Unprecedented High Probe-Reported Polarity of Deep Eutectic Solvents Composed of Lanthanide Salts and Urea
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef] [PubMed]
- Wagle, D.V.; Zhao, H.; Baker, G.A. Deep Eutectic Solvents: Sustainable Media for Nanoscale and Functional Materials. Acc. Chem. Res. 2014, 47, 2299–2308. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.-N.; Pauli, G.F. Natural Deep Eutectic Solvents: Properties, Applications, and Perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Mbous, Y.P.; Hayyan, M.; Hayyan, A.; Wong, W.F.; Hashim, M.A.; Looi, C.Y. Applications of Deep Eutectic Solvents in Biotechnology and Bioengineering—Promises and Challenges. Biotechnol. Adv. 2017, 35, 105–134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; De Oliveira Vigier, K.; Royer, S.; Jérôme, F. Deep Eutectic Solvents: Syntheses, Properties and Applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef] [PubMed]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural Deep Eutectic Solvents—Solvents for the 21st Century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Zainal-Abidin, M.H.; Hayyan, M.; Ngoh, G.C.; Wong, W.F. Doxorubicin Loading on Functional Graphene as a Promising Nanocarrier Using Ternary Deep Eutectic Solvent Systems. ACS Omega 2020, 5, 1656–1668. [Google Scholar] [CrossRef] [PubMed]
- Ruesgas-Ramón, M.; Figueroa-Espinoza, M.C.; Durand, E. Application of Deep Eutectic Solvents (DES) for Phenolic Compounds Extraction: Overview, Challenges, and Opportunities. J. Agric. Food Chem. 2017, 65, 3591–3601. [Google Scholar] [CrossRef]
- van Osch, D.J.G.P.; Dietz, C.H.J.T.; Warrag, S.E.E.; Kroon, M.C. The Curious Case of Hydrophobic Deep Eutectic Solvents: A Story on the Discovery, Design, and Applications. ACS Sustain. Chem. Eng. 2020, 8, 10591–10612. [Google Scholar] [CrossRef]
- Tomé, L.C.; Mecerreyes, D. Emerging Ionic Soft Materials Based on Deep Eutectic Solvents. J. Phys. Chem. B 2020, 124, 8465–8478. [Google Scholar] [CrossRef]
- Pandey, A.; Pandey, S. Solvatochromic Probe Behavior within Choline Chloride-Based Deep Eutectic Solvents: Effect of Temperature and Water. J. Phys. Chem. B 2014, 118, 14652–14661. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Su, E. Hydrophobic Deep Eutectic Solvents: The New Generation of Green Solvents for Diversified and Colorful Applications in Green Chemistry. J. Clean. Prod. 2021, 314, 127965. [Google Scholar] [CrossRef]
- Farooq, M.Q.; Abbasi, N.M.; Anderson, J.L. Deep Eutectic Solvents in Separations: Methods of Preparation, Polarity, and Applications in Extractions and Capillary Electrochromatography. J. Chromatogr. A 2020, 1633, 461613. [Google Scholar] [CrossRef] [PubMed]
- Khokhar, V.; Dhingra, D.; Pandey, S. Effect of Temperature and Composition on Density and Dynamic Viscosity of (Lanthanide Metal Salts + Urea) Deep Eutectic Solvents. J. Mol. Liq. 2022, 360, 119396. [Google Scholar] [CrossRef]
- Khalid, A.; Tahir, S.; Khalid, A.R.; Hanif, M.A.; Abbas, Q.; Zahid, M. Breaking New Grounds: Metal Salts Based-Deep Eutectic Solvents and Their Applications- a Comprehensive Review. Green Chem. 2024, 26, 2421–2453. [Google Scholar] [CrossRef]
- Xia, Q.; Liu, Y.; Meng, J.; Cheng, W.; Chen, W.; Liu, S.; Liu, Y.; Li, J.; Yu, H. Multiple Hydrogen Bond Coordination in Three-Constituent Deep Eutectic Solvents Enhances Lignin Fractionation from Biomass. Green Chem. 2018, 20, 2711–2721. [Google Scholar] [CrossRef]
- Tong, Z.; Meng, J.; Liu, S.; Liu, Y.; Zeng, S.; Wang, L.; Xia, Q.; Yu, H. Room Temperature Dissolving Cellulose with a Metal Salt Hydrate-Based Deep Eutectic Solvent. Carbohydr. Polym. 2021, 272, 118473. [Google Scholar] [CrossRef] [PubMed]
- Huo, D.; Sun, Y.; Yang, Q.; Zhang, F.; Fang, G.; Zhu, H.; Liu, Y. Selective Degradation of Hemicellulose and Lignin for Improving Enzymolysis Efficiency via Pretreatment Using Deep Eutectic Solvents. Bioresour. Technol. 2023, 376, 128937. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Sun, C.; Hu, Y.; Xia, C.; Sun, F.; Zhang, Z. Reaction Characteristics of Metal-Salt Coordinated Deep Eutectic Solvents during Lignocellulosic Pretreatment. J. Environ. Chem. Eng. 2023, 11, 109531. [Google Scholar] [CrossRef]
- Khokhar, V.; Kumar, M.; Pandey, S. Pyrene Aggregation at Unprecedented Low Concentrations in (Lanthanide Metal Salt + Urea) Deep Eutectic Solvents. Phys. Chem. Chem. Phys. 2023, 25, 64–68. [Google Scholar] [CrossRef]
- Kamlet, M.J.; Abboud, J.L.; Taft, R.W. The Solvatochromic Comparison Method. 6. The π* Scale of Solvent Polarities. J. Am. Chem. Soc. 1977, 99, 6027–6038. [Google Scholar] [CrossRef]
- Madeira, P.P.; Passos, H.; Gomes, J.; Coutinho, J.A.P.; Freire, M.G. Alternative Probe for the Determination of the Hydrogen-Bond Acidity of Ionic Liquids and Their Aqueous Solutions. Phys. Chem. Chem. Phys. 2017, 19, 11011–11016. [Google Scholar] [CrossRef] [PubMed]
- Kamlet, M.J.; Taft, R.W. The Solvatochromic Comparison Method. I. The β-Scale of Solvent Hydrogen-Bond Acceptor (HBA) Basicities. J. Am. Chem. Soc. 1976, 98, 377–383. [Google Scholar] [CrossRef]
- Schneider, H.D.; Badrieh, Y.; Migron, Y.; Marcus, Y. Hydrogen Bond Donation Properties of Organic Solvents and Their Aqueous Mixtures from 13C NMR Data of Pyridine-N-Oxide. Z. Phys. Chem. 1992, 177, 143–156. [Google Scholar] [CrossRef]
- Wojeicchowski, J.P.; Abranches, D.O.; Ferreira, A.M.; Mafra, M.R.; Coutinho, J.A.P. Using COSMO-RS to Predict Solvatochromic Parameters for Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2021, 9, 10240–10249. [Google Scholar] [CrossRef]
- Kalyanasundaram, K.; Thomas, J.K. Solvent-Dependent Fluorescence of Pyrene-3-carboxaldehyde and Its Applications in the Estimation of Polarity at Micelle-Water Interfaces. J. Phys. Chem. 1977, 81, 2176–2180. [Google Scholar] [CrossRef]
- Street, K.W.; Acree, W.E. Estimation of the Effective Dielectric Constant of Cyclodextrin Cavities Based on the Fluorescence Properties of Pyrene-3-Carboxaldehyde. Appl Spectrosc 1988, 42, 1315–1318. [Google Scholar] [CrossRef]
- Pandey, A.; Rai, R.; Pal, M.; Pandey, S. How Polar Are Choline Chloride-Based Deep Eutectic Solvents? Phys. Chem. Chem. Phys. 2014, 16, 1559–1568. [Google Scholar] [CrossRef]
- Dutta, A.K.; Kamada, K.; Ohta, K. Spectroscopic Studies of Nile Red in Organic Solvents and Polymers. J. Photochem. Photobiol. A Chem. 1996, 93, 57–64. [Google Scholar] [CrossRef]
- Kurniasih, I.N.; Liang, H.; Mohr, P.C.; Khot, G.; Rabe, J.P.; Mohr, A. Nile Red Dye in Aqueous Surfactant and Micellar Solution. Langmuir 2015, 31, 2639–2648. [Google Scholar] [CrossRef]
- Mishra, A.; Behera, R.K.; Behera, P.K.; Mishra, B.K.; Behera, G.B. Cyanines during the 1990s: A Review. Chem. Rev. 2000, 100, 1973–2012. [Google Scholar] [CrossRef]
- Greenspan, P.; Fowler, S.D. Spectrofluorometric Studies of the Lipid Probe, Nile Red. J. Lipid Res. 1985, 26, 781–789. [Google Scholar] [CrossRef]
- Tolbert, L.M.; Solntsev, K.M. Excited-State Proton Transfer: From Constrained Systems to “Super” Photoacids to Superfast Proton Transfer. Acc. Chem. Res. 2002, 35, 19–27. [Google Scholar] [CrossRef]
- Ireland, J.F.; Wyatt, P.A.H. Acid-Base Properties of Electronically Excited States of Organic Molecules. Adv. Phys. Org. Chem. 1976, 12, 131–221. [Google Scholar]
- Förster, T. Primary Photophysical Processes. Pure Appl. Chem. 1973, 34, 225–234. [Google Scholar] [CrossRef]
- Htun, T. Excited-State Proton Transfer in Nonaqueous Solvent. J. Fluoresc. 2003, 13, 323–329. [Google Scholar] [CrossRef]
- Grdadolnik, J.; Maréchal, Y. Urea and Urea–Water Solutions—An Infrared Study. J. Mol. Struct. 2002, 615, 177–189. [Google Scholar] [CrossRef]
- Nelson, D.L.; Irish, D.E. Interactions in Lanthanide Systems. I. A Raman and Infrared Study of Aqueous Gadolinium Nitrate. J. Chem. Phys. 1971, 54, 4479–4489. [Google Scholar] [CrossRef]
- Hammond, O.S.; Bowron, D.T.; Edler, K.J. Structure and Properties of “Type IV” Lanthanide Nitrate Hydrate:Urea Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2019, 7, 4932–4940. [Google Scholar] [CrossRef]

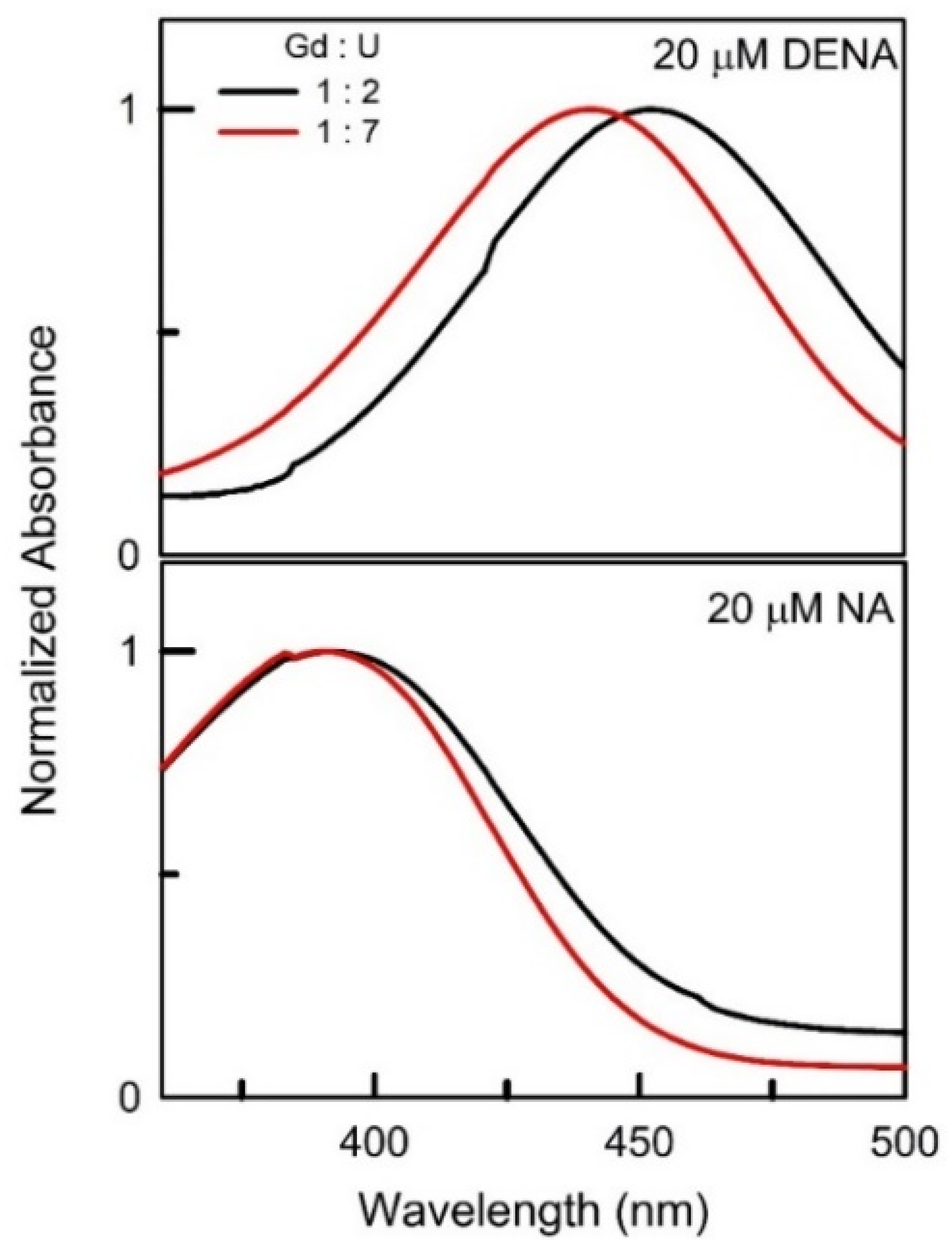


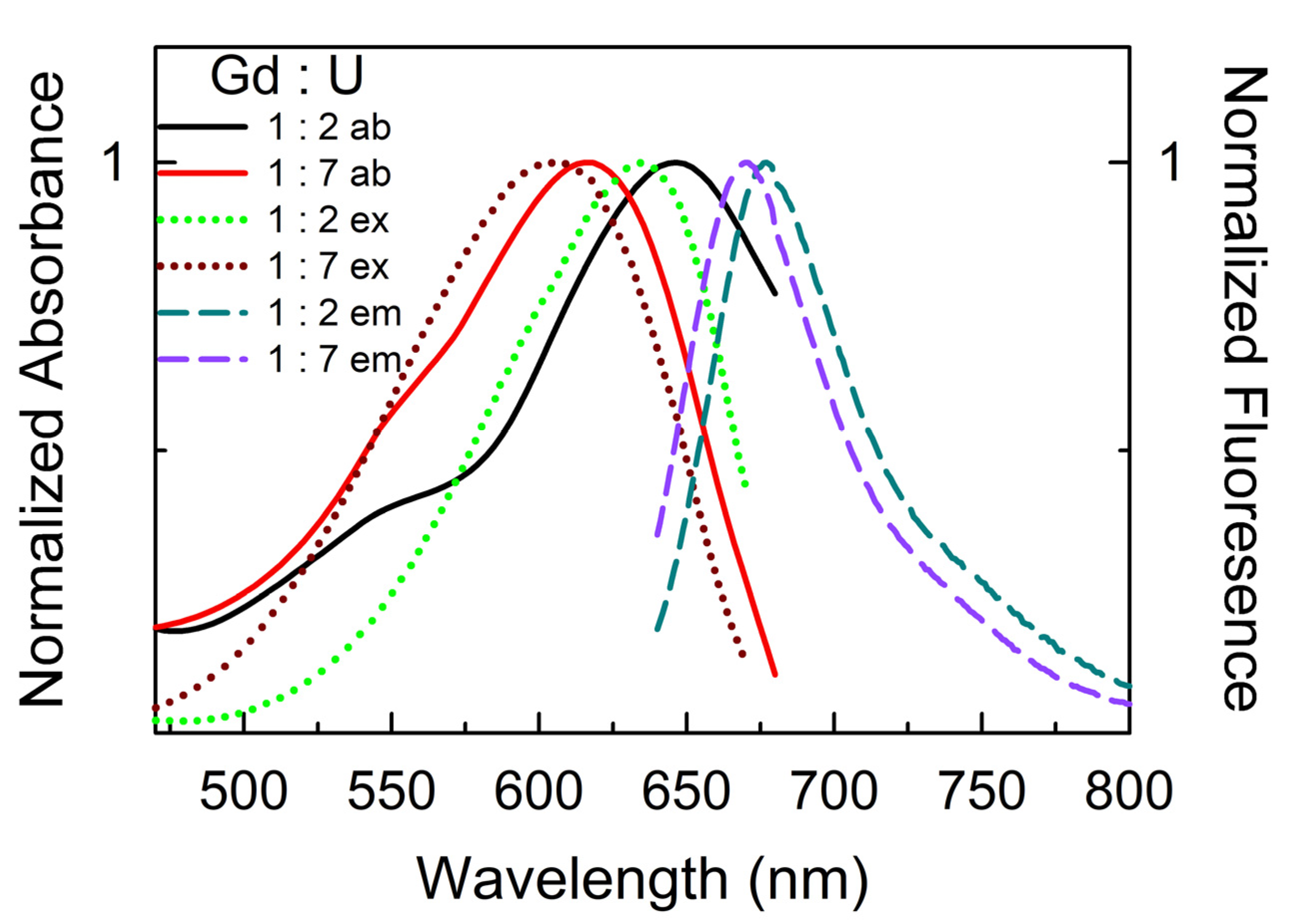


| DESs | Ratio | π* | β |
|---|---|---|---|
| La : U | 1 : 3 | 1.64 | 0.07 |
| 1 : 4 | 1.62 | 0.10 | |
| 1 : 5 | 1.59 | 0.13 | |
| 1 : 6 | 1.54 | 0.19 | |
| 1 : 7 | 1.53 | 0.20 | |
| Ce : U | 1 : 3.5 | 1.61 | 0.30 |
| 1 : 4 | 1.61 | 0.30 | |
| 1 : 5 | 1.58 | 0.33 | |
| 1 : 6 | 1.54 | 0.38 | |
| 1 : 7 | 1.50 | 0.43 | |
| Gd : U | 1 : 2 | 1.70 | −0.01 |
| 1 : 3 | 1.62 | 0.08 | |
| 1 : 4 | 1.59 | 0.12 | |
| 1 : 5 | 1.58 | 0.13 | |
| 1 : 6 | 1.54 | 0.18 | |
| 1 : 7 | 1.53 | 0.19 |
| DES | Ratio | α24 | αRD | ||
|---|---|---|---|---|---|
| La : U | 1 : 3 | 1.55 | 1.77 | 1.42 | 76.67 |
| 1 : 4 | 1.51 | 1.71 | 1.39 | 75.64 | |
| 1 : 5 | 1.46 | 1.66 | 1.35 | 74.54 | |
| 1 : 6 | 1.39 | 1.58 | 1.29 | 72.65 | |
| 1 : 7 | 1.38 | 1.56 | 1.29 | 72.35 |
| Ratio | La : U | Ce : U | Gd : U |
|---|---|---|---|
| 1 : 7 | 479 | 474 | 472 |
| 1 : 6 | 482 | 476 | 477 |
| 1 : 5 | 482 | 477 | 477 |
| 1 : 4 | 483 | 479 | 483 |
| 1 : 3.5 | 482 | ||
| 1 : 3 | 486 | 484 | |
| 1 : 2 | 486 |
| Ratio | La : U | Ce : U | Gd : U | ||||||
|---|---|---|---|---|---|---|---|---|---|
| nm | nm | nm | nm | nm | nm | nm | nm | nm | |
| 1 : 7 | 616 | 617 | 672 | 617 | 590 | 658 | 618 | 607 | 670 |
| 1 : 6 | 620 | 618 | 672 | 622 | 592 | 661 | 621 | 608 | 670 |
| 1 : 5 | 623 | 619 | 674 | 625 | 602 | 663 | 628 | 612 | 671 |
| 1 : 4 | 632 | 627 | 674 | 628 | 607 | 663 | 630 | 618 | 672 |
| 1 : 3.5 | 634 | 614 | 664 | ||||||
| 1 : 3 | 632 | 631 | 675 | 636 | 619 | 675 | |||
| 1 : 2 | 646 | 632 | 677 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patra, A.; Khokhar, V.; Pandey, S. Unprecedented High Probe-Reported Polarity of Deep Eutectic Solvents Composed of Lanthanide Salts and Urea. Liquids 2024, 4, 505-517. https://doi.org/10.3390/liquids4030028
Patra A, Khokhar V, Pandey S. Unprecedented High Probe-Reported Polarity of Deep Eutectic Solvents Composed of Lanthanide Salts and Urea. Liquids. 2024; 4(3):505-517. https://doi.org/10.3390/liquids4030028
Chicago/Turabian StylePatra, Anushis, Vaishali Khokhar, and Siddharth Pandey. 2024. "Unprecedented High Probe-Reported Polarity of Deep Eutectic Solvents Composed of Lanthanide Salts and Urea" Liquids 4, no. 3: 505-517. https://doi.org/10.3390/liquids4030028
APA StylePatra, A., Khokhar, V., & Pandey, S. (2024). Unprecedented High Probe-Reported Polarity of Deep Eutectic Solvents Composed of Lanthanide Salts and Urea. Liquids, 4(3), 505-517. https://doi.org/10.3390/liquids4030028










