Green Plasticizers from Dimer Acids with Selected Esters Classified Through the Nile Red [E(NR)] Polarity Scale
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Equipment
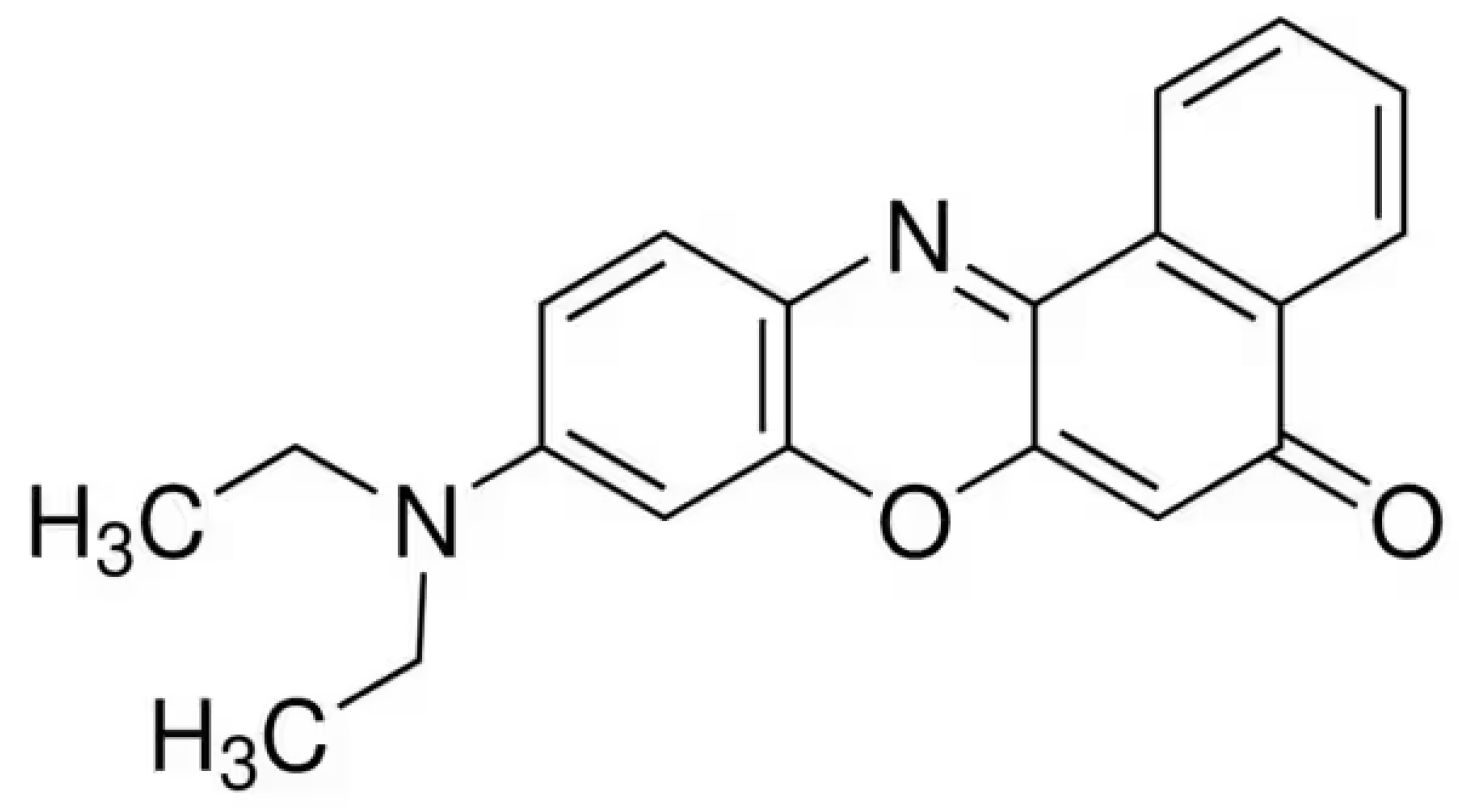
2.2. Determination of the Maximum Absorbance with Solvatochromic Dyes in Liquid Samples
3. Results and Discussion
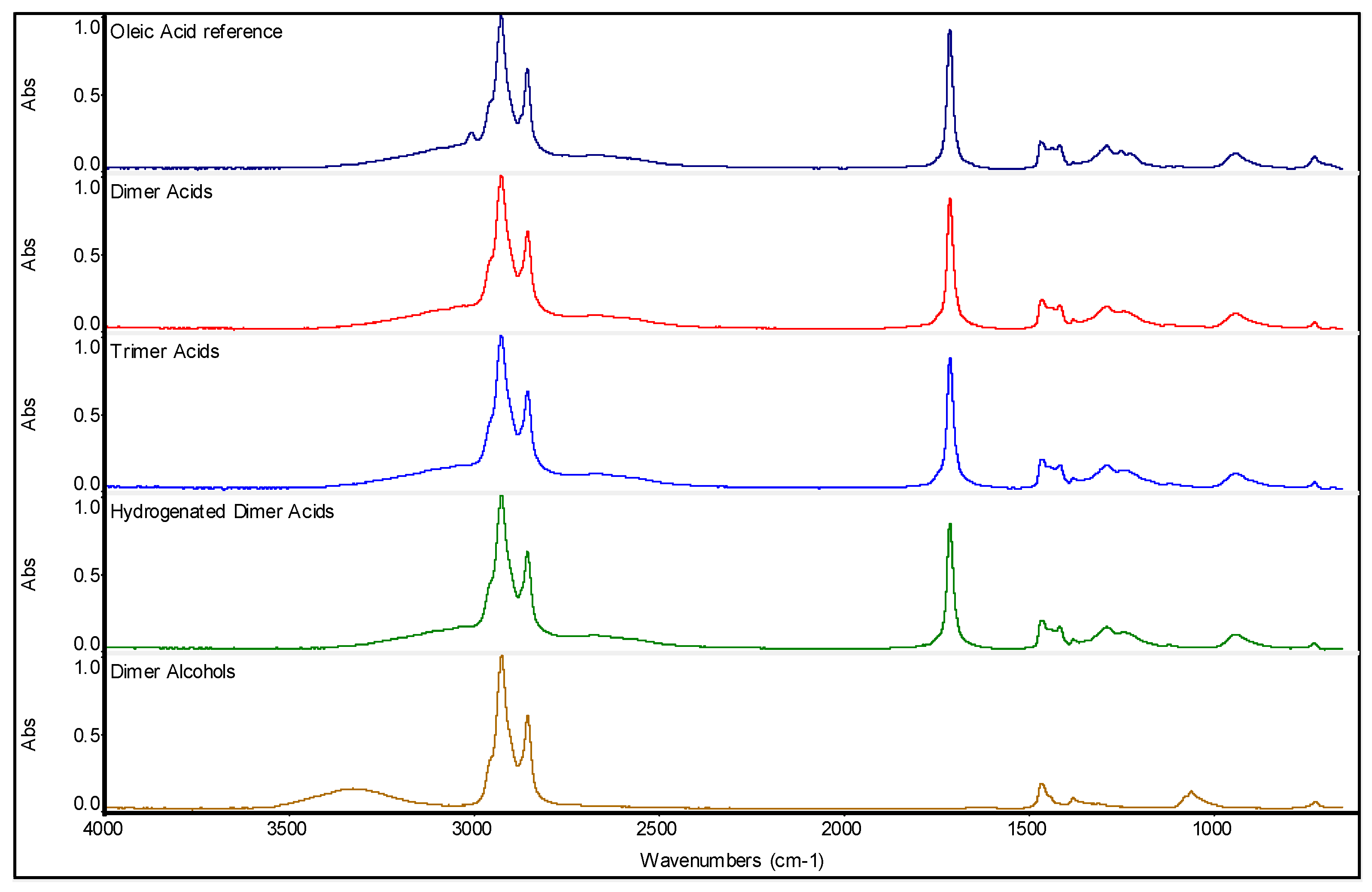
3.1. Electronic Absorption Spectra of Dimer Acids and Hydrogenated Dimer Acids
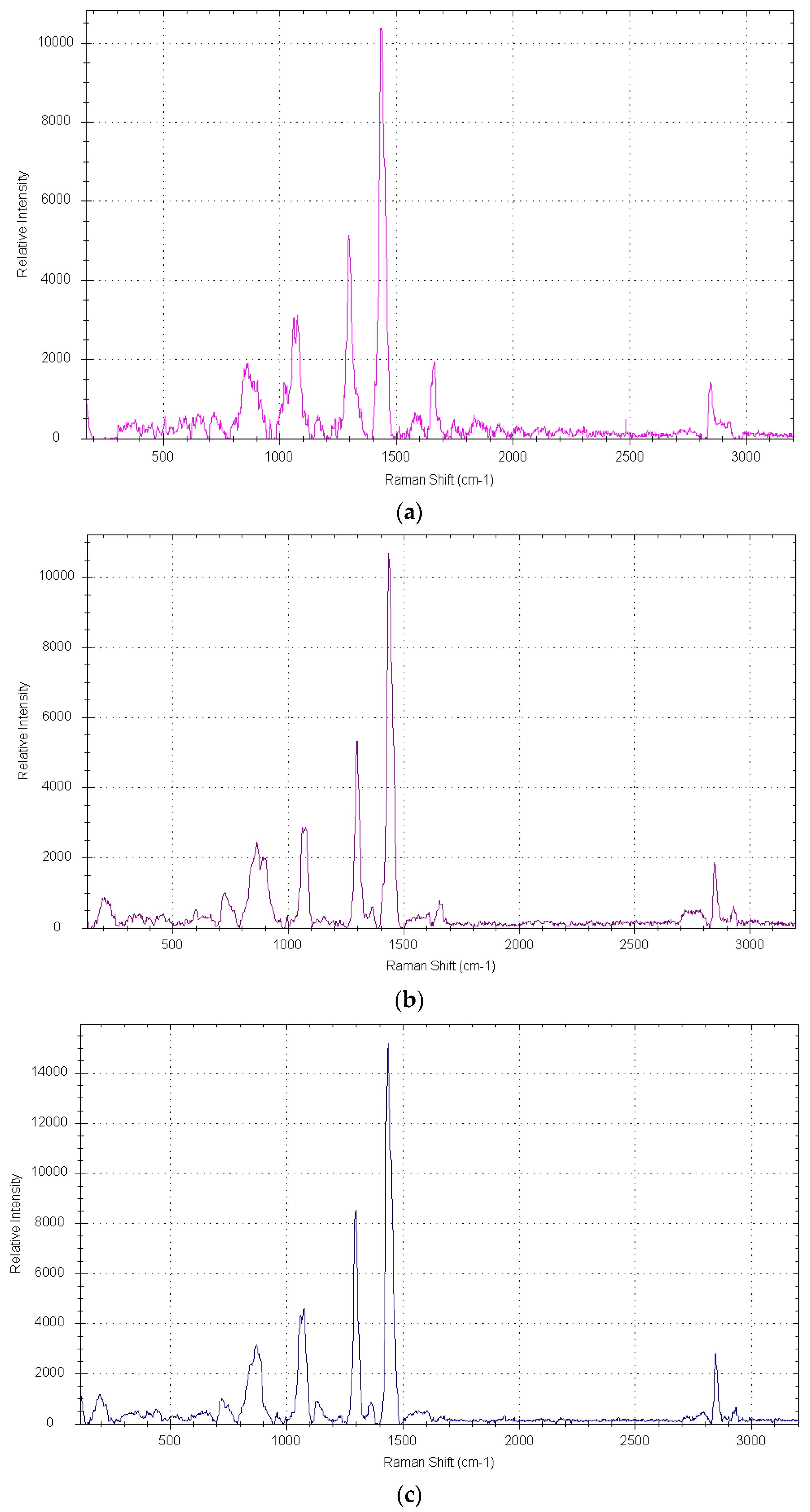
3.2. Raman Spectroscopy on Dimer Acids and Hydrogenated Dimer Acids
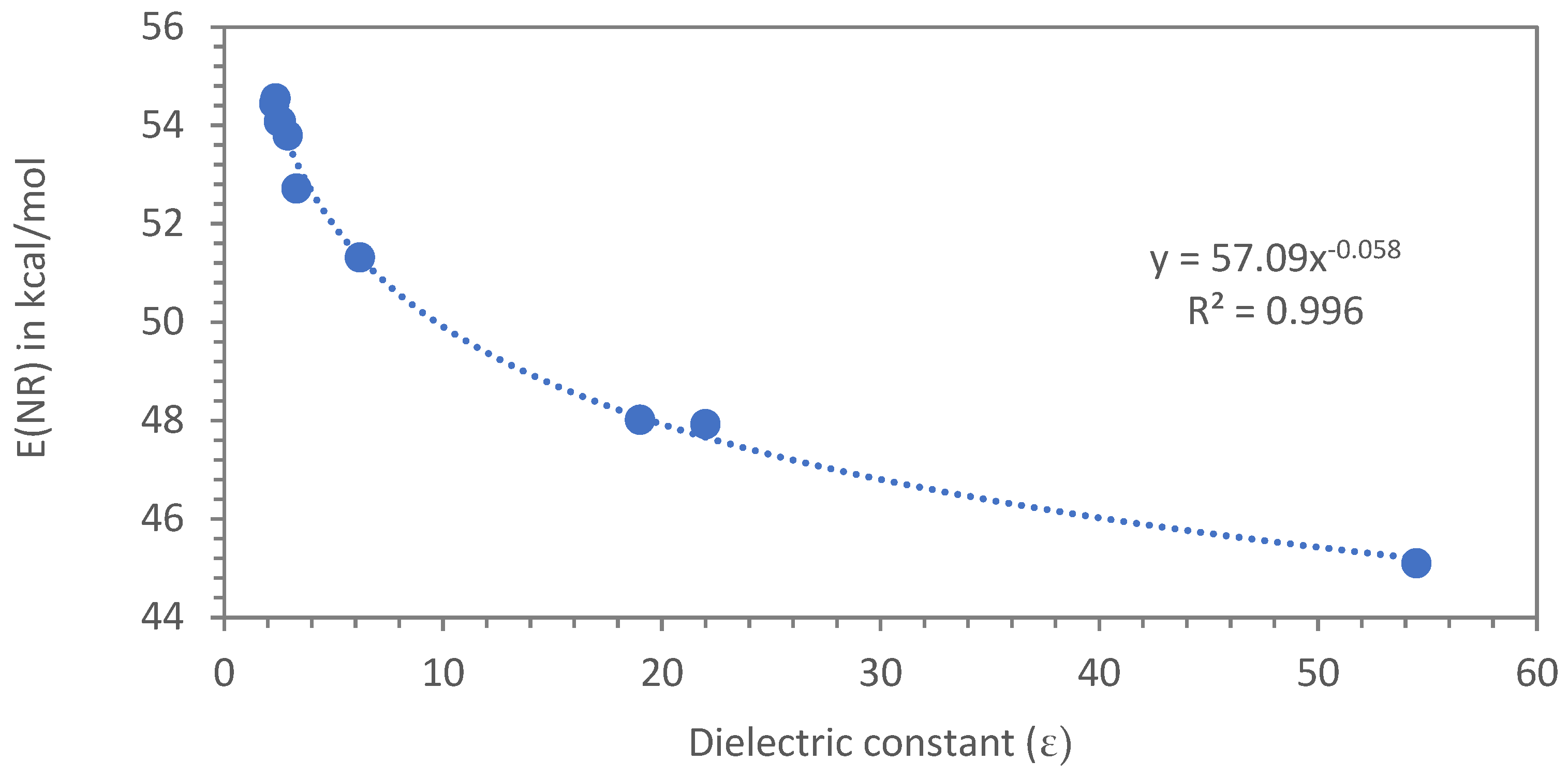
3.3. Dimer Acids and Their Esters as Plasticizers Evaluated in the Nile Red Polarity Scale E(NR)
3.3.1. Dimer Acids and Other Carboxylic Acids Evaluated with the Nile Red Polarity Scale E(NR)
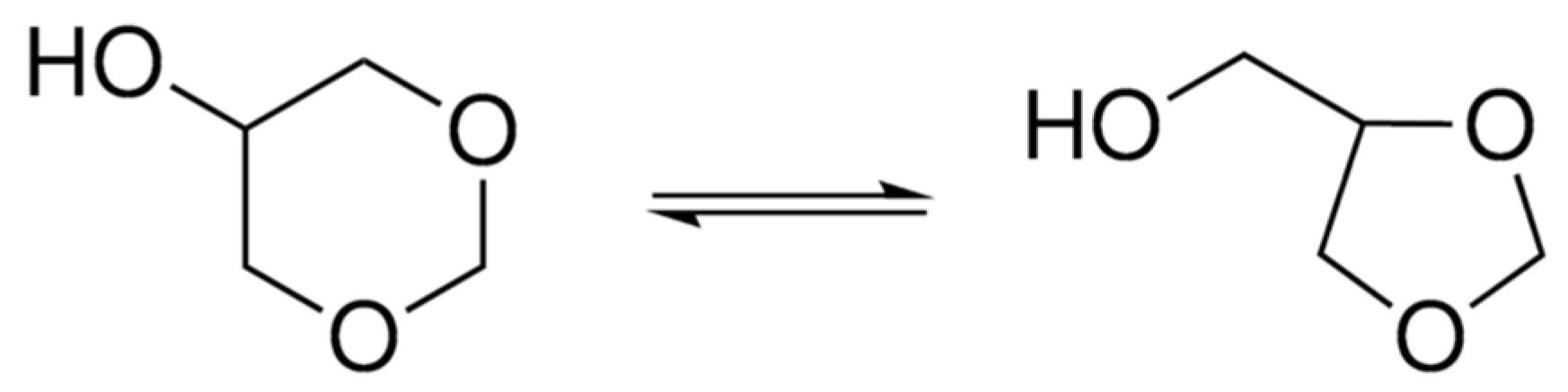
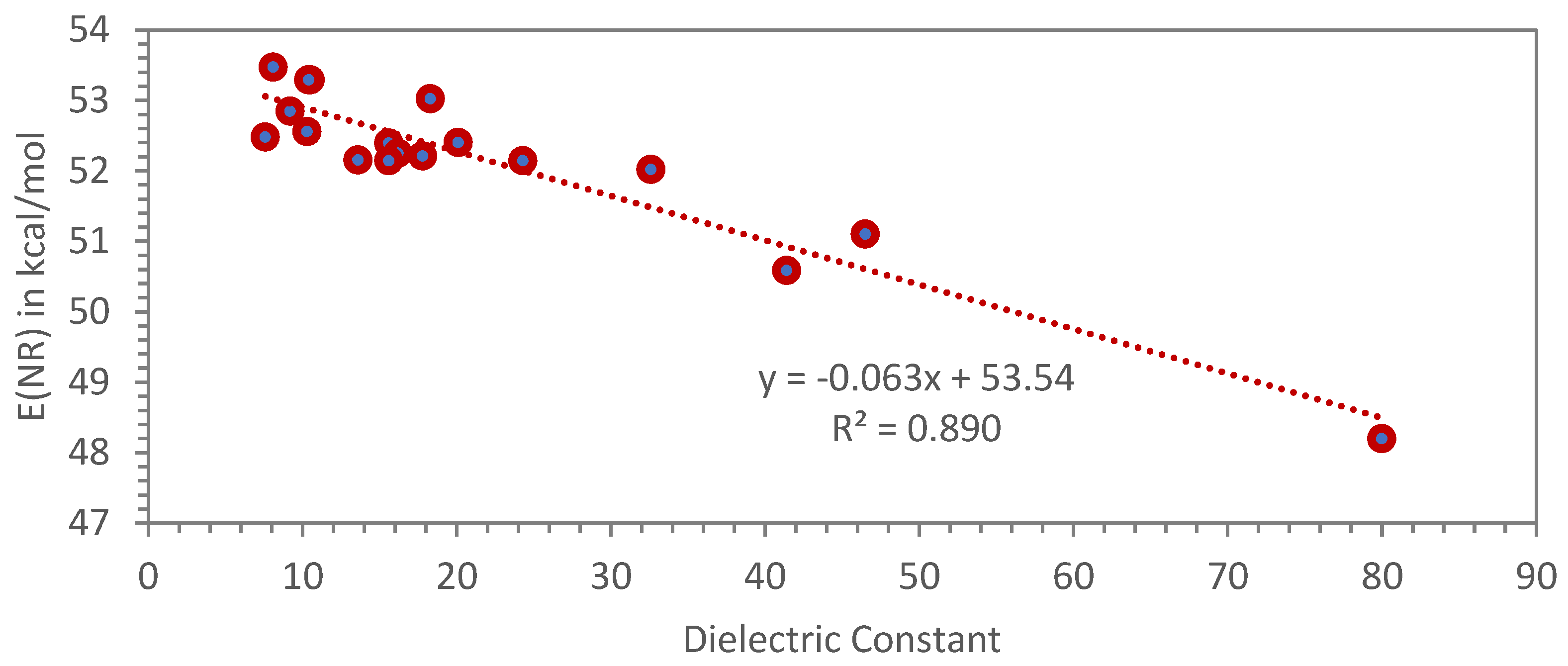
3.3.2. Dimer Alcohols and Other Alcohols Evaluated with the Nile Red Polarity Scale E(NR)
| Alcohol | λ (nm) | E(NR) kcal/mol | References or Notes on the E(NR) Values | Dielectric Constant ε (*) |
|---|---|---|---|---|
| 1-Dodecanol | 534.7 | 53.47 | this work | 8.1 |
| 2-Methyl-2-propanol (t-butanol) (***) | 536.5 | 53.29 | this work | 10.5 |
| Dimer alcohol | 536.5 | 53.29 | this work | 10.4 (**) |
| 2-Propanol (isopropanol) (***) | 539.2 | 53.03 | Ref. [27] | 18.3 |
| 1-Nonanol | 541.0 | 52.85 | this work | 9.2 |
| 1-Octanol | 544.0 | 52.56 | Ref. [27] | 10.3 |
| 2-Ethylhexanol (***) | 544.8 | 52.48 | this work | 7.6 |
| 1-Propanol | 545.6 | 52.40 | Ref. [27] | 20.1 |
| 3-Methyl-1-butanol (***) | 545.7 | 52.39 | this work | 15.6 |
| 1-Pentanol | 547.2 | 52.25 | this work | 16.2 |
| 1-Butanol | 547.6 | 52.21 | Ref. [27] | 17.8 |
| Tetrahydrofurfuryl alcohol (***) | 548.2 | 52.16 | this work | 13.6 |
| Ethanol | 548.3 | 52.15 | Ref. [27] | 24.3 |
| 2-Methyl-1-butanol (***) | 548.3 | 52.15 | this work | 15.6 |
| Methanol | 549.6 | 52.02 | Ref. [27] | 32.6 |
| Glycerol formal | 559.5 | 51.10 | this work | 46.5 (**) |
| Ethylene glycol | 565.2 | 50.59 | Ref. [27] | 41.4 |
| Water (***) | 593.2 | 48.20 | Ref. [27] | 80.0 |
3.3.3. Plasticizers Evaluated with the Nile Red Polarity Scale E(NR)
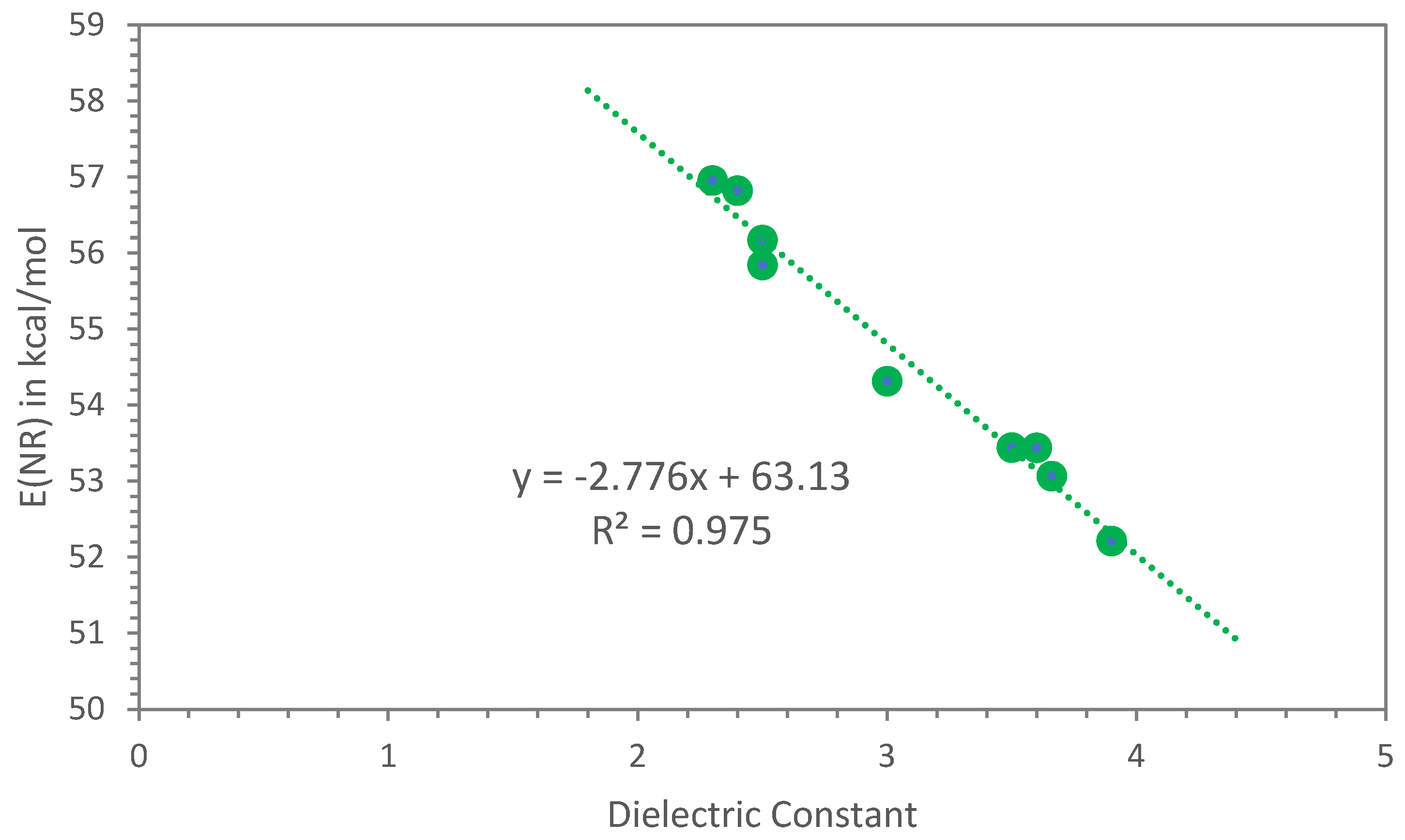
4. Discussion of the Application of Dimerates, Trimerates and Other Esters
| Polymers | λ (nm) | E(NR) kcal/mol | References or Notes on the E(NR) Values | Dielectric Constant ε (*) |
|---|---|---|---|---|
| Polibutadiene (BR) | 502.0 | 56.96 | Ref. [1] | 2.3 |
| Polyisoprene (IR or NR) | 503.2 | 56.82 | Ref. [1] | 2.4 |
| Styrene–butadiene copolymer (S-SBR) with styrene 21% & vinyl 50% | 509.0 | 56.17 | Ref. [1] | 2.5 |
| Polystyrene (PS) | 512.0 | 55.84 | Ref. [1] | 2.5 |
| Epoxidized natural rubber (ENR-25) | 526.4 | 54.32 | Ref. [1] | 3.0 |
| Polymethylmethacrylate film (PMMA) | 535.0 | 53.44 | Ref. [1] | 3.6 |
| Poly(lactic acid) (PLLA) | 535.0 | 53.44 | Ref. [1] | 3.5 (**) |
| Nitrile rubber (NBR) with 33% ACN | 538.8 | 53.07 | Ref. [1] | 3.66 |
| Polyvinylchloride (PVC) | 547.6 | 52.21 | This work | 3.7 |
5. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cataldo, F. Conventional and Green Rubber Plasticizers Classified through Nile Red [E(NR)] and Reichardt’s Polarity Scale [ET(30)]. Liquids 2024, 4, 305–321. [Google Scholar] [CrossRef]
- Sandstrom, P.H.; Rodewald, S.; Ramanathan, A. Rubber Composition and Tire with Component Comprised of Polyisoprene Rubber and Soybean Oil. US Patent N°20140135424A1, 15 May 2014. [Google Scholar]
- Brunelet, T.; Dinh, M.; Favrot, J.M.; Labrunie, P.; Lopitaux, G.; Royet, J.G. Plasticizing System for Rubber Composition. U.S. Patent N°7834074B2, 16 November 2010. [Google Scholar]
- Mohamed, N.R.; Othman, N.; Shuib, R.K. Synergistic effect of sunflower oil and soybean oil as alternative processing oil in the development of greener tyre tread compound. J. Rubber Res. 2022, 25, 239–249. [Google Scholar] [CrossRef]
- Cataldo, F.; Ursini, O.; Angelini, G. Biodiesel as a plasticizer of a SBR-based tire tread formulation. ISRN Polym. Sci. 2013, 2013, 340426. [Google Scholar] [CrossRef]
- Cowan, J.C. Dimer acids. J. Am. Oil Chem. Soc. 1962, 39, 534–545. [Google Scholar] [CrossRef]
- Den Otter, M.J.A.M. The dimerization of oleic acid with a montmorillonite catalyst I: Important process parameters; some main reactions. Fette Seifen Anstrichm. 1970, 72, 667–673. [Google Scholar] [CrossRef]
- Den Otter, M.J.A.M. The Dimerization of Oleic Acid with a Montmorillonite Catalyst II: GLC Analysis of the Monomer; theStructure of the Dimer; a Reaction Model. Fette Seifen Anstrichm. 1970, 72, 875–883. [Google Scholar] [CrossRef]
- Den Otter, M.J.A.M. The Dimerization of Oleic Acid with a Montmorillonite Catalyst III: Test of the Reaction Model. Fette Seifen Anstrichm. 1970, 72, 1056–1066. [Google Scholar] [CrossRef]
- McMahon, D.H.; Crowell, E.P. Characterization of products from clay catalyzed polymerization of tall oil fatty acids. J. Am. Oil Chem. Soc. 1974, 51, 522–527. [Google Scholar] [CrossRef]
- Leonard, E.C. The Dimer Acids: The Chemical and Physical Properties, Reactions, and Applications of Polymerized Fatty Acids; Humko Sheffield Chemical: Memphis, TN, USA, 1975. [Google Scholar]
- Leonard, E.C. Polymerization-dimer acids. J. Am. Oil Chem. Soc. 1979, 56, 782A–785A. [Google Scholar] [CrossRef]
- Balogh, M.; Laszlo, P. Organic Chemistry Using Clays; Springer: Berlin/Heidelberg, Germany, 1993; p. 113. [Google Scholar]
- Park, K.J.; Kim, M.; Seok, S.; Kim, Y.W. Quantitative analysis of cyclic dimer fatty acid content in the dimerization product by proton NMR spectroscopy. Spectrochim. Acta Part A Molec. Biomolec. Spectros. 2015, 149, 402–407. [Google Scholar] [CrossRef]
- Zhang, J.; Nuñez, A.; Strahan, G.D.; Ashby, R.; Huang, K.; Moreau, R.A.; Yan, Z.; Chen, L.; Ngo, H. An advanced process for producing structurally selective dimer acids to meet new industrial uses. Ind. Crop. Prod. 2020, 146, 112132. [Google Scholar] [CrossRef]
- Frihart, C.R. Chemistry of Dimer Acid Production from Fatty acids and the Structure–Property Relationships of Polyamides Made from These Dimer Acids. Polymers 2023, 15, 3345. [Google Scholar] [CrossRef] [PubMed]
- Cornils, B.; Lappe, P. Dicarboxylic Acids, Aliphatics. In Ullmanns Encyclopedia of Industrial Chemistry, 5th ed.; Gerhartz, W., Ed.; VCH: Weinheim, Germany, 1987; Volume A8, p. 535. [Google Scholar]
- Johnson, R.W.; Valdospino, J.M.; Gordon, R.L.; Miller, G.E.; Knight, R.W. Polyamides from fatty acids. In Encyclopedia of Polymer Science and Engineering, 2nd ed.; Kroschwitz, J.I., Ed.; Wiley: New York, NY, USA, 1986; Volume 11, pp. 476–489. [Google Scholar]
- McAdams, L.V.; Gannon, J.A. Epoxy Resins. In Encyclopedia of Polymer Science and Engineering, 2nd ed.; Kroschwitz, J.I., Ed.; Wiley: New York, NY, USA, 1986; Volume 6, p. 352. [Google Scholar]
- Bajpai, U.D.N.; Nivedita, M. Dimer Acids-based Polyesters. In Concise Polymeric Materials Encyclopedia; Salamone, J.C., Ed.; CRC Press: Boca Raton, FL, USA, 1999; pp. 385–386. [Google Scholar]
- Cataldo, F.; Plancq, L.; Genin, F.; Arora, Z. Rubber Composition Comprising Esters from Renewable Sources as Plasticizers. U.S. Patent US11945952B2, 2 April 2020. [Google Scholar]
- Santiago, C.; Gandour, R.W.; Houk, K.N.; Nutakul, W.; Cravey, W.E.; Thummel, R.P. Photoelectron and ultraviolet spectra of small-ring fused aromatic molecules as probes of aromatic ring distortions. J. Am. Chem. Soc. 1978, 100, 3730–3737. [Google Scholar] [CrossRef]
- Breuer, T.E. Dimer acids. In Kirk-Othmer Encyclopedia of Chemical Technology; Wiley & Sons: New York, NY, USA, 2001. [Google Scholar] [CrossRef]
- Perkampus, H.H. UV-VIS Atlas of Organic Compounds, 2nd ed.; VCH: Weinheim, Germany, 1992; Spectrum D2/10. [Google Scholar]
- Czamara, K.; Majzner, K.; Pacia, M.Z.; Kochan, K.; Kaczor, A.; Baranska, M. Raman spectroscopy of lipids: A review. J. Raman Spectrosc. 2015, 46, 4–20. [Google Scholar] [CrossRef]
- Acree Jr, W.E.; Lang, A.S. Reichardt’s Dye-Based Solvent Polarity and Abraham Solvent Parameters: Examining Correlations and Predictive Modeling. Liquids 2023, 3, 303–313. [Google Scholar] [CrossRef]
- Deye, J.F.; Berger, T.A.; Anderson, A.G. Nile Red as a solvatochromic dye for measuring solvent strength in normal liquids and mixtures of normal liquids with supercritical and near critical fluids. Anal. Chem. 1990, 62, 615–622. [Google Scholar] [CrossRef]
- Dean, J.A. (Ed.) Lange’s Handbook of Chemistry, 15th ed.; McGraw-Hill: New York, NY, USA, 1999; Section 5-105. [Google Scholar]
- Weast, R.C. (Ed.) CRC Handbook of Chemistry and Physics, 68th ed.; CRC Press: Boca Raton, FL, USA, 1987; Section E-49. [Google Scholar]
- Chiaradia, G. Plasticizers for Polymers. US Patent 2016026 4758A1, 2016.
- Chiaradia, G.; Cataldo, F.; Bastiaensen, E.; Colombo, D.; Turesso, C. Esters from Vegetable Oils as Process Oils for the Production of Elastomers. Italian patent Demand 102024000007495, 2024. [Google Scholar]
- Randles, S.J. Synthetic Lubricants and High-Performance Functional Fluids, 2nd ed.; Rudnick, L.R., Shubkin, R.L., Eds.; CRC Press: Boca Raton, FL, USA, 1999; Chapter 3; p. 69, Table 6. [Google Scholar]
- Falbe, J.; Lipps, W.; Mayer, D. Alcohols, Aliphatic. In Ullmanns Encyclopedia of Industrial Chemistry, 5th ed.; Gerhartz, W., Ed.; VCH: Weinheim, Germany, 1985; Volume A1, p. 291. [Google Scholar]
- Tavassoli-Kafrani, M.H.; Foley, P.; Kharraz, E.; Curtis, J.M. Quantification of nonanal and oleic acid formed during the ozonolysis of vegetable oil free fatty acids or fatty acid methyl esters. J. Am. Oil Chem. Soc. 2016, 93, 303–310. [Google Scholar] [CrossRef]
- Aries, R.S. Alcohol, Industrial. In Encyclopedia of Chemical Technology; Kirk, R.E., Othmer, D., Eds.; Interscience Publishers: New York, NY, USA, 1952; Volume 1, pp. 262–264. [Google Scholar]
- Davis, B.J.; Maronpot, R.R.; Heindel, J.J. Di-(2-ethylhexyl) phthalate suppresses estradiol and ovulation in cycling rats. Toxicol. Appl. Pharmacol. 1994, 128, 216–223. [Google Scholar] [CrossRef]
- Specht, I.O.; Toft, G.; Hougaard, K.S.; Lindh, C.H.; Lenters, V.; Jönsson, B.A.; Bonde, J.P.E. Associations between serum phthalates and biomarkers of reproductive function in 589 adult men. Environ. Int. 2014, 66, 146–156. [Google Scholar] [CrossRef]
- Brandrup, J.; Immergut, E.H.; Grulke, E.A. (Eds.) Polymer Handbook, 4th ed.; Wiley & Sons: New York, NY, USA, 1999; Section V. [Google Scholar]
- Boussatour, G.; Cresson, P.Y.; Genestie, B.; Joly, N.; Lasri, T. Dielectric characterization of polylactic acid substrate in the fre quency band 0.5–67 GHz. IEEE Microw. Wirel. Comp. Lett. 2018, 28, 374–376. [Google Scholar] [CrossRef]
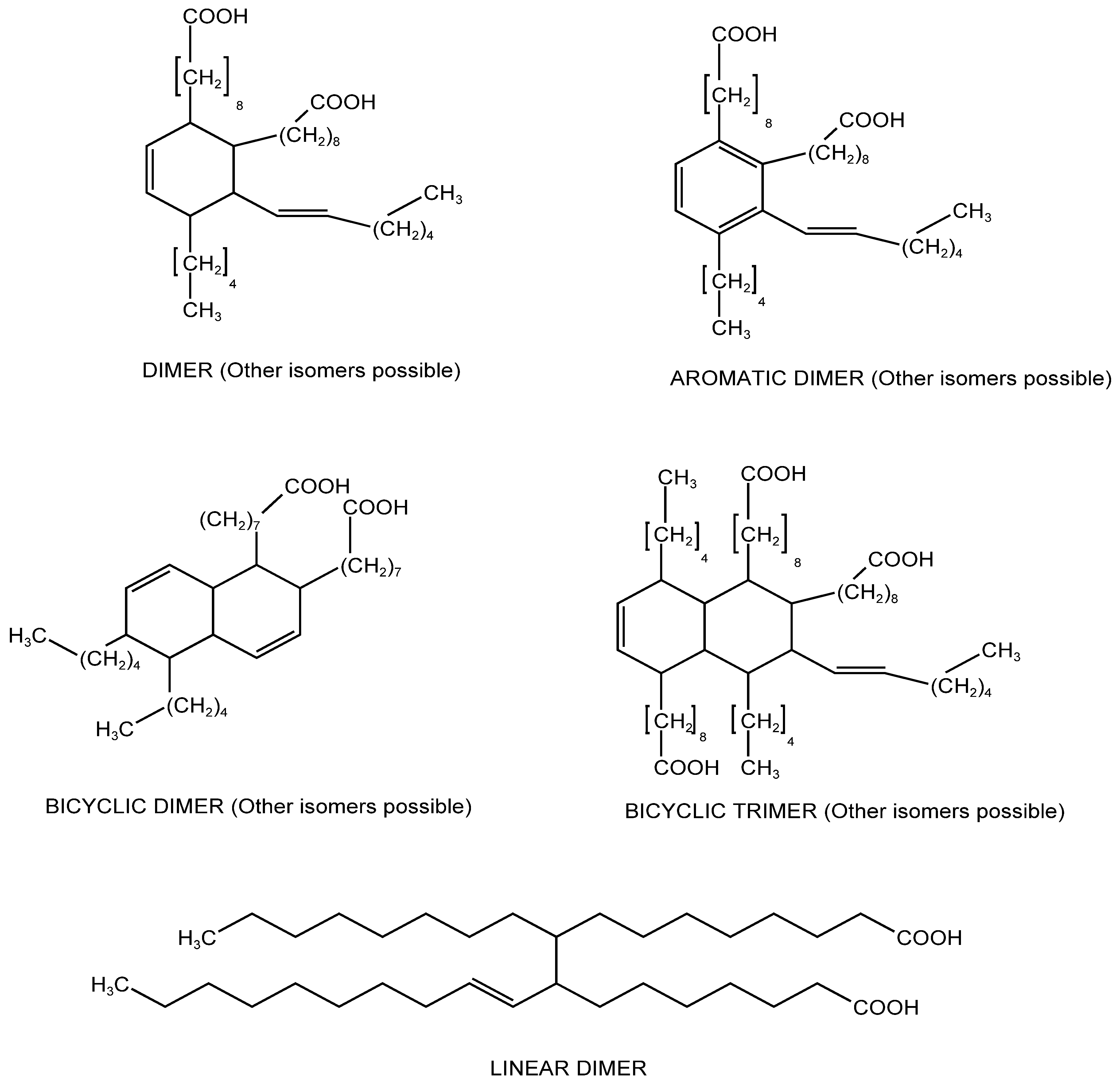
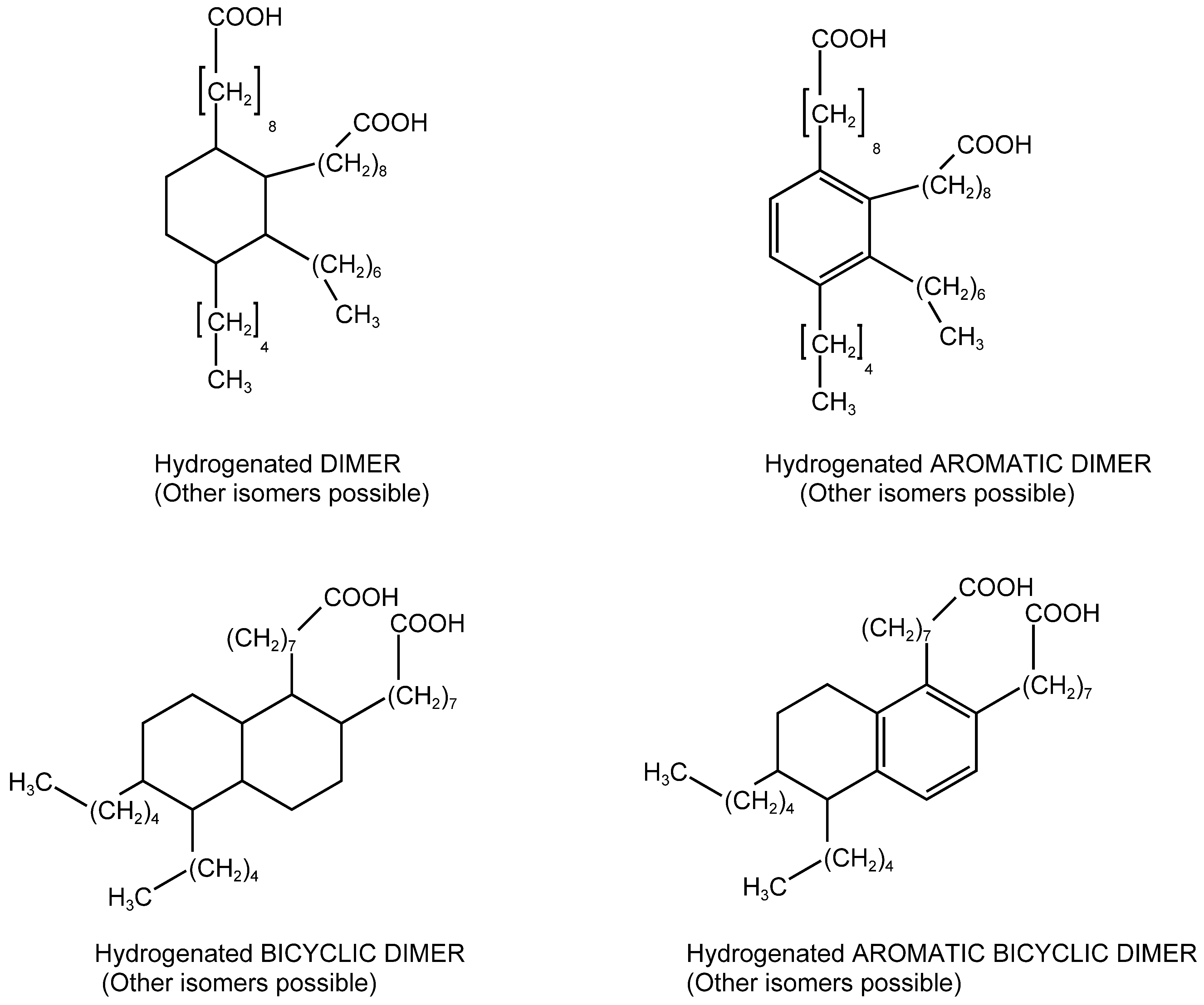
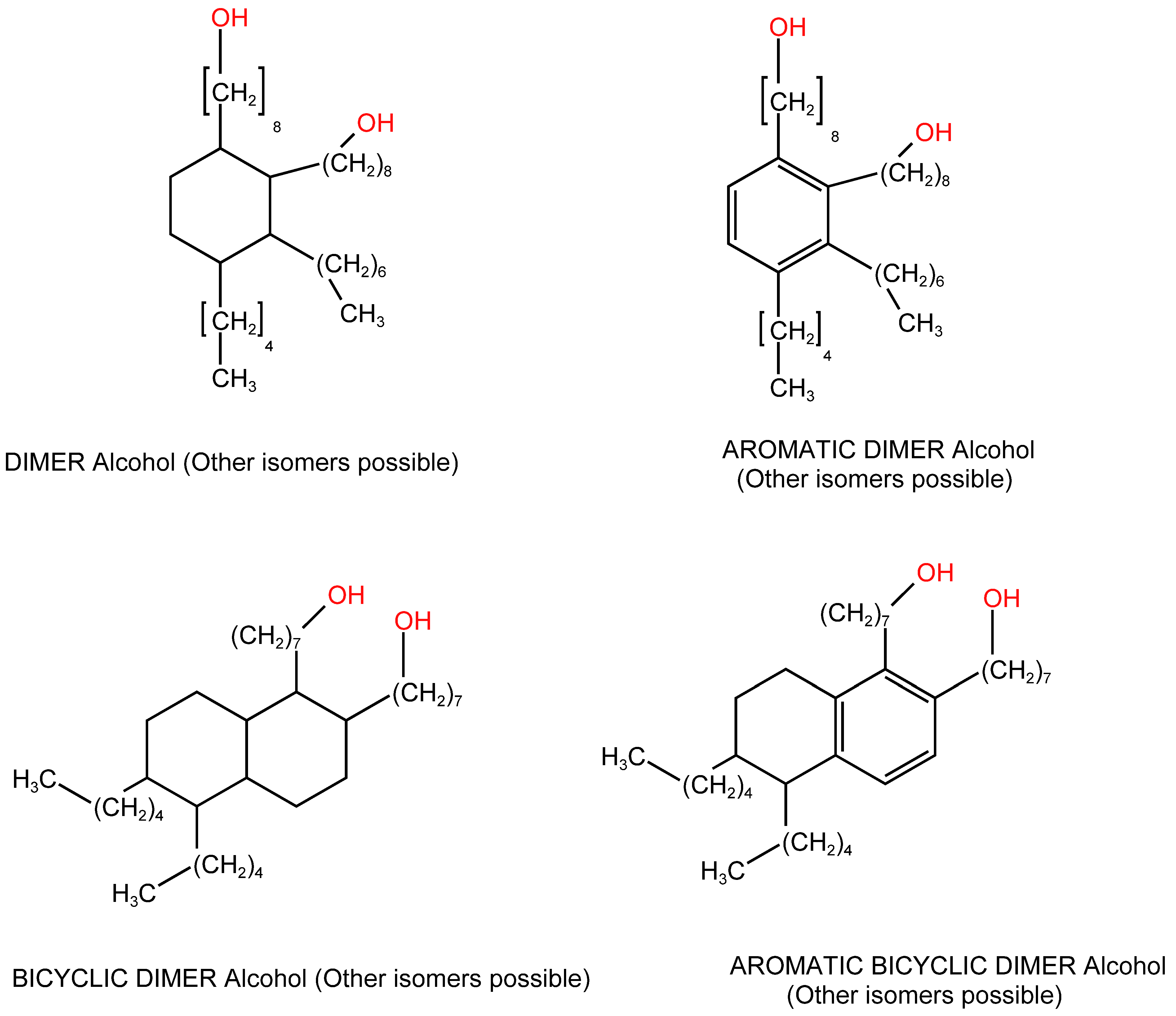

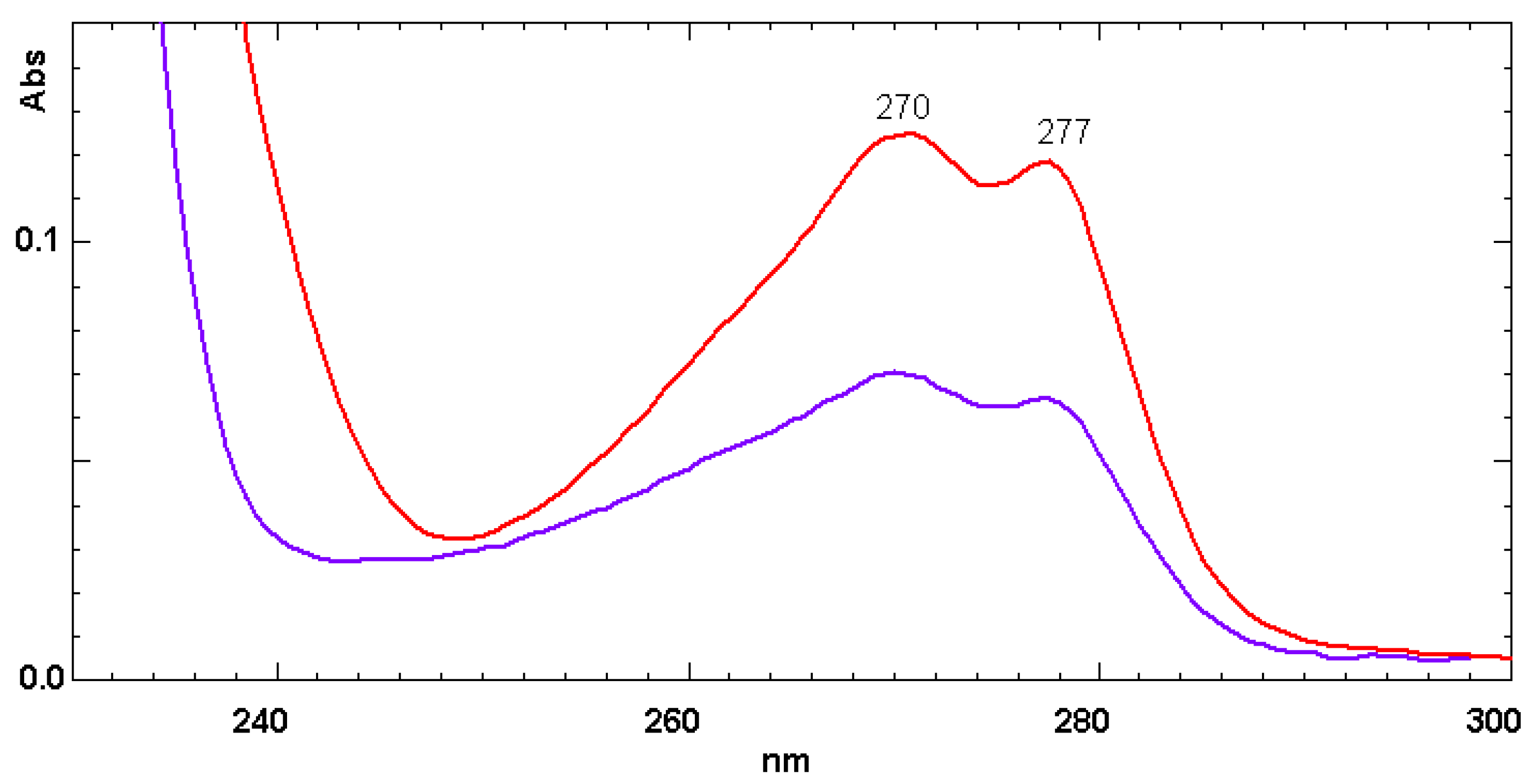


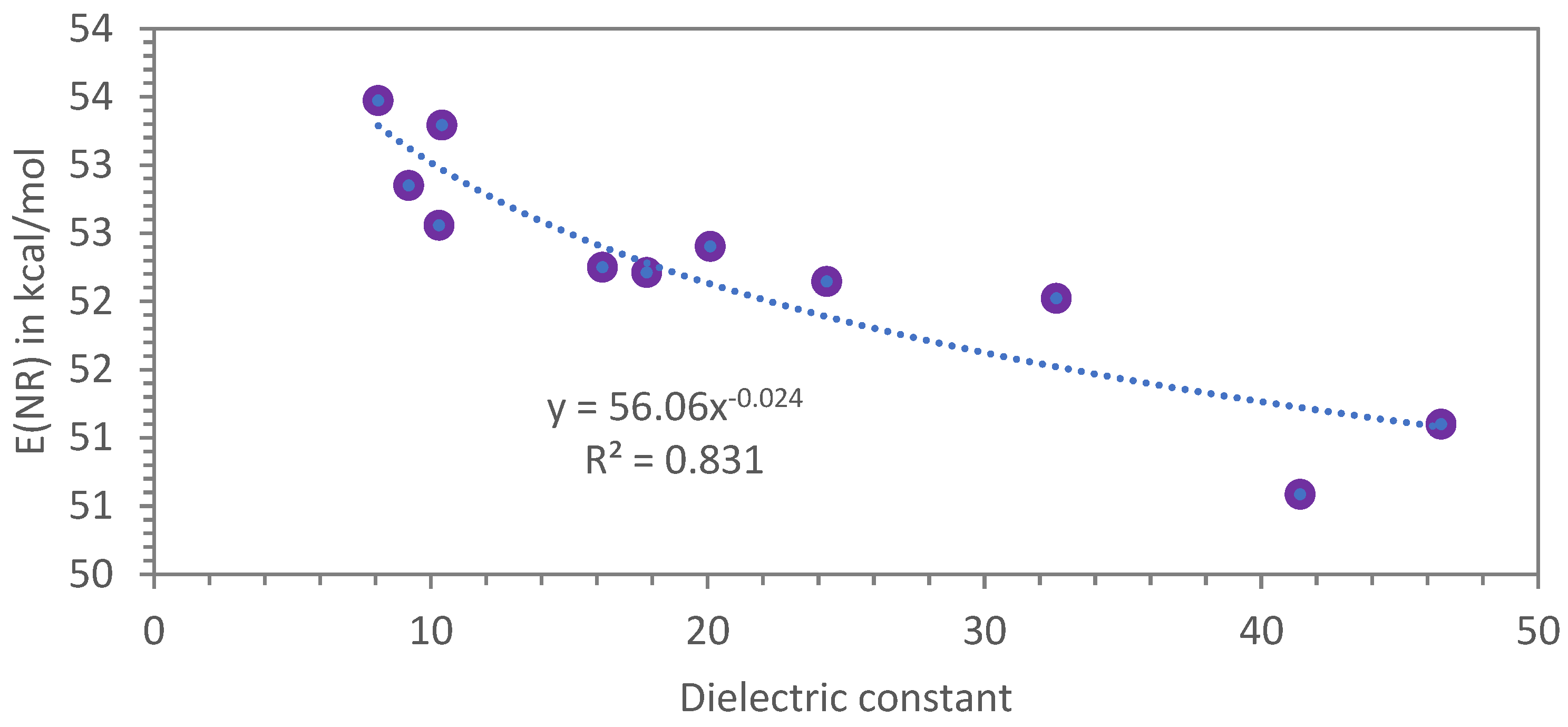

| Liquid Carboxylic Acid | λ (nm) | E(NR) kcal/mol | References or Notes on the E(NR) Values | Dielectric Constant ε (*) |
|---|---|---|---|---|
| Oleic acid | 524.2 | 54.54 | this work | 2.34 |
| Isostearic acid | 525.2 | 54.44 | this work | 2.3 (**) |
| Dimer acids | 528.7 | 54.08 | this work | 2.6 (**) |
| Hydrogenated dimer acids | 528.7 | 54.08 | this work | 2.5 (**) |
| Trimer acids | 531.5 | 53.79 | this work | 2.9 (**) |
| Propionic acid | 542.4 | 52.71 | Ref. [27] | 3.3 |
| Acetic acid | 557.2 | 51.31 | Ref. [27] | 6.2 |
| Levulinic acid (γ-ketovaleric acid) | 595.5 | 48.01 | this work | 19 |
| Lactic acid 90% | 596.6 | 47.92 | this work | 22 |
| Formic acid | 634.0 | 45.10 | Ref. [27] | 54.5 |
| Plasticizers | λ (nm) | E(NR) kcal/mol | References or Notes on the E(NR) Values |
|---|---|---|---|
| T-DAE (treated distillate aromatic extract) | -- | 56.20 | Ref. [1] |
| Oleyl oleate | 508.1 | 56.27 | Ref. [1] |
| Ethyl oleate | 521.3 | 54.85 | Ref. [1] |
| MES (mild extract solvate) | 521.5 | 54.83 | Ref. [1] |
| Methyl esters of fatty acids (brassica) | 522.0 | 54.77 | Ref. [1] |
| Methyl esters of fatty acids (cocco) | 522.7 | 54.70 | Ref. [1] |
| Dioctyl adipate (diethylhexyl adipate) | 523.0 | 54.67 | Ref. [1] |
| Soybean oil | 524.1 | 54.55 | Ref. [1] |
| NYTEX Bio (Naphthenic oil and vegetable oil mixture) | 525.4 | 54.42 | Ref. [1] |
| Lauryl dimerate | 526.0 | 54.36 | This work |
| Nonyl dimerate | 526.2 | 54.34 | This work |
| Lauryl trimerate | 526.2 | 54.34 | This work |
| Amyl dimerate | 526.6 | 54.29 | This work |
| Ethylhexyl dimerate | 526.7 | 54.28 | This work |
| Glycerolformal esters of brassica fatty acids | 526.8 | 54.27 | This work |
| Isoamyl dimerate | 527.1 | 54.24 | This work |
| Butyl dimerate | 527.1 | 54.24 | This work |
| Ethyl dimerate | 527.7 | 54.18 | This work |
| Sunflower oil (high oleic) | 528.2 | 54.13 | Ref. [1] |
| Ethyl trimerate | 528.4 | 54.11 | This work |
| Diisododecyl adipate | 528.5 | 54.10 | Ref. [1] |
| Dimer acids | 528.7 | 54.08 | This work |
| Hydrogenated dimer acids | 528.7 | 54.08 | This work |
| Diethyl azelate | 528.7 | 54.08 | Ref. [1] |
| Methyl undecenoate | 529.6 | 53.99 | Ref. [1] |
| PEG dioleate | 531.5 | 53.79 | Ref. [1] |
| Tetrahydrofurfuryl dimerate | 531.9 | 53.75 | This work |
| PEG monoleate | 533.4 | 53.60 | Ref. [1] |
| Ethyl levulinate | 536.4 | 53.30 | Ref. [1] |
| Dimer alcohol | 536.5 | 53.29 | This work |
| Di(ethylhexyl)phthalate | 537.0 | 53.24 | Ref. [1] |
| Epoxidized glycerolformal ester brassica fatty acids | 538.2 | 53.12 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cataldo, F. Green Plasticizers from Dimer Acids with Selected Esters Classified Through the Nile Red [E(NR)] Polarity Scale. Liquids 2025, 5, 6. https://doi.org/10.3390/liquids5010006
Cataldo F. Green Plasticizers from Dimer Acids with Selected Esters Classified Through the Nile Red [E(NR)] Polarity Scale. Liquids. 2025; 5(1):6. https://doi.org/10.3390/liquids5010006
Chicago/Turabian StyleCataldo, Franco. 2025. "Green Plasticizers from Dimer Acids with Selected Esters Classified Through the Nile Red [E(NR)] Polarity Scale" Liquids 5, no. 1: 6. https://doi.org/10.3390/liquids5010006
APA StyleCataldo, F. (2025). Green Plasticizers from Dimer Acids with Selected Esters Classified Through the Nile Red [E(NR)] Polarity Scale. Liquids, 5(1), 6. https://doi.org/10.3390/liquids5010006








