Magnetic Micro and Nano Sensors for Continuous Health Monitoring
Abstract
:1. Introduction
2. Physical Effects Used in Magnetic Micro and Nano Sensors
2.1. Different Magnetic Arrangements
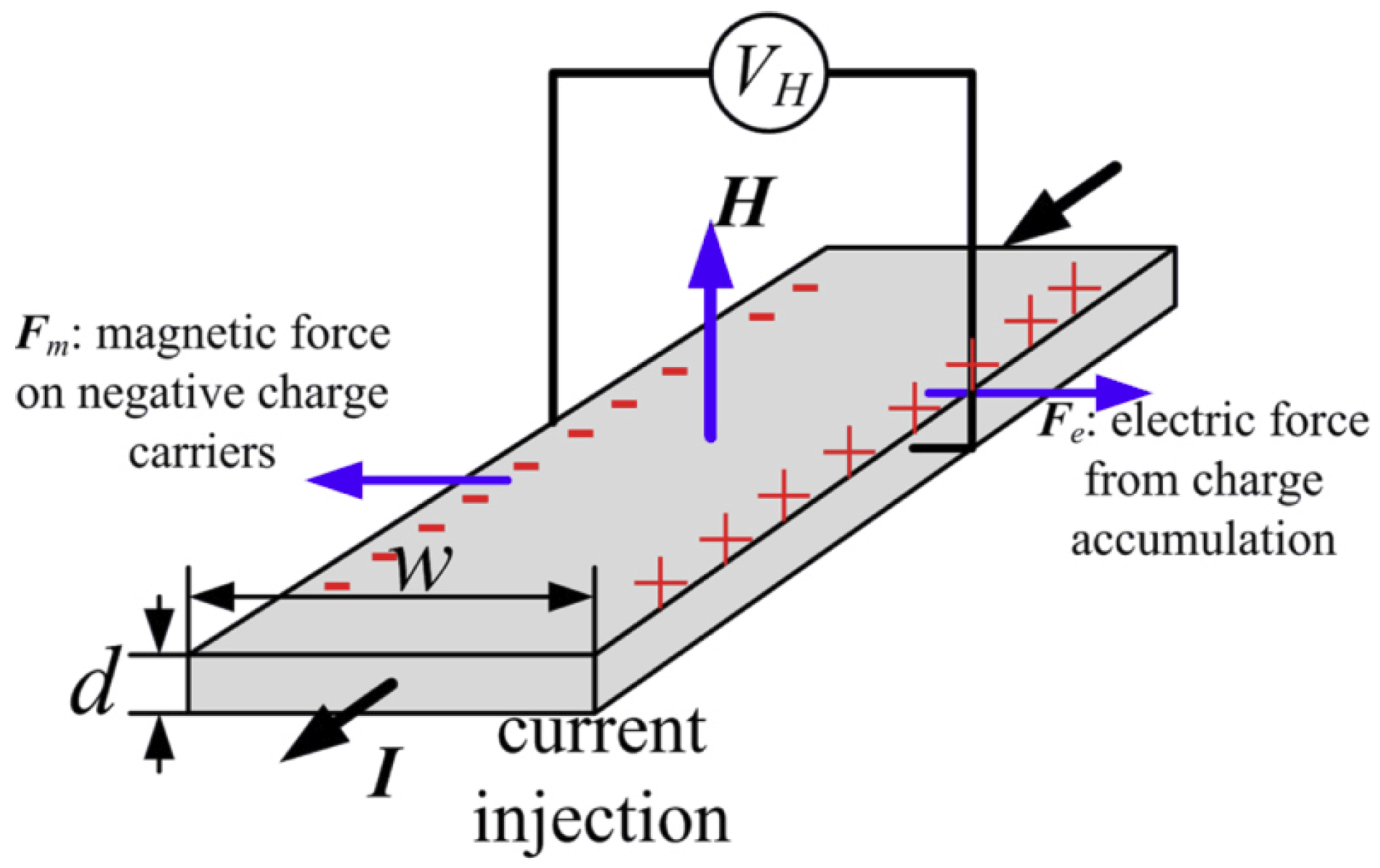
2.2. Hall Effect
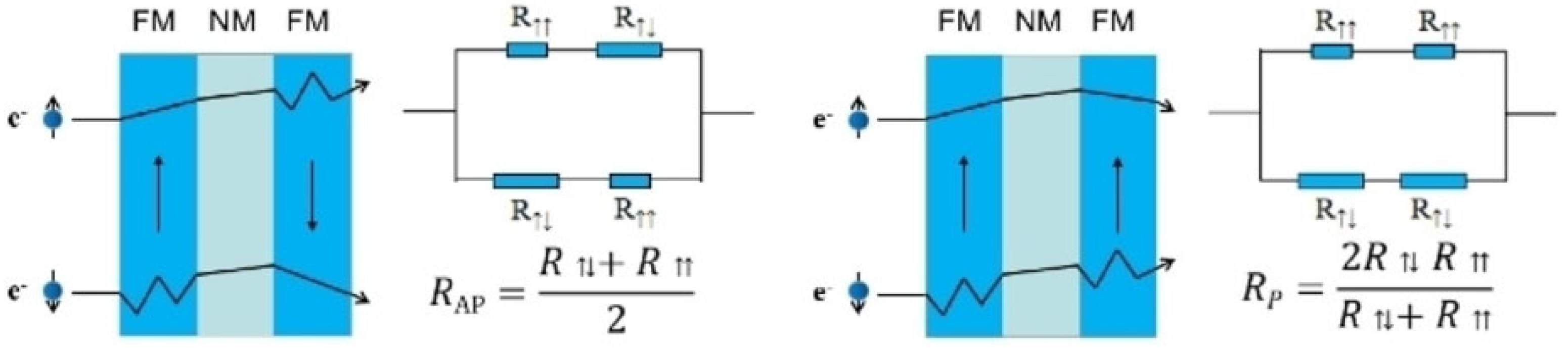
2.3. Giant Magnetoresistance
2.4. Tunnel Magnetoresistance
2.5. Anisotropic and Colossal Magnetoresistance
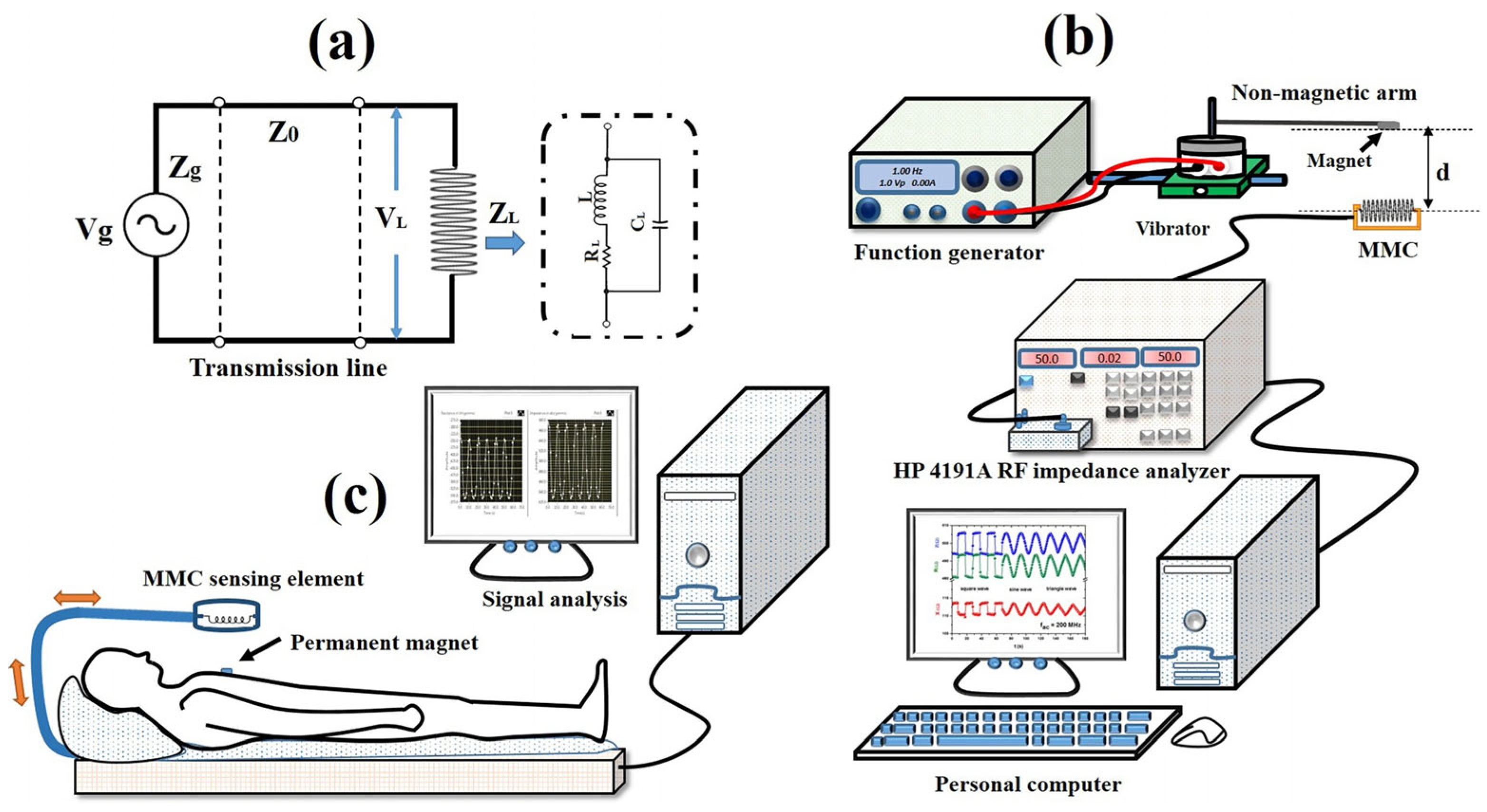
2.6. Giant Magnetoimpedance
2.7. Other Magnetic Effects and Sensors
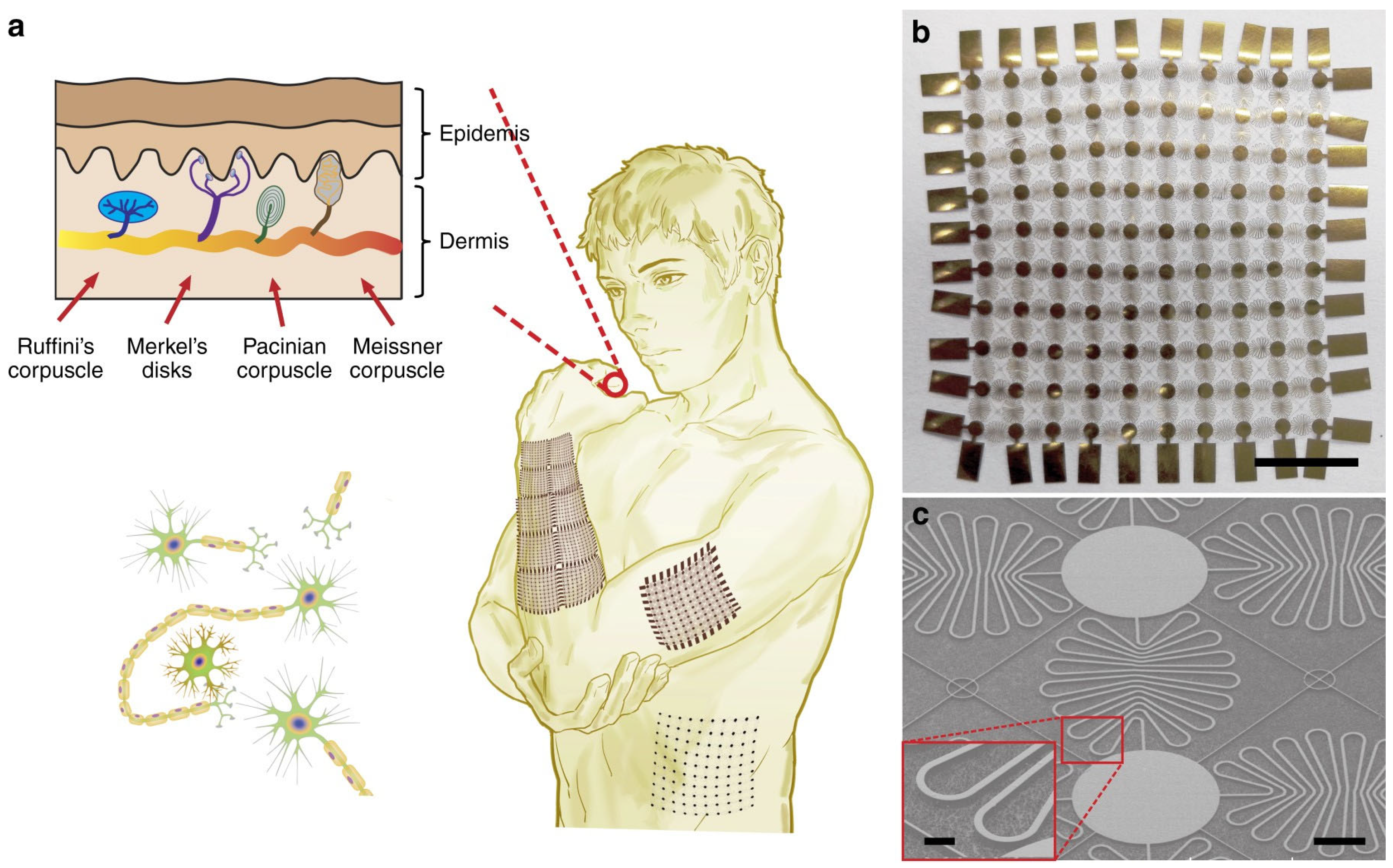
3. Positioning Magnetic Micro and Nano Sensors for Continuous Health Monitoring
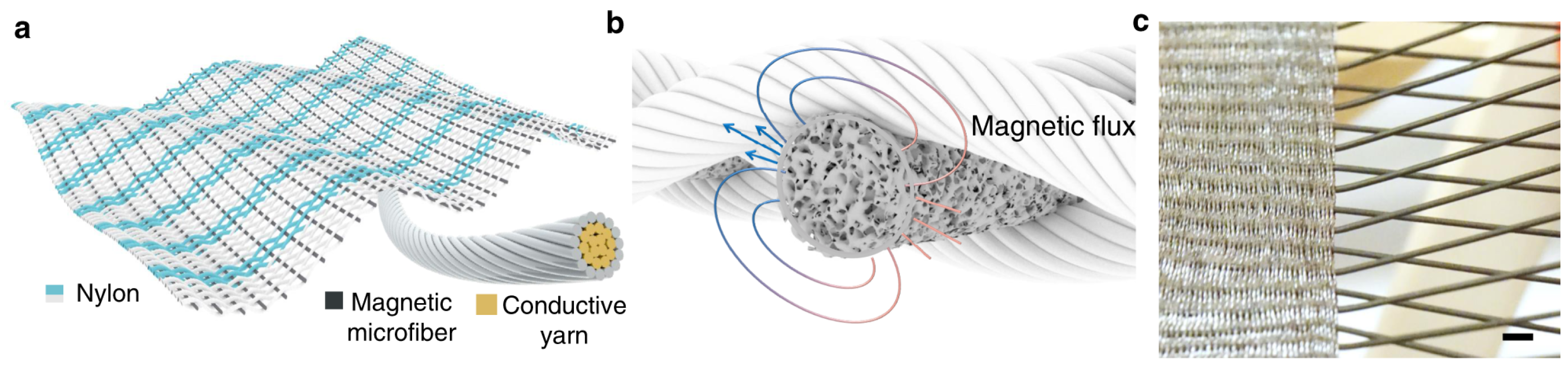
3.1. Magnetic Sensors in Smart Textiles
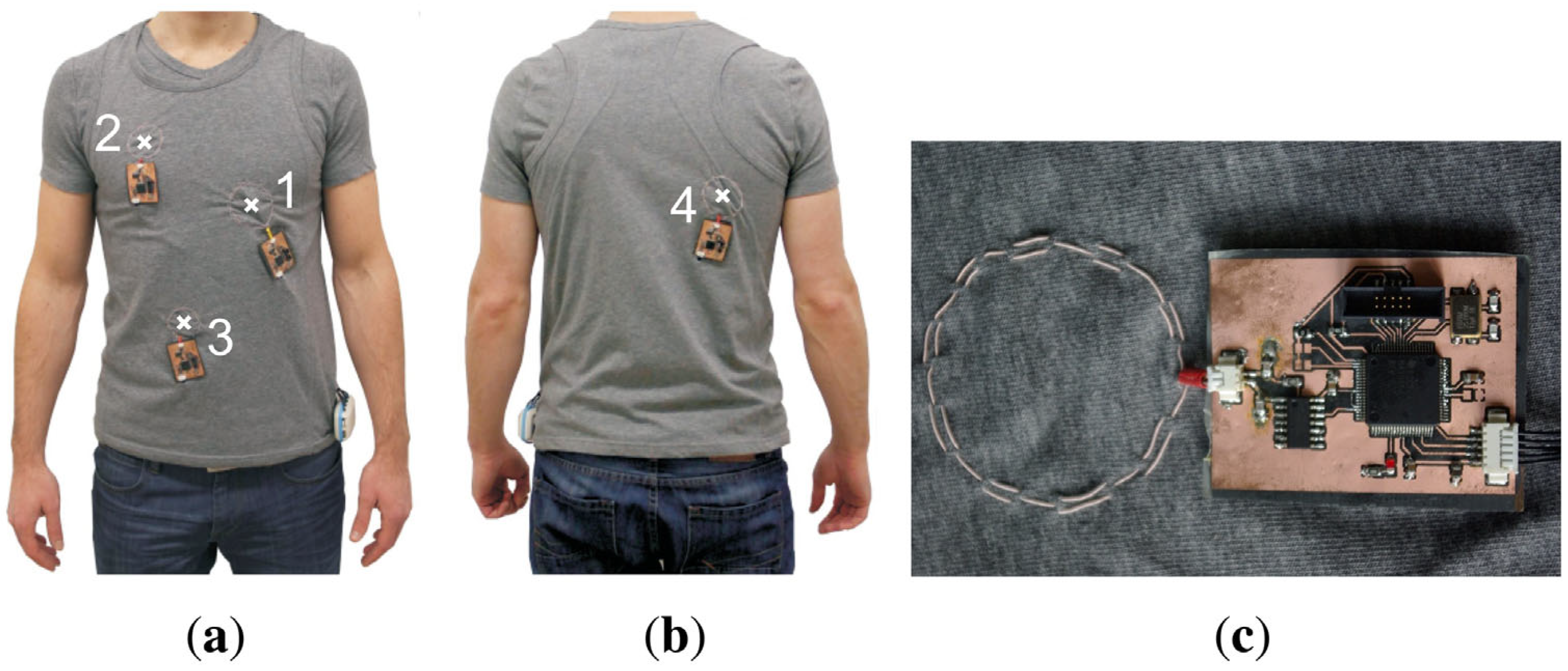
3.2. Magnetic Sensors in Rigid Wearables
3.3. Magnetic Sensors Glued on the Skin
3.4. Magnetic Sensors in Medical Nano Capsules and for Micro-Deliveries
3.5. Magnetic Capsule Endoscopy
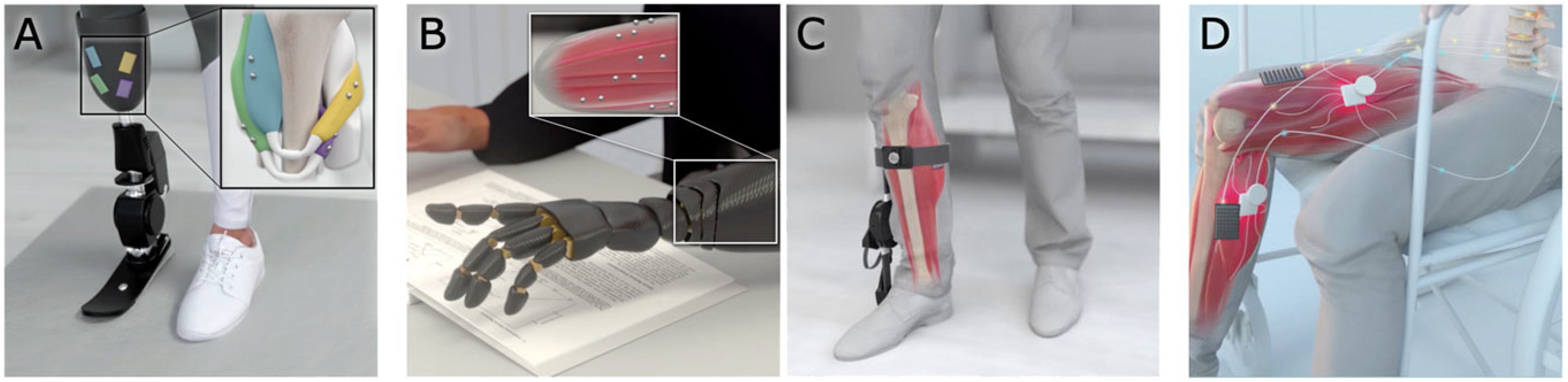
3.6. Implanted Magnetic Sensors
4. Hall Effect Health Monitoring
5. Giant Magnetoresistance Health Monitoring
6. Tunnel Magnetoresistance Health Monitoring
7. Anisotropic Magnetoresistance Health Monitoring
8. Colossal Magnetoresistance Health Monitoring
9. Giant Magnetoimpedance Health Monitoring
10. Other Magnetic Effects and Sensors for Health Monitoring
11. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Teixeira, E.; Fonseca, H.; Diniz-Sousa, F.; Veras, L.; Boppre, G.; Oliveira, J.; Pinto, D.; Alves, A.J.; Barbosa, A.; Mendes, R.; et al. Wearable Devices for Physical Activity and Healthcare Monitoring in Elderly People: A Critical Review. Geriatrics 2021, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ballesteros, R.; Robine, J.M.; Walker, A.; Kalache, A. Active Aging: A Global Goal. Curr. Gerontol. Geriatr. Res. 2013, 2013, 298012. [Google Scholar] [CrossRef] [PubMed]
- Koch, S. Healthy ageing supported by technology—A cross-disciplinary research challenge. Inform. Health Soc. Care 2010, 35, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Souza do Nascimento, L.M.; Bonfati, L.V.; La Banca Freitas, M.; Alves Mendes, J.J., Jr.; Siqueira, H.V.; Stevan, S.L., Jr. Sensors and Systems for Physical Rehabilitation and Health Monitoring—A Review. Sensors 2020, 20, 4063. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shrestha, A.; Heidari, H.; Kernec, J.L.; Fioranelli, F. A Multisensory Approach for Remote Health Monitoring of Older People. IEEE J. Electromagn. RF Microw. Med. Biol. 2018, 2, 102–108. [Google Scholar] [CrossRef]
- Chen, Y.; Shen, C. Performance Analysis of Smartphone-Sensor Behavior for Human Activity Recognition. IEEE Access 2017, 5, 3095–3110. [Google Scholar] [CrossRef]
- Chen, S.W.; Qi, J.M.; Fan, S.C.; Qiao, Z.; Yeo, J.C.; Lim, C.T. Flexible Wearable Sensors for Cardiovascular Health Monitoring. Adv. Healthc. Mater. 2021, 10, 2100116. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Wang, T.S.; Liu, G.Z.; Liu, H.; Xie, X.Y.; Wie, Z.; Li, J.; Jiang, C.; He, Y.; Xu, F. Materials with Tunable Optical Properties for Wearable Epidermal Sensing in Health Monitoring. Adv. Mater. 2022, 34, 2109055. [Google Scholar] [CrossRef]
- Anikwe, C.V.; Nweke, H.F.; Ikegwu, A.C.; Egwuonwu, C.A.; Onu, F.U.; Alo, U.R.; The, Y.W. Mobile and wearable sensors for data-driven health monitoring system: State-of-the-art and future prospect. Expert Syst. Appl. 2022, 202, 117362. [Google Scholar] [CrossRef]
- Henderson, J.; Condell, J.; Connolly, J.; Kelly, D.; Curran, K. Review of Wearable Sensor-Based Health Monitoring Glove Devices for Rheumatoid Arthritis. Sensors 2021, 21, 1576. [Google Scholar] [CrossRef]
- Li, W.-D.; Ke, K.; Jia, J.; Pu, J.-H.; Zhao, X.; Bao, R.-Y.; Liu, Z.-Y.; Bai, L.; Zhang, K.; Yang, M.-B.; et al. Recent Advances in Multiresponsive Flexible Sensors towards E-skin: A Delicate Design for Versatile Sensing. Small 2022, 18, 2103734. [Google Scholar] [CrossRef] [PubMed]
- Sujith, A.V.L.N.; Sajja, G.S.; Mahalakshmi, V.; Nuhmani, S.; Prasanalakshmi, B. Systematic review of smart health monitoring using deep learning and Artificial intelligence. Neurosci. Inform. 2022, 2, 1000028. [Google Scholar] [CrossRef]
- AlShorman, O.; AlShorman, B.; Al-khassaweneh, M.; Alkahtani, F. A review of internet of medical things (IoMT)-based remote health monitoring through wearable sensors: A case study for diabetic patients. Indones. J. Electr. Eng. Comput. Sci. 2020, 20, 414–422. [Google Scholar] [CrossRef]
- Kim, J.Y.; Khan, S.; Wu, P.; Park, S.J.; Park, H.J.; Yu, C.H.; Kim, W.C. Self-charging wearables for continuous health monitoring. Nano Energy 2021, 79, 105419. [Google Scholar] [CrossRef]
- Zhang, X.; Ai, J.W.; Zou, R.P.; Su, B. Compressible and Stretchable Magnetoelectric Sensors Based on Liquid Metals for Highly Sensitive, Self-Powered Respiratory Monitoring. ACS Appl. Mater. Interfaces 2021, 13, 15727–15737. [Google Scholar] [CrossRef]
- Nasiri, S.; Khosravani, M.R. Progress and challenges in fabrication of wearable sensors for health monitoring. Sens. Actuators A Phys. 2020, 312, 112105. [Google Scholar] [CrossRef]
- Sreenilayam, S.P.; Ul Ahad, I.; Nicolosi, V.; Garzon, V.A.; Brabazon, D. Advanced materials of printed wearables for physiological parameter monitoring. Mater. Today 2020, 32, 147–177. [Google Scholar] [CrossRef]
- Peng, B.; Zhao, F.N.; Ping, J.F.; Ying, Y.B. Recent Advances in Nanomaterial-Enabled Wearable Sensors: Material Synthesis, Sensor Design, and Personal Health Monitoring. Small 2020, 16, 2002681. [Google Scholar] [CrossRef] [PubMed]
- Khoshmanesh, F.; Thurgood, P.; Pirogova, E.; Nahavandi, S.; Baratchi, S. Wearable sensors: At the frontier of personalised health monitoring, smart prosthetics and assistive technologies. Biosens. Bioelectron. 2021, 176, 112946. [Google Scholar] [CrossRef]
- Zuo, S.M.; Heidari, H.; Farina, D.; Nazarpour, K. Miniaturized Magnetic Sensors for Implantable Magnetomyography. Adv. Mater. Technol. 2020, 5, 2000185. [Google Scholar] [CrossRef]
- Mostufa, S.; Yari, P.; Rezaei, B.; Xu, K.L.; Wu, K. Flexible Magnetic Field Nanosensors for Wearable Electronics: A Review. ACS Appl. Nano Mater. 2023, 6, 13732–13765. [Google Scholar] [CrossRef]
- Murzin, D.; Mapps, D.J.; Levada, K.; Belyaev, V.; Omelyanchik, A.; Panina, L.; Rodionova, V. Ultrasensitive Magnetic Field Sensors for Biomedical Applications. Sensors 2020, 20, 1569. [Google Scholar] [CrossRef]
- Reis, M. Fundamentals of Magnetism; Elsevier: Oxford, UK, 2013. [Google Scholar]
- Popovic, R.S. Hall Effect Devices; Institute of Physics Publishing: Bristol, UK; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Khan, M.A.; Sun, J.; Li, B.D.; Przybysz, A.; Kosel, J. Magnetic sensors-A review and recent technologies. Eng. Res. Express 2021, 3, 022005. [Google Scholar] [CrossRef]
- Thompson, S.M. The discovery, development and future of GMR: The Nobel Prize 2007. J. Phys. D Appl. Phys. 2008, 41, 093001. [Google Scholar] [CrossRef]
- Garcia, N.; Munoz, M.; Zhao, Y.W. Magnetoresistance in excess of 200% in Ballistic Ni Nanocontacts at Room Temperature and 100 Oe. Phys. Rev. Lett. 1999, 82, 2923. [Google Scholar] [CrossRef]
- Parkin, S.S.P.; Li, Z.G.; Smith, D.J. Giant magnetoresistance in antiferromagnetic Co/Cu multilayers. Appl. Phys. Lett. 1991, 58, 2710–2712. [Google Scholar] [CrossRef]
- Zhu, Y.N.; Jiang, Q.L.; Zhang, J.; Ma, Y.G. Recent Progress of Organic Semiconductor Materials in Spintronics. Chem.–Asian J. 2023, 18, e202201125. [Google Scholar] [CrossRef] [PubMed]
- Zutic, I.; Fabian, J.; Das Sarma, S. Spintronics: Fundamentals and applications. Rev. Mod. Phys. 2004, 76, 323. [Google Scholar] [CrossRef]
- Yuasa, S.; Fukushima, A.; Nagahama, T.; Ando, K.; Suzuki, Y. High Tunnel Magnetoresistance at Room Temperature in Fully Epitaxial Fe/MgO/Fe Tunnel Junctions due to Coherent Spin-Polarized Tunneling. Jpn. J. Appl. Phys. 2004, 43, L588. [Google Scholar] [CrossRef]
- Parkin, S.S.P.; Kaiser, C.; Panchula, A.; Rice, P.M.; Hughes, B.; Samant, M.; Yang, S.-H. Giant tunnelling magnetoresistance at room temperature with MgO (100) tunnel barriers. Nat. Mater. 2004, 3, 862–867. [Google Scholar] [CrossRef]
- Bowen, M.; Cros, V.; Petroff, F.; Fert, A.; Martínez Boubeta, C.; Costa-Krämer, J.L.; Anguita, J.V.; Cebollada, A.; Briones, F.; de Teresa, J.M.; et al. Large magnetoresistance in Fe/MgO/FeCo(001) epitaxial tunnel junctions on GaAs(001). Appl. Phys. Lett. 2001, 79, 1655–1657. [Google Scholar] [CrossRef]
- Djayaprawira, D.D.; Tsunekawa, K.; Nagai, M.; Maehara, H.; Yamagata, S.; Watanabe, N.; Yuasa, S.; Suzuki, Y.; Ando, K. 230% room-temperature magnetoresistance in CoFeB/MgO/CoFeB magnetic tunnel junctions. Appl. Phys. Lett. 2005, 86, 09202. [Google Scholar] [CrossRef]
- Lv, Y.-Y.; Zhang, B.-B.; Li, X.; Yao, S.-H.; Chen, Y.B.; Zhou, J.; Zhang, S.-T.; Lu, M.-H.; Chen, Y.-F. Extremely large and significantly anisotropic magnetoresistance in ZrSiS single crystals. Appl. Phys. Lett. 2016, 108, 244101. [Google Scholar] [CrossRef]
- Zhao, C.-J.; Ding, L.; HuangFu, J.-S.; Zhang, J.-Y.; Yu, G.-H. Research progress in anisotropic magnetoresistance. Rare Met. 2013, 32, 213–224. [Google Scholar] [CrossRef]
- Fina, I.; Marti, X.; Yi, D.; Liu, J.; Chu, J.H.; Rayan-Serrao, C.; Suresha, S.; Shick, A.B.; Zelezny, J.; Junghwirth, T.; et al. Anisotropic magnetoresistance in an antiferromagnetic semiconductor. Nat. Commun. 2014, 5, 4671. [Google Scholar] [CrossRef] [PubMed]
- Yau, J.-B.; Hong, X.; Posadas, A.; Ahn, C.H.; Gao, W.; Altman, E.; Bason, Y.; Klein, L.; Sidorov, M.; Krivokapic, Z. Anisotropic magnetoresistance in colossal magnetoresistive La1-xSrxnO3 thin films. J. Appl. Phys. 2007, 102, 103901. [Google Scholar] [CrossRef]
- Knobel, M.; Vázquez, M.; Kraus, L. Giant Magnetoimpedance. Handb. Magn. Mater. 2003, 15, 497–563. [Google Scholar]
- Phan, M.-H.; Peng, H.-X. Giant magnetoimpedance materials: Fundamentals and applications. Prog. Mater. Sci. 2008, 53, 323–420. [Google Scholar] [CrossRef]
- Mooney, J.W.; Ghasemi-Roudsari, S.; Banham, E.R.; Symonds, C.; Pawlowski, N.; Varcoe, B.T.H. A portable diagnostic device for cardiac magnetic field mapping. Biomed. Phys. Eng. Express 2017, 3, 015008. [Google Scholar] [CrossRef]
- Clarke, J.; Weinstock, H. SQUID Sensors: Fundamentals, Fabrication and Applications, 1st ed.; Weinstock, H., Ed.; Springer: Berlin/Heidelberg, Germany, 1996. [Google Scholar]
- Wang, Y.J.; Gao, J.Q.; Li, M.H.; Shen, Y.; Hasanyan, D.; Li, J.F.; Viehland, D. A review on equivalent magnetic noise of magnetoelectric laminate sensors. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2014, 372, 20120455. [Google Scholar] [CrossRef]
- Li, B.-B.; Bulla, D.; Prakash, V.; Forstne, S.; Dehghan-Manshadi, A.; Dunlop, H.R.; Foster, S.; Bowen, W.P. Scalable high-sensitivity optomechanical magnetometers on a chip. APL Photonics 2018, 3, 120806. [Google Scholar] [CrossRef]
- Ren, L.; Yu, K.; Tan, Y. Wireless and Passive Magnetoelastic-Based Sensor for Force Monitoring of Artificial Bone. IEEE Sens. J. 2019, 19, 2096–2104. [Google Scholar] [CrossRef]
- Blachowicz, T.; Ehrmann, G.; Ehrmann, A. Textile-Based Sensors for Biosignal Detection and Monitoring. Sensors 2021, 21, 6042. [Google Scholar] [CrossRef] [PubMed]
- Singha, K.; Kumar, J.; Pandit, P. Recent Advancements in Wearable & Smart Textiles: An Overview. Mater. Today Proc. 2019, 16, 1518–1523. [Google Scholar]
- Trummer, S.; Ehrmann, A.; Büsgen, A. Development of underwear with integrated 12 channel ECG for men and women. AUTEX Res. J. 2017, 17, 344–349. [Google Scholar] [CrossRef]
- Massaroni, C.; Saccomandi, P.; Schena, E. Medical Smart Textiles Based on Fiber Optic Technology: An Overview. J. Funct. Biomater. 2015, 6, 204–221. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhou, Y.H.; Xu, J.; Chen, G.R.; Fang, Y.S.; Tat, T.; Xiao, X.; Song, Y.; Li, S.; Chen, J. Soft fibers with magnetoelasticity for wearable electronics. Nat. Commun. 2021, 12, 6755. [Google Scholar] [CrossRef]
- Ding, L.; Xuan, S.H.; Feng, J.B.; Gong, X.L. Magnetic/conductive composite fibre: A multifunctional strain sensor with magnetically driven property. Compos. Part A Appl. Sci. Manuf. 2017, 100, 97–105. [Google Scholar] [CrossRef]
- Teichmann, D.; Kuhn, A.; Leonhardt, S.; Walter, M. The MAIN Shirt: A Textile-Integrated Magnetic Induction Sensor Array. Sensors 2014, 14, 1039–1056. [Google Scholar] [CrossRef]
- Zhang, W.G.; Guo, Q.H.; Duan, Y.; Xing, C.Y.; Peng, Z.C. A Textile Proximity/Pressure Dual-Mode Sensor Based on Magneto-Straining and Piezoresistive Effects. IEEE Sens. J. 2022, 22, 10420–10427. [Google Scholar] [CrossRef]
- Chen, L.M.; Lu, M.Y.; Wang, Y.Q.; Huang, Y.H.; Zhu, S.; Tang, J.W.; Zhu, C.; Liu, X.Q.; Yin, W.L. Whole System Design of a Wearable Magnetic Induction Sensor for Physical Rehabilitation. Adv. Intell. Syst. 2019, 1, 1900037. [Google Scholar] [CrossRef]
- Mecnika, V.; Hoerr, M.; Krievins, I.; Jockenhoevel, S.; Gries, T. Technical Embroidery for Smart Textiles: Review. Mater. Sci. Text. Cloth. Technol. 2014, 9, 56–63. [Google Scholar] [CrossRef]
- Gi, S.O.; Lee, Y.J.; Koo, H.R.; Khang, S.N.; Kim, K.-N.; Kang, S.-J.; Lee, J.H.; Lee, J.-W. Application of a Textile-based Inductive Sensor for the Vital Sign Monitoring. J. Electr. Eng. Technol. 2014, 9, 742–749. [Google Scholar] [CrossRef]
- Grabham, N.J.; Li, Y.; Clare, L.R.; Stark, B.H.; Beeby, S.P. Fabrication Techniques for Manufacturing Flexible Coils on Textiles for Inductive Power Transfer. IEEE Sens. J. 2018, 18, 2599–2606. [Google Scholar] [CrossRef]
- Kunze, K.; Bahle, G.; Lukowicz, P.; Partidge, K. Can magnetic field sensors replace gyroscopes in wearable sensing applications? In Proceedings of the International Symposium on Wearable Computers (ISWC) 2010, Seoul, Republic of Korea, 10–13 October 2010; pp. 1–4. [Google Scholar]
- Bian, S.Z.; Zhou, B.; Lukowicz, P. Social Distance Monitor with a Wearable Magnetic Field Proximity Sensor. Sensors 2020, 20, 5101. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chen, H.K.; Lin, S. MagTrack: Enabling Safe Driving Monitoring with Wearable Magnetics. In Proceedings of the MobiSys ‘19: Proceedings of the 17th Annual International Conference on Mobile Systems, Applications, and Services, Seoul, Republic of Korea, 17–21 June 2019; pp. 326–339. [Google Scholar]
- Friedman, N.; Rowe, J.B.; Reinkensmeyer, D.J.; Bachmann, J. The Manumeter: A Wearable Device for Monitoring Daily Use of the Wrist and Fingers. IEEE J. Biomed. Health Inform. 2014, 18, 1804–1812. [Google Scholar] [CrossRef]
- Poh, M.-Z.; Swenson, N.C.; Picard, R.W. Motion-tolerant magnetic earring sensor and wireless earpiece for wearable photoplethysmography. IEEE Trans. Inf. Technol. Biomed. 2010, 14, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.D.; Hou, W.S.; Peng, C.L.; Zheng, X.L.; Fang, X.; He, J. Wearable magnetic locating and tracking system for MEMS medical capsule. Sens. Actuators A Phys. 2008, 141, 432–439. [Google Scholar] [CrossRef]
- Fu, Y.M.; Guo, Y.-X. Wearable Permanent Magnet Tracking System for Wireless Capsule Endoscope. IEEE Sens. J. 2022, 22, 8113–8122. [Google Scholar] [CrossRef]
- Almansouri, A.S.; Alsharif, N.A.; Khan, M.A.; Swanepoel, L.; Kaidarova, A.; Salama, K.N.; Kosel, J. An Imperceptible Magnetic Skin. Adv. Mater. Technol. 2019, 4, 1900493. [Google Scholar] [CrossRef]
- Wang, C.G.; Liu, C.; Shang, F.F.; Niu, S.Y.; Ke, L.N.; Zhang, N.; Ma, B.B.; Li, R.Z.; Sun, X.; Zhang, S. Tactile sensing technology in bionic skin: A review. Biosens. Bioelectron. 2023, 220, 114882. [Google Scholar] [CrossRef]
- Hellebrekers, T.; Kroemer, O.; Majidi, C. Soft Magnetic Skin for Continuous Deformation Sensing. Adv. Intell. Syst. 2019, 1, 1900025. [Google Scholar] [CrossRef]
- Li, S.B.; Wu, Y.Z.; Asghar, W.; Li, F.L.; Zhang, Y.; He, Z.D.; Liu, J.Y.; Wang, Y.W.; Liao, M.Y.; Shang, J.; et al. Wearable Magnetic Field Sensor with Low Detection Limit and Wide Operation Range for Electronic Skin Applications. Adv. Sci. 2023, 10, 2304525. [Google Scholar]
- Yan, Y.C.; Hu, Z.; Yang, Z.B.; Yuan, W.H.; Song, C.Y.; Pan, J.; Shen, Y.J. Soft magnetic skin for super-resolution tactile sensing with force self-decoupling. Sci. Robot. 2021, 6, eabc8801. [Google Scholar] [CrossRef] [PubMed]
- Roberts, P.; Zadan, M.; Majidi, C. Soft Tactile Sensing Skins for Robotics. Curr. Robot. Rep. 2021, 2, 343–354. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Y.; Zhou, Y.; Man, Q.; Hu, C.; Asghar, W.; Li, F.; Yu, Z.; Shang, J.; Liu, G.; et al. A skin-inspired tactile sensor for smart prosthetics. Sci. Robot. 2018, 3, eaat0429. [Google Scholar] [CrossRef] [PubMed]
- Weathersby, E.J.; Gurrey, C.J.; McLean, J.B.; Sanders, B.N.; Larsen, B.G.; Carter, R.; Garbini, J.L.; Sanders, J.E. Thin Magnetically Permeable Targets for Inductive Sensing: Application to Limb Prosthetics. Sensors 2019, 19, 4041. [Google Scholar] [CrossRef]
- Hua, Q.L.; Sun, J.L.; Liu, H.T.; Bao, R.R.; Yu, R.M.; Zhai, J.Y.; Pan, C.F.; Wang, Z.L. Skin-inspired highly stretchable and conformable matrix networks for multifunctional sensing. Nat. Commun. 2018, 9, 244. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.; Bao, B.; Karnaushenko, D.D.; Bandari, V.K.; Rivkin, B.; Li, Z.; Faghih, M.; Karnaushenko, D.; Schmidt, O.G. A new dimension for magnetosensitive e-skins: Active matrix integrated micro-origami sensor arrays. Nat. Commun. 2022, 13, 2121. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, J.B.; Soulez, G.; Martel, S. Potential Applications of Untethered Microdevices in the Blood Vessels within the Constraints of an MRI System. In Proceedings of the 2005 IEEE Engineering in Medicine and Biology 27th Annual Conference, Shanghai, China, 17–18 January 2005; pp. 4850–4853. [Google Scholar]
- Benhal, P.; Broda, A.; Najafali, D.; Malik, P.; Mohammed, A.; Ramaswamy, B.; Depireux, D.A.; Shimoji, M.; Shukoor, M.; Shapiro, B. On-chip testing of the speed of magnetic nano- and micro-particles under a calibrated magnetic gradient. J. Magn. Magn. Mat. 2019, 474, 187–198. [Google Scholar] [CrossRef]
- Xie, A.; Hanif, S.; Ouyang, J.; Tang, Z.; Kong, N.; Kim, N.Y.; Qi, B.; Patel, D.; Shi, B.; Tao, W. Stimuli-responsive prodrug-based cancer nanomedicine. eBioMedicine 2020, 56, 102821. [Google Scholar] [CrossRef]
- Kainz, Q.M.; Reiser, O. Polymer- and Dendrimer-Coated Magnetic Nanoparticles as Versatile Supports for Catalysts, Scavengers, and Reagents. Acc. Chem. Res. 2014, 47, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-W.; Hua, M.-Y.; Liu, H.-L.; Huang, C.-Y.; Tsai, R.-Y.; Lu, Y.-J.; Chen, H.-J.; Tang, H.-J.; Hsien, H.-Y.; Chang, Y.-S.; et al. Self-protecting core-shell magnetic nanoparticles for targeted, traceable, long half-life delivery of BCNU to gliomas. Biomaterials 2011, 32, 6523–6532. [Google Scholar] [CrossRef] [PubMed]
- Han Kim, S.; Chun Jai, H. Capsule Endoscopy: Pitfalls and Approaches to Overcome. Diagnostics 2021, 11, 1765. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, F.; McNamara, D. Small-Bowel Capsule Endoscopy—Optimizing Capsule Endoscopy in Clinical Practice. Diagnostics 2021, 11, 2139. [Google Scholar] [CrossRef]
- Solitano, V.; Zilli, A.; Franchellucci, G.; Alloca, M.; Fiorino, G.; Furfaro, F.; D’Amico, F.; Danase, S.; Al Awadhi, S. Artificial Endoscopy and Inflammatory Bowel Disease: Welcome to the Future. J. Clinic. Med. 2022, 11, 569. [Google Scholar] [CrossRef] [PubMed]
- Estevinho, M.M.; Pinho, R.; Rodrigues, A.; Ponte, A.; Correia, J.; Mesquita, P.; Freitas, T. Capsule Enteroscopy Using the Mirocam® versus OMOM® Systems: A Matched Case–Control Study. Life 2023, 13, 1809. [Google Scholar] [CrossRef] [PubMed]
- Levartovsky, A.; Eliakim, R. Video Capsule Endoscopy Plays an Important Role in the Management of Crohn’s Disease. Diagnostics 2023, 13, 1507. [Google Scholar] [CrossRef] [PubMed]
- Tziortziotis, I.; Laskaratos, F.-M.; Coda, S. Role of Artificial Intelligence in Video Capsule Endoscopy. Diagnostics 2021, 11, 1192. [Google Scholar] [CrossRef]
- Moen, S.; Vuik, S.E.R.; Kuipers, E.J.; Spaander, M.C.W. Artificial Intelligence in Colon Capsule Endoscopy—A Systematic Review. Diagnostics 2022, 12, 1994. [Google Scholar] [CrossRef]
- Mascarenhas, M.; Martins, M.; Afonso, J.; Ribeiro, T.; Cardoso, P.; Mendes, F.; Andrade, P.; Cardoso, H.; Ferreira, J.; Macedo, G. The Future of Minimally Invasive Capsule Panendoscopy: Robotic Precision, Wireless Imaging and AI-Driven Insights. Cancers 2023, 15, 5861. [Google Scholar] [CrossRef]
- Tomek, J.; Mlejnek, P.; Janásek, V.; Ripka, P.; Kaspar, P.; Chen, J. Gastric Motility and Volume Sensing by Implanted Magnetic Sensors. Sens. Lett. 2007, 5, 276–278. [Google Scholar] [CrossRef]
- Tomek, J.; Mlejnek, P.; Janásek, V.; Ripka, P.; Kaspar, P.; Chen, J. The precision of gastric motility and volume sensing by implanted magnetic sensors. Sens. Actuators A Phys. 2008, 142, 34–39. [Google Scholar] [CrossRef]
- Veletic, M.; Apu, E.H.; Simic, M.; Bergsland, J.; Balasingham, I.; Contag, C.H.; Ashammakhi, N. Implants with Sensing Capabilities. Chem. Rev. 2022, 122, 16329–16363. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, C.; Kiourti, A. Wireless Sensors for Smart Orthopedic Implants. J. Bio-Tribo-Corros. 2017, 3, 20. [Google Scholar] [CrossRef]
- Han, W.; Chau, K.T.; Jiang, C.Q.; Liu, W. Accurate Position Detection in Wireless Power Transfer Using Magnetoresistive Sensors for Implant Applications. EEE Trans. Magn. 2018, 54, 4001205. [Google Scholar]
- Weir, R.F.; Troyk, P.R.; DeMichele, G.A.; Kerns, D.A.; Schorsch, J.F.; Maas, H. Implantable Myoelectric Sensors (IMESs) for Intramuscular Electromyogram Recording. IEEE Trans. Biomed. Eng. 2009, 56, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.R.; Clark, W.H.; Clarrissimeaux, E.G.; Yeon, S.H.; Carty, M.J.; Lipsitz, S.R.; Bronson, R.T.; Roberts, T.J.; Herr, H.M. Clinical viability of magnetic bead implants in muscle. Front. Bioeng. Biotechnol. 2022, 10, 1010276. [Google Scholar] [CrossRef] [PubMed]
- Hofe, R.; Ell, S.R.; Fagan, M.J.; Gilbert, J.M.; Green, P.D.; Moore, R.K.; Rybchenko, S.I. Small-vocabulary speech recognition using a silent speech interface based on magnetic sensing. Speech Commun. 2013, 55, 22–32. [Google Scholar] [CrossRef]
- Angelopoulos, S.; Misiaris, D.; Banis, G.; Liang, K.; Tsarabaris, P.; Ktena, A.; Hristoforou, E. Steel health monitoring device based on Hall sensors. J. Magn. Magn. Mater. 2020, 515, 167304. [Google Scholar] [CrossRef]
- Addabbo, T.; Fort, A.; Mugnaini, M.; Panzardi, E.; Pozzebon, A.; Tani, M.; Vignoli, V. A low cost distributed measurement system based on Hall effect sensors for structural crack monitoring in monumental architecture. Measurement 2018, 116, 652–657. [Google Scholar] [CrossRef]
- Verma, A.K.; Akkulu, P.; Padmanabhan, S.V.; Radhika, S. Automatic Condition Monitoring of Industrial Machines Using FSA-Based Hall-Effect Transducer. IEEE Sens. J. 2021, 21, 1072–1081. [Google Scholar] [CrossRef]
- Liu, X.Q.; Ye, C.; Li, X.Q.; Cui, N.Y.; Wu, T.Z.; Du, S.Y.; Wie, Q.P.; Fu, L.; Yin, J.C.; Lin, C.-T. Highly Sensitive and Selective Potassium Ion Detection Based on Graphene Hall Effect Biosensors. Materials 2018, 11, 399. [Google Scholar] [CrossRef] [PubMed]
- Cui, N.Y.; Wang, F.; Ding, H.Y. Graphene-Based Hall Effect Biosensor for Improved Specificity and Sensitivity of Label-Free DNA Detection. Nano 2020, 15, 2050088. [Google Scholar] [CrossRef]
- Noordin, M.K.; Amran, M.E.; Bani, N.A.; Ahmad Kamil, A.S.; Md Nasir, A.N.; Arsat, M. Failure Prediction for Hemodialysis Units Using Machine Learning and Hall Effect Sensors. In Proceedings of the 2023 IEEE 2nd National Biomedical Engineering Conference (NBEC), Melaka, Malaysia, 5–7 September 2023; pp. 170–175. [Google Scholar]
- Chheng, C.; Wilson, D. Abnormal Gait Detection Using Wearable Hall-Effect Sensors. Sensors 2021, 21, 1206. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.; Wang, L.F.; Ghanbari, A.; Vardakastani, V.; Kedgley, A.E.; Gardiner, M.D.; Vincent, T.L.; Culmer, P.R.; Alazmani, A. Design and Evaluation of Magnetic Hall Effect Tactile Sensors for Use in Sensorized Splints. Sensors 2020, 20, 1123. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-S.; Choi, J.-G.; Son, I.-H.; Kim, K.-H.; Nam, D.-H.; Hong, Y.-S.; Lee, W.-B.; Hwang, D.-G.; Rhee, J.-R. Fabrication and Characterization of a Wrist Wearable Cuffless Pulsimeter by Using the Hall Effect Device. J. Magn. 2011, 16, 449–452. [Google Scholar] [CrossRef]
- Son, I.-H.; Kim, K.-H.; Choi, J.-G.; Nam, D.-H.; Lee, S.-S. Measurement and Analysis of Pulse Wave Using a Clamping Pulsimeter Equipped With Hall Effect Device. IEEE Trans. Magn. 2011, 47, 3063–3065. [Google Scholar] [CrossRef]
- Delmas, A.; Belguerras, L.; Weber, N.; Odille, F.; Pasquier, C.; Felblinger, J.; Vuissoz, P.-A. Calibration and non-orthogonality correction of three-axis Hall sensors for the monitoring of MRI workers’ exposure to static magnetic fields. Bio Electro Magn. 2018, 39, 108–119. [Google Scholar] [CrossRef]
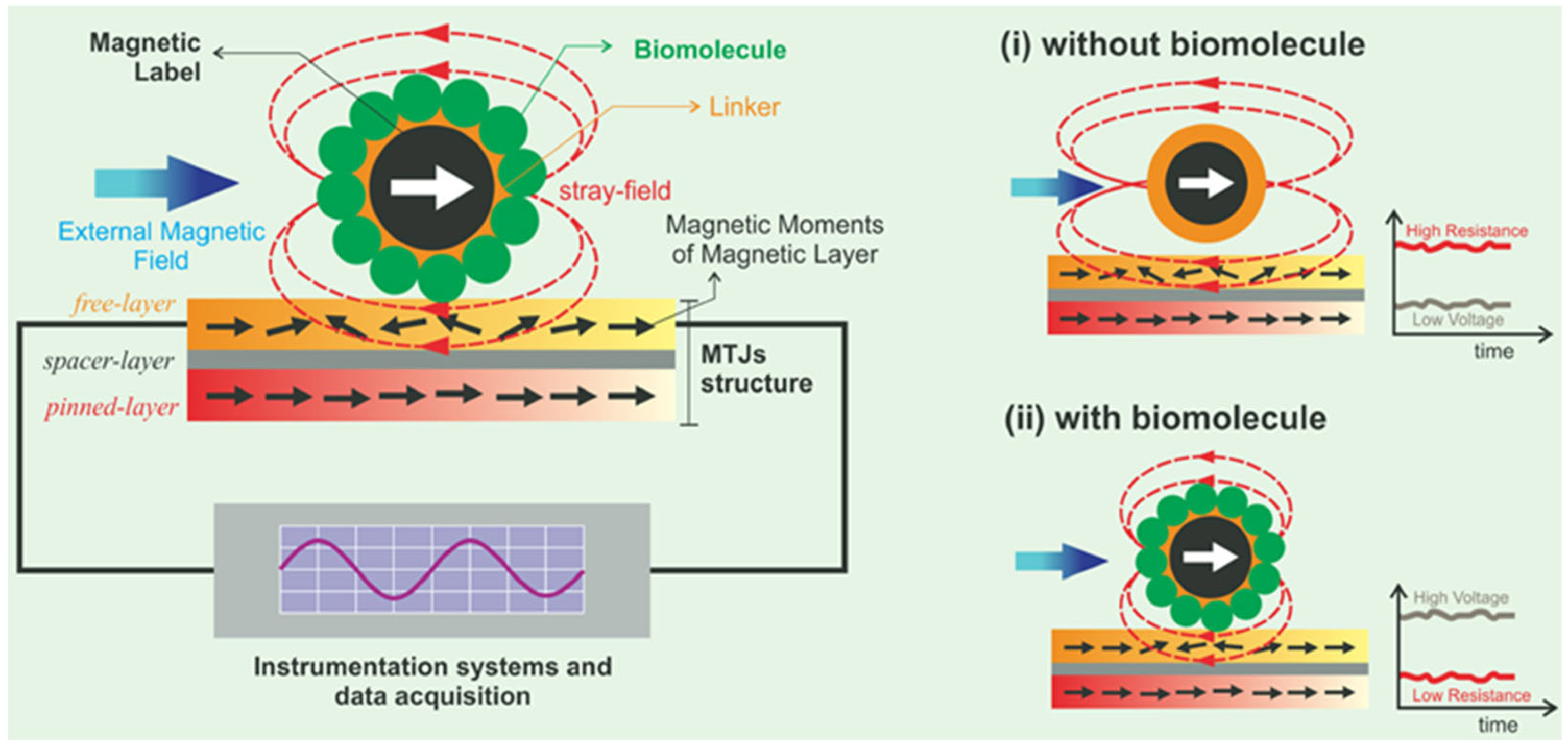
- Wu, K.; Tonini, D.; Liang, S.; Saha, R.; Chugh, V.K.; Wang, J.-P. Giant Magnetoresistance Biosensors in Biomedical Applications. ACS Appl. Mater. Interfaces 2022, 14, 9945–9969. [Google Scholar] [CrossRef]
- Weiss, R.; Mattheis, R.; Reiss, G. Advanced giant magnetoresistance technology for measurement applications. Meas. Sci. Technol. 2013, 24, 082001. [Google Scholar] [CrossRef]
- Antarnusa, G.; Elda Swastika, P.; Suharyadi, E. Wheatstone bridge-giant magnetoresistance (GMR) sensors based on Co/Cu multilayers for bio-detection applications. J. Phys. Conf. Ser. 2018, 1011, 012061. [Google Scholar] [CrossRef]
- Krishna, V.D.; Wu, K.; Perez, A.M.; Wang, J.-P. Giant Magnetoresistance-based Biosensor for Detection of Influenza A Virus. Front. Microbiol. 2016, 7, 400. [Google Scholar] [CrossRef]
- Mostufa, S.; Rezaei, B.; Yari, P.; Xu, K.L.; Gómez-Pastora, J.; Sun, J.J.; Shi, Z.Q.; Wu, K. Giant Magnetoresistance Based Biosensors for Cancer Screening and Detection. ACS Appl. Bio Mater. 2023, 6, 4042–4059. [Google Scholar] [CrossRef]
- Nesvet, J.C.; Antilla, K.A.; Pancirer, D.S.; Lozano, A.X.; Preiss, J.S.; Ma, W.J.; Fu, A.H.; Park, S.-M.; Gambhir, S.S.; Fan, A.C.; et al. Giant Magnetoresistive Nanosensor Analysis of Circulating Tumor DNA Epidermal Growth Factor Receptor Mutations for Diagnosis and Therapy Response Monitoring. Clin. Chem. 2021, 67, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, N.A.; Riyanto, C.A.; Suharyadi, E.; Sabarman, H. Giant Magnetoresistance Sensor for Rapid and Simple Bovine Serum Albumin Assay With Ag-Functionalized Iron Oxide Nanoparticles Label. IEEE Sens. J. 2023, 23, 9204–9209. [Google Scholar] [CrossRef]
- Meng, F.; Zhang, L.; Huo, W.S.; Lian, J.; Jesorka, A.; Shi, X.Z.; Gao, Y.H. Dynamic Range Expansion of the C-Reactive Protein Quantification with a Tandem Giant Magnetoresistance Biosensor. ACS Omega 2021, 6, 12923–12930. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, W.; Yu, L.; Tu, L.; Feng, Y.L.; Klein, T.; Wang, J.-P. Giant magnetoresistive-based biosensing probe station system for multiplex protein assays. Biosens. Bioelectron. 2015, 70, 61–68. [Google Scholar] [CrossRef]
- Ger, T.-R.; Wu, P.-S.; Wang, W.-J.; Chen, C.-A.; Abu, P.A.R.; Chen, S.-L. Development of a Microfluidic Chip System with Giant Magnetoresistance Sensor for High-Sensitivity Detection of Magnetic Nanoparticles in Biomedical Applications. Biosensors 2023, 13, 807. [Google Scholar] [CrossRef]
- Ha, M.J.; Canón Bermúdez, G.S.; Kosub, T.; Mönch, I.; Zabila, Y.; Oliveros Mata, E.S.; Illing, R.; Wang, Y.K.; Fassbender, J.; Makarov, D. Printable and Stretchable Giant Magnetoresistive Sensors for Highly Compliant and Skin-Conformal Electronics. Adv. Mater. 2021, 33, 2005521. [Google Scholar] [CrossRef]
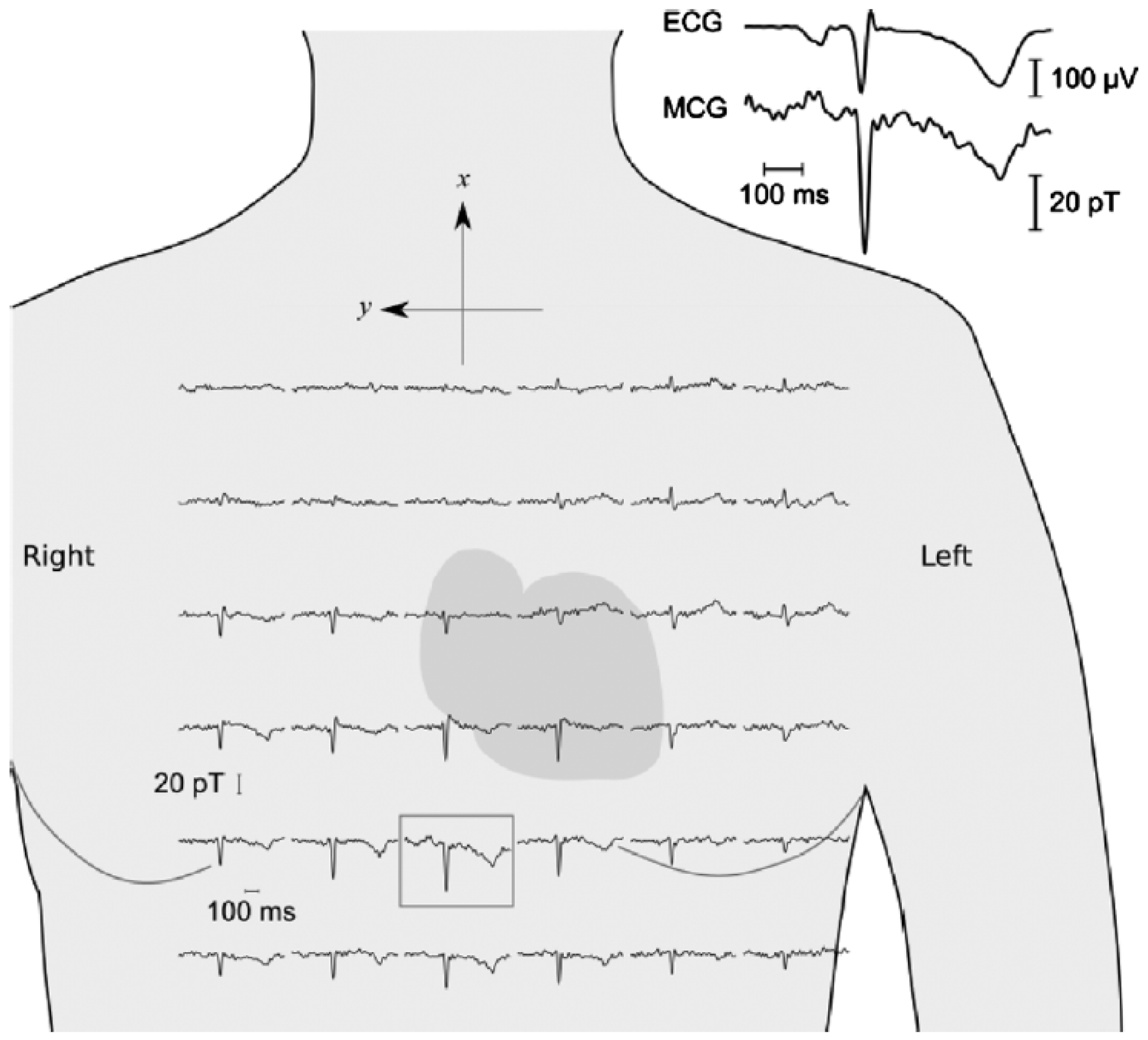
- Pannetier-Lecoeur, M.; Parkkonen, L.; Sergeeva-Chollet, N.; Polovy, H.; Fermon, C.; Fowley, C. Magnetocardiography with sensors based on giant magnetoresistance. Appl. Phys. Lett. 2011, 98, 153705. [Google Scholar] [CrossRef]
- Kalyan, K.; Chugh, V.K.; Anoop, C.S. Non-invasive heart rate monitoring system using giant magneto resistance sensor. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 4873–4876. [Google Scholar]
- Jishnu, K.; Anoop, C.S. A Simple Bio-Instrumentation Platform for Vital-Sign Estimation Using MagnetoPleythsmography. In Proceedings of the 2023 International Conference on Power, Instrumentation, Energy and Control (PIECON), Aligarh, India, 10–12 February 2023; pp. 1–5. [Google Scholar]
- Sarkar, S. Design of Magnetic Sensor Based All-in-One Cardiorespiratory Health Monitoring System. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; pp. 4660–4663. [Google Scholar]
- Chugh, V.K.; Kalyan, K.; Anoop, C.S. Feasibility study of a giant Magneto-Resistance based respiration rate monitor. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 2327–2330. [Google Scholar]
- Chugh, V.K.; Kalyan, K.; Anoop, C.S.; Patra, A.; Negi, S. Analysis of a GMR-based plethysmograph transducer and its utility for real-time Blood Pressure measurement. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Jeju, Republic of Korea, 11–15 July 2017; pp. 1704–1707. [Google Scholar]
- Gooneratne, C.P.; Kurnicki, A.; Yamada, S.; Mukhopadhyay, S.C.; Kosel, J. Analysis of the Distribution of Magnetic Fluid inside Tumors by a Giant Magnetoresistance Probe. PLoS ONE 2013, 8, e81227. [Google Scholar] [CrossRef]
- Caruso, L. Giant magnetoresistance Based Sensors for Local Magnetic Detection of Neuronal Currents. Ph.D. Thesis, Université Pierre et Marie Curie—Paris VI, Paris, France, 2015. [Google Scholar]
- Ren, C.H.; Bayin, Q.G.; Feng, S.L.; Fu, Y.S.; Ma, X.; Guo, J.H. Biomarkers detection with magnetoresistance-based sensors. Biosens. Bioelectron. 2020, 165, 112340. [Google Scholar] [CrossRef]
- Wibowo, N.A.; Kurniawan, C.; Kusumahastuti, D.K.A.; Setiawan, A.; Suharyadi, E. Review—Potential of Tunneling Magnetoresistance Coupled to Iron Oxide Nanoparticles as a Novel Transducer for Biosensors-on-Chip. J. Electrochem. Soc. 2024, 171, 017512. [Google Scholar] [CrossRef]
- Lei, H.M.; Wang, K.; Ji, X.J.; Cui, D.X. Contactless Measurement of Magnetic Nanoparticles on Lateral Flow Strips Using Tunneling Magnetoresistance (TMR) Sensors in Differential Configuration. Sensors 2016, 16, 2130. [Google Scholar] [CrossRef]
- Wu, Y.Z.; Liu, Y.W.; Zhan, Q.F.; Liu, J.P.; Li, R.-W. Rapid detection of Escherichia coli O157:H7 using tunneling magnetoresistance biosensor. AIP Adv. 2017, 7, 056658. [Google Scholar] [CrossRef]
- Mu, X.-H.; Liu, H.-F.; Tong, Z.-Y.; Du, B.; Liu, S.; Liu, B.; Liu, Z.-W.; Gao, C.; Wang, J.; Dong, H. A new rapid detection method for ricin based on tunneling magnetoresistance biosensor. Sens. Actuators B Chem. 2019, 284, 638–649. [Google Scholar] [CrossRef]
- Albon, C.; Weddemann, A.; Auge, A.; Rott, K.; Hütten, A. Tunneling magnetoresistance sensors for high resolutive particle detection. Appl. Phys. Lett. 2009, 95, 023101. [Google Scholar] [CrossRef]
- Amara, S.; Bu, R.; Alawein, M.; Alsharif, N.; Khan, M.A.; Wen, Y.; Zhang, X.X.; Kosel, J.; Fariborzi, H. Highly-Sensitive Magnetic Tunnel Junction Based Flow Cytometer. In Proceedings of the 2018 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Rome, Italy, 11–13 June 2018; pp. 1–5. [Google Scholar]
- Lian, J.; Chen, S.; Qiu, Y.Q.; Zhang, S.H.; Shi, S.; Gao, Y.H. A fully automated in vitro diagnostic system based on magnetic tunnel junction arrays and superparamagnetic particles. J. Appl. Phys. 2012, 111, 07B315. [Google Scholar] [CrossRef]
- Amara, S.; Aljedaibi, A.; Alrashoudi, A.; Mbarek, S.B.; Khan, D.; Massoud, Y. High-performance MTJ-based sensors for monitoring of atmospheric pollution. AIP Adv. 2023, 13, 035329. [Google Scholar] [CrossRef]
- Ghemes, C.; Dragos-Pinzaru, O.-G.; Tibu, M.; Lostun, M.; Lupu, N.; Chiriac, H. Tunnel Magnetoresistance-Based Sensor for Biomedical Application: Proof-of-Concept. Coatings 2023, 13, 227. [Google Scholar] [CrossRef]
- Tanwear, A.; Heidari, H.; Paz, E.; Böhnert, T.; Ferreira, R. Eyelid Gesture Control using Wearable Tunnelling Magnetoresistance Sensors. In Proceedings of the 2020 27th IEEE International Conference on Electronics, Circuits and Systems (ICECS), Glasgow, UK, 23–25 November 2020; pp. 1–4. [Google Scholar]
- Tanwear, A.; Liang, X.P.; Liu, Y.C.; Vuckovic, A.; Ghannam, R.; Böhnert, T.; Paz, E.; Freitas, P.P.; Ferreira, R.; Heidari, H. Spintronic Sensors Based on Magnetic Tunnel Junctions for Wireless Eye Movement Gesture Control. IEEE Trans. Biomed. Circuits Syst. 2020, 14, 1299–1310. [Google Scholar] [CrossRef]
- Khokle, R.P.; Franco, F.; de Freitas, S.C.; Esselle, K.P.; Heimlich, M.C.; Bokor, D.J. Eddy Current–Tunneling Magneto-Resistive Sensor for Micromotion Detection of a Tibial Orthopaedic Implant. IEEE Sens. J. 2019, 19, 1285–1292. [Google Scholar] [CrossRef]
- Cousins, A.; Balalis, G.L.; Thompson, S.K.; Forero Morales, D.; Mohtar, A.; Wedding, A.B.; Thierry, B. Novel Handheld Magnetometer Probe Based on Magnetic Tunnelling Junction Sensors for Intraoperative Sentinel Lymph Node Identification. Sci. Rep. 2015, 5, 10842. [Google Scholar] [CrossRef]
- Cañón Bermúdez, G.S.; Fuchs, H.; Bischoff, L.; Fassbender, J.; Makarov, D. Electronic-skin compasses for geomagnetic field-driven artificial magnetoreception and interactive electronics. Nat. Electron. 2018, 1, 589. [Google Scholar] [CrossRef]
- Ge, J.; Wang, X.; Drack, M.; Volkov, O.; Liang, M.; Cañón Bermúdez, G.S.; Illing, R.; Wang, C.; Zhou, S.; Fassbender, J.; et al. A bimodal soft electronic skin for tactile and touchless interaction in real time. Nat. Commun. 2019, 10, 4405. [Google Scholar] [CrossRef]
- Wang, C.; Su, W.; Pu, J.; Hu, Z.; Liu, M. A Self-biased Anisotropic Magnetoresistive (AMR) Magnetic Field Sensor on Flexible Kapton. In Proceedings of the 2018 IEEE International Magnetics Conference (INTERMAG), Singapore, 23–27 April 2018; p. 1. [Google Scholar]
- Oliveros Mata, E.S.; Canón Bermúdaz, G.S.; Ha, M.J.; Kosub, T.; Zabila, Y.; Fassbender, J.; Makarov, D. Printable anisotropic magnetoresistance sensors for highly compliant electronics. Appl. Phys. A 2021, 127, 280. [Google Scholar] [CrossRef]
- Wang, Z.G.; Wang, X.J.; Li, M.H.; Gao, Y.; Hu, Z.Q.; Nan, T.X.; Liang, X.F.; Chen, H.H.; Yang, J.; Cash, S.; et al. Highly Sensitive Flexible Magnetic Sensor Based on Anisotropic Magnetoresistance Effect. Adv. Mater. 2016, 28, 9370–9377. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.N.; Zhao, D.Y.; Shao, J.; Fu, Z.; Wang, C.Y.; Wang, S.P.; Du, J.; Zhong, M.C.; Duan, J.B.; Li, Y.; et al. Highly flexible anisotropic magnetoresistance sensor for wearable electronics. Rev. Sci. Instrum. 2023, 94, 045005. [Google Scholar] [CrossRef]
- Su, G.-Y.; You, M.-C.; Chuang, K.-W.; Wu, M.-H.; Hsieh, C.-H.; Lin, C.-Y.; Yang, C.-Y.; Anbalagan, A.K.; Lee, C.-H. Investigating Anisotropic Magnetoresistance in Epitaxially Strained CoFe Thin Films on a Flexible Mica. Nanomaterials 2023, 13, 3154. [Google Scholar] [CrossRef]
- Sorli, B.; Vena, A.; Belaizi, Y.; Balde, M. UHF RFID anisotropic magnetoresistance sensor for human motion monitoring. In Proceedings of the 2015 IEEE International Instrumentation and Measurement Technology Conference (I2MTC) Proceedings, Pisa, Italy, 11–14 May 2015; pp. 1165–1168. [Google Scholar]
- Vera, A.; Martínez, I.; Enger, L.G.; Guillet, B.; Guerrero, R.; Diez, J.M.; Rousseau, O.; Chok Sing, M.L.; Pierron, V.; Perna, P.; et al. High-Performance Implantable Sensors based on Anisotropic Magnetoresistive La0.67Sr0.33MnO3 for Biomedical Applications. ACS Biomater. Sci. Eng. 2023, 9, 1020–1029. [Google Scholar] [CrossRef]
- Weitschies, W.; Hartmann, V.; Grützmann, R.; Breitkreutz, J. Determination of the disintegration behavior of magnetically marked tablets. Eur. J. Pharm. Biopharm. 2001, 52, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Andrä, W.; Danan, H.; Kirmsse, W.; Kramer, H.H.; Saupe, P.; Schmieg, R.; Bellemann, M.E. A novel method for real-time magnetic marker monitoring in the gastrointestinal tract. Phys. Med. Biol. 2000, 45, 3081–3093. [Google Scholar] [CrossRef] [PubMed]
- Weitschies, W.; Blume, H.; Mönnikes, H. Magnetic marker monitoring: High resolution real-time tracking of oral solid dosage forms in the gastrointestinal tract. Eur. J. Pharm. Biopharm. 2010, 74, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Placidi, G.; Franchi, D.; Gallo, P.; Sotgiu, A. Design of a Magnetic Localisation System for In-Vivo Endovascular Interventions. In Proceedings of the 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 22–26 August 2007; pp. 499–503. [Google Scholar]
- Paixao, F.C.; Corá, L.A.; Américo, M.F.; de Oliveira, R.B.; Baffa, O.; Miranda, J.R.A. Development of an AMR-ACB Array for Gastrointestinal Motility Studies. IEEE Trans. Biomed. Eng. 2012, 59, 2737–2743. [Google Scholar] [CrossRef] [PubMed]
- Arami, A.; Simoncini, M.; Atasoy, O.; Ali, S.; Hasenkamp, W.; Bertsch, A.; Meurville, E.; Tanner, S.; Renaud, P.; Dehollain, C.; et al. Instrumented Knee Prosthesis for Force and Kinematics Measurements. IEEE Trans. Autom. Sci. Eng. 2013, 10, 615–624. [Google Scholar] [CrossRef]
- De, D.; Mandal, S.M.; Gauri, S.G.; Bhattacharya, R.; Ram, S.; Roy, S.K. Antibacterial Effect of Lanthanum Calcium Manganate (La0.67Ca0.33MnO3) Nanoparticles Against Pseudomonas aeruginosa ATCC 27853. J. Biomed. Nanotechnol. 2010, 6, 138–144. [Google Scholar] [CrossRef]
- Hou, W.X.; Yao, Y.F.; Li, Y.J.; Peng, B.; Shi, K.Q.; Zhou, Z.Y.; Pan, J.Y.; Liu, M.; Hu, J.F. Linearly shifting ferromagnetic resonance response of La0.7Sr0.3MnO3 thin film for body temperature sensors. Front. Mater. Sci. 2022, 16, 220589. [Google Scholar] [CrossRef]
- Wu, S.; Fadil, D.; Liu, S.; Aryan, A.; Renault, B.; Routoure, J.-M.; Guillet, B.; Flament, S.; Langlois, P.; Méchin, L. La0.7Sr0.3MnO3 Thin Films for Magnetic and Temperature Sensors at Room Temperature. Sens. Transducers 2012, 253–265. [Google Scholar]
- Carlier, T.; Ferri, A.; Saitzek, S.; Huvé, M.; Bayart, A.; Da Costa, A.; Desfeux, R.; Tebano, A. Microstructure and local electrical behavior in [(Nd2Ti2O7)4/(SrTiO3)n]10 (n = 4–8) superlattices. RSC Adv. 2018, 8, 11262–11271. [Google Scholar] [CrossRef]
- Jimenez, V.O.; Hwang, K.Y.; Nguyen, D.; Rahman, Y.; Albrecht, C.; Senator, B.; Thiabgoh, O.; Devkota, J.; Bui, V.D.A.; Lam, D.S.; et al. Magnetoimpedance Biosensors and Real-Time Healthcare Monitors: Progress, Opportunities, and Challenges. Biosensors 2022, 12, 517. [Google Scholar] [CrossRef]
- Karnaushenko, D.; Karnaushenko, D.D.; Makarov, D.; Baunack, S.; Schäfer, R.; Schmidt, O.G. Self-Assembled On-Chip-Integrated Giant Magneto-Impedance Sensorics. Adv. Mater. 2015, 27, 6582–6589. [Google Scholar] [CrossRef]
- Thiabgoh, O.; Eggers, T.; Phan, M.-H. A new contactless magneto-LC resonance technology for real-time respiratory motion monitoring. Sens. Actuators A Phys. 2017, 265, 120–126. [Google Scholar] [CrossRef]
- Kobayashi, K.; Iwai, M.; Tanaka, T.; Hata, Y.; Ogata, Y.; Kakinuma, B. Magnetocardiogram measurement using SQUID magnetometer and Magneto-Impedance sensor. J. Magn. Soc. Jpn. 2018, 42, 61. Available online: https://www.magnetics.jp/kouenkai/2018/doc/program/06ALL.pdf (accessed on 2 February 2024).
- Kurlyandskaya, G.V.; Sanchez, M.L.; Hernando, B.; Prida, V.M.; Gorria, P.; Tejedor, M. Giant-magnetoimpedance-based sensitive element as a model for biosensors. Appl. Phys. Lett. 2003, 82, 3053–3055. [Google Scholar] [CrossRef]
- Kurlyandskaya, G.V. Giant magnetoimpedance for biosensing: Advantages and shortcomings. J. Magn. Magn. Mater. 2009, 321, 659–662. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, Q.; Li, X.; Pan, H.L.; Wang, J.T.; Zhao, Z.J. Detection of AFP with an ultra-sensitive giant magnetoimpedance biosensor. Sens. Actuators B Chem. 2019, 293, 53–58. [Google Scholar] [CrossRef]
- Wang, T.; Chen, Y.Y.; Wang, B.C.; He, Y.; Li, H.Y.; Liu, M.; Rao, J.J.; Wu, Z.Z.; Xie, S.R.; Luo, J. A giant magnetoimpedance-based separable-type method for supersensitive detection of 10 magnetic beads at high frequency. Sens. Actuators A Phys. 2019, 300, 111656. [Google Scholar] [CrossRef]
- Blanc-Béguin, F.; Nabily, S.; Gieraltowski, J.; Turzo, A.; Querellou, S.; Salaun, P.Y. Cytotoxicity and GMI bio-sensor detection of maghemite nanoparticles internalized into cells. J. Magn. Magn. Mater. 2009, 321, 192–197. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, Y.; Lei, c.; Sun, X.-C.; Zhou, Y. A flexible giant magnetoimpedance-based biosensor for the determination of the biomarker C-reactive protein. Microchim. Acta 2015, 182, 2411–2417. [Google Scholar] [CrossRef]
- Yang, H.; Chen, L.; Lei, C.; Zhang, J.; Li, D.; Zhou, Z.-M.; Bao, C.-C.; Hu, H.-Y.; Chen, X.; Cui, F.; et al. Giant magnetoimpedance-based microchannel system for quick and parallel genotyping of human papilloma virus type 16/18. Appl. Phys. Lett. 2010, 97, 043702. [Google Scholar] [CrossRef]
- Mahdavi, H.; Rosell-Ferrer, J. In-bed vital signs monitoring system based on unobtrusive magnetic induction method with a concentric planar gradiometer. Physiol. Meas. 2017, 38, 1226. [Google Scholar] [CrossRef] [PubMed]
- Scharfetter, H.; Lackner, H.K.; Rosell, J. Magnetic induction tomography: Hardware for multi-frequency measurements in biological tissues. Physiol. Meas. 2001, 22, 131. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Soleimani, M. Magnetic induction tomography methods and applications: A review. Meas. Sci. Technol. 2017, 28, 072001. [Google Scholar] [CrossRef]
- Leontiev, V.S.; Lobekin, V.N.; Saplev, A.F.; Zueva, E.A.; Evasheva, E.E.; Bichurin, M.I. Application of magnetoelectric sensors in biomedicine. J. Phys. Conf. Ser. 2021, 2052, 012022. [Google Scholar] [CrossRef]
- Dong, C.Z.; Liang, X.F.; Gao, J.Y.; Chen, H.H.; He, Y.F.; Wei, Y.Y.; Zaeimbashi, M.; Matyushov, A.; Sun, C.X.; Sun, N.X. Thin Film Magnetoelectric Sensors Toward Biomagnetism: Materials, Devices, and Applications. Adv. Electron. Mater. 2022, 8, 2200013. [Google Scholar] [CrossRef]
- Lobekin, V.N.; Petrov, R.V.; Bichurin, M.I.; Rebinok, A.V.; Sulimanov, R.A. Magnetoelectric sensor for measuring weak magnetic biological fields. IOP Conf. Ser. Mater. Sci. Eng. 2018, 441, 012035. [Google Scholar] [CrossRef]
- Zuo, S.M.; Schmalz, J.; Özden, M.-Ö.; Gerken, M.; Su, J.X.; Niekiel, F.; Lofink, F.; Nazarpour, K.; Heidari, H. Ultrasensitive Magnetoelectric Sensing System for Pico-Tesla MagnetoMyoGraphy. IEEE Trans. Biomed. Circuits Syst. 2020, 14, 971–984. [Google Scholar] [CrossRef] [PubMed]
- Apu, E.H.; Nafiujjaman, M.; Sandeep, S.; Makela, A.V.; Khaleghi, A.; Vainio, S.; Contag, C.H.; Li, J.X.; Balagingham, I.; Kim, T.H.; et al. Biomedical applications of multifunctional magnetoelectric nanoparticles. Mater. Chem. Front. 2022, 6, 1368–1390. [Google Scholar]
- Das, D.; Xu, Z.Y.; Nasrollahpour, M.; Martos-Repath, I.; Zaeimbashi, M.; Khalifa, A.; Mittal, A.; Cash, S.S.; Sun, N.X.; Shrivastava, A.; et al. Circuit-Level Modeling and Simulation of Wireless Sensing and Energy Harvesting With Hybrid Magnetoelectric Antennas for Implantable Neural Devices. IEEE Open J. Circuits Syst. 2023, 4, 139–155. [Google Scholar] [CrossRef]
- Sharma, S.D.; Fischer, R.; Schoennagel, B.P.; Nielsen, P.; Kooijman, H.; Yamamura, J.; Adam, G.; Bannas, P.; Hernando, D.; Reeder, S.B. MRI-based quantitative susceptibility mapping (QSM) and R2* mapping of liver iron overload: Comparison with SQUID-based biomagnetic liver susceptometry. Magn. Reson. Med. 2017, 78, 264–270. [Google Scholar] [CrossRef]
- Li, B.-B.; Ou, L.F.; Lei, Y.C.; Liu, Y.-C. Cavity optomechanical sensing. Nanophotonics 2021, 10, 2799–2832. [Google Scholar] [CrossRef]




| Physical Effect | Application |
|---|---|
| Hall effect | Biosensor—detection of DNA, etc. |
| Hall effect | Gait detection, hand movement, pulsimeter, magnetic field exposure |
| GMR | Biotechnological assays—biomolecule concentration with magnetic labels |
| GMR | Magnetocardiography, respiration rate, blood pressure |
| GMR | Magnetic fluid detection in hyperthermia therapy |
| TMR | Biotechnology—biomarker detection with magnetic beads as labels |
| TMR | Detection of magnetic nanoparticles in hyperthermia therapy |
| TMR | Detection of implants |
| AMR | Movement detection during rehabilitation |
| AMR | Detection of neural activity (implanted) |
| AMR | Kinematic information about prosthesis |
| GMI | Measuring biomagnetic fields of muscles, heart or brain, breathing sensors |
| GMI | Finger motion detection during rehabilitation |
| GMI | Biosensors for label-free detection of cancer biomarkers |
| GMI | Detection of magnetically labeled biomolecules |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blachowicz, T.; Kola, I.; Ehrmann, A.; Guenther, K.; Ehrmann, G. Magnetic Micro and Nano Sensors for Continuous Health Monitoring. Micro 2024, 4, 206-228. https://doi.org/10.3390/micro4020015
Blachowicz T, Kola I, Ehrmann A, Guenther K, Ehrmann G. Magnetic Micro and Nano Sensors for Continuous Health Monitoring. Micro. 2024; 4(2):206-228. https://doi.org/10.3390/micro4020015
Chicago/Turabian StyleBlachowicz, Tomasz, Ilda Kola, Andrea Ehrmann, Karoline Guenther, and Guido Ehrmann. 2024. "Magnetic Micro and Nano Sensors for Continuous Health Monitoring" Micro 4, no. 2: 206-228. https://doi.org/10.3390/micro4020015
APA StyleBlachowicz, T., Kola, I., Ehrmann, A., Guenther, K., & Ehrmann, G. (2024). Magnetic Micro and Nano Sensors for Continuous Health Monitoring. Micro, 4(2), 206-228. https://doi.org/10.3390/micro4020015







