Porous Inorganic Nanomaterials: Their Evolution towards Hierarchical Porous Nanostructures
Abstract
1. Introduction
2. Description of Porosity
3. Towards Engineering Porous Nanomaterials
3.1. Porous Nanomaterial Preparation Techniques
3.1.1. Template-Assisted Pore Formation
3.1.2. Template-Free Pathways to Form Porous Materials
4. The Rise of Hierarchical Nanostructures
- An increased surface area and pore volume, enabling species to interact and functionalize pore walls;
- Providing more accessible mass transport pathways within the structure framework and allowing more molecules to flow within or out of the porous matrices;
- A more effective diffusion and possibly simultaneous loading of target/adsorbing molecules.
4.1. Porous Oxide Nanostructures and Derivatives
4.1.1. Porous Metal Oxides, Phosphides, Nitrides and Other Derivatives
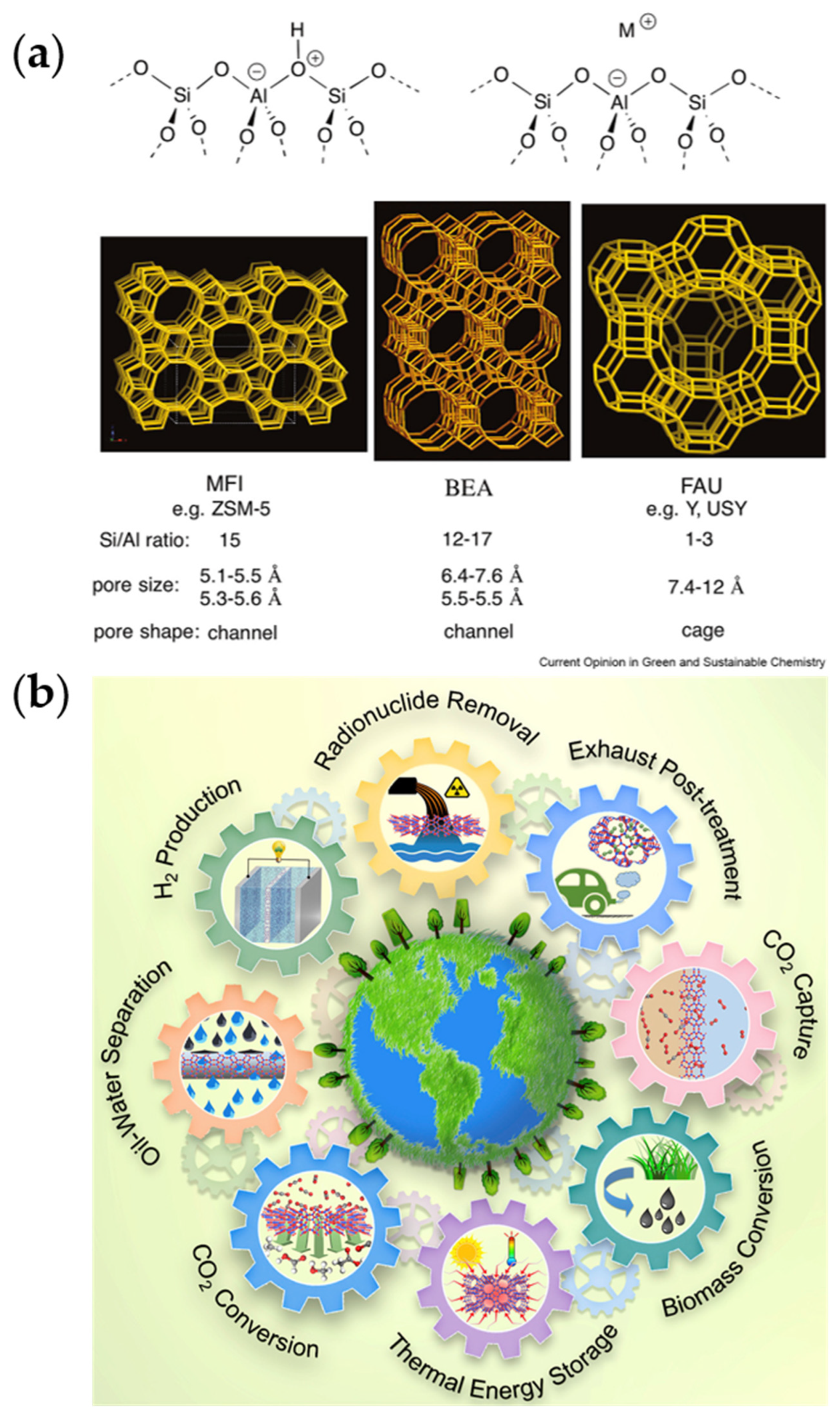
4.1.2. Zeolites
4.1.3. Porous Silica
4.1.4. Porous Amorphous Oxides
4.1.5. Porous Nanomaterial Ordered Structures and Arrays of Oxides and Derivatives
4.2. Porous Metallic Nanostructures
4.2.1. Porous Gold Nanostructures and Other Precious Metals
4.2.2. Porous Non-Precious Metals, MOFs, and Their Nanostructures
- Having a small size, suitable for use in biomedicine;
- Their assembly into different possible nanostructures for separation applications, energy conversion and storage, and related devices (possible also due to their small size);
- Enhanced kinetics related to adsorption–desorption;
- Better active site accessibility resulting in enhanced catalytic performance.
4.3. Porous Carbon Nanostructures and Derivatives
5. Technological Advancement and (Hierarchical) Porous Nanomaterials
6. Conclusions
- Small (nano) size;
- Large surface area;
- Accessibility of more active/adsorption sites due to the pores/porous network:
- Relatively more reactive/active sites due to the exposed surface (uncoordinated atoms);
- Lightweight (compared to bulk; both due to the dimension and presence of voids).
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, D.-J.; Ding, Y. Porous Nanostructured Metals for Electrocatalysis. Electroanalysis 2012, 24, 2035–2043. [Google Scholar] [CrossRef]
- Sheta, S.M.; El-Sheikh, S.M. Nanomaterials and Metal-Organic Frameworks for Biosensing Applications of Mutations of the Emerging Viruses. Anal. Biochem. 2022, 648, 114680. [Google Scholar] [CrossRef] [PubMed]
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A Review of Synthesis Methods, Properties, Recent Progress, and Challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Datt, A.; Ndiege, N.; Larsen, S.C. Development of Porous Nanomaterials for Applications in Drug Delivery and Imaging. In Nanomaterials for Biomedicine; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2012; Volume 1119, pp. 239–258. ISBN 978-0-8412-2718-7. [Google Scholar]
- Quesada-González, D.; Merkoçi, A. Nanomaterial-Based Devices for Point-of-Care Diagnostic Applications. Chem. Soc. Rev. 2018, 47, 4697–4709. [Google Scholar] [CrossRef] [PubMed]
- Wen, N.; Zhang, L.; Jiang, D.; Wu, Z.; Li, B.; Sun, C.; Guo, Z. Emerging Flexible Sensors Based on Nanomaterials: Recent Status and Applications. J. Mater. Chem. A 2020, 8, 25499–25527. [Google Scholar] [CrossRef]
- Kianfar, E.; Sayadi, H. Recent Advances in Properties and Applications of Nanoporous Materials and Porous Carbons. Carbon Lett. 2022, 32, 1645–1669. [Google Scholar] [CrossRef]
- Du, C.; Li, P.; Zhuang, Z.; Fang, Z.; He, S.; Feng, L.; Chen, W. Highly Porous Nanostructures: Rational Fabrication and Promising Application in Energy Electrocatalysis. Coord. Chem. Rev. 2022, 466, 214604. [Google Scholar] [CrossRef]
- Gleiter, H. Nanostructured Materials: Basic Concepts and Microstructure. Acta Mater. 2000, 48, 1–29. [Google Scholar] [CrossRef]
- Cai, X.; Xie, Z.; Li, D.; Kassymova, M.; Zang, S.Q.; Jiang, H.L. Nano-Sized Metal-Organic Frameworks: Synthesis and Applications. Coord. Chem. Rev. 2020, 417, 213366. [Google Scholar] [CrossRef]
- Querebillo, C.J. A Review on Nano Ti-Based Oxides for Dark and Photocatalysis: From Photoinduced Processes to Bioimplant Applications. Nanomaterials 2023, 13, 982. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-Y.; Léonard, A.; Lemaire, A.; Tian, G.; Su, B.-L. Self-Formation Phenomenon to Hierarchically Structured Porous Materials: Design, Synthesis, Formation Mechanism and Applications. Chem. Commun. 2011, 47, 2763–2786. [Google Scholar] [CrossRef] [PubMed]
- Pavlenko, V.; Khosravi, H.S.; Żółtowska, S.; Haruna, A.B.; Zahid, M.; Mansurov, Z.; Supiyeva, Z.; Galal, A.; Ozoemena, K.I.; Abbas, Q.; et al. A Comprehensive Review of Template-Assisted Porous Carbons: Modern Preparation Methods and Advanced Applications. Mater. Sci. Eng. R Rep. 2022, 149, 100682. [Google Scholar] [CrossRef]
- Scandura, G.; Kumari, P.; Palmisano, G.; Karanikolos, G.N.; Orwa, J.; Dumée, L.F. Nanoporous Dealloyed Metal Materials Processing and Applications—A Review. Ind. Eng. Chem. Res. 2023, 62, 1736–1763. [Google Scholar] [CrossRef]
- Fang, B.; Kim, J.H.; Kim, M.-S.; Yu, J.-S. Hierarchical Nanostructured Carbons with Meso–Macroporosity: Design, Characterization, and Applications. Acc. Chem. Res. 2013, 46, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Charkhi, A.; Kazemeini, M.; Ahmadi, S.J.; Kazemian, H. Fabrication of Granulated NaY Zeolite Nanoparticles Using a New Method and Study the Adsorption Properties. Powder Technol. 2012, 231, 1–6. [Google Scholar] [CrossRef]
- Dzyazko, Y.S.; Rozhdestvenska, L.M.; Kudelko, K.O.; Fedina, I.V.; Ponomaryova, L.M.; Nikovska, G.M.; Dzyazko, O.G. Hydrated Iron Oxide Embedded to Natural Zeolite: Effect of Nanoparticles and Microparticles on Sorption Properties of Composites. Water Air Soil Pollut. 2022, 233, 205. [Google Scholar] [CrossRef]
- Zou, L.; Ge, M.; Zhao, C.; Meng, Q.; Wang, H.; Liu, X.; Lin, C.-H.; Xiao, X.; Lee, W.-K.; Shen, Q.; et al. Designing Multiscale Porous Metal by Simple Dealloying with 3D Morphological Evolution Mechanism Revealed via X-Ray Nano-Tomography. ACS Appl. Mater. Interfaces 2020, 12, 2793–2804. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, Y.; Kim, D.H.; Moon, J.H. Carbon-Deposited TiO2 3D Inverse Opal Photocatalysts: Visible-Light Photocatalytic Activity and Enhanced Activity in a Viscous Solution. ACS Appl. Mater. Interfaces 2013, 5, 12526–12532. [Google Scholar] [CrossRef] [PubMed]
- Wharry, J.P.; Nemets, G.; Marrero, E.; Emerson, J.; Gehmlich, N.; Okuniewski, M.A.; Clement, C.D.; Mao, K.S. Multimodal Characterization of Porosity in Advanced Manufactured and Welded Nuclear Structural Alloys. Microsc. Microanal. 2023, 29, 1536–1537. [Google Scholar] [CrossRef]
- Zhao, B.; Collinson, M.M. Well-Defined Hierarchical Templates for Multimodal Porous Material Fabrication. Chem. Mater. 2010, 22, 4312–4319. [Google Scholar] [CrossRef]
- Smått, J.-H.; Schunk, S.; Lindén, M. Versatile Double-Templating Synthesis Route to Silica Monoliths Exhibiting a Multimodal Hierarchical Porosity. Chem. Mater. 2003, 15, 2354–2361. [Google Scholar] [CrossRef]
- Hessien, M.; Prouzet, E. Synthesis of Hierarchical Porous Silica by Sol-Gel of Sodium Silicate and Nanoemulsion Templating: Effective Combination Conditions. ChemistrySelect 2021, 6, 1440–1447. [Google Scholar] [CrossRef]
- Chen, L.-H.; Li, Y.; Su, B.-L. Hierarchy in Materials for Maximized Efficiency. Natl. Sci. Rev. 2020, 7, 1626–1630. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.-C.; Tsao, C.-S.; Jao, M.-H.; Shyue, J.-J.; Hsu, C.-P.; Huang, Y.-C.; Tian, K.-Y.; Chen, C.-Y.; Su, C.-J.; Su, W.-F. Hierarchical i–p and i–n Porous Heterojunction in Planar Perovskite Solar Cells. J. Mater. Chem. A 2015, 3, 10526–10535. [Google Scholar] [CrossRef]
- Rabiee, N.; Atarod, M.; Tavakolizadeh, M.; Asgari, S.; Rezaei, M.; Akhavan, O.; Pourjavadi, A.; Jouyandeh, M.; Lima, E.C.; Hamed Mashhadzadeh, A.; et al. Green Metal-Organic Frameworks (MOFs) for Biomedical Applications. Microporous Mesoporous Mater. 2022, 335, 111670. [Google Scholar] [CrossRef]
- Chattopadhyay, K.; Mandal, M.; Maiti, D.K. Smart Metal-Organic Frameworks for Biotechnological Applications: A Mini-Review. ACS Appl. Bio Mater. 2021, 4, 8159–8171. [Google Scholar] [CrossRef] [PubMed]
- Viciano-Chumillas, M.; Liu, X.; Leyva-Pérez, A.; Armentano, D.; Ferrando-Soria, J.; Pardo, E. Mixed Component Metal-Organic Frameworks: Heterogeneity and Complexity at the Service of Application Performances. Coord. Chem. Rev. 2022, 451, 214273. [Google Scholar] [CrossRef]
- Lahcen, A.A.; Surya, S.G.; Beduk, T.; Vijjapu, M.T.; Lamaoui, A.; Durmus, C.; Timur, S.; Shekhah, O.; Mani, V.; Amine, A.; et al. Metal–Organic Frameworks Meet Molecularly Imprinted Polymers: Insights and Prospects for Sensor Applications. ACS Appl. Mater. Interfaces 2022, 14, 49399–49424. [Google Scholar] [CrossRef]
- Beg, S.; Rahman, M.; Jain, A.; Saini, S.; Midoux, P.; Pichon, C.; Ahmad, F.J.; Akhter, S. Nanoporous Metal Organic Frameworks as Hybrid Polymer–Metal Composites for Drug Delivery and Biomedical Applications. Drug Discov. Today 2017, 22, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hu, Q.; Liu, J.; Zhang, P.; Fu, S.; Wu, S. Iron–Organic Framework Nanoparticle-Supported Tungstosilicic Acid as a Catalyst for the Biginelli Reaction. ACS Appl. Nano Mater. 2022, 5, 16987–16995. [Google Scholar] [CrossRef]
- Kalaj, M.; Bentz, K.C.; Ayala, S.; Palomba, J.M.; Barcus, K.S.; Katayama, Y.; Cohen, S.M. MOF-Polymer Hybrid Materials: From Simple Composites to Tailored Architectures. Chem. Rev. 2020, 120, 8267–8302. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zou, J.; Wang, Y.; Fu, A.; Guo, Y.-G.; Li, H. (Ni)MOF Interpenetrated with MnO2 Nanothorns Anchored on Porous Carbon Cloth as Self-Standing Cathode for High-Performance Supercapacitors. Int. J. Electrochem. Sci. 2022, 17, 220843. [Google Scholar] [CrossRef]
- McCue, I.; Benn, E.; Gaskey, B.; Erlebacher, J. Dealloying and Dealloyed Materials. Annu. Rev. Mater. Res. 2016, 46, 263–286. [Google Scholar] [CrossRef]
- Gu, D.; Schüth, F. Synthesis of Non-Siliceous Mesoporous Oxides. Chem. Soc. Rev. 2013, 43, 313–344. [Google Scholar] [CrossRef] [PubMed]
- Cundy, C.S.; Cox, P.A. The Hydrothermal Synthesis of Zeolites: History and Development from the Earliest Days to the Present Time. Chem. Rev. 2003, 103, 663–702. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Ran, R.; Zhong, Y.; Zhou, W.; Ni, M.; Shao, Z. Advances in Porous Perovskites: Synthesis and Electrocatalytic Performance in Fuel Cells and Metal–Air Batteries. Energy Environ. Mater. 2020, 3, 121–145. [Google Scholar] [CrossRef]
- Derakhshankhah, H.; Jafari, S.; Sarvari, S.; Barzegari, E.; Moakedi, F.; Ghorbani, M.; Varnamkhasti, B.S.; Jaymand, M.; Izadi, Z.; Tayebi, L. Biomedical Applications of Zeolitic Nanoparticles, with an Emphasis on Medical Interventions. Int. J. Nanomed. 2020, 15, 363–386. [Google Scholar] [CrossRef] [PubMed]
- Sastre, G.; Daeyaert, F.F. (Eds.) AI-Guided Design and Property Prediction for Zeolites and Nanoporous Materials; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 2023; ISBN 978-1-119-81975-2. [Google Scholar]
- Xu, D.; Lv, H.; Liu, B. Encapsulation of Metal Nanoparticle Catalysts Within Mesoporous Zeolites and Their Enhanced Catalytic Performances: A Review. Front. Chem. 2018, 6, 550. [Google Scholar] [CrossRef] [PubMed]
- Schlumberger, C.; Thommes, M. Characterization of Hierarchically Ordered Porous Materials by Physisorption and Mercury Porosimetry—A Tutorial Review. Adv. Mater. Interfaces 2021, 8, 2002181. [Google Scholar] [CrossRef]
- Van der Zalm, J.; Chen, S.; Huang, W.; Chen, A. Review—Recent Advances in the Development of Nanoporous Au for Sensing Applications. J. Electrochem. Soc. 2020, 167, 037532. [Google Scholar] [CrossRef]
- Xiao, S.; Wang, S.; Wang, X.; Xu, P. Nanoporous Gold: A Review and Potentials in Biotechnological and Biomedical Applications. Nano Sel. 2021, 2, 1437–1458. [Google Scholar] [CrossRef]
- Song, M.; Kim, Y.; Baek, D.S.; Kim, H.Y.; Gu, D.H.; Li, H.; Cunning, B.V.; Yang, S.E.; Heo, S.H.; Lee, S.; et al. 3D Microprinting of Inorganic Porous Materials by Chemical Linking-Induced Solidification of Nanocrystals. Nat. Commun. 2023, 14, 8460. [Google Scholar] [CrossRef] [PubMed]
- Muldoon, K.; Song, Y.; Ahmad, Z.; Chen, X.; Chang, M.-W. High Precision 3D Printing for Micro to Nano Scale Biomedical and Electronic Devices. Micromachines 2022, 13, 642. [Google Scholar] [CrossRef] [PubMed]
- Kausar, A. Scientific Worth of Polymer and Graphene Foam-Based Nanomaterials. J. Chin. Adv. Mater. Soc. 2018, 6, 779–800. [Google Scholar] [CrossRef]
- Chabot, V.; Higgins, D.; Yu, A.; Xiao, X.; Chen, Z.; Zhang, J. A Review of Graphene and Graphene Oxide Sponge: Material Synthesis and Applications to Energy and the Environment. Energy Environ. Sci. 2014, 7, 1564. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Klobes, P.; Meyer, K.; Munro, R.G. Porosity and Specific Surface Area Measurements for Solid Materials; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2006. [Google Scholar]
- Zdravkov, B.; Čermák, J.; Šefara, M.; Janků, J. Pore Classification in the Characterization of Porous Materials: A Perspective. Open Chem. 2007, 5, 385–395. [Google Scholar] [CrossRef]
- Du, X.; He, J. Hierarchically Mesoporous Silica Nanoparticles: Extraction, Amino-Functionalization, and Their Multipurpose Potentials. Langmuir 2011, 27, 2972–2979. [Google Scholar] [CrossRef] [PubMed]
- Wawrzyńczak, A.; Nowak, I.; Woźniak, N.; Chudzińska, J.; Feliczak-Guzik, A. Synthesis and Characterization of Hierarchical Zeolites Modified with Polysaccharides and Its Potential Role as a Platform for Drug Delivery. Pharmaceutics 2023, 15, 535. [Google Scholar] [CrossRef] [PubMed]
- Mansoori, G.A.; Soelaiman, T.F. Nanotechnology—An Introduction for the Standards Community. J. ASTM Int. 2005, 2, JAI13110. [Google Scholar] [CrossRef]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The History of Nanoscience and Nanotechnology: From Chemical–Physical Applications to Nanomedicine. Molecules 2019, 25, 112. [Google Scholar] [CrossRef]
- Mekuye, B.; Abera, B. Nanomaterials: An Overview of Synthesis, Classification, Characterization, and Applications. Nano Sel. 2023, 4, 486–501. [Google Scholar] [CrossRef]
- Feynman, R.P. There’s Plenty of Room at the Bottom. Eng. Sci. 1960, 23, 22–36. [Google Scholar]
- Taniguchi, N. On The Basic Concept of Nano-Technology. In Proceedings of the International Conference on Production Engineering, Tokyo, Japan, 26–29 August 1974; pp. 18–23. [Google Scholar]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and Nanoparticles: Sources and Toxicity. Biointerphases 2007, 2, MR17–MR71. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Jin, Z.; Dai, Z.; Chang, Y.; Jiang, X.; Wang, H. Porous Carbons Synthesized by Templating Approach from Fluid Precursors and Their Applications in Environment and Energy Storage: A Review. Carbon 2020, 170, 100–118. [Google Scholar] [CrossRef]
- Polarz, S.; Smarsly, B. Nanoporous Materials. J. Nanosci. Nanotechnol. 2002, 2, 581–612. [Google Scholar] [CrossRef]
- Manzano, M.; Vallet-Regí, M. Mesoporous Silica Nanoparticles for Drug Delivery. Adv. Funct. Mater. 2020, 30, 1902634. [Google Scholar] [CrossRef]
- Innocenzi, P.; Costacurta, S.; Kidchob, T.; Malfatti, L.; Falcaro, P.; Soler-Illia, G. Mesoporous Thin Films: Properties and Applications. In Proceedings of the Sol-Gel Methods for Materials Processing; Innocenzi, P., Zub, Y.L., Kessler, V.G., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 105–123. [Google Scholar]
- Grosso, D.; Cagnol, F.; Soler-Illia, G.J.d.A.A.; Crepaldi, E.L.; Amenitsch, H.; Brunet-Bruneau, A.; Bourgeois, A.; Sanchez, C. Fundamentals of Mesostructuring Through Evaporation-Induced Self-Assembly. Adv. Funct. Mater. 2004, 14, 309–322. [Google Scholar] [CrossRef]
- Sakatani, Y.; Boissière, C.; Grosso, D.; Nicole, L.; Soler-Illia, G.J.A.A.; Sanchez, C. Coupling Nanobuilding Block and Breath Figures Approaches for the Designed Construction of Hierarchically Templated Porous Materials and Membranes. Chem. Mater. 2008, 20, 1049–1056. [Google Scholar] [CrossRef]
- Ahn, S.H.; Kim, D.J.; Chi, W.S.; Kim, J.H. Hierarchical Double-Shell Nanostructures of TiO2 Nanosheets on SnO2 Hollow Spheres for High-Efficiency, Solid-State, Dye-Sensitized Solar Cells. Adv. Funct. Mater. 2014, 24, 5037–5044. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, Q.; Zhao, J.; Guan, Y.; Liu, J.; An, Z.; Xu, B. Nitrogen-Doped Hierarchical Porous Carbon for Supercapacitors with High Rate Performance. Microporous Mesoporous Mater. 2019, 279, 439–445. [Google Scholar] [CrossRef]
- Qiu, D.; Cao, T.; Zhang, J.; Zhang, S.-W.; Zheng, D.; Wu, H.; Lv, W.; Kang, F.; Yang, Q.-H. Precise Carbon Structure Control by Salt Template for High Performance Sodium-Ion Storage. J. Energy Chem. 2019, 31, 101–106. [Google Scholar] [CrossRef]
- Chen, N.; Xiao, T.-H.; Luo, Z.; Kitahama, Y.; Hiramatsu, K.; Kishimoto, N.; Itoh, T.; Cheng, Z.; Goda, K. Porous Carbon Nanowire Array for Surface-Enhanced Raman Spectroscopy. Nat. Commun. 2020, 11, 4772. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Bhushan, P.; Bhattacharya, S. Fabrication of Nanostructures with Bottom-up Approach and Their Utility in Diagnostics, Therapeutics, and Others. In Environmental, Chemical and Medical Sensors; Bhattacharya, S., Agarwal, A.K., Chanda, N., Pandey, A., Sen, A.K., Eds.; Energy, Environment, and Sustainability; Springer: Singapore, 2018; pp. 167–198. ISBN 978-981-10-7750-0. [Google Scholar]
- Galarneau, A.; Mehlhorn, D.; Guenneau, F.; Coasne, B.; Villemot, F.; Minoux, D.; Aquino, C.; Dath, J.-P. Specific Surface Area Determination for Microporous/Mesoporous Materials: The Case of Mesoporous FAU-Y Zeolites. Langmuir 2018, 34, 14134–14142. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Malik, M.M.; Purohit, R. Synthesis of High Surface Area Mesoporous Silica Materials Using Soft Templating Approach. Mater. Today Proc. 2018, 5, 4128–4133. [Google Scholar] [CrossRef]
- Yang, P.; Zhao, D.; Margolese, D.I.; Chmelka, B.F.; Stucky, G.D. Generalized Syntheses of Large-Pore Mesoporous Metal Oxides with Semicrystalline Frameworks. Nature 1998, 396, 152–155. [Google Scholar] [CrossRef]
- Yang, P.; Deng, T.; Zhao, D.; Feng, P.; Pine, D.; Chmelka, B.F.; Whitesides, G.M.; Stucky, G.D. Hierarchically Ordered Oxides. Science 1998, 282, 2244–2246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Dai, H.; Du, Y.; Zhang, L.; Deng, J.; Xia, Y.; Zhao, Z.; Meng, X.; Liu, Y. P123-PMMA Dual-Templating Generation and Unique Physicochemical Properties of Three-Dimensionally Ordered Macroporous Iron Oxides with Nanovoids in the Crystalline Walls. Inorg. Chem. 2011, 50, 2534–2544. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; Yun, J.; Lee, J.; Lee, K.; Jang, J. Hierarchical Mesoporous Silica Nanoparticles as Superb Light Scattering Materials. Chem. Commun. 2016, 52, 2165–2168. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhao, G.; Wang, G.; Irvine, J.T.S. Synthesis and Applications of Nanoporous Perovskite Metal Oxides. Chem. Sci. 2018, 9, 3623–3637. [Google Scholar] [CrossRef]
- Suzuki, N.; Osada, M.; Billah, M.; Alothman, Z.A.; Bando, Y.; Yamauchi, Y.; Hossain, M.S.A. Origin of Thermally Stable Ferroelectricity in a Porous Barium Titanate Thin Film Synthesized through Block Copolymer Templating. APL Mater. 2017, 5, 076111. [Google Scholar] [CrossRef]
- Li, K.; Yang, J.; Huang, R.; Lin, S.; Gu, J. Ordered Large-Pore MesoMOFs Based on Synergistic Effects of TriBlock Polymer and Hofmeister Ion. Angew. Chem. 2020, 132, 14228–14232. [Google Scholar] [CrossRef]
- Li, K.; Yang, J.; Gu, J. Hierarchically Porous MOFs Synthesized by Soft-Template Strategies. Acc. Chem. Res. 2022, 55, 2235–2247. [Google Scholar] [CrossRef]
- Szczęśniak, B.; Choma, J.; Jaroniec, M. Recent Advances in Mechanochemical Synthesis of Mesoporous Metal Oxides. Mater. Adv. 2021, 2, 2510–2523. [Google Scholar] [CrossRef]
- Bashir, J.; Chowdhury, M.B.; Kathak, R.R.; Dey, S.; Tasnim, A.T.; Amin, M.A.; Kaneti, Y.V.; Masud, M.K.; Hossain, M.S.A. Electrochemical Fabrication of Mesoporous Metal-Alloy Films. Mater. Adv. 2023, 4, 408–431. [Google Scholar] [CrossRef]
- Iqbal, M.; Li, C.; Wood, K.; Jiang, B.; Takei, T.; Dag, Ö.; Baba, D.; Nugraha, A.S.; Asahi, T.; Whitten, A.E.; et al. Continuous Mesoporous Pd Films by Electrochemical Deposition in Nonionic Micellar Solution. Chem. Mater. 2017, 29, 6405–6413. [Google Scholar] [CrossRef]
- Li, C.; Jiang, B.; Wang, Z.; Li, Y.; Hossain, M.S.A.; Kim, J.H.; Takei, T.; Henzie, J.; Dag, Ö.; Bando, Y.; et al. First Synthesis of Continuous Mesoporous Copper Films with Uniformly Sized Pores by Electrochemical Soft Templating. Angew. Chem. Int. Ed. 2016, 55, 12746–12750. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Steinmiller, E.M.P.; Choi, K.-S. Electrochemical Tailoring of Lamellar-Structured ZnO Films by Interfacial Surfactant Templating. Langmuir 2005, 21, 9618–9624. [Google Scholar] [CrossRef] [PubMed]
- Jiokeng, S.L.Z.; Tonle, I.K.; Walcarius, A. Amino-Attapulgite/Mesoporous Silica Composite Films Generated by Electro-Assisted Self-Assembly for the Voltammetric Determination of Diclofenac. Sens. Actuators B Chem. 2019, 287, 296–305. [Google Scholar] [CrossRef]
- Lytle, J.C.; Yan, H.; Turgeon, R.T.; Stein, A. Multistep, Low-Temperature Pseudomorphic Transformations of Nanostructured Silica to Titania via a Titanium Oxyfluoride Intermediate. Chem. Mater. 2004, 16, 3829–3837. [Google Scholar] [CrossRef]
- Wen, X.; Zhang, D.; Yan, T.; Zhang, J.; Shi, L. Three-Dimensional Graphene-Based Hierarchically Porous Carbon Composites Prepared by a Dual-Template Strategy for Capacitive Deionization. J. Mater. Chem. A 2013, 1, 12334–12344. [Google Scholar] [CrossRef]
- Chen, Y.; Su, X.; Esmail, D.; Buck, E.; Tran, S.D.; Szkopek, T.; Cerruti, M. Dual-Templating Strategy for the Fabrication of Graphene Oxide, Reduced Graphene Oxide and Composite Scaffolds with Hierarchical Architectures. Carbon 2022, 189, 186–198. [Google Scholar] [CrossRef]
- Hou, J.; Tu, X.; Wu, X.; Shen, M.; Wang, X.; Wang, C.; Cao, C.; Pang, H.; Wang, G. Remarkable Cycling Durability of Lithium-Sulfur Batteries with Interconnected Mesoporous Hollow Carbon Nanospheres as High Sulfur Content Host. Chem. Eng. J. 2020, 401, 126141. [Google Scholar] [CrossRef]
- Trovagunta, R.; Kelley, S.S.; Lavoine, N. Dual-Templating Approach for Engineering Strong, Biodegradable Lignin-Based Foams. ACS Sustain. Chem. Eng. 2022, 10, 15058–15067. [Google Scholar] [CrossRef]
- Inagaki, M.; Toyoda, M.; Soneda, Y.; Tsujimura, S.; Morishita, T. Templated Mesoporous Carbons: Synthesis and Applications. Carbon 2016, 107, 448–473. [Google Scholar] [CrossRef]
- Liu, H.-J.; Cui, W.-J.; Jin, L.-H.; Wang, C.-X.; Xia, Y.-Y. Preparation of Three-Dimensional Ordered Mesoporous Carbon Sphere Arrays by a Two-Step Templating Route and Their Application for Supercapacitors. J. Mater. Chem. 2009, 19, 3661–3667. [Google Scholar] [CrossRef]
- Kumar, A.; Bhattacharyya, S. Porous NiFe-Oxide Nanocubes as Bifunctional Electrocatalysts for Efficient Water-Splitting. ACS Appl. Mater. Interfaces 2017, 9, 41906–41915. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Dhainaut, J.; Rochard, G.; Dacquin, J.-P.; Mamede, A.-S.; Giraudon, J.-M.; Lamonier, J.-F.; Zhang, H.; Royer, S. Hierarchical Porous ε-MnO2 from Perovskite Precursor: Application to the Formaldehyde Total Oxidation. Chem. Eng. J. 2020, 388, 124146. [Google Scholar] [CrossRef]
- Yang, W.; Li, J.; Wang, Y.; Zhu, F.; Shi, W.; Wan, F.; Xu, D. A Facile Synthesis of Anatase TiO2 Nanosheets -Based Hierarchical Spheres with over 90% {001} Facets for Dye -Sensitized Solar Cells. Chem. Commun. 2011, 47, 1809–1811. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.; Wang, C.; Chen, Z.; Wang, T.; Chen, T.; Shi, R.; Yu, S.; Liu, J. Investigation of the TiO2 Nanoparticles Aggregation with High Light Harvesting for High-Efficiency Dye-Sensitized Solar Cells. Mater. Res. Bull. 2021, 135, 111148. [Google Scholar] [CrossRef]
- Chaida-Chenni, F.Z.; Belhadj, F.; Grande Casas, M.S.; Márquez-Álvarez, C.; Hamacha, R.; Bengueddach, A.; Pérez-Pariente, J. Synthesis of Mesoporous-Zeolite Materials Using Beta Zeolite Nanoparticles as Precursors and Their Catalytic Performance in m-Xylene Isomerization and Disproportionation. Appl. Catal. A Gen. 2018, 568, 148–156. [Google Scholar] [CrossRef]
- Nguyen, K.; Hoa, N.D.; Hung, C.M.; Le, D.T.T.; Duy, N.V.; Hieu, N.V. A Comparative Study on the Electrochemical Properties of Nanoporous Nickel Oxide Nanowires and Nanosheets Prepared by a Hydrothermal Method. RSC Adv. 2018, 8, 19449–19455. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Zhang, Y.; Heard, C.J.; Gołąbek, K.; Ju, X.; Čejka, J.; Mazur, M. Encapsulating Metal Nanoparticles into a Layered Zeolite Precursor with Surface Silanol Nests Enhances Sintering Resistance**. Angew. Chem. Int. Ed. 2023, 62, e202213361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ouyang, B.; Xu, J.; Chen, S.; Rawat, R.S.; Fan, H.J. 3D Porous Hierarchical Nickel–Molybdenum Nitrides Synthesized by RF Plasma as Highly Active and Stable Hydrogen-Evolution-Reaction Electrocatalysts. Adv. Energy Mater. 2016, 6, 1600221. [Google Scholar] [CrossRef]
- Liu, G.; Chen, H.; Xia, L.; Wang, S.; Ding, L.-X.; Li, D.; Xiao, K.; Dai, S.; Wang, H. Hierarchical Mesoporous/Macroporous Perovskite La0.5Sr0.5CoO3–x Nanotubes: A Bifunctional Catalyst with Enhanced Activity and Cycle Stability for Rechargeable Lithium Oxygen Batteries. ACS Appl. Mater. Interfaces 2015, 7, 22478–22486. [Google Scholar] [CrossRef] [PubMed]
- Pang, C.; Zhu, S.; Xu, W.; Liang, Y.; Li, Z.; Wu, S.; Jiang, H.; Wang, H.; Cui, Z. Self-Standing Mo-NiO/Ni Electrocatalyst with Nanoporous Structure for Hydrogen Evolution Reaction. Electrochim. Acta 2023, 439, 141621. [Google Scholar] [CrossRef]
- Chuang, A.; Erlebacher, J. Challenges and Opportunities for Integrating Dealloying Methods into Additive Manufacturing. Materials 2020, 13, 3706. [Google Scholar] [CrossRef] [PubMed]
- Graf, M.; Roschning, B.; Weissmüller, J. Nanoporous Gold by Alloy Corrosion: Method-Structure-Property Relationships. J. Electrochem. Soc. 2017, 164, C194. [Google Scholar] [CrossRef]
- Weissmüller, J.; Sieradzki, K. Dealloyed Nanoporous Materials with Interface-Controlled Behavior. MRS Bull. 2018, 43, 14–19. [Google Scholar] [CrossRef]
- Bao, J.; Chen, H.; Yang, S.; Zhang, P. Mechanochemical Redox-Based Synthesis of Highly Porous CoxMn1-xOy Catalysts for Total Oxidation. Chin. J. Catal. 2020, 41, 1846–1854. [Google Scholar] [CrossRef]
- Friščić, T.; Mottillo, C.; Titi, H.M. Mechanochemistry for Synthesis. Angew. Chem. Int. Ed. 2020, 59, 1018–1029. [Google Scholar] [CrossRef] [PubMed]
- Rahmanivahid, B.; Pinilla-de Dios, M.; Haghighi, M.; Luque, R. Mechanochemical Synthesis of CuO/MgAl2O4 and MgFe2O4 Spinels for Vanillin Production from Isoeugenol and Vanillyl Alcohol. Molecules 2019, 24, 2597. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, R.; Chakraborty, S.; Shukla, R.; Bahadur, J.; Ram, R.; Mazumder, S.; Sarma, H.D.; Kumar Tyagi, A.; Dash, A. Mechanochemical Synthesis of Mesoporous Tin Oxide: A New Generation Nanosorbent for 68 Ge/68 Ga Generator Technology. Dalton Trans. 2016, 45, 13361–13372. [Google Scholar] [CrossRef]
- Plowman, B.J.; Jones, L.A.; Bhargava, S.K. Building with Bubbles: The Formation of High Surface Area Honeycomb-like Films via Hydrogen Bubble Templated Electrodeposition. Chem. Commun. 2015, 51, 4331–4346. [Google Scholar] [CrossRef] [PubMed]
- Cherevko, S.; Chung, C.-H. Direct Electrodeposition of Nanoporous Gold with Controlled Multimodal Pore Size Distribution. Electrochem. Commun. 2011, 13, 16–19. [Google Scholar] [CrossRef]
- Dorofeeva, T.S.; Seker, E. Electrically Tunable Pore Morphology in Nanoporous Gold Thin Films. Nano Res. 2015, 8, 2188–2198. [Google Scholar] [CrossRef]
- Wittstock, G.; Bäumer, M.; Dononelli, W.; Klüner, T.; Lührs, L.; Mahr, C.; Moskaleva, L.V.; Oezaslan, M.; Risse, T.; Rosenauer, A.; et al. Nanoporous Gold: From Structure Evolution to Functional Properties in Catalysis and Electrochemistry. Chem. Rev. 2023, 123, 6716–6792. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Barad, H.-N.; Silva Olaya, A.R.; Alarcón-Correa, M.; Hahn, K.; Richter, G.; Wittstock, G.; Fischer, P. Ultra-Pure Nanoporous Gold Films for Electrocatalysis. ACS Catal. 2023, 13, 11656–11665. [Google Scholar] [CrossRef]
- Ankah, G.N.; Pareek, A.; Cherevko, S.; Topalov, A.A.; Rohwerder, M.; Renner, F.U. The Influence of Halides on the Initial Selective Dissolution of Cu3Au (111). Electrochim. Acta 2012, 85, 384–392. [Google Scholar] [CrossRef]
- Xiao, X.; Ou, W.; Du, P.; Lyu, F.; Diao, Y.; Lu, J.; Li, Y.Y. Ultrafine Nanoporous Gold via Thiol Compound-Mediated Chemical Dealloying. J. Phys. Chem. C 2020, 124, 10026–10031. [Google Scholar] [CrossRef]
- Xu, C.; Wang, R.; Chen, M.; Zhang, Y.; Ding, Y. Dealloying to Nanoporous Au/Pt Alloys and Their Structure Sensitive Electrocatalytic Properties. Phys. Chem. Chem. Phys. 2010, 12, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.Y.; Wei, Q.; Xu, C.; Li, H.; Wu, D.; Cai, Y.; Mao, K.; Cui, Z.; Du, B. Label-Free Electrochemical Immunosensor for Sensitive Detection of Kanamycin. Sens. Actuators B Chem. 2011, 155, 618–625. [Google Scholar] [CrossRef]
- Qian, L.H.; Yan, X.Q.; Fujita, T.; Inoue, A.; Chen, M.W. Surface Enhanced Raman Scattering of Nanoporous Gold: Smaller Pore Sizes Stronger Enhancements. Appl. Phys. Lett. 2007, 90, 153120. [Google Scholar] [CrossRef]
- Chen, C.; Liu, Z.; Cai, C.; Qi, Z. Facile Fabrication of Nanoporous Gold Films for Surface Plasmon Resonance (SPR) Sensing and SPR-Based SERS. J. Mater. Chem. C 2021, 9, 6815–6822. [Google Scholar] [CrossRef]
- Morrish, R.; Dorame, K.; Muscat, A.J. Formation of Nanoporous Au by Dealloying AuCu Thin Films in HNO3. Scr. Mater. 2011, 64, 856–859. [Google Scholar] [CrossRef]
- Kim, J.; Song, J.T.; Oh, J. Facile Electrochemical Synthesis of Dilute AuCu Alloy Nanostructures for Selective and Long-Term Stable CO2 Electrolysis. J. Chem. Phys. 2020, 153, 054702. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.Y.; Shi, S.S.; Qiu, Y.D.; Xie, X.F.; Ruan, H.H.; Gu, J.F.; Pan, D. Pore-Size Tuning and Optical Performances of Nanoporous Gold Films. Microporous Mesoporous Mater. 2015, 202, 50–56. [Google Scholar] [CrossRef]
- Huang, W.; Wang, M.; Zheng, J.; Li, Z. Facile Fabrication of Multifunctional Three-Dimensional Hierarchical Porous Gold Films via Surface Rebuilding. J. Phys. Chem. C 2009, 113, 1800–1805. [Google Scholar] [CrossRef]
- Sulka, G.D. (Ed.) Chapter One—Introduction to Anodization of Metals. In Nanostructured Anodic Metal Oxides; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–34. ISBN 978-0-12-816706-9. [Google Scholar]
- Reghunath, S.; Pinheiro, D.; Kr, S.D. A Review of Hierarchical Nanostructures of TiO2: Advances and Applications. Appl. Surf. Sci. Adv. 2021, 3, 100063. [Google Scholar] [CrossRef]
- Lee, J.; Hwan Ko, S. Fundamentals of Hierarchical Nanostructures. In Hierarchical Nanostructures for Energy Devices; Ko, S.H., Grigoropoulos, C.P., Eds.; The Royal Society of Chemistry: London, UK, 2014; pp. 7–25. ISBN 978-1-84973-628-2. [Google Scholar]
- Qin, Z.; Buehler, M.J. Hierarchical Nanostructures for Functional Materials. Nanotechnology 2018, 29, 280201. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Currás, N.; Scida, K.; Ploense, K.L.; Kippin, T.E.; Plaxco, K.W. High Surface Area Electrodes Generated via Electrochemical Roughening Improve the Signaling of Electrochemical Aptamer-Based Biosensors. Anal. Chem. 2017, 89, 12185–12191. [Google Scholar] [CrossRef] [PubMed]
- Downs, A.M.; Gerson, J.; Hossain, M.N.; Ploense, K.; Pham, M.; Kraatz, H.-B.; Kippin, T.; Plaxco, K.W. Nanoporous Gold for the Miniaturization of In Vivo Electrochemical Aptamer-Based Sensors. ACS Sens. 2021, 6, 2299–2306. [Google Scholar] [CrossRef]
- Antonelli, D.M.; Ying, J.Y. Synthesis of Hexagonally Packed Mesoporous TiO2 by a Modified Sol–Gel Method. Angew. Chem. Int. Ed. Engl. 1995, 34, 2014–2017. [Google Scholar] [CrossRef]
- Gong, D.; Grimes, C.A.; Varghese, O.K.; Hu, W.; Singh, R.S.; Chen, Z.; Dickey, E.C. Titanium Oxide Nanotube Arrays Prepared by Anodic Oxidation. J. Mater. Res. 2001, 16, 3331–3334. [Google Scholar] [CrossRef]
- Macak, J.M.; Zlamal, M.; Krysa, J.; Schmuki, P. Self-Organized TiO2 Nanotube Layers as Highly Efficient Photocatalysts. Small 2007, 3, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Teodorescu-Soare, C.T.; Catrinescu, C.; Dobromir, M.; Stoian, G.; Arvinte, A.; Luca, D. Growth and Characterization of TiO2 Nanotube Arrays under Dynamic Anodization. Photocatalytic Activity. J. Electroanal. Chem. 2018, 823, 388–396. [Google Scholar] [CrossRef]
- Querebillo, C.J.; Öner, I.H.; Hildebrandt, P.; Ly, K.H.; Weidinger, I.M. Accelerated Photo-Induced Degradation of Benzidine-p-Aminothiophenolate Immobilized at Light-Enhancing TiO2 Nanotube Electrodes. Chem. A Eur. J. 2019, 25, 16048–16053. [Google Scholar] [CrossRef] [PubMed]
- Oener, H.I.; Querebillo, C.J.; David, C.; Gernert, U.; Walter, C.; Driess, M.; Leimkühler, S.; Ly, K.H.; Weidinger, I.M. High Electromagnetic Field Enhancement of TiO2 Nanotubes Electrodes. Angew. Chem. Int. Ed. 2018, 57, 7225–7229. [Google Scholar] [CrossRef] [PubMed]
- Kapusta-Kołodziej, J.; Chudecka, A.; Sulka, G.D. 3D Nanoporous Titania Formed by Anodization as a Promising Photoelectrode Material. J. Electroanal. Chem. 2018, 823, 221–233. [Google Scholar] [CrossRef]
- Zhang, C.; Smirnov, A.I.; Hahn, D.; Grebel, H. Surface Enhanced Raman Scattering of Biospecies on Anodized Aluminum Oxide Films. Chem. Phys. Lett. 2007, 440, 239–243. [Google Scholar] [CrossRef]
- Huang, X.; Mutlu, H.; Théato, P. The Toolbox of Porous Anodic Aluminum Oxide–Based Nanocomposites: From Preparation to Application. Colloid Polym. Sci. 2021, 299, 325–341. [Google Scholar] [CrossRef]
- Poinern, G.E.J.; Ali, N.; Fawcett, D. Progress in Nano-Engineered Anodic Aluminum Oxide Membrane Development. Materials 2011, 4, 487–526. [Google Scholar] [CrossRef] [PubMed]
- Macak, J.M.; Tsuchiya, H.; Taveira, L.; Ghicov, A.; Schmuki, P. Self-Organized Nanotubular Oxide Layers on Ti-6Al-7Nb and Ti-6Al-4V Formed by Anodization in NH4F Solutions. J. Biomed. Mater. Res. Part A 2005, 75A, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Roshchina, M.Y.; Querebillo, C.J.; Dmitrieva, E.; Voss, A.; Israel, N.; Gemming, T.; Giebeler, L.; Pilz, S.; Roeher, S.; Hoffmann, V.; et al. Corrosion Behavior of an Oxide Nanotube-Coated β-Type Ti-45Nb Implant Alloy in a Simulated Inflammatory Solution. Corros. Sci. 2024, 227, 111767. [Google Scholar] [CrossRef]
- Kumaravel, V.; Nair, K.M.; Mathew, S.; Bartlett, J.; Kennedy, J.E.; Manning, H.G.; Whelan, B.J.; Leyland, N.S.; Pillai, S.C. Antimicrobial TiO2 Nanocomposite Coatings for Surfaces, Dental and Orthopaedic Implants. Chem. Eng. J. 2021, 416, 129071. [Google Scholar] [CrossRef] [PubMed]
- O’Regan, B.; Grätzel, M. A Low-Cost, High-Efficiency Solar Cell Based on Dye-Sensitized Colloidal TiO2 Films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, Y.; Xue, G.; Yu, T.; Liu, J.; Zou, Z. Fabrication of Hierarchically Assembled Microspheres Consisting of Nanoporous ZnO Nanosheets for High-Efficiency Dye-Sensitized Solar Cells. J. Mater. Chem. 2012, 22, 14341–14345. [Google Scholar] [CrossRef]
- Yan, G.; Li, G.; Tan, H.; Gu, Y.; Li, Y. Spinel-Type Ternary Multimetal Hybrid Oxides with Porous Hierarchical Structure Grown on Ni Foam as Large-Current-Density Water Oxidation Electrocatalyst. J. Alloys Compd. 2020, 838, 155662. [Google Scholar] [CrossRef]
- Singh, K.P.; Shin, C.-H.; Lee, H.-Y.; Razmjooei, F.; Sinhamahapatra, A.; Kang, J.; Yu, J.-S. TiO2/ZrO2 Nanoparticle Composites for Electrochemical Hydrogen Evolution. ACS Appl. Nano Mater. 2020, 3, 3634–3645. [Google Scholar] [CrossRef]
- Vij, V.; Sultan, S.; Harzandi, A.M.; Meena, A.; Tiwari, J.N.; Lee, W.-G.; Yoon, T.; Kim, K.S. Nickel-Based Electrocatalysts for Energy-Related Applications: Oxygen Reduction, Oxygen Evolution, and Hydrogen Evolution Reactions. ACS Catal. 2017, 7, 7196–7225. [Google Scholar] [CrossRef]
- Brezesinski, T.; Groenewolt, M.; Antonietti, M.; Smarsly, B. Crystal-to-Crystal Phase Transition in Self-Assembled Mesoporous Iron Oxide Films. Angew. Chem. Int. Ed. 2006, 45, 781–784. [Google Scholar] [CrossRef]
- Tian, B.; Liu, X.; Yang, H.; Xie, S.; Yu, C.; Tu, B.; Zhao, D. General Synthesis of Ordered Crystallized Metal Oxide Nanoarrays Replicated by Microwave-Digested Mesoporous Silica. Adv. Mater. 2003, 15, 1370–1374. [Google Scholar] [CrossRef]
- Zakaria, M.B.; Belik, A.A.; Liu, C.-H.; Hsieh, H.-Y.; Liao, Y.-T.; Malgras, V.; Yamauchi, Y.; Wu, K.C.-W. Prussian Blue Derived Nanoporous Iron Oxides as Anticancer Drug Carriers for Magnetic-Guided Chemotherapy. Chem. Asian J. 2015, 10, 1457–1462. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Peng, M.; Wu, L.; Dong, Y.; You, G.; Duan, Y.; Yang, W.; He, L.; Liu, X. Progress in Iron Oxides Based Nanostructures for Applications in Energy Storage. Nanoscale Res. Lett. 2021, 16, 138. [Google Scholar] [CrossRef] [PubMed]
- Prakasam, H.E.; Varghese, O.K.; Paulose, M.; Mor, G.K.; Grimes, C.A. Synthesis and Photoelectrochemical Properties of Nanoporous Iron (III) Oxide by Potentiostatic Anodization. Nanotechnology 2006, 17, 4285. [Google Scholar] [CrossRef]
- Fu, K.; Chen, W.; Jiang, F.; Chen, X.; Liu, J. Research Progress of Perovskite-Based Bifunctional Oxygen Electrocatalyst in Alkaline Conditions. Molecules 2023, 28, 7114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, Y.; Zhao, X.; Liu, Z.; Chen, W. Porous Perovskite LaNiO3 Nanocubes as Cathode Catalysts for Li-O2 Batteries with Low Charge Potential. Sci. Rep. 2014, 4, 6005. [Google Scholar] [CrossRef]
- Hussain, S.; Javed, M.S.; Ullah, N.; Shaheen, A.; Aslam, N.; Ashraf, I.; Abbas, Y.; Wang, M.; Liu, G.; Qiao, G. Unique Hierarchical Mesoporous LaCrO3 Perovskite Oxides for Highly Efficient Electrochemical Energy Storage Applications. Ceram. Int. 2019, 45, 15164–15170. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, W.; Wang, H.; Yang, B. Two-Dimensional Perovskite LaNiO3 Nanosheets with Hierarchical Porous Structure for High-Rate Capacitive Energy Storage. Electrochim. Acta 2017, 258, 561–570. [Google Scholar] [CrossRef]
- Jiang, S.; Liang, F.; Zhou, W.; Shao, Z. Hierarchical Porous Cobalt-Free Perovskite Electrode for Highly Efficient Oxygen Reduction. J. Mater. Chem. 2012, 22, 16214–16218. [Google Scholar] [CrossRef]
- Cuan, J.; Zhang, D.; Xing, W.; Han, J.; Zhou, H.; Zhou, Y. Confining CsPbX3 Perovskites in a Hierarchically Porous MOF as Efficient and Stable Phosphors for White LED. Chem. Eng. J. 2021, 425, 131556. [Google Scholar] [CrossRef]
- Hu, E.; Feng, Y.; Nai, J.; Zhao, D.; Hu, Y.; Lou, X.W.D. Construction of Hierarchical Ni–Co–P Hollow Nanobricks with Oriented Nanosheets for Efficient Overall Water Splitting. Energy Environ. Sci. 2018, 11, 872–880. [Google Scholar] [CrossRef]
- Chassaing, S.; Bénéteau, V.; Pale, P. Green Catalysts Based on Zeolites for Heterocycle Synthesis. Curr. Opin. Green Sustain. Chem. 2018, 10, 35–39. [Google Scholar] [CrossRef]
- Rahimi, M.; Ng, E.-P.; Bakhtiari, K.; Vinciguerra, M.; Ahmad, H.A.; Awala, H.; Mintova, S.; Daghighi, M.; Bakhshandeh Rostami, F.; de Vries, M.; et al. Zeolite Nanoparticles for Selective Sorption of Plasma Proteins. Sci. Rep. 2015, 5, 17259. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, L.; Yu, J. Applications of Zeolites in Sustainable Chemistry. Chem 2017, 3, 928–949. [Google Scholar] [CrossRef]
- Zimmermann, N.E.R.; Haranczyk, M. History and Utility of Zeolite Framework-Type Discovery from a Data-Science Perspective. Cryst. Growth Des. 2016, 16, 3043–3048. [Google Scholar] [CrossRef]
- Fan, W.; Snyder, M.A.; Kumar, S.; Lee, P.-S.; Yoo, W.C.; McCormick, A.V.; Lee Penn, R.; Stein, A.; Tsapatsis, M. Hierarchical Nanofabrication of Microporous Crystals with Ordered Mesoporosity. Nat. Mater. 2008, 7, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Seljak, K.B.; Kocbek, P.; Gašperlin, M. Mesoporous Silica Nanoparticles as Delivery Carriers: An Overview of Drug Loading Techniques. J. Drug Deliv. Sci. Technol. 2020, 59, 101906. [Google Scholar] [CrossRef]
- Rastegari, E.; Hsiao, Y.-J.; Lai, W.-Y.; Lai, Y.-H.; Yang, T.-C.; Chen, S.-J.; Huang, P.-I.; Chiou, S.-H.; Mou, C.-Y.; Chien, Y. An Update on Mesoporous Silica Nanoparticle Applications in Nanomedicine. Pharmaceutics 2021, 13, 1067. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, T.; Shimizu, T.; Kuroda, K.; Kato, C. The Preparation of Alkyltrimethylammonium–Kanemite Complexes and Their Conversion to Microporous Materials. Bull. Chem. Soc. Jpn. 1990, 63, 988–992. [Google Scholar] [CrossRef]
- Pal, N.; Lee, J.-H.; Cho, E.-B. Recent Trends in Morphology-Controlled Synthesis and Application of Mesoporous Silica Nanoparticles. Nanomaterials 2020, 10, 2122. [Google Scholar] [CrossRef] [PubMed]
- McGrath, K.M.; Dabbs, D.M.; Yao, N.; Edler, K.J.; Aksay, I.A.; Gruner, S.M. Silica Gels with Tunable Nanopores through Templating of the L3 Phase. Langmuir 2000, 16, 398–406. [Google Scholar] [CrossRef]
- Izquierdo-Barba, I.; Ruiz-González, L.; Doadrio, J.C.; González-Calbet, J.M.; Vallet-Regí, M. Tissue Regeneration: A New Property of Mesoporous Materials. Solid State Sci. 2005, 7, 983–989. [Google Scholar] [CrossRef]
- Huang, L.; Ma, J.; Wang, X.; Zhang, P.; Yu, L.; Zhang, S. Mesoporous Silica Nanoparticles-Loaded Methyl 3-(3,5-Di- Tert -Butyl-4-Hydroxyphenyl) Propanoate as a Smart Antioxidant of Synthetic Ester Oil. Tribol. Int. 2018, 121, 114–120. [Google Scholar] [CrossRef]
- Liu, M.; Li, T.; Zhang, C.; Zheng, Y.; Wu, C.; Zhang, J.; Zhang, K.; Zhang, Z. Fluorescent Carbon Dots Embedded in Mesoporous Silica Nanospheres: A Simple Platform for Cr(VI) Detection in Environmental Water. J. Hazard. Mater. 2021, 415, 125699. [Google Scholar] [CrossRef] [PubMed]
- Pal, N.; Cho, E.-B.; Kim, D. Synthesis of Ordered Mesoporous Silica/Ceria–Silica Composites and Their High Catalytic Performance for Solvent-Free Oxidation of Benzyl Alcohol at Room Temperature. RSC Adv. 2014, 4, 9213–9222. [Google Scholar] [CrossRef]
- Mikšík, F.; Miyazaki, T.; Inada, M. Detailed Investigation on Properties of Novel Commercial Mesoporous Silica Materials. Microporous Mesoporous Mater. 2019, 289, 109644. [Google Scholar] [CrossRef]
- Armstrong, E.; O’Dwyer, C. Artificial Opal Photonic Crystals and Inverse Opal Structures—Fundamentals and Applications from Optics to Energy Storage. J. Mater. Chem. C 2015, 3, 6109–6143. [Google Scholar] [CrossRef]
- Fathi, F.; Monirinasab, H.; Ranjbary, F.; Nejati-Koshki, K. Inverse Opal Photonic Crystals: Recent Advances in Fabrication Methods and Biological Applications. J. Drug Deliv. Sci. Technol. 2022, 72, 103377. [Google Scholar] [CrossRef]
- Jiang, Y.; Zheng, P.; Li, D.; Zhou, L.; Tian, L.; Wang, J.; Yang, B.; Wang, X.; Zhang, X.; Gao, J. Enzyme-Containing Silica Inverse Opals Prepared by Using Water-Soluble Colloidal Crystal Templates: Characterization and Application. Biochem. Eng. J. 2016, 112, 123–129. [Google Scholar] [CrossRef]
- Pham, K.; Temerov, F.; Saarinen, J.J. Multicompound Inverse Opal Structures with Gold Nanoparticles for Visible Light Photocatalytic Activity. Mater. Des. 2020, 194, 108886. [Google Scholar] [CrossRef]
- Sakamoto, J.S.; Dunn, B. Hierarchical Battery Electrodes Based on Inverted Opal Structures. J. Mater. Chem. 2002, 12, 2859–2861. [Google Scholar] [CrossRef]
- Von Freymann, G.; Kitaev, V.; Lotsch, B.V.; Ozin, G.A. Bottom-up Assembly of Photonic Crystals. Chem. Soc. Rev. 2013, 42, 2528–2554. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, H.; Lin, R.; Ai, Y.; Lan, K.; Duan, L.; Chen, W.; Duan, X.; Ma, B.; Wang, C.; et al. Remodeling Nanodroplets into Hierarchical Mesoporous Silica Nanoreactors with Multiple Chambers. Nat. Commun. 2022, 13, 6136. [Google Scholar] [CrossRef] [PubMed]
- Brothers, A.H.; Dunand, D.C. Porous and Foamed Amorphous Metals. MRS Bull. 2007, 32, 639–643. [Google Scholar] [CrossRef][Green Version]
- Jones, J.R.; Lee, P.D.; Hench, L.L. Hierarchical Porous Materials for Tissue Engineering. Phil. Trans. R. Soc. A 2006, 364, 263–281. [Google Scholar] [CrossRef] [PubMed]
- Li, T.H.; Wong, P.C.; Chang, S.F.; Tsai, P.H.; Jang, J.S.C.; Huang, J.C. Biocompatibility Study on Ni-Free Ti-Based and Zr-Based Bulk Metallic Glasses. Mater. Sci. Eng. C 2017, 75, 1–6. [Google Scholar] [CrossRef]
- Du, P.; Li, B.; Chen, J.; Li, K.; Xie, G. Novel Ti-Based Bulk Metallic Glass Free of Toxic and Noble Elements for Bio-Implant Applications. J. Alloys Compd. 2023, 934, 167996. [Google Scholar] [CrossRef]
- Trexler, M.M.; Thadhani, N.N. Mechanical Properties of Bulk Metallic Glasses. Prog. Mater. Sci. 2010, 55, 759–839. [Google Scholar] [CrossRef]
- Inoue, A.; Takeuchi, A. Recent Development and Application Products of Bulk Glassy Alloys. Acta Mater. 2011, 59, 2243–2267. [Google Scholar] [CrossRef]
- Liu, Y.; Pang, S.; Li, H.; Hu, Q.; Chen, B.; Zhang, T. Formation and Properties of Ti-Based Ti–Zr–Cu–Fe–Sn–Si Bulk Metallic Glasses with Different (Ti + Zr)/Cu Ratios for Biomedical Application. Intermetallics 2016, 72, 36–43. [Google Scholar] [CrossRef]
- Wang, C.; Hua, N.; Liao, Z.; Yang, W.; Pang, S.; Liaw, P.K.; Zhang, T. Ti-Cu-Zr-Fe-Sn-Si-Ag-Pd Bulk Metallic Glasses with Potential for Biomedical Applications. Met. Mater. Trans A 2021, 52, 1559–1567. [Google Scholar] [CrossRef]
- Liens, A.; Etiemble, A.; Rivory, P.; Balvay, S.; Pelletier, J.-M.; Cardinal, S.; Fabrègue, D.; Kato, H.; Steyer, P.; Munhoz, T.; et al. On the Potential of Bulk Metallic Glasses for Dental Implantology: Case Study on Ti40Zr10Cu36Pd14. Materials 2018, 11, 249. [Google Scholar] [CrossRef] [PubMed]
- Biały, M.; Hasiak, M.; Łaszcz, A. Review on Biocompatibility and Prospect Biomedical Applications of Novel Functional Metallic Glasses. J. Funct. Biomater. 2022, 13, 245. [Google Scholar] [CrossRef] [PubMed]
- Gostin, P.F.; Addison, O.; Morrell, A.P.; Zhang, Y.; Cook, A.J.M.C.; Liens, A.; Stoica, M.; Ignatyev, K.; Street, S.R.; Wu, J.; et al. In Situ Synchrotron X-Ray Diffraction Characterization of Corrosion Products of a Ti-Based Metallic Glass for Implant Applications. Adv. Healthc. Mater. 2018, 7, 1800338. [Google Scholar] [CrossRef] [PubMed]
- Blanquer, A.; Hynowska, A.; Nogués, C.; Ibáñez, E.; Sort, J.; Baró, M.D.; Özkale, B.; Pané, S.; Pellicer, E.; Barrios, L. Effect of Surface Modifications of Ti40Zr10Cu38Pd12 Bulk Metallic Glass and Ti-6Al-4V Alloy on Human Osteoblasts In Vitro Biocompatibility. PLoS ONE 2016, 11, e0156644. [Google Scholar] [CrossRef] [PubMed]
- Oak, J.-J.; Inoue, A.; Rao, K.V.; Chun, H.-H.; Park, Y.H. Surface Modified Ti Based Metallic Glasses for Bioactivation by Electrochemical Treatment Technique. J. Alloys Compd. 2014, 615, S136–S141. [Google Scholar] [CrossRef]
- Sharma, A.; Zadorozhnyy, V. Review of the Recent Development in Metallic Glass and Its Composites. Metals 2021, 11, 1933. [Google Scholar] [CrossRef]
- Zhang, C.; Li, X.; Liu, S.-Q.; Liu, H.; Yu, L.-J.; Liu, L. 3D Printing of Zr-Based Bulk Metallic Glasses and Components for Potential Biomedical Applications. J. Alloys Compd. 2019, 790, 963–973. [Google Scholar] [CrossRef]
- Shen, X.-J.; Zhang, C.; Yang, Y.-G.; Liu, L. On the Microstructure, Mechanical Properties and Wear Resistance of an Additively Manufactured Ti64/Metallic Glass Composite. Addit. Manuf. 2019, 25, 499–510. [Google Scholar] [CrossRef]
- Du, P.; Xiang, T.; Yang, X.; Xie, G. Optimization of Bioactivity and Antibacterial Properties of Porous Ti-Based Bulk Metallic Glass through Chemical Treatment. Ceram. Int. 2023, 49, 13960–13971. [Google Scholar] [CrossRef]
- Du, P.; Xiang, T.; Yang, X.; Xie, G. Enhanced Mechanical and Antibacterial Properties of Cu-Bearing Ti-Based Bulk Metallic Glass by Controlling Porous Structure. J. Alloys Compd. 2022, 904, 164005. [Google Scholar] [CrossRef]
- Zhang, P.; Tan, J.; Tian, Y.; Yan, H.; Yu, Z. Research Progress on Selective Laser Melting (SLM) of Bulk Metallic Glasses (BMGs): A Review. Int. J. Adv. Manuf. Technol. 2022, 118, 2017–2057. [Google Scholar] [CrossRef]
- Kunz, C.; Müller, F.; Gräf, S. Multifunctional Hierarchical Surface Structures by Femtosecond Laser Processing. Materials 2018, 11, 789. [Google Scholar] [CrossRef] [PubMed]
- Badria, A. Click Chemistry: A Promising Tool for Building Hierarchical Structures. Polymers 2022, 14, 4077. [Google Scholar] [CrossRef] [PubMed]
- Mishnaevsky, L.; Tsapatsis, M. Hierarchical Materials: Background and Perspectives. MRS Bull. 2016, 41, 661–664. [Google Scholar] [CrossRef]
- Huang, Y.; Zha, G.; Luo, Q.; Zhang, J.; Zhang, F.; Li, X.; Zhao, S.; Zhu, W.; Li, X. The Construction of Hierarchical Structure on Ti Substrate with Superior Osteogenic Activity and Intrinsic Antibacterial Capability. Sci. Rep. 2014, 4, 6172. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, D.; Wang, T. Hierarchical Structures of Bone and Bioinspired Bone Tissue Engineering. Small 2016, 12, 4611–4632. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Li, Y.; Liang, J.; Jiang, P.; Huang, Q.; Yang, Y.; Duan, H.; Dong, X.; Rui, G.; et al. Cell Osteogenic Bioactivity Mediated Precisely by Varying Scaled Micro-Pits on Ordered Micro/Nano Hierarchical Structures of Titanium. Regen. Biomater. 2022, 9, rbac046. [Google Scholar] [CrossRef] [PubMed]
- Öner, I.H.; David, C.; Querebillo, C.J.; Weidinger, I.M.; Ly, K.H. Electromagnetic Field Enhancement of Nanostructured TiN Electrodes Probed with Surface-Enhanced Raman Spectroscopy. Sensors 2022, 22, 487. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Zhu, Y.; Zhong, Y.; Miao, J.; Lin, B.; Zhou, W.; Shao, Z. Enabling High and Stable Electrocatalytic Activity of Iron-Based Perovskite Oxides for Water Splitting by Combined Bulk Doping and Morphology Designing. Adv. Mater. Interfaces 2019, 6, 1801317. [Google Scholar] [CrossRef]
- Hou, X.; Pan, L.; Huang, S.; Wei, O.-Y.; Chen, X. Enhanced Efficiency and Stability of Perovskite Solar Cells Using Porous Hierarchical TiO2 Nanostructures of Scattered Distribution as Scaffold. Electrochim. Acta 2017, 236, 351–358. [Google Scholar] [CrossRef]
- Lombardi, J.R.; Birke, R.L. Theory of Surface-Enhanced Raman Scattering in Semiconductors. J. Phys. Chem. C 2014, 118, 11120–11130. [Google Scholar] [CrossRef]
- Yang, H.-Y.; Rho, W.-Y.; Lee, S.; Kim, S.; Hahn, Y.-B. TiO2 Nanoparticles/Nanotubes for Efficient Light Harvesting in Perovskite Solar Cells. Nanomaterials 2019, 9, 326. [Google Scholar] [CrossRef] [PubMed]
- Pérez León, C.; Kador, L.; Peng, B.; Thelakkat, M. Characterization of the Adsorption of Ru-Bpy Dyes on Mesoporous TiO2 Films with UV-Vis, Raman, and FTIR Spectroscopies. J. Phys. Chem. B 2006, 110, 8723–8730. [Google Scholar] [CrossRef] [PubMed]
- Blackbourn, R.L.; Johnson, C.S.; Hupp, J.T. Surface Intervalence Enhanced Raman Scattering from Ferrocyanide on Colloidal Titanium Dioxide. A Mode-by-Mode Description of the Franck-Condon Barrier to Interfacial Charge Transfer. J. Am. Chem. Soc. 1991, 113, 1060–1062. [Google Scholar] [CrossRef]
- Qi, D.; Lu, L.; Wang, L.; Zhang, J. Improved SERS Sensitivity on Plasmon-Free TiO2 Photonic Microarray by Enhancing Light-Matter Coupling. J. Am. Chem. Soc. 2014, 136, 9886–9889. [Google Scholar] [CrossRef] [PubMed]
- Öner, I.H.; Querebillo, C.J.; David, C.; Gernert, U.; Walter, C.; Driess, M.; Leimkühler, S.; Ly, K.H.; Weidinger, I.M. Hohe Elektromagnetische Feldverstärkung in Nanotubularen TiO2-Elektroden. Angew. Chem. 2018, 130, 7344–7348. [Google Scholar] [CrossRef]
- Gesesse, G.D.; Li, C.; Paineau, E.; Habibi, Y.; Remita, H.; Colbeau-Justin, C.; Ghazzal, M.N. Enhanced Photogenerated Charge Carriers and Photocatalytic Activity of Biotemplated Mesoporous TiO2 Films with a Chiral Nematic Structure. Chem. Mater. 2019, 31, 4851–4863. [Google Scholar] [CrossRef]
- Zhang, X.; John, S. Enhanced Photocatalysis by Light-Trapping Optimization in Inverse Opals. J. Mater. Chem. A 2020, 8, 18974–18986. [Google Scholar] [CrossRef]
- Huo, J.; Yuan, C.; Wang, Y. Nanocomposites of Three-Dimensionally Ordered Porous TiO2 Decorated with Pt and Reduced Graphene Oxide for the Visible-Light Photocatalytic Degradation of Waterborne Pollutants. ACS Appl. Nano Mater. 2019, 2, 2713–2724. [Google Scholar] [CrossRef]
- Chen, J.I.L.; Von Freymann, G.; Choi, S.Y.; Kitaev, V.; Ozin, G.A. Slow Photons in the Fast Lane in Chemistry. J. Mater. Chem. 2008, 18, 369–373. [Google Scholar] [CrossRef]
- Sordello, F.; Duca, C.; Maurino, V.; Minero, C. Photocatalytic Metamaterials: TiO2 Inverse Opals. Chem. Commun. 2011, 47, 6147–6149. [Google Scholar] [CrossRef] [PubMed]
- Maznichenko, D.; Venkatakrishnan, K.; Tan, B. Stimulating Multiple SERS Mechanisms by a Nano Fi Brous Three- Dimensional Network Structure of Titanium Dioxide (TiO2). J. Phys. Chem. C 2013, 117, 578–583. [Google Scholar] [CrossRef]
- Han, X.X.; Köhler, C.; Kozuch, J.; Kuhlmann, U.; Paasche, L.; Sivanesan, A.; Weidinger, I.M.; Hildebrandt, P. Potential-Dependent Surface-Enhanced Resonance Raman Spectroscopy at Nanostructured TiO2: A Case Study on Cytochrome B5. Small 2013, 9, 4175–4181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lei, L.; Zhang, J.; Chen, Q.; Bao, J.; Fang, B. A Novel CdS/S-TiO2 Nanotubes Photocatalyst with High Visible Light Activity. Sep. Purif. Technol. 2009, 66, 417–421. [Google Scholar] [CrossRef]
- Shin, S.W.; Lee, J.Y.; Ahn, K.-S.; Kang, S.H.; Kim, J.H. Visible Light Absorbing TiO2 Nanotube Arrays by Sulfur Treatment for Photoelectrochemical Water Splitting. J. Phys. Chem. C 2015, 119, 13375–13383. [Google Scholar] [CrossRef]
- Feng, X.; Sloppy, J.D.; LaTempa, T.J.; Paulose, M.; Komarneni, S.; Bao, N.; Grimes, C.A. Synthesis and Deposition of Ultrafine Pt Nanoparticles within High Aspect Ratio TiO2 Nanotube Arrays: Application to the Photocatalytic Reduction of Carbon Dioxide. J. Mater. Chem. 2011, 21, 13429. [Google Scholar] [CrossRef]
- Teng, X.; Wei, M.; Yi, W.; Li, J.; Yin, M.; Xi, G. General Synthesis of Large-Area Transition Metal Nitride Porous Arrays for Highly Sensitive Surface-Enhanced Raman Scattering Substrates with Ultrahigh Durability. Small Struct. 2023, 4, 2300127. [Google Scholar] [CrossRef]
- Lu, X.; Chen, J.; Skrabalak, S.E.; Xia, Y. Galvanic Replacement Reaction: A Simple and Powerful Route to Hollow and Porous Metal Nanostructures. Proc. Inst. Mech. Eng. Part N J. Nanoeng. Nanosyst. 2007, 221, 1–16. [Google Scholar] [CrossRef]
- Fujita, T.; Qian, L.-H.; Inoke, K.; Erlebacher, J.; Chen, M.-W. Three-Dimensional Morphology of Nanoporous Gold. Appl. Phys. Lett. 2008, 92, 251902. [Google Scholar] [CrossRef]
- Carabineiro, S.A.C. Supported Gold Nanoparticles as Catalysts for the Oxidation of Alcohols and Alkanes. Front. Chem. 2019, 7, 702. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Lu, Y.; Liu, J.; Zhang, S.; Zhang, X. In Situ Monitoring of Catalytic Reaction on Single Nanoporous Gold Nanowire with Tuneable SERS and Catalytic Activity. Talanta 2020, 218, 121181. [Google Scholar] [CrossRef]
- Detsi, E.; Sellès, M.S.; Onck, P.R.; De Hosson, J.T.M. Nanoporous Silver as Electrochemical Actuator. Scr. Mater. 2013, 69, 195–198. [Google Scholar] [CrossRef]
- Jin, H.-J.; Wang, X.-L.; Parida, S.; Wang, K.; Seo, M.; Weissmüller, J. Nanoporous Au−Pt Alloys As Large Strain Electrochemical Actuators. Nano Lett. 2010, 10, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Bai, Q.; Zhang, Z. Dealloying-Driven Nanoporous Palladium with Superior Electrochemical Actuation Performance. Nanoscale 2016, 8, 7287–7295. [Google Scholar] [CrossRef] [PubMed]
- Yogeswaran, U.; Chen, S.-M. A Review on the Electrochemical Sensors and Biosensors Composed of Nanowires as Sensing Material. Sensors 2008, 8, 290–313. [Google Scholar] [CrossRef]
- Klinghammer, S.; Uhlig, T.; Patrovsky, F.; Böhm, M.; Schütt, J.; Pütz, N.; Baraban, L.; Eng, L.M.; Cuniberti, G. Plasmonic Biosensor Based on Vertical Arrays of Gold Nanoantennas. ACS Sens. 2018, 3, 1392–1400. [Google Scholar] [CrossRef] [PubMed]
- Bollella, P. Porous Gold: A New Frontier for Enzyme-Based Electrodes. Nanomaterials 2020, 10, 722. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.K.; Lee, M.-J.; Lee, S.N.; Kim, T.-H.; Oh, B.-K. Noble Metal Nanomaterial-Based Biosensors for Electrochemical and Optical Detection of Viruses Causing Respiratory Illnesses. Front. Chem. 2021, 9, 672739. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, D.; Bourgeois, L.; Wang, H.; Webley, P.A. Direct Electrodeposition of Porous Gold Nanowire Arrays for Biosensing Applications. ChemPhysChem 2009, 10, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E.; Rabaey, K. Conversion of Wastes into Bioelectricity and Chemicals by Using Microbial Electrochemical Technologies. Science 2012, 337, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Ortac, I.; Simberg, D.; Yeh, Y.; Yang, J.; Messmer, B.; Trogler, W.C.; Tsien, R.Y.; Esener, S. Dual-Porosity Hollow Nanoparticles for the Immunoprotection and Delivery of Nonhuman Enzymes. Nano Lett. 2014, 14, 3023–3032. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Sarwar, Y.; Abbasi, R.; Ishaq, H.M.; Iftikhar, M.; Hussain, I.; Demirdogen, R.E.; Ihsan, A. Nanogold Morphologies with the Same Surface Chemistry Provoke a Different Innate Immune Response: An in-Vitro and in-Vivo Study. NanoImpact 2022, 28, 100419. [Google Scholar] [CrossRef] [PubMed]
- Abeyweera, S.C.; Yu, J.; Perdew, J.P.; Yan, Q.; Sun, Y. Hierarchically 3D Porous Ag Nanostructures Derived from Silver Benzenethiolate Nanoboxes: Enabling CO2 Reduction with a Near-Unity Selectivity and Mass-Specific Current Density over 500 A/g. Nano Lett. 2020, 20, 2806–2811. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Lührs, L. Robust Metallic Actuators Based on Nanoporous Gold Rapidly Dealloyed from Gold–Nickel Precursors. Adv. Funct. Mater. 2021, 31, 2107241. [Google Scholar] [CrossRef]
- Pareek, A.; Ankah, G.N.; Cherevko, S.; Ebbinghaus, P.; Mayrhofer, K.J.J.; Erbe, A.; Renner, F.U. Effect of Thiol Self-Assembled Monolayers and Plasma Polymer Films on Dealloying of Cu–Au Alloys. RSC Adv. 2013, 3, 6586. [Google Scholar] [CrossRef]
- Ateya, B.G.; Geh, G.; Carim, A.H.; Pickering, H.W. Selective Dissolution below the Critical Potential and Back Alloying in Copper-Gold Alloy. J. Electrochem. Soc. 2002, 149, B27. [Google Scholar] [CrossRef]
- Erlebacher, J.; Aziz, M.J.; Karma, A.; Dimitrov, N.; Sieradzki, K. Evolution of Nanoporosity in Dealloying. Nature 2001, 410, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Ankah, G.N.; Pareek, A.; Cherevko, S.; Zegenhagen, J.; Renner, F.U. Hierarchical Nanoporous Films Obtained by Surface Cracking on Cu-Au and Ethanethiol on Au(001). Electrochim. Acta 2014, 140, 352–358. [Google Scholar] [CrossRef]
- Ding, Y.; Chen, M. Nanoporous Metals for Catalytic and Optical Applications. MRS Bull. 2009, 34, 569–576. [Google Scholar] [CrossRef]
- Sun, H.; Mowbray, D.J.; Migani, A.; Zhao, J.; Petek, H.; Rubio, A. Comparing Quasiparticle H2O Level Alignment on Anatase and Rutile TiO2. ACS Catal. 2015, 5, 4242–4254. [Google Scholar] [CrossRef]
- Collinson, M.M. Nanoporous Gold Electrodes and Their Applications in Analytical Chemistry. ISRN Anal. Chem. 2013, 2013, 692484. [Google Scholar] [CrossRef]
- Qi, Z.; Weissmüller, J. Hierarchical Nested-Network Nanostructure by Dealloying. ACS Nano 2013, 7, 5948–5954. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.-F.; Han, D.-Q.; Wu, Y.-F.; Guan, Y.; Bao, N.; Xu, X.-H. Fabrication and Electrochemical Property of Hierarchically Porous Au-Cu Films. Mater. Lett. 2012, 71, 108–110. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Yu, B.; Yin, K.; Zhang, Z. Hierarchically Structured Black Gold Film with Ultrahigh Porosity for Solar Steam Generation. Adv. Mater. 2022, 34, 2200108. [Google Scholar] [CrossRef] [PubMed]
- Sohn, Y.; Joo, J.B.; Kim, P. Chemically Dealloyed Pt–Au–Cu Ternary Electrocatalysts with Enhanced Stability in Electrochemical Oxygen Reduction. Res. Chem. Intermed. 2018, 44, 3697–3712. [Google Scholar] [CrossRef]
- Lee, M.N.; Santiago-Cordoba, M.A.; Hamilton, C.E.; Subbaiyan, N.K.; Duque, J.G.; Obrey, K.A.D. Developing Monolithic Nanoporous Gold with Hierarchical Bicontinuity Using Colloidal Bijels. J. Phys. Chem. Lett. 2014, 5, 809–812. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Erlebacher, J. Nanoporous Metals with Controlled Multimodal Pore Size Distribution. J. Am. Chem. Soc. 2003, 125, 7772–7773. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Searson, P.C. Single Nanoporous Gold Nanowire Sensors. J. Phys. Chem. B 2006, 110, 4318–4322. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.-H.; Park, S. Platinum-Coated, Nanoporous Gold Nanorod Arrays: Synthesis and Characterization. Adv. Mater. 2007, 19, 1612–1615. [Google Scholar] [CrossRef]
- Samanman, S.; Thammakhet, C.; Kanatharana, P.; Buranachai, C.; Thavarungkul, P. Novel Template-Assisted Fabrication of Porous Gold Nanowire Arrays Using a Conductive-Layer-Free Anodic Alumina Oxide Membrane. Electrochim. Acta 2013, 102, 342–350. [Google Scholar] [CrossRef]
- Wu, Y.; Markmann, J.; Lilleodden, E.T. Electro-Chemo-Mechanical Coupling of Nanoporous Gold at the Microscale. Appl. Phys. Lett. 2019, 115, 251602. [Google Scholar] [CrossRef]
- Schubert, I.; Huck, C.; Kröber, P.; Neubrech, F.; Pucci, A.; Toimil-Molares, M.E.; Trautmann, C.; Vogt, J. Porous Gold Nanowires: Plasmonic Response and Surface-Enhanced Infrared Absorption. Adv. Opt. Mater. 2016, 4, 1838–1845. [Google Scholar] [CrossRef]
- Awada, C.; Dab, C.; Grimaldi, M.G.; Alshoaibi, A.; Ruffino, F. High Optical Enhancement in Au/Ag Alloys and Porous Au Using Surface-Enhanced Raman Spectroscopy Technique. Sci. Rep. 2021, 11, 4714. [Google Scholar] [CrossRef] [PubMed]
- Wiriyakun, N.; Pankhlueab, K.; Boonrungsiman, S.; Laocharoensuk, R. Site-Selective Controlled Dealloying Process of Gold-Silver Nanowire Array: A Simple Approach towards Long-Term Stability and Sensitivity Improvement of SERS Substrate. Sci. Rep. 2016, 6, 39115. [Google Scholar] [CrossRef] [PubMed]
- Chandra, D.; Yang, S. Capillary-Force-Induced Clustering of Micropillar Arrays: Is It Caused by Isolated Capillary Bridges or by the Lateral Capillary Meniscus Interaction Force? Langmuir 2009, 25, 10430–10434. [Google Scholar] [CrossRef]
- Ou, F.S.; Hu, M.; Naumov, I.; Kim, A.; Wu, W.; Bratkovsky, A.M.; Li, X.; Williams, R.S.; Li, Z. Hot-Spot Engineering in Polygonal Nanofinger Assemblies for Surface Enhanced Raman Spectroscopy. Nano Lett. 2011, 11, 2538–2542. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Li, J.; Ma, C.; Wang, P.; Sun, X.; Fang, J. An Ordered Mesoporous Ag Superstructure Synthesized via a Template Strategy for Surface-Enhanced Raman Spectroscopy. Nanoscale 2015, 7, 12318–12324. [Google Scholar] [CrossRef] [PubMed]
- Capaccio, A.; Sasso, A.; Rusciano, G. A Simple and Reliable Approach for the Fabrication of Nanoporous Silver Patterns for Surface-Enhanced Raman Spectroscopy Applications. Sci. Rep. 2021, 11, 22295. [Google Scholar] [CrossRef]
- Lee, H.; Habas, S.E.; Kweskin, S.; Butcher, D.; Somorjai, G.A.; Yang, P. Morphological Control of Catalytically Active Platinum Nanocrystals. Angew. Chem. Int. Ed. 2006, 45, 7824–7828. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Malgras, V.; Alshehri, S.M.; Kim, J.H.; Yamauchi, Y. Electrochemical Synthesis of Mesoporous Pt Nanowires with Highly Electrocatalytic Activity toward Methanol Oxidation Reaction. Electrochim. Acta 2015, 183, 107–111. [Google Scholar] [CrossRef]
- Halder, A.; Patra, S.; Viswanath, B.; Munichandraiah, N.; Ravishankar, N. Porous, Catalytically Active Palladium Nanostructures by Tuning Nanoparticle Interactions in an Organic Medium. Nanoscale 2011, 3, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Weissmüller, J.; Viswanath, R.N.; Kramer, D.; Zimmer, P.; Würschum, R.; Gleiter, H. Charge-Induced Reversible Strain in a Metal. Science 2003, 300, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Jia, H.; An, C.; Wu, S.; Zhao, S.; Hu, N. Progress and Prospective of Electrochemical Actuator Materials. Compos. Part A Appl. Sci. Manuf. 2023, 165, 107336. [Google Scholar] [CrossRef]
- Kramer, D.; Viswanath, R.N.; Weissmüller, J. Surface-Stress Induced Macroscopic Bending of Nanoporous Gold Cantilevers. Nano Lett. 2004, 4, 793–796. [Google Scholar] [CrossRef]
- Zheng, G.; Pastoriza-Santos, I.; Pérez-Juste, J.; Liz-Marzán, L.M. Plasmonic Metal-organic Frameworks. SmartMat 2021, 2, 446–465. [Google Scholar] [CrossRef]
- Hansen, J.N.; Prats, H.; Toudahl, K.K.; Mørch Secher, N.; Chan, K.; Kibsgaard, J.; Chorkendorff, I. Is There Anything Better than Pt for HER? ACS Energy Lett. 2021, 6, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, X.; Rui, Y.; Wang, R.; Li, X. Recent Advances in Non-Precious Metal Electrocatalysts for pH-Universal Hydrogen Evolution Reaction. Green Energy Environ. 2021, 6, 458–478. [Google Scholar] [CrossRef]
- Cao, X.; Wang, T.; Jiao, L. Transition-Metal (Fe, Co, and Ni)-Based Nanofiber Electrocatalysts for Water Splitting. Adv. Fiber Mater. 2021, 3, 210–228. [Google Scholar] [CrossRef]
- Xie, Y.; Zhao, B.; Tang, K.; Qin, W.; Tan, C.; Yao, J.; Li, Y.; Jiang, L.; Wang, X.; Sun, Y. In-Situ Phase Transition Induced Nanoheterostructure for Overall Water Splitting. Chem. Eng. J. 2021, 409, 128156. [Google Scholar] [CrossRef]
- Zhang, M.; Shao, X.; Liu, L.; Xu, X.; Pan, J.; Hu, J. 3d Transition Metal Doping Induced Charge Rearrangement and Transfer to Enhance Overall Water-Splitting on Ni3S2 (101) Facet: A First-Principles Calculation Study. RSC Adv. 2022, 12, 26866–26874. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Z.; Ji, Y.; Yang, J.; Fan, K.; Ma, X.; Wang, C.; Shu, R.; Chen, Y. Surface Engineering Induced Hierarchical Porous Ni12P5-Ni2P Polymorphs Catalyst for Efficient Wide pH Hydrogen Production. Appl. Catal. B Environ. 2021, 282, 119609. [Google Scholar] [CrossRef]
- Baghban, A.; Habibzadeh, S.; Ashtiani, F.Z. New Insights into the Hydrogen Evolution Reaction Using Ni-ZIF8/67-Derived Electrocatalysts. Sci. Rep. 2023, 13, 8359. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Sun, Y.; Zhu, C.; Li, C.; Zhang, X.; Chen, Y. Bimetallic Ni–Mo Nitride Nanotubes as Highly Active and Stable Bifunctional Electrocatalysts for Full Water Splitting. J. Mater. Chem. A 2017, 5, 13648–13658. [Google Scholar] [CrossRef]
- Zhou, W.; Lu, X.-F.; Chen, J.-J.; Zhou, T.; Liao, P.-Q.; Wu, M.; Li, G.-R. Hierarchical Porous Prism Arrays Composed of Hybrid Ni–NiO–Carbon as Highly Efficient Electrocatalysts for Overall Water Splitting. ACS Appl. Mater. Interfaces 2018, 10, 38906–38914. [Google Scholar] [CrossRef]
- Peng, L.; Liang, Y.; Wu, S.; Li, Z.; Sun, H.; Jiang, H.; Zhu, S.; Cui, Z.; Li, L. Nanoporous Ni/NiO Catalyst for Efficient Hydrogen Evolution Reaction Prepared by Partial Electro-Oxidation after Dealloying. J. Alloys Compd. 2022, 911, 165061. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, R.; Zheng, Y.; Zhang, L.; Jiao, T.; Peng, Q.; Liu, Z. Facile Preparation of Self-Assembled Ni/Co Phosphates Composite Spheres with Highly Efficient HER Electrocatalytic Performances. Appl. Surf. Sci. 2020, 509, 145383. [Google Scholar] [CrossRef]
- Pham, N.N.T.; Kang, S.G.; Kim, H.-J.; Pak, C.; Han, B.; Lee, S.G. Catalytic Activity of Ni3Mo Surfaces for Hydrogen Evolution Reaction: A Density Functional Theory Approach. Appl. Surf. Sci. 2021, 537, 147894. [Google Scholar] [CrossRef]
- Kong, Q.; Lian, L.; Liu, Y.; Zhang, J. Hierarchical Porous Copper Materials: Fabrication and Characterisation. Micro Nano Lett. 2013, 8, 432–435. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, X.; Nomura, N.; Fujita, T. Hierarchical Nanoporous Copper Architectures via 3D Printing Technique for Highly Efficient Catalysts. Small 2019, 15, 1805432. [Google Scholar] [CrossRef] [PubMed]
- Grosu, Y.; Zaki, A.; Faik, A. Trimodal Hierarchical Nanoporous Copper with Tunable Porosity Prepared by Dealloying Mg-Cu Alloys of Close-to-Eutectic Compositions. Appl. Surf. Sci. 2019, 475, 748–753. [Google Scholar] [CrossRef]
- Liu, W.B.; Zhang, S.C.; Li, N.; Zheng, J.W.; An, S.S.; Xing, Y.L. A General Dealloying Strategy to Nanoporous Intermetallics, Nanoporous Metals with Bimodal, and Unimodal Pore Size Distributions. Corros. Sci. 2012, 58, 133–138. [Google Scholar] [CrossRef]
- Su, Y.-R.; Wu, T.-H.; Cheng, I.-C. Synthesis and Catalytical Properties of Hierarchical Nanoporous Copper from θ and η Phases in CuAl Alloys. J. Phys. Chem. Solids 2021, 151, 109915. [Google Scholar] [CrossRef]
- Li, X.; Huang, B.; Qiu, C.; Li, Z.; Shao, L.-H.; Liu, H. Hierarchical Nested-Network Porous Copper Fabricated by One-Step Dealloying for Glucose Sensing. J. Alloys Compd. 2016, 681, 109–114. [Google Scholar] [CrossRef]
- Chen, X.; Tan, F.; Wang, J.; Zhao, K.; Wang, Y.; Zhang, J.; Liu, H. The Electrochemical Actuation Performances of Nanoporous Ternary AlCoCu Alloy with a Unique Nanosheet Structure. Materials 2023, 16, 6942. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Zheng, B.; Chen, L.; Zhang, J.; Zhuang, Z.; Chen, B. MOF-Derived Formation of Ni2P–CoP Bimetallic Phosphides with Strong Interfacial Effect toward Electrocatalytic Water Splitting. ACS Appl. Mater. Interfaces 2017, 9, 23222–23229. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, Y.; Zhou, S.; Ye, Q.; Zan, X.; He, Y. Metal-Organic Framework-Based Composites for Protein Delivery and Therapeutics. ACS Biomater. Sci. Eng. 2022, 8, 4028–4038. [Google Scholar] [CrossRef] [PubMed]
- Falcaro, P.; Hill, A.J.; Nairn, K.M.; Jasieniak, J.; Mardel, J.I.; Bastow, T.J.; Mayo, S.C.; Gimona, M.; Gomez, D.; Whitfield, H.J.; et al. A New Method to Position and Functionalize Metal-Organic Framework Crystals. Nat. Commun. 2011, 2, 237. [Google Scholar] [CrossRef]
- Stoeckli, H.F.; Kraehenbuehl, F. The External Surface of Microporous Carbons, Derived from Adsorption and Immersion Studies. Carbon 1984, 22, 297–299. [Google Scholar] [CrossRef][Green Version]
- Perret, R.; Ruland, W. The Microstructure of PAN-Base Carbon Fibres. J. Appl. Crystallogr. 1970, 3, 525–532. [Google Scholar] [CrossRef]
- Boehm, H.P. Book Review: Active Carbon. Manufacture, Properties and Applications. By M. Smišek and S. Černý. Angew. Chem. Int. Ed. Engl. 1971, 10, 360. [Google Scholar] [CrossRef]
- Pitchaiya, S.; Eswaramoorthy, N.; Natarajan, M.; Santhanam, A.; Asokan, V.; Madurai Ramakrishnan, V.; Rangasamy, B.; Sundaram, S.; Ravirajan, P.; Velauthapillai, D. Perovskite Solar Cells: A Porous Graphitic Carbon Based Hole Transporter/Counter Electrode Material Extracted from an Invasive Plant Species Eichhornia Crassipes. Sci. Rep. 2020, 10, 6835. [Google Scholar] [CrossRef]
- Mukai, K.; Asaka, K.; Sugino, T.; Kiyohara, K.; Takeuchi, I.; Terasawa, N.; Futaba, D.N.; Hata, K.; Fukushima, T.; Aida, T. Highly Conductive Sheets from Millimeter-Long Single-Walled Carbon Nanotubes and Ionic Liquids: Application to Fast-Moving, Low-Voltage Electromechanical Actuators Operable in Air. Adv. Mater. 2009, 21, 1582–1585. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Y.; Cebeci, H.; De Villoria, R.G.; Lin, J.; Wardle, B.L.; Zhang, Q.M. High Electromechanical Response of Ionic Polymer Actuators with Controlled-Morphology Aligned Carbon Nanotube/Nafion Nanocomposite Electrodes. Adv. Funct. Mater. 2010, 20, 3266–3271. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Liu, J.; Hu, Y.; Zhang, Y.; Randriamahazaka, H.; Chen, W. Highly Stable Air Working Bimorph Actuator Based on a Graphene Nanosheet/Carbon Nanotube Hybrid Electrode. Adv. Mater. 2012, 24, 4317–4321. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Hu, Y.; Liu, Y.; Zhao, J.; Chen, X.; Whoehling, V.; Plesse, C.; Nguyen, G.T.M.; Vidal, F.; Chen, W. Graphitic Carbon Nitride Nanosheet Electrode-Based High-Performance Ionic Actuator. Nat. Commun. 2015, 6, 7258. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Ungureanu, S.; Birot, M.; Deleuze, H.; Schmitt, V.; Mano, N.; Backov, R. Triple Hierarchical Micro–Meso–Macroporous Carbonaceous Foams Bearing Highly Monodisperse Macroporosity. Carbon 2015, 91, 311–320. [Google Scholar] [CrossRef]
- Meng, Y.; Gu, D.; Zhang, F.; Shi, Y.; Cheng, L.; Feng, D.; Wu, Z.; Chen, Z.; Wan, Y.; Stein, A.; et al. A Family of Highly Ordered Mesoporous Polymer Resin and Carbon Structures from Organic−Organic Self-Assembly. Chem. Mater. 2006, 18, 4447–4464. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, A.; Fierro, V.; Izquierdo, M.T.; Celzard, A. Functionalized, Hierarchical and Ordered Mesoporous Carbons for High-Performance Supercapacitors. J. Mater. Chem. A 2016, 4, 6140–6148. [Google Scholar] [CrossRef]
- Zhou, P.; Zhang, J.; Song, Z.; Wang, L.; Zhang, Q. Ordered Mesoporous Carbon with Hierarchical Pore Structure for High-Efficiency Electromagnetic Wave Absorber under Thin Matching Thickness. J. Mater. Res. Technol. 2023, 25, 1560–1569. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, H.; Zhou, Y.; Qiao, H.; Gurung, A.; Naderi, R.; Elbohy, H.; Smirnova, A.L.; Lu, H.; Chen, S.; et al. Binder Free Hierarchical Mesoporous Carbon Foam for High Performance Lithium Ion Battery. Sci. Rep. 2017, 7, 1440. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Wang, H.; Liu, J.; Sun, P.; Chen, T. Hierarchically Porous and Orderly Mesostructured Carbon Nanorods with Excellent Supercapacitive Performance. ACS Appl. Nano Mater. 2022, 5, 13384–13394. [Google Scholar] [CrossRef]
- Wang, J.; Tang, J.; Ding, B.; Malgras, V.; Chang, Z.; Hao, X.; Wang, Y.; Dou, H.; Zhang, X.; Yamauchi, Y. Hierarchical Porous Carbons with Layer-by-Layer Motif Architectures from Confined Soft-Template Self-Assembly in Layered Materials. Nat. Commun. 2017, 8, 15717. [Google Scholar] [CrossRef] [PubMed]
- Bu, F.; Zagho, M.M.; Ibrahim, Y.; Ma, B.; Elzatahry, A.; Zhao, D. Porous MXenes: Synthesis, Structures, and Applications. Nano Today 2020, 30, 100803. [Google Scholar] [CrossRef]
- Ren, C.E.; Zhao, M.; Makaryan, T.; Halim, J.; Boota, M.; Kota, S.; Anasori, B.; Barsoum, M.W.; Gogotsi, Y. Porous Two-Dimensional Transition Metal Carbide (MXene) Flakes for High-Performance Li-Ion Storage. ChemElectroChem 2016, 3, 689–693. [Google Scholar] [CrossRef]
- Wang, X.; Raghupathy, R.K.M.; Querebillo, C.J.; Liao, Z.; Li, D.; Lin, K.; Hantusch, M.; Sofer, Z.; Li, B.; Zschech, E.; et al. Interfacial Covalent Bonds Regulated Electron-Deficient 2D Black Phosphorus for Electrocatalytic Oxygen Reactions. Adv. Mater. 2021, 33, 2008752. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Liu, Y.; Baek, J.-B.; Dai, L. Nitrogen-Doped Graphene as Efficient Metal-Free Electrocatalyst for Oxygen Reduction in Fuel Cells. ACS Nano 2010, 4, 1321–1326. [Google Scholar] [CrossRef]
- Jeon, I.; Zhang, S.; Zhang, L.; Choi, H.; Seo, J.; Xia, Z.; Dai, L.; Baek, J. Edge-Selectively Sulfurized Graphene Nanoplatelets as Efficient Metal-Free Electrocatalysts for Oxygen Reduction Reaction: The Electron Spin Effect. Adv. Mater. 2013, 25, 6138–6145. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Jiang, S.; Zhao, Y.; Zhu, L.; Chen, S.; Wang, X.; Wu, Q.; Ma, J.; Ma, Y.; Hu, Z. Boron-Doped Carbon Nanotubes as Metal-Free Electrocatalysts for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2011, 50, 7132–7135. [Google Scholar] [CrossRef]
- Paek, E.; Pak, A.J.; Kweon, K.E.; Hwang, G.S. On the Origin of the Enhanced Supercapacitor Performance of Nitrogen-Doped Graphene. J. Phys. Chem. C 2013, 117, 5610–5616. [Google Scholar] [CrossRef]
- Lee, W.J.; Maiti, U.N.; Lee, J.M.; Lim, J.; Han, T.H.; Kim, S.O. Nitrogen-Doped Carbon Nanotubes and Graphene Composite Structures for Energy and Catalytic Applications. Chem. Commun. 2014, 50, 6818. [Google Scholar] [CrossRef]
- Pei, Y.; Zhao, J.; Shi, R.; Wang, X.; Li, Z.; Ren, J. Hierarchical Porous Carbon-Supported Copper Nanoparticles as an Efficient Catalyst for the Dimethyl Carbonate Synthesis. Catal. Lett. 2019, 149, 3184–3193. [Google Scholar] [CrossRef]
- Tang, M.; Deng, J.; Li, M.; Li, X.; Li, H.; Chen, Z.; Wang, Y. 3D-Interconnected Hierarchical Porous N-Doped Carbon Supported Ruthenium Nanoparticles as an Efficient Catalyst for Toluene and Quinoline Hydrogenation. Green Chem. 2016, 18, 6082–6090. [Google Scholar] [CrossRef]
- Ramalingam, M.; Jaisankar, A.; Cheng, L.; Krishnan, S.; Lan, L.; Hassan, A.; Sasmazel, H.T.; Kaji, H.; Deigner, H.-P.; Pedraz, J.L.; et al. Impact of Nanotechnology on Conventional and Artificial Intelligence-Based Biosensing Strategies for the Detection of Viruses. Discov. Nano 2023, 18, 58. [Google Scholar] [CrossRef]
- Tan, P.; Chen, X.; Zhang, H.; Wei, Q.; Luo, K. Artificial Intelligence Aids in Development of Nanomedicines for Cancer Management. Semin. Cancer Biol. 2023, 89, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Cui, H.; Zhang, H.; Wang, F.; Zhan, X.; Mayer, F.; Nestler, B.; Wegener, M.; Levkin, P.A. 3D Printing of Inherently Nanoporous Polymers via Polymerization-Induced Phase Separation. Nat. Commun. 2021, 12, 247. [Google Scholar] [CrossRef]
- Mayer, F.; Ryklin, D.; Wacker, I.; Curticean, R.; Čalkovský, M.; Niemeyer, A.; Dong, Z.; Levkin, P.A.; Gerthsen, D.; Schröder, R.R.; et al. 3D Two-Photon Microprinting of Nanoporous Architectures. Adv. Mater. 2020, 32, 2002044. [Google Scholar] [CrossRef] [PubMed]
- Demirörs, A.F.; Poloni, E.; Chiesa, M.; Bargardi, F.L.; Binelli, M.R.; Woigk, W.; De Castro, L.D.C.; Kleger, N.; Coulter, F.B.; Sicher, A.; et al. Three-Dimensional Printing of Photonic Colloidal Glasses into Objects with Isotropic Structural Color. Nat. Commun. 2022, 13, 4397. [Google Scholar] [CrossRef]
- Moore, D.G.; Barbera, L.; Masania, K.; Studart, A.R. Three-Dimensional Printing of Multicomponent Glasses Using Phase-Separating Resins. Nat. Mater. 2020, 19, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Duda, T.; Raghavan, L.V. 3D Metal Printing Technology: The Need to Re-Invent Design Practice. AI Soc. 2018, 33, 241–252. [Google Scholar] [CrossRef]
- Park, S.H.; Su, R.; Jeong, J.; Guo, S.; Qiu, K.; Joung, D.; Meng, F.; McAlpine, M.C. 3D Printed Polymer Photodetectors. Adv. Mater. 2018, 30, 1803980. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.L.; Tamargo, I.A.; Kim, H.; Johnson, B.N.; Gupta, M.K.; Koh, T.-W.; Chin, H.-A.; Steingart, D.A.; Rand, B.P.; McAlpine, M.C. 3D Printed Quantum Dot Light-Emitting Diodes. Nano Lett. 2014, 14, 7017–7023. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Zhang, B.; Wang, W.; Ye, F.; Yue, S.; Guo, H.; Gao, G.; Zhao, Y.; Fang, Q.; Nguyen, C.; et al. 3D-Printed Silica with Nanoscale Resolution. Nat. Mater. 2021, 20, 1506–1511. [Google Scholar] [CrossRef]
- Zhou, N.; Bekenstein, Y.; Eisler, C.N.; Zhang, D.; Schwartzberg, A.M.; Yang, P.; Alivisatos, A.P.; Lewis, J.A. Perovskite Nanowire–Block Copolymer Composites with Digitally Programmable Polarization Anisotropy. Sci. Adv. 2019, 5, eaav8141. [Google Scholar] [CrossRef] [PubMed]
- Aubert, T.; Huang, J.-Y.; Ma, K.; Hanrath, T.; Wiesner, U. Porous Cage-Derived Nanomaterial Inks for Direct and Internal Three-Dimensional Printing. Nat. Commun. 2020, 11, 4695. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-Y.; Xu, H.; Peretz, E.; Wu, D.-Y.; Ober, C.K.; Hanrath, T. Three-Dimensional Printing of Hierarchical Porous Architectures. Chem. Mater. 2019, 31, 10017–10022. [Google Scholar] [CrossRef]
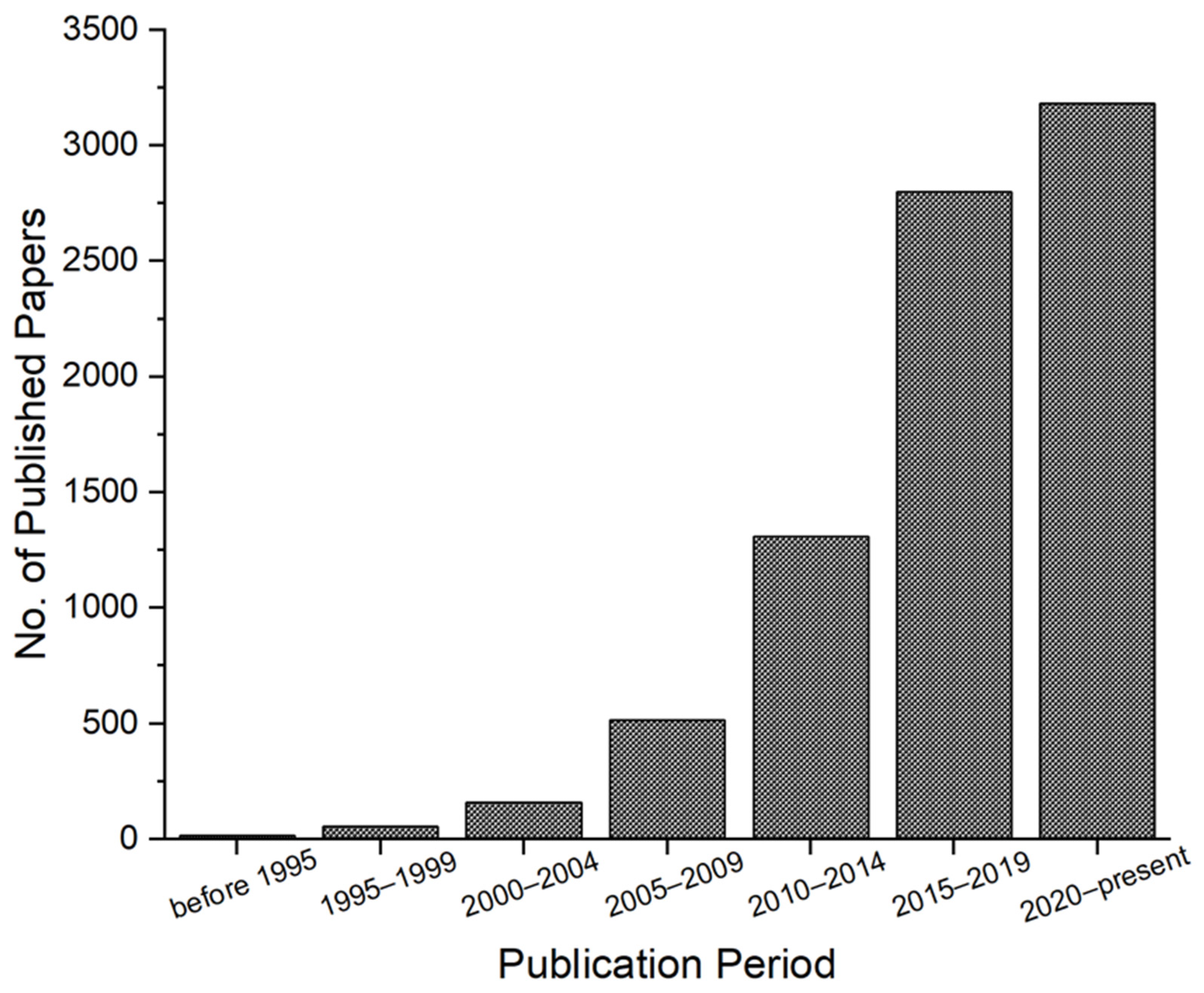
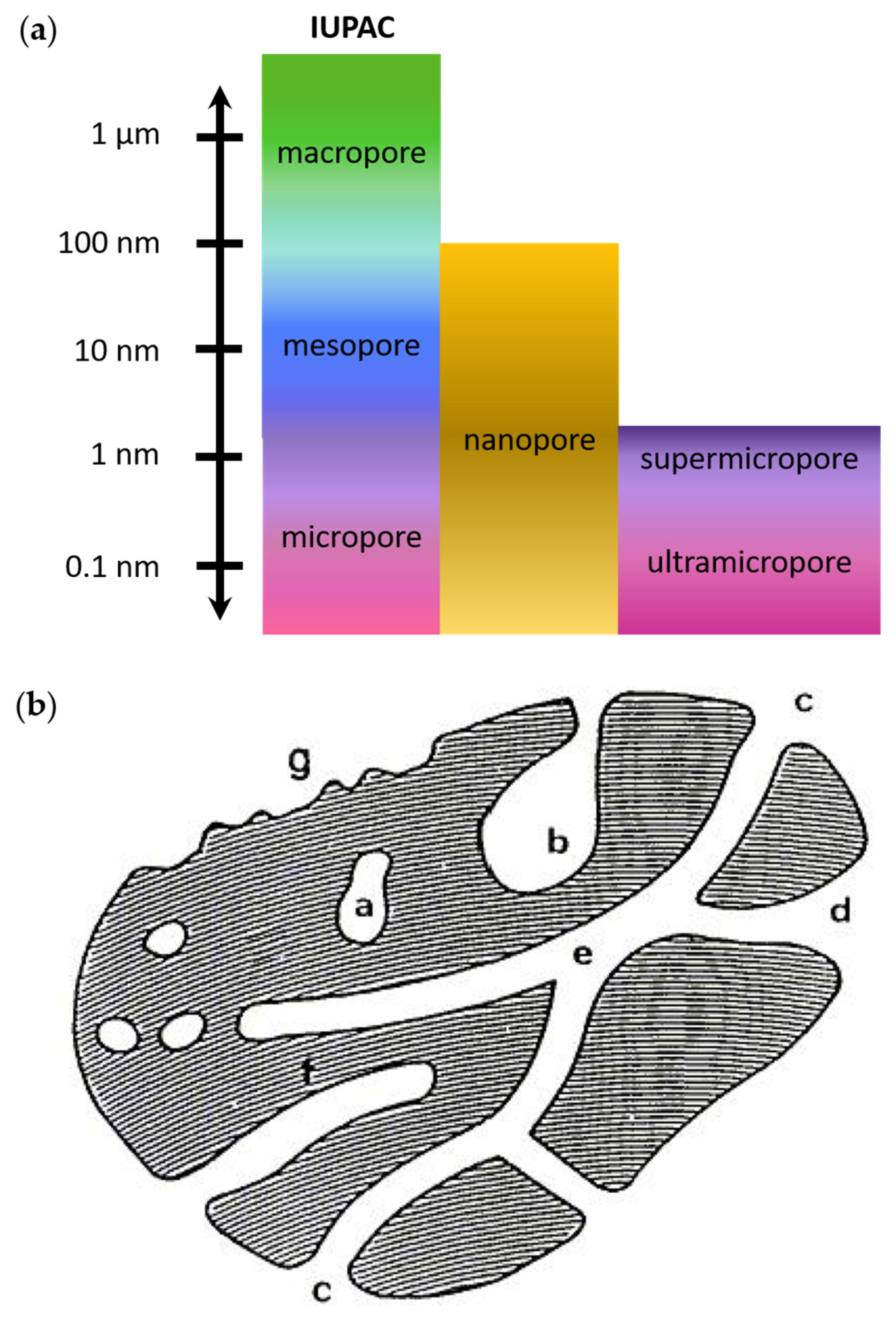
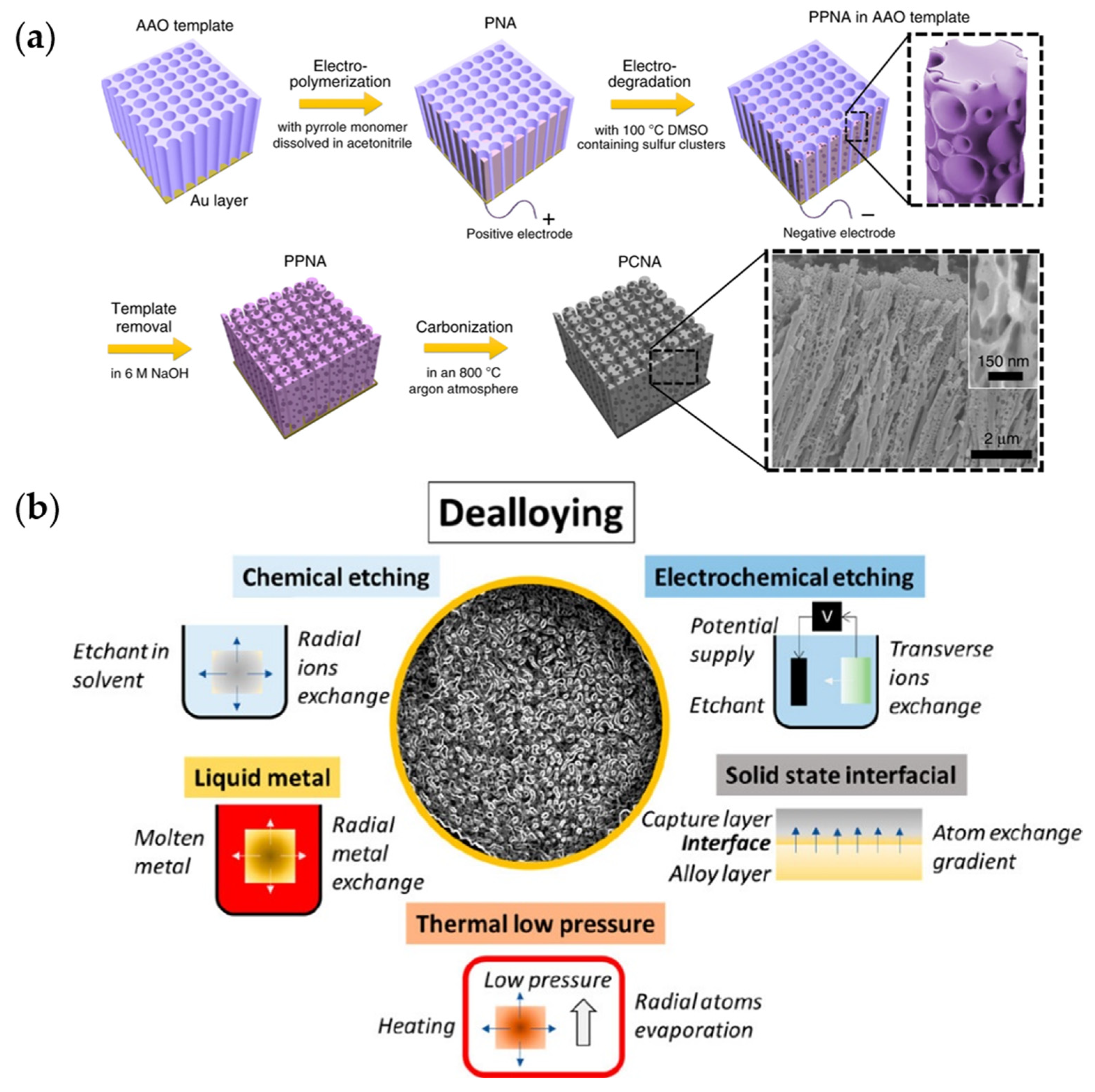



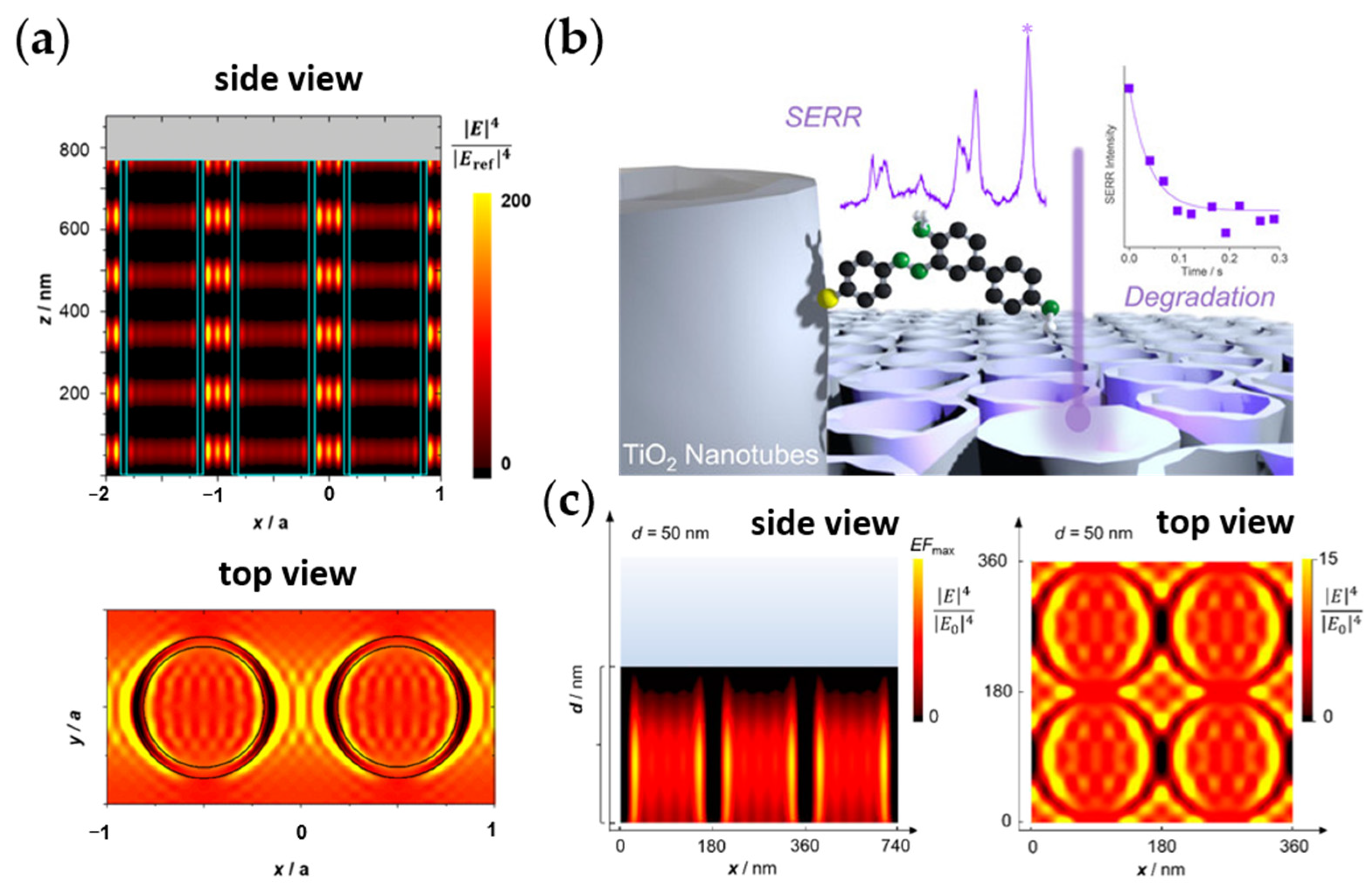
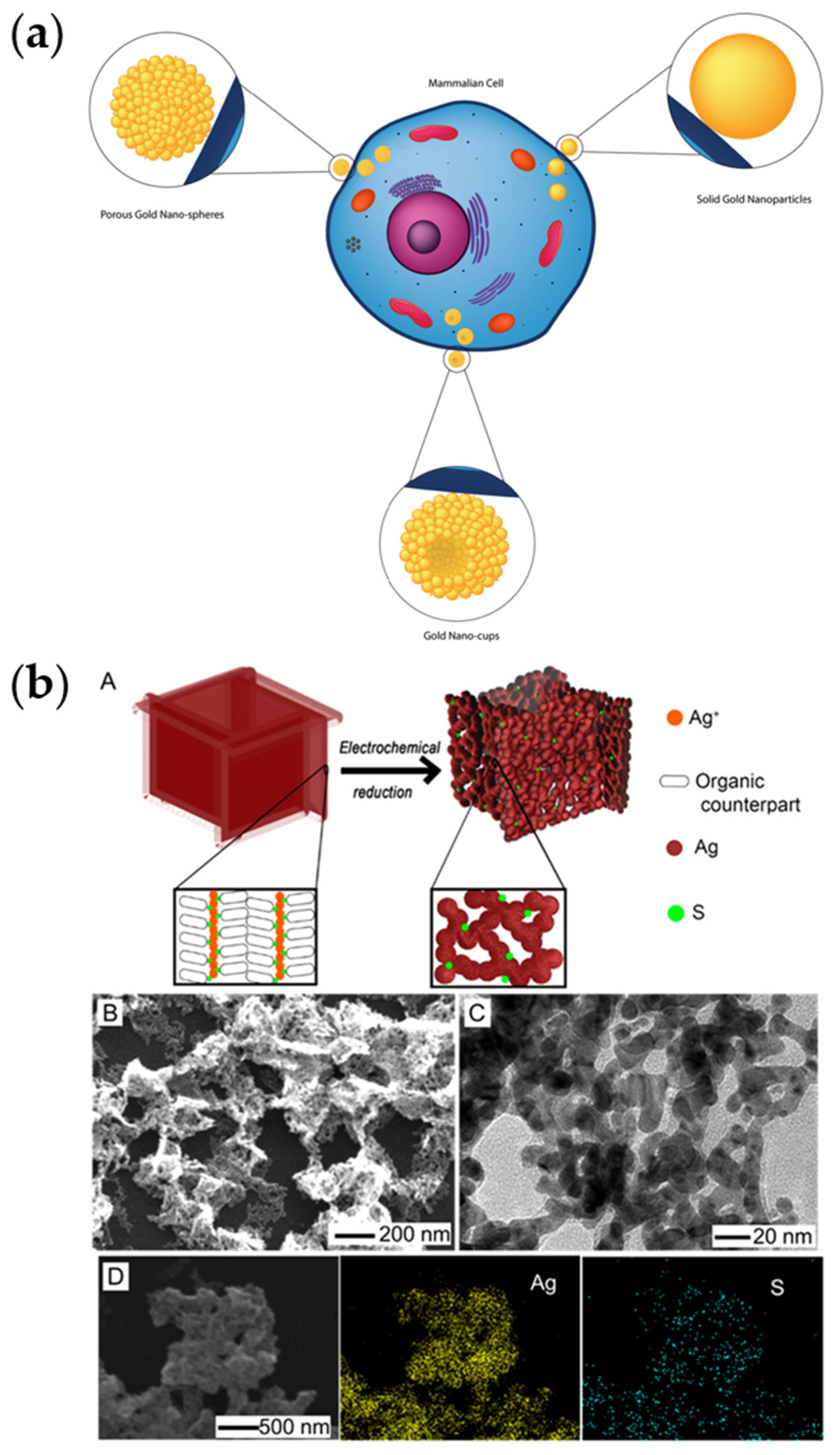
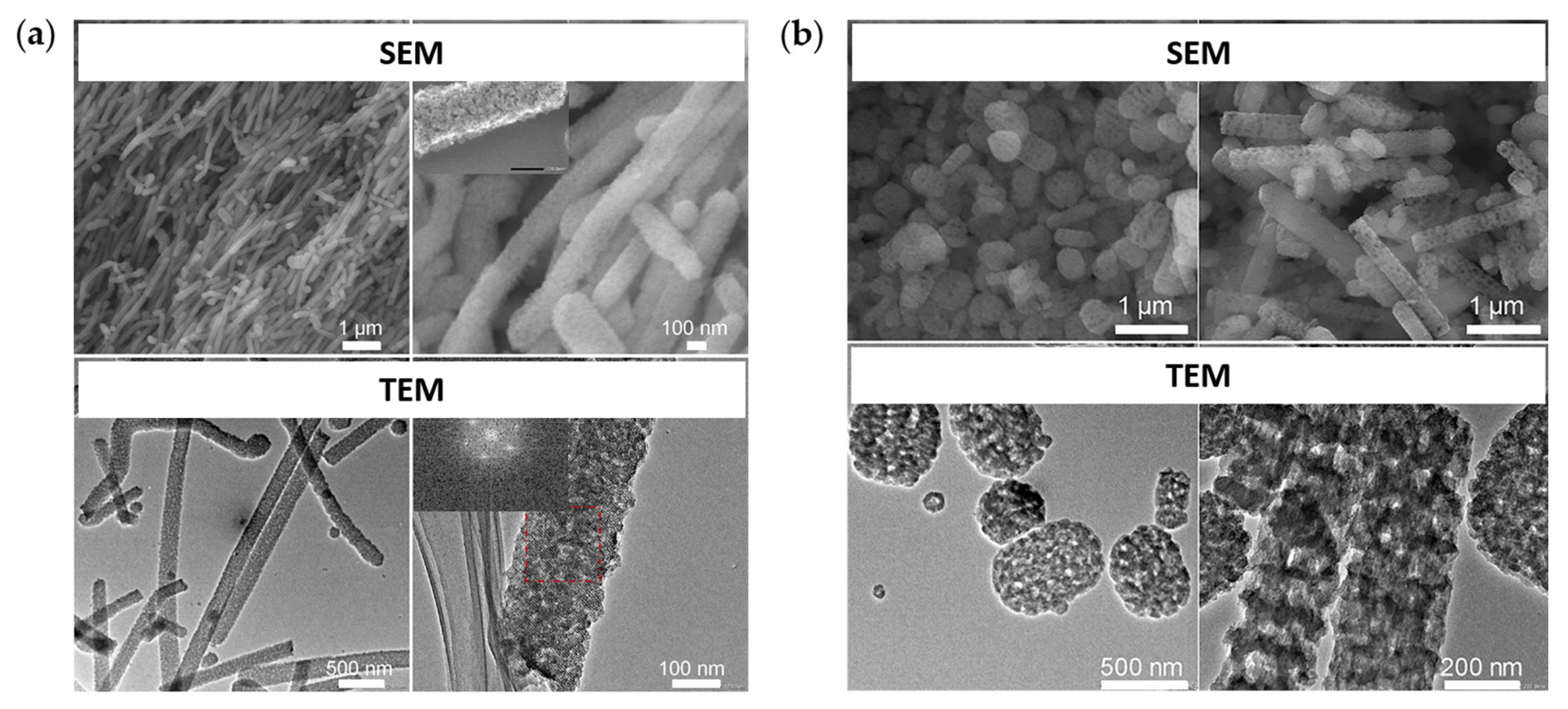
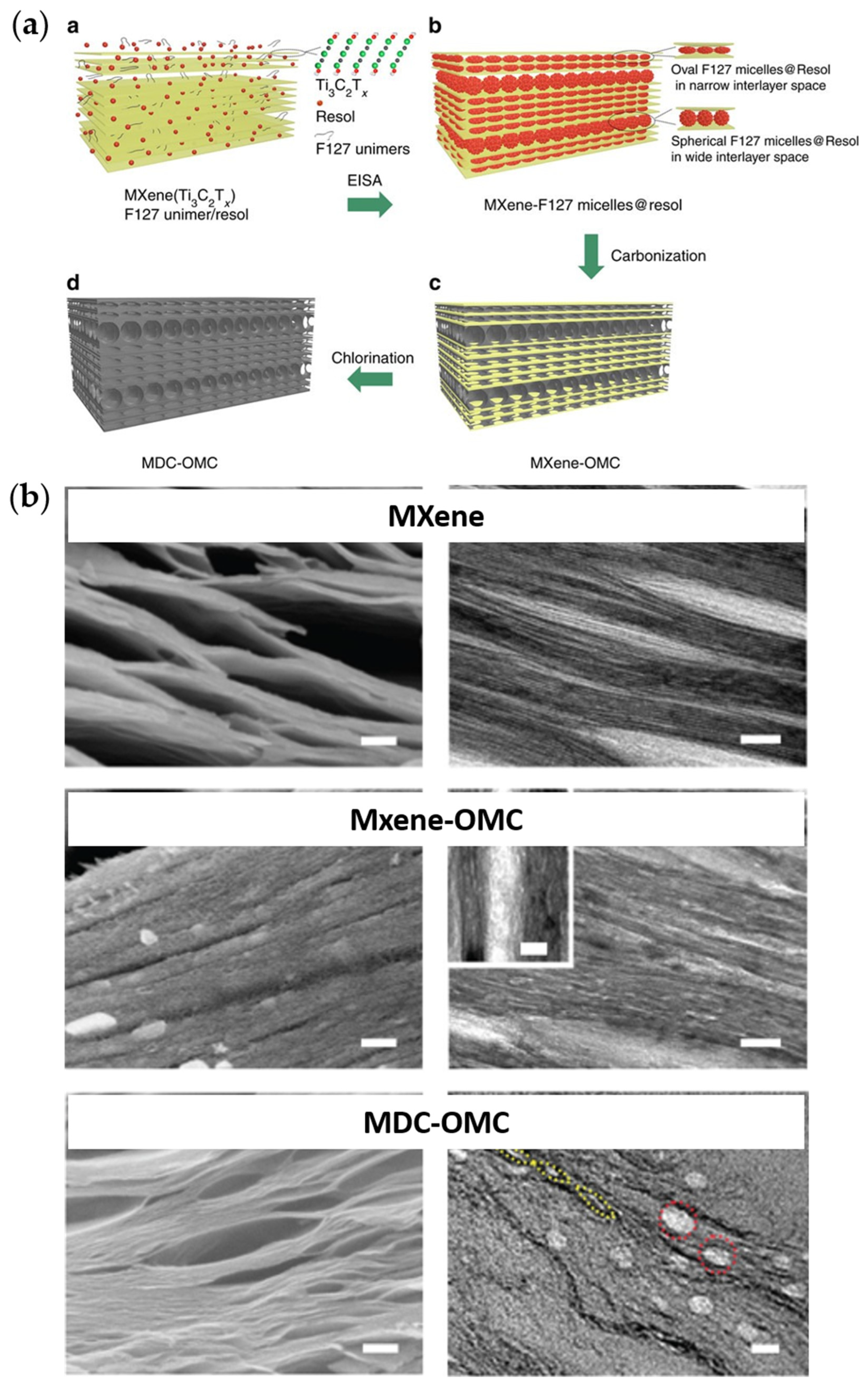
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jose, A.; Mathew, T.; Fernández-Navas, N.; Querebillo, C.J. Porous Inorganic Nanomaterials: Their Evolution towards Hierarchical Porous Nanostructures. Micro 2024, 4, 229-280. https://doi.org/10.3390/micro4020016
Jose A, Mathew T, Fernández-Navas N, Querebillo CJ. Porous Inorganic Nanomaterials: Their Evolution towards Hierarchical Porous Nanostructures. Micro. 2024; 4(2):229-280. https://doi.org/10.3390/micro4020016
Chicago/Turabian StyleJose, Anitta, Tom Mathew, Nora Fernández-Navas, and Christine Joy Querebillo. 2024. "Porous Inorganic Nanomaterials: Their Evolution towards Hierarchical Porous Nanostructures" Micro 4, no. 2: 229-280. https://doi.org/10.3390/micro4020016
APA StyleJose, A., Mathew, T., Fernández-Navas, N., & Querebillo, C. J. (2024). Porous Inorganic Nanomaterials: Their Evolution towards Hierarchical Porous Nanostructures. Micro, 4(2), 229-280. https://doi.org/10.3390/micro4020016






