Revision and Extension of a Generally Applicable Group Additivity Method for the Calculation of the Refractivity and Polarizability of Organic Molecules at 298.15 K
Abstract
:1. Introduction
2. Method
2.1. Definition of the Atom Groups
2.2. Calculation of the Atom Group Contributions
2.3. Calculation of the Refractivity
2.4. Cross-Validation Calculations
2.5. Calculation of the Polarizability
3. Sources of Refractivity and Polarizability Data
4. Results
4.1. Refractivity
4.1.1. Ionic Liquids
4.1.2. Silanes, Silanols, Siloxanes, Silazanes, and Silicates
4.1.3. Boranes, Borines, Borazines, Boronates, and Borates
4.2. Polarizability
4.3. Refractivity/Polarizability and Molecular Volume
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naef, R. A Generally Applicable Computer Algorithm Based on the Group Additivity Method for the Calculation of Seven Molecular Descriptors: Heat of Combustion, LogPO/W, LogS, Refractivity, Polarizability, Toxicity and LogBB of Organic Compounds; Scope and Limits of Applicability. Molecules 2015, 20, 18279–18351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denbigh, K.G. The Polarizabilities of Bonds—I. Trans. Faraday Soc. 1940, 36, 936–948. [Google Scholar] [CrossRef]
- Ghose, A.K.; Crippen, G.M. Atomic physicochemical parameters for three-dimensional structure-directed quantitative structure-activity relationships I. Partition coefficients as a measure of hydrophobicity. J. Comput. Chem. 1986, 7, 565–577. [Google Scholar] [CrossRef] [Green Version]
- Miller, K.J.; Savchik, J.A. A new empirical Method to calculate Average Molecular Polarizabilities. J. Am. Chem. Soc. 1979, 101, 7206–7213. [Google Scholar] [CrossRef]
- Miller, K.J. Additivity methods in molecular polarizability. J. Am. Chem. Soc. 1990, 112, 8533–8542. [Google Scholar] [CrossRef]
- Brink, T.; Murray, J.S.; Politzer, P. Polarizability and volume. J. Chem. Phys. 1993, 98, 4305–4306. [Google Scholar] [CrossRef]
- Naef, R. Calculation of the isobaric heat capacities of the liquid and solid phase of organic compounds at and around 298.15 K based on their “True” molecular volume. Molecules 2019, 24, 1626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naef, R.; Acree, W.E. Application of a General Computer Algorithm Based on the Group-Additivity Method for the Calculation of Two Molecular Descriptors at Both Ends of Dilution: Liquid Viscosity and Activity Coefficient in Water at Infinite Dilution. Molecules 2018, 23, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardtwig, E. Fehler- Und Ausgleichsrechnung; Bibliographisches Institut AG: Mannheim, Germany, 1968. [Google Scholar]
- Kim, K.-S.; Shin, B.-K.; Lee, H.; Ziegler, F. Refractive index and heat capacity of 1-butyl-3-methylimidazolium bromide and 1-butyl-3-methylimidazolium tetrafluoroborate, and vapor pressure of binary systems for 1-butyl-3-methylimidazolium bromide + trifluoroethanol and 1-butyl-3-methylimidazolium tetrafluoroborate + trifluoroethanol. Fluid Phase Equil. 2004, 218, 215–220. [Google Scholar] [CrossRef]
- Lide, D.R. (Ed.) Physical Constants of Organic Compounds. In CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2005; pp. 3-1–3-740. [Google Scholar]
- Ghose, A.K.; Crippen, G.M. Atomic Physicochemical Parameters for Three-Dimensional-Structure-Directed Quantitative Structure-Activity Relationships. 2. Modeling Dispersive and Hydrophobic Interactions. J. Chem. Inf. Comput. Sci. 1987, 27, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Perlman, D.; Davidson, D.; Bogert, M.T. The Synthesis of Phenanthrenes from Hydroxyl Derivatives of beta-Phenylethylcyclohexanes and the Nature of the By-Product. J. Org. Chem. 1936, 01, 288–299. [Google Scholar] [CrossRef]
- Drake, N.L.; Welsh, L.H. 2,2,3,4-Tetramethylhexane and 3,3,5-trimethylheptane. J. Am. Chem. Soc. 1938, 60, 488–489. [Google Scholar] [CrossRef]
- Petrov, A.D.; Baidanov, A.P.; Zakotin, N.N.; Suntsov, P.I. The synthesis and properties of α-methylheptylbenzene, α-butylamylbenzene and α-hexylheptylbenzene. Zh. Obsh. Khim. 1939, 9, 509–512. [Google Scholar]
- Matui, E. Styrene substitutes and their polymers. II. p-Ethylstyrene and its polymers. Kogyo Kagaku Zasshi 1941, 44, 107–108. [Google Scholar]
- Matui, E. Substituted styrenes and their polymers. III. p-Isopropylstyrene and its polymer. Kogyo Kagaku Zasshi 1941, 44, 284–286. [Google Scholar]
- Petrov, A.D.; Pavlov, A.M.; Makarov, Y.A. Synthesis of 3-ethyldecane and of 2,5-dimethylhendecane. Zh. Obsh. Khim. 1941, 2, 1104–1106. [Google Scholar]
- Petrov, A.D.; Chel’tsova, M.A. Synthesis and properties of hydrocarbons of aromatic and naphthenic series of the composition C19-C26. II. Zh. Obsh. Khim. 1942, 12, 87–94. [Google Scholar]
- Petrov, A.D.; Kaplan, E.P. Synthesis and properties of isoparaffin hydrocarbons of the composition C12–C22. II. Zh. Obsh. Khim. 1942, 12, 99–103. [Google Scholar]
- Petrov, A.D.; Shchupina, Z.K.; Ol’dekop, Y.A. Synthesis of 9,10-dimethyloctadecane and 9,10-di-propyloctadecane. Zh. Obsh. Khim. 1944, 14, 490–500. [Google Scholar]
- Petrov, A.D.; Vittikh, M.V. Synthesis and properties of isoparaffinic hydrocarbons of the composition C13–C24. Izvest. Akad. Nauk SSSR Ser. Khim. 1944, 238–242. [Google Scholar]
- Petrov, A.D.; Krutov, K.M.; Khrenov, I.M. Synthesis and properties of cyclohexylhexylmethanol and 3-cyclohexyl-2-methylnonane. Zh. Obsh. Khim. 1945, 15, 799–801. [Google Scholar]
- Lunshof, H.J.; van Stenis, J.; Waterman, H.I. Preparation of some Physical Constants of 2-Methyltetradecane and 3-Methylpentadecane. Rec. Trav. Chim. Pays Bas 1947, 66, 348–352. [Google Scholar] [CrossRef]
- Petrov, A.D.; Ol’dekop, Y.A. Synthesis and properties of higher isoparaffin hydrocarbons of composition C20-C34 (7,8-diisopropyltetradecane, 7,8-diisoamyltetradecane, 10,11-dipropyleicosane, 11,12-dipropyldocosane, 9,10-dioctyloctadecane, and 9,10,11,12-tetrapropyleicosane). Zh. Obsh. Khim. 1948, 18, 859–864. [Google Scholar]
- Petrov, A.D.; Kaplan, E.P. The synthesis and the physical properties of C22-branched hydrocarbons. Izvest. Akad. Nauk SSSR Ser. Khim. 1949, 539–544. [Google Scholar]
- Romadane, I. Alkylation of naphthalene with iso alcohols in the presence of boron trifluoride. Zh. Obsh. Khim. 1957, 27, 1939–1941. [Google Scholar]
- Petrov, A.A.; Mingaleva, K.S.; Kupin, B.S. Dipole moments and reactivity of vinylacetylenic hydrocarbons. Dokl. Akad. Nauk SSSR 1958, 123, 298–300. [Google Scholar]
- Terres, E.; Brinkmann, L.; Fischer, D.; Hüllstrung, D.; Lorz, W.; Weisbrod, G. Synthese und physikalische Daten einiger Isoparaffinreihen mit 11 bis 24 C-Atomen. Brennstoff Chem. 1959, 40, 279–280. [Google Scholar]
- Petrov, A.A.; Sergienko, S.R.; Nechitailo, N.A.; Tsedilina, A.L. Synthesis and properties of C12—C16 monomethylalkanes. Russ. Chem. Bull. 1959, 8, 1091–1097. [Google Scholar] [CrossRef]
- Leibnitz, E.; Hager, W.; Winkler, R. Studien zur Chemie der Paraffine und Paraffingatsche. V. Synthese und physikalische Eigenschaften einiger 4n-Propyl- und 2-Methyl-3-isopropyl-Alkane mit mehr als 10 C-Atomen. J. Prakt. Chem. 1959, 9, 275–288. [Google Scholar] [CrossRef]
- Levina, R.Y.; Kostin, V.N.; Gembitskii, P.A.; Treshchova, E.G. Cyclopropanes and cyclobutanes. XVII. Reduction of arylcyclopropanes with metals in liquid ammonia and with methyl alcohol. Zh. Obsh. Khim. 1960, 31, 829–836. [Google Scholar]
- Terres, E.; Paulsen, S.R.; Huellstrung, D. Isoparaffins. Erdoel Kohle 1960, 13, 323–325. [Google Scholar]
- Xiaomei, Q.; Xiaofang, C.; Yongsheng, G.; Li, X.; Shenlin, H.; Wenjun, F. Density, Viscosity, Surface Tension, and Refractive Index for Binary Mixtures of 1,3-Dimethyladamantane with Four C10 Alkanes. J. Chem. Eng. Data 2014, 59, 775–783. [Google Scholar]
- Skita, A.; Faust, W. Velocities of formation of the stereomeric methylcyclohexanols. Ber. Dt. Chem. Ges. 1931, 64B, 2878–2892. [Google Scholar] [CrossRef]
- Viktorova, E.A.; Shuikin, N.I.; Karakhanov, E.A. Catalytic alkylation of p-cresol by dipropenyl. Izvest. Akad. Nauk SSSR Ser. Khim. 1963, 12, 2226–2227. [Google Scholar] [CrossRef]
- Viktorova, E.A.; Karakhanov, E.A.; Shuikin, A.N.; Shuikin, N.I. Alkylation of phenols by compounds with two functions. II. Alkylation of p-cresol by diene hydrocarbons with isolated double bonds. Izvest. Akad. Nauk SSSR Ser. Khim. 1966, 3, 523–527. [Google Scholar]
- Viktorova, E.A.; Shuikin, N.I.; Karakahanov, E.A. Alkylation of phenols with bifunctional compounds. XII. Catalytic alkenylation of o- and p-cresols with butadiene. Izvest. Akad. Nauk SSSR Ser. Khim. 1966, 5, 915–918. [Google Scholar]
- Mori, S. Response Correction of Differential Refractometer for Polyethylene Glycols in Size Exclusion Chromatography. Anal. Chem. 1978, 50, 1639–1643. [Google Scholar] [CrossRef]
- Teregulova, G.T. Synthesis of 1,3-dioxolanes containing aromatic fragments. Zh. Priklad Khim. 1990, 63, 1383–1386. [Google Scholar]
- Crespo, E.A.; Costa, J.M.L.; Hanafiah, Z.B.M.A.; Kurnia, K.A.; Oliveira, M.B.; Lovell, F.; Vega, L.F.; Carvalho, P.J.; Coutinho, J.A.P. New measurements and modeling of high pressure thermodynamic properties of glycols. Fluid Phase Equil. 2017, 436, 113–123. [Google Scholar] [CrossRef]
- Chaudhary, N.; Nain, A.K. Densities, speeds of sound, refractive indices, excess and partial molar properties of polyethylene glycol 200 + benzyl methacrylate binary mixtures at temperatures from 293.15 to 318.15 K. J. Mol. Liq. 2021, 346, 117923. [Google Scholar] [CrossRef]
- Mottier, M. Sur La Méthylene-Pyrocatéchine. Arch. Sci. Phys. Nat. 1935, 17, 289–291. [Google Scholar]
- Jacobs, T.L.; Cramer, R.; Hanson, J.E. Acetylenic ethers. II. Ethoxy- and butoxyacetylene. J. Am. Chem. Soc. 1942, 64, 223–226. [Google Scholar] [CrossRef]
- Dandegaonker, S.H.; Gerrard, W.; Lappert, M.F. Reactions of phenylboron dichloride with ethers. J. Chem. Soc. 1957, 2893–2897. [Google Scholar] [CrossRef]
- Kalabina, A.V.; Shergina, S.I.; Shergina, N.I. Synthesis and properties of the cis and trans isomers of β-bromo vinyl aryl ethers. Izvest. Vyssnikh Ucheb. Zavedenii Khim Khim. Tekhnol. 1959, 2, 545–549. [Google Scholar]
- Dai, F.; Xin, K.; Song, Y.; Shi, M.; Yu, Y.; Li, Q. Liquid-liquid equilibria for the ternary system containing 1-Butanol + methoxy-(methoxymethoxy)methane + water at temperatures of 303.15, 323.15 and 343.15 K. Fluid Phase Equil. 2016, 409, 466–471. [Google Scholar] [CrossRef]
- Berinde, Z.M. QSPR Models for the Molar Refraction, Polarizability and Refractive Index of Aliphatic Carboxylic Acids Using the ZEP Topological Index. Symmetry 2021, 13, 2359. [Google Scholar] [CrossRef]
- West, C.D. Crystal Form of Sucrose Octaacetate. J. Am. Chem. Soc. 1941, 63, 630. [Google Scholar] [CrossRef]
- Rehberg, C.E.; Faucette, W.A. Preparation and Polymerization of Cycloalkyl Acrylates. J. Am. Chem. Soc. 1950, 72, 4307. [Google Scholar] [CrossRef]
- Alexander, E.R.; Busch, H.M. A convenient synthesis of orthoformic esters. J. Am. Chem. Soc. 1952, 74, 554–555. [Google Scholar] [CrossRef]
- Satta, V.; Fein, M.L.; Filachtone, E.M. Some Esters of Unsaturated Acids. J. Am. Chem. Soc. 1953, 75, 4101. [Google Scholar] [CrossRef]
- Arbuzov, B.A.; Shavsha-Tolkacheva, T.G. Dipole moments of esters of orthopropionic and orthoformic acids. Russ. Chem. Bull. 1954, 3, 525–530. [Google Scholar] [CrossRef]
- Shigley, J.W.; Bonhorst, C.W.; Liang, C.C.; Althouse, P.M.; Triebold, H.O. Physical Characterization of a) a Series of Ethyl Esters and b) a Series of Ethanoate Esters. J. Am. Oil Chem. Soc. 1955, 32, 213–215. [Google Scholar] [CrossRef]
- Grzeskowiak, R.; Jeffrey, G.H.; Vogel, A.I. Physical Properties and Chemical Constitution. Part XXIX. Acetylenic Compounds. J. Chem. Soc. 1960, 4719–4722. [Google Scholar] [CrossRef]
- Mekhtiev, S.D.; Sharifova, S.M.; Smirnova, V.P. Esterification of terephthalic and isophthalic acids by aliphatic alcohols. Azerbaidzhan. Khim. Zh. 1965, 3, 67–72. [Google Scholar]
- Freidlin, G.N.; Bushinskii, V.I. Physical Properties of Monoalkyl Esters of Adipic Acid. Zh. Prikl. Khim. 1971, 44, 944–945. [Google Scholar]
- Ortega, J. Measurements of Excess Enthalpies of {a Methyl n-Alkanoate (from n-Hexanoate to n-Pentadecanoate) + n-Pentadeane} at 298.15 K. J. Chem. Thermodyn. 1990, 22, 1165–1170. [Google Scholar] [CrossRef]
- De Lorenzi, L.; Fermeglia, M.; Torriani, G. Density, Refractive Index, and Kinematic Viscosity of Diesters and Triesters. J. Chem. Eng. Data 1997, 42, 919–923. [Google Scholar] [CrossRef]
- De Lorenzi, L.; Fermeglia, M.; Torriani, G. Density, Kinematic Viscosity, and Refractive Index for Bis(2-ethylhexyl) Adipate, Tris(2-ethylhexyl) Trimellitate, and Diisononyl Phthalate. J. Chem. Eng. Data 1998, 43, 183–186. [Google Scholar] [CrossRef]
- Oswal, S.L.; Oswal, P.; Modi, P.S.; Dave, J.P.; Gardas, R.L. Acoustic, volumetric, compressibility and refractivity properties and Flory’s reduction parameters of some homologous series of alkyl alkanoates from 298.15 to 333.15 K. Thermochim. Acta 2004, 410, 1–14. [Google Scholar] [CrossRef]
- Anton, V.; Munoz-Embid, J.; Gascon, I.; Artal, M.; Lafuente, C. Thermophysical Characterization of Furfuryl Esters: Experimental and Modeling. Energy Fuels 2017, 31, 4143–4154. [Google Scholar] [CrossRef]
- Bogdanova, A.V.; Shostakovskii, M.F.; Plotnikova, G.I. Synthesis of unsaturated ether acetals, thioether acetals, and mercaptals. Dokl Akad. Nauk SSSR 1960, 134, 587–590. [Google Scholar]
- Makin, S.M.; Sudakova, V.S. Telomerization of vinyl ethyl ether with acetaldehyde acetal. Synthesis of 1-alkoxypolyenes. Zh. Obsh. Khim. 1962, 32, 3161–3166. [Google Scholar]
- Vogel, A.I. Physical Properties and Chemical Constitution. Part XI. Ketones. J. Chem. Soc. 1948, 610–615. [Google Scholar] [CrossRef]
- Overberger, C.G.; Frazier, C.; Mandelman, J.; Smith, H.F. The Preparation and Polymerization of p-Alkylstyrenes. Effect of Structure on the Transition Temperatures of the Polymers. J. Am. Chem. Soc. 1953, 75, 3326–3330. [Google Scholar] [CrossRef]
- Medwedew, S.S.; Alexejewa, E.N.; Organische Peroxyde, I. Mitteil.: Propyl- und Isopropyl-hydroperoxyd. Ber. Dt. Chem. Ges. 1932, 65, 133–137. [Google Scholar] [CrossRef]
- Harris, E.J. Decomposition of alkyl peroxides: Propyl peroxide, ethyl hydrogen peroxide and propyl hydrogen peroxide. Proc. R. Soc. A 1939, 173, 126–146. [Google Scholar] [CrossRef]
- Milas, N.A.; Surgenor, D.M. Organic peroxides. X. t-Amyl hydroperoxide and di-t-amyl peroxide. J. Am. Chem. Soc. 1946, 68, 643–644. [Google Scholar] [CrossRef]
- Lindstrom, E.G. Preparation of normal and secondary butyl hydroperoxides. J. Am. Chem. Soc. 1953, 75, 5123–5124. [Google Scholar] [CrossRef]
- Williams, H.R.; Mosher, H.S. Peroxides. I. n-Alkyl Hydroperoxides. J. Am. Chem. Soc. 1954, 76, 2984–2987. [Google Scholar] [CrossRef]
- Sanz, L.F.; Gonzalez, J.A.; de la Garcia Fuente, I.; Cobos, J.C. Thermodynamics of mixtures with strongly negative deviations from Raoult’s law. XII. Densities, viscosities and refractive indices at T = (293.15 to 303.15) K for (1-heptanol, or 1-decanol + cyclohexylamine) systems. Application of the ERAS model to (1-alkanol + cyclohexylamine) mixtures. J. Chem. Thermodyn. 2015, 80, 161–171. [Google Scholar] [CrossRef]
- Ioffe, B.V. Synthesis of unsymmetric dialkylhydrazines. Zh. Obsh. Khim. 1958, 28, 1296–1302. [Google Scholar]
- Legrand, R. Dimethylaminobenzylidenemalononitrile. Bull. Soc. Chim. Belges 1944, 53, 166–177. [Google Scholar]
- Paul, R.; Tchelitcheff, S. Synthesis of w-dinitriles and w-chlorinated nitriles from acetonitrile. Bull. Soc. Chim. Fr. 1949, 16, 470–475. [Google Scholar]
- Kuhn, L.P.; DeAngelis, L. The thermal decomposition of dinitrites. I. Vicinal dinitrites. J. Am. Chem. Soc. 1954, 76, 328–329. [Google Scholar] [CrossRef]
- Toops, E.E., Jr. Physical Properties of Eight High-Purity Nitroparaffins. J. Phys. Chem. 1956, 60, 304–306. [Google Scholar] [CrossRef]
- Oswal, S.L.; Oswal, P.; Gardas, R.L.; Patel, S.G.; Shinde, R.G. Acoustic, volumetric, compressibility and refractivity properties and reduction parameters for the ERAS and Flory models of some homologous series of amines from 298.15 to 328.15 K. Fluid Phase Equil. 2004, 216, 33–45. [Google Scholar] [CrossRef]
- McCusker, P.A.; Ashby, E.C.; Makowski, H.S. Organoboron compounds. III. Preparation and properties of alkyldichloroboranes. J. Am. Chem. Soc. 1957, 79, 5182–5184. [Google Scholar] [CrossRef]
- Christopher, P.M.; Tully, T.J. Some Octet and Bond Refractivities Involving Boron. J. Am. Chem. Soc. 1958, 80, 6516–6519. [Google Scholar] [CrossRef]
- Aubrey, D.W.; Lappert, M.F. 586. Cyclic organic boron compounds. Part IV. B-amino- and B-alkoxy-borazoles and their precursors the tris(primary amino)borons and (primary amino)boron alkoxides. J. Chem. Soc. 1959, 2927–2931. [Google Scholar] [CrossRef]
- Mikhailov, B.M.; Bazhenova, A.V. Organoboron compounds. 29. Cyclohexaneboronic acid and its derivatives. Russ. Chem. Bull. 1959, 8, 68–71. [Google Scholar] [CrossRef]
- Mikhailov, B.M.; Bubnov, Y.N. Organoboron compounds. XXXVIII. Reaction of trialkylboron with sulfur. Synthesis of esters of dialkylthioboronic acids. Zh. Obsh. Khim. 1959, 29, 1648–1650. [Google Scholar]
- Mikhailov, B.M.; Aronovich, P.M. Orgonoboron compounds. XXXV. Alkylphenylboronic acids and their anhydrides. Zh. Obsh. Khim. 1959, 29, 1257–1262. [Google Scholar]
- Mikhailov, B.M.; Shchegoleva, T.A. Synthesis of bis(alkylthio) boranes and trialkyl thioborates. Izvest. Akad. Nauk SSSR Ser. Khim. 1959, 8, 1868. [Google Scholar]
- Mikhailov, B.M.; Shchegoleva, T.A.; Blokhina, A.N. Reaction of tetra-n-butylmercaptodiborane with unsaturated compounds. Russ. Chem. Bull. 1960, 9, 1218–1219. [Google Scholar] [CrossRef]
- Mikhailov, B.M.; Shchegoleva, T.A. Synthesis and some transformations of alkylthiodiboranes. Dokl. Akad. Nauk SSSR 1960, 131, 834–836. [Google Scholar]
- Mikhailov, B.M.; Dorokhov, V.A. Organoboron compounds. LXXXVI. Alkylthio(diethylamino) boranes. Zh. Obsh. Khim. 1961, 31, 3750–3756. [Google Scholar]
- Mikhailov, B.M.; Bubnov, Y.N. Organoboron compounds. LXV. Synthesis of esters of dialkylthioboronic acids by the action of mercaptans on trialkylboron. Zh. Obsh. Khim. 1961, 31, 160–166. [Google Scholar]
- Shchegoleva, T.A.; Belyavskaya, E.M. Organoboron compounds. Synthesis and some properties of tris(ethylthio) diborane. Dokl Akad. Nauk SSSR 1961, 136, 638–641. [Google Scholar]
- Mikhailov, B.M.; Shchegoleva, T.A.; Shashkova, E.M. The synthesis of esters of alkyl thioboronic acids from trialkylboron and thioborates. Russ. Chem. Bull. 1961, 10, 845–847. [Google Scholar] [CrossRef]
- Mikhailov, B.M.; Kozminskaya, T.K. Organoboron Compounds. 90. Alkanehalothioboronic esters. Russ. Chem. Bull. 1962, 11, 234–237. [Google Scholar] [CrossRef]
- Mikhailov, B.M.; Dorokhov, V.A. Organoboron compounds. XCIV. Bis(dialkylamino) boranes, and bis(monoalkylamino) boranes. Zh. Obsh. Khim. 1962, 32, 1511–1514. [Google Scholar]
- Mikhailov, B.M.; Fedotov, N.S. The mechanism of nucleophilic substitution at the boron atom in organoboron compounds. Dokl. Akad. Nauk SSSR 1964, 154, 1128–1131. [Google Scholar]
- Mikhailov, B.M.; Vasil’ev, L.S. Organoboron compounds. CLII. Mutual exchange of alkoxy and alkylthio groups in organoboron compounds. Zh. Obsh. Khim. 1965, 35, 1073–1078. [Google Scholar]
- Zakharkin, L.I.; Kovredov, A.I. Compounds produced from products of 1,3-butadiene hydroboronation. Zh. Obsh. Khim. 1966, 36, 2153–2170. [Google Scholar]
- Jackson, I.K.; Davies, W.C.; Jones, W.J. Tertiary arylalkylphosphines. I. J. Chem. Soc. 1930, 2298–2301. [Google Scholar] [CrossRef]
- Kosolapoff, G.M. Isomerization of alkyl phosphites. III. Synthesis of alkylphosphonic acids. J. Am. Chem. Soc. 1945, 67, 1180–1182. [Google Scholar] [CrossRef]
- Jones, W.J.; Davies, W.C.; Bowden, S.T.; Edwards, C.; Davis, V.E.; Thomas, L.H. Preparation and properties of allyl phosphines, arsines, and stannanes. J. Chem. Soc. 1947, 1446–1450. [Google Scholar] [CrossRef]
- Knunyants, I.L.; Sterlin, R.N. Reactions between organic oxides and phosphine. Comptes Rendus (Dokl.) Acad. Des Sci. URSS 1947, 56, 49–52. [Google Scholar]
- Fox, R.B. Organophosphorus compounds. Alkyldichlorophosphines. J. Am. Chem. Soc. 1950, 72, 4147–4149. [Google Scholar] [CrossRef]
- Razumov, A.I.; Mukhacheva, O.A.; Khen, S.-D. Certain alkylphosphonothionic, alkylphosphonoselenonic, dialkylphosphinic, and alkylphosphonous esters, and the mechanism of addition to alkylphosphonous esters. Russ. Chem. Bull. 1952, 1, 797–802. [Google Scholar] [CrossRef]
- Pudovik, A.N.; Yarmukhametova, D.K. New synthesis of esters of phosphonic and thiophosphonic acids. XV. Addition of esters of phenyl- and alkylphosphonous acids to esters of methacrylic and acrylic acids. Izvest. Akad. Nauk SSSR Ser. Khim. 1952, 902–907. [Google Scholar]
- Yakubovich, A.Y.; Motsarev, G.V. Synthesis of hetero-organic compounds of the aromatic series by the reaction of arylsilanes with aluminum chloride and halides of various elements. I. Organophosphorus compounds. Zh. Obsh. Khim. 1953, 23, 1547–1552. [Google Scholar]
- Arbuzov, B.A.; Rizpolozhenskii, N.I. Esters of diethylphosphinous acid. Dokl. Akad. Nauk SSSR 1953, 89, 291–292. [Google Scholar]
- Anlsimov, K.N.; Nesmeyanov, A.N. Derivatives of unsaturated phosphonic acids. Russ. Chem. Bull. 1955, 4, 915–917. [Google Scholar] [CrossRef]
- Anisimov, K.N.; Kolobova, N.E.; Nesmeyanov, A.N. Derivatives of unsaturated phosphonic acids. IX. Neutral esters of 2-alkoxy (or phenoxy)vinylthiophosphonic acids. Izvest. Akad. Nauk SSSR Ser. Khim. 1955, 669–671. [Google Scholar]
- Razumov, A.I.; Mukhacheva, O.A. Derivatives of alkylphosphonous and dialkylphosphinic acids. III. Atomic refraction of phosphorus in esters of alkylphosphonous acids. Zh. Obsh. Khim. 1956, 26, 1436–1440. [Google Scholar]
- Razumov, A.I.; Mukhacheva, O.A. Derivatives of alkylphosphonous and dialkylphosphinic acids. IV. Reactions of addition and isomerization of esters of alkylphosphonous acids. Zh. Obsh. Khim. 1956, 26, 2463–2468. [Google Scholar]
- Anisimov, K.N.; Nesmeyanov, A.N. Derivatives of unsaturated phosphonic acids. XVII. Derivatives of β-phenylvinylphosphonic acid. Izvest. Akad. Nauk SSSR Ser. Khim. 1956, 19–22. [Google Scholar]
- Anisimov, K.N.; Kolobova, N.E.; Nesmeyanov, A.N. Derivatives of unsaturated phosphonic acids. XVIII. Chlorides of alkylthiovinylphosphonic acids and their derivatives. Izvest. Akad. Nauk SSSR Ser. Khim. 1956, 23–26. [Google Scholar]
- Lenard-Borecka, B.; Michalski, J. Organophosphorus compounds of sulfur and selenium. VII. Dialkoxyphosphinylsulfenyl chlorides. Rocz. Chem. 1957, 31, 1167–1176. [Google Scholar]
- Razumov, A.I.; Mukhacheva, O.A.; Markovich, E.A. Derivatives of alkylphosphonous and phosphonic acids. VIII. Synthesis and properties of some alkylated amides of alkylphosphonic chlorides. Zh. Obsh. Khim. 1958, 28, 194–197. [Google Scholar]
- Yamasaki, T. Preparation and properties of alkyl phosphonothionates, (RO)2P(S)H. Sci. Repts. Res. Insts. Tohoku Univ. Ser. A 1959, 11, 73–79. [Google Scholar]
- Kukhtin, V.A.; Abramov, V.S.; Orekhova, K.M. Rearrangement of esters of x-hydroxyalkylphosphonic acids into isomeric phosphates. Dokl. Akad. Nauk SSSR 1959, 128, 1198–1200. [Google Scholar]
- Grechkin, N.P.; Shagidullin, R.R. Organophosphorus derivatives of ethylenimine. Russ. Chem. Bull. 1960, 9, 1978–1982. [Google Scholar] [CrossRef]
- Grechkin, N.P.; Shagidullin, R.R. Organophosphorus derivatives of ethylenimine. III. Addition of acids to ethylenamides of phosphorus acids. Izvest. Akad. Nauk SSSR Ser. Khim. 1960, 2135–2139. [Google Scholar]
- Stolzer, C.; Simon, A. Fluorophosphorus compounds. III. Symmetrical diphosphoryl difluoride dichloride, P2O3Cl2F2. Chem. Ber. 1961, 94, 1976–1979. [Google Scholar] [CrossRef]
- Pass, F.; Steininger, E.; Zorn, H. Organic phosphorus compounds III. A new method for the preparation of primary phosphines. Monats. Chem. 1962, 93, 230–236. [Google Scholar] [CrossRef]
- Pass, F.; Steininger, E.; Zorn, H. Eine neue Methode zur Darstellung primärer Phosphine. Mon. Chem. Und Verwandte Teile And. Wiss. 1962, 93, 230–236. [Google Scholar] [CrossRef]
- Kabachnik, M.I.; Tsvetkov, E.N. Lower dialkylphosphinous acids (secondary phosphine oxides) and some of their properties. Russ. Chem. Bull. 1963, 12, 1120–1124. [Google Scholar] [CrossRef]
- Boerner, K.B.; Stoelzer, C.; Simon, A. Fluorophosphorus compounds. IX. The catalytic hydrogenation of fluorophosphoric acid phenyl esters. Chem. Ber. 1963, 96, 1328–1334. [Google Scholar]
- Stoelzer, C.; Simon, A. Fluorophosphorus compounds. VI. Alkylamides of fluorodiphosphoric acids. Chem. Ber. 1963, 96, 881–895. [Google Scholar]
- Stoelzer, C.; Simon, A. Fluorophosphorus compounds. X. Results of refractometric investigations of fluorophosphoric acid derivatives. Chem. Ber. 1963, 96, 1335–1340. [Google Scholar]
- Arbuzov, B.A.; Vinokurova, G.M. Synthesis of bffunctional organophosphorus compounds. II. Addition of butylphosphine to unsaturated compounds. Izvest. Akad. Nauk SSSR Ser. Khim. 1963, 3, 502–506. [Google Scholar]
- Voigt, D.; Labarre, M.C. Synthesis and magneto-optical study of some trialkylated trithiophosphites. Compt. Rend. 1964, 259, 4632–4634. [Google Scholar]
- Zhmurova, I.N.; Voitsekhovskaya, I.Y. Alkyltetrachlorophosphoranes. Zh. Obsh. Khim. 1965, 35, 2197–2200. [Google Scholar]
- Grishina, O.N.; Bezzubova, L.M. Alkylthiophosphine sulfides. III. O-Alkyl alkylphosphonodithioates. Izvest. Akad. Nauk SSSR Ser. Khim. 1966, 9, 1617–1620. [Google Scholar]
- Neimysheva, A.A.; Knunyants, I.L. Nucleophilic displacement in the series of derivatives of acids of phosphorus. I. Kinetics of hydrolysis of chlorides of di-alkylphosphinic acids. Zh. Obsh. Khim. 1966, 36, 1090–1098. [Google Scholar]
- Foxton, A.A.; Jeffrey, G.H.; Vogel, A.I. Physical properties and chemical constitution. Part XLIX. The refractivities, densities, and surface tensions of some organophosphorus compounds. J. Chem. Soc. A 1966, 249–253. [Google Scholar] [CrossRef]
- Akamsin, V.D.; Rizpolozhenskii, N.I. Esters of phosphorus(III) thioacids. V. New method for preparation of thiophosphinous acid esters. Izvest. Akad. Nauk SSSR Ser. Khim. 1967, 9, 1987–1989. [Google Scholar]
- Buina, N.A.; Nuretdinov, I.A.; Grechkin, N.P. Ethylenimides of arylphosphorous and thiophosphoric acids. Izvest. Akad. Nauk SSSR Ser. Khim. 1967, 1, 217–220. [Google Scholar] [CrossRef]
- Voigt, D.; Turpin, R.; Torres, M. Magnetooptical study of some dialkylphosphines. Comptes Rendus. Seances Acad. Sci. Ser. C Sc. Chim. 1967, 265, 884–887. [Google Scholar]
- Grishina, O.N.; Potekhina, M.I. Synthesis of O-alkylalkyldithiophosphinic aids from products of oxidative phosphination of hydrocarbons of petroleum fractions. Neftekhim 1968, 8, 111–117. [Google Scholar]
- Kas’yanova, E.F.; Gurvich, S.M. Synthesis of some derivatives of monothiophosphoric acid for the flotation of ores of heavy nonferrous metals. Zh. Obsh. Khim. 1969, 39, 365–366. [Google Scholar]
- Fushimi, T.; Allcock, H.R. Cyclotriphosphazenes with sulfur-containing side groups: Refractive index and optical dispersion. Dalton Trans. 2009, 14, 2477–2481. [Google Scholar] [CrossRef]
- Noller, C.R.; Gordon, J.J. The Preparation of Some Higher Aliphatic Sulfonic Acids. J. Am. Chem. Soc. 1933, 55, 1090–1094. [Google Scholar] [CrossRef]
- Allen, P., Jr. The Preparation of Some Normal Aliphatic Thiocyanates. J. Am. Chem. Soc. 1935, 57, 198–199. [Google Scholar] [CrossRef]
- Post, H.W. The reaction of certain orthoesters with aldehydes. J. Org. Chem. 1940, 5, 244–249. [Google Scholar] [CrossRef]
- Hall, W.P.; Reid, E.E. A Series of α,w-Dimercaptans. J. Am. Chem. Soc. 1943, 65, 1466–1468. [Google Scholar] [CrossRef]
- Whitehead, E.V.; Dean, R.A.; Fidler, F.A. The preparation and properties of sulfur compounds related to petroleum. II. Cyclic sulfides. J. Am. Chem. Soc. 1951, 73, 3632–3635. [Google Scholar] [CrossRef]
- Cairns, T.L.; Evans, G.L.; Larchar, A.W.; McKusick, B.C. Gem-Dithiols. J. Am. Chem. Soc. 1952, 74, 3982–3989. [Google Scholar] [CrossRef]
- Birch, S.F.; Cullum, T.V.; Dean, R.A. The preparation and properties of dialkyl di- and polysulfides. Some disproportionation reactions. J. Inst. Pet. 1953, 39, 206–219. [Google Scholar]
- Cope, A.C.; Farkas, E. Cleavage of carbon-sulfur bonds by catalytic hydrogenation. J. Org. Chem. 1954, 19, 385–390. [Google Scholar] [CrossRef]
- Backer, H.J.; Kloosterziel, H. Thiolsulfinic esters. Rec. Trav. Chim. Pays Bas Belg. 1954, 73, 129–139. [Google Scholar] [CrossRef]
- Kabachnik, M.I.; Golubeva, E.I. Addition of sulfur to dialkyl phosphites. Dokl. Akad. Nauk SSSR 1955, 105, 1258–1261. [Google Scholar]
- Haines, W.E.; Helm, B.V.; Cook, G.L.; Ball, J.S. Purification and Properties of Ten Organic Sulfur Compounds—Second Series. Phys. Chem. 1956, 60, 549–555. [Google Scholar] [CrossRef]
- Freidlina, R.K.; Chukovskaya, E.T. Reaction of mercuric acetate with esters of xanthic acids. Izvest. Akad. Nauk SSSR Ser. Khim. 1957, 6, 187–193. [Google Scholar]
- Boonstra, H.J.; Brandsma, L.; Wiegman, A.M.; Arens, J.F. Chemistry of acetylenic ethers. XXXVI. Preparation and properties of some 1-alkylthio-1-alkynes. Rec. Trav. Chim. Pays Bas Belg. 1959, 78, 252–264. [Google Scholar] [CrossRef]
- Jeffrey, G.H.; Parker, R.; Vogel, A.I. 113. Physical properties and chemical constitution. Part XXXII. Thiophen compounds. J. Chem. Soc. 1961, 570–575. [Google Scholar] [CrossRef]
- Mathias, S.; de Carvalho, E., Jr.; Cecchini, R.G. The Dipole Moments of Cyclohexanethiol, a-Toluenethiol and Benzenethiol. J. Phys. Chem. 1961, 65, 425–427. [Google Scholar] [CrossRef]
- Hine, J.; Bayer, R.P.; Hammer, G.G. Formation of Bis-(Methylthio)-Methylene from Methyl Orthothioformate and Potassium Amide. J. Am. Chem. Soc. 1962, 84, 1751–1752. [Google Scholar] [CrossRef]
- Volynskii, N.P.; Gal’pern, G.D.; Smolyaninov, V.V. Synthesis of 2-substituted thiacyclohexanes. Neftekhim 1963, 3, 482–487. [Google Scholar] [CrossRef]
- Shostakovskii, M.F.; Atavin, A.S.; Dmitrieva, L.P.; Vasil’ev, N.P.; Gladkova, G.A. Reaction of 2,2-dialkyl-4-vinyloxymethyl-1,3-dioxolanes with thiols. Zh. Obsh. Khim. 1966, 2, 209–212. [Google Scholar]
- Shostakovskii, M.F.; Atabin, A.S.; Mikhaleva, A.I.; Vasil’ev, N.P.; Dmitrieva, L.P. Synthesis of 2,2-bis(alkthio)propyl vinyl ethers. Russ. Chem. Bull. 1967, 16, 1337–1338. [Google Scholar] [CrossRef]
- Nakhmanovich, A.S.; Skvortsova, G.G.; Shostakovskii, M.F.; Shulyak, L.A. Synthesis of vinyl esters of α-thienylcarbinols. Khim. Atset. Dokl. Vsesoyuz. Nauch. Konf. Khim. Atset. Ego Proiz. 1968, 256–259. [Google Scholar]
- Bittell, J.E.; Speier, J.L. Synthesis of Thiols and Polysulfides from Alkyl Halides, Hydrogen Sulfide, Ammonia, and Sulfur. J. Org. Chem. 1978, 43, 1687–1689. [Google Scholar] [CrossRef]
- Taganliev, A.; Rol’nik, L.Z.; Lapuka, L.F.; Rol’nik, L.Z.; Kirilyuk, G.G.; Pastushchenko, E.V.; Khekimov, Y.K. Structure and physicochemical properties of thioorthoformates. Izvest Akad. Nauk Turkm. SSR Ser. Fiz. Tekhn. Khim. Geolog. Nauk 1986, 1, 60–64. [Google Scholar]
- Khekimov, Y.K.; Taganlyev, A.; Kurbanov, D.; Khodzhalyev, T.K.; Kurbanov, I. Homolytic isomerization of 1,1,1-tris(ethylthio) ethane. Izvest Akad. Nauk Turkm. SSR Ser. Fiz. Tekhn. Khim. Geolog. Nauk 1987, 4, 105–106. [Google Scholar]
- Gilani, H.G.; Gilani, A.G.; Shekarsaree, S. Solubility and tie line data of the water–phosphoric acid–solvents at T = 303.2, 313.2, and 323.2 K: An experimental and correlational study. Thermochim. Acta 2013, 558, 36–45. [Google Scholar] [CrossRef]
- Vaughn, T.H. 1-Propyl-2-iodoacetylene. J. Am. Chem. Soc. 1933, 55, 1293. [Google Scholar] [CrossRef]
- Vaughn, T.H. Direct iodination of monosubstituted acetylenes. J. Am. Chem. Soc. 1933, 55, 2150–2153. [Google Scholar] [CrossRef]
- Bachman, G.B. Dehalogenation of aliphatic bromo acids. The bromo- and dibromo lefins. J. Am. Chem. Soc. 1933, 55, 4279–4284. [Google Scholar] [CrossRef]
- Vaughn, T.H.; Nieuwland, J.A. Synthesis and properties of 2-iodo-1-vinylacetylene. J. Chem. Soc. 1933, 741–743. [Google Scholar] [CrossRef]
- Desreux, V. Further study of alkyl fluorides. Bull. Cl. Sci. Acad. Royale Belg. 1934, 20, 457–476. [Google Scholar]
- Audsley, A.; Goss, F.R. The magnitude of the solvent effect in dipole-moment measurements. V. The solvent-effect constant and the moments of alkyl iodides. J. Chem. Soc. 1942, 358, 358–366. [Google Scholar] [CrossRef]
- Schmerling, L. Condensation of saturated halides with unsaturated compounds. II. Condensation of alkyl halides with monohaloo lefins. J. Am. Chem. Soc. 1946, 68, 1650–1654. [Google Scholar] [CrossRef]
- Mousseron, M.; Winternitz, F.; Jacquier, R. Some alicyclic chloro epoxides. Compt. Rend. 1946, 223, 1014–1015. [Google Scholar]
- Luciens, H.W.; Mason, C.T. The Preparation and Properties of Some Branched-Chain Alkyl Bromomethyl Ethers. J. Am. Chem. Soc. 1949, 71, 258–260. [Google Scholar] [CrossRef]
- Hoffmann, F.W. Aliphatic fluorides. I. ω, ω’-Difluoroalkanes. J. Org. Chem. 1949, 14, 105–110. [Google Scholar] [CrossRef]
- Coffman, D.D.; Raasch, M.S.; Rigby, G.W.; Barrick, P.L.; Hanford, W.E. Addition reactions of tetrafluoroethylene. J. Org. Chem. 1949, 14, 747–753. [Google Scholar] [CrossRef]
- Roe, A.; Cheek, P.H.; Hawkins, G.F. The Synthesis of 2-Fluoro-4- and 2-Fluoro-6-pyridinecarboxylic Acid and Derivatives. J. Am. Chem. Soc. 1949, 71, 4152–4153. [Google Scholar] [CrossRef]
- Stone, H.; Shechter, H. A new method for the preparation of organic iodides. J. Org. Chem. 1950, 15, 491–495. [Google Scholar] [CrossRef]
- Norton, T.R. New synthesis of ethyl trifluoroacetate. J. Am. Chem. Soc. 1950, 72, 3527–3528. [Google Scholar] [CrossRef]
- Hauptschein, M.; Grosse, A.V. Perfluoroalkyl Halides Prepared from Silver Perfluoro-fatty Acid Salts. I. Perfluoroalkyl Iodides. J. Am. Chem. Soc. 1951, 73, 2461–2463. [Google Scholar] [CrossRef]
- Douglass, I.B.; Martin, F.T.; Addor, R. Sulfenyl Chloride Studies. II. Mono-, Di-, and Tri-Chloromethanesulfenyl Chlorides and Certain of their Derivatives. J. Org. Chem. 1951, 16, 1297–1302. [Google Scholar] [CrossRef]
- Hauptschein, M.; Stokes, C.S.; Grosse, A.V. The properties and reactions of perfluorobutyrolactone. J. Am. Chem. Soc. 1952, 74, 1974–1976. [Google Scholar] [CrossRef]
- Douglass, I.B.; Osborne, C.E. The anhydrous chlorination of thioesters and related compounds. J. Am. Chem. Soc. 1953, 75, 4582–4583. [Google Scholar] [CrossRef]
- Yagupol’skii, L.M. Synthesis of derivatives of phenyl trifluoromethyl ether. Dokl. Akad. Nauk SSSR 1955, 105, 100–102. [Google Scholar]
- Stevens, C.L.; Mukherjee, T.K.; Traynelis, V. gem-Dihalides from the Hofmann degradation of α-haloamides. J. Am. Chem. Soc. 1956, 78, 2264–2267. [Google Scholar] [CrossRef]
- Douglass, I.B.; Warner, G.H. Methyl and ethyl trichloromethyl ethers. J. Am. Chem. Soc. 1956, 78, 6070–6071. [Google Scholar] [CrossRef]
- Douglass, I.B.; Poole, D.R. A New Method for the Preparation of Sulfinyl Chlorides. J. Org. Chem. 1957, 22, 536–537. [Google Scholar] [CrossRef]
- Yarovenko, N.N.; Vasil’eva, A.S. New method of introduction of the trihalomethyl group into organic compounds. Zh. Obsh. Khim. 1958, 28, 2502–2504. [Google Scholar]
- Soborovskii, L.Z.; Gladshtein, B.M.; Kiseleva, M.I.; Chernetskii, V.N. Organic compounds of sulfur. I. Synthesis of fluorides of alkanesulfonic acids and their halogen derivatives. Zh. Obsh. Khim. 1958, 28, 1866–1870. [Google Scholar]
- Bissell, E.R.; Spengler, R.E. Styrene-p-carboxylic acid. J. Org. Chem. 1959, 24, 1146–1147. [Google Scholar] [CrossRef]
- Macey, W.A.T. The Physical Properties of Certain Organic Fluorides. J. Phys. Chem. 1960, 64, 254–257. [Google Scholar] [CrossRef]
- Sadykh-Zade, S.I.; Sultanov, N.T. A new synthesis of α- and β-chlorostyrenes by direct chlorination of styrene. Azerbaid. Khim. Zh. 1960, 5, 33–36. [Google Scholar]
- Stolzer, C.; Simon, A. Fluorophosphorus compounds. I. Chem. Ber. 1960, 93, 1323–1331. [Google Scholar]
- Stolzer, C.; Simon, A. Fluorophosphorus compounds. II. Esters of fluorodiphosphoric acids. Chem. Ber. 1960, 93, 2578–2590. [Google Scholar]
- Bergel’son, L.D. Stereochemistry of addition reactions at a triple bond. VII. Stereochemistry of hydrobromination of bromoacetylenes under radical conditions. Izvest. Akad. Nauk SSSR Ser. Khim. 1960, 9, 1235–1240. [Google Scholar]
- Kost, V.N.; Freidlina, R.K. Telomerization of ethylene with polychloroalkanes containing the CCl2Br group. Izvest. Akad. Nauk SSSR Ser. Khim. 1961, 10, 1252–1256. [Google Scholar] [CrossRef]
- Gubanov, V.A.; Tumanova, A.V.; Dolgopol’skii, I.M.; Shcherbakov, V.A. Reaction of perfluoromethyl perfluorovinyl ether with hydrogen halides. Zh. Obsh. Khim. 1964, 34, 2802–2803. [Google Scholar]
- Ol’dekop, Y.A.; Kaberdin, R.V. Acyl peroxides. IX. Reaction of acetyl peroxide with cis-1-2dichloroethylene. Zh. Organ. Khim. 1965, 1, 873–876. [Google Scholar]
- Knunyants, I.L.; Krasuskaya, M.P.; Del’tsova, D.P. Di (1, 2, 4-oxadiazolyl) polydifluoromethylenes. Izvest. Akad. Nauk SSSR Ser. Khim. 1966, 577–579. [Google Scholar]
- Tataurov, G.P.; Sokolov, S.V. Synthesis and properties of octafluoroanisole. Zh. Obsh. Khim. 1966, 36, 537–540. [Google Scholar]
- Vipinchandra, A.R.; Hemalkumar, P.V.; Hemant, A.C. Static Permittivity and Refractive Index of Binary Mixtures of 3-Bromoanisole and 1-Propanol at Different Temperatures. J. Chem. Eng. Data 2015, 60, 3113–3119. [Google Scholar] [CrossRef]
- Gierut, J.A.; Sowa, F.J.; Nieuwland, J.A. Organic reactions with silicon compounds. II. The reaction of silicon tetrafluoride with the Grignard reagent. J. Am. Chem. Soc. 1936, 58, 897–898. [Google Scholar] [CrossRef]
- Gilman, H.; Clark, R.N. Some steric effects of the isopropyl group in organosilicon compounds. J. Am. Chem. Soc. 1947, 69, 1499–1500. [Google Scholar] [CrossRef]
- Petrov, A.D.; Shchukovskaya, L.L. Synthesis and properties of symmetric acetylenic disilanes. Dokl. Akad. Nauk SSSR 1952, 86, 551–553. [Google Scholar]
- Takatani, T. Silicic acid esters. V. Some physical properties of the silicates of aliphatic alcohols. Nippon Kagaku Zasshi 1953, 74, 948–950. [Google Scholar] [CrossRef]
- Petrov, A.D.; Ponomarenko, V.A. Synthesis and properties of disilylmethane, 1,2-disilylethane, 1,3-disilylpropane, and 1,3,5-trisilacyclohexane. Dokl. Akad. Nauk SSSR 1953, 90, 387–390. [Google Scholar]
- Zimmermann, W. Stability of chlorinated methylchlorosilane and chlorinated methylsiloxanes. Chem. Ber. 1954, 87, 887–891. [Google Scholar] [CrossRef]
- Batuev, M.I.; Shostakovskii, M.F.; Belyaev, V.I.; Matveeva, A.D.; Dubrova, E.V. Chemical and physical properties of the hydroxyl group in trimethylsilanol. Dokl. Akad. Nauk SSSR 1954, 95, 531–534. [Google Scholar]
- Dolgov, B.N.; Kharitonov, N.P.; Voronkov, M.G. Reaction of triethylsilane with ammonia and amines. Zh. Obsh. Khim. 1954, 24, 678–683. [Google Scholar]
- Voronkov, M.G.; Dolgov, B.N. Isothiocyano-substituted silanes. Zh. Obsh. Khim. 1954, 24, 1082–1087. [Google Scholar]
- Petrov, A.D.; Chernysheva, T.I. Synthesis of tetraisobutyl, tetraisopropyl, tetracyclohexyl, and tetra-1-naphthylsilanes. Zh. Obsh. Khim. 1954, 24, 1189–1192. [Google Scholar]
- Shostakovskii, M.F.; Shikhiev, I.A.; Kochkin, D.A.; Belyaev, V.I. Oxygen-containing organosilicon compounds. III. Preparation of trimethyl- and triethylsilanols and their transformations. Zh. Obsh. Khim. 1954, 24, 2202–2206. [Google Scholar]
- Petrov, A.D.; Shchukovskaya, L.L. Behavior toward chemical reagents of the silicon-carbon bond in α-alkynyl- and β-alkenylsilanes. Zh. Obsh. Khim. 1955, 25, 1128–1136. [Google Scholar]
- Shostakovskii, M.F.; Malinovskii, M.S.; Romantsevich, M.K.; Kochkin, D.A. Synthesis and transformations of oxygen-containing organosilicon compounds. III. Reactions of propylene oxide with alkyl(aryl)chlorosilanes. Izvest. Akad. Nauk SSSR Ser. Khim. 1956, 632–634. [Google Scholar]
- Voronkov, M.G.; Khudobin, Y.I. Reaction of trialkylsilanes with iodine and hydrogen iodide. Akad. Nauk SSSR Ser. Khim. 1956, 5, 805–810. [Google Scholar] [CrossRef]
- Shostakovskii, M.F.; Kochkin, D.A.; Rogov, V.M. 102. Synthesis and transformation of oxygen-containing organosilicon compounds. VI. Preparation of secondary dialkyl(aryl)chlorosilanes, dialkyl(aryl)silanols, and some of their transformations. Akad. Nauk SSSR Ser. Khim. 1956, 1062–1069. [Google Scholar]
- Shostakovskii, M.F.; Kochkin, D.A.; Vinogradov, V.L.; Neterman, V.A. Synthesis and transformation of oxygen-containing organosilicon compounds. VI. Reaction of hydrogen containing alkyl(aryl)dichlorosilanes with alcohols. Akad. Nauk SSSR Ser. Khim. 1956, 1269–1271. [Google Scholar]
- Dolgov, B.N.; Borisov, S.N.; Voronkov, M.G. Reaction of alkylhalosilanes with trialkylsilanes. Zh. Obsh. Khim. 1957, 27, 2692–2697. [Google Scholar]
- Kaufman, H.C.; Douthett, O.R. Preparaton and Comparison of the Physical Properties of Alkyl and Alkforyl Silicates. Ind. Eng. Chem. 1958, 3, 324–327. [Google Scholar]
- Voronkov, M.G.; Shabarova, Z.I. Alkoxysilanes. XIV. Cleavage of organosiloxanes by alcohols as a method of synthesis of organoalkoxysilanes. Zh. Obsh. Khim. 1959, 29, 1528–1534. [Google Scholar]
- Duffaut, N.; Calas, R.; Mace, J.C. The oxidation of trialkyl and triaryl silanes by oxygen containing silver compounds. Bull. Soc. Chim. Fr. 1959, 1971–1973. [Google Scholar]
- Wilson, G.R.; Smith, A.G. Preparation of Decamethyltetrasilane and Its Lower Homologs. J. Org. Chem. 1961, 26, 557–559. [Google Scholar] [CrossRef]
- Sergeeva, S.I.; Chien, H.-C.; Tsitovich, D.D. Synthesis of alkyl- and dialkylbis(1,1-dialkylhydrazino)silane. Zh. Obsh. Khim. 1960, 30, 694–695. [Google Scholar]
- Voronkov, M.G.; Rabkina, S.M. Alkoxysilanes. XVI. Reaction of tetraalkoxysilanes with ketones. Zh. Obsh. Khim. 1961, 31, 1259–1265. [Google Scholar]
- Sergeeva, Z.I.; Hsieh, C.-L. A new method of synthesis of organosilicon hydrazines. Zh. Obsh. Khim. 1962, 32, 1987–1993. [Google Scholar]
- Stolberg, U.G. Octamethyltrisilane and decamethyltetrasilane. Angew. Chem. 1962, 74, 696. [Google Scholar]
- Sergeeva, Z.I.; Hsieh, C.-L. Reaction of nonsymmetric dialkylhydrazines with alkylchloro-silanes. Zh. Obsh. Khim. 1963, 33, 1874–1878. [Google Scholar]
- Shostakovskii, M.F.; Sokolov, B.A.; Dmitrieva, G.V.; Alekseeva, G.M. Addition reaction of hydrosilanes with vinyl ethers. Zh. Obsh. Khim. 1964, 34, 2839–2842. [Google Scholar]
- Cudlin, J.; Schraml, J.; Chvalovsky, V. Organosilicon compounds. XXXV. Addition of dichloromethylene to isomeric bis(trimethylsilyl) ethylenes. Coll. Czech. Chem. Comm. 1964, 29, 1476–1483. [Google Scholar] [CrossRef]
- Voronkov, M.G.; Skorik, Y.I. Synthesis of organofluorosilanes. Akad. Nauk SSSR Ser. Khim. 1964, 7, 1215–1221. [Google Scholar] [CrossRef]
- Giao, D.-H. New cleavage method for tetrabutylated derivatives of Si, Ge, and Sn. Compt. Rend. 1965, 260, 6937–6938. [Google Scholar]
- Prejzner, J. Organoxyisocyanatosilanes. I. Preparation and properties of phenoxyisocyanatosilanes. Rocz. Chem. 1965, 39, 747–753. [Google Scholar]
- Bolotov, B.A.; Kharitonov, N.P.; Batyaev, E.A.; Rumyantseva, E.G. Destructive hydrogenation of trialkylalkoxysilanes. Zh. Obsh. Khim. 1967, 37, 2113–2117. [Google Scholar]
- Cer, L.; Vaisarova, V.; Chvalovsky, V. Organosilicon compounds. LIII. Dipole moments of benzylmethylethoxysilanes. Coll. Czech. Chem. Comm. 1967, 32, 3784–3786. [Google Scholar] [CrossRef]
- Kannengiesser, G.; Damm, F. Preparation of some tetrakis(dialkylamino)silanes. Bull. Soc. Chim. Fr. 1967, 7, 2492–2495. [Google Scholar]
- Khudobin, Y.I.; Voronkov, M.G.; Kharitonov, N.P. Trialkyl(aryl)bromosilanes. Latv. PSR Zinat. Akad. Vest. Kim. Ser. 1967, 5, 595–600. [Google Scholar]
- Zhinkin, D.Y.; Mal’nova, G.N.; Gorislavskaya, Z.V. Ammonolysis of triorganochlorosilanes, their coammonolysis with trimethylchlorosilane, and coammonolysis of some tri- and diorganochlorosilanes. Zh. Obsh. Khim. 1968, 38, 2800–2807. [Google Scholar]
- Feher, F.; Hädicke, P.; Frings, H. Beiträge zur Chemie des Siliciums und Germaniums, XXIII (1) Physikalisch-Chemische Eigenschaften der Silane von Trisilan bis Heptasilan. Inorg. Nucl. Chem. Lett. 1973, 9, 931–936. [Google Scholar] [CrossRef]
- Boredau, M.; Dédier, J.; Frainnet, E. Etude Structurale d’Hexaalkyldisiloxanes et de Trialkylalkoxy- ou Aryloxysilanes. I. Determination par Dipolemetrie d’Angles SiOC et SiOSi. J. Organomet. Chem. 1973, 59, 125–139. [Google Scholar]
- Moerlein, S.M. Synthesis and Spectroscopic Characteristics of Aryltrimethyl-Silicon, -Germanium, and Tin Compounds. J. Organomet. Chem. 1987, 319, 29–39. [Google Scholar] [CrossRef]
- Alagar, M.; Krishnasami, V. Ultrasonic Properties of Tetraalkoxysilanes. Ultrasonics 1987, 25, 283–287. [Google Scholar] [CrossRef]
- Alagar, M.; Ponnusamy, M.; Amsavel, A. Studies on Dielectric Behaviour of Dimethyldialkoxysilanes. Hung. J. Ind. Chem. 1993, 21, 19–22. [Google Scholar]
- Yu, L.; Hu, Y.; Dong, H.; Wu, C. The physicochemical properties of 1,3,5-trimethyl-1,3,5-tris(3,3,3-trifluoropropyl)cyclotrisiloxane with various aromatic hydrocarbons at T = (308.15 to 323.15) K. J. Chem. Thermodyn. 2016, 96, 117–126. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, C.; Dong, H.; Wu, C. Excess molar volume along with refractive index for binary systems of dimethoxymethylphenylsilane with dimethyldimethoxysilane, dimethyldiethoxylsilane, methylvinyldiethoxysilane and ethenyltrimethoxysilane. J. Chem. Thermodyn. 2017, 109, 82–90. [Google Scholar] [CrossRef]
- Jin, J.; Shao, F.; Dong, H.; ·Wu, C. Volumetric and Optical Properties of Dimethylvinylethoxysilane with n-Alkanes (C7 to C10) at Temperatures in the Range (298.15 to 318.15) K. J. Sol. Chem. 2019, 48, 82–103. [Google Scholar] [CrossRef]
- Terent’ev, A.P.; Yanovskaya, L.A. Sulfonation and sulfonic acids of acidophobic substances. XVI. Sulfonation of some indole derivatives. Zh. Obsh. Khim. 1951, 21, 1295–1297. [Google Scholar]
- Leonard, N.J.; Ryder, B.L. The stereoisomers of vic-dialkylpiperidines. The 2-butyl-3-methylpiperidines. J. Org. Chem. 1953, 18, 598–608. [Google Scholar] [CrossRef]
- Mikhailov, G.I. 2-Bromopyridine. Zh. Prikl. Khim. 1954, 27, 349–351. [Google Scholar]
- Shuikin, N.I.; Bel’skii, I.F.; Skobtsova, G.E. Catalytic synthesis of higher homologs of pyrrole and pyrrolidine from α-furylalkylamines. Izvest. Akad. Nauk SSSR Ser. Khim. 1963, 9, 1678–1680. [Google Scholar] [CrossRef]
- Bel’skii, I.F.; Shuikin, N.I.; Skobtsova, G.E. Conjugated hydrogenolysis in the synthesis of pyrrolidine homologs. Izvest. Akad. Nauk SSSR Ser. Khim. 1963, 9, 1675–1678. [Google Scholar] [CrossRef]
- Shuikin, N.I.; Bel’skii, I.F.; Karakhanov, R.A.; Kozma, B.; Bartok, M. Investigations in the field of diols and cyclic ethers. V. Preparation of 2-monosubstituted derivatives of tetrahydropyran. Acta Phys. Chem. 1963, 9, 37–42. [Google Scholar]
- Bel’skii, I.F.; Shuikin, N.I.; Skobtsova, G.E. Catalytic conversion of furan amines into 2,4-dialkylpyrroles. Izvest. Akad. Nauk SSSR Ser. Khim. 1964, 6, 1118–1120. [Google Scholar]
- Shuikin, N.I.; Bel’skii, I.F.; Karakhanov, R.A.; Kozma, B.; Bartok, M. Isomerization of tetrahydropyrans. Izvest. Akad. Nauk SSSR Ser. Khim. 1964, 4, 747–750. [Google Scholar] [CrossRef]
- Shuikin, N.I.; Bel’skii, I.F.; Barkovskaya, L.Y.; Dronov, V.I.; Alalykina, L.A. Synthesis of 2,4- and 2,5-dialkylthiophanes. Khim. Sera Organ. Soedin. Soderzhashch. Neff. Nefteprod. Akad. Nauk SSSR Bashkirsk. Filial 1964, 6, 197–200. [Google Scholar]
- Shuikin, N.I.; Bel’skii, I.F.; Barkovskaya, L.Y.; Gerasimov, M.M. Synthesis of some cyclic sulfides. Khim. Sera Organ. Soedin. Soderzhashch. Neff. Nefteprod. Akad. Nauk SSSR Bashkirsk. Filial 1964, 7, 58–60. [Google Scholar]
- Bel’skii, I.F.; Shuikin, N.I.; Karakhanov, R.A. Synthesis of γ-oxo alcohols and dihydrofurans from 1-furyl-3alkanols. Izvest. Akad Nauk SSSR Ser. Khim. 1964, 2, 326–331. [Google Scholar]
- Bel’skii, I.F.; Shuikin, N.I.; Skobtsova, G.E. Catalytic synthesis of pyrrole and pyrrolidine homologs. Probl. Organ. Sinteza Akad. Nauk SSSR Otd. Obshch. Tekhn. Khim. 1965, 186–189. [Google Scholar]
- Shuikin, N.I.; Bel’skii, I.F.; Abgaforova, G.E. Conjugated hydrogenolysis in the synthesis of 2,5-dialkylpyrroles. Izvest. Akad. Nauk SSSR Ser. Khim. 1965, 1, 163–165. [Google Scholar]
- Bel’skii, I.F.; Khar’kov, S.N.; Shuikin, N.I. Synthesis of homologs of 1,4-dioxane and 1,4-dioxene. Dokl. Akad. Nauk SSSR 1965, 165, 821–823. [Google Scholar]
- Abgaforova, G.E.; Shuikin, N.I.; Bel’skii, I.F. Synthesis of trialkyl derivatives of pyrrole and pyrrolidine. Izvest. Akad. Nauk SSSR Ser. Khim. 1965, 4, 734–736. [Google Scholar] [CrossRef]
- Shuikin, N.I.; Lebedev, B.L. Alkylation of thiophene by isobutylene. Izvest. Akad. Nauk SSSR Ser. Khim. 1967, 5, 1154–1155. [Google Scholar] [CrossRef]
- Shuikin, N.I.; Lebedev, B.L.; Nikol’skii, V.G.; Korytina, O.A.; Kessenikh, A.V.; Prokof’ev, E.P. Direction of furan alkylation with isobutylene. Izvest. Akad. Nauk SSSR Ser. Khim. 1967, 7, 1618–1620. [Google Scholar]
- Fedorov, E.I.; Mikhant’ev, B.I. Crotylation of sodium 2-pyridinolate. Khim. Geterotsikl. Soedin. 1969, 6, 1022–1023. [Google Scholar]
- Anton, V.; Munoz-Embid, J.; Artigas, H.; Artal, M.; Lafuente, C. Thermophysical properties of oxygenated thiophene derivatives: Experimental data and modelling. J. Chem. Thermodyn. 2017, 113, 330–339. [Google Scholar] [CrossRef]
- Baird, Z.S.; Dahlberg, A.; Uusi-Kyyny, P.; Osmanbegovic, N.; Witos, J.; Helminen, J.; Cederkrantz, D.; Hyväri, P.; Alopaeus, V.; Kilpeläinen, I.; et al. Physical Properties of 7-Methyl-1,5,7-triazabicyclo[4.4.0]dec-5-ene (mTBD). Int. J. Thermophys. 2019, 40, 71. [Google Scholar] [CrossRef] [Green Version]
- Gomez, E.; Gonzalez, B.; Dominguez, A.; Tojo, E.; Tojo, J. Dynamic Viscosities of a Series of 1-Alkyl-3-methylimidazolium Chloride Ionic Liquids and Their Binary Mixtures with Water at Several Temperatures. J. Chem. Eng. Data 2006, 51, 696–701. [Google Scholar] [CrossRef]
- Pereiro, A.B.; Tojo, E.; Rodriguez, A.; Canosa, J.; Tojo, J. Properties of ionic liquid HMIMPF6 with carbonates, ketones and alkyl acetates. J. Chem. Thermodyn. 2006, 38, 651–661. [Google Scholar] [CrossRef]
- Pereiro, A.B.; Santamarta, F.; Tojo, E.; Rodriguez, A.; Tojo, J. Temperature Dependence of Physical Properties of Ionic Liquid 1,3-Dimethylimidazolium Methyl Sulfate. J. Chem. Eng. Data 2006, 51, 952–954. [Google Scholar] [CrossRef]
- Pereiro, A.B.; Verdia, P.; Tojo, E.; Rodriguez, A. Physical Properties of 1-Butyl-3-methylimidazolium Methyl Sulfate as a Function of Temperature. J. Chem. Eng. Data 2007, 52, 377–380. [Google Scholar] [CrossRef]
- Pereiro, A.B.; Legido, J.L.; Rodriguez, A. Physical properties of ionic liquids based on 1-alkyl-3-methylimidazolium cation and hexafluorophosphate as anion and temperature dependence. J. Chem. Thermodyn. 2007, 39, 1168–1175. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Z.; Zhang, J.; Zhang, S.; Zhu, L.; Yang, J.; Zhang, X.; Deng, Y. Physicochemical Properties of Nitrile-Functionalized Ionic Liquids. J. Phys. Chem. B 2007, 111, 2864–2872. [Google Scholar] [CrossRef]
- Pereiro, A.B.; Rodriguez, A. Thermodynamic Properties of Ionic Liquids in Organic Solvents from (293.15 to 303.15) K. J. Chem. Eng. Data 2007, 52, 600–608. [Google Scholar] [CrossRef]
- Mokhtarain, B.; Mojtahedi, M.M.; Mortaheb, H.R.; Mafi, M.; Yazdani, F.; Sadeghian, F. Densities, Refractive Indices, and Viscosities of the Ionic Liquids 1-Methyl-3-octylimidazolium Tetrafluoroborate and 1-Methyl-3-butylimidazolium Perchlorate and Their Binary Mixtures with Ethanol at Several Temperatures. J. Chem. Eng. Data 2008, 53, 677–682. [Google Scholar] [CrossRef]
- Ortega, J.; Vreekamp, R.; Penco, E.; Marrero, E. Mixing thermodynamic properties of 1-butyl-4-methylpyridinium tetrafluoroborate [b4mpy][BF4] with water and with an alkan-1ol (methanol to pentanol). J. Chem. Thermodyn. 2008, 40, 1087–1094. [Google Scholar] [CrossRef]
- Gonzalez, B.; Calvar, N.; Gomez, E.; Macedo, E.A.; Dominguez, A. Synthesis and Physical Properties of 1-Ethyl 3-methylpyridinium Ethylsulfate and Its Binary Mixtures with Ethanol and Water at Several Temperatures. J. Chem. Eng. Data 2008, 53, 1824–1828. [Google Scholar] [CrossRef]
- Muhammad, A.; Mutalib, M.I.A.; Wilfred, C.D.; Murugesn, T.; Shafeeq, A. Thermophysical properties of 1-hexyl-3-methyl imidazolium based ionic liquids with tetrafluoroborate, hexafluorophosphate and bis(trifluoromethylsulfonyl)imide anions. J. Chem. Thermodyn. 2008, 40, 1433–1438. [Google Scholar] [CrossRef]
- Malham, I.B.; Turmine, M. Viscosities and refractive indices of binary mixtures of 1-butyl-3-methylimidazolium tetrafluoroborate and 1-butyl-2,3-dimethylimidazolium tetrafluoroborate with water at 298 K. J. Chem. Thermodyn. 2008, 40, 718–723. [Google Scholar] [CrossRef]
- Tariq, M.; Forte, P.A.S.; Gomes, M.F.C.; Lopes, J.N.C.; Rebelo, L.P.N. Densities and refractive indices of imidazolium- and phosphonium-based ionic liquids: Effect of temperature, alkyl chain length, and anion. J. Chem. Thermodyn. 2009, 41, 790–798. [Google Scholar] [CrossRef]
- Torrecilla, J.S.; Palomar, J.; Garcia, J.; Rodriguez, F. Effect of Cationic and Anionic Chain Lengths on Volumetric, Transport, and Surface Properties of 1-Alkyl-3-methylimidazolium Alkylsulfate Ionic Liquids at (298.15 and 313.15) K. J. Chem. Eng. Data 2009, 54, 1297–1301. [Google Scholar] [CrossRef]
- Pereiro, A.B.; Veiga, H.I.M.; Esperanca, J.M.S.S.; Rodriguez, A. Effect of temperature on the physical properties of two ionic liquids. J. Chem. Thermodyn. 2009, 41, 1419–1423. [Google Scholar] [CrossRef]
- Calvar, N.; Gomez, E.; Gonzalez, B.; Dominguez, A. Experimental Determination, Correlation, and Prediction of Physical Properties of the Ternary Mixtures Ethanol and 1-Propanol + Water + 1-Ethyl-3-methylpyridinium Ethylsulfate at 298.15 K. J. Chem. Eng. Data 2009, 54, 2229–2234. [Google Scholar] [CrossRef]
- Navas, A.; Ortega, J.; Vreekamp, R.; Marrero, E.; Palomar, J. Experimental Thermodynamic Properties of 1-Butyl-2-methylpyridinium Tetrafluoroborate [b2mpy][BF4] with Water and with Alkan-1-ol and Their Interpretation with the COSMO-RS Methodology. Ind. Eng. Chem. Res. 2009, 48, 2678–2690. [Google Scholar] [CrossRef]
- Soriano, A.N.; Doma, B.T., Jr.; Li, M.-H. Measurements of the density and refractive index for 1-n-butyl-3-methylimidazolium-based ionic liquids. J. Chem. Thermodyn. 2009, 41, 301–307. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, S.; Li, Z.; Li, J.; Chen, Z.; Wang, R.; Lu, L.; Deng, Y. Novel Cyclic Sulfonium-Based Ionic Liquids: Synthesis, Characterization, and Physicochemical Properties. Chem. Eur. J. 2009, 15, 765–778. [Google Scholar] [CrossRef]
- Pereiro, A.B.; Rodriguez, A. Separation of Ethanol-Heptane Azeotropic Mixtures by Solvent Extraction with an Ionic Liquid. Ind. Eng. Chem. Res. 2009, 48, 1579–1585. [Google Scholar] [CrossRef]
- Gonzalez, R.; Calvar, N.; Gomez, E.; Dominguez, I.; Dominguez, A. Synthesis and Physical Properties of 1-Ethylpyridinium Ethylsulfate and its Binary Mixtures with Ethanol and 1-Propanol at Several Temperatures. J. Chem. Eng. Data 2009, 54, 1353–1358. [Google Scholar] [CrossRef]
- Singh, T.; Kumar, A. Temperature Dependence of Physical Properties of Imidazolium Based Ionic Liquids: Internal Pressure and Molar Refraction. J. Solut. Chem. 2009, 38, 1043–1053. [Google Scholar] [CrossRef]
- Bandres, I.; Giner, B.; Artigas, H.; Lafuente, C.; Royo, F.M. Thermophysical Properties of N-Octyl-3-methylpyridinium Tetrafluoroborate. J. Chem. Eng. Data 2009, 54, 236–240. [Google Scholar] [CrossRef]
- Bandres, I.; Pera, G.; Martin, S.; Castro, M.; Lafuente, C. Thermophysical Study of 1-Butyl-2-Methylpyridinium Tetrafluoroborate Ionic Liquid. J. Phys. Chem. B 2009, 113, 11936–11942. [Google Scholar] [CrossRef] [PubMed]
- Bandres, I.; Royo, F.; Gascon, I.; Castro, M.; Lafuente, C. Anion Influence on Thermophysical Properties of Ionic Liquids: 1-Butylpyridinium Tetrafluoroborate and 1-Butylpyridinium Triflate. J. Phys. Chem. B 2010, 114, 3601–3607. [Google Scholar] [CrossRef] [PubMed]
- Arce, A.; Francisco, M.; Soto, A. Evaluation of the polysubstituted pyridinium ionic liquid [hmmpy][Ntf2] as a suitable solvent for desulfurization: Phase equilibria. J. Chem. Thermodyn. 2010, 42, 712–718. [Google Scholar] [CrossRef]
- Shekaari, H.; Armanfar, E. Physical Properties of Aqueous Solutions of Ionic Liquid, 1-Propyl-3-methylimidazolium Methyl Sulfate, at T) (298.15 to 328.15) K. J. Chem. Eng. Data 2010, 55, 765–772. [Google Scholar] [CrossRef]
- Brigouleix, C.; Arnouti, M.; Jacquemin, J.; Caillon-Caravanier, M.; Galiano, H.; Lemordant, D. Physicochemical Characterization of Morpholinium Cation Based Protic Ionic Liquids Used As Electrolytes. J. Phys. Chem. B 2010, 114, 1757–1766. [Google Scholar] [CrossRef]
- Alvarez, V.H.; Mattedi, S.; Martin-Pastor, M.; Aznar, M.; Iglesias, M. Synthesis and thermophysical properties of two new protic long-chain ionic liquids with the oleate anion. Fluid Phase Equil. 2010, 299, 42–50. [Google Scholar] [CrossRef]
- Yunus, N.M.; Mutalib, M.I.A.; Man, Z.; Bustam, M.A.; Murugesan, T. Thermophysical properties of 1-alkylpyridinum bis(trifluoromethylsulfonyl)imide ionic liquids. J. Chem. Thermodyn. 2010, 42, 491–495. [Google Scholar] [CrossRef]
- Ziyada, A.K.; Wilfred, C.D.; Bustam, M.A.; Man, Z.; Murugesan, T. Thermophysical Properties of 1-Propyronitrile-3-alkylimidazolium Bromide Ionic Liquids at Temperatures from (293.15 to 353.15) K. J. Chem. Eng. Data 2010, 55, 3886–3890. [Google Scholar] [CrossRef]
- Yu, Z.; Gao, H.; Wang, H.; Chen, L. Densities, Excess Molar Volumes, and Refractive Properties of the Binary Mixtures of the Amino Acid Ionic Liquid [bmim][Gly] with 1-Butanol or Isopropanol at T = (298.15 to 313.15) K. J. Chem. Eng. Data 2011, 56, 4295–4300. [Google Scholar] [CrossRef]
- Kurnia, K.A.; Taib, M.M.; Mutalib, M.I.A.; Murugesan, T. Densities, refractive indices and excess molar volumes for binary mixtures of protic ionic liquids with methanol at T = 293.15 to 313.15 K. J. Mol. Liq. 2011, 159, 211–219. [Google Scholar] [CrossRef]
- Ziyada, A.K.; Bustam, M.A.; Wilfred, C.D.; Murugesan, T. Densities, Viscosities, and Refractive Indices of 1-Hexyl-3-propanenitrile Imidazolium Ionic Liquids Incorporated with Sulfonate-Based Anions. J. Chem. Eng. Data 2011, 56, 2343–2348. [Google Scholar] [CrossRef]
- Yu, Z.; Gao, H.; Wang, H.; Chen, L. Densities, Viscosities, and Refractive Properties of the Binary Mixtures of the Amino Acid Ionic Liquid [bmim][Ala] with Methanol or Benzylalcohol at T = (298.15 to 313.15) K. J. Chem. Eng. Data 2011, 56, 2877–2883. [Google Scholar] [CrossRef]
- Taib, M.M.; Murugesan, T. Density, Refractive Index, and Excess Properties of 1-Butyl-3-methylimidazolium Tetrafluoroborate with Water and Monoethanolamine. J. Chem. Eng. Data 2012, 57, 120–126. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, X.; Liu, S.; Yang, B.; Lu, L.; He, Y.; Deng, Y. Hydrophobic 1-allyl-3-alkylimidazolium dicyanamide ionic liquids with low densities. J. Mater. Chem. 2011, 21, 6864–6868. [Google Scholar] [CrossRef]
- de Hadlich Oliveira, L.; Aznar, M. Liquid_Liquid Equilibria for {1-Ethyl-3-methylimidazolium Diethylphosphate or 1-Ethyl-3-methylimidazolium Ethylsulfate} + 4,6-Dimethyldibenzothiophene + Dodecane Systems at 298.2 K and 313.2 K. J. Chem. Eng. Data 2011, 56, 2005–2012. [Google Scholar] [CrossRef]
- Tsunashima, K.; Kawabata, A.; Matsumiya, M.; Kodama, S.; Enomoto, R.; Sugiya, M.; Kunugi, Y. Low viscous and highly conductive phosphonium ionic liquids based on bis(fluorosulfonyl)amide anion as potential electrolytes. Electrochem. Comm. 2011, 13, 178–181. [Google Scholar] [CrossRef]
- Gonzalez, B.; Gomez, E.; Dominguez, A.; Vilas, M.; Tojo, E. Physicochemical Characterization of New Sulfate Ionic Liquids. J. Chem. Eng. Data 2011, 56, 14–20. [Google Scholar] [CrossRef]
- Vercher, E.; Llopis, F.J.; Gonzalez-Alfaro, V.; Miguel, P.J.; Martinez-Andreu, A. Refractive Indices and Deviations in Refractive Indices of Trifluoromethanesulfonate-Based Ionic Liquids in Water. J. Chem. Eng. Data 2011, 56, 4499–4504. [Google Scholar] [CrossRef]
- Wu, T.Y.; Su, S.G.; Gung, S.T.; Lin, M.W.; Ouyang, W.C.; Sun, I.W.; Lai, C.A. Synthesis and Characterization of Protic Ionic liquids Containing Cyclic Amine Cations and Tetrafluoroborate Anion. J. Iran. Chem. Soc. 2011, 8, 149–165. [Google Scholar] [CrossRef]
- Muhammad, N.; Man, Z.B.; Bustam, M.A.; Mutalib, M.I.A.; Wilfred, C.D.; Rafiq, S. Synthesis and Thermophysical Properties of Low Viscosity Amino Acid-Based Ionic Liquids. J. Chem. Eng. Data 2011, 56, 3157–3162. [Google Scholar] [CrossRef]
- Hossain, M.I.; Babaa, M.-R.; El-Harbawi, M.; Man, Z.; Hefter, G.; Yin, C.-Y. Synthesis, Characterization, Physical Properties, and Cytotoxicities of 1-(6-Hydroxyhexyl)-3-alkylimidazolium Chloride Ionic Liquids. J. Chem. Eng. Data 2011, 56, 4188–4193. [Google Scholar] [CrossRef]
- Freire, M.G.; Teles, A.R.R.; Rocha, M.A.A.; Schröder, B.; Neves, C.M.S.S.; Carvalho, P.J.; Evtuguin, D.V.; Santos, L.M.N.B.F.; Coutinho, J.A.P. Thermophysical Characterization of Ionic Liquids Able To Dissolve Biomass. J. Chem. Eng. Data 2011, 56, 4813–4822. [Google Scholar] [CrossRef]
- Larriba, M.; Garcia, S.; Garcia, J.; Torrecilla, J.S.; Rodriguez, F. Thermophysical Properties of 1-Ethyl-3-methylimidazolium 1,1,2,2-Tetrafluoroethanesulfonate and 1-Ethyl-3-methylimidazolium Ethylsulfate Ionic Liquids as a Function of Temperature. J. Chem. Eng. Data 2011, 56, 3589–3597. [Google Scholar] [CrossRef]
- Deive, F.J.; Rivas, M.A.; Rodriguez, A. Thermophysical properties of two ionic liquids based on benzyl imidazolium cation. J. Chem. Thermodyn. 2011, 43, 487–491. [Google Scholar] [CrossRef]
- Seki, S.; Tsuzuki, S.; Hayamizu, K.; Umebayashi, Y.; Serizawa, N.; Takei, K.; Miyashiro, H. Comprehensive Refractive Index Property for Room-Temperature Ionic Liquids. J. Chem. Eng. Data 2012, 57, 2211–2216. [Google Scholar] [CrossRef]
- Xu, W.-G.; Li, L.; Ma, X.-X.; Wei, J.; Duan, W.-B.; Guan, W.; Yang, J.-Z. Density, Surface Tension, and Refractive Index of Ionic Liquids Homologue of 1-Alkyl-3-methylimidazolium Tetrafluoroborate [Cnmim][BF4] (n = 2,3,4,5,6). J. Chem. Eng. Data 2012, 57, 2177–2184. [Google Scholar] [CrossRef]
- Gomez, E.; Calvar, N.; Macedo, E.A.; Dominguez, A. Effect of the temperature on the physical properties of pure 1-propyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide and characterization of its binary mixtures with alcohols. J. Chem. Thermodyn. 2012, 45, 9–15. [Google Scholar] [CrossRef]
- Gonzalez, E.J.; Dominguez, A.; Macedo, E.A. Physical and Excess Properties of Eight Binary Mixtures Containing Water and Ionic Liquids. J. Chem. Eng. Data 2012, 57, 2165–2176. [Google Scholar] [CrossRef]
- Larriba, M.; Garcia, S.; Navarro, P.; Garcia, J.; Rodriguez, F. Physical Properties of N-Butylpyridinium Tetrafluoroborate and N-Butylpyridinium Bis(trifluoromethylsulfonyl)imide Binary Ionic Liquid Mixtures. J. Chem. Eng. Data 2012, 57, 1318–1325. [Google Scholar] [CrossRef]
- Trivedi, T.J.; Bharmoria, P.; Singh, T.; Kumar, A. Temperature Dependence of Physical Properties of Amino Acid Ionic Liquid Surfactants. J. Chem. Eng. Data 2012, 57, 317–323. [Google Scholar] [CrossRef]
- Bandres, I.; Lopez, M.C.; Castro, M.; Barbera, J.; Lafuente, C. Thermophysical properties of 1-propylpyridinium tetrafluoroborate. J. Chem. Thermodyn. 2012, 44, 148–153. [Google Scholar] [CrossRef] [Green Version]
- Machanova, K.; Boisset, A.; Sedlakova, Z.; Anouti, M.; Bendova, M.; Jacquemin, J. Thermophysical Properties of Ammonium-Based Bis{(trifluoromethyl)sulfonyl}imide Ionic Liquids: Volumetric and Transport Properties. J. Chem. Eng. Data 2012, 57, 2227–2235. [Google Scholar] [CrossRef] [Green Version]
- Muhammad, N.; Man, Z.; Ziyada, A.K.; Bustam, M.A.; Mutalib, M.I.A.; Wilfred, C.D.; Rafiq, S.; Tan, I.M. Thermophysical Properties of Dual Functionalized Imidazolium-Based Ionic Liquids. J. Chem. Eng. Data 2012, 57, 737–743. [Google Scholar] [CrossRef]
- Almeida, H.F.D.; Passos, H.; Lopes-da-Silva, J.A.; Fernandes, A.M.; Freire, M.G.; Coutinho, J.A.P. Thermophysical Properties of Five Acetate-Based Ionic Liquids. J. Chem. Eng. Data 2012, 57, 3005–3013. [Google Scholar] [CrossRef]
- Reddy, P.; Siddiqi, M.A.; Atakan, B.; Diedenhofen, M.; Ramjugernath, D. Activity coefficients at infinite dilution of organic solutes in the ionic liquid PEG-5 cocomonium methylsulfate at T = (313.15, 323.15, 333.15, and 343.15) K: Experimental results and COSMO-RS predictions. J. Chem. Thermodyn. 2013, 58, 322–329. [Google Scholar] [CrossRef]
- Ma, X.-X.; Wei, J.; Zhang, Q.-B.; Tian, F.; Feng, Y.-Y.; Guan, W. Prediction of Thermophysical Properties of Acetate-Based Ionic Liquids Using Semiempirical Methods. Ind. Eng. Chem. Res. 2013, 52, 9490–9496. [Google Scholar] [CrossRef]
- Neves, C.M.S.S.; Kurnia, K.A.; Coutinho, J.A.P.; Marrucho, I.M.; Lopes, J.N.C.; Freire, M.G.; Rebelo, L.P.N. Systematic Study of the Thermophysical Properties of Imidazolium-Based Ionic Liquids with Cyano-Functionalized Anions. J. Phys. Chem. B 2013, 117, 10271–10283. [Google Scholar] [CrossRef]
- Rocha, M.A.A.; Ribeiro, F.M.S.; Loobo Ferreira, A.I.M.C.; Coutinho, J.A.P.; Santos, L.M.N.B.F. Thermophysical properties of [CN − 1C1im][PF6] ionic liquids. J. Mol. Liq. 2013, 188, 196–202. [Google Scholar] [CrossRef]
- Koller, T.M.; Rausch, M.H.; Ramos, J.; Schulz, P.S.; Wasserscheid, P.; Economou, I.G.; Fröba, A.P. Thermophysical Properties of the Ionic Liquids [EMIM][B(CN)4] and [HMIM][B(CN)4]. J. Phys. Chem. B 2013, 117, 8512–8523. [Google Scholar] [CrossRef]
- Koller, T.M.; Schmid, S.R.; Sachnov, S.J.; Rausch, M.H.; Wasserscheid, P.; Fröba, A.P. Measurement and Prediction of the Thermal Conductivity of Tricyanomethanide- and Tetracyanoborate-Based Imidazolium Ionic Liquids. Int. J. Thermophys. 2014, 35, 195–217. [Google Scholar] [CrossRef]
- Oezdemir, M.C.; Oezgün, B. Phenyl/alkyl-substituted-3,5-dimethylpyrazolium ionic liquids. J. Mol. Liq. 2014, 200, 129–135. [Google Scholar] [CrossRef]
- Seki, S.; Tsuzuki, S.; Hayamizu, K.; Serizawa, N.; Ono, S.; Takei, K.; Doi, H.; Umebayashi, Y. Static and Transport Properties of Alkyltrimethylammonium Cation-Based Room-Temperature Ionic Liquids. J. Phys. Chem. B 2014, 118, 4590–4599. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Ma, T.; Ma, X.; Guan, W.; Liu, Q.; Yang, J. Study on thermodynamic properties and estimation of polarity of ionic liquids {[Cnmmim]-[NTf2] (n = 2, 4)}. RSC Adv. 2014, 4, 30725–30732. [Google Scholar] [CrossRef]
- Meng, Y.; Liu, J.; Li, Z.; Wei, H. Synthesis and Physicochemical Properties of Two SO3H-Functionalized Ionic Liquids with Hydrogen Sulfate Anion. J. Chem. Eng. Data 2014, 59, 2186–2195. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Carvalho, P.J.; Coutinho, J.A.P. The effect of the cation aromaticity upon the thermophysicalproperties of piperidinium- and pyridinium-based ionic liquids. Fluid Phase Equil. 2014, 375, 80–88. [Google Scholar] [CrossRef]
- Umapathi, R.; Attri, P.; Venkatesu, P. Thermophysical Properties of Aqueous Solution of Ammonium-Based Ionic Liquids. J. Phys. Chem. B 2014, 118, 5971–5982. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Luis, A.; Santos, J.H.; Lopes-da-Silva, J.A.; Freire, M.G.; Carvalho, P.J.; Coutinho, J.A.P. Thermophysical properties of sulfonium- and ammonium-based ionic liquids. Fluid Phase Equil. 2014, 381, 36–45. [Google Scholar] [CrossRef] [Green Version]
- Pinto, R.R.; Santos, D.; Mattedi, S.; Aznar, M. Density, refractive index, apparent volumes and excess molar Volumes of four protic ionic liquids + water at T = 298.15 and 323.15 K. Braz. J. Chem. Eng. 2015, 32, 671–682. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, A.S.M.C.; Rocha, M.A.A.; Almeida, H.F.D.; Neves, C.M.S.S.; Lopes-da-Silva, J.A.; Freire, M.G.; Coutinho, J.A.P.; Santos, L.M.N.B.F. Effect of the Methylation and N−H Acidic Group on the Physicochemical Properties of Imidazolium-Based Ionic Liquids. J. Phys. Chem. B 2015, 119, 8781–8792. [Google Scholar] [CrossRef] [Green Version]
- Gonfa, G.; Bustam, M.A.; Muhammad, N.; Khan, A.S. Evaluation of Thermophysical Properties of Functionalized Imidazolium Thiocyanate Based Ionic Liquids. Ind. Eng. Chem. Res. 2015, 54, 12428–12437. [Google Scholar] [CrossRef]
- Rao, S.G.; Mohan, T.M.; Krishna, T.V.; Raju, K.T.S.S.; Rao, B.S. Excess thermodynamic properties of ionic liquid 1-butyl-3-methylimidazolium tetrafluoroborate and N-octyl-2-pyrrolidone from T = (298.15 to 323.15) K at atmospheric pressure. J. Chem. Thermodyn. 2015, 89, 286–295. [Google Scholar]
- Sattari, M.; Kamari, A.; Mohammadi, A.H.; Ramjugernath, D. Prediction of refractive indices of ionic liquids—A quantitative structure-property relationship based model. J. Taiwan Inst. Chem. Eng. 2015, 52, 165–180. [Google Scholar] [CrossRef]
- Soucknova, M.; Klomfar, J.; Patek, J. Surface tension and 0.1 MPa density data for 1-Cn-3-methylimidazolium iodides with n = 3, 4, and 6, validated using a parachor and group contribution model. J. Chem. Thermodyn. 2015, 83, 52–60. [Google Scholar] [CrossRef]
- Vatascin, E.; Dohnal, V. Thermophysical properties of aqueous solutions of the 1-ethyl-3-methylimidazolium tricyanomethanide ionic liquid. J. Chem. Thermodyn. 2015, 89, 169–176. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Lopes-da-Silva, J.A.; Freire, M.G.; Coutinho, J.A.P.; Carvalho, P.J. Thermophysical properties of phosphonium-based ionic liquids. Fluid Phase Equil. 2015, 400, 103–113. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharjee, A.; Coutinho, J.A.P.; Freire, M.G.; Carvalho, P.J. Thermophysical Properties of Two Ammonium-Based Protic Ionic Liquids. J. Solut. Chem. 2015, 44, 703–717. [Google Scholar] [CrossRef] [Green Version]
- Akbar, M.M.; Chemat, F.; Arunagiri, A.; Murugesan, T. Density and excess properties of N-methyldiethanolamine (MDEA) with 1-hexyl-3-methylimidazolium tris(pentafluoroethyl)trifluorophosphate [hmim][FAP]. J. Therm. Anal. Calorim. 2016, 123, 785–791. [Google Scholar] [CrossRef]
- Gouveia, A.S.L.; Tome, L.C.; Marrucho, I.M. Density, Viscosity, and Refractive Index of Ionic Liquid Mixtures Containing Cyano and Amino Acid-Based Anions. J. Chem. Eng. Data 2016, 61, 83–93. [Google Scholar] [CrossRef]
- Xue, L.; Gurung, E.; Tamas, G.; Koh, Y.P.; Shadeck, M.; Simon, S.l.; Maroncelli, M.; Quitevis, E.L. Effect of Alkyl Chain Branching on Physicochemical Properties of Imidazolium-Based Ionic Liquids. J. Chem. Eng. Data 2016, 61, 1078–1091. [Google Scholar] [CrossRef]
- Kumar, A.V.; Redhi, G.G.; Gengan, R.M. Influence of Epoxy Group in 2-Pyrrolidonium Ionic Liquid Interactions and Thermo-Physical Properties with Ethanoic or Propanoic Acid at Various Temperatures. ACS Sustain. Chem. Eng. 2016, 4, 4951–4964. [Google Scholar] [CrossRef]
- Chen, F.-F.; Dong, Y.; Sang, X.-Y.; Zhou, Y.; Tao, D.-J. Physicochemical Properties and CO2 Solubility of Tetrabutylphosphonium Carboxylate Ionic Liquids. Acta Phys. Chim. Sin. 2016, 32, 605–610. [Google Scholar] [CrossRef]
- Ye, F.; Zhu, J.; Yu, K.; Zhu, R.; Xu, Y.; Chen, J.; Chen, L. Physicochemical properties of binary mixtures of 1,1,3,3-tetramethylguanidine imidazolide ionic liquid with water and alcohols. J. Chem. Thermodyn. 2016, 97, 39–47. [Google Scholar] [CrossRef]
- Segade, L.; Cabanas, M.; Dominguez-Perez, M.; Rilo, E.; Garcia-Garabal, S.; Turmine, M.; Varela, L.M.; Gomez-Gonzalez, V.; Docampo-Alvarez, B.; Cabeza, O. Surface and bulk characterisation of mixtures containing alkylammonium nitrates and water or ethanol: Experimental and simulated properties at 298.15 K. J. Mol. Liq. 2016, 222, 663–670. [Google Scholar] [CrossRef]
- Rodrigues, A.S.M.C.; Almeida, H.F.D.; Freire, M.G.; Lopes-da-Silva, J.A.; Coutinho, J.A.P.; Santos, L.M.N.B.F. The effect of n vs. iso isomerization on the thermophysical properties of aromatic and non-aromatic ionic liquids. Fluid Phase Equil. 2016, 423, 190–202. [Google Scholar] [CrossRef] [Green Version]
- Xing, N.; Dai, B.; Ma, X.; Wei, J.; Pan, Y.; Guan, W. The molar surface Gibbs energy and prediction of surface tension of [Cnpy][DCA] (n = 3,4,5). J. Chem. Thermodyn. 2016, 95, 21–25. [Google Scholar] [CrossRef]
- Shekaari, H.; Zafarani-Moattar, M.T.; Niknam, M. Thermodynamic evaluation of imidazolium based ionic liquids with thiocyanate anion as effective solvent to thiophene extraction. J. Mol. Liq. 2016, 219, 975–984. [Google Scholar] [CrossRef]
- Garcia-Andreu, M.; Castro, M.; Gascon, I.; Lafuente, C. Thermophysical characterization of 1-ethylpyridinium triflate and comparison with similar ionic liquids. J. Chem. Thermodyn. 2016, 103, 395–402. [Google Scholar] [CrossRef]
- Xu, Y. CO2 absorption behavior of azole-based protic ionic liquids: Influence of the alkalinity and physicochemical properties. J. CO2 Utiliz. 2017, 19, 1–8. [Google Scholar] [CrossRef]
- Nazet, A.; Sokolov, S.; Sonnleitner, T.; Friesen, S.; Buchner, R. Densities, Refractive Indices, Viscosities, and Conductivities of Non-Imidazolium Ionic Liquids [Et3S][TFSI], [Et2MeS][TFSI], [BuPy][TFSI], [N8881][TFA], and [P14][DCA]. J. Chem. Eng. Data 2017, 62, 2549–2561. [Google Scholar] [CrossRef]
- Lee, K.-H.; Park, S.-J.; Choi, Y.-Y. Density, refractive index and kinematic viscosity of MIPK, MEK and phosphonium-based ionic liquids and the excess and deviation properties of their binary systems. Korean J. Chem. Eng. 2017, 34, 214–224. [Google Scholar] [CrossRef]
- Sardar, S.; Wilfred, C.D.; Mumtaz, A.; Leveque, J.-M. Investigation of the Thermophysical Properties of AMPS-Based Aprotic Ionic Liquids for Potential Application in CO2 Sorption Processes. J. Chem. Eng. Data 2017, 62, 4160–4168. [Google Scholar] [CrossRef]
- Zhang, D.; Gong, Y.-Y.; Tong, J.; Fang, D.-W.; Tong, J. Physicochemical properties of [C n mim][Thr](n = 3,5,6) amino acid ionic liquids. J. Chem. Thermodyn. 2017, 115, 47–51. [Google Scholar] [CrossRef]
- Arosa, Y.; Fernandez, C.D.R.; Lago, E.L.; Amigo, A.; Varela, L.M.; Cabeza, O.; de la Fuente, R. Refractive index measurement of imidazolium based ionic liquids in the Vis-NIR. Opt. Mat. 2017, 73, 647–657. [Google Scholar] [CrossRef]
- Arumugam, V.; Redhi, G.G.; Gengan, R.M. Synthesis, characterization and thermophysical properties of novel 2′,3′-N-epoxypropyl-N-methyl-2-oxopyrrolidinium acetate ionic liquid and their binary mixture with water or methanol. J. Mol. Liq. 2017, 242, 1215–1227. [Google Scholar] [CrossRef]
- Pereiro, A.B.; Llovell, F.; Araujo, J.M.M.; Santos, A.S.S.; Rebelo, L.P.N.; Pineiro, M.M.; Vega, L.F. Thermophysical Characterization of Ionic Liquids Based on the Perfluorobutanesulfonate Anion: Experimental and Soft-SAFT Modeling Results. ChemPhysChem 2017, 18, 2012–2023. [Google Scholar] [CrossRef]
- Venkatraman, V.; Raj, J.J.; Evjen, S.; Lethesh, K.C.; Fiksdahl, A. In silico prediction and experimental verification of ionic liquid refractive indices. J. Mol. Liq. 2018, 264, 563–570. [Google Scholar] [CrossRef]
- Marcinkowski, L.; Szepiński, E.; Milewska, M.J.; Kloskowski, A. Density, sound velocity, viscosity, and refractive index of new morpholinium ionic liquids with amino acid-based anions: Effect of temperature, alkyl chain length, and anion. J. Mol. Liq. 2019, 284, 557–568. [Google Scholar] [CrossRef]
- Ferreira, M.L.; Araújo, J.M.M.; Vega, L.F.; Llovell, F.; Pereiro, A.B. Functionalization of fluorinated ionic liquids: A combined experimental-theoretical study. J. Mol. Liq. 2020, 302, 112489. [Google Scholar] [CrossRef]
- Wu, X.-L.; Sang, X.-Y.; Li, Z.-M.; Tao, D.-J. Study on physicochemical properties and basicity of carbanionfunctionalized ionic liquids. J. Mol. Liq. 2020, 312, 113405. [Google Scholar] [CrossRef]
- Song, Z.; Yan, Q.; Xia, M.; Qi, X.; Zhang, Z.; Wei, J.; Fang, D.; Ma, X. Physicochemical properties of N-alkylpyridine trifluoroacetate ionic liquids [Cn Py][TFA] (n = 2–6). J. Chem. Thermodyn. 2021, 155, 106366. [Google Scholar] [CrossRef]
- Fei, Y.; Chen, Z.; Zhang, J.; Yu, M.; Kong, J.; Wu, Z.; Cao, J.; Zhang, J. Thiazolium-based ionic liquids: Synthesis, characterization and physicochemical properties. J. Mol. Liq. 2021, 342, 117553. [Google Scholar] [CrossRef]
- Aljasmi, A.; Aljimaz, A.S.; Alkhaldi, K.H.A.E.; AlTuwaim, M.S. Dependency of Physicochemical Properties of Imidazolium Bis(Trifluoromethylsulfonyl)Imide-Based Ionic Liquids on Temperature and Alkyl Chain. J. Chem. Eng. Data 2022, 67, 858–868. [Google Scholar] [CrossRef]
- Kong, J.; Liu, L.; Li, X.; Yang, Y.; Chen, X.; Fei, Y.; Xu, L.; Chen, Z. Dimethylthioformamide-derived ionic liquids: Synthesis, characterization and application as supercapacitor electrolyte. J. Mol. Liq. 2022, 365, 120114. [Google Scholar] [CrossRef]
- Vogel, A.I. 369. Physical properties and chemical constitution. Part XXIII. Miscellaneous compounds. Investigation of the so-called co-ordinate or dative link in esters of oxy-acids and in nitro-paraffins by molecular refractivity determinations. atomic, structural, and group parachors and refractivities. J. Chem. Soc. 1948, 1833–1855. [Google Scholar] [CrossRef]
- Vogel, A.I.; Cresswell, W.T.; Jeffrey, G.H.; Leicester, J. 98. Physical properties and chemical constitution. Part XXIV. Aliphatic aldoximes, ketoximes, and ketoxime O-alkyl ethers, NN-dialkylhydrazines, aliphatic ketazines, mono- and di-alkylaminopropionitriles, alkoxypropionitriles, dialkyl azodiformates, and dialkyl carbonates. Bond parachors, bond refractions, and bond refraction coefficients. J. Chem. Soc. 1952, 514–549. [Google Scholar] [CrossRef]
- Shuikin, N.I.; Bel’skii, I.F. Catalytic hydrogenolysis in the series of furan compounds. Zh. Obsh. Khim. 1957, 27, 402–406. [Google Scholar]
- Yur’ev, Y.K.; Novitskii, K.Y.; Bolesov, I.G. Furan series. I. Synthesis of N-(β-hydroxyalkyl)furfurylamines. Zh. Obsh. Khim. 1959, 29, 2951–2954. [Google Scholar]
- Petrov, A.A.; Mingaleva, K.S.; Zavgorodnii, V.S. Chemistry of unsaturated stannyl hydrocarbons. IV. Dipole moments of alkyl-, alkenyl-, and phenylacetylenic stannyl hydrocarbons. Zh. Obsh. Khim. 1964, 34, 533–535. [Google Scholar]
- Bel’skii, I.F.; Shuikin, N.I.; Vol’nova, Z. K Synthesis and isomerization of 2,2-dialkyl-5-propyltetrahydrofurans. Izvest Akad. Nauk SSSR Ser Khim. 1964, 2, 369–371. [Google Scholar] [CrossRef]
- Shuikin, N.I.; Lebedev, B.L.; Nikol’skii, V.G. Vinylation of cyclanes and cyclic ethers. Dokl. Akad. Nauk SSSR 1964, 158, 692–693. [Google Scholar]
- Arrowsmith, G.B.; Jeffery, G.H. Vogel, Physical Properties and Chemical Constitution. Part XLI. Naphthalene Compounds. J. Chem. Soc. 1965, 2072–2078. [Google Scholar] [CrossRef]
- Bel’skii, I.F.; Khar’kov, S.N.; Shuikin, N.I. Synthesis of 3-oxa-5-oxo alcohols and a study of their tautomeric transformations to form dioxenes. Dokl. Akad. Nauk SSSR 1965, 165, 1071–1074. [Google Scholar]
- Wiswanadhan, V.N.; Ghose, A.K.; Revankar, G.R.; Robins, R.K. Atomic Physicochemical Parameters for Three Dimensional Structure Directed Quantitative Structure-Activity Relationships. 4. Additional Parameters for Hydrophobic and Dispersive Interactions and Their Application for an Automated Superposition of Certain Naturally Occurring Nucleoside Antibiotics. J. Chem. Inf. Comput. Sci. 1989, 29, 163–172. [Google Scholar]
- Pacak, P. Refractivity and density of some organic solvents. Chem. Pap. 1991, 45, 227–232. [Google Scholar]
- Oswal, S.; Patel, A.T. Speeds of Sound, Isentropic Compressibilities, and Excess Volumes of Binary Mixtures. 1. Tri-n-alkylamines with Cyclohexane and Benzene. J. Chem. Eng. Data 1994, 39, 366–371. [Google Scholar] [CrossRef]
- Gao, D.; Zhu, D.; Zhang, L.; Guan, H.; Sun, H.; Chen, H.; Si, J. Isobaric Vapor-Liquid Equilibria for Binary and Ternary Mixtures of Propanal, Propanol, and Propanoic Acid. J. Chem. Eng. Data 2010, 55, 5887–5895. [Google Scholar] [CrossRef]
- Chen, K.-D.; Lin, Y.-F.; Tu, C.-H. Densities, Viscosities, Refractive Indexes, and Surface Tensions for Mixtures of Ethanol, Benzyl Acetate, and Benzyl Alcohol. J. Chem. Eng. Data 2012, 57, 1118–1127. [Google Scholar] [CrossRef]
- Kumar, S.; Jeevanandham, P. Densities, viscosities, refractive indices and excess properties of aniline and o-anisidine with 2-alkoxyethanols at 303.15 K. J. Mol. Liq. 2012, 174, 34–41. [Google Scholar] [CrossRef]
- Jeevanandham, P.; Kumar, S.; Periyasamy, P. Densities, viscosities, refractive indices and excess properties of ortho- and meta-chloroaniline with 2-alkoxyethanols at 303.15 K. J. Mol. Liq. 2013, 188, 203–209. [Google Scholar] [CrossRef]
- Cai, C.; Marsh, A.; Zhang, Y.-H.; Reid, J.P. Group Contribution Approach To Predict the Refractive Index of Pure Organic Components in Ambient Organic Aerosol. Environ. Sci. Technol. 2017, 51, 9683–9690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dantas, C.E.S.; Ceriani, R. Liquid−Liquid Equilibria of Ternary Mixtures Containing n-Tetradecane + γ-Valerolactone + Aldehyde [Butanal or Pentanal or (E)-2-Undecenal] at 298.15 K. J. Chem. Eng. Data 2022, 67, 393–403. [Google Scholar] [CrossRef]
- Sema, T.; Gao, H.; Liang, Z.; Tontiwachwuthikul, P.; Idem, R.O. New Insights and Updated Correlations for Density, Viscosity, Refractive Index, and Associated Properties of Aqueous 4-Diethyl-Amino-2-Butanol Solution. Fluid Phase Equil. 2022, 562, 113565. [Google Scholar] [CrossRef]
- Applequist, J.; Carl, J.R.; Fung, K.-K. An Atom Dipole Interaction Model for Molecular Polarizability. Application to Polyatomic Molecules and Determination of Atom Polarizabilities. J. Am. Chem. Soc. 1972, 94, 2952–2960. [Google Scholar] [CrossRef]
- Thole, B.T. Molecular polarizabilities calculated with a modified dipole interaction. Chem. Phys. 1981, 59, 341–350. [Google Scholar] [CrossRef]
- Nagle, J.K. Atomic Polarizability and Electronegativity. J. Am. Chem. Soc. 1990, 112, 4741–4747. [Google Scholar] [CrossRef]
- Miller, K.J. Calculation of the Molecular Polarizability Tensor. J. Am. Chem. Soc. 1990, 112, 8543–8551. [Google Scholar] [CrossRef]
- Cheng, L.-T.; Tam, W.; Stevenson, S.H.; Meredith, G.R.; Rikken, G.; Marder, S.R. Experimental Investigations of Organic Molecular Nonlinear Optical Polarizabilities. 1. Methods and Results on Benzene and Stilbene Derivatives. J. Phys. Chem. 1991, 95, 10631–10643. [Google Scholar] [CrossRef]
- Lu, Y.-J.; Lee, S.-L. Semi-empirical calculations of the nonlinear optical properties of polycyclic aromatic compounds. Chem. Phys. 1994, 179, 431–444. [Google Scholar] [CrossRef]
- Schürer, G.; Gedeck, P.; Gottschalk, M.; Clark, T. Accurate Parametrized Variational Calculations of the Molecular Electronic Polarizability by NDDO-Based Methods. Intern. J. Quantum Chem. 1999, 75, 17–31. [Google Scholar] [CrossRef]
- Bosque, R.; Sales, J. Polarizabilities of Solvents from the Chemical Composition. J. Chem. Inf. Comput. Sci. 2002, 42, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xie, X.-Q.; Hou, T.; Xu, X. Fast Approaches for Molecular Polarizability Calculations. Phys. Chem. A 2007, 111, 4443–4448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murthy, A.R.; Giridhar, G.; Rangacharyulu, M. Molecular Polarizabilities of Some Liquid Crystal Compounds. Chin. J. Phys. 2008, 46, 54–62. [Google Scholar]
- Cao, X.; Hancock, B.C.; Leyva, N.; Becker, J.; Yu, W.; Masterson, V.M. Estimating the refractive index of pharmaceutical solids using predictive methods. Int. J. Pharm. 2009, 368, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Naef, R.; Acree, W.E., Jr. Revision and Extension of a Generally Applicable Group-Additivity Method for the Calculation of the Standard Heat of Combustion and Formation of Organic Molecules. Molecules 2021, 26, 6101. [Google Scholar] [CrossRef] [PubMed]
- Naef, R.; Acree, W.E., Jr. Calculation of Five Thermodynamic Molecular Descriptors by Means of a General Computer Algorithm Based on the Group-Additivity Method: Standard Enthalpies of Vaporization, Sublimation and Solvation, and Entropy of Fusion of Ordinary Organic Molecules and Total Phase-Change Entropy of Liquid Crystals. Molecules 2017, 22, 1059. [Google Scholar] [CrossRef]
- Naef, R.; Acree, W.E., Jr. Calculation of the Surface Tension of Ordinary Organic and Ionic Liquids by Means of a Generally Applicable Computer Algorithm Based on the Group-Additivity Method. Molecules 2018, 23, 1224. [Google Scholar] [CrossRef] [Green Version]
- Naef, R. Calculation of the Isobaric Heat Capacities of the Liquid and Solid Phase of Organic Compounds at 298.15K by Means of the Group-Additivity Method. Molecules 2020, 25, 1147. [Google Scholar] [CrossRef] [Green Version]
- Naef, R.; Acree, W.E., Jr. Calculation of the Vapour Pressure of Organic Molecules by Means of a Group-Additivity Method and Their Resultant Gibbs Free Energy and Entropy of Vaporization at 298.15 K. Molecules 2021, 26, 1045. [Google Scholar] [CrossRef]
- Gough, K.M. Theoretical analysis of molecular polarizabilities and polarizability derivatives in hydrocarbons. J. Chem. Phys. 1989, 91, 2424. [Google Scholar] [CrossRef]
- Laidig, K.E.; Bader, R.F.W. Properties of atoms in molecules: Atomic polarizabilities. J. Chem. Phys. 1990, 93, 7213. [Google Scholar] [CrossRef]



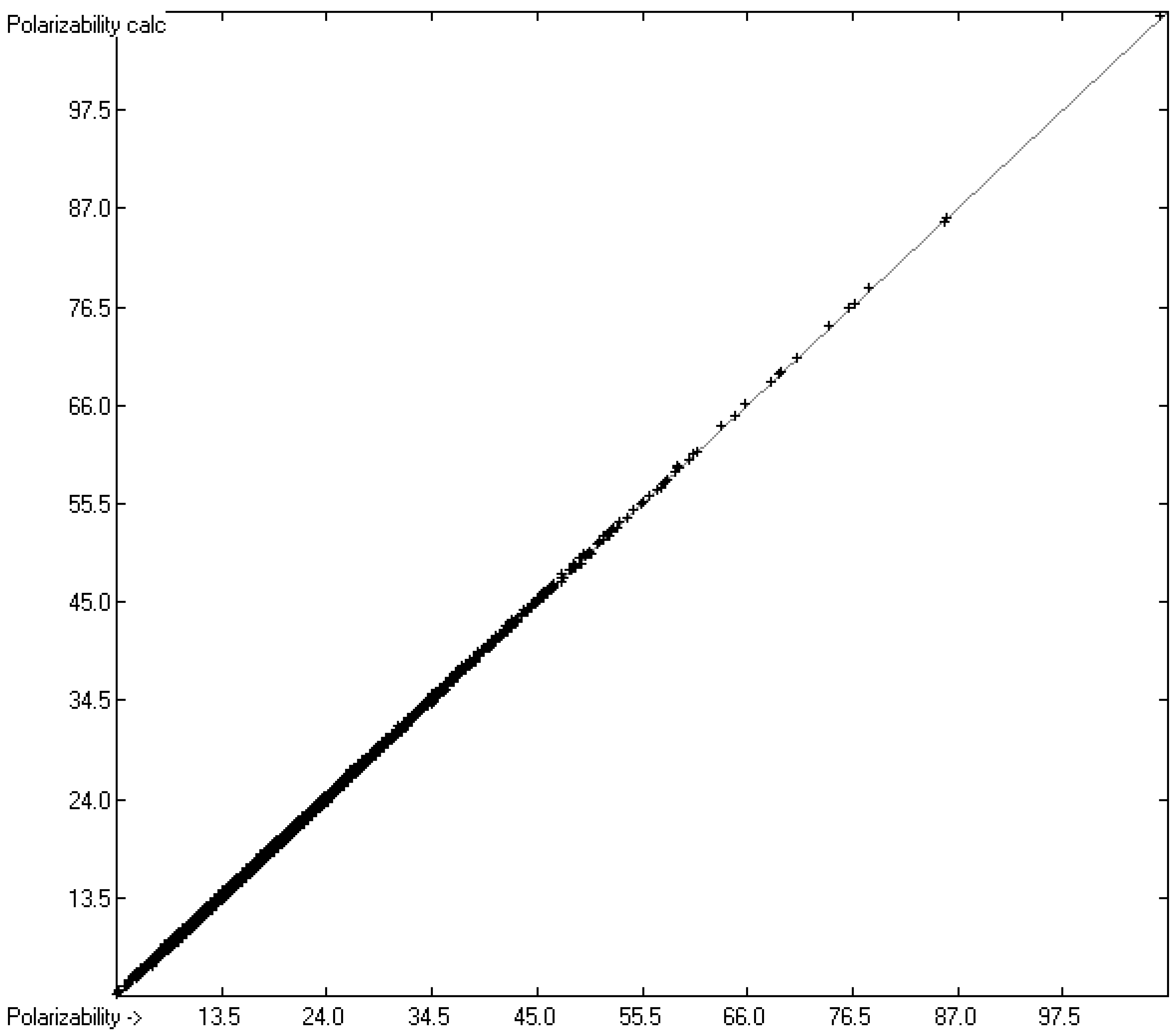



| No | Atom Type | Neighbors | Meaning | Example |
|---|---|---|---|---|
| 1 | B | HN2 | HBN2 | Bis(butylamino)borane |
| 7 | B | CO2 | CBO2 | Phenyl dimethoxyborine |
| 21 | B | O3 | BO3 | Triethoxyborine |
| 25 | B(−) | C4 | BC4− | Tetracyanoborate |
| 152 | C(−) sp3 | C3 | C–C−(C)–C | Tricyanomethanide |
| 245 | C aromatic | H:C:N(+) | C:CH:N+ | C2 in pyridinium |
| 272 | C(+) aromatic | H:N2 | N:C+(H):N | C2 in imidazolium |
| 280 | C sp | C#N(−) | N#C–C− | Tricyanomethanide |
| 290 | C sp | N#N(−) | N#C–N− | Dicyanoamide |
| 295 | C sp | =N=S(−) | N=C=S− | Thiocyanate |
| 316 | N sp3 | HSi2 | HNSi2 | Bis(trimethoxysilyl)amine |
| 352 | N(+) sp3 | C4 | NC4+ | Tetraalkylammonium |
| 359 | N(+) sp2 | O2=O(−) | NO3− | Nitrate |
| 363 | N aromatic | C2:C(+) | C–N(C):C+ | N1 in 1-alkylimidazolium |
| 365 | N(+) aromatic | C:C2 | C:N+(C):C | N in 1-alkylpyridinium |
| 370 | N(−) | C2 | C–N−–C | Dicyanoamide |
| 398 | O | CSi | COSi | Bis(trimethoxysilyl)amine |
| 502 | S4 | CO=O2(−) | C–SO3− | Methylsulfonate |
| 512 | S4 | O2=O2(−) | SO4− | Hydrosulfate |
| 533 | Si | C3O | C3SiO | Methoxytrimethylsilane |
| 539 | Si | C2N2 | C2SiN2 | Diethyldiisothiocyanatosilane |
| 554 | Si | NO3 | NSiO3 | Bis(trimethoxysilyl)amine |
| Entry | Atom Type | Neighbors | Contribution | Occurrences | Molecules |
|---|---|---|---|---|---|
| 1 | B | HN2 | 39.13 | 15 | 11 |
| 2 | B | HNS | 46.42 | 2 | 2 |
| 3 | B | HO2 | 28.1 | 1 | 1 |
| 4 | B | HS2 | 52.49 | 5 | 5 |
| 5 | B | C3 | 66.64 | 4 | 4 |
| 6 | B | C2N | 62.25 | 8 | 8 |
| 7 | B | C2O | 64.24 | 22 | 15 |
| 8 | B | C2S | 69.73 | 10 | 10 |
| 9 | B | CN2 | 58.53 | 2 | 2 |
| 10 | B | CNO | 59.28 | 1 | 1 |
| 11 | B | CNS | 65.63 | 1 | 1 |
| 12 | B | CO2 | 61.69 | 31 | 26 |
| 13 | B | COCl | 48.7 | 4 | 4 |
| 14 | B | CS2 | 72.57 | 8 | 8 |
| 15 | B | CSCl | 53.86 | 2 | 2 |
| 16 | B | CSBr | 56.93 | 4 | 4 |
| 17 | B | CCl2 | 36.22 | 8 | 6 |
| 18 | B | N3 | 54.93 | 4 | 2 |
| 19 | B | N2O | 56.24 | 4 | 2 |
| 20 | B | NO2 | 58.3 | 1 | 1 |
| 21 | B | O3 | 59.3 | 21 | 21 |
| 22 | B | O2Cl | 46.04 | 8 | 8 |
| 23 | B | OCl2 | 33.44 | 6 | 6 |
| 24 | B | S3 | 75.69 | 3 | 3 |
| 25 | B(−) | C4 | 74.59 | 5 | 5 |
| 26 | B(−) | O2F2 | 63.86 | 4 | 4 |
| 27 | B(−) | F4 | −2.41 | 18 | 18 |
| 28 | C sp3 | H3C | 5.7 | 9421 | 4296 |
| 29 | C sp3 | H3C(+) | 58.97 | 3 | 3 |
| 30 | C sp3 | H3N | 12.24 | 387 | 246 |
| 31 | C sp3 | H3N(+) | 19.98 | 76 | 55 |
| 32 | C sp3 | H3O | 11.65 | 505 | 359 |
| 33 | C sp3 | H3S | 11.49 | 79 | 55 |
| 34 | C sp3 | H3S(+) | 16.94 | 2 | 2 |
| 35 | C sp3 | H3P | 14.04 | 17 | 16 |
| 36 | C sp3 | H3P(+) | 11.23 | 2 | 2 |
| 37 | C sp3 | H3Si | 10.79 | 626 | 180 |
| 38 | C sp3 | H2BC | −16.41 | 97 | 53 |
| 39 | C sp3 | H2C2 | 4.63 | 15,752 | 3454 |
| 40 | C sp3 | H2CN | 11.13 | 1078 | 561 |
| 41 | C sp3 | H2CN(+) | 18.6 | 223 | 129 |
| 42 | C sp3 | H2CO | 10.61 | 2562 | 1560 |
| 43 | C sp3 | H2CS | 10.63 | 395 | 253 |
| 44 | C sp3 | H2CS(+) | 15.8 | 13 | 5 |
| 45 | C sp3 | H2CP | 12.84 | 239 | 171 |
| 46 | C sp3 | H2CP(+) | 12.03 | 46 | 12 |
| 47 | C sp3 | H2CF | 5.62 | 33 | 28 |
| 48 | C sp3 | H2CCl | 10.5 | 229 | 190 |
| 49 | C sp3 | H2CBr | 13.44 | 144 | 127 |
| 50 | C sp3 | H2CJ | 18.58 | 39 | 35 |
| 51 | C sp3 | H2CSi | 9.66 | 345 | 154 |
| 52 | C sp3 | H2N2 | 17.9 | 2 | 2 |
| 53 | C sp3 | H2NO | 18.9 | 1 | 1 |
| 54 | C sp3 | H2NS | 17.11 | 1 | 1 |
| 55 | C sp3 | H2O2 | 16.37 | 19 | 18 |
| 56 | C sp3 | H2OCl | 16.45 | 8 | 7 |
| 57 | C sp3 | H2OBr | 20.6 | 7 | 7 |
| 58 | C sp3 | H2S2 | 16.43 | 10 | 5 |
| 59 | C sp3 | H2SCl | 16.52 | 4 | 4 |
| 60 | C sp3 | H2SJ | 21.65 | 2 | 2 |
| 61 | C sp3 | H2SiCl | 15.47 | 6 | 5 |
| 62 | C sp3 | H2SiBr | 18.54 | 4 | 3 |
| 63 | C sp3 | H2Si2 | 14.42 | 5 | 3 |
| 64 | C sp3 | HBC2 | −17.46 | 10 | 7 |
| 65 | C sp3 | HC3 | 3.52 | 1516 | 1044 |
| 66 | C sp3 | HC2N | 9.99 | 135 | 114 |
| 67 | C sp3 | HC2N(+) | 17.27 | 13 | 13 |
| 68 | C sp3 | HC2O | 9.55 | 561 | 462 |
| 69 | C sp3 | HC2P | 11.84 | 23 | 21 |
| 70 | C sp3 | HC2S | 9.55 | 75 | 53 |
| 71 | C sp3 | HC2F | 8.42 | 2 | 2 |
| 72 | C sp3 | HC2Cl | 9.39 | 84 | 74 |
| 73 | C sp3 | HC2Br | 12.42 | 80 | 70 |
| 74 | C sp3 | HC2J | 17.84 | 7 | 7 |
| 75 | C sp3 | HC2Si | 8.64 | 29 | 15 |
| 76 | C sp3 | HCN2(+) | 31.68 | 1 | 1 |
| 77 | C sp3 | HCNCl(+) | 23.09 | 2 | 2 |
| 78 | C sp3 | HCO2 | 15.36 | 53 | 45 |
| 79 | C sp3 | HCOF | 11.6 | 2 | 2 |
| 80 | C sp3 | HCOCl | 15.53 | 14 | 9 |
| 81 | C sp3 | HCOBr | 20.2 | 1 | 1 |
| 82 | C sp3 | HCS2 | 15.5 | 11 | 11 |
| 83 | C sp3 | HCSCl | 14.69 | 1 | 1 |
| 84 | C sp3 | HCF2 | 5.59 | 29 | 19 |
| 85 | C sp3 | HCFCl | 10.61 | 7 | 6 |
| 86 | C sp3 | HCFBr | 13.45 | 1 | 1 |
| 87 | C sp3 | HCCl2 | 15.36 | 33 | 32 |
| 88 | C sp3 | HCClBr | 18.18 | 5 | 5 |
| 89 | C sp3 | HCClJ | 22.97 | 2 | 2 |
| 90 | C sp3 | HCBr2 | 20.98 | 13 | 12 |
| 91 | C sp3 | HCBrJ | 25.95 | 1 | 1 |
| 92 | C sp3 | HCJ2 | 31.39 | 2 | 2 |
| 93 | C sp3 | HNO2 | 21.67 | 2 | 2 |
| 94 | C sp3 | HO3 | 21.51 | 9 | 9 |
| 95 | C sp3 | HOF2 | 11.17 | 1 | 1 |
| 96 | C sp3 | HOCl2 | 21.99 | 1 | 1 |
| 97 | C sp3 | HS3 | 22.99 | 2 | 2 |
| 98 | C sp3 | HSiCl2 | 20.41 | 5 | 4 |
| 99 | C sp3 | C4 | 2.47 | 299 | 251 |
| 100 | C sp3 | C3N | 8.92 | 25 | 21 |
| 101 | C sp3 | C3N(+) | 16.1 | 2 | 2 |
| 102 | C sp3 | C3O | 8.42 | 125 | 113 |
| 103 | C sp3 | C3S | 8.86 | 21 | 14 |
| 104 | C sp3 | C3P | 11.38 | 1 | 1 |
| 105 | C sp3 | C3F | 3.41 | 5 | 4 |
| 106 | C sp3 | C3Cl | 8.47 | 7 | 7 |
| 107 | C sp3 | C3Br | 11.46 | 7 | 7 |
| 108 | C sp3 | C3J | 16.82 | 3 | 3 |
| 109 | C sp3 | C3Si | 8.05 | 1 | 1 |
| 110 | C sp3 | C2NCl(+) | 22.78 | 1 | 1 |
| 111 | C sp3 | C2O2 | 14.18 | 15 | 15 |
| 112 | C sp3 | C2OCl | 14.76 | 4 | 4 |
| 113 | C sp3 | C2OS | 14 | 1 | 1 |
| 114 | C sp3 | C2OP | 17.43 | 3 | 3 |
| 115 | C sp3 | C2S2 | 14.73 | 14 | 14 |
| 116 | C sp3 | C2Si2 | 10.98 | 1 | 1 |
| 117 | C sp3 | C2F2 | 5.01 | 145 | 40 |
| 118 | C sp3 | C2FCl | 9.6 | 3 | 3 |
| 119 | C sp3 | C2Cl2 | 14.26 | 29 | 26 |
| 120 | C sp3 | C2ClBr | 17.66 | 3 | 3 |
| 121 | C sp3 | C2Br2 | 20.24 | 6 | 6 |
| 122 | C sp3 | C2J2 | 30.57 | 1 | 1 |
| 123 | C sp3 | CNF2 | 11.26 | 8 | 3 |
| 124 | C sp3 | CNF2(+) | 19.26 | 2 | 1 |
| 125 | C sp3 | CO3 | 19.63 | 7 | 7 |
| 126 | C sp3 | CO2Si | 17.49 | 1 | 1 |
| 127 | C sp3 | COF2 | 11.48 | 9 | 8 |
| 128 | C sp3 | COFCl | 16.44 | 1 | 1 |
| 129 | C sp3 | CS3 | 25.53 | 2 | 2 |
| 130 | C sp3 | CSF2 | 10.98 | 4 | 3 |
| 131 | C sp3 | CSCl2 | 22.95 | 1 | 1 |
| 132 | C sp3 | CSiBr2 | 32.18 | 1 | 1 |
| 133 | C sp3 | CF3 | 5.98 | 98 | 75 |
| 134 | C sp3 | CF2Cl | 10.67 | 16 | 14 |
| 135 | C sp3 | CF2Br | 13.41 | 6 | 5 |
| 136 | C sp3 | CF2J | 18.51 | 4 | 3 |
| 137 | C sp3 | CPF2(−) | 8.9 | 6 | 2 |
| 138 | C sp3 | CFCl2 | 15.39 | 8 | 6 |
| 139 | C sp3 | CCl3 | 20.42 | 34 | 31 |
| 140 | C sp3 | CCl2Br | 23.23 | 3 | 3 |
| 141 | C sp3 | CBr3 | 29.63 | 4 | 3 |
| 142 | C sp3 | N2F2(+) | 32.23 | 1 | 1 |
| 143 | C sp3 | O4 | 25.69 | 3 | 3 |
| 144 | C sp3 | OSCl2 | 28.62 | 3 | 3 |
| 145 | C sp3 | OF3 | 11.23 | 7 | 7 |
| 146 | C sp3 | OF2Cl | 16.36 | 1 | 1 |
| 147 | C sp3 | OCl3 | 26.18 | 5 | 5 |
| 148 | C sp3 | SF3 | 11.78 | 134 | 72 |
| 149 | C sp3 | SCl3 | 28.7 | 1 | 1 |
| 150 | C sp3 | SiCl3 | 25.66 | 1 | 1 |
| 151 | C(−) sp3 | HC2 | 8.26 | 2 | 2 |
| 152 | C(−) sp3 | C3 | 24.54 | 5 | 5 |
| 153 | C sp2 | H2=C | 5.49 | 497 | 428 |
| 154 | C sp2 | HC=C | 4.53 | 1415 | 860 |
| 155 | C sp2 | HC=N | 8.09 | 22 | 21 |
| 156 | C sp2 | HC=N(+) | 16.04 | 1 | 1 |
| 157 | C sp2 | H=CN | 9.75 | 238 | 132 |
| 158 | C sp2 | H=CN(+) | 17.95 | 3 | 3 |
| 159 | C sp2 | H=CN(−) | −7.22 | 4 | 4 |
| 160 | C sp2 | HC=O | 6.22 | 99 | 97 |
| 161 | C sp2 | H=CO | 3.36 | 131 | 120 |
| 162 | C sp2 | H=CP | 14.75 | 25 | 25 |
| 163 | C sp2 | H=CS | 10.03 | 79 | 72 |
| 164 | C sp2 | H=CF | 5.13 | 1 | 1 |
| 165 | C sp2 | H=CCl | 10.29 | 26 | 23 |
| 166 | C sp2 | H=CBr | 13.14 | 15 | 13 |
| 167 | C sp2 | H=CJ | 18.02 | 2 | 2 |
| 168 | C sp2 | H=CSi | 9.49 | 21 | 15 |
| 169 | C sp2 | HN=N | 13.1 | 7 | 7 |
| 170 | C sp2 | HN=N(−) | 5.23 | 2 | 2 |
| 171 | C sp2 | HN=O | 11.36 | 11 | 11 |
| 172 | C sp2 | H=NO | 6.76 | 3 | 3 |
| 173 | C sp2 | H=NS | 13.17 | 2 | 2 |
| 174 | C sp2 | H=NS(+) | −1.5 | 11 | 11 |
| 175 | C sp2 | HN=S | 20.09 | 1 | 1 |
| 176 | C sp2 | HO=O | 5.11 | 25 | 23 |
| 177 | C sp2 | HO=O(−) | −0.62 | 4 | 4 |
| 178 | C sp2 | H=OS | 12.42 | 2 | 2 |
| 179 | C sp2 | C2=C | 3.53 | 377 | 295 |
| 180 | C sp2 | C2=N | 6.82 | 41 | 34 |
| 181 | C sp2 | C2=N(+) | 25.75 | 5 | 5 |
| 182 | C sp2 | C2=O | 4.9 | 341 | 330 |
| 183 | C sp2 | C2=O(−) | 0 | 4 | 2 |
| 184 | C sp2 | C2=S | 11.71 | 1 | 1 |
| 185 | C sp2 | C=CN | 8.66 | 44 | 33 |
| 186 | C sp2 | C=CN(+) | 16.87 | 3 | 3 |
| 187 | C sp2 | C=CO | 2.4 | 94 | 88 |
| 188 | C sp2 | C=CS | 9.32 | 40 | 39 |
| 189 | C sp2 | C=CF | 4.43 | 6 | 4 |
| 190 | C sp2 | C=CCl | 9.45 | 51 | 39 |
| 191 | C sp2 | C=CBr | 11.94 | 15 | 15 |
| 192 | C sp2 | C=CJ | 18.24 | 1 | 1 |
| 193 | C sp2 | CN=N | 12.41 | 3 | 3 |
| 194 | C sp2 | CN=N(+) | −2.61 | 4 | 4 |
| 195 | C sp2 | CN=O | 10.15 | 76 | 71 |
| 196 | C sp2 | C=NO | 5.46 | 11 | 11 |
| 197 | C sp2 | CN=O(+) | 22.4 | 3 | 3 |
| 198 | C sp2 | =CNO(+) | 18.51 | 1 | 1 |
| 199 | C sp2 | C=NS | 12.19 | 3 | 3 |
| 200 | C sp2 | CO=O | 3.91 | 1115 | 877 |
| 201 | C sp2 | CO=O(−) | −2.03 | 50 | 50 |
| 202 | C sp2 | C=OP | 13.82 | 1 | 1 |
| 203 | C sp2 | C=OS | 10.82 | 8 | 8 |
| 204 | C sp2 | C=OF | 4.46 | 2 | 2 |
| 205 | C sp2 | C=OCl | 11.16 | 64 | 55 |
| 206 | C sp2 | C=OBr | 14.11 | 4 | 4 |
| 207 | C sp2 | C=OJ | 20.45 | 1 | 1 |
| 208 | C sp2 | CS=S | 19.39 | 1 | 1 |
| 209 | C sp2 | =CO2 | 1.21 | 2 | 2 |
| 210 | C sp2 | =COS | 8 | 3 | 3 |
| 211 | C sp2 | =COCl | 7.75 | 1 | 1 |
| 212 | C sp2 | =COBr | 10.45 | 1 | 1 |
| 213 | C sp2 | =COJ | 15.61 | 1 | 1 |
| 214 | C sp2 | =CSCl | 14.74 | 6 | 4 |
| 215 | C sp2 | =CSBr | 17.68 | 4 | 3 |
| 216 | C sp2 | =CSJ | 22.25 | 1 | 1 |
| 217 | C sp2 | =CSiBr | 17.13 | 1 | 1 |
| 218 | C sp2 | =CF2 | 5.09 | 7 | 7 |
| 219 | C sp2 | =CFCl | 10.2 | 3 | 2 |
| 220 | C sp2 | =CCl2 | 15.3 | 15 | 13 |
| 221 | C sp2 | =CClJ | 22.87 | 1 | 1 |
| 222 | C sp2 | =CBr2 | 20.76 | 7 | 7 |
| 223 | C sp2 | =CBrJ | 25.82 | 1 | 1 |
| 224 | C sp2 | =CJ2 | 30.75 | 1 | 1 |
| 225 | C sp2 | N2=N | 16.88 | 2 | 2 |
| 226 | C sp2 | N2=O | 14.77 | 10 | 10 |
| 227 | C sp2 | N2=S | 21.5 | 2 | 2 |
| 228 | C sp2 | NO=O | 9.3 | 19 | 16 |
| 229 | C sp2 | NO=S | 18.4 | 1 | 1 |
| 230 | C sp2 | N=OS | 17.32 | 2 | 2 |
| 231 | C sp2 | N=OCl | 16.29 | 1 | 1 |
| 232 | C sp2 | =NOCl | 10.4 | 1 | 1 |
| 233 | C sp2 | =NS2 | 20.87 | 3 | 3 |
| 234 | C sp2 | =NSCl | 17.86 | 1 | 1 |
| 235 | C sp2 | =NSBr | 21.65 | 1 | 1 |
| 236 | C sp2 | O2=O | 2.9 | 21 | 20 |
| 237 | C sp2 | O=OS | −14.19 | 1 | 1 |
| 238 | C sp2 | O=OCl | 10 | 13 | 12 |
| 239 | C sp2 | =OS2 | 17.71 | 1 | 1 |
| 240 | C sp2 | OS=S | 18.21 | 8 | 8 |
| 241 | C sp2 | S2=S | 26.78 | 2 | 2 |
| 242 | C sp2 | =OSCl | 17.33 | 1 | 1 |
| 243 | C aromatic | H:C2 | 4.42 | 6519 | 1357 |
| 244 | C aromatic | H:C:N | 6.45 | 139 | 92 |
| 245 | C aromatic | H:C:N(+) | 3.24 | 62 | 33 |
| 246 | C aromatic | H:N2 | 7.84 | 3 | 3 |
| 247 | C aromatic | B:C2 | −16.99 | 46 | 37 |
| 248 | C aromatic | :C3 | 4.56 | 251 | 119 |
| 249 | C aromatic | C:C2 | 3.53 | 1300 | 909 |
| 250 | C aromatic | C:C:N | 5.73 | 52 | 43 |
| 251 | C aromatic | C:C:N(+) | 2.09 | 6 | 5 |
| 252 | C aromatic | :C2N | 9.79 | 158 | 143 |
| 253 | C aromatic | :C2N(+) | 18.25 | 40 | 35 |
| 254 | C aromatic | :C2:N | 6.2 | 11 | 11 |
| 255 | C aromatic | :C2O | 2.8 | 359 | 287 |
| 256 | C aromatic | :C2P | 11.21 | 35 | 34 |
| 257 | C aromatic | :C2S | 9.77 | 43 | 40 |
| 258 | C aromatic | :C2F | 4.32 | 119 | 64 |
| 259 | C aromatic | :C2Cl | 9.24 | 123 | 99 |
| 260 | C aromatic | :C2Br | 12.01 | 60 | 54 |
| 261 | C aromatic | :C2J | 16.99 | 19 | 18 |
| 262 | C aromatic | :C2Si | 8.57 | 79 | 52 |
| 263 | C aromatic | :CN:N | 11.15 | 3 | 2 |
| 264 | C aromatic | C:N2 | 8.01 | 4 | 2 |
| 265 | C aromatic | :C:NO | 5.39 | 4 | 4 |
| 266 | C aromatic | :C:NF | 6.07 | 5 | 4 |
| 267 | C aromatic | :C:NCl | 11.5 | 3 | 3 |
| 268 | C aromatic | :C:NBr | 13.99 | 2 | 2 |
| 269 | C aromatic | :C:NJ | 20.37 | 1 | 1 |
| 270 | C aromatic | N:N2 | 15.39 | 5 | 2 |
| 271 | C aromatic | :N2Cl | 12.28 | 1 | 1 |
| 272 | C(+) aromatic | H:N2 | −10.11 | 95 | 95 |
| 273 | C(+) aromatic | C:N2 | −64.74 | 3 | 3 |
| 274 | C(+) aromatic | :N3 | −8.28 | 5 | 5 |
| 275 | C sp | B#N(−) | −14.24 | 20 | 5 |
| 276 | C sp | H#C | 4.41 | 84 | 77 |
| 277 | C sp | =C2 | 4.9 | 9 | 9 |
| 278 | C sp | C#C | 3.88 | 200 | 138 |
| 279 | C sp | C#N | 5.49 | 149 | 132 |
| 280 | C sp | C#N(−) | −3.13 | 15 | 5 |
| 281 | C sp | =C=O | 5.84 | 4 | 3 |
| 282 | C sp | #CO | 3.2 | 6 | 6 |
| 283 | C sp | #CS | 9.65 | 1 | 1 |
| 284 | C sp | #CSi | 8.08 | 6 | 3 |
| 285 | C sp | #CCl | 10.37 | 3 | 3 |
| 286 | C sp | #CBr | 12.31 | 4 | 4 |
| 287 | C sp | #CJ | 17.16 | 6 | 6 |
| 288 | C sp | =N2 | 11.03 | 1 | 1 |
| 289 | C sp | N#N | 10.62 | 3 | 3 |
| 290 | C sp | N#N(−) | 1.34 | 26 | 13 |
| 291 | C sp | =N=O | 8.24 | 18 | 14 |
| 292 | C sp | #NO | 5.66 | 1 | 1 |
| 293 | C sp | #NP | −2.29 | 1 | 1 |
| 294 | C sp | =N=S | 15.8 | 34 | 20 |
| 295 | C sp | =N=S(−) | 7.85 | 4 | 4 |
| 296 | C sp | #NS | 11.5 | 12 | 12 |
| 297 | N sp3 | H2B | −12.94 | 1 | 1 |
| 298 | N sp3 | H2C | −2 | 148 | 133 |
| 299 | N sp3 | H2C(pi) | −1.3 | 77 | 69 |
| 300 | N sp3 | H2N | −5.01 | 13 | 13 |
| 301 | N sp3 | H2Si | 1.97 | 4 | 4 |
| 302 | N sp3 | HC2 | −9.63 | 110 | 108 |
| 303 | N sp3 | HBC | −20.05 | 18 | 11 |
| 304 | N sp3 | HBC(pi) | −18.67 | 6 | 5 |
| 305 | N sp3 | HC2(pi) | −8.63 | 54 | 53 |
| 306 | N sp3 | HC2(2pi) | −7.37 | 37 | 34 |
| 307 | N sp3 | HCN | −2.94 | 9 | 5 |
| 308 | N sp3 | HCN(pi) | −1.31 | 2 | 2 |
| 309 | N sp3 | HCN(+)(pi) | 4.48 | 2 | 2 |
| 310 | N sp3 | HCN(2pi) | −3.61 | 3 | 3 |
| 311 | N sp3 | HCO | −2.67 | 2 | 2 |
| 312 | N sp3 | HCP | 0.72 | 3 | 3 |
| 313 | N sp3 | HCSi | −5.5 | 6 | 6 |
| 314 | N sp3 | HCSi(pi) | −4.28 | 1 | 1 |
| 315 | N sp3 | HNSi | −8.36 | 34 | 19 |
| 316 | N sp3 | HSi2 | −1.34 | 19 | 15 |
| 317 | N sp3 | B2C | −38.6 | 12 | 4 |
| 318 | N sp3 | BC2 | −27.51 | 16 | 11 |
| 319 | N sp3 | BC2(pi) | −26.43 | 2 | 1 |
| 320 | N sp3 | C3 | −16.75 | 123 | 110 |
| 321 | N sp3 | C3(pi) | −15.83 | 67 | 61 |
| 322 | N sp3 | C3(2pi) | −14.97 | 22 | 22 |
| 323 | N sp3 | C3(3pi) | −15.13 | 3 | 3 |
| 324 | N sp3 | C2N | −0.73 | 45 | 30 |
| 325 | N sp3 | C2N(pi) | −0.19 | 14 | 14 |
| 326 | N sp3 | C2N(2pi) | −10.64 | 4 | 4 |
| 327 | N sp3 | C2N(3pi) | −9.23 | 2 | 2 |
| 328 | N sp3 | C2N(+)(pi) | −2.99 | 2 | 2 |
| 329 | N sp3 | C2N(+)(2pi) | −1.57 | 2 | 2 |
| 330 | N sp3 | C2N(+)(3pi) | −40.57 | 5 | 5 |
| 331 | N sp3 | C2O | −10.11 | 4 | 4 |
| 332 | N sp3 | C2P | −6.55 | 86 | 54 |
| 333 | N sp3 | C2Si | −12.89 | 27 | 12 |
| 334 | N sp3 | CCl2(pi) | 11.05 | 1 | 1 |
| 335 | N sp2 | H=C | 1.03 | 8 | 8 |
| 336 | N sp2 | C=C | −6.59 | 63 | 58 |
| 337 | N sp2 | C=N | −2.23 | 11 | 6 |
| 338 | N sp2 | C=N(+) | 0.41 | 5 | 5 |
| 339 | N sp2 | =CN | −0.2 | 19 | 13 |
| 340 | N sp2 | =CN(−) | 13.22 | 2 | 2 |
| 341 | N sp2 | =CO | −2.21 | 34 | 33 |
| 342 | N sp2 | =CP | 2.65 | 1 | 1 |
| 343 | N sp2 | =CS | 4.72 | 3 | 2 |
| 344 | N sp2 | =CSi | −2.01 | 26 | 12 |
| 345 | N sp2 | N=N | 3.04 | 1 | 1 |
| 346 | N sp2 | N=O | −4.93 | 12 | 12 |
| 347 | N sp2 | O=O | 3.41 | 18 | 15 |
| 348 | N sp2 | P=P | 4.02 | 9 | 3 |
| 349 | N(+) sp3 | H3C | −0.39 | 13 | 13 |
| 350 | N(+) sp3 | H2C2 | −15.27 | 4 | 4 |
| 351 | N(+) sp3 | HC3 | −30.15 | 8 | 8 |
| 352 | N(+) sp3 | C4 | −47.94 | 46 | 46 |
| 353 | N(+) sp2 | HC=C | 12.52 | 4 | 4 |
| 354 | N(+) sp2 | C2=C | −5.91 | 11 | 11 |
| 355 | N(+) sp2 | C=CN | 0 | 5 | 5 |
| 356 | N(+) sp2 | C=NO(−) | −4.48 | 2 | 2 |
| 357 | N(+) sp2 | CO=O(−) | −7.16 | 75 | 65 |
| 358 | N(+) sp2 | NO=O(−) | 0 | 6 | 6 |
| 359 | N(+) sp2 | O2=O(−) | 0.2 | 27 | 20 |
| 360 | N aromatic | H2:C(+) | 0 | 5 | 5 |
| 361 | N aromatic | HC:C(+) | 10.05 | 1 | 1 |
| 362 | N aromatic | :C2 | −1.94 | 120 | 105 |
| 363 | N aromatic | C2:C(+) | 1.26 | 205 | 103 |
| 364 | N aromatic | :C:N | 0.2 | 6 | 3 |
| 365 | N(+) aromatic | C:C2 | −1.86 | 33 | 33 |
| 366 | N(+) aromatic | :C2O(−) | 8.85 | 1 | 1 |
| 367 | N(+) sp | C#C(−) | −7.99 | 3 | 3 |
| 368 | N(+) sp | =C=N(−) | −3.91 | 1 | 1 |
| 369 | N(+) sp | =N2(−) | 2.99 | 3 | 3 |
| 370 | N(−) | C2 | 4.69 | 15 | 15 |
| 371 | N(−) | CN | −12.52 | 2 | 2 |
| 372 | N(−) | S2 | 1.69 | 69 | 69 |
| 373 | O | HB | −17.09 | 3 | 3 |
| 374 | O | B2 | −35.49 | 6 | 6 |
| 375 | O | HC | −3.5 | 602 | 527 |
| 376 | O | HC(pi) | 3.39 | 266 | 250 |
| 377 | O | HN | 2.35 | 4 | 4 |
| 378 | O | HN(pi) | 4.33 | 14 | 14 |
| 379 | O | HO | 2.75 | 15 | 15 |
| 380 | O | HS | 7.17 | 5 | 4 |
| 381 | O | HP | 5.14 | 26 | 23 |
| 382 | O | HSi | 0.8 | 7 | 7 |
| 383 | O | BC | −23.16 | 165 | 75 |
| 384 | O | BC(pi) | −11.65 | 2 | 1 |
| 385 | O | BC(−)(pi) | −31.4 | 8 | 4 |
| 386 | O | C2 | −10.27 | 617 | 420 |
| 387 | O | C2(pi) | −3.51 | 1209 | 958 |
| 388 | O | C2(2pi) | 3.23 | 137 | 135 |
| 389 | O | CN | −4.7 | 2 | 2 |
| 390 | O | CN(pi) | −2.22 | 31 | 28 |
| 391 | O | CN(+)(pi) | 2.51 | 24 | 17 |
| 392 | O | CN(2pi) | 3.03 | 5 | 5 |
| 393 | O | CO | −3.92 | 31 | 23 |
| 394 | O | CO(pi) | 3.65 | 2 | 2 |
| 395 | O | CP | −1.46 | 471 | 249 |
| 396 | O | CP(pi) | 4.87 | 50 | 30 |
| 397 | O | CS | −0.98 | 48 | 37 |
| 398 | O | CSi | −6.31 | 244 | 103 |
| 399 | O | CSi(pi) | 0.28 | 29 | 19 |
| 400 | O | CCl | 2.14 | 1 | 1 |
| 401 | O | N2(2pi) | 3.14 | 1 | 1 |
| 402 | O | P2 | 7.2 | 23 | 19 |
| 403 | O | Si2 | −2.39 | 116 | 35 |
| 404 | P3 | H2C | 2.85 | 17 | 17 |
| 405 | P3 | HC2 | −6.24 | 3 | 3 |
| 406 | P3 | C3 | −15.17 | 18 | 18 |
| 407 | P3 | C2O | −10.92 | 10 | 10 |
| 408 | P3 | C2S | −3.08 | 3 | 3 |
| 409 | P3 | C2Cl | −6.82 | 16 | 16 |
| 410 | P3 | CO2 | −6.82 | 15 | 15 |
| 411 | P3 | COCl | −2.2 | 6 | 6 |
| 412 | P3 | CS2 | 17.67 | 1 | 1 |
| 413 | P3 | CCl2 | 12.05 | 8 | 8 |
| 414 | P3 | N3 | −4.26 | 1 | 1 |
| 415 | P3 | N2O | −2.29 | 3 | 3 |
| 416 | P3 | NO2 | −1.46 | 3 | 3 |
| 417 | P3 | O3 | −1.41 | 26 | 26 |
| 418 | P3 | O2Cl | 6.11 | 1 | 1 |
| 419 | P3 | OCl2 | 16.38 | 1 | 1 |
| 420 | P3 | S3 | 21.45 | 4 | 4 |
| 421 | P4 | HC2=O | −7.52 | 2 | 2 |
| 422 | P4 | HO2=O | 2.18 | 17 | 17 |
| 423 | P4 | HO2=S | 8.79 | 3 | 3 |
| 424 | P4 | C3=O | −17.18 | 3 | 3 |
| 425 | P4 | C3=S | −8.85 | 3 | 3 |
| 426 | P4 | C2O=O | −12.11 | 10 | 10 |
| 427 | P4 | C2O=O(−) | −15.97 | 1 | 1 |
| 428 | P4 | C2=OS | −4.17 | 1 | 1 |
| 429 | P4 | C2O=S | −4.41 | 1 | 1 |
| 430 | P4 | C2S=S | −0.38 | 1 | 1 |
| 431 | P4 | C2=SCl | 5.18 | 1 | 1 |
| 432 | P4 | CN2=O | −10.68 | 10 | 10 |
| 433 | P4 | CNO=O | 8.01 | 1 | 1 |
| 434 | P4 | CN=OF | −5.4 | 4 | 4 |
| 435 | P4 | CN=OCl | 0.56 | 5 | 5 |
| 436 | P4 | CO2=O | −7.79 | 42 | 42 |
| 437 | P4 | CO2=O(−) | −10.36 | 1 | 1 |
| 438 | P4 | CO2=S | 0.26 | 8 | 8 |
| 439 | P4 | C=OS2 | 8.61 | 2 | 2 |
| 440 | P4 | COS=S | 7.49 | 30 | 30 |
| 441 | P4 | C=OF2 | 0.52 | 5 | 5 |
| 442 | P4 | C=OCl2 | 11.1 | 9 | 9 |
| 443 | P4 | CS2=S | 16.66 | 6 | 3 |
| 444 | P4 | C=SCl2 | 19.41 | 5 | 5 |
| 445 | P4 | N3=O | −6.3 | 1 | 1 |
| 446 | P4 | N2O=O | −5 | 5 | 5 |
| 447 | P4 | N=NO2 | −3.25 | 6 | 2 |
| 448 | P4 | N2O=S | 3.2 | 2 | 2 |
| 449 | P4 | N2=OF | −0.37 | 1 | 1 |
| 450 | P4 | N=NS2 | 12.33 | 3 | 1 |
| 451 | P4 | NO2=O | −4.03 | 3 | 3 |
| 452 | P4 | NO2=S | 4.11 | 6 | 6 |
| 453 | P4 | NO=OF | 0.12 | 7 | 6 |
| 454 | P4 | NO=SF | 7.56 | 5 | 4 |
| 455 | P4 | N=OF2 | 4.05 | 1 | 1 |
| 456 | P4 | N=OFCl | 9.6 | 2 | 2 |
| 457 | P4 | N=OFBr | 12.55 | 1 | 1 |
| 458 | P4 | N=OCl2 | 14.9 | 1 | 1 |
| 459 | P4 | N=SFCl | 17.24 | 1 | 1 |
| 460 | P4 | N=SFBr | 20.83 | 1 | 1 |
| 461 | P4 | N=SCl2 | 22.78 | 1 | 1 |
| 462 | P4 | O3=O | −2.5 | 33 | 26 |
| 463 | P4 | O3=O(−) | −6.33 | 3 | 3 |
| 464 | P4 | O3=S | 5.03 | 16 | 14 |
| 465 | P4 | O2=OS | 15.08 | 4 | 4 |
| 466 | P4 | O2=OF | 1.56 | 16 | 12 |
| 467 | P4 | O2=OCl | 6.38 | 2 | 2 |
| 468 | P4 | O2S=S | 12.87 | 2 | 2 |
| 469 | P4 | O2=SF | 9.65 | 2 | 1 |
| 470 | P4 | O2=SCl | 14.28 | 3 | 3 |
| 471 | P4 | O=OF2 | 5.45 | 1 | 1 |
| 472 | P4 | O=OFCl | 10.51 | 7 | 6 |
| 473 | P4 | O=OFBr | 13.17 | 1 | 1 |
| 474 | P4 | O=OCl2 | 15.86 | 1 | 1 |
| 475 | P4 | O=SF2 | 12.58 | 1 | 1 |
| 476 | P4 | O=SFCl | 18.16 | 1 | 1 |
| 477 | P4 | O=SFBr | 21.4 | 1 | 1 |
| 478 | P4 | O=SCl2 | 23.03 | 1 | 1 |
| 479 | P(−) | C3F3 | −11.9 | 2 | 2 |
| 480 | P(−) | F6 | 1.32 | 6 | 6 |
| 481 | P(+) | C4 | −14.3 | 12 | 12 |
| 482 | S2 | HC | 2.74 | 78 | 58 |
| 483 | S2 | HC(pi) | 2.64 | 8 | 7 |
| 484 | S2 | HP | 5.84 | 27 | 27 |
| 485 | S2 | BC | −21.7 | 51 | 32 |
| 486 | S2 | BC(pi) | −21.88 | 3 | 3 |
| 487 | S2 | C2 | −4.08 | 157 | 98 |
| 488 | S2 | C2(pi) | −3.93 | 77 | 73 |
| 489 | S2 | C2(2pi) | −4.31 | 70 | 70 |
| 490 | S2 | CP | −1.63 | 34 | 18 |
| 491 | S2 | CS | 2.06 | 38 | 21 |
| 492 | S2 | CS(pi) | −12.06 | 2 | 1 |
| 493 | S2 | CCl | 6.16 | 5 | 5 |
| 494 | S2 | N2(2pi) | −5.14 | 1 | 1 |
| 495 | S2 | PCl | 0 | 4 | 4 |
| 496 | S2 | P2 | 0 | 6 | 3 |
| 497 | S2 | S2 | 8.87 | 6 | 5 |
| 498 | S4 | C2=O | −3.2 | 4 | 4 |
| 499 | S4 | C2=O2 | −3.24 | 12 | 12 |
| 500 | S4 | CN=O2(−) | −0.22 | 126 | 63 |
| 501 | S4 | CO=O2 | −1.48 | 11 | 11 |
| 502 | S4 | CO=O2(−) | −3.78 | 24 | 24 |
| 503 | S4 | C=OCl | 9.42 | 6 | 6 |
| 504 | S4 | C=OS | 3.69 | 4 | 4 |
| 505 | S4 | C=O2F | 4.69 | 8 | 8 |
| 506 | S4 | C=O2Cl | 8.91 | 8 | 8 |
| 507 | S4 | N=O2F(−) | 5.76 | 12 | 6 |
| 508 | S4 | N=O2Cl | 10.28 | 1 | 1 |
| 509 | S4 | O=OCl | 11.86 | 1 | 1 |
| 510 | S4 | O2=O | 1.31 | 7 | 7 |
| 511 | S4 | O2=O2 | 1.17 | 4 | 4 |
| 512 | S4 | O2=O2(−) | −2.26 | 16 | 16 |
| 513 | S4 | O=O2Cl | 11.45 | 2 | 2 |
| 514 | S4 | O=O2F | 5.93 | 1 | 1 |
| 515 | S(+) | C3 | −19.18 | 5 | 5 |
| 516 | Si | H3C | 6.42 | 8 | 7 |
| 517 | Si | H3Si | 12.32 | 19 | 8 |
| 518 | Si | H2C2 | 0.06 | 12 | 10 |
| 519 | Si | H2CN | 6.65 | 1 | 1 |
| 520 | Si | H2CCl | 10.82 | 1 | 1 |
| 521 | Si | H2Si2 | 12.04 | 18 | 7 |
| 522 | Si | HC3 | −6.7 | 14 | 14 |
| 523 | Si | HC2N | 0.57 | 5 | 4 |
| 524 | Si | HC2O | −0.27 | 11 | 7 |
| 525 | Si | HC2Cl | 3.98 | 4 | 4 |
| 526 | Si | HCN2 | 7.39 | 6 | 6 |
| 527 | Si | HCO2 | 5.89 | 18 | 9 |
| 528 | Si | HCOCl | 10.13 | 1 | 1 |
| 529 | Si | HN3 | 14.51 | 1 | 1 |
| 530 | Si | HSi3 | 12.05 | 3 | 3 |
| 531 | Si | C4 | −12.83 | 49 | 40 |
| 532 | Si | C3N | −6.05 | 42 | 32 |
| 533 | Si | C3O | −6.7 | 84 | 61 |
| 534 | Si | C3F | −7.57 | 5 | 5 |
| 535 | Si | C3Cl | −2.55 | 12 | 12 |
| 536 | Si | C3Br | 0.52 | 3 | 3 |
| 537 | Si | C3J | 6.46 | 2 | 2 |
| 538 | Si | C3Si | −6.69 | 6 | 3 |
| 539 | Si | C2N2 | 1.02 | 12 | 10 |
| 540 | Si | C2O2 | −0.54 | 100 | 39 |
| 541 | Si | C2Si2 | −0.39 | 3 | 2 |
| 542 | Si | C2SiCl | 3.56 | 2 | 1 |
| 543 | Si | C2F2 | −2.89 | 6 | 6 |
| 544 | Si | C2Cl2 | 8.03 | 13 | 13 |
| 545 | Si | C2Br2 | 14.34 | 1 | 1 |
| 546 | Si | C2J2 | 26 | 1 | 1 |
| 547 | Si | CN3 | 7.74 | 6 | 6 |
| 548 | Si | CN2Cl | 11.22 | 2 | 2 |
| 549 | Si | CO3 | 5.81 | 30 | 30 |
| 550 | Si | COCl2 | 14.59 | 4 | 4 |
| 551 | Si | CF3 | 2.56 | 3 | 3 |
| 552 | Si | CCl3 | 18.65 | 17 | 16 |
| 553 | Si | CBr3 | 27.76 | 1 | 1 |
| 554 | Si | NO3 | 12.84 | 6 | 4 |
| 555 | Si | N2O2 | 4.42 | 1 | 1 |
| 556 | Si | N3O | 0.43 | 1 | 1 |
| 557 | Si | N4 | 14.64 | 4 | 4 |
| 558 | Si | O4 | 12.5 | 15 | 15 |
| 559 | Si | O3Cl | 16.78 | 1 | 1 |
| 560 | Si | OCl3 | 25.14 | 2 | 2 |
| 561 | Chloride | −1 | 7 | 7 | |
| 562 | Bromide | 3.11 | 3 | 3 | |
| A | Based on | Valid groups | 382 | 5988 | |
| B | Goodness of fit | R2 | 0.9997 | 5763 | |
| C | Deviation | Average | 0.29 | 5763 | |
| D | Deviation | Standard | 0.38 | 5763 | |
| E | K-fold cv | K | 10 | 5572 | |
| F | Goodness of fit | Q2 | 0.9997 | 5572 | |
| G | Deviation | Average (cv) | 0.31 | 5572 | |
| H | Deviation | Standard (cv) | 0.41 | 5572 |
| Atom Type Neighbors | B(−) F4 | C sp3 H3C | C sp3 H3N | C sp3 H2C2 | C sp3 H2CN | C sp2 H=CN | C(+) Aromatic HN2 | N Aromatic C2:C(+) | Sum |
|---|---|---|---|---|---|---|---|---|---|
| Contribution | −2.41 | 5.7 | 12.24 | 4.63 | 11.13 | 9.75 | −10.11 | 1.26 | |
| n Groups | 1 | 1 | 1 | 2 | 1 | 2 | 1 | 2 | |
| n × Contribution | −2.41 | 5.7 | 12.24 | 9.26 | 11.13 | 19.5 | −10.11 | 2.52 | 47.83 |
| Molecule Name | Refractivity Exp. | Refractivity Calc. | Deviation | Dev. in % |
|---|---|---|---|---|
| 1-(2-Cyanoethyl)-3-(2-hydroxyethyl)-imidazolium chloride | 51.06 | 50.40 | 0.66 | 1.29 |
| 1-(2-Cyanoethyl)-3-(2-propen-1-yl)-imidazolium chloride | 53.46 | 53.31 | 0.15 | 0.28 |
| 1-(2-Cyanoethyl)-3-octylimidazolium 3-sulfobenzoate | 111.59 | 112.27 | −0.68 | −0.61 |
| 1-(2-Hydroxyethyl)-3-methylimidazolium perfluoropentanoate | 62.30 | 61.37 | 0.93 | 1.49 |
| 1-(3-Cyanopropyl)-3-methylimidazolium bis(trifluoromethylsulfonyl) amide | 74.64 | 74.84 | −0.20 | −0.27 |
| 1-(3-Cyanopropyl)-3-methylimidazolium dicyanamide | 56.96 | 57.40 | −0.44 | −0.77 |
| 1-(3-Cyanopropyl)-3-methylimidazolium tetrafluoroborate | 46.88 | 47.62 | −0.74 | −1.58 |
| 1-(3-Cyanopropyl)-pyridinium bis(trifluoromethylsulfonyl) amide | 75.74 | 76.04 | −0.30 | −0.40 |
| 1-(3-Cyanopropyl)-pyridinium dicyanamide | 57.75 | 58.60 | −0.85 | −1.47 |
| 1,1,3,3-Tetramethylguanidinium butanoate | 56.23 | 56.13 | 0.10 | 0.18 |
| 1,1,3,3-Tetramethylguanidinium heptanoate | 70.63 | 70.02 | 0.61 | 0.86 |
| 1,1,3,3-Tetramethylguanidinium hexanoate | 64.74 | 65.39 | −0.65 | −1.00 |
| 1,1,3,3-Tetramethylguanidinium octanoate | 74.36 | 74.65 | −0.29 | −0.39 |
| 1,1,3,3-Tetramethylguanidinium pentanoate | 60.85 | 60.76 | 0.09 | 0.15 |
| 1,2-Diethylpyridinium ethylsulfate | 64.48 | 64.43 | 0.05 | 0.08 |
| 1,3-Diethylimidazolium bis(trifluoromethylsulfonyl) amide | 70.29 | 70.38 | −0.09 | −0.13 |
| 1,3-Dimethylimidazolium bis(trifluoromethylsulfonyl) amide | 60.56 | 61.20 | −0.64 | −1.06 |
| 1,3-Dimethylimidazolium methosulfate | 44.78 | 44.80 | −0.02 | −0.04 |
| 1,3-Dipropylimidazolium bis(trifluoromethylsulfonyl) amide | 79.71 | 79.64 | 0.07 | 0.09 |
| 1-Benzyl-3-methylimidazolium bis(trifluoromethylsulfonyl) amide | 85.86 | 85.72 | 0.14 | 0.16 |
| 1-Butyl-1-methylpiperidinium bis(trifluoromethylsulfonyl)amide | 81.48 | 81.50 | −0.02 | −0.02 |
| 1-Butyl-1-methylpyrrolidinium 2-acryloamido-2-methylpropanesulfonate | 91.47 | 90.77 | 0.70 | 0.77 |
| 1-Butyl-1-methylpyrrolidinium acetate | 55.00 | 55.73 | −0.73 | −1.33 |
| 1-Butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)amide | 76.89 | 76.87 | 0.02 | 0.03 |
| 1-Butyl-1-methylpyrrolidinium dicyanamide | 60.12 | 59.43 | 0.69 | 1.15 |
| 1-Butyl-1-methylpyrrolidinium methylsulfate | 60.93 | 60.47 | 0.46 | 0.75 |
| 1-Butyl-1-methylpyrrolidinium trifluoromethanesulfonate | 60.44 | 60.06 | 0.38 | 0.63 |
| 1-Butyl-2,3-dimethylimidazolium bis(trifluoromethanesulfonyl) amide | 79.33 | 79.39 | −0.06 | −0.08 |
| 1-Butyl-2,3-dimethylimidazolium tetrafluoroborate | 52.38 | 52.17 | 0.21 | 0.40 |
| 1-Butyl-2-methylpyridinium tetrafluoroborate | 53.45 | 53.58 | −0.13 | −0.24 |
| 1-Butyl-3-(2-cyanoethyl)-imidazolium chloride | 57.93 | 58.25 | −0.32 | −0.55 |
| 1-Butyl-3-(2-cyanoethyl)imidazolium thiocyanate | 67.06 | 67.10 | −0.04 | −0.06 |
| 1-Butyl-3-ethylimidazolium bis(trifluoromethylsulfonyl) amide | 79.44 | 79.64 | −0.20 | −0.25 |
| 1-Butyl-3-ethylimidazolium triflate | 63.01 | 62.83 | 0.18 | 0.29 |
| 1-Butyl-3-methylimidazolium 2-acryloamido-2-methylpropanesulfonate | 88.52 | 88.95 | −0.43 | −0.49 |
| 1-Butyl-3-methylimidazolium acetate | 54.18 | 53.91 | 0.27 | 0.50 |
| 1-Butyl-3-methylimidazolium bis(perfluorobutanesulfonyl) amide | 105.39 | 105.45 | −0.06 | −0.06 |
| 1-Butyl-3-methylimidazolium bis(trifluoromethanesulfonyl) amide | 74.99 | 75.05 | −0.06 | −0.08 |
| 1-Butyl-3-methylimidazolium dicyanoamide | 57.80 | 57.61 | 0.19 | 0.33 |
| 1-Butyl-3-methylimidazolium glycine | 58.39 | 57.34 | 1.05 | 1.80 |
| 1-Butyl-3-methylimidazolium hexafluorophosphate | 51.46 | 51.56 | −0.10 | −0.19 |
| 1-Butyl-3-methylimidazolium methosulfate | 58.58 | 58.65 | −0.07 | −0.12 |
| 1-Butyl-3-methylimidazolium octylsulfate | 91.49 | 91.09 | 0.40 | 0.44 |
| 1-Butyl-3-methylimidazolium perfluorobutylsulfonate | 73.34 | 73.44 | −0.10 | −0.14 |
| 1-Butyl-3-methylimidazolium tetracyanoborate | 67.83 | 67.87 | −0.04 | −0.06 |
| 1-Butyl-3-methylimidazolium tetrafluoroborate | 47.81 | 47.83 | −0.02 | −0.04 |
| 1-Butyl-3-methylimidazolium thiocyanate | 57.79 | 58.09 | −0.30 | −0.52 |
| 1-Butyl-3-methylimidazolium threoninate | 68.48 | 67.95 | 0.53 | 0.77 |
| 1-Butyl-3-methylimidazolium tricyanomethanide | 65.33 | 65.39 | −0.06 | −0.09 |
| 1-Butyl-3-methylimidazolium trifluoromethylsulfonate | 57.91 | 58.24 | −0.33 | −0.57 |
| 1-Butyl-4-methylpyridinium tetrafluoroborate | 54.00 | 53.84 | 0.16 | 0.30 |
| 1-Butylpyridinium 2-acryloamido-2-methylpropanesulfonate | 91.01 | 90.15 | 0.86 | 0.94 |
| 1-Butylpyridinium bis(fluorosulfonyl) amide | 64.60 | 64.65 | −0.05 | −0.08 |
| 1-Butylpyridinium dicyanamide | 58.87 | 58.81 | 0.06 | 0.10 |
| 1-Butylpyridinium tetrafluoroborate | 48.99 | 49.03 | −0.04 | −0.08 |
| 1-Butyltetrahydrothiophenium dicyanamide | 59.59 | 59.81 | −0.22 | −0.37 |
| 1-Decyl-3-methylimidazolium bis(trifluoromethanesulfonyl) amide | 102.90 | 102.83 | 0.07 | 0.07 |
| 1-Decyl-3-methylimidazolium tetracyanoborate | 95.66 | 95.65 | 0.01 | 0.01 |
| 1-Decyl-3-methylimidazolium tricyanomethanide | 93.23 | 93.17 | 0.06 | 0.06 |
| 1-Dodecyl-3-methylimidazolium bis(trifluoromethanesulfonyl) amide | 112.08 | 112.09 | −0.01 | −0.01 |
| 1-Ethyl-2,3-dimethylimidazolium bis(trifluoromethanesulfonyl) amide | 69.94 | 70.13 | −0.19 | −0.27 |
| 1-Ethyl-3-methylimidazolium 1,1,2,2-tetrafluoroethanesulfonate | 53.91 | 53.77 | 0.14 | 0.26 |
| 1-Ethyl-3-methylimidazolium acetate | 44.95 | 44.65 | 0.30 | 0.67 |
| 1-Ethyl-3-methylimidazolium aminoacetate | 47.68 | 48.08 | −0.40 | −0.84 |
| 1-Ethyl-3-methylimidazolium bis(trifluoromethanesulfonyl) amide | 65.77 | 65.79 | −0.02 | −0.03 |
| 1-Ethyl-3-methylimidazolium dicyanamide | 48.35 | 48.35 | 0.00 | 0.00 |
| 1-Ethyl-3-methylimidazolium diethylphosphate | 64.56 | 64.35 | 0.21 | 0.33 |
| 1-Ethyl-3-methylimidazolium dimethylphosphate | 55.17 | 55.03 | 0.14 | 0.25 |
| 1-Ethyl-3-methylimidazolium ethosulfate | 54.16 | 54.05 | 0.11 | 0.20 |
| 1-Ethyl-3-methylimidazolium imidodisulfurylfluoride | 54.05 | 54.19 | −0.14 | −0.26 |
| 1-Ethyl-3-methylimidazolium l-alanine | 52.10 | 52.64 | −0.54 | −1.04 |
| 1-Ethyl-3-methylimidazolium l-proline | 58.84 | 59.70 | −0.86 | −1.46 |
| 1-Ethyl-3-methylimidazolium l-serine | 54.05 | 54.05 | 0.00 | 0.00 |
| 1-Ethyl-3-methylimidazolium methanesulfonate | 48.45 | 48.69 | −0.24 | −0.50 |
| 1-Ethyl-3-methylimidazolium methylsulfate | 48.62 | 49.39 | −0.77 | −1.58 |
| 1-Ethyl-3-methylimidazolium taurinate | 56.41 | 56.96 | −0.55 | −0.98 |
| 1-Ethyl-3-methylimidazolium tetracyanoborate | 58.77 | 58.61 | 0.16 | 0.27 |
| 1-Ethyl-3-methylimidazolium tetrafluoroborate | 38.70 | 38.57 | 0.13 | 0.34 |
| 1-Ethyl-3-methylimidazolium thiocyanate | 48.31 | 48.83 | −0.52 | −1.08 |
| 1-Ethyl-3-methylimidazolium threoninate | 58.86 | 58.69 | 0.17 | 0.29 |
| 1-Ethyl-3-methylimidazolium tricyanomethide | 55.87 | 56.13 | −0.26 | −0.47 |
| 1-Ethyl-3-methylimidazolium trifluoromethylsulfonate | 48.91 | 48.98 | −0.07 | −0.14 |
| 1-Ethyl-3-methylpyridinium bis(fluorosulfonyl) amide | 60.16 | 60.20 | −0.04 | −0.07 |
| 1-Ethyl-3-methylpyridinium ethylsulfate | 60.18 | 60.06 | 0.12 | 0.20 |
| 1-Ethyl-3-propylimidazolium bis(trifluoromethanesulfonyl) amide | 74.95 | 75.01 | −0.06 | −0.08 |
| 1-Ethylmorpholinium tetrafluoroborate | 40.81 | 39.89 | 0.92 | 2.25 |
| 1-Ethylpyridinium bis(fluorosulfonyl) amide | 55.18 | 55.39 | −0.21 | −0.38 |
| 1-Ethylpyridinium ethylsulfate | 55.29 | 55.25 | 0.04 | 0.07 |
| 1-Ethylpyridinium triflate | 50.05 | 50.18 | −0.13 | −0.26 |
| 1-Ethyltetrahydrothiophenium dicyanamide | 50.87 | 50.55 | 0.32 | 0.63 |
| 1-Heptyl-3-methylimidazolium bis(trifluoromethanesulfonyl) amide | 88.14 | 88.94 | −0.80 | −0.91 |
| 1-Heptyl-3-methylimidazolium hexafluorophosphate | 65.44 | 65.45 | −0.01 | −0.02 |
| 1-Hexyl-1-methylpyrrolidinium bis(trifluoromethanesulfonyl) amide | 87.26 | 86.13 | 1.13 | 1.29 |
| 1-Hexyl-3,5-dimethylpyridinium bis(trifluoromethylsulfonyl)amide | 95.56 | 95.13 | 0.43 | 0.45 |
| 1-Hexyl-3-methylimidazolium acetate | 63.54 | 63.17 | 0.37 | 0.58 |
| 1-Hexyl-3-methylimidazolium bis(trifluoromethanesulfonyl) amide | 84.23 | 84.31 | −0.08 | −0.09 |
| 1-Hexyl-3-methylimidazolium chloride | 58.80 | 58.50 | 0.30 | 0.51 |
| 1-Hexyl-3-methylimidazolium dicyanoamide | 67.19 | 66.87 | 0.32 | 0.48 |
| 1-Hexyl-3-methylimidazolium hexafluorophosphate | 60.69 | 60.82 | −0.13 | −0.21 |
| 1-Hexyl-3-methylimidazolium tetracyanoborate | 76.88 | 77.13 | −0.25 | −0.33 |
| 1-Hexyl-3-methylimidazolium tetrafluoroborate | 56.91 | 57.09 | −0.18 | −0.32 |
| 1-Hexyl-3-methylimidazolium thiocyanate | 68.17 | 67.35 | 0.82 | 1.20 |
| 1-Hexyl-3-methylimidazolium tricyanomethanide | 74.67 | 74.65 | 0.02 | 0.03 |
| 1-Hexylpyridinium bis(fluorosulfonyl) amide | 73.94 | 73.91 | 0.03 | 0.04 |
| 1-Isobutyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)amide | 77.02 | 76.83 | 0.19 | 0.25 |
| 1-Isobutyl-3-methylimidazolium bis(trifluoromethanesulfonyl) amide | 74.92 | 75.01 | −0.09 | −0.12 |
| 1-Isobutyl-3-methylpyridinium bis(trifluoromethylsulfonyl)amide | 81.23 | 81.02 | 0.21 | 0.26 |
| 1-Methyl-1-(2′,3′-epoxypropyl)-2-oxopyrrolidinium chloride | 49.38 | 49.79 | −0.41 | −0.83 |
| 1-Methyl-1-decylpyrrolidinium bis(trifluoromethanesulfonyl) amide | 104.90 | 104.65 | 0.25 | 0.24 |
| 1-Methyl-1-propylpiperidinium bis(trifluoromethylsulfonyl)amide | 76.90 | 76.87 | 0.03 | 0.04 |
| 1-Methyl-1-propylpyrrolidinium bis(fluorosulfonyl) amide | 61.04 | 60.64 | 0.40 | 0.66 |
| 1-Methyl-1-propylpyrrolidinium bis(trifluoromethanesulfonyl) amide | 72.50 | 72.24 | 0.26 | 0.36 |
| 1-Methyl-2-pyrrolidonium tetrafluoroborate | 37.04 | 37.68 | −0.64 | −1.73 |
| 1-Methyl-3-hexylimidazolium threoninate | 77.73 | 77.21 | 0.52 | 0.67 |
| 1-Methyl-3-pentylimidazolium threoninate | 73.07 | 72.58 | 0.49 | 0.67 |
| 1-Methyl-3-propylimidazolium threoninate | 63.75 | 63.32 | 0.43 | 0.67 |
| 1-Methylmorpholinium tetrafluoroborate | 35.90 | 35.57 | 0.33 | 0.92 |
| 1-Methylpiperidinium tetrafluoroborate | 37.84 | 38.51 | −0.67 | −1.77 |
| 1-Methylpyridinium methylsulfate | 46.05 | 46.27 | −0.22 | −0.48 |
| 1-Methylpyrrolidinium tetrafluoroborate | 34.55 | 33.88 | 0.67 | 1.94 |
| 1-Nonyl-3-methylimidazolium bis(trifluoromethanesulfonyl) amide | 98.06 | 98.20 | −0.14 | −0.14 |
| 1-Nonyl-3-methylimidazolium hexafluorophosphate | 74.74 | 74.71 | 0.03 | 0.04 |
| 1-Octyl-3-methylimidazolium bis(trifluoromethanesulfonyl) amide | 93.61 | 93.57 | 0.04 | 0.04 |
| 1-Octyl-3-methylimidazolium chloride | 67.80 | 67.76 | 0.04 | 0.06 |
| 1-Octyl-3-methylimidazolium hexafluorophosphate | 70.30 | 70.08 | 0.22 | 0.31 |
| 1-Octyl-3-methylimidazolium tetracyanoborate | 86.41 | 86.39 | 0.02 | 0.02 |
| 1-Octyl-3-methylimidazolium tetrafluoroborate | 66.24 | 66.35 | −0.11 | −0.17 |
| 1-Octyl-3-methylimidazolium tricyanomethanide | 84.09 | 83.91 | 0.18 | 0.21 |
| 1-Octyl-3-methylpyridinium tetrafluoroborate | 72.74 | 72.36 | 0.38 | 0.52 |
| 1-Octylpyridinium bis(trifluoromethylsulfonyl)amide | 95.19 | 94.77 | 0.42 | 0.44 |
| 1-Pentyl-3-methylimidazolium acetate | 59.01 | 58.54 | 0.47 | 0.80 |
| 1-Pentyl-3-methylimidazolium bis(trifluoromethanesulfonyl) amide | 79.57 | 79.68 | −0.11 | −0.14 |
| 1-Pentyl-3-methylimidazolium hexafluorophosphate | 56.11 | 56.19 | −0.08 | −0.14 |
| 1-Pentyl-3-methylimidazolium tetrafluoroborate | 52.25 | 52.46 | −0.21 | −0.40 |
| 1-Pentylpyridinium dicyanamide | 63.44 | 63.44 | 0.00 | 0.00 |
| 1-Phenyl-2,3,5-trimethylpyrazolium methylsulfonate | 68.77 | 69.35 | −0.58 | −0.84 |
| 1-Phenyl-2-butyl-3,5-dimethylpyrazolium methylsulfonate | 82.67 | 82.93 | −0.26 | −0.31 |
| 1-Phenyl-2-heptyl-3,5-dimethylpyrazolium methylsulfonate | 97.61 | 96.82 | 0.79 | 0.81 |
| 1-Phenyl-2-hexyl-3,5-dimethylpyrazolium methylsulfonate | 92.64 | 92.19 | 0.45 | 0.49 |
| 1-Phenyl-2-pentyl-3,5-dimethylpyrazolium methylsulfonate | 87.17 | 87.56 | −0.39 | −0.45 |
| 1-Propyl-3-methylimidazolium acetate | 49.37 | 49.28 | 0.09 | 0.18 |
| 1-Propyl-3-methylimidazolium bis(trifluoromethanesulfonyl) amide | 70.48 | 70.42 | 0.06 | 0.09 |
| 1-Propyl-3-methylimidazolium tetrafluoroborate | 43.09 | 43.20 | −0.11 | −0.26 |
| 1-Propylpyridinium dicyanamide | 54.24 | 54.18 | 0.06 | 0.11 |
| 1-Propylpyridinium tetrafluoroborate | 44.35 | 44.40 | −0.05 | −0.11 |
| 1-Propyronitrile-3-butylimidazolium bromide | 62.60 | 62.36 | 0.24 | 0.38 |
| 1-Propyronitrile-3-hexylimidazolium bromide | 71.74 | 71.62 | 0.12 | 0.17 |
| 1-Propyronitrile-3-octylimidazolium bromide | 80.51 | 80.88 | −0.37 | −0.46 |
| 2-Hydroxyethylammonium acetate | 29.31 | 28.99 | 0.32 | 1.09 |
| 2-hydroxyethylammonium butanoate | 38.53 | 38.25 | 0.28 | 0.73 |
| 2-Hydroxyethylammonium formate | 24.85 | 24.70 | 0.15 | 0.60 |
| 2-Hydroxyethylammonium hexanoate | 47.80 | 47.51 | 0.29 | 0.61 |
| 2-Hydroxyethylammonium lactate | 34.69 | 35.04 | −0.35 | −1.01 |
| 2-Hydroxyethylammonium pentanoate | 43.12 | 42.88 | 0.24 | 0.56 |
| 2-Hydroxyethylammonium propionate | 33.29 | 33.62 | −0.33 | −0.99 |
| 3-Methyl-1-propylpyridinium bis(trifluoromethylsulfonyl)amide | 76.40 | 76.43 | −0.03 | −0.04 |
| Bis(2-hydroxyethyl)ammonium hexanoate | 58.49 | 58.34 | 0.15 | 0.26 |
| Butylammonium nitrate | 33.34 | 33.37 | −0.03 | −0.09 |
| Dimethyl butyl isopropylammonium bis(trifluoromethylsulfonyl)amide | 78.81 | 79.06 | −0.25 | −0.32 |
| Dimethyl hexyl isopropylammonium bis(trifluoromethylsulfonyl)amide | 89.14 | 88.32 | 0.82 | 0.92 |
| Dimethylpropylisopropylammonium bis(trifluoromethylsulfonyl)amide | 73.56 | 74.43 | −0.87 | −1.18 |
| Ethylammonium nitrate | 24.14 | 24.11 | 0.03 | 0.12 |
| l-Alanine 1-methylethyl ester dodecyl sulfate | 102.88 | 103.30 | −0.42 | −0.41 |
| l-Alanine 2-methylpropyl ester dodecyl sulfate | 107.40 | 107.88 | −0.48 | −0.45 |
| l-Proline 1-methylethyl ester dodecyl sulfate | 110.32 | 110.58 | −0.26 | −0.24 |
| l-Valine 1-methylethyl ester dodecyl sulfate | 112.65 | 112.52 | 0.13 | 0.12 |
| Morpholinium formate | 32.20 | 32.26 | −0.06 | −0.19 |
| N-(2′,3′-Epoxypropyl)-N-methylpyrrolidonium acetate | 55.50 | 54.46 | 1.04 | 1.87 |
| N-Butyl-3-methylpyridinium bis(trifluoromethylsulfonyl)amide | 80.49 | 81.06 | −0.57 | −0.71 |
| N-Butyl-3-methylpyridinium trifluoromethanesulfonate | 64.26 | 64.25 | 0.01 | 0.02 |
| N-Butyl-4-methylthiazolium bis(trifluoromethylsulfonyl) amide | 78.68 | 79.25 | −0.57 | −0.72 |
| N-Butyl-4-methylthiazolium difluoro(oxalato)borate | 63.24 | 63.32 | −0.08 | −0.13 |
| N-Butyl-N-methylmorpholinium N-acetylalaninate | 74.32 | 74.63 | −0.31 | −0.42 |
| N-Butyl-N-methylmorpholinium N-acetylleucinate | 88.43 | 88.48 | −0.05 | −0.06 |
| N-Butyl-N-methylmorpholinium N-acetylvalinate | 83.98 | 83.85 | 0.13 | 0.15 |
| N-Butylpyridinium bis(trifluoromethanesulfonyl) amide | 76.22 | 76.25 | −0.03 | −0.04 |
| N-Butylpyridinium triflate | 59.25 | 59.44 | −0.19 | −0.32 |
| N-Butylpyridinium trifluoroacetate | 54.87 | 55.39 | −0.52 | −0.95 |
| N-Butylthiazolium bis(trifluoromethylsulfonyl) amide | 73.89 | 74.63 | −0.74 | −1.00 |
| N-Butylthiazolium difluoro(oxalato)borate | 58.43 | 58.70 | −0.27 | −0.46 |
| N-Decylpyridinium bis(trifluoromethanesulfonyl) amide | 104.52 | 104.03 | 0.49 | 0.47 |
| N-Dodecylpyridinium bis(trifluoromethanesulfonyl) amide | 113.68 | 113.29 | 0.39 | 0.34 |
| N-Ethyl-2-methylpyridinium bis(trifluoromethylsulfonyl)amide | 71.54 | 71.54 | 0.00 | 0.00 |
| N-Ethylmorpholinium formate | 41.07 | 41.68 | −0.61 | −1.49 |
| N-Ethyl-N-methylmorpholinium N-acetylalaninate | 65.23 | 65.37 | −0.14 | −0.21 |
| N-Ethyl-N-methylmorpholinium N-acetylisoleucinate | 78.62 | 79.22 | −0.60 | −0.76 |
| N-Ethyl-N-methylmorpholinium N-acetylleucinate | 78.65 | 79.22 | −0.57 | −0.72 |
| N-Ethyl-N-methylmorpholinium N-acetylvalinate | 74.01 | 74.59 | −0.58 | −0.78 |
| N-Hexyl-N-methylmorpholinium N-acetylalaninate | 83.42 | 83.89 | −0.47 | −0.56 |
| N-Hexyl-N-methylmorpholinium N-acetylleucinate | 97.24 | 97.74 | −0.50 | −0.51 |
| N-Hexyl-N-methylmorpholinium N-acetylvalinate | 92.26 | 93.11 | −0.85 | −0.92 |
| N-Isobutyl-3-sulfopropan-1-aminium hydrogen sulfate | 62.85 | 62.71 | 0.14 | 0.22 |
| N-Methyl-N-(2,3-dihydroxypropyl)pyrrolidinium bis(trifluoromethanesulfonyl) amide | 74.88 | 75.07 | −0.19 | −0.25 |
| N-Octyl-N-methylmorpholinium N-acetylalaninate | 92.82 | 93.15 | −0.33 | −0.36 |
| N-Octyl-N-methylmorpholinium N-acetylvalinate | 102.12 | 102.37 | −0.25 | −0.24 |
| N-Propyl-2-methylpyridinium bis(trifluoromethylsulfonyl)amide | 76.28 | 76.17 | 0.11 | 0.14 |
| N-Propyl-N-methylmorpholinium N-acetylalaninate | 69.57 | 70.00 | −0.43 | −0.62 |
| N-Propyl-N-methylmorpholinium N-acetylleucinate | 83.88 | 83.85 | 0.03 | 0.04 |
| N-Propyl-N-methylmorpholinium N-acetylvalinate | 79.02 | 79.22 | −0.20 | −0.25 |
| Propylammonium nitrate | 28.78 | 28.74 | 0.04 | 0.14 |
| S-Butyl-dimethylthioformamidium bis(trifluoromethylsulfonyl) amide | 79.26 | 79.02 | 0.24 | 0.30 |
| S-Butyl-dimethylthioformamidium difluorooxalylborate | 63.42 | 63.09 | 0.33 | 0.52 |
| S-Ethyl-dimethylthioformamidium bis(trifluoromethylsulfonyl) amide | 69.84 | 69.76 | 0.08 | 0.11 |
| S-Ethyl-dimethylthioformamidium difluorooxalylborate | 53.86 | 53.83 | 0.03 | 0.06 |
| S-Ethyl-dimethylthioformamidium trifluoromethylsulfonate | 53.62 | 52.95 | 0.67 | 1.25 |
| S-Methyl-dimethylthioformamidium bis(trifluoromethylsulfonyl) amide | 64.95 | 64.92 | 0.03 | 0.05 |
| S-Methyl-dimethylthioformamidium trifluoromethylsulfonate | 48.32 | 48.11 | 0.21 | 0.43 |
| Tetrabutylphosphonium acetate | 97.22 | 97.33 | −0.11 | −0.11 |
| Tetrabutylphosphonium formate | 93.57 | 93.04 | 0.53 | 0.57 |
| Tetrabutylphosphonium propanoate | 102.16 | 101.96 | 0.20 | 0.20 |
| Tetradecyl trihexylphosphonium bis(trifluoromethylsulfonyl)amide | 192.57 | 192.55 | 0.02 | 0.01 |
| Tetradecyl trihexylphosphonium chloride | 166.32 | 166.74 | −0.42 | −0.25 |
| Tributylmethylammonium bis(trifluoromethylsulfonyl)amide | 97.59 | 97.53 | 0.06 | 0.06 |
| Triethylammonium acetate | 46.76 | 46.42 | 0.34 | 0.73 |
| Triethyldecylammonium bis(trifluoromethylsulfonyl) amide | 111.38 | 111.11 | 0.27 | 0.24 |
| Triethyldodecylammonium bis(trifluoromethylsulfonyl) amide | 120.72 | 120.37 | 0.35 | 0.29 |
| Triethylheptylammonium bis(trifluoromethylsulfonyl) amide | 97.05 | 97.22 | −0.17 | −0.18 |
| Triethylhexylammonium bis(trifluoromethylsulfonyl) amide | 92.72 | 92.59 | 0.13 | 0.14 |
| Triethyloctylammonium bis(trifluoromethylsulfonyl) amide | 101.96 | 101.85 | 0.11 | 0.11 |
| Triethylsulfonium bis(trifluoromethylsulfonyl) amide | 70.04 | 70.13 | −0.09 | −0.13 |
| Triethyltetradecylammonium bis(trifluoromethylsulfonyl) amide | 129.84 | 129.63 | 0.21 | 0.16 |
| Trihexyl tetradecyl phosphonium dicyanamide | 174.84 | 175.11 | −0.27 | −0.15 |
| Trihexyl tetradecyl phosphonium trifluoromethylsulfonate | 175.69 | 175.74 | −0.05 | −0.03 |
| Trimethyl butylammonium bis(trifluoromethylsulfonyl)amide | 70.29 | 70.37 | −0.08 | −0.11 |
| Trimethyl hexylammonium bis(trifluoromethylsulfonyl)amide | 79.64 | 79.63 | 0.01 | 0.01 |
| Trimethyl octylammonium bis(trifluoromethylsulfonyl)amide | 88.97 | 88.89 | 0.08 | 0.09 |
| Trimethyl pentylammonium bis(trifluoromethylsulfonyl)amide | 75.01 | 75.00 | 0.01 | 0.01 |
| Trimethyl propylammonium bis(trifluoromethylsulfonyl)amide | 65.65 | 65.74 | −0.09 | −0.14 |
| Molecule Name | Refractivity Exp. | Refractivity Calc. | Deviation | Dev. in % |
|---|---|---|---|---|
| (2-Phenylethyl)trichlorosilane | 58.62 | 58.57 | 0.05 | 0.09 |
| (3-Chloropropyl)trichlorosilane | 43.26 | 43.44 | −0.18 | −0.42 |
| (3-Chloropropyl)trimethoxysilane | 46.53 | 46.62 | −0.09 | −0.19 |
| (3-Chloropropyl)trimethylsilane | 44.47 | 44.33 | 0.14 | 0.31 |
| (4-Bromophenoxy)trimethylsilane | 58.55 | 58.44 | 0.11 | 0.19 |
| (4-Chlorophenyl)trichlorosilane | 55.04 | 54.14 | 0.90 | 1.64 |
| (4-Methoxyphenyl)trimethylsilane | 56.94 | 56.73 | 0.21 | 0.37 |
| (4-Methylphenyl)trimethylsilane | 54.82 | 55.02 | −0.20 | −0.36 |
| (Bromomethyl)chlorodimethylsilane | 37.57 | 37.57 | 0.00 | 0.00 |
| (Bromomethyl)trimethylsilane | 38.09 | 38.08 | 0.01 | 0.03 |
| (Chloromethyl)trichlorosilane | 34.10 | 34.12 | −0.02 | −0.06 |
| (Chloromethyl)trimethylsilane | 35.13 | 35.01 | 0.12 | 0.34 |
| (Dichloromethyl)trichlorosilane | 39.36 | 39.06 | 0.30 | 0.76 |
| (Diethylamino)trimethylsilane | 47.33 | 47.09 | 0.24 | 0.51 |
| 1,1,1,3,5,5,5-Heptamethyltrisiloxane | 63.17 | 63.24 | −0.07 | −0.11 |
| 1,1,1,3,5,7,7,7-Octamethyltetrasiloxane | 77.46 | 77.53 | −0.07 | −0.09 |
| 1,1,3,3-Tetramethyl-1,3-diphenyldisiloxane | 88.87 | 88.71 | 0.16 | 0.18 |
| 1,1,3,3-Tetramethyldisiloxane | 40.19 | 40.23 | −0.04 | −0.10 |
| 1,2-Bis(tributylsilyl)acetylene | 138.06 | 138.22 | −0.16 | −0.12 |
| 1,2-Bis(triethylsilyl)ethane | 85.59 | 85.82 | −0.23 | −0.27 |
| 1,2-Bis(trimethylsilyl)acetylene | 55.18 | 55.24 | −0.06 | −0.11 |
| 1,2-Dis(trimethylsilyl)-ethylene | 58.50 | 58.06 | 0.44 | 0.75 |
| 1,3,5-Trisilacyclohexane | 43.69 | 43.44 | 0.25 | 0.57 |
| 1,3-Bis(bromomethyl)tetramethyldisiloxane | 64.41 | 64.45 | −0.04 | −0.06 |
| 1,3-Bis(chloromethyl)tetramethyldisiloxane | 58.30 | 58.31 | −0.01 | −0.02 |
| 1,3-Bis(dichloromethyl)tetramethyldisiloxane | 68.07 | 68.19 | −0.12 | −0.18 |
| 1,3-Bis(trimethylsiloxy)benzene | 75.39 | 75.18 | 0.21 | 0.28 |
| 1,3-Divinyl-1,1,3,3-tetramethyldisiloxane | 57.23 | 57.33 | −0.10 | −0.17 |
| 1-Heptyltrifluorosilane | 41.32 | 41.07 | 0.25 | 0.61 |
| 2,2,4,4,6,6-Hexamethylcyclotrisilazane | 63.90 | 63.78 | 0.12 | 0.19 |
| 2,4,6,8,10,12-Hexamethylcyclohexasiloxane | 85.86 | 85.74 | 0.12 | 0.14 |
| 2,4,6,8-Tetramethylcyclotetrasiloxane | 57.13 | 57.16 | −0.03 | −0.05 |
| 2,4,6-Trimethyl-2,4,6-triphenylcyclotrisiloxane | 115.86 | 115.59 | 0.27 | 0.23 |
| 2-Butylsilane | 31.02 | 31.09 | −0.07 | −0.23 |
| 2-Pentyloxytrimethylsilane | 49.75 | 49.57 | 0.18 | 0.36 |
| 2-Silylpentasilane | 73.17 | 73.09 | 0.08 | 0.11 |
| 2-Silyltetrasilane | 61.12 | 61.05 | 0.07 | 0.11 |
| 2-Silyltrisilane | 48.88 | 49.01 | −0.13 | −0.27 |
| 3-(Triethoxysilyl)-1-propanamine | 59.24 | 59.23 | 0.01 | 0.02 |
| 3-(Trimethylsilyl)-1-propanol | 41.55 | 40.94 | 0.61 | 1.47 |
| 3-Mercaptopropyl-trimethoxysilane | 49.46 | 49.49 | −0.03 | −0.06 |
| 4-Bromophenyltrimethylsilane | 57.19 | 57.80 | −0.61 | −1.07 |
| 4-Chlorophenoxytriethylsilane | 69.66 | 69.38 | 0.28 | 0.40 |
| 4-Fluorophenyltrimethylsilane | 50.34 | 50.11 | 0.23 | 0.46 |
| 4-Trifluoromethylphenyltrimethylsilane | 54.63 | 55.30 | −0.67 | −1.23 |
| 4-Trimethylsiloxyphenyltrimethylsilane | 74.63 | 74.54 | 0.09 | 0.12 |
| Allylchlorodimethylsilane | 37.98 | 38.71 | −0.73 | −1.92 |
| Allylmethyldichlorosilane | 38.14 | 38.50 | −0.36 | −0.94 |
| Allyltrichlorosilane | 38.97 | 38.33 | 0.64 | 1.64 |
| Allyltriethoxysilane | 55.73 | 55.49 | 0.24 | 0.43 |
| Allyltrimethylsilane | 39.14 | 39.22 | −0.08 | −0.20 |
| Amylsilane | 35.64 | 35.67 | −0.03 | −0.08 |
| Benzyltrimethylsilane | 55.11 | 54.83 | 0.28 | 0.51 |
| Bis(butylthio) 2-(diethylmethylsilyl)ethyl borane | 101.77 | 102.28 | −0.51 | −0.50 |
| Bis(diethyl)disiloxane | 58.54 | 58.51 | 0.03 | 0.05 |
| Bis(diethylmethylsilyl)amine | 69.24 | 69.58 | −0.34 | −0.49 |
| Bis(diethylsilyl)amine | 61.40 | 61.24 | 0.16 | 0.26 |
| Bis(dimethylamino)bis(diethylamino)silane | 79.50 | 79.36 | 0.14 | 0.18 |
| Bis(dimethylphenylsilyl)amine | 91.08 | 91.06 | 0.02 | 0.02 |
| Bis(ethyldimethylsilyl)amine | 61.01 | 60.44 | 0.57 | 0.93 |
| Bis(ethylisobutyl)disiloxane | 76.95 | 76.95 | 0.00 | 0.00 |
| Bis(ethylphenyl)disiloxane | 88.84 | 89.13 | −0.29 | −0.33 |
| Bis(trichlorosilyl)methane | 51.08 | 51.72 | −0.64 | −1.25 |
| Bis(triethylsilyl)amine | 78.33 | 78.72 | −0.39 | −0.50 |
| Bis(trihexylsilyl)acetylene | 193.90 | 193.78 | 0.12 | 0.06 |
| Bis(trimethoxysilyl)amine | 56.30 | 56.38 | −0.08 | −0.14 |
| Bis(trimethoxysilylamino)dimethylsilane | 77.61 | 77.64 | −0.03 | −0.04 |
| Bis(trimethylsilylamino)dimethylsilane | 71.81 | 72.56 | −0.75 | −1.04 |
| Bromotriethylsilane | 46.44 | 46.60 | −0.16 | −0.34 |
| Bromotrimethylsilane | 32.92 | 32.89 | 0.03 | 0.09 |
| Butoxytrimethylsilane | 44.88 | 44.93 | −0.05 | −0.11 |
| Butylsilane | 30.90 | 31.04 | −0.14 | −0.45 |
| Butyltrichlorosilane | 43.18 | 43.27 | −0.09 | −0.21 |
| Butyltriisothiocyanatosilane | 73.92 | 73.73 | 0.19 | 0.26 |
| Chloro(chloromethyl)dimethylsilane | 34.43 | 34.50 | −0.07 | −0.20 |
| Chloro(dichloromethyl)dimethylsilane | 39.39 | 39.44 | −0.05 | −0.13 |
| Chlorodiethylsilane | 34.92 | 34.70 | 0.22 | 0.63 |
| Chlorodimethylphenylsilane | 49.33 | 49.70 | −0.37 | −0.75 |
| Chlorodimethylsilane | 25.90 | 25.56 | 0.34 | 1.31 |
| Chloroethyldimethylsilane | 35.15 | 34.39 | 0.76 | 2.16 |
| Chloromethylphenylsilane | 45.46 | 45.44 | 0.02 | 0.04 |
| Chlorotriethylsilane | 43.54 | 43.53 | 0.01 | 0.02 |
| Chlorotrimethylsilane | 29.88 | 29.82 | 0.06 | 0.20 |
| Chlorovinyldimethylsilane | 34.49 | 34.01 | 0.48 | 1.39 |
| cis-1,2-Bis(trimethylsilyl)-3,3-dichlorocyclopropane | 71.37 | 70.62 | 0.75 | 1.05 |
| cis-1,2-Dis(trimethylsilyl)-ethylene | 57.88 | 58.06 | −0.18 | −0.31 |
| Cyclohexyloxytrimethylsilane | 52.12 | 52.06 | 0.06 | 0.12 |
| Cyclohexyltrifluorosilane | 34.26 | 34.35 | −0.09 | −0.26 |
| Decamethylcyclopentasiloxane | 93.33 | 93.25 | 0.08 | 0.09 |
| Decamethyltetrasiloxane | 86.18 | 86.25 | −0.07 | −0.08 |
| Decyldiphenylnonylsilane | 148.69 | 148.68 | 0.01 | 0.01 |
| Decyldiphenylsilane | 106.74 | 107.04 | −0.30 | −0.28 |
| Decyloxytrimethylsilane | 72.71 | 72.71 | 0.00 | 0.00 |
| Di(cyclohexyloxy)dimethylsilane | 74.01 | 73.82 | 0.19 | 0.26 |
| Diallyldimethylsilane | 48.34 | 48.11 | 0.23 | 0.48 |
| Diamylsilane | 58.60 | 58.56 | 0.04 | 0.07 |
| Dibutyldiisothiocyanatosilane | 77.77 | 77.84 | −0.07 | −0.09 |
| Dibutyldinonylsilane | 131.54 | 131.95 | −0.41 | −0.31 |
| Dibutylmethylsilyl bromide | 60.64 | 60.55 | 0.09 | 0.15 |
| Dibutylnonylsilane | 90.16 | 90.31 | −0.15 | −0.17 |
| Dibutylsilane | 49.39 | 49.30 | 0.09 | 0.18 |
| Dichloro(chloromethyl)methylsilane | 34.17 | 34.29 | −0.12 | −0.35 |
| Dichloro(dichloromethyl)methylsilane | 39.13 | 39.23 | −0.10 | −0.26 |
| Dichlorodiethylsilane | 38.71 | 38.75 | −0.04 | −0.10 |
| Dichlorodimethylsilane | 29.65 | 29.61 | 0.04 | 0.13 |
| Dichlorodiphenylsilane | 69.99 | 69.37 | 0.62 | 0.89 |
| Dichloromethylphenylsilane | 48.81 | 49.49 | −0.68 | −1.39 |
| Dicosamethyldecasiloxane | 197.51 | 198.15 | −0.64 | −0.32 |
| Didecyldiphenylsilane | 153.40 | 153.31 | 0.09 | 0.06 |
| Diethoxydimethylsilane | 40.99 | 41.04 | −0.05 | −0.12 |
| Diethoxydiphenylsilane | 81.07 | 80.80 | 0.27 | 0.33 |
| Diethoxymethylphenylsilane | 60.85 | 60.92 | −0.07 | −0.12 |
| Diethoxymethylsilane | 36.84 | 36.68 | 0.16 | 0.43 |
| Diethyl 2,2-diethylhydrazinosilane | 55.43 | 55.86 | −0.43 | −0.78 |
| Diethyl 2,2-dimethylhydrazinosilane | 46.39 | 46.68 | −0.29 | −0.63 |
| Diethyl bis(2,2-diethylhydrazino)silane | 81.18 | 80.88 | 0.30 | 0.37 |
| Diethyl bis(2,2-dimethylhydrazino)silane | 62.47 | 62.52 | −0.05 | −0.08 |
| Diethyl diethylaminosilane | 52.34 | 52.06 | 0.28 | 0.53 |
| Diethyldifluorosilane | 27.74 | 27.83 | −0.09 | −0.32 |
| Diethyldiisothiocyanatosilane | 59.55 | 59.32 | 0.23 | 0.39 |
| Diethylmethylchlorosilane | 38.62 | 38.96 | −0.34 | −0.88 |
| Diethylmethylsilanol | 35.43 | 35.61 | −0.18 | −0.51 |
| Diethylnonylsilane | 71.60 | 71.79 | −0.19 | −0.27 |
| Diethyloctylsilane | 67.23 | 67.16 | 0.07 | 0.10 |
| Diethylphenyl 1-isopropoxyethoxysilane | 80.26 | 80.12 | 0.14 | 0.17 |
| Diethylsilane | 30.71 | 30.78 | −0.07 | −0.23 |
| Diethylsilanol | 31.62 | 31.25 | 0.37 | 1.17 |
| Difluorodiphenylsilane | 58.69 | 58.45 | 0.24 | 0.41 |
| Dimethoxydiphenylsilane | 71.70 | 71.48 | 0.22 | 0.31 |
| Dimethyl bis(2,2-diethylhydrazino)silane | 71.55 | 71.74 | −0.19 | −0.27 |
| Dimethyl bis(2,2-dimethylhydrazino)silane | 53.54 | 53.38 | 0.16 | 0.30 |
| Dimethyl bis(2-chloropropoxy)silane | 59.83 | 59.82 | 0.01 | 0.02 |
| Dimethyldi(2-ethylbutoxy)silane | 78.27 | 78.00 | 0.27 | 0.34 |
| Dimethyldi(2-octyloxy)silane | 96.44 | 96.62 | −0.18 | −0.19 |
| Dimethyldi-2-butoxysilane | 59.68 | 59.58 | 0.10 | 0.17 |
| Dimethyldi-2-pentoxysilane | 68.61 | 68.84 | −0.23 | −0.34 |
| Dimethyldiacetoxysilane | 40.81 | 40.82 | −0.01 | −0.02 |
| Dimethyldibutoxysilane | 59.63 | 59.56 | 0.07 | 0.12 |
| Dimethyldidodecyloxysilane | 133.43 | 133.64 | −0.21 | −0.16 |
| Dimethyldiheptoxysilane | 87.25 | 87.34 | −0.09 | −0.10 |
| Dimethyldihexoxysilane | 77.46 | 78.08 | −0.62 | −0.80 |
| Dimethyldiisopropoxysilane | 49.53 | 50.32 | −0.79 | −1.59 |
| Dimethyldiisothiocyanatosilane | 49.92 | 50.18 | −0.26 | −0.52 |
| Dimethyldimethoxysilane | 31.51 | 31.72 | −0.21 | −0.67 |
| Dimethyldinonyloxysilane | 105.67 | 105.86 | −0.19 | −0.18 |
| Dimethyldioctoxysilane | 96.50 | 96.60 | −0.10 | −0.10 |
| Dimethyldipentoxysilane | 68.38 | 68.82 | −0.44 | −0.64 |
| Dimethyldiphenoxysilane | 71.56 | 71.40 | 0.16 | 0.22 |
| Dimethyldiphenylsilane | 70.04 | 70.09 | −0.05 | −0.07 |
| Dimethyldipropoxysilane | 50.94 | 50.30 | 0.64 | 1.26 |
| Dimethylethylsilanol | 30.79 | 31.04 | −0.25 | −0.81 |
| Dimethylphenylsilane | 45.04 | 45.55 | −0.51 | −1.13 |
| Dimethylvinylethoxysilane | 39.82 | 39.86 | −0.04 | −0.10 |
| Dinonyldiphenylsilane | 144.50 | 144.05 | 0.45 | 0.31 |
| Diphenylmethylsilane | 65.28 | 65.43 | −0.15 | −0.23 |
| Diphenylmethylsilylamine | 68.03 | 68.05 | −0.02 | −0.03 |
| Diphenylnonylsilane | 102.30 | 102.41 | −0.11 | −0.11 |
| Diphenylsilane | 61.53 | 61.40 | 0.13 | 0.21 |
| Dipropyldifluorosilane | 37.24 | 37.09 | 0.15 | 0.40 |
| Dipropylsilane | 40.08 | 40.04 | 0.04 | 0.10 |
| Disilanomethane | 27.16 | 27.26 | −0.10 | −0.37 |
| Dodecamethylcyclohexasiloxane | 111.89 | 111.90 | −0.01 | −0.01 |
| Dodecamethylpentasiloxane | 104.79 | 104.90 | −0.11 | −0.10 |
| Eicosamethylnonasiloxane | 179.30 | 179.50 | −0.20 | −0.11 |
| Ethoxytriethylsilane | 49.10 | 49.38 | −0.28 | −0.57 |
| Ethoxytrihexylsilane | 105.58 | 104.94 | 0.64 | 0.61 |
| Ethoxytrimethylsilane | 35.67 | 35.67 | 0.00 | 0.00 |
| Ethyl bis(2,2-diethylhydrazino)silane | 71.76 | 71.89 | −0.13 | −0.18 |
| Ethyl bis(2,2-dimethylhydrazino)silane | 53.67 | 53.53 | 0.14 | 0.26 |
| Ethyl bis(2-chloropropoxy)silane | 59.73 | 60.03 | −0.30 | −0.50 |
| Ethyl bis(diethylamino)silane | 64.26 | 64.29 | −0.03 | −0.05 |
| Ethyl isobutyl 2-chloropropoxysilane | 59.24 | 59.06 | 0.18 | 0.30 |
| Ethyl tris(2,2-diethylhydrazino)silane | 96.93 | 96.81 | 0.12 | 0.12 |
| Ethylaminotriethylsilane | 51.46 | 51.36 | 0.10 | 0.19 |
| Ethylcyclohexyldifluorosilane | 44.31 | 44.26 | 0.05 | 0.11 |
| Ethyldibutoxysilane | 59.63 | 59.77 | −0.14 | −0.23 |
| Ethyldimethyl 1-(3-pentoxy)ethoxysilane | 64.89 | 64.93 | −0.04 | −0.06 |
| Ethylisobutylsilanol | 40.40 | 40.47 | −0.07 | −0.17 |
| Ethylphenylchlorosilane | 49.44 | 50.01 | −0.57 | −1.15 |
| Ethyltributoxysilane | 78.67 | 78.95 | −0.28 | −0.36 |
| Ethyltrichlorosilane | 33.83 | 34.01 | −0.18 | −0.53 |
| Ethyltriethoxysilane | 51.50 | 51.17 | 0.33 | 0.64 |
| Ethyltriisothiocyanatosilane | 64.17 | 64.47 | −0.30 | −0.47 |
| Ethyltrimethoxysilane | 37.01 | 37.19 | −0.18 | −0.49 |
| Fluoroethyldiisopropylsilane | 47.71 | 47.87 | −0.16 | −0.34 |
| Fluorotributylsilane | 66.71 | 66.29 | 0.42 | 0.63 |
| Fluorotriethylsilane | 38.10 | 38.51 | −0.41 | −1.08 |
| Fluorotripentylsilane | 80.30 | 80.18 | 0.12 | 0.15 |
| Fluorotripropylsilane | 52.33 | 52.40 | −0.07 | −0.13 |
| Heptasilane | 84.77 | 84.84 | −0.07 | −0.08 |
| Heptyloxytrimethylsilane | 58.80 | 58.82 | −0.02 | −0.03 |
| Hexadecamethylheptasiloxane | 142.31 | 142.20 | 0.11 | 0.08 |
| Hexaethyldisiloxane | 75.92 | 76.37 | −0.45 | −0.59 |
| Hexamethyldisilane | 51.42 | 51.36 | 0.06 | 0.12 |
| Hexamethyldisiloxane | 48.94 | 48.95 | −0.01 | −0.02 |
| Hexapropyldisiloxane | 104.05 | 104.15 | −0.10 | −0.10 |
| Hexasilane | 72.78 | 72.80 | −0.02 | −0.03 |
| Hexyloxytrimethylsilane | 54.16 | 54.19 | −0.03 | −0.06 |
| Hexylsilane | 40.34 | 40.30 | 0.04 | 0.10 |
| HMDS | 51.55 | 51.30 | 0.25 | 0.48 |
| iso-Butylsilane | 31.05 | 31.00 | 0.05 | 0.16 |
| Isopentyloxytrimethylsilane | 49.54 | 49.52 | 0.02 | 0.04 |
| Isopropylmethylsilane | 30.66 | 30.89 | −0.23 | −0.75 |
| Isopropyltriisothiocyanatosilane | 69.52 | 69.15 | 0.37 | 0.53 |
| Isopropyltrimethylsilane | 38.47 | 39.58 | −1.11 | −2.89 |
| Methoxytriethylsilane | 44.49 | 44.72 | −0.23 | −0.52 |
| Methoxytrimethylsilane | 30.80 | 31.01 | −0.21 | −0.68 |
| Methoxytripropylsilane | 58.64 | 58.61 | 0.03 | 0.05 |
| Methyl bis(2,2-diethylhydrazino)silane | 67.18 | 67.32 | −0.14 | −0.21 |
| Methyl bis(2,2-dimethylhydrazino)silane | 48.80 | 48.96 | −0.16 | −0.33 |
| Methyl bis(2-chloropropoxy)silane | 55.37 | 55.46 | −0.09 | −0.16 |
| Methyl bis(diethylamino)silane | 59.99 | 59.72 | 0.27 | 0.45 |
| Methyl tris(2,2-diethylhydrazino)silane | 92.26 | 92.24 | 0.02 | 0.02 |
| Methylcyclohexyldifluorosilane | 39.68 | 39.69 | −0.01 | −0.03 |
| Methyldibutoxysilane | 54.87 | 55.20 | −0.33 | −0.60 |
| Methyldichloro-2-(2,4-dichlorophenoxy)ethylsilane | 70.31 | 70.12 | 0.19 | 0.27 |
| Methyldichloro-2-(4-chlorophenoxy)ethylsilane | 65.49 | 65.30 | 0.19 | 0.29 |
| Methyldichloro-2,4-dichlorophenoxysilane | 60.94 | 60.20 | 0.74 | 1.21 |
| Methyldichloro-2-butoxyethylsilane | 55.11 | 54.39 | 0.72 | 1.31 |
| Methyldichloro-2-isobutoxyethylsilane | 54.52 | 54.35 | 0.17 | 0.31 |
| Methyldichloro-2-phenoxyethylsilane | 60.39 | 60.48 | −0.09 | −0.15 |
| Methyldichloro-4-chlorophenoxysilane | 55.08 | 55.38 | −0.30 | −0.54 |
| Methyldichlorobutoxysilane | 44.66 | 44.64 | 0.02 | 0.04 |
| Methyldichlorophenoxysilane | 50.14 | 50.56 | −0.42 | −0.84 |
| Methyldiethyl-2-phenoxyethylsilane | 70.07 | 70.34 | −0.27 | −0.39 |
| Methyldiethylphenoxysilane | 60.29 | 59.99 | 0.30 | 0.50 |
| Methylnonylphenylsilane | 82.90 | 82.53 | 0.37 | 0.45 |
| Methyloctylphenylsilane | 78.18 | 77.90 | 0.28 | 0.36 |
| Methylphenyldifluorosilane | 38.26 | 38.57 | −0.31 | −0.81 |
| Methylphenylsilane | 40.82 | 41.52 | −0.70 | −1.71 |
| Methylpropylsilane | 30.73 | 30.84 | −0.11 | −0.36 |
| Methylsilanetriol triacetate | 46.27 | 46.27 | 0.00 | 0.00 |
| Methyltri(2-octyloxy)silane | 129.85 | 129.97 | −0.12 | −0.09 |
| Methyltributoxysilane | 74.39 | 74.38 | 0.01 | 0.01 |
| Methyltrichlorosilane | 29.13 | 29.44 | −0.31 | −1.06 |
| Methyltriheptyloxysilane | 116.01 | 116.05 | −0.04 | −0.03 |
| Methyltrihexyloxysilane | 102.11 | 102.16 | −0.05 | −0.05 |
| Methyltriisopentyloxysilane | 88.26 | 88.15 | 0.11 | 0.12 |
| Methyltripentyloxysilane | 88.20 | 88.27 | −0.07 | −0.08 |
| Methyltriphenoxysilane | 91.85 | 92.14 | −0.29 | −0.32 |
| Methylvinyldichlorosilane | 33.33 | 33.80 | −0.47 | −1.41 |
| N-(1,1-Dimethylpropyl)aminotriethylsilane | 64.79 | 65.18 | −0.39 | −0.60 |
| N-(3-(Trimethoxysilyl)propyl)-1,2-ethanediamine | 58.21 | 57.88 | 0.33 | 0.57 |
| N,N-Dibutylaminotriethylsilane | 78.65 | 79.32 | −0.67 | −0.85 |
| N,N-Diethylaminotriethylsilane | 60.99 | 60.80 | 0.19 | 0.31 |
| N,N-Diisobutylaminotriethylsilane | 78.67 | 79.24 | −0.57 | −0.72 |
| N-Fenchylaminotriethylsilane | 83.74 | 83.97 | −0.23 | −0.27 |
| N-Isopropylaminotriethylsilane | 56.05 | 55.92 | 0.13 | 0.23 |
| Nonyloxytrimethylsilane | 68.07 | 68.08 | −0.01 | −0.01 |
| N-Propylaminotriethylsilane | 56.11 | 55.99 | 0.12 | 0.21 |
| N-t-Butylaminotriethylsilane | 60.63 | 60.55 | 0.08 | 0.13 |
| Octadecamethyloctasiloxane | 160.19 | 160.85 | −0.66 | −0.41 |
| Octadecyltrichlorosilane | 108.02 | 108.09 | −0.07 | −0.06 |
| Octamethylcyclotetrasiloxane | 74.68 | 74.60 | 0.08 | 0.11 |
| Octamethyltrisiloxane | 67.44 | 67.60 | −0.16 | −0.24 |
| Octyloxytrimethylsilane | 63.43 | 63.45 | −0.02 | −0.03 |
| Pentasilane | 60.80 | 60.76 | 0.04 | 0.07 |
| Pentyloxytrimethylsilane | 49.52 | 49.56 | −0.04 | −0.08 |
| Pentyltrichlorosilane | 48.79 | 47.90 | 0.89 | 1.82 |
| Phenoxytriethylsilane | 64.47 | 64.56 | −0.09 | −0.14 |
| Phenoxytrimethylsilane | 51.17 | 50.85 | 0.32 | 0.63 |
| Phenoxytripropylsilane | 78.41 | 78.45 | −0.04 | −0.05 |
| Phenylsilane | 37.44 | 37.09 | 0.35 | 0.93 |
| Phenyltri(2-octyloxy)silane | 149.82 | 149.85 | −0.03 | −0.02 |
| Phenyltri(cyclohexyloxy)silane | 115.70 | 115.65 | 0.05 | 0.04 |
| Phenyltrichlorosilane | 48.92 | 49.32 | −0.40 | −0.82 |
| Phenyltrifluorosilane | 33.09 | 33.23 | −0.14 | −0.42 |
| Phenyltriisopentyloxysilane | 108.25 | 108.03 | 0.22 | 0.20 |
| Phenyltrimethylsilane | 49.88 | 50.21 | −0.33 | −0.66 |
| Propyltrichlorosilane | 38.46 | 38.64 | −0.18 | −0.47 |
| Propyltriisothiocyanatosilane | 68.69 | 69.10 | −0.41 | −0.60 |
| t-Butoxytriethylsilane | 58.54 | 58.59 | −0.05 | −0.09 |
| t-Butoxytrimethylsilane | 44.98 | 44.88 | 0.10 | 0.22 |
| t-Butoxytripropylsilane | 72.68 | 72.48 | 0.20 | 0.28 |
| Tetra(1H,1H,3H-perfluoropropyl)silicate | 72.19 | 72.10 | 0.09 | 0.12 |
| Tetra(1H,1H,5H-perfluoropentyl)silicate | 111.97 | 112.18 | −0.21 | −0.19 |
| Tetra(2-ethylbutyl) silicate | 125.53 | 126.42 | −0.89 | −0.71 |
| Tetra(diethylamino)silane | 97.33 | 97.72 | −0.39 | −0.40 |
| Tetra(dimethylamino)silane | 61.29 | 61.00 | 0.29 | 0.47 |
| Tetra-2-butoxysilane | 90.48 | 89.58 | 0.90 | 0.99 |
| Tetra-2-methyl-1-propoxysilane | 88.88 | 89.38 | −0.50 | −0.56 |
| Tetra-2-pentoxysilane | 108.98 | 108.10 | 0.88 | 0.81 |
| Tetra-3-methyl-1-butoxysilane | 107.23 | 107.90 | −0.67 | −0.62 |
| Tetrabutoxysilane | 88.88 | 89.54 | −0.66 | −0.74 |
| Tetracosamethylhendecasiloxane | 217.25 | 216.80 | 0.45 | 0.21 |
| Tetradecamethylcycloheptasiloxane | 130.84 | 130.55 | 0.29 | 0.22 |
| Tetradecamethylhexasiloxane | 123.45 | 123.55 | −0.10 | −0.08 |
| Tetraethoxysilane | 53.33 | 52.50 | 0.83 | 1.56 |
| Tetraethylsilane | 48.37 | 48.61 | −0.24 | −0.50 |
| Tetrahexoxysilane | 127.50 | 126.58 | 0.92 | 0.72 |
| Tetraisobutylsilane | 86.00 | 85.49 | 0.51 | 0.59 |
| Tetraisopropoxysilane | 70.82 | 71.06 | −0.24 | −0.34 |
| Tetraisopropylsilane | 66.92 | 67.33 | −0.41 | −0.61 |
| Tetramethylsilane | 29.95 | 30.33 | −0.38 | −1.27 |
| Tetramethyltetraphenylcyclotetrasiloxane | 154.31 | 154.12 | 0.19 | 0.12 |
| Tetramethyoxysilane | 33.51 | 33.86 | −0.35 | −1.04 |
| Tetraoctoxysilane | 163.90 | 163.62 | 0.28 | 0.17 |
| Tetrapentoxysilane | 107.38 | 108.06 | −0.68 | −0.63 |
| Tetrapropoxysilane | 70.65 | 71.02 | −0.37 | −0.52 |
| Tetrasilane | 48.89 | 48.72 | 0.17 | 0.35 |
| Tetravinylsilane | 46.88 | 47.09 | −0.21 | −0.45 |
| trans-1,2-Bis(trimethylsilyl)-3,3-dichlorocyclopropane | 71.02 | 70.62 | 0.40 | 0.56 |
| Tributylisothiocyanatosilane | 82.05 | 81.60 | 0.45 | 0.55 |
| Tributylsilane | 67.51 | 67.16 | 0.35 | 0.52 |
| Tributylsilanol | 67.79 | 67.96 | −0.17 | −0.25 |
| Tributylsilylamine | 69.61 | 69.78 | −0.17 | −0.24 |
| Trichloro-2-phenoxyethylsilane | 60.44 | 60.31 | 0.13 | 0.22 |
| Trichlorovinylsilane | 33.54 | 33.63 | −0.09 | −0.27 |
| Triethoxymethylsilane | 46.50 | 46.60 | −0.10 | −0.22 |
| Triethoxypentylsilane | 64.96 | 65.06 | −0.10 | −0.15 |
| Triethoxyphenylsilane | 66.15 | 66.48 | −0.33 | −0.50 |
| Triethyl 1-propoxyethoxysilane | 65.15 | 64.80 | 0.35 | 0.54 |
| Triethyl 2,2-diethylhydrazinosilane | 64.50 | 64.60 | −0.10 | −0.16 |
| Triethyl 2,2-dimethylhydrazinosilane | 55.48 | 55.42 | 0.06 | 0.11 |
| Triethylisothiocyanatosilane | 53.85 | 53.82 | 0.03 | 0.06 |
| Triethylsilane | 39.60 | 39.38 | 0.22 | 0.56 |
| Triethylsilanol | 40.59 | 40.18 | 0.41 | 1.01 |
| Triethylsilylamine | 42.07 | 42.00 | 0.07 | 0.17 |
| Triethylsilyloxytripropylsilane | 90.19 | 90.26 | −0.07 | −0.08 |
| Triethyltriphenylcyclotrisiloxane | 129.17 | 129.30 | −0.13 | −0.10 |
| Triisopropoxyvinylsilane | 65.03 | 64.71 | 0.32 | 0.49 |
| Triisopropylethoxysilane | 63.56 | 63.42 | 0.14 | 0.22 |
| Triisopropylphenylsilane | 77.50 | 77.96 | −0.46 | −0.59 |
| Triisopropylsilane | 53.57 | 53.42 | 0.15 | 0.28 |
| Triisopropylsilyl chloride | 57.72 | 57.57 | 0.15 | 0.26 |
| Trimethoxymethylsilane | 32.24 | 32.62 | −0.38 | −1.18 |
| Trimethoxyphenylsilane | 52.32 | 52.50 | −0.18 | −0.34 |
| Trimethoxysilylamine | 30.95 | 30.83 | 0.12 | 0.39 |
| Trimethyl 1-ethoxyethoxysilane | 46.52 | 46.46 | 0.06 | 0.13 |
| Trimethyl 1-isopropoxyethoxysilane | 51.15 | 51.10 | 0.05 | 0.10 |
| Trimethyl 2,2-diethylhydrazinosilane | 50.99 | 50.89 | 0.10 | 0.20 |
| Trimethyl 2,2-dimethylhydrazinosilane | 41.77 | 41.71 | 0.06 | 0.14 |
| Trimethylisothiocyanatosilane | 40.13 | 40.11 | 0.02 | 0.05 |
| Trimethylsilanol | 26.22 | 26.47 | −0.25 | −0.95 |
| Trimethylsilylaminodimethylphenylsilane | 71.09 | 71.18 | −0.09 | −0.13 |
| Trimethylsilylaminodiphenylmethylsilane | 91.05 | 91.06 | −0.01 | −0.01 |
| Trimethylsilylaminotriethylsilane | 65.00 | 65.01 | −0.01 | −0.02 |
| Trimethylsilylaminotrimethoxysilane | 53.70 | 53.84 | −0.14 | −0.26 |
| Trimethylsilylaminotriphenylsilane | 111.69 | 110.94 | 0.75 | 0.67 |
| Trimethylsilyloxytriethylsilane | 62.45 | 62.66 | −0.21 | −0.34 |
| Trimethylsilyloxytripropylsilane | 76.55 | 76.55 | 0.00 | 0.00 |
| Trimethylvinylsilane | 34.55 | 34.52 | 0.03 | 0.09 |
| Tripentylsilane | 81.54 | 81.05 | 0.49 | 0.60 |
| Tripropylsilane | 52.75 | 53.27 | −0.52 | −0.99 |
| Tris(dimethylamino)dibutylaminosilane | 88.58 | 88.70 | −0.12 | −0.14 |
| Trisilane | 36.61 | 36.68 | −0.07 | −0.19 |
| Vinyl tris(2-chloropropoxy)silane | 79.31 | 78.96 | 0.35 | 0.44 |
| Vinyldiethoxymethylsilane | 45.09 | 45.23 | −0.14 | −0.31 |
| Vinyltriacetoxysilane | 50.55 | 50.46 | 0.09 | 0.18 |
| Vinyltriethoxysilane | 50.75 | 50.79 | −0.04 | −0.08 |
| Vinyltrimethoxysilane | 36.41 | 36.81 | −0.40 | −1.10 |
| Molecule Name | Refractivity Exp. | Refractivity Calc. | Deviation | Dev. in % |
|---|---|---|---|---|
| 1,2-Bis(dipentylborinoxy)ethane | 116.12 | 116.10 | 0.02 | 0.02 |
| 1,3,5-Tributylborazine | 79.59 | 79.86 | −0.27 | −0.34 |
| 1,3,5-Triethylborazine | 52.48 | 52.08 | 0.40 | 0.76 |
| 1,3-Bis(dibutoxyboryl)propane | 104.73 | 104.83 | −0.10 | −0.10 |
| 1,3-Bis(dichloroboryl)propane | 44.12 | 44.25 | −0.13 | −0.29 |
| 1,3-Bis(dimethoxyboryl)propane | 48.96 | 49.15 | −0.19 | −0.39 |
| 1,4-Bis(dichloroboryl)butane | 48.79 | 48.88 | −0.09 | −0.18 |
| 1,4-Bis(diethoxyboryl)butane | 73.12 | 72.42 | 0.70 | 0.96 |
| 1,5-Bis(diethoxyboryl)pentane | 77.13 | 77.05 | 0.08 | 0.10 |
| 1,6-Bis(diethoxyboryl)hexane | 81.81 | 81.68 | 0.13 | 0.16 |
| 2-Butoxy-2-bora-1,3-dioxacyclopentane | 37.13 | 36.61 | 0.52 | 1.40 |
| 2-Tolyl 3-tolyl propoxyborine | 82.11 | 81.86 | 0.25 | 0.30 |
| 2-Tolyl 4-tolyl propoxyborine | 82.26 | 81.86 | 0.40 | 0.49 |
| 2-Tolyl dibutoxyborine | 76.18 | 76.43 | −0.25 | −0.33 |
| 2-Tolyl diethoxyborine | 57.69 | 57.91 | −0.22 | −0.38 |
| 2-Tolyl dimethoxyborine | 48.14 | 48.59 | −0.45 | −0.93 |
| 2-Tolyl dipropoxyborine | 66.97 | 67.17 | −0.20 | −0.30 |
| 3-Tolyl dibutoxyborine | 76.48 | 76.43 | 0.05 | 0.07 |
| 3-Tolyl diethoxyborine | 58.08 | 57.91 | 0.17 | 0.29 |
| 3-Tolyl dimethoxyborine | 48.38 | 48.59 | −0.21 | −0.43 |
| 3-Tolyl dipropoxyborine | 67.26 | 67.17 | 0.09 | 0.13 |
| 4-Tolyl dibutoxyborine | 76.67 | 76.43 | 0.24 | 0.31 |
| 4-Tolyl diethoxyborine | 58.04 | 57.91 | 0.13 | 0.22 |
| 4-Tolyl dimethoxyborine | 48.49 | 48.59 | −0.10 | −0.21 |
| 4-Tolyl dipropoxyborine | 67.42 | 67.17 | 0.25 | 0.37 |
| Bis(2-tolyl)-N-ethylaminoborane | 79.12 | 78.87 | 0.25 | 0.32 |
| Bis(2-tolyl)-N-isobutylaminoborane | 88.08 | 88.09 | −0.01 | −0.01 |
| Bis(2-tolyl)-N-methylaminoborane | 74.00 | 74.28 | −0.28 | −0.38 |
| Bis(allylamino)borane | 40.89 | 41.33 | −0.44 | −1.08 |
| Bis(butylamino)borane | 51.24 | 51.21 | 0.03 | 0.06 |
| Bis(butylthio) 2-(diethylmethylsilyl)ethyl borane | 101.77 | 102.28 | −0.51 | −0.50 |
| Bis(butylthio) hexyl borane | 88.58 | 88.16 | 0.42 | 0.47 |
| Bis(butylthio) octyl borane | 98.30 | 97.42 | 0.88 | 0.90 |
| Bis(butylthio)borane | 60.17 | 60.27 | −0.10 | −0.17 |
| Bis(diallylamino)borane | 67.84 | 68.71 | −0.87 | −1.28 |
| Bis(diethylamino)borane | 51.54 | 51.43 | 0.11 | 0.21 |
| Bis(di-isoamylamino)borane | 107.11 | 106.83 | 0.28 | 0.26 |
| Bis(diisobutylamino)borane | 88.26 | 88.31 | −0.05 | −0.06 |
| Bis(ethylthio) butylborane | 60.19 | 60.38 | −0.19 | −0.32 |
| Bis(ethylthio) isobutylborane | 60.30 | 60.34 | −0.04 | −0.07 |
| Bis(ethylthio) isopropylborane | 55.34 | 55.77 | −0.43 | −0.78 |
| Bis(ethylthio) octyl borane | 78.77 | 78.90 | −0.13 | −0.17 |
| Bis(ethylthio) propylborane | 55.66 | 55.75 | −0.09 | −0.16 |
| Bis(ethylthio)borane | 41.16 | 41.75 | −0.59 | −1.43 |
| Bis(isobutyl) cyclohexylboronate | 72.44 | 72.12 | 0.32 | 0.44 |
| Bis(isopropylthio)borane | 51.08 | 50.99 | 0.09 | 0.18 |
| Bis(propylthio)borane | 50.77 | 51.01 | −0.24 | −0.47 |
| Bis(t-butylthio)borane | 61.77 | 61.01 | 0.76 | 1.23 |
| Bromo butylthio isopentylborane | 64.00 | 63.96 | 0.04 | 0.06 |
| Bromo ethylthio isopentylborane | 54.51 | 54.70 | −0.19 | −0.35 |
| Bromo ethylthio isopropylborane | 45.04 | 45.50 | −0.46 | −1.02 |
| Bromo ethylthio phenylborane | 57.27 | 56.67 | 0.60 | 1.05 |
| Butoxy chloro phenylborine | 56.56 | 56.22 | 0.34 | 0.60 |
| Butyl butoxy chloroborine | 49.43 | 49.66 | −0.23 | −0.47 |
| Butyl dibutoxyborine | 64.87 | 65.06 | −0.19 | −0.29 |
| Butylphenylboronic acid | 50.06 | 50.81 | −0.75 | −1.50 |
| Butylthiodiethylborane | 52.20 | 52.20 | 0.00 | 0.00 |
| Chloro 2-octoxy phenylborine | 74.42 | 74.75 | −0.33 | −0.44 |
| Chloro di(1,2,2-trimethylpropoxy)borine | 69.31 | 69.36 | −0.05 | −0.07 |
| Chloro di(2-octoxy)borine | 87.34 | 87.92 | −0.58 | −0.66 |
| Chloro di(sec-butoxy)borine | 50.69 | 50.88 | −0.19 | −0.37 |
| Chloro dibutoxyborine | 51.03 | 50.86 | 0.17 | 0.33 |
| Chloro diisobutoxyborine | 50.35 | 50.78 | −0.43 | −0.85 |
| Chloro dineopentoxyborine | 60.34 | 60.08 | 0.26 | 0.43 |
| Chloro dioctoxyborine | 88.29 | 87.90 | 0.39 | 0.44 |
| Chloro dipropoxyborine | 41.83 | 41.60 | 0.23 | 0.55 |
| Cyclohexyldichloroborane | 41.98 | 41.91 | 0.07 | 0.17 |
| Dibutoxy 2-hydroxyethoxy borine | 59.48 | 58.68 | 0.80 | 1.34 |
| Dibutylboronic anhydride | 86.44 | 87.19 | −0.75 | −0.87 |
| Dibutylbutylaminoborane | 65.68 | 65.39 | 0.29 | 0.44 |
| Dibutylethylthioborane | 61.49 | 61.46 | 0.03 | 0.05 |
| Dibutylphenylaminoborane | 72.53 | 72.57 | −0.04 | −0.06 |
| Dibutylphenylthioborane | 76.46 | 76.82 | −0.36 | −0.47 |
| Dichloro 4-chlorobutoxyborine | 40.73 | 40.65 | 0.08 | 0.20 |
| Dichloro butoxyborine | 36.03 | 35.85 | 0.18 | 0.50 |
| Dichloro isobutoxyborine | 36.55 | 35.81 | 0.74 | 2.02 |
| Dichloro neopentoxyborine | 40.51 | 40.46 | 0.05 | 0.12 |
| Dichloro octoxyborine | 53.85 | 54.37 | −0.52 | −0.97 |
| Dichloro propoxyborine | 30.62 | 31.22 | −0.60 | −1.96 |
| Diisoamylbutylthioborane | 79.99 | 79.90 | 0.09 | 0.11 |
| Diisobutylboronic anhydride | 87.73 | 87.03 | 0.70 | 0.80 |
| Diisobutylbutylthioborane | 71.06 | 70.64 | 0.42 | 0.59 |
| Diisopentylboronic anhydride | 105.56 | 105.55 | 0.01 | 0.01 |
| Diisopropylboronic anhydride | 68.78 | 68.75 | 0.03 | 0.04 |
| Dioctyl butoxyborine | 100.02 | 100.79 | −0.77 | −0.77 |
| Dipropylbutylthioborane | 61.51 | 61.46 | 0.05 | 0.08 |
| Dipropylethylthioborane | 52.48 | 52.20 | 0.28 | 0.53 |
| Dipropylisobutylaminoborane | 55.99 | 56.09 | −0.10 | −0.18 |
| Dipropylphenylaminoborane | 63.31 | 63.31 | 0.00 | 0.00 |
| Dipropylphenylthioborane | 67.67 | 67.56 | 0.11 | 0.16 |
| Ethyl phenyl butoxyborine | 61.09 | 61.05 | 0.04 | 0.07 |
| Ethylphenylboronic acid | 42.13 | 41.55 | 0.58 | 1.38 |
| Ethylphenylboronic anhydride | 82.57 | 81.79 | 0.78 | 0.94 |
| Ethylthio dioctyl borane | 98.03 | 98.50 | −0.47 | −0.48 |
| Ethylthiodiethylborane | 42.82 | 42.94 | −0.12 | −0.28 |
| Hexyldichloroborane | 44.07 | 44.03 | 0.04 | 0.09 |
| Isobutyl cyclohexylchloroboronate | 56.96 | 56.76 | 0.20 | 0.35 |
| o-Tolylamino(diethylamino)borane | 63.48 | 63.31 | 0.17 | 0.27 |
| Pentyldichloroborane | 39.49 | 39.40 | 0.09 | 0.23 |
| Phenyl 2-tolyl propoxyborine | 77.12 | 77.05 | 0.07 | 0.09 |
| Phenyl di(sec-butoxy)borine | 71.45 | 71.64 | −0.19 | −0.27 |
| Phenyl di(sec-octoxy)borine | 108.55 | 108.68 | −0.13 | −0.12 |
| Phenyl dibutoxyborine | 71.32 | 71.62 | −0.30 | −0.42 |
| Phenyl diethoxyborine | 52.87 | 53.10 | −0.23 | −0.44 |
| Phenyl diisobutoxyborine | 71.44 | 71.54 | −0.10 | −0.14 |
| Phenyl dimethoxyborine | 43.45 | 43.78 | −0.33 | −0.76 |
| Phenyl dipropoxyborine | 62.40 | 62.36 | 0.04 | 0.06 |
| Phenylamino(diethylamino)borane | 58.85 | 58.50 | 0.35 | 0.59 |
| Phenylboron dichloride | 41.64 | 41.33 | 0.31 | 0.74 |
| Phenylpropylboronic acid | 46.40 | 46.18 | 0.22 | 0.47 |
| Phenylpropylboronic anhydride | 90.30 | 91.05 | −0.75 | −0.83 |
| Tri(1,2,2-trimethylpropoxy)borine | 93.61 | 94.28 | −0.67 | −0.72 |
| Tri(1,3-dichloro-2-propoxy)borine | 81.12 | 81.47 | −0.35 | −0.43 |
| Tri(1-chloroethoxy)borine | 52.99 | 53.51 | −0.52 | −0.98 |
| Tri(1-ethoxycarbonylethoxy)borine | 85.93 | 85.70 | 0.23 | 0.27 |
| Tri(1-methoxyethoxy)borine | 57.42 | 57.14 | 0.28 | 0.49 |
| Tri(2-ethoxycarbonylethoxy)borine | 84.97 | 85.67 | −0.70 | −0.82 |
| Tri(2-pentoxy)borine | 80.11 | 80.45 | −0.34 | −0.42 |
| Tri(butylthio)borane | 87.47 | 87.36 | 0.11 | 0.13 |
| Tri(ethoxycarbonylmethoxy)borine | 71.14 | 71.78 | −0.64 | −0.90 |
| Tri(propylthio)borane | 73.47 | 73.47 | 0.00 | 0.00 |
| Tri(t-butoxy)borine | 66.77 | 66.38 | 0.39 | 0.58 |
| Tributyl borate | 66.65 | 66.53 | 0.12 | 0.18 |
| Tridecyl borate | 149.41 | 149.87 | −0.46 | −0.31 |
| Triethyl borate | 38.69 | 38.75 | −0.06 | −0.16 |
| Triethylborane | 33.72 | 34.51 | −0.79 | −2.34 |
| Triheptyl borate | 109.18 | 108.20 | 0.98 | 0.90 |
| Triisobutylborane | 62.31 | 62.17 | 0.14 | 0.22 |
| Triisopentoxyborine | 80.14 | 80.30 | −0.16 | −0.20 |
| Triisopentylborane | 76.54 | 76.06 | 0.48 | 0.63 |
| Triisopropyl borate | 52.52 | 52.67 | −0.15 | −0.29 |
| Trimethoxyborine | 24.86 | 24.77 | 0.09 | 0.36 |
| Trioctyl borate | 122.30 | 122.09 | 0.21 | 0.17 |
| Tripentoxyborine | 80.42 | 80.42 | 0.00 | 0.00 |
| Tripropyl borate | 52.55 | 52.64 | −0.09 | −0.17 |
| Tripropylborane | 48.54 | 48.40 | 0.14 | 0.29 |
| Tris(ethylthio)borane | 59.41 | 59.58 | −0.17 | −0.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naef, R.; Acree, W.E., Jr. Revision and Extension of a Generally Applicable Group Additivity Method for the Calculation of the Refractivity and Polarizability of Organic Molecules at 298.15 K. Liquids 2022, 2, 327-377. https://doi.org/10.3390/liquids2040020
Naef R, Acree WE Jr. Revision and Extension of a Generally Applicable Group Additivity Method for the Calculation of the Refractivity and Polarizability of Organic Molecules at 298.15 K. Liquids. 2022; 2(4):327-377. https://doi.org/10.3390/liquids2040020
Chicago/Turabian StyleNaef, Rudolf, and William E. Acree, Jr. 2022. "Revision and Extension of a Generally Applicable Group Additivity Method for the Calculation of the Refractivity and Polarizability of Organic Molecules at 298.15 K" Liquids 2, no. 4: 327-377. https://doi.org/10.3390/liquids2040020
APA StyleNaef, R., & Acree, W. E., Jr. (2022). Revision and Extension of a Generally Applicable Group Additivity Method for the Calculation of the Refractivity and Polarizability of Organic Molecules at 298.15 K. Liquids, 2(4), 327-377. https://doi.org/10.3390/liquids2040020







