Journal Description
Liquids
Liquids
is an international, peer-reviewed, open access journal on all aspects of liquid material research published quarterly online by MDPI.
- Open Access— free for readers, with article processing charges (APC) paid by authors or their institutions.
- High Visibility: indexed within ESCI (Web of Science), Scopus, AGRIS, and other databases.
- Rapid Publication: manuscripts are peer-reviewed and a first decision is provided to authors approximately 34.9 days after submission; acceptance to publication is undertaken in 4.6 days (median values for papers published in this journal in the first half of 2025).
- Recognition of Reviewers: APC discount vouchers, optional signed peer review, and reviewer names published annually in the journal.
Latest Articles
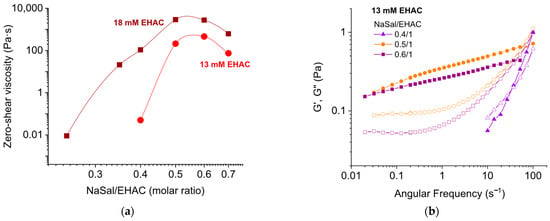
Tuning of the Viscosity Maximum and the Temperature Effect on Wormlike Micelle Solutions Using Hydrotropic and Inorganic Salts
Liquids 2025, 5(4), 28; https://doi.org/10.3390/liquids5040028 (registering DOI) - 26 Oct 2025
Abstract
The rheological properties of aqueous solutions of wormlike micelles (WLMs) of cationic surfactant erucyl bis(hydroxyethyl)methylammonium chloride (EHAC) in the presence of hydrotropic salt sodium salicylate (NaSal) and inorganic salt sodium chloride (NaCl) have been studied. The conditions for maximum zero-shear viscosity at fixed
[...] Read more.
The rheological properties of aqueous solutions of wormlike micelles (WLMs) of cationic surfactant erucyl bis(hydroxyethyl)methylammonium chloride (EHAC) in the presence of hydrotropic salt sodium salicylate (NaSal) and inorganic salt sodium chloride (NaCl) have been studied. The conditions for maximum zero-shear viscosity at fixed surfactant concentration were investigated. It has been shown that charged WLMs in the presence of NaSal have higher viscosities than well-screened micelles in the presence of NaCl. This is because the adsorption of hydrophobic salicylate ions onto the micelles increases their length more significantly than the presence of a large amount of sodium ions in the solution. It was discovered that the effect of temperature on the rheological properties depends on both the type of salt used and the salt/surfactant molar ratio. An unusual increase in zero-shear viscosity and elastic modulus was observed at a NaSal concentration that corresponds to the maximum zero-shear viscosity when the WLMs are linear, charged, and “unbreakable”. These results expand the possibilities of using hydrotropic salts to create stable, highly viscous systems in various fields, and opening up new horizons for applications in oil production, cosmetics, and household chemicals.
Full article
(This article belongs to the Section Chemical Physics of Liquids)
►
Show Figures
Open AccessArticle
Mesoscopic Liquids Emit Thermal Waves Under Shear Strain or Microflow
by
Laurence Noirez, Eni Kume and Patrick Baroni
Liquids 2025, 5(4), 27; https://doi.org/10.3390/liquids5040027 - 9 Oct 2025
Abstract
Liquids like water are not expected to produce a thermal change under shear strain or flow (away from extreme conditions). In this study, we reveal experimental conditions for which the conventional athermal hydrodynamic assumption is no longer valid. We highlight the establishment of
[...] Read more.
Liquids like water are not expected to produce a thermal change under shear strain or flow (away from extreme conditions). In this study, we reveal experimental conditions for which the conventional athermal hydrodynamic assumption is no longer valid. We highlight the establishment of non-equilibrium hot and cold thermal states occurring when a mesoscopic confined liquid is set in motion. Two stress situations are considered: low-frequency shear stress at large strain amplitude and microfluidic transport (pressure gradient). Two liquids are tested: water and glycerol at room temperature. In confined conditions (submillimeter scale), these liquids exhibit stress-induced thermal waves. We interpret the emergence of non-equilibrium temperatures as a consequence of the solicitation of the mesoscopic liquid elasticity. In analogy with elastic deformation, the mesoscopic volume decreases or increases slightly, which leads to a change in temperature (thermo-mechanical energy conversion). The energy acquired or released is converted to heat or cold, respectively. To account for these non-equilibrium temperatures, the mesoscopic flow is no longer considered as a complete dissipative process but as a way of propagating shear and thus compressive waves. This conclusion is consistent with recent theoretical developments showing that liquids propagate shear elastic waves at small scales.
Full article
(This article belongs to the Section Physics of Liquids)
►▼
Show Figures

Figure 1
Open AccessArticle
Hydroxyl Radical Formation and Its Mechanism in Cavitation Bubble Plasma-Treated Water: A Chemical Probe Study
by
Kotaro Kawano and Yoshihiro Oka
Liquids 2025, 5(4), 26; https://doi.org/10.3390/liquids5040026 - 1 Oct 2025
Abstract
This study investigates the formation of hydroxyl radicals (OH radicals) in cavitation bubble plasma-treated water (CBPTW) using a chemical probe method. CBPTW samples were prepared with different electrode materials (W, Fe, Cu, and Ag), and the chemical scavenger was added two minutes after
[...] Read more.
This study investigates the formation of hydroxyl radicals (OH radicals) in cavitation bubble plasma-treated water (CBPTW) using a chemical probe method. CBPTW samples were prepared with different electrode materials (W, Fe, Cu, and Ag), and the chemical scavenger was added two minutes after the completion of cavitation and plasma treatments. The concentrations of metal ions and hydrogen peroxide (H2O2) generated in the CBPTW were also measured over time. This study reveals a novel mechanism whereby metal nanoparticles and ions released from electrodes catalyze the continuous generation of hydroxyl radicals in CBPTW, which has not been fully addressed in previous studies. The results suggest a continuous generation of OH radicals in CBPTW prepared with W, Fe, and Cu electrodes, with the amount of OH radicals produced in the order Cu > Fe > W. The study reveals a correlation between OH radical production and electrode wear, suggesting that the continuous generation of OH radicals in CBPTW results from the catalytic decomposition of H2O2 by metal nanoparticles or ions released from the electrodes. It should be noted that cavitation bubble plasma (CBP) is fundamentally different from sonochemistry. While sonochemistry utilizes ultrasound-induced cavitation to generate radicals, CBP relies on plasma discharge generated inside cavitation bubbles. No ultrasound was applied in this study; therefore, all observed radical formation is attributable exclusively to plasma processes rather than sonochemical effects. However, the precise mechanism of continuous OH radical formation in CBPTW remains unclear and requires further investigation. These findings provide new insights into the role of electrode materials in continuous OH radical generation in cavitation bubble plasma treated water, offering potential applications in water purification and sterilization technologies.
Full article
(This article belongs to the Section Molecular Liquids)
►▼
Show Figures

Figure 1
Open AccessArticle
Dispersion, Polar, and Hydrogen-Bonding Contributions to Solvation Free Energies
by
William E. Acree, Jr. and Costas Panayiotou
Liquids 2025, 5(4), 25; https://doi.org/10.3390/liquids5040025 - 25 Sep 2025
Abstract
A new method is presented for the estimation of contributions to solvation free energy from dispersion, polar, and hydrogen-bonding (HB) intermolecular interactions. COSMO-type quantum chemical solvation calculations are used for the development of four new molecular descriptors of solutes for their electrostatic interactions.
[...] Read more.
A new method is presented for the estimation of contributions to solvation free energy from dispersion, polar, and hydrogen-bonding (HB) intermolecular interactions. COSMO-type quantum chemical solvation calculations are used for the development of four new molecular descriptors of solutes for their electrostatic interactions. The new model needs one to three solvent-specific parameters for the prediction of solvation free energies. The widely used Abraham’s LSER model is used for providing the reference solvation free energy data for the determination of the solvent-specific parameters. Extensive calculations in 80 solvent systems have verified the good performance of the model. The very same molecular descriptors are used for the calculation of solvation enthalpies. The advantages of the present model over Abraham’s LSER model are discussed along with the complementary character of the two models. Enthalpy and free-energy solvation information for pure solvents is translated into partial solvation parameters (PSP) analogous to the widely used Hansen solubility parameters and enlarge significantly their range of applications. The potential and the perspectives of the new approach for further molecular thermodynamic developments are discussed.
Full article
(This article belongs to the Special Issue Energy Transfer in Liquids)
Open AccessArticle
Thermodynamic Constraints on the “Hidden” Folding Intermediates
by
Timur A. Mukhametzyanov, Mikhail I. Yagofarov and Christoph Schick
Liquids 2025, 5(3), 24; https://doi.org/10.3390/liquids5030024 - 13 Sep 2025
Abstract
Experimental data on the folding and unfolding of small globular proteins are often well described assuming a two-state equilibrium process. It means that after careful analysis by a combination of experimental techniques, only folded and unfolded states of the protein are found to
[...] Read more.
Experimental data on the folding and unfolding of small globular proteins are often well described assuming a two-state equilibrium process. It means that after careful analysis by a combination of experimental techniques, only folded and unfolded states of the protein are found to be populated under various external conditions with no detectable intermediates. One of the consequences of the two-state behavior is that the equilibrium ratio of the folded to unfolded protein states follows a simple thermodynamic relation, and the enthalpy difference between states can be obtained from the temperature dependence of the equilibrium constant. In this paper, we theoretically investigate the criteria for the two-state equilibrium behavior and discuss the thermodynamic constraint on the properties of the “hidden” folding intermediates. The literature data on the folding mechanism of lysozyme in water and glycerol, which follows a two-state equilibrium behavior but includes kinetic intermediates, is analysed in light of this constraint.
Full article
(This article belongs to the Section Chemical Physics of Liquids)
►▼
Show Figures

Figure 1
Open AccessArticle
Bifunctional HLD–NAC for Clove Oil Microemulsions
by
Jia-Xin Tan and Edgar Acosta
Liquids 2025, 5(3), 23; https://doi.org/10.3390/liquids5030023 - 8 Sep 2025
Abstract
Clove oil is an essential oil used in food and pharmaceutical applications, with a market value of 300+ million dollars per year. Microemulsions have been used as effective clove oil delivery vehicles and could also be used to develop new extraction processes from
[...] Read more.
Clove oil is an essential oil used in food and pharmaceutical applications, with a market value of 300+ million dollars per year. Microemulsions have been used as effective clove oil delivery vehicles and could also be used to develop new extraction processes from clove buds. Eugenol, the main component of clove oil, is a polar oil that behaves as a surfactant and as an oil. This bifunctional behavior makes formulating clove oil microemulsions a challenging task. Here, we used a version of the Hydrophilic–Lipophilic Difference (HLD) + Net-Average Curvature (NAC) model that incorporates the bifunctional polar oil model to predict and fit the phase behavior of lecithin (surfactant) + polyglycerol-10 caprylate (hydrophilic linker) microemulsions using mixtures of heptane and clove oil as the oil phase. Using HLD-NAC parameters from the literature, the predicted HLD-NAC curves reproduced the expected phase transitions and the trends in Eugenol segregation toward the surfactant layer. Using these literature parameters as an initial guess to fit the experimental phase volumes produced accurate calculated phase volumes, and predicted interfacial tensions. This work demonstrates the application of heuristics and databases of HLD-NAC parameters in predicting the complex phase behavior of surfactant–oil–water (SOW) systems.
Full article
(This article belongs to the Collection Feature Papers in Solutions and Liquid Mixtures Research)
►▼
Show Figures

Figure 1
Open AccessArticle
Microheterogeneous Polymeric Solvent Systems
by
Thomas J. Malinski, Ying-Hua Fu, Sopida Thavornpradit, Yu Ching Wong, Yunnuen Avila-Martinez, William Dow and David E. Bergbreiter
Liquids 2025, 5(3), 22; https://doi.org/10.3390/liquids5030022 - 8 Sep 2025
Abstract
This paper shows that low concentrations of either a low-molecular-weight or a recyclable polymeric cosolvent can be used to design recyclable, tunable alkane polymeric solvent systems. We have shown that dyes experience a microheterogeneous environment that is ca. 40–50% like that of a
[...] Read more.
This paper shows that low concentrations of either a low-molecular-weight or a recyclable polymeric cosolvent can be used to design recyclable, tunable alkane polymeric solvent systems. We have shown that dyes experience a microheterogeneous environment that is ca. 40–50% like that of a polar solvent with as little as 0.1 M added cosolvent. Dyes like Nile red or a polyisobutylene (PIB)-bound dansyl fluorophore both detected microheterogeneity in macrohomogeneous mixtures of heptane or a poly(α-olefin) (PAO) with 0.1–2.0 M added polar solvents. H-Bonding cosolvents have greater effects than cosolvents that only interact with dyes by dipole–dipole interactions. Microheterogeneity was also seen when a PIB-bound version of a low-molecular-weight solvent is used as the added polar cosolvent. These microheterogeneous environments can advantageously be used in synthetic and catalytic reactions. This was demonstrated in transesterification and SN2 chemistry. Reactions in PAO solutions polarized by 2 M added THF or by 0.5 M of a PIB-bound HMPA analog both had enhanced reactivity versus reactions in a PAO solution without added cosolvent. In the latter case, the catalyst, the PAO solvent, and the PIB-bound cosolvent were all fully recyclable.
Full article
(This article belongs to the Section Molecular Liquids)
►▼
Show Figures

Figure 1
Open AccessArticle
Entropy-Based Solubility Parameter-Translated Peng–Robinson Equation of State (eSPT-PR EoS)
by
Masaki Ota, Naishu Yang, Hiroyuki Komatsu, Hiroshi Inomata and Richard Lee Smith, Jr.
Liquids 2025, 5(3), 21; https://doi.org/10.3390/liquids5030021 - 25 Aug 2025
Abstract
Peng–Robinson equation of state (PR EoS) has good prediction accuracy for phase diagrams of pure substances or mixtures, but liquid density, especially for high polar substances, is known to be ~20% lower value compared with experimental data at standard atmospheric temperature and pressure
[...] Read more.
Peng–Robinson equation of state (PR EoS) has good prediction accuracy for phase diagrams of pure substances or mixtures, but liquid density, especially for high polar substances, is known to be ~20% lower value compared with experimental data at standard atmospheric temperature and pressure (SATP) conditions. To overcome this issue, translation via entropy-based solubility parameter (eSP) Peng–Robinson EoS (eSPT-PR EoS) is proposed in this work. The technique uses eSP for the liquid phase at SATP conditions and correlates the ideal value and a constant C for each substance as a correction. As a result, the C value can be linearly correlated with critical compressibility factor (ZC). Finally, the liquid density was improved and gave an average relative deviation (ARD) value of 4.2% for the generally used 27 chemicals selected at SATP condition. Furthermore, critical density was also improved and gave ARD values of 3.9% compared with the original PR EoS of 21.8%. Thus, a universal calculation method based on PR EoS was developed for improving liquid density representation with the eSPT-PR EoS.
Full article
(This article belongs to the Special Issue Energy Transfer in Liquids)
►▼
Show Figures

Figure 1
Open AccessArticle
Empirical Rules in Thermochemistry: Overlooked Overestimations of the Liquid- and Crystal-Phase Heat Capacities of α,ω-Alkanediols and Their Consequences
by
Riko Siewert, Vladimir V. Emelyanov, Artemiy A. Samarov, Matthis Richter, Karsten Müller and Sergey P. Verevkin
Liquids 2025, 5(3), 20; https://doi.org/10.3390/liquids5030020 - 13 Aug 2025
Abstract
The utilisation of empirical correlations for the estimation of thermodynamic functions is a valuable approach for reducing experimental effort and for validating existing data. Established correlations and group contribution methods provide reliable heat capacity estimates for simple organic compounds. The present work assesses
[...] Read more.
The utilisation of empirical correlations for the estimation of thermodynamic functions is a valuable approach for reducing experimental effort and for validating existing data. Established correlations and group contribution methods provide reliable heat capacity estimates for simple organic compounds. The present work assesses the extent of deviations introduced by employing conventional heat capacity correlations for diols. For this purpose, heat capacity differences between the solid, liquid and gas phases are evaluated based on experimentally determined vapour pressures, enthalpies of vaporisation, heat capacities in the solid and liquid phases, and quantum chemical calculations. It is demonstrated that the structural characteristics of diols result in a significant overestimation of heat capacities when conventional empirical methods are applied. Deviations in the range of 30–50 J·K−1·mol−1 were observed when compared to consistent experimental data. As part of the evaluation, new group contribution parameters were developed for calculating heat capacities in the solid and liquid phases. Based on these improved data, inconsistencies in literature values for enthalpies of vaporisation (on the order of 10–15 kJ mol−1) could be resolved. Furthermore, a new correlation was derived that allows for the reliable prediction of enthalpies of vaporisation for α,ω-alkanediols from pentanediol to decanediol. The resulting data provide significant advantages for the design of technical processes involving diols as renewable sources and for the accurate modelling of their phase behaviour.
Full article
(This article belongs to the Special Issue Thermodynamics of Molecular Complexation and Hydrogen Bonding in Solution Chemistry—A Themed Issue Honoring Professor Dr. Boris N. Solomonov)
►▼
Show Figures

Figure 1
Open AccessReview
Changes in Thermodynamic Parameters Induced by Pyrimidine Nucleic Bases Forming Complexes with Amino Acids and Peptides in a Buffer Solution at pH = 7.4
by
Elena Yu. Tyunina, Vladimir P. Barannikov and Igor N. Mezhevoi
Liquids 2025, 5(3), 19; https://doi.org/10.3390/liquids5030019 - 22 Jul 2025
Abstract
This article presents a mini-review of the available data on the thermodynamics of the complexation of amino acids and peptides with some nucleic bases in a buffer medium. Data on changes in thermodynamic parameters (binding constants, Gibbs energy, enthalpy, entropy) during the complexation
[...] Read more.
This article presents a mini-review of the available data on the thermodynamics of the complexation of amino acids and peptides with some nucleic bases in a buffer medium. Data on changes in thermodynamic parameters (binding constants, Gibbs energy, enthalpy, entropy) during the complexation of nucleic bases with amino acids and peptides as a function of physicochemical properties are given at T = 298.15 K. The effects of complexation on the volumetric properties of nucleic bases, including apparent molar volumes, standard molar volumes, and limiting molar expansibility, over a temperature range of 288.15 to 313.15 K are considered in detail. Differences in the behavior of amino acids and peptides caused by different modes of coordination with nucleic bases are noted. These manifest in the stoichiometry of the formed complexes, the relationship with the acid dissociation constants of carboxyl and amino groups, enthalpy–entropy compensation in the complexation process, the temperature dependence of the transfer volumes, and the effect of hydrophobicity on volumetric characteristics.
Full article
(This article belongs to the Special Issue Thermodynamics of Molecular Complexation and Hydrogen Bonding in Solution Chemistry—A Themed Issue Honoring Professor Dr. Boris N. Solomonov)
►▼
Show Figures

Figure 1
Open AccessCommunication
Dynamic Behaviors of Concentrated Colloidal Silica Suspensions: Dancing, Bouncing, Solidifying, and Melting Under Vibration
by
Motoyoshi Kobayashi, Takuya Sugimoto, Ryoichi Ishibashi and Shunsuke Sato
Liquids 2025, 5(3), 18; https://doi.org/10.3390/liquids5030018 - 11 Jul 2025
Abstract
Concentrated suspensions exhibit intriguing behaviors under external forces, including vibration and shear. While previous studies have focused primarily on cornstarch suspensions, this paper reports a novel observation that colloidal silica suspensions also exhibit dancing, bouncing, solidification, and melting under vertical vibration. Unlike cornstarch,
[...] Read more.
Concentrated suspensions exhibit intriguing behaviors under external forces, including vibration and shear. While previous studies have focused primarily on cornstarch suspensions, this paper reports a novel observation that colloidal silica suspensions also exhibit dancing, bouncing, solidification, and melting under vertical vibration. Unlike cornstarch, silica particles offer high stability, controlled size distribution, and tunable surface properties, making them an ideal system for investigating these phenomena. The 70 wt.% aqueous suspensions of spherical silica particles with a diameter of 0.55 μm were subjected to controlled vertical vibration (60–100 Hz, 100–500 m/s2). High-speed video analysis revealed dynamic transitions, including melting, fingering, squirming, fragmentation, and jumping. The solidified suspension retained its shape after vibration ceased but melted upon weak vibration. This study demonstrates that such dynamic state transitions are not exclusive to starch-based suspensions but can also occur in well-defined colloidal suspensions. Our findings provide a new platform for investigating shear-thickening, jamming, and vibrational solidification in suspensions with controllable parameters. Further work is required to elucidate the underlying mechanisms.
Full article
(This article belongs to the Section Physics of Liquids)
►▼
Show Figures

Figure 1
Open AccessArticle
Compensation Relationships in the Solvation Thermodynamics of Proton Acceptors in Aliphatic Alcohols
by
Boris N. Solomonov, Mansur B. Khisamiev and Mikhail I. Yagofarov
Liquids 2025, 5(2), 17; https://doi.org/10.3390/liquids5020017 - 13 Jun 2025
Abstract
Solvent association and solute–solvent complexation are known to influence the relationship between the thermodynamic functions of solvation, known as the compensation relationship. Here, we accomplish a series of works devoted to the analysis of Gibbs energy–enthalpy relations in the systems with different capabilities
[...] Read more.
Solvent association and solute–solvent complexation are known to influence the relationship between the thermodynamic functions of solvation, known as the compensation relationship. Here, we accomplish a series of works devoted to the analysis of Gibbs energy–enthalpy relations in the systems with different capabilities of hydrogen bonding. The data on proton acceptors solvated in alcohols were collected, and the quantitative regularities in their solvation thermodynamics were established, depending on the binding degree in solution. The equations connecting the Gibbs energies and enthalpies of solvation in the systems with competition for hydrogen bonding sites were derived from the previously found correlation between the thermodynamic functions of complexation and solvation in simpler solutions. These equations enabled the successful prediction of the solvation enthalpies of 56 proton acceptors in alcohols (RMSD = 1.8 kJ·mol−1). Together with the results of the previous works, the general linear equation connecting the Gibbs energies and enthalpies of solvation in various solute–solvent systems has been obtained. This finding led us to reshaping common understanding of the compensation relationship phenomenon.
Full article
(This article belongs to the Special Issue Thermodynamics of Molecular Complexation and Hydrogen Bonding in Solution Chemistry—A Themed Issue Honoring Professor Dr. Boris N. Solomonov)
►▼
Show Figures

Figure 1
Open AccessArticle
Machine Learning Prediction of Henry’s Law Constant for CO2 in Ionic Liquids and Deep Eutectic Solvents
by
Dmitriy M. Makarov, Yuliya A. Fadeeva and Arkadiy M. Kolker
Liquids 2025, 5(2), 16; https://doi.org/10.3390/liquids5020016 - 30 May 2025
Cited by 3
Abstract
Ionic liquids (ILs) and deep eutectic solvents (DESs) have been extensively studied as absorbents for CO2 capture, demonstrating high efficiency in this role. To optimize the search for compounds with superior absorption properties, theoretical approaches, including machine learning methods, are highly relevant.
[...] Read more.
Ionic liquids (ILs) and deep eutectic solvents (DESs) have been extensively studied as absorbents for CO2 capture, demonstrating high efficiency in this role. To optimize the search for compounds with superior absorption properties, theoretical approaches, including machine learning methods, are highly relevant. In this study, machine learning models were developed and applied to predict Henry’s law constants for CO2 in ILs and DESs, aiming to identify systems with the best absorption performance. The accuracy of the models was assessed in interpolation tasks within the training set and extrapolation beyond its domain. The optimal predictive models were built using the CatBoost algorithm, leveraging CDK molecular descriptors for ILs and RDKit descriptors for DESs. To define the applicability domain of the models, the SHAP-based leverage method was employed, providing a quantitative characterization of the descriptor space where predictions remain reliable. The developed models have been integrated into the web platform chem-predictor, where they can be utilized for predicting absorption properties.
Full article
(This article belongs to the Special Issue Thermodynamics of Molecular Complexation and Hydrogen Bonding in Solution Chemistry—A Themed Issue Honoring Professor Dr. Boris N. Solomonov)
►▼
Show Figures

Figure 1
Open AccessArticle
Measurement and Modelling of Carbon Dioxide in Triflate-Based Ionic Liquids: Imidazolium, Pyridinium, and Pyrrolidinium
by
Raheem Akinosho, Amr Henni and Farhan Shaikh
Liquids 2025, 5(2), 15; https://doi.org/10.3390/liquids5020015 - 30 May 2025
Abstract
►▼
Show Figures
Carbon dioxide, the primary greenhouse gas responsible for global warming, represents today a critical environmental challenge for humans. Mitigating CO2 emissions and other greenhouse gases is a pressing global concern. The primary goal of this study is to investigate the potential of
[...] Read more.
Carbon dioxide, the primary greenhouse gas responsible for global warming, represents today a critical environmental challenge for humans. Mitigating CO2 emissions and other greenhouse gases is a pressing global concern. The primary goal of this study is to investigate the potential of particular ionic liquids (ILs) in capturing CO2 for the sweetening of natural and other gases. The solubility of CO2 was measured in three distinct ILs, which shared a common anion (triflate, TfO) but differed in their cations. The selected ionic liquids were {1-butyl-3-methylimidazolium triflate [BMIM][TfO], 1-butyl-1-methylpyrrolidinium triflate [BMP][TfO], and 1-butyl-4-methylpyridium triflate [MBPY][TfO]}. The solvents were screened based on results from a molecular computational study that predicted low CO2 Henry’s Law constants. Solubility measurements were conducted at 303.15 K, 323.15 K, and 343.15 K and pressures up to 1.5 MPa using a gravimetric microbalance (IGA-003). The CO2 experimental results were modeled using the Peng–Robinson Equation of state with three mixing rules: van der Waals one (vdWI), van der Waals two (vdWII), and the non-random two-liquid (NRTL) Wong–Sandler (WS) mixing rule. For the three ILs, the NRTL-WS mixing rule regressed the data with the lowest average deviation percentage of 1.24%. The three solvents had similar alkyl chains but slightly different polarities. [MBPY][TfO], with the largest size, exhibited the highest CO2 solubility at all three temperatures. Calculation of its relative polarity descriptor (N) shows it was the least polar of the three ILs. Conversely, [BMP][TfO] showed the highest Henry’s Law constant (lowest solubility) across the studied temperature range. Comparing the results to published data, the study concludes that triflate-based ionic liquids with three fluorine atoms had lower capacity for CO2 compared to bis(trifluoromethylsulfonyl) imide (Tf2N)-based ionic liquids with six fluorine atoms. Additionally, the study provided data on the enthalpy and entropy of absorption. A final comparison shows that the ILs had a lower CO2 capacity than Selexol, a solvent widely used in commercial carbon capture operations. Compared to other ILs, the results confirm that the type of anion had a more significant impact on solubility than the cation.
Full article

Figure 1
Open AccessArticle
Molecular Dynamics Study on Complexation of Uranyl and Zinc Ions with Fatty Acid Bound Human Serum Albumin
by
Vijayakriti Mishra, Pramilla D. Sawant and Arup Kumar Pathak
Liquids 2025, 5(2), 14; https://doi.org/10.3390/liquids5020014 - 16 May 2025
Abstract
Nuclear technology, while offering significant benefits across various sectors, poses potential health risks due to uranium (U) contamination, particularly through its internalization and subsequent interactions with biological systems. This study investigates the binding of uranyl (UO22+) and zinc (Zn2+
[...] Read more.
Nuclear technology, while offering significant benefits across various sectors, poses potential health risks due to uranium (U) contamination, particularly through its internalization and subsequent interactions with biological systems. This study investigates the binding of uranyl (UO22+) and zinc (Zn2+) ions to Human Serum Albumin (HSA) that is already bound to fatty acids (FAs), using all-atom molecular dynamics (MD) simulations. The analysis focuses on the structural and dynamic alterations in the protein’s multi-metal binding site (MBS-A) caused by FA binding. Results reveal that FA binding induces a conformational change in HSA, disrupting the pre-formed MBS-A binding site, while still allowing uranyl and zinc ions to interact with residue D249 through strong Coulombic interactions. Secondary binding sites, associated with calcium and zinc binding, remain largely unaffected by FAs, providing alternative coordination for metal ions. This study also explores the binding and unbinding pathways of the metal ions using well-tempered meta-dynamics (WT-MtD), showing that while FA binding disrupts the primary metal binding site, it does not completely inhibit the binding of both uranyl and zinc ions. These findings offer new insights into the nature of uranium’s interactions with blood serum proteins and the role of fatty acids in modulating these interactions, which may help in designing future strategies for managing uranium contamination in biological systems.
Full article
(This article belongs to the Special Issue Thermodynamics of Molecular Complexation and Hydrogen Bonding in Solution Chemistry—A Themed Issue Honoring Professor Dr. Boris N. Solomonov)
►▼
Show Figures

Graphical abstract
Open AccessArticle
Experimental Liquid Densities of Red Palm Oil at Pressures up to 150 MPa from (312 to 352) K and Dynamic Viscosities at 0.1 MPa from (293 to 353) K
by
Jia Lin Lee, Gun Hean Chong, Yuya Hiraga, Yoshiyuki Sato, Masaki Ota and Richard Lee Smith, Jr.
Liquids 2025, 5(2), 13; https://doi.org/10.3390/liquids5020013 - 13 May 2025
Abstract
►▼
Show Figures
Density and viscosity are fundamental properties necessary for processing of red palm oil (RPO). The main fatty acid constituents of RPO were determined to be palmitic acid (C16:0), oleic acid (C18:1), and linoleic acid (C18:2). Rheology measurements
[...] Read more.
Density and viscosity are fundamental properties necessary for processing of red palm oil (RPO). The main fatty acid constituents of RPO were determined to be palmitic acid (C16:0), oleic acid (C18:1), and linoleic acid (C18:2). Rheology measurements confirmed that RPO behaved as a Newtonian fluid. Viscosities and atmospheric densities of RPO were measured at 0.1 MPa and (293 K to 413) K and correlated with the Rodenbush model (0.05% deviation). Dynamic viscosities of RPO were correlated with the Vogel–Fulcher–Tammann model (0.06% deviation) and Doolittle free volume model (0.04% deviation). High-pressure densities of RPO were measured at (10 to 150) MPa and (312 to 352) K. The Tait equation could correlate the high-pressure densities of RPO to within 0.021% deviation and was used to estimate the thermal expansion as 5.1 × 10−4 K−1 (at 312 K, 150 MPa) to 4.8 × 10−4 K−1 (at 352 K, 150 MPa) and isothermal compressibility as 7.3 × 10−4 MPa−1 (at 352 K, 0.1 MPa) to 3.5 × 10−4 MPa−1 (at 352 K, 150 MPa). Parameters for the perturbed-chain statistical associating fluid theory equation of state were determined and gave an average of 0.143% deviation in density. The data and equations developed should be useful in high-pressure food processing as well as in applications considering vegetable oils as heat transfer fluids or as lubricants.
Full article

Figure 1
Open AccessArticle
Prediction of Hydrogen-Bonding Interaction Free Energies with Two New Molecular Descriptors
by
William E. Acree, Jr. and Costas Panayiotou
Liquids 2025, 5(2), 12; https://doi.org/10.3390/liquids5020012 - 17 Apr 2025
Cited by 1
Abstract
This work is a continuation of our recent work on the prediction of hydrogen-bonding (HB) interaction enthalpies. In the present work, a simple method is proposed for the prediction of the HB interaction free energies. Quantum chemical (QC) calculations are combined with the
[...] Read more.
This work is a continuation of our recent work on the prediction of hydrogen-bonding (HB) interaction enthalpies. In the present work, a simple method is proposed for the prediction of the HB interaction free energies. Quantum chemical (QC) calculations are combined with the Linear Solvation Energy Relationship (LSER) approach for the determination of novel QC-LSER molecular descriptors and the development of the method. Each hydrogen-bonded molecule is characterized by an acidity or proton donor capacity,
(This article belongs to the Special Issue Thermodynamics of Molecular Complexation and Hydrogen Bonding in Solution Chemistry—A Themed Issue Honoring Professor Dr. Boris N. Solomonov)
►▼
Show Figures

Figure 1
Open AccessArticle
Enhancing Physiological Realism in Nasal Spray Deposition Studies: Synthetic Mucus Properties and Interactions with Saline Solutions and Stereolithography Resin
by
Amr Seifelnasr, Farhad Zare, Xiuhua Si and Jinxiang Xi
Liquids 2025, 5(2), 11; https://doi.org/10.3390/liquids5020011 - 7 Apr 2025
Cited by 3
Abstract
This study investigated the role of synthetic mucus coatings in enhancing the physiological relevance of in vitro nasal spray deposition assessments using 3D-printed nasal cavity models. Synthetic mucus solutions, representing normal (0.25% w/v xanthan gum) and diseased (1% w/v
[...] Read more.
This study investigated the role of synthetic mucus coatings in enhancing the physiological relevance of in vitro nasal spray deposition assessments using 3D-printed nasal cavity models. Synthetic mucus solutions, representing normal (0.25% w/v xanthan gum) and diseased (1% w/v xanthan gum) nasal conditions, were developed to mimic the viscoelastic properties of human nasal mucus. Their physical properties, including viscosity, surface tension, contact angle, and adhesivity on dry and synthetic mucus-coated stereolithography (SLA) surfaces, were systematically characterized. Comparative experiments evaluated the behavior of saline drops and liquid films on dry versus synthetic mucus-coated SLA surfaces at inclinations of 30°, 45°, and 60°. Observational deposition experiments using anatomically accurate nasal models were conducted under a 45° backward-tilted head position with gentle sniff airflow across uncoated, 0.25% w/v mucus-coated, and 1% w/v mucus-coated surfaces. Synthetic mucus coatings significantly influenced saline spray deposition patterns. On uncoated surfaces, deposition consisted of scattered droplets and limited film formation, mainly in the anterior and turbinate regions. In contrast, synthetic mucus coatings facilitated broader and more uniform liquid distribution due to diffusion and lubrication effects. These findings highlight the value of synthetic mucus coatings for better simulating nasal environments, offering insights to optimize nasal spray formulations and delivery devices.
Full article
(This article belongs to the Section Physics of Liquids)
►▼
Show Figures

Figure 1
Open AccessArticle
Influence of Ion Generation–Recombination on Dielectric Relaxation Time in Electrolytes
by
Ioannis Lelidis and Giovanni Barbero
Liquids 2025, 5(2), 10; https://doi.org/10.3390/liquids5020010 - 3 Apr 2025
Abstract
The well-known Poisson–Nernst–Planck model is a classical approach usedto describe ion transport in liquids. Extended versions of this model account for thegeneration–recombination of ions at equilibrium. In this paper, we investigate the influenceof the generation–recombination term on dielectric relaxation in an electrolytic cell
[...] Read more.
The well-known Poisson–Nernst–Planck model is a classical approach usedto describe ion transport in liquids. Extended versions of this model account for thegeneration–recombination of ions at equilibrium. In this paper, we investigate the influenceof the generation–recombination term on dielectric relaxation in an electrolytic cell shapedlike a slab, bounded by two parallel blocking electrodes. We show that in the adiabaticlimit—which holds when the reaction time is much longer than the dielectric relaxationtime—the electric current in the external circuit does not follow a simple relaxation mechanism.Instead, it is characterized by two distinct relaxation times: a short relaxationtime associated with dielectric relaxation and a longer relaxation time related to the iondissociation–association process. Conversely, this information could be used to assess thepresence and/or significance of the generation–recombination effect in an electrolytic cell.
Full article
(This article belongs to the Section Physics of Liquids)
►▼
Show Figures

Figure 1
Open AccessArticle
Progress in the Understanding of Liquids Dynamics via a General Theory of Correlation Functions
by
Eleonora Guarini, Ubaldo Bafile, Daniele Colognesi, Alessandro Cunsolo, Alessio De Francesco and Ferdinando Formisano
Liquids 2025, 5(2), 9; https://doi.org/10.3390/liquids5020009 - 26 Mar 2025
Cited by 1
Abstract
This work provides a comprehensive picture of the advances that the exponential expansion theory (EET) of autocorrelation functions relevant to liquids dynamics made possible in the last decade up to very recent times. The role of both longitudinal and transverse collective excitations in
[...] Read more.
This work provides a comprehensive picture of the advances that the exponential expansion theory (EET) of autocorrelation functions relevant to liquids dynamics made possible in the last decade up to very recent times. The role of both longitudinal and transverse collective excitations in liquids is investigated by studying the main autocorrelation functions typically obtained either experimentally (when possible) or through molecular dynamics simulations. Examples for some classes of liquids are provided, especially intended for the understanding of dispersion curves, i.e., the collective mode frequencies as a function of the wavevector Q, which is inversely proportional to the length scale at which microscopic processes are probed. The main result of this work is the ubiquitous observation that the EET method works extremely well for all considered autocorrelation functions or spectra, either experimental or simulated. This paper provides also, in its final part, important hints for future research, based on an integration of the EET lineshape description within Bayesian inference analysis.
Full article
(This article belongs to the Section Physics of Liquids)
►▼
Show Figures

Figure 1
Highly Accessed Articles
Latest Books
E-Mail Alert
News
Topics

Special Issues
Special Issue in
Liquids
Energy Transfer in Liquids
Guest Editor: Darin J. UlnessDeadline: 31 December 2025
Special Issue in
Liquids
Electrolytes for High-Performance Energy Storage
Guest Editor: Dmitrii RakovDeadline: 31 October 2026
Special Issue in
Liquids
Hydration of Ions in Aqueous Solution, 2nd Edition
Guest Editor: Cory PyeDeadline: 31 December 2026
Topical Collections
Topical Collection in
Liquids
Feature Papers in Solutions and Liquid Mixtures Research
Collection Editors: Enrico Bodo, Federico Marini