × 106 and 2.26 × 106 on day 90, respectively. Despite the variations in the different treatments, the landfarming assisted with the bioaugmentation method (LB) had the highest alkB copy number (Figure 3).
Analysis of variance (two-way ANOVA) revealed significant differences in alkB and 16S rRNA gene copy numbers, as well as in the alkB/16S ratio, among soil samples from the different treatments (p < 0.00001). The copy numbers of alkB, 16S, and the alkB/16S ratio also varied significantly with treatment time (p < 0.0001). Furthermore, the interaction between soil samples and incubation time had a significant effect on the copy numbers of the alkB and 16S rRNA genes, as well as the alkB/16S ratio (p < 0.00001) (Figure 3).
4.2. GC-MS Analysis
The study utilized GC-MS analysis to assess the extent of hydrocarbon reduction in the contaminated soil and the effectiveness of the different treatments. The experiment lasted for 90 days, and the analysis focused on the residual concentration of n-alkanes, which are more prone to biodegradation among petroleum hydrocarbons [44,45]. Data were obtained by analyzing duplicate samples collected at each time point and for each treatment and expressed as mean values.
The results revealed a reduction in the light and medium fractions of n-alkanes (n-C11 to n-C17) in all treatments. Specifically, the compounds n-C11 and n-C12 disappeared completely in the bioaugmentation with fresh beads (BA) treatment. However, in the other two treatments, natural attenuation (NA) and bioaugmentation with lyophilized beads (LA), there were substantial reductions of 79.8% and 66.1% for n-C11 and 89% and 78.7% for n-C12, respectively.
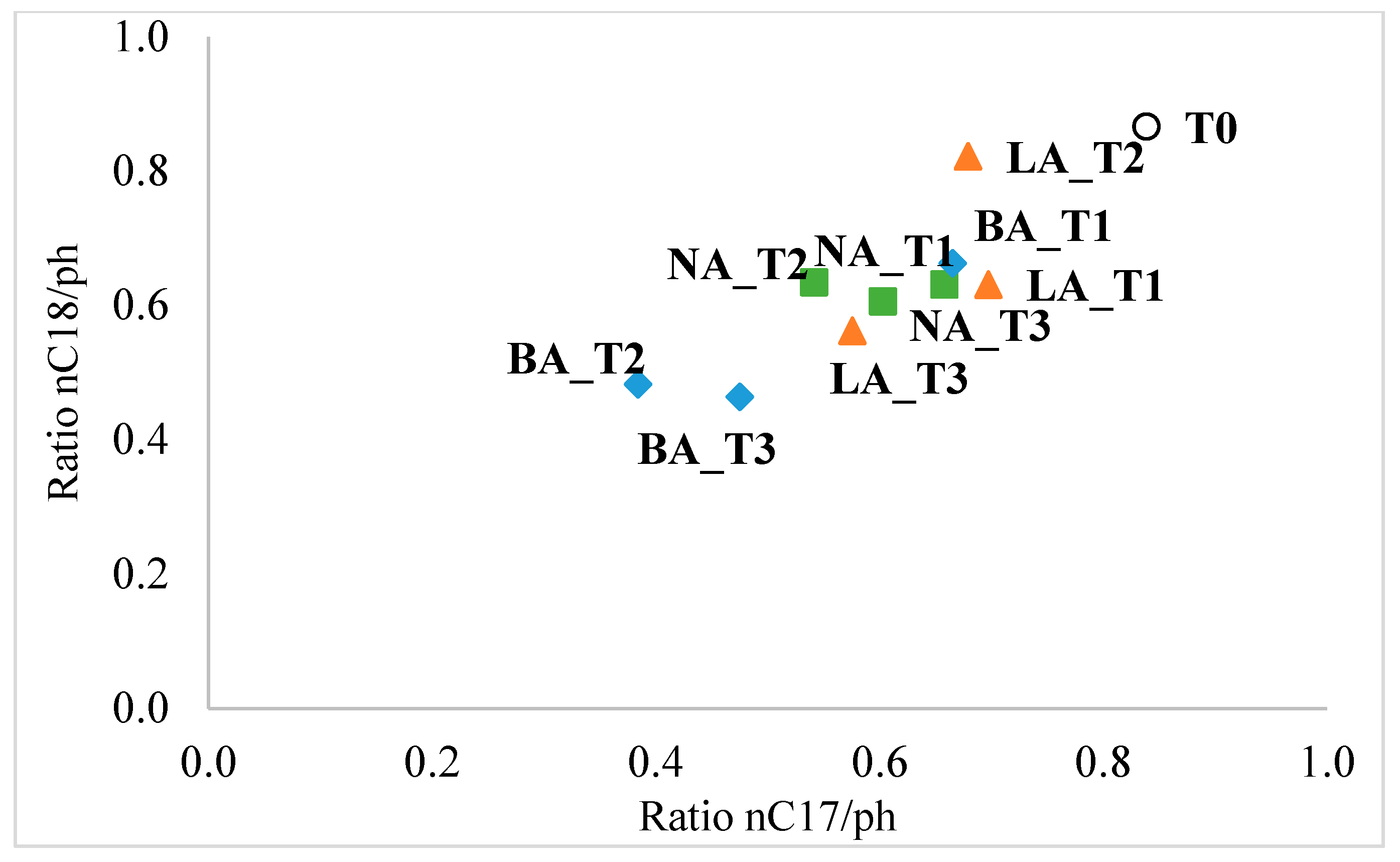
For the n-C13 to n-C17 compounds, decreases were observed in all three treatments, ranging from 16.6% (n-C16) to 71.6% (n-C14) in NA, 13.5% (n-C16) to 69.4% (n-C14) in BA, and 2.9% (n-C17) to 54.8% (n-C14) in LA. Interestingly, the heavier fraction of n-alkanes (n-C18 to n-C27) showed an increase in concentration. Notably, the LA treatment resulted in the complete degradation of n-C27 hydrocarbons. The study also used n-C17/pristane (n-C17/pr) and n-C18/phytane (n-C18/ph) ratios as biomarkers to assess biodegradation. These ratios are helpful because n-alkanes are more susceptible to biodegradation than isoprenoids. The results showed that active biodegradation was occurring in all treatments. The n-C17/pr ratio decreased from 0.84 (initial value in the untreated soil) to 0.6, 0.47, and 0.58 in the natural attenuation (NA), bioaugmentation with fresh beads (BA), and bioaugmentation with lyophilized beads (LA) treatments after 90 days, respectively. Similarly, the n-C18/ph ratio decreased from 0.87 (initial value) to 0.61, 0.46, and 0.56 in NA, BA, and LA at the end of the experiment.
During the remediation process, the BA treatment consistently had the lowest biomarker ratios on day 60 and day 90, reaching 0.38 and 0.47 for n-C17/pr, and 0.48 (day 60) and 0.46 (day 90) for n-C18/ph. Additionally, the LA treatment showed lower biomarker ratios compared to NA only at the end of the experiment.
When plotting the biomarker ratios, two distinct clusters were observed. The biodegradation in LA treatment throughout the entire experiment (LA_T1 to LA_T3) resembled the degradation in natural attenuation at the beginning and the end of the process (NA_1 and NA_T3). Moreover, the BA treatment was similar to NA only during the first 30 days, while at day 60 and day 90, it formed a separate cluster, indicating a significant difference from the other treatment (Figure 4).
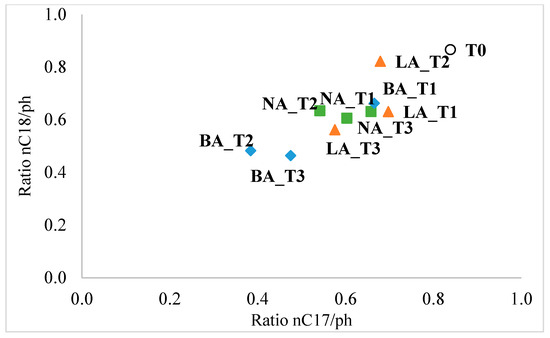
Figure 4.
Ratio plot of n-C17/pristane and n-C18/phytane in the different soil treatments and the original untreated soil. T0, T1, T2, T3 indicate initial (untreated soil), 30 days, 60 days, and 90 days sampling times, respectively. NA: natural attenuation (blue); BA: fresh alginate beads treatment (green); LA: lyophilized beads treatment (orange).
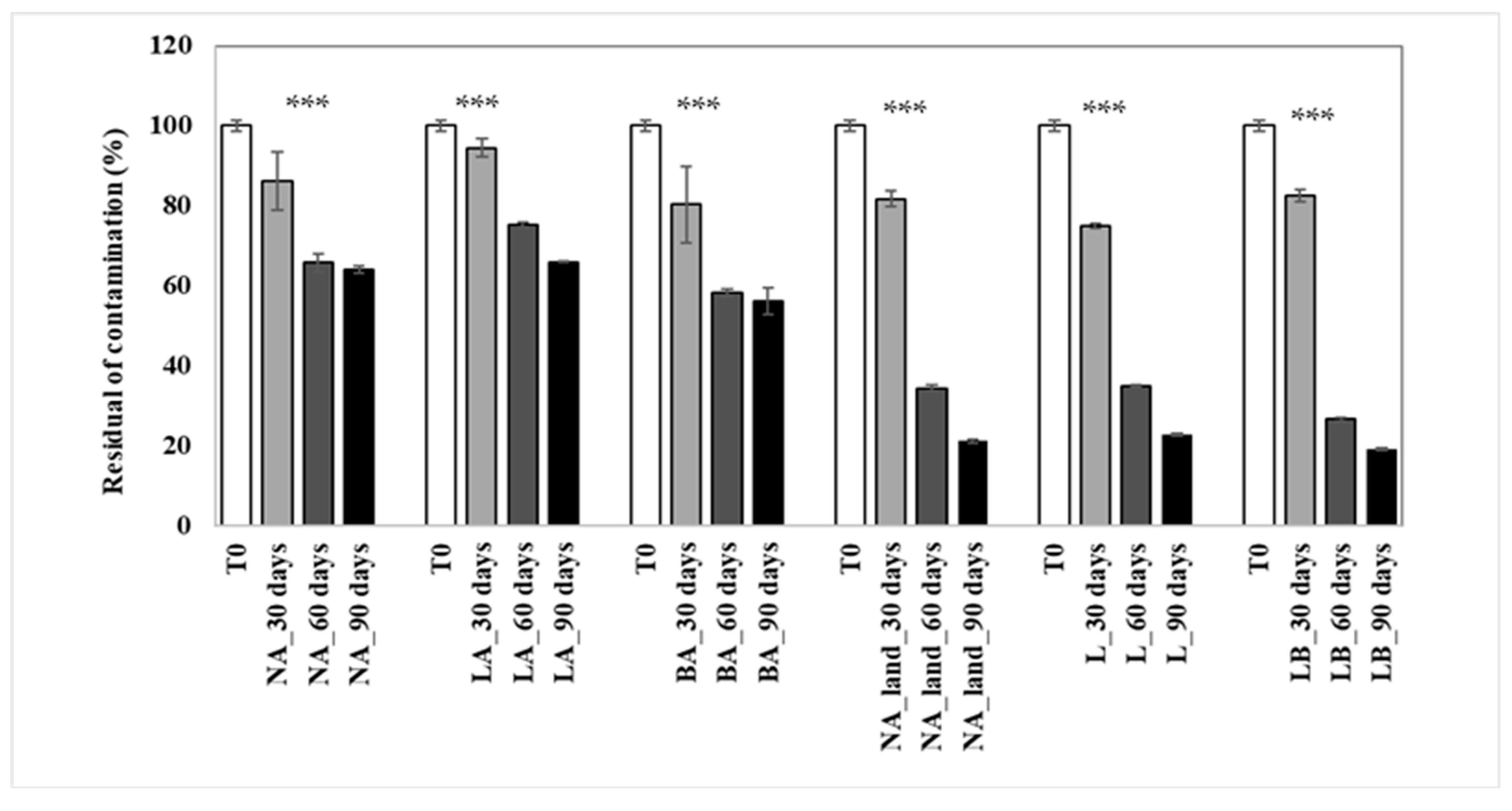
The findings aligned with the overall decrease in total petroleum hydrocarbons (TPH) in the treatments. Natural attenuation (NA) and bioaugmentation with lyophilized beads (LA) showed similar performance, with 36% and 34% TPH biodegradation, respectively, while bioaugmentation with fresh beads (BA) was the most effective, depleting almost half (44%) of the TPH (Figure 5). This indicates that BA resulted in about 10% higher hydrocarbon depletion compared to NA. During the initial two months of the experiment, all approaches showed an exponential increase in biodegradation, reaching 42%, 34%, and 25% for NA, BA, and LA, respectively. However, after day 60, the biodegradation rate in NA reached a plateau and diminished in BA, also showing a plateau tendency. In contrast, the biodegradation rate in LA continued to grow even in the last experimental period, from day 60 to day 90 (Figure 5). The n-alkane chromatograms after 90 days of treatment are given in Supplementary Figure S2.
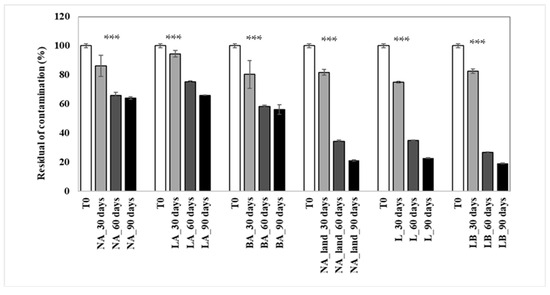
Figure 5.
Residual TPH concentration in the soil during the 90-day period treatment. NA: natural attenuation; LA: bioaugmentation with consortium entrapped in lyophilized beads; BA: bioaugmentation with consortium entrapped in fresh beads; NA_land: natural attenuation in the landfarming experiment; L: landfarming; LB: landfarming + bioaugmentation. 30, 60 and 90 days indicate the sampling times. The *** mark indicates significant differences observed (p < 0.001 ***).
The hydrocarbons’ half-life degradation was determined using the equation
where k represents the kinetic constant given by
t1/2 = ln2/k,
k = ln (T0/T)/t.
Here, T0 stands for the initial concentration of hydrocarbons, T denotes the hydrocarbon concentration at a specific time, and t represents the time duration.
The calculated half-life degradation revealed that natural attenuation would require approximately 140 days for the reduction in half the initial contamination. Conversely, for treatments involving bioaugmentation with fresh beads (BA) and with lyophilized beads (LA), the corresponding periods were projected to be 108 days and 150 days, respectively.
In the context of the landfarming experiments, the outcomes indicated a substantial biodegradation across all treatments, encompassing Natural Attenuation (NA), Landfarming (L), and Landfarming with Bioaugmentation (LB). Notably, nearly the entire content of total petroleum hydrocarbons (TPH) in the soil was degraded. After 90 days (as depicted in Figure 5), residual contamination levels were measured at 21% for NA, 23% for L, and 19% for LB treatments.
Within the initial 30 days of the experiment, the Landfarming approach (L) exhibited a notably accelerated biodegradation rate of 25% compared to the other two treatments. Subsequently, the integration of bioaugmentation with landfarming (LB treatment) seemed to exert a pronounced influence on the degradation process. The degradation rate escalated from 17% to an impressive 81% by the conclusion of the study.
The differences in residual contamination observed between the NA, BA, and LA treatments were statistically significant, as were the differences within each treatment strategy (p ˂ 0.0001).
5. Discussion
Research on bioremediation strategies has significantly expanded in recent years to address oil contamination. Although several commercial formulations are already available [24], further research is needed, particularly in the field of bioaugmentation using allochthonous bacteria, to ensure their effectiveness across a wide range of environmental conditions [23,46]. Ultimately, bioremediation efforts should aim to equip decision-makers with sustainable, efficient tools and strategies, enabling them to respond swiftly and effectively to recurring hydrocarbon pollution events and minimize their impacts.
In this work, we investigated the effectiveness of a mixed bacterial consortium consisting of strains Shinella zoogloeoides LFG9, Achromobacter pestifer S-BM4, Pseudomonas benzenivorans S-AR5, and Rhodococcus qingshengii C-M4 originating from different geographical regions [30] when applied as a bioaugmentation agent for oil recovery in short-term contaminated soil. These strains were selected based on their possession of the alkB gene and their capacity to produce biosurfactant-like molecules or at least one of these characteristics. Together, they formed the consortium COBIONA, which was immobilized in alginate beads. The bioaugmentation process involved inoculating the immobilized consortium into the contaminated soil, along with the addition of calcium peroxide (CaO2) as an oxygen supplier. Indeed, previous research has demonstrated the effectiveness of CaO2 in oil remediation. For instance, [33], applied CaO2 capsules for benzene removal (50 mg/L in groundwater) and achieved 100% efficiency within 60 days.
In the present study, bioaugmentation proved effective in the reclamation of hydrocarbons, achieving depletion rates of 44% and 34% for the fresh (BA treatment) and lyophilized (LA treatment) alginate beads containing the consortium, respectively. In comparison, the natural attenuation (NA) condition resulted in a TPH depletion of 36%. These results indicate the superiority of the BA treatment, showing a 10% higher efficiency compared to natural attenuation. The occurrence of natural hydrocarbon biodegradation in the NA condition is not surprising, as oil contamination often selects hydrocarbon degraders in impacted areas [24,29]. Moreover, the presence of a high number of alkB gene copies in the soil supports the degradation of alkanes. It is worth noting that over time, a more pronounced difference in hydrocarbon degradation between the two treatments could be observed, as natural attenuation requires 32 days longer than BA for the bioremediation of half the contamination. Previous studies have consistently reported enhanced hydrocarbon degradation in bioaugmentation treatments compared to control systems without amendments [10]. The outcomes of this study underscore the effectiveness and viability of our approach in accelerating hydrocarbon remediation in different areas, considering that half the strains forming the consortium were exogenous to the studied soil. In previous research, [46] reported the absence of petroleum hydrocarbon remediation during bioaugmentation with an exogenous consortium in laboratory conditions. The authors obtained 15% hydrocarbon degradation enhancement compared to natural attenuation after 9 months in the field exposed to good oxygenation, only by using nutrients and surfactants in addition to the consortium. Even though the entrapment technique was not employed by [46], it seems reasonable to assert that this experimental approach holds significant value and offers greater efficiency in enhancing this type of bioremediation. Achromobacter, Rhodococcus, and Pseudomonas, as genera used in our consortium, are known alkane degraders with a wide spectrum of action [4,18,19,44]. Moreover, the other genus Shinella has been shown to have versatile hydrocarbon degradation abilities [30,47].
It seemed that the immobilized consortium, in addition to the aeration provided by the CaO2, favored the biodegradation of those compounds by promoting the growth of indigenous bacteria from the order Actinomycetales, especially Gordonia and Microbacterium genera. In fact, Actinomycetales remained dominant in all treatments throughout the remediation process, indicating that this taxon was well established and acclimatized to the soil environmental conditions. Actinomycetales have been reported to be among the dominant phyla of bacterial communities in crude oil-contaminated soil and to harbor aerobic members [10,24,29,48]. Bacillales was the other well-promoted order, also comprising known hydrocarbon-degrading members [27,49,50].
The observed changes paralleled a significant increase in both bacterial biomass and alkB gene copies in the BA treatment, in contrast to the NA conditions. This outcome is consistent with the expected structural changes and succession of hydrocarbon-degrading taxa within the microbial communities during a remediation process [10,24,29]. Previous studies have also highlighted alterations in the bacterial community in hydrocarbon-contaminated soil amended with oxidants such as hydrogen peroxide H2O2 [51]. Furthermore, it has been reported that the introduction of immobilized strains in hydrocarbon-contaminated environments leads to higher degradation of hydrocarbons and an increase in the size of the bacterial community [10,11]. This is mainly attributed to an increase in the proportions of taxa that promote the degradation efficiency of the introduced immobilized strains [11]. Additionally, the rise in biomass is often associated with elevated microbial activity in bioaugmented environments [10,11], which is induced by the immobilized strains and results in increased functional gene abundances [11]. A reverse observation was made for the other taxa of interest in this study. Indeed, Achromobacter and Pseudomonas were not detected in BA treatment, like in the untreated soil, while the abundance of Rhodococcus considerably diminished at the end of the experiment (by three times compared to T0). Low abundances of bioaugmented genera, especially Achromobacter and Rhodococcus, suggested that the indigenous bacteria outcompeted those taxa after their release from the protective alginate niche or could not cope with the environmental conditions after promoting the growth of indigenous bacteria. Indeed, it is thought in this study that Achromobacter and Rhodococcus members are not key hydrocarbons-degrading players since they were likely not naturally selected in accordance with their low abundance and decrease in the NA treatment as well. Other authors also noticed interferences in the development of certain groups of bacteria during bioaugmentation and low abundance of the consortium members used, due to poor survival [10]. Although similar results were obtained with LA treatment regarding Achromobacter and Rhodococcus, the lyophilized consortium induced a different behavior regarding Pseudomonas. Indeed, the genus rose from an undetectable level in T0 to 14% of relative abundance at the end of the remediation process. This suggested that freeze-drying provided enhanced survival to Pseudomonas strains compared to fresh alginate beads. We hypothesized that this genus was naturally selected at the beginning of the remediation process. Indeed, it expanded in the natural attenuation treatment until 60 days with the same abundances as those registered in the LA treatment. In contrast, while the genus declined in NA, it kept increasing in LA treatment during the last phase of the experiment process, showing the advantage conferred by lyophilization for survival and growth. Previous studies have reported the benefits of freeze-drying, especially regarding increased viability and survival of Pseudomonas species [31]. In addition, some undetectable taxa or with very low abundance can proliferate after the occurrence of pollution [10,24] or during a remediation process [45], which phenomenon could explain the increase in Pseudomonas species in LA treatment. The rise in Pseudomonas during the last 30 days of the experimentation was concomitant with a recrudescence of the copy numbers of the alkB gene after a decrease on day 60. This augmentation was opposed to the decrease in the gene copies observed in BA and NA treatments during the same last period of the experimentation.
The microbial degradation of alkanes was further assessed by the ratios of the biomarkers n-C17/pristane and n-C18/phytane. The significance of these biomarker ratios lies in their capacity to indicate the overall degradation efficiency of alkanes. The isoprenoids pristane and phytane are considered more recalcitrant compounds compared to n-alkanes with similar carbon numbers. As a result, the decline in the ratios of n-C17/pristane and n-C18/phytane suggests the successful degradation of the more recalcitrant compounds. If the more recalcitrant compounds are degraded, it likely means that their n-alkane counterparts have been degraded, which will translate into an overall increase in alkanes degradation. Here, the ratios of n-C17/pristane and n-C18/phytane in LA treatment decreased significantly under the natural attenuation (NA) values during the last 30 days of the experimentation. The decrease in the ratios indicates variations in microbial degradation performance between the two treatments. This indicates that the microbial community in the LA treatment exhibited higher activity and efficiency in degrading alkanes, particularly the more resistant components, pristane, and phytane [45]. Additionally, there was a concurrent increase in the abundance of Pseudomonas bacteria in the LA treatment, while a decrease was observed in the NA treatment. These observations also suggest a potential association between the particular increase in Pseudomonas in LA treatment, the decrease in the biomarker ratios, and the efficiency of alkane degradation. The findings corroborate the research by [45], suggesting that pristane and phytane, being more recalcitrant compounds than n-alkanes, serve as reliable biomarkers for assessing microbial degradation performance. The genetic basis behind alkane degradation is essential for comprehending the underlying mechanisms responsible for the observed changes in biomarker ratios. The alkB gene, which codes for an alkane monooxygenase enzyme, plays a pivotal role in initiating the degradation of alkanes. The enzyme catalyzes the oxidation of n-alkanes by incorporating oxygen into the molecule. This initial step is vital in breaking down alkanes into primary alcohols, which are further metabolized into alkyl aldehydes and carboxylic acids, eventually integrating into the central metabolism [52]. Recent studies have highlighted other genetic determinants and enzymatic systems involved in alkane degradation, such as the ladA gene encoding a flavin-dependent long-chain alkane monooxygenase [53]. Based on these results, it was hypothesized that the lyophilization-induced changes would result in a more sustained hydrocarbon degradation over time, despite the initial lower performance compared to the other treatments. A previous study supports this assumption, showing that after an initial lag phase required for the reactivation of freeze-dried cells, bacterial growth accelerated, leading to higher oil degradation, including improved breakdown of alkanes such as phytane and pristane [54]. The potential higher performance of the lyophilized consortium in the long term is further supported by the marked increase in total bacterial abundance (as indicated by 16S rRNA gene copy numbers) and the enrichment in alkB gene copies at the end of the experiment. Indeed, as reported by [55], biodegradation efficiency is closely linked to the abundance and activity of degrading microorganisms. Biomass plays a critical role in hydrocarbon removal processes [49]. Our results also align with the findings of [55] who observed that the dominance of Pseudomonas species in bioaugmented soils significantly enhanced hydrocarbon degradation compared to non-bioaugmented soils and natural attenuation. Additionally, our findings showed that community shifts induced by lyophilization appeared more pronounced at lower taxonomic levels (family and genus). For instance, the abundant families in LA such as Enterobacteriaceae and Pseudomonadaceae, were present at very low abundance or undetectable in BA while the opposite trend was observed for Gordoniaceae and Microbacteriaceae which were more abundant in BA treatment.
Regarding the landfarming experiment, all treatments demonstrated high efficiency, with an average of 80% of total petroleum hydrocarbons (TPH) depletion after 90 days. This underscores the overall effectiveness of the methods applied. Regular soil turning, employed to enhance oxygen availability for microbial populations, likely contributed to this performance, as previously reported [49,55]. The similar degradation rates across treatments may be attributed to the relatively comparable microbial communities present in the soils.
Nonetheless, the combination of bioaugmentation with landfarming (LB) was expected to yield enhanced degradation compared to landfarming alone (L). Indeed, several studies have highlighted the synergistic effect of these approaches. For instance, [54] reported an 86% reduction in TPH after 90 days using bioaugmentation-assisted landfarming with an autochthonous bacterial consortium.
Overall, although the landfarming and the entrapped consortium experiments cannot be directly compared, a common conclusion from both assays is the presence of an efficient bacterial community well-adapted to petroleum hydrocarbon degradation. Especially, the two assays revealed that bacteria belonging to the order Actinomycetales were the dominant dwelling hydrocarbon-degrading community.
Landfarming thus appears to be an effective strategy for hydrocarbon remediation in soils, particularly when combined with bioaugmentation using autochthonous strains. In the present study, this combined approach (LB) not only enhanced TPH removal but also stimulated microbial biomass and increased the alkB gene abundance, both of which are key indicators of hydrocarbon degradation potential. Similar benefits were observed with the alginate bead-entrapped consortium (COBIONA), combined with calcium peroxide, which led to a two-order increase in bacterial biomass and a doubling of alkB gene copies. However, when considering all the features associated with the two techniques and the broader aspects of field implementation, including effectiveness, operational feasibility, and environmental sustainability, bioaugmentation using the lyophilized consortium (LA) appears more advantageous than the LB strategy. Indeed, soil disturbance is being minimized in bioremediation to better reflect natural conditions and reduce environmental impacts. In this context, soil turning for aeration, though effective, can generate additional pollution due to logistical demands, ultimately increasing the environmental footprint of the intervention [56]. The use of oxygen-releasing compounds, such as CaO2, offers a more sustainable alternative to physical soil manipulation.
While the effectiveness of the methodology is acknowledged, it is also worth noting that introducing allochthonous bacteria into contaminated environments may entail ecological risks. These organisms can compete with native microorganisms for nutrients, potentially altering community composition and ecosystem functions. They may also influence biogeochemical cycles and resource availability, or engage in horizontal gene transfer, which could occasionally result in the emergence of undesirable traits. From a “One Health” perspective, such shifts could affect microbial, plant, animal, and even human health [57,58]. In the present work, the proposed bioaugmentation strategy was first evaluated at a small scale to better assess potential impacts. This stepwise approach provides an initial safeguard, enabling optimization of the method while minimizing ecological risks before any broader application.
6. Conclusions
This study developed and validated a methodological framework to implement exogenous bioaugmentation using an immobilized mixed bacterial consortium. The approach was designed to overcome site-specific limitations and support broader applications across various contaminated environments. Structured as a five-step process, it integrates the identification of a functional gene marker adapted to the contamination profile, in this case, alkB, to guide strain selection and monitor bioremediation performance. Additional elements, such as microbial immobilization and oxygen supplementation, were incorporated to enhance microbial activity and hydrocarbon degradation. The experimental application in a 3-month petroleum-contaminated soil showed promising outcomes. The alginate bead-entrapped consortium COBIONA, supplemented with calcium peroxide, achieved 44% reduction in total petroleum hydrocarbons, a rate 10% higher than natural attenuation. Substantial degradation of n-alkanes, including complete removal of C11 and C12 compounds, was observed. These results confirm the relevance of targeting alkB-carrying strains and highlight the gene’s suitability as both a functional and monitoring tool. Furthermore, community analysis suggested that the introduced bacteria may have stimulated indigenous alkB-positive populations, probably through horizontal gene transfer. Lyophilized formulations of the consortium also emerged as a promising option for long-term application owing to their stability and practicality. While further optimization is required, such as adjusting calcium peroxide dosage and strain ratios or assessing single-strain immobilization, this study establishes a solid foundation for designing adaptable microbial cocktails for oil bioremediation. This framework may also be tailored for older contamination scenarios by fine-tuning microbial features and gene targets. The use of other carriers can further be explored. Overall, the methodology developed here represents a promising approach for in situ remediation of hydrocarbon-contaminated soils. While biopiles and ex situ treatments remain the most controllable setups, the integration of immobilized consortia with oxygen-releasing compounds offers a feasible strategy for field-scale applications in heterogeneous soils where excavation is not practical.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/applmicrobiol5040102/s1, Figure S1: Rarefaction curves of the microbial community in the soil samples. a, b, and c represent the individual rarefaction of exogenous bioaugmentation experiments at 30 days, 60 days, and 90 days respectively while d represents the rarefaction curves of the landfarming soil samples; Figure S2: n-alkanes chromatograms.
Author Contributions
E.J.C.D.G.-T.—Conceptualization; Investigation; Methodology; Writing—original draft; Writing—review & editing; I.P.—Investigation; Methodology; Writing—review & editing; A.C.—Formal analysis; N.C.—Formal analysis; S.P.C.—Supervision; Validation. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by ENI SpA under the Eni award Young Talents from Africa 2018.
Data Availability Statement
The data used in this study are available in Genbank (https://www.ncbi.nlm.nih.gov/nucleotide/) under the accession numbers PRJNA1000427 (exogenous bioaugmentation) and PRJNA1000434 (landfarming).
Acknowledgments
The authors thank Elisabbetta Franchi, Luca Paolo Serbolisca and Anna Cardiaci for previous analyses.
Conflicts of Interest
Author Ilaria Pietrini, Alessandro Conte, and Neria Costa were employed by the company ENI SpA. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Chicca, I.; Becarelli, S.; Di, G.S. Microbial Involvement in the Bioremediation of Total Petroleum Hydrocarbon Polluted Soils: Challenges and Perspectives. Environments. Environments 2022, 9, 52. [Google Scholar] [CrossRef]
- Sattar, S.; Hussain, R.; Shah, S.M.; Bibi, S.; Ahmad, S.R. Composition, impacts, and removal of liquid petroleum waste through bioremediation as an alternative clean-up technology: A review. Heliyon 2022, 8, e11101. [Google Scholar] [CrossRef]
- Gros, J.; Reddy, C.M.; Aeppli, C.; Nelson, R.K.; Carmichael, C.A.; Arey, J.S. Resolving Biodegradation Patterns of Persistent Saturated Hydrocarbons in Weathered Oil Samples from the Deepwater Horizon Disaster. Environ. Sci. Technol. 2014, 48, 1628–1637. [Google Scholar] [CrossRef]
- Li, Q. Mining secondary metabolism of Achromobacter and analysis of key genes of petroleum degradation. IOP Conf. Ser. Earth Environ. Sci. 2021, 692, 042032. [Google Scholar] [CrossRef]
- Cappelletti, M.; Fedi, S.; Zannoni, D. Degradation of Alkanes in Rhodococcus. Microbiol. Monogr. 2019, 16, 147–175. [Google Scholar] [CrossRef]
- Maqsood, Q.; Waseem, R.; Sumrin, A.; Wajid, A.; Tariq, M.R.; Ali, S.W.; Mahnoor, M. Recent trends in bioremediation and bioaugmentation strategies for mitigation of marine based pollutants: Current perspectives and future outlook. Discov. Sustain. 2024, 5, 524. [Google Scholar] [CrossRef]
- Goma-Tchimbakala, E.J.C.D.; Pietrini, I.; Goma-Tchimbakala, J.; Corgnati, S.P. Use of Shotgun Metagenomics to Assess the Microbial Diversity and Hydrocarbons Degrading Functions of Auto-Mechanic Workshops Soils Polluted with Gasoline and Diesel Fuel. Microorganisms 2023, 11, 722. [Google Scholar] [CrossRef]
- Mekonnen, B.A.; Aragaw, T.A.; Genet, M.B. Bioremediation of petroleum hydrocarbon contaminated soil: A review on principles, degradation mechanisms, and advancements. Front. Environ. Sci. 2024, 12, 1354422. [Google Scholar] [CrossRef]
- Ntroumpogianni, G.C.; Giannoutsou, E.; Karagouni, A.D.; Savvides, A.L. Bacterial Isolates from Greek Sites and Their Efficacy in Degrading Petroleum. Sustainability 2022, 14, 9562. [Google Scholar] [CrossRef]
- Dellagnezze, B.M.; Vasconcellos, S.P.; Angelim, A.L.; Melo, V.M.M.; Santisi, S.; Cappello, S.; Oliveira, V.M. Bioaugmentation strategy employing a microbial consortium immobilized in chitosan beads for oil degradation in mesocosm scale. Mar. Pollut. Bull. 2016, 107, 107–117. [Google Scholar] [CrossRef]
- Dou, R.; Sun, J.; Lu, J.; Deng, F.; Yang, C.; Lu, G.; Dang, Z. Bacterial communities and functional genes stimulated during phenanthrene degradation in soil by bio-microcapsules. Ecotoxicol. Environ. Saf. 2021, 212, 111970. [Google Scholar] [CrossRef]
- Xue, J.; Shi, K.; Chen, C.; Bai, Y.; Cui, Q.; Li, N.; Fu, X.; Qiao, Y. Evaluation of response of dynamics change in bioaugmentation process in diesel-polluted seawater via high-throughput sequencing: Degradation characteristic, community structure, functional genes. J. Hazard. Mater. 2021, 403, 123569. [Google Scholar] [CrossRef]
- Jurelevicius, D.; Alvarez, V.M.; Peixoto, R.; Rosado, A.S.; Seldin, L. The use of a combination of alkB primers to better characterize the distribution of alkane-degrading bacteria. PLoS ONE 2013, 8, e66565. [Google Scholar] [CrossRef]
- Muter, O. Current Trends in Bioaugmentation Tools for Bioremediation: A Critical Review of Advances and Knowledge Gaps. Microorganisms 2023, 11, 710. [Google Scholar] [CrossRef]
- Wu, M.; Wu, J.; Zhang, X.; Ye, X. Effect of bioaugmentation and biostimulation on hydrocarbon degradation and microbial community composition in petroleum-contaminated loessal soil. Chemosphere 2019, 237, 124456. [Google Scholar] [CrossRef]
- Nwankwegu, A.S.; Zhang, L.; Xie, D.; Onwosi, C.O.; Muhammad, W.I.; Odoh, C.K.; Kabari, S.K.; Idenyi, J.N. Bioaugmentation as a green technology for hydrocarbon pollution remediation. Problems and prospects. J. Environ. Manag. 2022, 304, 114313. [Google Scholar] [CrossRef]
- Nejidat, A.; Meshulam, M.; Diaz-Reck, D.; Ronen, Z. Emergence of hydrocarbon-degrading bacteria in crude oil-contaminated soil in a hyperarid ecosystem: Effect of phosphate addition and augmentation with nitrogen-fixing cyanobacteria on oil bioremediation. Int. Biodeterior. Biodegrad. 2023, 178, 105556. [Google Scholar] [CrossRef]
- Kumari, S.; Regar, R.K.; Manickam, N. Improved polycyclic aromatic hydrocarbon degradation in a crude oil by individual and a consortium of bacteria. Bioresour. Technol. 2018, 254, 174–179. [Google Scholar] [CrossRef]
- Okafor, C.P.; Udemang, N.L.; Chikere, C.B.; Akaranta, O.; Ntushelo, K. Indigenous microbial strains as bioresource for remediation of chronically polluted Niger Delta soils. Sci. Afr. 2021, 11, e00682. [Google Scholar] [CrossRef]
- Pozdnyakova, N.; Muratova, A.; Turkovskaya, O. Degradation of Polycyclic Aromatic Hydrocarbons by Co-Culture of Pleurotus ostreatus Florida and Azospirillum brasilense. Appl. Microbiol. 2022, 2, 735–748. [Google Scholar] [CrossRef]
- Ssenku, J.E.; Walusansa, A.; Oryem-Origa, H.; Ssemanda, P.; Ntambi, S.; Omujal, F.; Mustafa, A.S. Bacterial community and chemical profiles of oil-polluted sites in selected cities of Uganda: Potential for developing a bacterial-based product for remediation of oil-polluted sites. BMC Microbiol. 2022, 22, 120. [Google Scholar] [CrossRef]
- Behera, I.D.; Nayak, M.; Biswas, S.; Meikap, B.C.; Sen, R. Enhanced biodegradation of total petroleum hydrocarbons by implementing a novel two-step bioaugmentation strategy using indigenous bacterial consortium. J. Environ. Manag. 2021, 292, 112746. [Google Scholar] [CrossRef]
- Al-Mailem, D.M.; Kansour, M.K.; Radwan, S.S. Correction: Cross-Bioaugmentation Among Four Remote Soil Samples Contaminated with Oil Exerted Just Inconsistent Effects on Oil-Bioremediation. Front. Microbiol. 2020, 11, 211. [Google Scholar] [CrossRef]
- Radwan, S.S.; Al-Mailem, D.M.; Kansour, M.K. Bioaugmentation failed to enhance oil bioremediation in three soil samples from three different continents. Sci. Rep. 2019, 9, 19508. [Google Scholar] [CrossRef]
- Perdigão, R.; Almeida, C.M.R.; Magalhães, C.; Ramos, S.; Carolas, A.L.; Ferreira, B.S.; Carvalho, M.F.; Mucha, A.P. Bioremediation of Petroleum Hydrocarbons in Seawater: Prospects of Using Lyophilized Native Hydrocarbon-Degrading Bacteria. Microorganisms 2021, 9, 2285. [Google Scholar] [CrossRef]
- Rivelli, V.; Franzetti, A.; Gandolfi, I.; Cordoni, S.; Bestetti, G. Persistence and degrading activity of free and immobilised allochthonous bacteria during bioremediation of hydrocarbon-contaminated soils. Biodegradation 2013, 24, 1–11. [Google Scholar] [CrossRef]
- Chen, Q.; Li, J.; Liu, M.; Sun, H.; Bao, M. Study on the biodegradation of crude oil by free and immobilized bacterial consortium in marine environment. PLoS ONE 2017, 12, e0174445. [Google Scholar] [CrossRef]
- Shan, L.; Gao, Y.; Zhang, Y.; Yu, W.; Yang, Y.; Shen, S.; Zhang, S.; Zhu, L.; Xu, L.; Tian, B.; et al. Fabrication and use of alginate-based cryogel delivery beads loaded with urea and phosphates as potential carriers for bioremediation. Ind. Eng. Chem. Res. 2016, 55, 7655–7660. [Google Scholar] [CrossRef]
- Khandelwal, A.; Sugavanam, R.; Ramakrishnan, B.; Dutta, A.; Varghese, E.; Nain, L.; Banerjee, T.; Singh, N. Free and immobilized microbial culture–mediated crude oil degradation and microbial diversity changes through taxonomic and functional markers in a sandy loam soil. Front. Environ. Sci. 2022, 9, 794303. [Google Scholar] [CrossRef]
- Goma-Tchimbakala, E.J.C.D.; Pietrini, I.; Dal Bello, F.; Goma-Tchimbakala, J.; Lo Russo, S.; Corgnati, S.P. Great abilities of Shinella zoogloeoides strain from a landfarming soil for crude oil degradation and a synergy model for alginate-bead-entrapped consortium efficiency. Microorganisms 2022, 10, 1361. [Google Scholar] [CrossRef]
- Cabrefiga, J.; Francés, J.; Montesinos, E.; Bonaterra, A. Improvement of a dry formulation of Pseudomonas fluorescens EPS62e for fire blight disease biocontrol by combination of culture osmoadaptation with a freeze-drying lyoprotectant. J. Appl. Microbiol. 2014, 117, 1122–1131. [Google Scholar] [CrossRef]
- Chevalier, L.R.; McCann, C. Feasibility of calcium peroxide as an oxygen releasing compound in treatment walls. Int. J. Environ. Pollut. 2006, 2, 245–256. [Google Scholar] [CrossRef]
- Mosmeri, H.; Alaie, E.; Shavandi, M.; Dastgheib, S.M.M.; Tasharrofi, S. Benzene-contaminated groundwater remediation using calcium peroxide nanoparticles: Synthesis and process optimization. Environ. Monit. Assess. 2017, 189, 452. [Google Scholar] [CrossRef]
- Gnida, A.; Turek-Szytow, J. Calcium preparation aided bioremediation of fluoranthene-contaminated soil. Water Air Soil Pollut. 2023, 234, 17. [Google Scholar] [CrossRef]
- Teran-Cuadrado, G.; Ortega-Vega, C.; Alves-de Brito, H.; Quiñones-Murillo, D.; Jiménez Rojas, M. Effectiveness of a bio-catalytic agent used in the bioremediation of crude oil-polluted seawater. Heliyon 2021, 7, e06926. [Google Scholar] [CrossRef]
- Das, N.; Chandran, P. Microbial degradation of petroleum hydrocarbon contaminants: An overview. Biotechnol. Res. Int. 2011, 2011, 941810. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.E.; Brant, J.L.; Campo, P.; Clark, D.R.; Coulon, F.; Gregson, B.H.; McGenity, T.J.; McKew, B.A. Effects of dispersants and biosurfactants on crude-oil biodegradation and bacterial community succession. Microorganisms 2021, 9, 1200. [Google Scholar] [CrossRef]
- Révész, F.; Figueroa-Gonzalez, P.A.; Probst, A.J.; Kriszt, B.; Banerjee, S.; Szoboszlay, S.; Maróti, G.; Táncsics, A. Microaerobic conditions caused the overwhelming dominance of Acinetobacter spp. and the marginalization of Rhodococcus spp. in diesel fuel/crude oil mixture-amended enrichment cultures. Arch. Microbiol. 2020, 202, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Gielnik, A.; Pechaud, Y.; Huguenot, D.; Cébron, A.; Esposito, G.; van Hullebusch, E.D. Functional potential of sewage sludge digestate microbes to degrade aliphatic hydrocarbons during bioremediation of a petroleum hydrocarbons contaminated soil. J. Environ. Manag. 2020, 271, 111648. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.-D. Biodegradation testing: So many tests but very little new innovation. Appl. Environ. Biotechnol. 2016, 1, 92–95. [Google Scholar] [CrossRef]
- Gao, L.; Gu, J.-D. A unified conceptual framework involving maintenance energy, metabolism and toxicity involved in research on degradation of environmental organic pollutants. Int. Biodeterior. Biodegrad. 2021, 162, 105253. [Google Scholar] [CrossRef]
- Bertolini, V.; Gandolfi, I.; Ambrosini, R.; Bestetti, G.; Innocente, E.; Rampazzo, G.; Franzetti, A. Temporal variability and effect of environmental variables on airborne bacterial communities in an urban area of Northern Italy. Appl. Microbiol. Biotechnol. 2013, 97, 6561–6570. [Google Scholar] [CrossRef]
- Vázquez-Baeza, Y.; Pirrung, M.; Gonzalez, A.; Knight, R. EMPeror: A tool for visualizing high-throughput microbial community data. Gigascience 2013, 2, 16. [Google Scholar] [CrossRef] [PubMed]
- Zampolli, J.; Collina, E.; Lasagni, M.; Di Gennaro, P. Biodegradation of variable-chain-length n-alkanes in Rhodococcus opacus R7 and the involvement of an alkane hydroxylase system in the metabolism. AMB Express 2014, 4, 73. [Google Scholar] [CrossRef] [PubMed]
- Aljandal, S.; Doyle, S.M.; Bera, G.; Wade, T.L.; Knap, A.H.; Sylvan, J.B. Mesopelagic microbial community dynamics in response to increasing oil and Corexit 9500 concentrations. PLoS ONE 2022, 23, e0263420. [Google Scholar] [CrossRef]
- Couto, M.N.; Monteiro, E.; Vasconcelos, M.T. Mesocosm trials of bioremediation of contaminated soil of a petroleum refinery: Comparison of natural attenuation, biostimulation and bioaugmentation. Environ. Sci. Pollut. Res. Int. 2010, 17, 1339–1346. [Google Scholar] [CrossRef]
- Ntougias, S.; Melidis, P.; Navrozidou, E.; Tzegkas, F. Diversity and efficiency of anthracene-degrading bacteria isolated from a denitrifying activated sludge system treating municipal wastewater. Int. Biodeterior. Biodegrad. 2015, 97, 151–158. [Google Scholar] [CrossRef]
- Abbasian, F.; Palanisami, T.; Megharaj, M.; Naidu, R.; Lockington, R.; Ramadass, K. Microbial diversity and hydrocarbon degrading gene capacity of a crude oil field soil as determined by metagenomics analysis. Biotechnol. Prog. 2016, 32, 638–648. [Google Scholar] [CrossRef]
- Franchi, E.; Cardaci, A.; Pietrini, I.; Fusini, D.; Conte, A.; De Folly D’Auris, A.; Grifoni, M.; Pedron, F.; Barbafieri, M.; Petruzzelli, G.; et al. Nature-Based Solutions for Restoring an Agricultural Area Contaminated by an Oil Spill. Plants 2022, 11, 2250. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Singh, V.; Pandit, S.; Thapa, B.S.; Pant, K.; Tusher, T.R. Isolation of Biosurfactant-Producing Bacteria and Their Co-Culture Application in Microbial Fuel Cell for Simultaneous Hydrocarbon Degradation and Power Generation. Sustainability 2022, 14, 15638. [Google Scholar] [CrossRef]
- Apul, O.G.; Arrowsmith, S.; Hall, C.A.; Miranda, E.M.; Alama, F.; Dahlen, P.; Sra, K.; Kamath, R.; McMillen, S.J.; Sihota, N.; et al. Biodegradation of petroleum hydrocarbons in a weathered, unsaturated soil is inhibited by peroxide oxidants. J. Hazard. Mater. 2022, 433, 128770. [Google Scholar] [CrossRef] [PubMed]
- Gregson, B.H.; Metodieva, G.; Metodiev, M.V.; Golyshin, P.N.; McKew, B.A. Differential Protein Expression During Growth on Medium Versus Long-Chain Alkanes in the Obligate Marine Hydrocarbon-Degrading Bacterium Thalassolituus oleivorans MIL-1. Front. Microbiol. 2018, 9, 3130. [Google Scholar] [CrossRef] [PubMed]
- Tourova, T.P.; Sokolova, D.S.; Semenova, E.M.; Shumkova, E.S.; Korshunova, A.V.; Babich, T.L.; Poltaraus, A.B.; Nazina, T.N. Detection of n-alkane biodegradation genes alkB and ladA in thermophilic hydrocarbon-oxidizing bacteria of the genera Aeribacillus and Geobacillus. Microbiology 2016, 85, 693–707. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Bao, M.; Li, S. Solid inoculants as a practice for bioaugmentation to enhance bioremediation of hydrocarbon contaminated areas. Chemosphere 2021, 263, 128175. [Google Scholar] [CrossRef]
- Guarino, C.; Spada, V.; Sciarrillo, R. Assessment of three approaches of bioremediation (Natural Attenuation, Landfarming and Bioaugmentation—Assisted Landfarming) for a petroleum hydrocarbon contaminated soil. Chemosphere 2017, 170, 10–16. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (EPA). How to Evaluate Alternative Cleanup Technologies for Underground Storage Tank Sites. 2017. Available online: https://www.epa.gov/sites/default/files/2014-03/documents/tum_ch5.pdf (accessed on 31 August 2025).
- Fodelianakis, S.; Antoniou, E.; Mapelli, F.; Magagnini, M.; Nikolopoulou, M.; Marasco, R.; Barbato, M.; Tsiola, A.; Tsikopoulou, I.; Giaccaglia, L.; et al. Allochthonous bioaugmentation in ex situ treatment of crude oil-polluted sediments in the presence of an effective degrading indigenous microbiome. J. Hazard. Mater. 2015, 287, 78–86. [Google Scholar] [CrossRef]
- Fragkou, E.; Antoniou, E.; Daliakopoulos, I.; Manios, T.; Theodorakopoulou, M.; Kalogerakis, N. In Situ Aerobic Bioremediation of Sediments Polluted with Petroleum Hydrocarbons: A Critical Review. J. Mar. Sci. Eng. 2021, 9, 1003. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).