Roles of Polymerization Temperature and Initiator Type on Thermal Properties of Rubitherm® 21 PCM Microcapsules
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
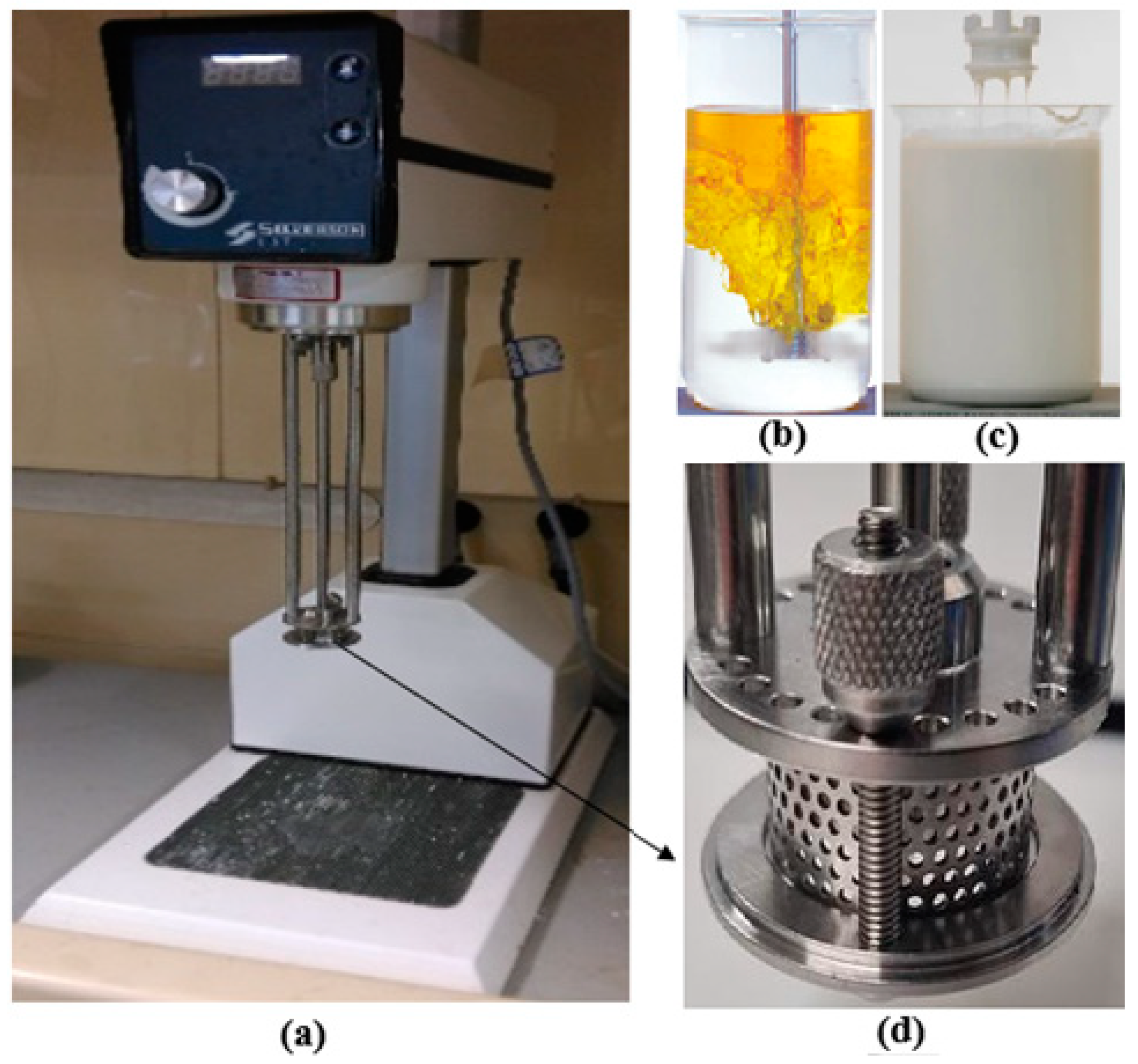
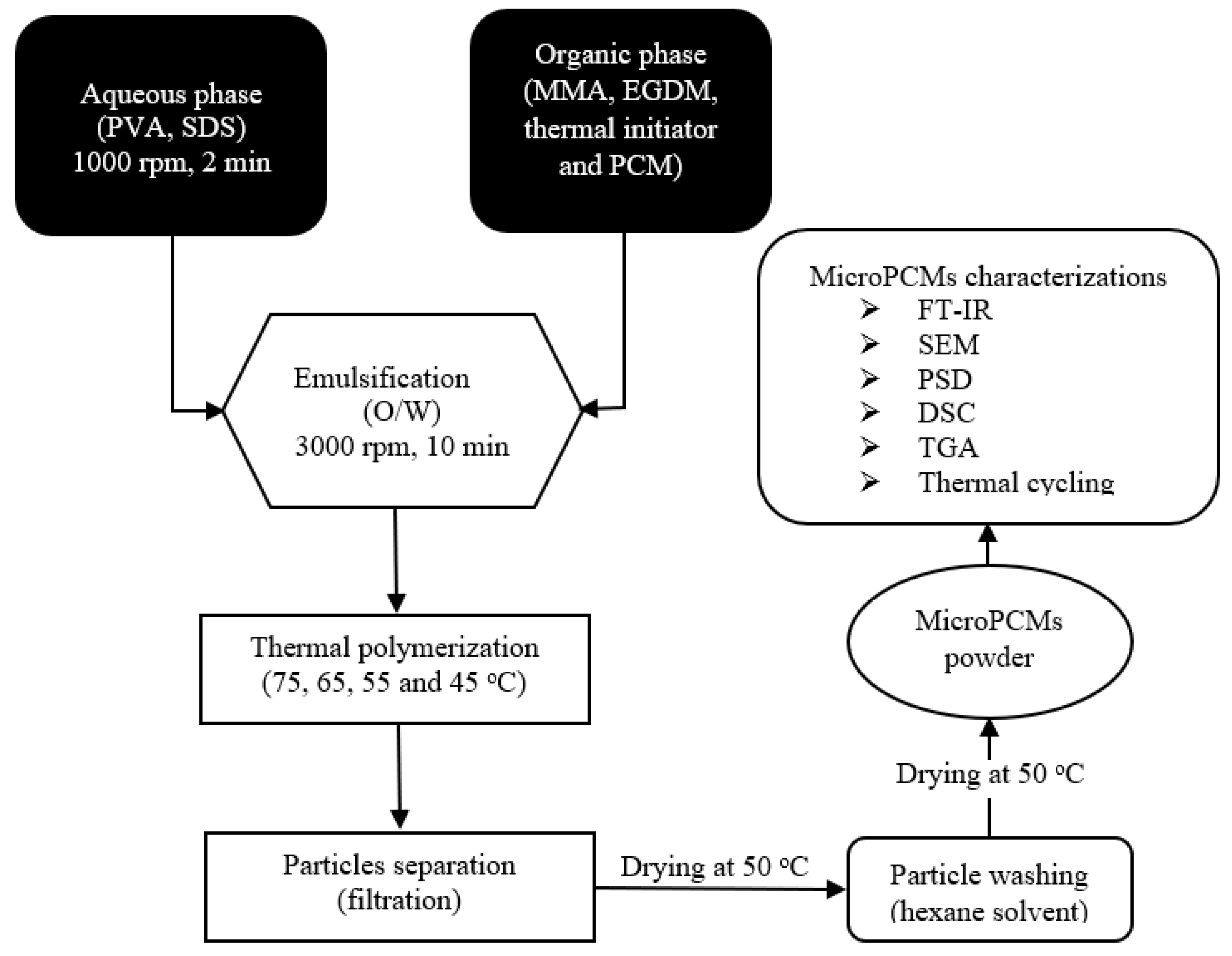
2.2. Preparation of MicroPCMs
2.2.1. Emulsification
2.2.2. Polymerization
2.3. Characterizations of MicroPCMs
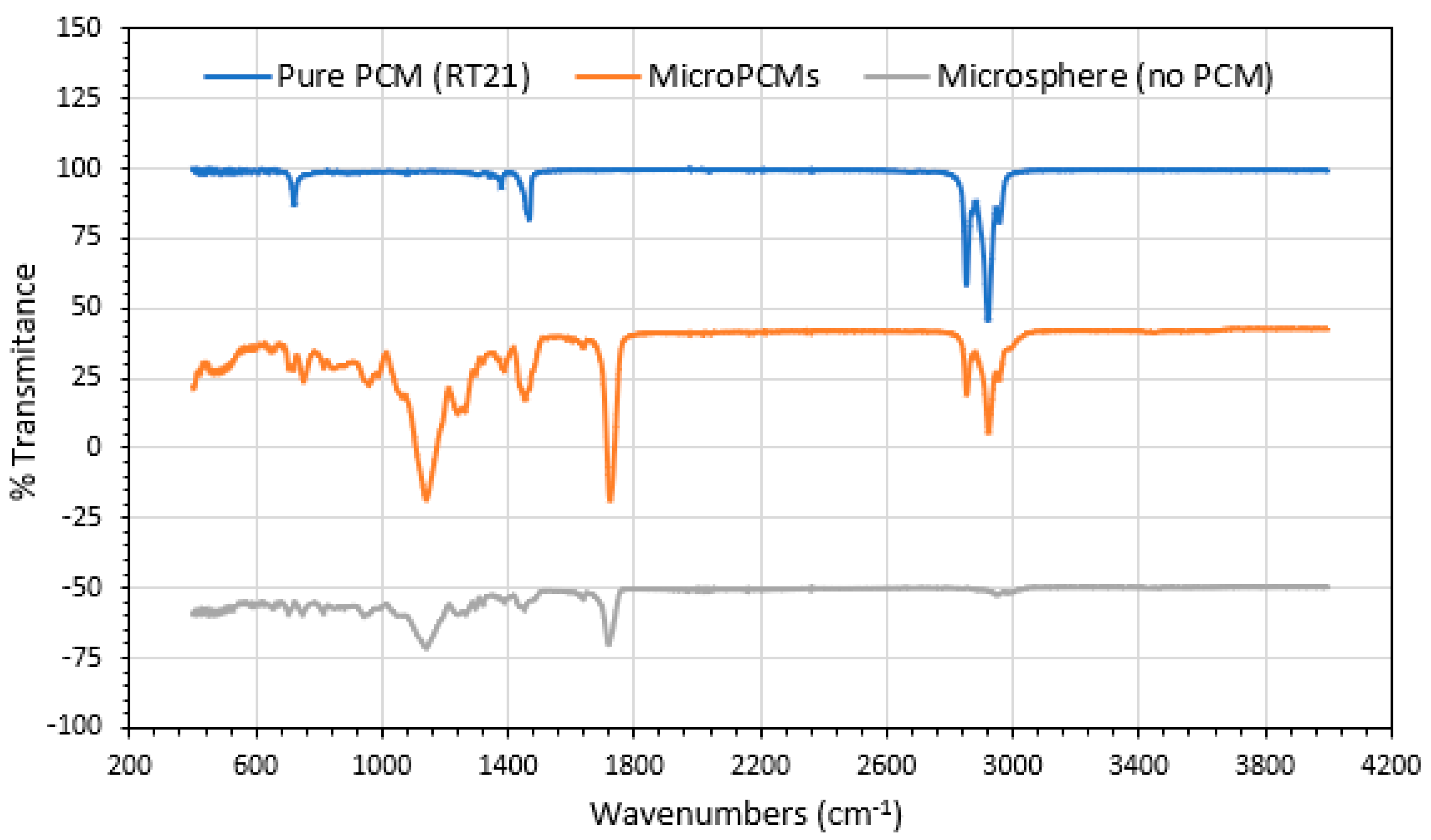
2.3.1. Fourier Transformed Infrared Spectroscopy (FT-IR)
2.3.2. Optical Microscopy (OP) and Scanning Electron Microscopy (SEM)
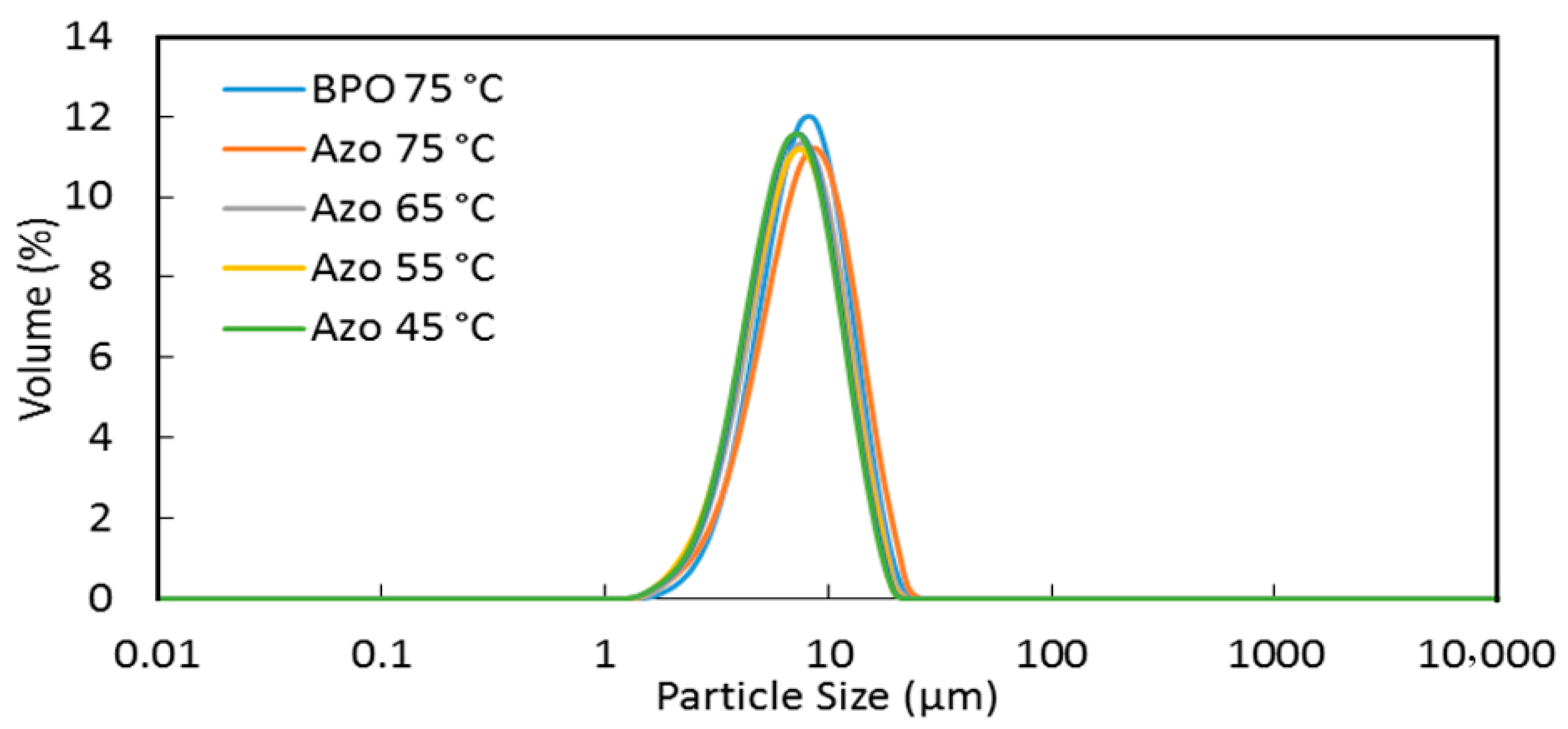
2.3.3. Particle Size Distribution (PSD)
2.3.4. Differential Scanning Calorimetry (DSC)
2.3.5. Thermal Gravimetric Analyzer (TGA)
2.3.6. Thermal Cycling
3. Results
3.1. MicroPCMs Formation
3.2. Morphology and Size of the MicroPCMs
3.3. Thermal Properties of the MicroPCMs
3.4. Thermal Stability and Core Content
3.5. Thermal Reliability
3.6. Comparing the Results of This Study with Related Publications
4. Applications of MicroPCMs
| Microencapsulation Technique | Core Material | Shell Material | Polymerization Temperature | Peak Melting Temperature (Tamp) °C | Latent Heat of Melting (J/g) | PCM Content (%) |
|---|---|---|---|---|---|---|
| in situ polymerization | eutectic mixture (75% SA + 25% CA) | melamine formaldehyde (MF) | 70 | 34.5 | 103.9 | 85.3 |
| in situ polymerization | C18 paraffin | melamine formaldehyde (MF) | 70 | 20.5 | 164.8 | 84.3 |
| interfacial polymerization and the sol–gel process | SCD-DHPD eutectic PCM | Inorganic Silica shell | 40 | 53.1 °C. | 97.7 | 45.8 |
| coacervation | poly (octadecyl acrylate) | natural chitosan | 85 | 32.9–47.3 | 92.9–131.4 | 49.8–69.0 |
| interfacial polymerization | butyl stearate | chitosan-based polyurethane (c-PU) | 50–90 | 21 | 106.3 | 71.4 |
| emulsion polymerization technique | paraffin | titanium dioxide (TiO2)-modified chitosan (CS) | 40 | 125.5 | 125.5 | 81.3 |
| suspension-like polymerization | n-octadecane | poly (methyl-methacrylate) doped with titanium dioxide nanoparticles | 80 | 4.5 | 89.0–153.8 | 43.10–73.20 |
| suspension-like polymerization | pure temp (PT) 6 | cross-linked polymethyl methacrylate | 30 | 8.2 | 131.10 | 87.40 |
| suspension-like polymerization | butyl stearate | comb-like acrylic co-polymer | 85 | 23.5 | 102.3 | 86.6 |
| in situ polymerization (commercially available microcapsules) | paraffin and bio-base methyl ester | melamine based capsules | 70 | −10, 6, 18, 24, 28, 29, 32, 37 | ~150–205 | - |
| suspension-like polymerization (This work) | paraffin (RT21) | cross-linked polymethyl methacrylate | 45 | 25.6 | 107.8 | 82.0 |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santamouris, M.; Vasilakopoulou, K. Present and future energy consumption of buildings: Challenges and opportunities towards decarbonisation. e-Prime—Adv. Electr. Eng. Electron. Energy 2021, 1, 100002. [Google Scholar] [CrossRef]
- González-Torres, M.; Pérez-Lombard, L.; Coronel, J.F.; Maestre, I.R.; Yan, D. A review on buildings energy information: Trends, end-uses, fuels and drivers. Energy Rep. 2022, 8, 626–637. [Google Scholar] [CrossRef]
- Yilin, L.; Han, M.; Liu, S.Y.; Chen, G.Q. Energy consumption and greenhouse gas emissions by buildings: A multi-scale perspective. Build. Environ. 2019, 151, 240–250. [Google Scholar] [CrossRef]
- Vakiloroaya, V.; Samali, B.; Fakhar, A.; Pishghadam, K. A review of different strategies for HVAC energy saving. Energy Convers. Manag. 2014, 77, 738–754. [Google Scholar] [CrossRef]
- Ürge-Vorsatz, D.; Cabeza, L.F.; Serrano, S.; Barreneche, C.; Petrichenko, K. Heating and cooling energy trends and drivers in buildings. Renew. Sustain. Energy Rev. 2015, 41, 85–98. [Google Scholar] [CrossRef]
- Junaid, M.F.; Rehman, Z.u.; Čekon, M.; Čurpek, J.; Farooq, R.; Cui, H.; Khan, I. Inorganic phase change materials in thermal energy storage: A review on perspectives and technological advances in building applications. Energy Build. 2021, 252, 111443. [Google Scholar] [CrossRef]
- Jha, S.K.; Sankar, A.; Zhou, Y.; Ghosh, A. Incorporation of Phase Change Materials in Buildings. Constr. Mater. 2024, 4, 676–703. [Google Scholar] [CrossRef]
- Farouk, N.; Alotaibi, A.; Alshahri, A.; Almitani, K. Using PCM in buildings to reduce HVAC energy usage taking into account Saudi Arabia climate region. J. Build. Eng. 2022, 50, 104073. [Google Scholar] [CrossRef]
- Abuelnuor, A.; Omara, A.; Saqr, K.; Elhag, I. Improving indoor thermal comfort by using phase change materials: A review. Int. J. Energy Res. 2018, 42, 2084–2103. [Google Scholar] [CrossRef]
- Al-Yasiri, Q.; Szabó, M. Energetic and thermal comfort assessment of phase change material passively incorporated building envelope in severe hot Climate: An experimental study. Appl. Energy 2022, 314, 118957. [Google Scholar] [CrossRef]
- Al-Shannaq, R.; Farid, M.M.; Ikutegbe, C.A. Methods for the Synthesis of Phase Change Material Microcapsules with Enhanced Thermophysical Properties—A State-of-the-Art Review. Micro 2022, 2, 426–474. [Google Scholar] [CrossRef]
- Ansari, J.A.; Al-Shannaq, R.; Kurdi, J.; Al-Muhtaseb, S.A.; Ikutegbe, C.A.; Farid, M.M. A Rapid Method for Low Temperature Microencapsulation of Phase Change Materials (PCMs) Using a Coiled Tube Ultraviolet Reactor. Energies 2021, 14, 7867. [Google Scholar] [CrossRef]
- Al-Shannaq, R.; Farid, M.M.; Wasi Ahmad, M.; Al-Muhtaseb, S.A.; Kurdi, J. Microencapsulation of phase change materials (PCMs) at low temperature using a novel thin film UV reactor. Chem. Eng. J. 2024, 493, 152807. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, H.; Xia, T.D.; Zhang, T.; Feng, H.X. Fabrication and performances of microencapsulated paraffin composites with polymethylmethacrylate shell based on ultraviolet irradiation-initiated. Mater. Chem. Phys. 2012, 135, 181–187. [Google Scholar] [CrossRef]
- Song, Y.; Qiu, X.; Liu, H.; Han, Y. Microencapsulated phase change materials (MicroPCMs) with TiO2-modified natural polymer shell and macrocapsules containing MicroPCMs for thermal energy storage and UV-shielding. Sol. Energy Mater. Sol. Cells 2024, 271, 112860. [Google Scholar] [CrossRef]
- Ikutegbe, C.A.; Al-Shannaq, R.; Farid, M.M. Microencapsulation of low melting phase change materials for cold storage applications. Appl. Energy 2022, 321, 119347. [Google Scholar] [CrossRef]
- Ma, S.; Song, G.; Li, W.; Fan, P.; Tang, G. UV irradiation-initiated MMA polymerization to prepare microcapsules containing phase change paraffin. Sol. Energy Mater. Sol. Cells 2010, 94, 1643–1647. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, M.; Zhang, Y.; Wang, Y. Microencapsulation of stearic acid with polymethylmethacrylate using iron (III) chloride as photo-initiator for thermal energy storage. Chin. J. Chem. Eng. 2017, 25, 1524–1532. [Google Scholar] [CrossRef]
- Yagci, Y.; Jockusch, S.; Turro, N. Photoinitiated Polymerization: Advances, Challenges, and Opportunities. Macromolecules 2010, 43, 6245–6260. [Google Scholar] [CrossRef]
- Decker, C. New Developments in UV-radiation curing of protective coatings. Surf. Coat. Int. Part B Coat. Trans. 2005, 88, 9–17. [Google Scholar] [CrossRef]
- Silva, M.R.F.; Alves, M.F.R.P.; Cunha, J.P.G.Q.; Costa, J.L.; Silva, C.A.; Fernandes, M.H.V.; Vilarinho, P.M.; Ferreira, P. Nanostructured transparent solutions for UV-shielding: Recent developments and future challenges. Mater. Today Phys. 2023, 35, 101131. [Google Scholar] [CrossRef]
- López-Alarcón, C.; Fuentes-Lemus, E.; Figueroa, J.D.; Dorta, E.; Schöneich, C.; Davies, M.J. Azocompounds as generators of defined radical species: Contributions and challenges for free radical research. Free Radic. Biol. Med. 2020, 160, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Wang, J.; Xie, H.; Guo, Z. Microencapsulated phase change n-Octadecane with high heat storage for application in building energy conservation. Appl. Energy 2023, 329, 120284. [Google Scholar] [CrossRef]
- Carreira, A.S.; Teixeira, R.F.A.; Beirão, A.; Vaz Vieira, R.; Figueiredo, M.M.; Gil, M.H. Preparation of acrylic based microcapsules using different reaction conditions for thermo-regulating textiles production. Eur. Polym. J. 2017, 93, 33–43. [Google Scholar] [CrossRef]
- Qiu, X.; Li, W.; Song, G.; Chu, X.; Tang, G. Fabrication and characterization of microencapsulated n-octadecane with different crosslinked methylmethacrylate-based polymer shells. Sol. Energy Mater. Sol. Cells 2012, 98, 283–293. [Google Scholar] [CrossRef]
- Su, W.; Zhou, T.; Li, Y.; Lv, Y. Development of microencapsulated phase change material with poly (methyl methacrylate) shell for thermal energy storage. Energy Procedia 2019, 158, 4483–4488. [Google Scholar] [CrossRef]
- Liu, S.-H.; Lu, Y.-M.; Chiang, C.-L.; Cao, C.-R. Determination of the thermal hazard and decomposition behaviors of 2,2′-azobis-(2,4-dimethylvaleronitrile). Process Saf. Environ. Prot. 2019, 131, 55–62. [Google Scholar] [CrossRef]
- Shen, J.; Ma, Y.; Zhou, F.; Sheng, X.; Chen, Y. Thermophysical properties investigation of phase change microcapsules with low supercooling and high energy storage capability: Potential for efficient solar energy thermal management. J. Mater. Sci. Technol. 2024, 191, 199–208. [Google Scholar] [CrossRef]
- Wang, X.; Gao, Y.; Han, N.; Zhang, X.; Li, W. Design and synthesis of microcapsules with cross-linking network supporting core for supercooling degree regulation. Energy Build. 2021, 253, 111437. [Google Scholar] [CrossRef]
- Zou, D.; Liu, X.; He, R.; Huang, L. High thermal response rate and super low supercooling degree microencapsulated phase change materials (MEPCM) developed by optimizing shell with various nanoparticles. Int. J. Heat Mass Transf. 2019, 140, 956–964. [Google Scholar] [CrossRef]
- Al-Shannaq, R.; Kurdi, J.; Al-Muhtaseb, S.; Dickinson, M.; Farid, M. Supercooling elimination of phase change materials (PCMs) microcapsules. Energy 2015, 87, 654–662. [Google Scholar] [CrossRef]
- Al-Shannaq, R.; Farid, M.; Al-Muhtaseb, S.; Kurdi, J. Emulsion stability and cross-linking of PMMA microcapsules containing phase change materials. Sol. Energy Mater. Sol. Cells 2015, 132, 311–318. [Google Scholar] [CrossRef]
- Christy, J.V.; Balwani, A.; Mehling, H.; Agrawal, N. Optimization of DSC measurements for organic phase change materials. J. Energy Storage 2023, 73, 109032. [Google Scholar] [CrossRef]
- Müller, L.; Rubio-Pérez, G.; Bach, A.; Muñoz-Rujas, N.; Aguilar, F.; Worlitschek, J. Consistent DSC and TGA Methodology as Basis for the Measurement and Comparison of Thermo-Physical Properties of Phase Change Materials. Materials 2020, 13, 4486. [Google Scholar] [CrossRef]
- Dou, G.; Xu, M.; Hu, Y.; Sun, Y.; Jiang, H.; Peng, G. Improving the mechanical performance of phase change microcapsule/epoxy composites via alternately enhancing interface bonding and strength of microcapsules. J. Energy Storage 2023, 73, 109212. [Google Scholar] [CrossRef]
- Hu, M.; Wang, D.; Kokogiannakis, G.; Darkwa, J.; Li, Y.; Wang, L.; Xu, Q.; Su, W. Enhancement of thermal and mechanical properties of microencapsulated phase change materials with graphene oxide. Chem. Eng. J. 2024, 479, 147855. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhou, L.; Sun, P.; Li, Z.; Kang, Y.; Guo, M.; Niu, Y.; Zhao, D. Regulation of mechanical properties of microcapsules and their applications. J. Control. Release 2024, 375, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Cao, V.D.; Pilehvar, S.; Salas-Bringas, C.; Szczotok, A.M.; Valentini, L.; Carmona, M.; Rodriguez, J.F.; Kjøniksen, A.-L. Influence of microcapsule size and shell polarity on thermal and mechanical properties of thermoregulating geopolymer concrete for passive building applications. Energy Convers. Manag. 2018, 164, 198–209. [Google Scholar] [CrossRef]
- Giro-Paloma, J.; Oncins, G.; Barreneche, C.; Martínez, M.; Fernández, A.I.; Cabeza, L.F. Physico-chemical and mechanical properties of microencapsulated phase change material. Appl. Energy 2013, 109, 441–448. [Google Scholar] [CrossRef]
- Suzuki, Y.; Mishima, R.; Matsumoto, A. Bulk polymerization kinetics of methyl methacrylate at broad temperature range investigated by differential scanning calorimetry. Int. J. Chem. Kinet. 2022, 54, 361–370. [Google Scholar] [CrossRef]
- Bayés-García, L.; Ventolà, L.; Cordobilla, R.; Benages, R.; Calvet, T.; Cuevas-Diarte, M.A. Phase Change Materials (PCM) microcapsules with different shell compositions: Preparation, characterization and thermal stability. Sol. Energy Mater. Sol. Cells 2010, 94, 1235–1240. [Google Scholar] [CrossRef]
- Deveci, S.S.; Basal, G. Preparation of PCM microcapsules by complex coacervation of silk fibroin and chitosan. Colloid Polym. Sci. 2009, 287, 1455–1467. [Google Scholar] [CrossRef]
- Ahangaran, F.; Navarchian, A.H.; Picchioni, F. Material encapsulation in poly(methyl methacrylate) shell: A review. J. Appl. Polym. Sci. 2019, 136, 48039. [Google Scholar] [CrossRef]
- Lygerakis, F.; Gioti, C.; Gournis, D.; Yentekakis, I.V.; Karakassides, M.; Kolokotsa, D. Enhancing Building Energy Efficiency with Innovative Paraffin-Based Phase Change Materials. Energies 2024, 17, 4155. [Google Scholar] [CrossRef]
- Abdullah, M.; Obayedullah, M.; Musfika, S.A. Recent Advances in Phase Change Energy Storage Materials: Developments and Applications. Int. J. Energy Res. 2025, 2025, 6668430. [Google Scholar] [CrossRef]
- Morciano, M.; Fasano, M.; Chiavazzo, E.; Mongibello, L. Trending applications of Phase Change Materials in sustainable thermal engineering: An up-to-date review. Energy Convers. Manag. X 2025, 25, 100862. [Google Scholar] [CrossRef]
- Gbekou, F.K.; Belloum, R.; Chennouf, N.; Agoudjil, B.; Boudenne, A.; Benzarti, K. Thermal performance of a building envelope including microencapsulated phase change materials (PCMs): A multiscale experimental and numerical investigation. Build. Environ. 2024, 253, 111294. [Google Scholar] [CrossRef]
- Naik, S.; Singh, L.P.; Tyagi, I.; Rawat, A.; Sinha, S. Microencapsulation of a eutectic PCM using in situ polymerization technique for thermal energy storage. Int. J. Energy Res. 2020, 44, 3854–3864. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Z.; Chang, T.; Wang, J.; Wang, X.; Zhou, G. Phase change material microcapsules with melamine resin shell via cellulose nanocrystal stabilized Pickering emulsion in-situ polymerization. Chem. Eng. J. 2022, 428, 131164. [Google Scholar] [CrossRef]
- Parsamanesh, M.; Shekarriz, S.; Montazer, M. Enhanced thermal stability of eutectic PCMs via microencapsulation: Inverse emulsion polymerization with silica shells. Therm. Sci. Eng. Prog. 2025, 60, 103420. [Google Scholar] [CrossRef]
- Huo, X.; Li, W.; Wang, Y.; Han, N.; Wang, J.; Wang, N.; Zhang, X. Chitosan composite microencapsulated comb-like polymeric phase change material via coacervation microencapsulation. Carbohydr. Polym. 2018, 200, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Geng, X.; Wang, X.; Han, N.; Zhang, X.; Li, W. Synthesis and characterization of microencapsulated phase change materials with chitosan-based polyurethane shell. Carbohydr. Polym. 2021, 273, 118629. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yang, Y.; Li, Y.; Zhao, L.; Wang, H.; Song, G.; Tang, G. Microencapsulated phase change materials with TiO2-doped PMMA shell for thermal energy storage and UV-shielding. Sol. Energy Mater. Sol. Cells 2017, 168, 62–68. [Google Scholar] [CrossRef]
- Ma, Y.; Xie, Q.; Wang, X.; Lu, J. Synthesis and characterization of microencapsulated phase change materials with comb-like acrylic co-polymer shell as thermal energy storage materials. Sol. Energy 2019, 179, 410–423. [Google Scholar] [CrossRef]
- Microtek Laboratories. Available online: https://www.microteklabs.com/microencapsulation-solutions (accessed on 18 March 2025).
- Roberts, N.S.; Al-Shannaq, R.; Kurdi, J.; Al-Muhtaseb, S.A.; Farid, M.M. Efficacy of using slurry of metal-coated microencapsulated PCM for cooling in a micro-channel heat exchanger. Appl. Therm. Eng. 2017, 122, 11–18. [Google Scholar] [CrossRef]
- Ghasemi, K.; Mahmud, S.; Tasnim, S. Optimization of microencapsulated phase change material slurry-based porous heat exchanger. J. Energy Storage 2022, 55, 105797. [Google Scholar] [CrossRef]
- Dubey, I.; Kadam, V.; Babel, S.; Jose, S.; Kumar, A. 1-Tetradecanol phase change material microcapsules coating on cotton fabric for enhanced thermoregulation. Int. J. Biol. Macromol. 2024, 280, 135926. [Google Scholar] [CrossRef]
- Huang, L.; Piontek, U. Improving Performance of Cold-Chain Insulated Container with Phase Change Material: An Experimental Investigation. Appl. Sci. 2017, 7, 1288. [Google Scholar] [CrossRef]


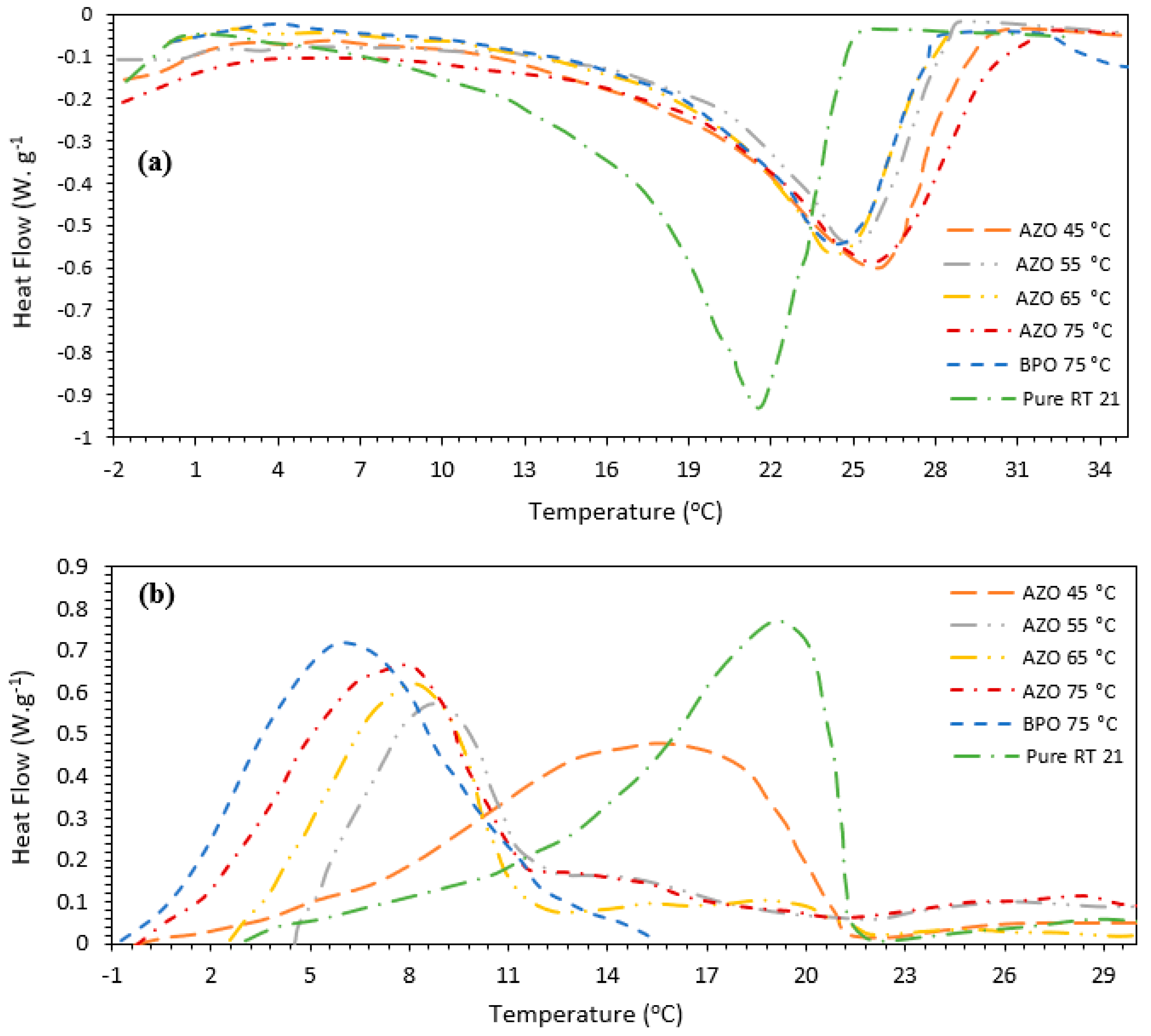


| Initiator | Tom (°C) | Tpm (°C) | Tem (°C) | ΔHm (J.g−1) | Toc (°C) | Tpc (°C) | Tec (°C) | ΔHc (J.g−1) | Core Content (%) |
|---|---|---|---|---|---|---|---|---|---|
| RT21 (PCM) | 16.53 | 22.10 | 23.85 | 132.53 | 21.38 | 19.39 | 14.50 | 134.48 | - |
| PBO (75 °C) | 19.55 | 24.49 | 27.92 | 99.69 | 10.42 | 6.51 | 2.56 | 108.33 | 77.9 |
| Azo-65 (75 °C) | 19.98 | 24.92 | 28.68 | 96.02 | 12.24 | 7.08 | 4.41 | 89.75 | 69.7 |
| Azo-65 (65 °C) | 19.05 | 25.58 | 30.36 | 94.21 | 11.51 | 7.83 | 2.99 | 95.34 | 71.0 |
| Azo-65 (55 °C) | 18.66 | 24.31 | 28.19 | 95.66 | 11.36 | 8.44 | 3.51 | 97.44 | 72.3 |
| Azo-65 (45 °C) | 17.59 | 25.63 | 29.05 | 107.76 | 21.29 | 15.66 | 4.67 | 111.18 | 82.0 |
| Initiator | Teo (°C) | (%) |
|---|---|---|
| MicroPCMs-PBO (75 °C) | 181.40 | 85 |
| MicroPCMs-Azo-65 (75 °C) | 187.72 | 84 |
| MicroPCMs-Azo-65 (65 °C) | 182.62 | 80 |
| MicroPCMs-Azo (55 °C) | 173.03 | 85 |
| MicroPCMs-Azo (45 °C) | 164.57 | 93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Shannaq, R.; Daoud, M.; Farid, M.; Ahmad, M.W.; Al-Muhtaseb, S.A.; Ul-Islam, M.; Al Saidi, A.; Zahid, I. Roles of Polymerization Temperature and Initiator Type on Thermal Properties of Rubitherm® 21 PCM Microcapsules. Micro 2025, 5, 19. https://doi.org/10.3390/micro5020019
Al-Shannaq R, Daoud M, Farid M, Ahmad MW, Al-Muhtaseb SA, Ul-Islam M, Al Saidi A, Zahid I. Roles of Polymerization Temperature and Initiator Type on Thermal Properties of Rubitherm® 21 PCM Microcapsules. Micro. 2025; 5(2):19. https://doi.org/10.3390/micro5020019
Chicago/Turabian StyleAl-Shannaq, Refat, Monzer Daoud, Mohammed Farid, Md Wasi Ahmad, Shaheen A. Al-Muhtaseb, Mazhar Ul-Islam, Abdullah Al Saidi, and Imran Zahid. 2025. "Roles of Polymerization Temperature and Initiator Type on Thermal Properties of Rubitherm® 21 PCM Microcapsules" Micro 5, no. 2: 19. https://doi.org/10.3390/micro5020019
APA StyleAl-Shannaq, R., Daoud, M., Farid, M., Ahmad, M. W., Al-Muhtaseb, S. A., Ul-Islam, M., Al Saidi, A., & Zahid, I. (2025). Roles of Polymerization Temperature and Initiator Type on Thermal Properties of Rubitherm® 21 PCM Microcapsules. Micro, 5(2), 19. https://doi.org/10.3390/micro5020019










