An Ab Initio Investigation of the Hydration of Lead(II)
Abstract
:1. Introduction
2. Materials and Methods
3. Results
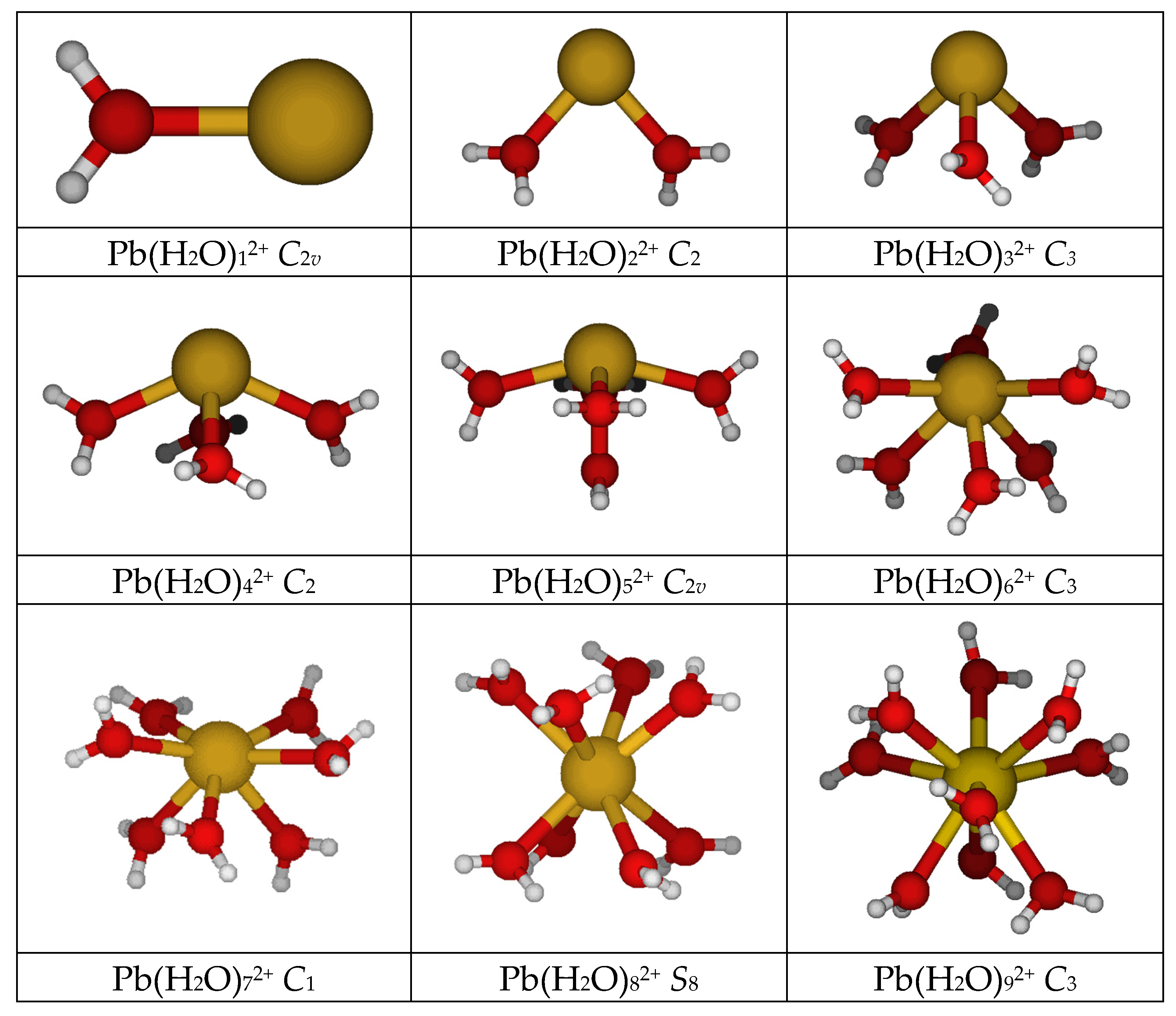
3.1. A Survey of Structures
- The bent C2 symmetry for diaqualead(II) is confirmed, with a Cs structure slightly higher and the linear D2d structure approximately 40 kJ/mol higher in energy. The related [1 + 1] and PbOH+ … H3O+ structures are 50–100 kJ/mol higher in energy. The use of either the SDD or LANL2DZ effective core potential on lead gives numbers on the lower end of this range. In some cases, the [1 + 1] structure does not exist, reverting to the PbOH+ … H3O+ (all MP2; HF/LANL2DZ) or [2 + 0] structure (HF/CEP-121G*);
- The pyramidal C3 symmetry for triaqualead(II) is confirmed, with the two C3v structures 10–20 kJ/mol higher in energy, and with the two D3h and one D3 planar structures approximately 40–60 kJ/mol higher in energy. The [2 + 1] C2v (HF/CEP-4G Cs) structure is 25–90 kJ/mol higher in energy, with the SDD or LANL2DZ effective core potential on lead giving numbers on the lower end of this range;
- The C2 see-saw structure for tetraaqualead(II) is confirmed at all levels except HF/LANL2MB (Cs), with the C2v #3 structure only slightly higher in energy. The distorted tetrahedral D2d #1, D2d #2, D2, and S4 structures are 20–40 kJ/mol higher in energy. The C2v #1 and 2 see-saw structures are approximately 10–20 kJ/mol higher in energy. The [3 + 1] Cs #2/C1 #1 structure tends to be about 25 kJ/mol higher in energy than the most stable [4 + 0] structure when using the CEP effective core potential/basis set combination, but is competitive in energy with the others (and even slightly lower for HF/LANL2DZ and HF/SDD), which may be a result of the lack of polarization functions in the basis set;
- The C2v #3/Cs square pyramidal structure is confirmed for pentaaqualead(II), with the other C2v structures (#1, #2, #4) being within 30 kJ/mol. The most stable [4 + 1] structure is either competitive in energy (Pb: SDD, LANL2DZ) or 10–25 kJ/mol less stable than the [5 + 0] structures;
- The C3 distorted octahedral structure is confirmed for hexaaqualead(II), with the undistorted Th structure being 2–30 kJ/mol higher in energy. For the CEP methods, the structure is competitive in energy with the [5 + 1] Cs structure, whereas for the LANL2DZ and SDD methods, the [5 + 1] is more stable by 10–25 kJ/mol;
- There are 16 possible C2v structures for heptaaqualead(II) spanning a range of 60 kJ/mol, with #1 and #16 being the most stable. In some cases, at some levels, these optimize to [6 + 1] or [5 + 2] structures. The C1 [7 + 0], [6 + 1], and [5 + 2] structures are competitive in energy. A stable [7 + 0] structure appears to be nonexistent at HF/LANL2MB, HF/LANL2DZ, HF/SDD, and HF/B. In some cases, the C2v [5 + 2] structures revert to [7 + 0] structures. Twelve C2v [5 + 2] structures and four C2v [6 + 1] structures were also located and desymmetrized. At least one stable structure was located for each by desymmetrization, except for the [6 + 1] structures at HF/LANL2MB, HF/LANL2DZ, and HF/SDD, which reverted to a [5 + 2] structure;
- For octaaqualead(II), the square prism D4h #1 and #2 and square antiprism D4d #1 and #2 have multiple imaginary frequencies, however the antiprism is much more stable. In some cases, stable S8 and D4 structures can be derived from these, and usually S8 is slightly more stable. Other possibilities include C4h, D2d, D2h, and C4v, although these are higher in energy. In some cases, a stable C4 structure forms from an unstable S8 (MP2/CEP-121G*, MP2/A), D4 (HF/SDD), or C4h structure (MP2/A+). Other C4, D2, S4, C2h, and C2v structures from desymmetrization are either not stable, or ascend in symmetry to give the S8 and D4 structure. Stable S4 structures exist at some of the MP2 levels. In all cases, the C1 #1 or #2 [7 + 1] structure is more stable than the S8, S4, or C4 [8 + 0] structures;
- For enneaaqualead(II), the tricapped trigonal prisms D3h #1–4 have multiple imaginary frequencies. Desymmetrization along A2′, A1”, and A2” modes led to C3h, D3, and C3v structures, respectively. These structures are unstable. The D3 #4 structure is often found, however the D3 #3 structure reverts to [6 + 3] in some cases. The C3h #3 and #4 coalesce to the corresponding #1 and #2 structures. In most cases, the C3v structures become [6 + 3]. To our surprise, the C3 #1–#3 structures formed from desymmetrization of D3 #4 (along A2), C3h #1, and #2 (along A”), respectively, were stable [9 + 0] structures if the split valence CEP basis sets were employed, although they usually reverted to stable [6 + 3] structures when using the LANL2DZ or SDD basis sets on lead. Desymmetrization along the E’ mode of the D3h structures gives C2v structures, whereas desymmetrization along the E” mode could give either a C2 or Cs structure. None of the C2v structures were stable, and many reverted to a [8 + 1], [7 + 2], [5 + 4], [5 + 2 + 2], or [4 + 5] structures. Desymmetrization of these along the A2 mode occasionally gave a stable C2 structure, whereas along the B1 or B2 modes gave either unstable Cs structures, ascension in symmetry to give C3h #1 or #2, or decoordination to give [8 + 1], [7 + 2], [6 + 3], [6 + 2 + 1], [5 + 4], [5 + 3 + 1], [4 + 5], or [4 + 3 + 2] structures.
3.2. The Pb-O Distance
3.3. The Pb-O Vibrational Frequency
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References and Notes
- Richens, D.T. The Chemistry of Aqua Ions; Wiley: Chichester, UK, 1997. [Google Scholar]
- Pye, C.C.; Gunasekara, C.M. An Ab Initio Investigation of the Hydration of Thallium(III) and Mercury(II). J. Solut. Chem. 2020, 49, 1419–1429. [Google Scholar] [CrossRef]
- Baes, C.F., Jr.; Mesmer, R.E. The Hydrolysis of Cations; Wiley: New York, NY, USA, 1976. [Google Scholar]
- Spiro, T.G.; Templeton, D.H.; Zalkin, A. The Crystal Structure of a Hexanuclear Basic Lead(II) Perchlorate Hydrate: Pb6O(OH)6(ClO4)4∙H2O. Inorg. Chem. 1968, 8, 856–861. [Google Scholar] [CrossRef]
- Olin, A.; Soderquist, R. The Crystal Structure of β-[Pb6O(OH)6] (ClO4)4∙H2O. Acta Chem. Scand. 1972, 26, 3505–3514. [Google Scholar] [CrossRef]
- Johansson, G.; Olin, A. On the Structures of the Dominating Hydrolysis Products of Lead(II) in Solution. Acta Chem. Scand. 1968, 22, 3197–3201. [Google Scholar] [CrossRef]
- Hong, S.-H.; Olin, A. On the Crystal Structure of [Pb4(OH)4]3(CO3)(ClO4)10∙6H2O. Acta Chem. Scand. 1973, 27, 2309–2320. [Google Scholar] [CrossRef]
- Hong, S.-H.; Olin, A. The Crystal Structure of [Pb4(OH)4](ClO4)4∙2H2O. Acta Chem. Scand. A 1974, 28, 233–238. [Google Scholar] [CrossRef]
- Cox, H.; Stace, A.J. Molecular View of the Anomalous Acidities of Sn2+, Pb2+, and Hg2+. J. Am. Chem. Soc. 2004, 126, 3939–3947. [Google Scholar] [CrossRef]
- Shi, T.; Orlova, G.; Guo, J.; Bohme, D.K.; Hopkinson, A.C.; Siu, K.W.M. Evidence of Doubly Charged Lead Monohydrate: Experimental Evidence and Theoretical Examination. J. Am. Chem. Soc. 2004, 126, 7975–7980. [Google Scholar] [CrossRef]
- Shi, T.; Zhao, J.; Hopkinson, A.C.; Siu, K.W.M. Formation of Abundant [Pb(H2O)]2+ by Ligand-Exchange Reaction between [Pb(N2)n]2+ (n = 1–3) and H2O. J. Phys. Chem. B 2005, 109, 10590–10593. [Google Scholar] [CrossRef]
- McQuinn, K.; Hof, F.; McIndoe, J.S.; Chen, X.; Wu, G.; Stace, A.J. Evidence of asymmetric cation solvation from the instability of [Pb(H2O)n]2+ complexes. Chem. Commun. 2009, 27, 4088–4090. [Google Scholar] [CrossRef]
- Gourlaouen, C.; Piquemal, J.-P.; Parisel, O. [Pb(H2O)]2+ and [Pb(OH)]+: Four-component density functional theory calculations, correlated scalar relativistic constrained-space orbital variation energy decompositions, and topological analysis. J. Chem. Phys. 2006, 124, 174311. [Google Scholar] [CrossRef] [PubMed]
- Arfa, M.; Olier, R.; Privat, M. Ab Initio Calculations on the Electronic Structure of the Divalent Lead-Water Complex. J. Phys. Chem. A 2008, 112, 6004–6008. [Google Scholar] [CrossRef] [PubMed]
- Hofer, T.S.; Rode, B.M. The solvation structure of Pb(II) in dilute aqueous solution: An ab initio quantum mechanical/molecular mechanical molecular dynamics approach. J. Chem. Phys. 2004, 121, 6406–6411. [Google Scholar] [CrossRef] [PubMed]
- Hofer, T.S.; Randolf, B.R.; Rode, B.M. The dynamics of the solvation of Pb(II) in aqueous solution obtained by an ab initio QM/MM MD approach. Chem. Phys. 2006, 323, 473–478. [Google Scholar] [CrossRef]
- Gourlaouen, C.; Gerard, H.; Parisel, O. Exploring the Hydration of Pb2+: Ab Initio Studies and First-Principles Molecular Dynamics. Chem. Eur. J. 2006, 12, 5024–5032. [Google Scholar] [CrossRef]
- Wander, M.C.F.; Clark, A.E. Hydration Properties of Aqueous Pb(II) Ion. Inorg. Chem. 2008, 47, 8233–8241. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Hofer, T.S.; Pribil, A.B.; Randolf, B.R.; Lim, L.H.V.; Lichtenberger, A.F.; Rode, B.M. Revisiting the Hydration of Pb(II): A QMCF MD Approach. J. Phys. Chem. B 2009, 113, 13007–13013. [Google Scholar] [CrossRef]
- Lei, X.L.; Pan, B.C. The Geometries and Proton Transfer of Hydrated Divalent Lead Ion Clusters [Pb(H2O)n]2+ (n = 1–17). J. Theor. Comput. Chem. 2012, 11, 1149–1164. [Google Scholar] [CrossRef]
- Wang, J.; Xia, S.; Yu, L. Hydration Structure of Pb(II) from Density Functional Theory Studies and First-Principles Molecular Dynamics. Acta Chim. Sin. 2013, 71, 1307–1312. (In Chinese)An English translation for ease of understanding is provided in the SM [Google Scholar] [CrossRef] [Green Version]
- Leon-Pimentel, C.I.; Amaro-Estrada, J.I.; Saint-Martin, H.; Ramirez-Solis, A. Born-Oppenheimer molecular dynamics studies of Pb(II) micro hydrated gas phase clusters. J. Chem. Phys. 2017, 146, 084307. [Google Scholar] [CrossRef]
- Kuznetsov, A.M.; Masliy, A.N.; Korshin, G.V. Quantum-chemical simulations of the hydration of Pb(II) ion: Structure, hydration energies, and pKa1 value. J. Mol. Model. 2018, 24, 193. [Google Scholar] [CrossRef] [PubMed]
- Leon-Pimentel, C.I.; Martinez-Jimenez, M.; Saint-Martin, H. Study of the Elusive Hydration of Pb2+ from the Gas Phase to the Liquid Aqueous Solution: Modeling the Hemidirected Solvation with a Polarizable MCDHO Force-Field. J. Phys. Chem. B 2019, 123, 9155–9166. [Google Scholar] [CrossRef] [PubMed]
- Tolbatov, I.; Marrone, A. Molecular dynamics simulation of the Pb(II) coordination in biological media via cationic dummy atom models. Theor. Chem. Acc. 2021, 140, 20. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Zakrzewski, V.G.; Montgomery, J.A., Jr.; Stratmann, R.E.; Burant, J.C.; et al. Gaussian 98, Revision A.9; Gaussian, Inc.: Pittsburgh, PA, USA, 1998. [Google Scholar]
- We thank an anonymous reviewer for carrying out some scalar relativistic (SR) and spin-orbit (SO) ZORA/BP86/DZP-small core Gibbs free energy of binding calculations using the Amsterdam Density Functional (ADF) package to confirm this: n = 1, −202.27 kJ/mol (SR), −205.13 kJ/mol; n = 2, −353.12 kJ/mol (SR), −356.05 kJ/mol (SO). The bond distances are: n = 1, 2.352 Å (SR and SO); n = 2, 2.394, 2.397 Å (both SR and SO). These compare favorably with our HF/C calculations.
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A., Jr.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03, Revision D.02; Gaussian, Inc.: Wallingford, CT, USA, 2004. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Pye, C.C.; Gunasekara, C.M.; Rudolph, W.W. An ab initio investigation of bismuth hydration. Can. J. Chem. 2007, 85, 945–950. [Google Scholar] [CrossRef]
- Pye, C.C.; Whynot, D.C.M.; Corbeil, C.R.; Mercer, D.J. Desymmetrization in geometry optimization: Application to an ab initio study of copper(I) hydration. Pure Appl. Chem. 2020, 92, 1643–1654. [Google Scholar] [CrossRef]
- Pye, C.C.; Gilbert, C.R. An ab initio investigation of the second hydration shell of metal cations. Comput. Appl. Chem. 2020, 208, 395–397. [Google Scholar]
- Pye, C.C.; Black, S.M.; Rudolph, W.W. An Ab Initio Investigation of Zinc Bromo Complexes. J. Sol. Chem. 2011, 40, 1932–1954, and references 7-16 therein. [Google Scholar] [CrossRef]
- Persson, I.; Lyczko, K.; Lundberg, D.; Eriksson, L.; Placzek, A. Coordination Chemistry Study of Hydrated and Solvated Lead(II) Ions in Solution and Solid State. Inorg. Chem. 2011, 50, 1058–1072. [Google Scholar] [CrossRef]
- Rode, B.M.; University of Innsbruck, Innsbruck, Austria. Personal communication, 2 February 2022.


| Level | [Pb(H2O)6]2+ C3 | [Pb(H2O)6]2+ Th | [Pb(H2O)18]2+ T |
|---|---|---|---|
| HF/CEP-4G | 355, 2.3663, 2.6202 | 307 *, 2.5401 | 366 *, 2.5123 |
| HF/CEP-31G* | 297, 2.4512, 2.6164 | 287 *, 2.5644 | 322 *, 2.5414 |
| HF/CEP-121G* | 296, 2.4450, 2.6167 | 286 *, 2.5619 | n/c, 2.5397 |
| HF/LANL2MB | 376, 2.2075, 2.4790 | 334 *, 2.4018 | 430 *, 2.3631 |
| HF/LANL2DZ | 277, 2.3813, 2.7737 | 252 *, 2.5948 | 297, 2.5610 |
| HF/SDD | 254, 2.5028, 2.8326 | 234 *, 2.6849 | 285, 2.6394 |
| MP2/CEP-31G* | 311, 2.4224, 2.5884 | 296 *, 2.5317 | n/c |
| MP2/CEP-121G* | 310, 2.4186, 2.5882 | 296 *, 2.5304 | n/c |
| HF/A | 311, 2.4136, 2.5928 | 298 *, 2.5384 | 336 *, 2.5157 |
| HF/B | 273, 2.4217, 2.7191 | 253 *, 2.5973 | 299 *, 2.5640 |
| HF/C | 253, 2.5149, 2.8082 | 235 *, 2.6820 | 284, 2.6408 |
| HF/A+ | 298, 2.4286, 2.6119 | 288 *, 2.5544 | 326, 2.5283 |
| HF/B+ | 252, 2.4609, 2.7373 | 238 *, 2.6192 | 284, 2.5869 |
| HF/C+ | 235, 2.5615, 2.8025 | 224 *, 2.6994 | 272, 2.6580 |
| MP2/A | 335, 2.3915, 2.5683 | 308 *, 2.5088 | n/c |
| MP2/A+ | 310, 2.4126, 2.5880 | 296 *, 2.5269 | n/c |
| Expt. | n/a, 2.528–2.543 [34] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pye, C.C.; Gunasekara, C.M. An Ab Initio Investigation of the Hydration of Lead(II). Liquids 2022, 2, 39-49. https://doi.org/10.3390/liquids2010004
Pye CC, Gunasekara CM. An Ab Initio Investigation of the Hydration of Lead(II). Liquids. 2022; 2(1):39-49. https://doi.org/10.3390/liquids2010004
Chicago/Turabian StylePye, Cory C., and Champika Mahesh Gunasekara. 2022. "An Ab Initio Investigation of the Hydration of Lead(II)" Liquids 2, no. 1: 39-49. https://doi.org/10.3390/liquids2010004






