Paracetamol Is Associated with a Lower Risk of COVID-19 Infection and Decreased ACE2 Protein Expression: A Retrospective Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. UK Biobank Data Sources
2.2. Cell Culture and Drug Treatments
2.3. Protein Extraction and Western Blotting
2.4. RNA Extraction and Quantitative Real-Time PCR
2.5. Statistical Analysis
3. Results
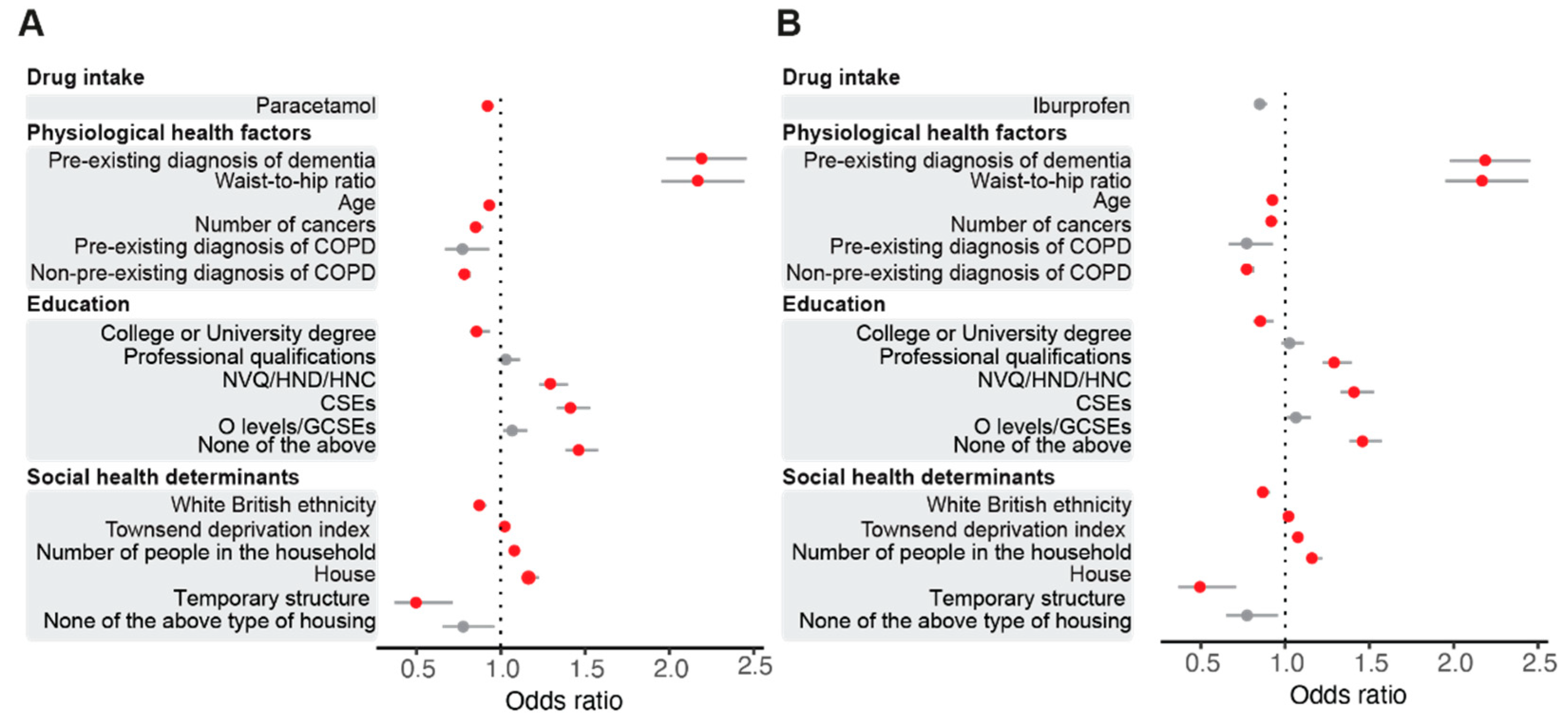
3.1. Regular Paracetamol Intake Results in a Lower Risk of SARS-CoV-2 Infection in the UK Biobank Cohort
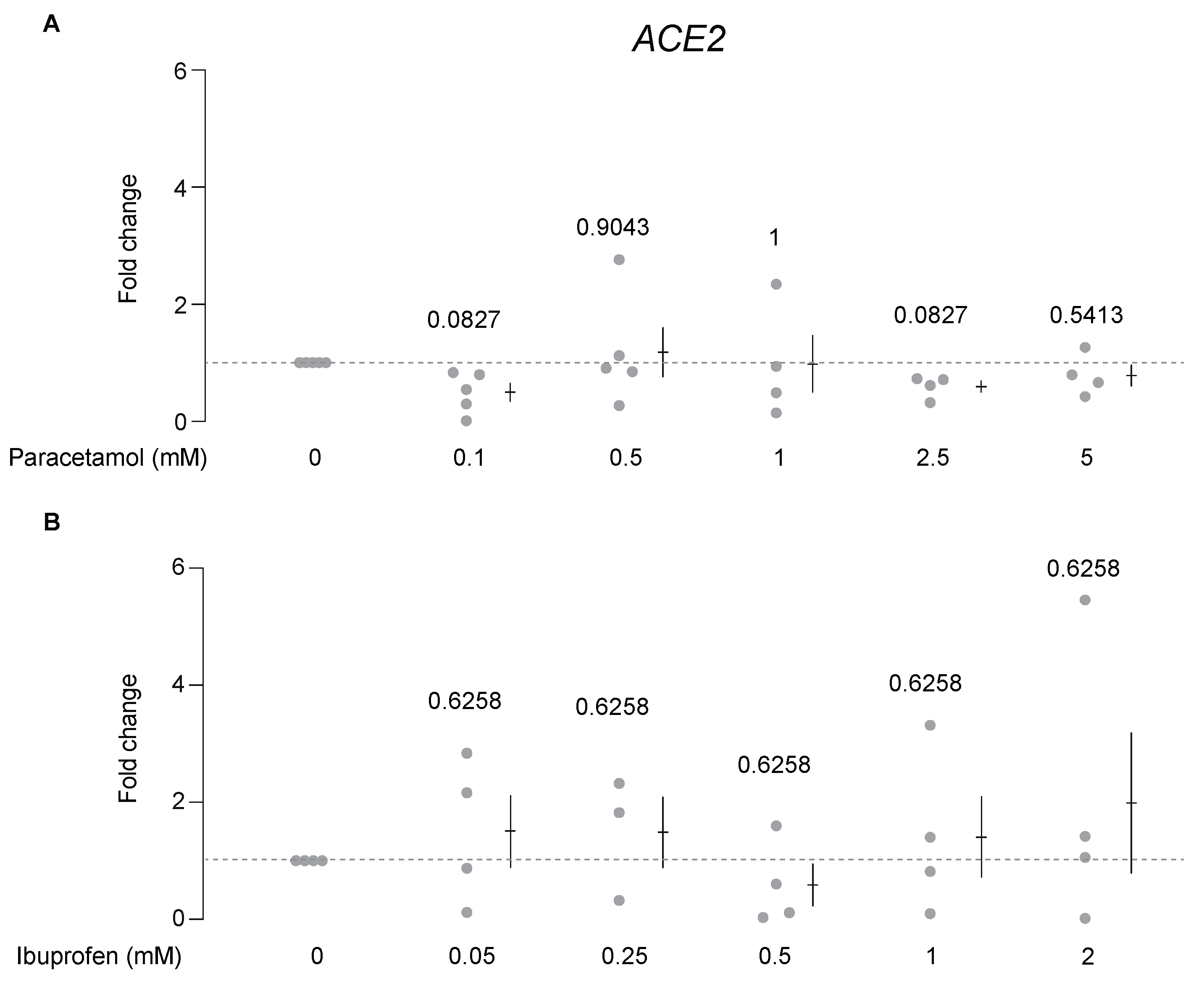
3.2. Paracetamol, but Not Ibuprofen, Decreases ACE2 Protein Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | arachidonic acid |
| ACE | angiotensin-converting enzyme |
| ACE2 | angiotensin-converting enzyme 2 |
| Ang I | angiotensin I |
| Ang II | angiotensin II |
| CI | confidence interval |
| COVID-19 | coronavirus disease 2019 |
| COX-1 | cyclooxygenase-1 |
| COX-2 | cyclooxygenase-2 |
| FBS | foetal bovine serum |
| mRNA | messenger RNA |
| NSAIDs | non-steroid anti-inflammatory drugs |
| OR | odds ratio |
| qRT-PCR | quantitative real-time PCR with reverse transcription |
| S | spike |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus 2 |
| s.e.m. | standard error of the mean |
References
- Wilcox, C.M.; Cryer, B.; Triadafilopoulos, G. Patterns of Use and Public Perception of Over-the-Counter Pain Relievers: Focus on Nonsteroidal Antiinflammatory Drugs. J. Rheumatol. 2005, 32, 2218–2224. [Google Scholar] [PubMed]
- Bushra, R.; Aslam, N. An Overview of Clinical Pharmacology of Ibuprofen. Oman Med. J. 2010, 25, 155–1661. [Google Scholar] [CrossRef]
- Jóźwiak-Bebenista, M.; Nowak, J.Z. Paracetamol: Mechanism of Action, Applications and Safety Concern. Acta Pol. Pharm. 2014, 71, 11–23. [Google Scholar]
- Harrison, A.G.; Lin, T.; Wang, P. Mechanisms of SARS-CoV-2 Transmission and Pathogenesis. Trends Immunol. 2020, 41, 1100–1115. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef] [PubMed]
- Bourgonje, A.R.; Abdulle, A.E.; Timens, W.; Hillebrands, J.-L.; Navis, G.J.; Gordijn, S.J.; Bolling, M.C.; Dijkstra, G.; Voors, A.A.; Osterhaus, A.D.M.E.; et al. Angiotensin-Converting Enzyme 2 (ACE2), SARS-CoV-2 and the Pathophysiology of Coronavirus Disease 2019 (COVID-19). J. Pathol. 2020, 251, 228–248. [Google Scholar] [CrossRef]
- Sanchis-Gomar, F.; Lavie, C.J.; Perez-Quilis, C.; Henry, B.M.; Lippi, G. Angiotensin-Converting Enzyme 2 and Antihypertensives (Angiotensin Receptor Blockers and Angiotensin-Converting Enzyme Inhibitors) in Coronavirus Disease 2019. Mayo Clin. Proc. 2020, 95, 1222–1230. [Google Scholar] [CrossRef] [PubMed]
- Karcioglu, O.; Bas, B.; Hosseinzadeh, M.; Kolahforoush, A.; Simsik, I.; Yeniocak, S. Commission of Human Medicines More Advice on the Use of Ibuprofen for COVID-19. Drug Ther. Bull. 2020, 58, 101. [Google Scholar] [CrossRef]
- Moore, N.; Carleton, B.; Blin, P.; Bosco-Levy, P.; Droz, C. Does Ibuprofen Worsen COVID-19? Drug Saf. 2020, 43, 611–614. [Google Scholar] [CrossRef]
- Powis, S. Message for All Clinical Staff: Anti-Inflammatory Medications. Available online: https://www.cas.mhra.gov.uk/ViewandAcknowledgment/ViewAlert.aspx?AlertID=103001 (accessed on 7 March 2021).
- Fang, L.; Karakiulakis, G.; Roth, M. Are Patients with Hypertension and Diabetes Mellitus at Increased Risk for COVID-19 Infection? Lancet Respir. Med. 2020, 8, e21. [Google Scholar] [CrossRef]
- Qiao, W.; Wang, C.; Chen, B.; Zhang, F.; Liu, Y.; Lu, Q.; Guo, H.; Yan, C.; Sun, H.; Hu, G.; et al. Ibuprofen Attenuates Cardiac Fibrosis in Streptozotocin-Induced Diabetic Rats. Cardiology 2015, 131, 97–106. [Google Scholar] [CrossRef]
- Rinott, E.; Kozer, E.; Shapira, Y.; Bar-Haim, A.; Youngster, I. Ibuprofen Use and Clinical Outcomes in COVID-19 Patients. Clin. Microbiol. Infect. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2020, 26, 1259.e5–1259.e7. [Google Scholar] [CrossRef] [PubMed]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK Biobank: An Open Access Resource for Identifying the Causes of a Wide Range of Complex Diseases of Middle and Old Age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, J.; Rudkin, J.K.; Allen, N.; Crook, D.W.; Wilson, D.J.; Wyllie, D.H.; O’Connell, A.M. Dynamic Linkage of COVID-19 Test Results between Public Health England’s Second Generation Surveillance System and UK Biobank. Microb. Genom. 2020, 6, mgen000397. [Google Scholar] [CrossRef] [PubMed]
- Behrends, V.; Giskeødegård, G.F.; Bravo-Santano, N.; Letek, M.; Keun, H.C. Acetaminophen Cytotoxicity in HepG2 Cells Is Associated with a Decoupling of Glycolysis from the TCA Cycle, Loss of NADPH Production, and Suppression of Anabolism. Arch. Toxicol. 2019, 93, 341–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierce, R.H.; Franklin, C.C.; Campbell, J.S.; Tonge, R.P.; Chen, W.; Fausto, N.; Nelson, S.D.; Bruschi, S.A. Cell Culture Model for Acetaminophen-Induced Hepatocyte Death in Vivo. Biochem. Pharmacol. 2002, 64, 413–424. [Google Scholar] [CrossRef]
- Prieto, P.; Hoffmann, S.; Tirelli, V.; Tancredi, F.; González, I.; Bermejo, M.; de Angelis, I. An Exploratory Study of Two Caco-2 Cell Models for Oral Absorption: A Report on Their within-Laboratory and between-Laboratory Variability, and Their Predictive Capacity. Altern. Lab. Anim. ATLA 2010, 38, 367–386. [Google Scholar] [CrossRef]
- Siissalo, S.; Laine, L.; Tolonen, A.; Kaukonen, A.M.; Finel, M.; Hirvonen, J. Caco-2 Cell Monolayers as a Tool to Study Simultaneous Phase II Metabolism and Metabolite Efflux of Indomethacin, Paracetamol and 1-Naphthol. Int. J. Pharm. 2010, 383, 24–29. [Google Scholar] [CrossRef]
- Vad, N.M.; Yount, G.; Moore, D.; Weidanz, J.; Moridani, M.Y. Biochemical Mechanism of Acetaminophen (APAP) Induced Toxicity in Melanoma Cell Lines. J. Pharm. Sci. 2009, 98, 1409–1425. [Google Scholar] [CrossRef] [Green Version]
- Bombardo, M.; Malagola, E.; Chen, R.; Rudnicka, A.; Graf, R.; Sonda, S. Ibuprofen and Diclofenac Treatments Reduce Proliferation of Pancreatic Acinar Cells upon Inflammatory Injury and Mitogenic Stimulation. Br. J. Pharmacol. 2018, 175, 335–347. [Google Scholar] [CrossRef] [Green Version]
- Hao, H.; Wang, G.; Sun, J.; Ding, Z.; Wu, X.; Roberts, M. Unidirectional Inversion of Ibuprofen in Caco-2 Cells: Developing a Suitable Model for Presystemic Chiral Inversion Study. Biol. Pharm. Bull. 2005, 28, 682–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heikkinen, A.T.; Mönkkönen, J.; Korjamo, T. Kinetics of Cellular Retention during Caco-2 Permeation Experiments: Role of Lysosomal Sequestration and Impact on Permeability Estimates. J. Pharmacol. Exp. Ther. 2009, 328, 882–892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leidgens, V.; Seliger, C.; Jachnik, B.; Welz, T.; Leukel, P.; Vollmann-Zwerenz, A.; Bogdahn, U.; Kreutz, M.; Grauer, O.M.; Hau, P. Ibuprofen and Diclofenac Restrict Migration and Proliferation of Human Glioma Cells by Distinct Molecular Mechanisms. PLoS ONE 2015, 10, e0140613. [Google Scholar] [CrossRef] [Green Version]
- Liao, C.-H.; Lin, L.-P.; Yu, T.-Y.; Hsu, C.-C.; Pang, J.-H.S.; Tsai, W.-C. Ibuprofen Inhibited Migration of Skeletal Muscle Cells in Association with Downregulation of P130cas and CrkII Expressions. Skelet. Muscle 2019, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing Real-Time PCR Data by the Comparative C(T) Method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.C.; Sausville, E.L.; Girish, V.; Yuan, M.L.; Vasudevan, A.; John, K.M.; Sheltzer, J.M. Cigarette Smoke Exposure and Inflammatory Signaling Increase the Expression of the SARS-CoV-2 Receptor ACE2 in the Respiratory Tract. Dev. Cell 2020, 53, 514–529.e3. [Google Scholar] [CrossRef]
- Travaglio, M.; Yu, Y.; Popovic, R.; Selley, L.; Leal, N.S.; Martins, L.M. Links between Air Pollution and COVID-19 in England. Environ. Pollut. 2021, 268, 115859. [Google Scholar] [CrossRef]
- Yu, Y.; Travaglio, M.; Popovic, R.; Leal, N.S.; Martins, L.M. Alzheimer’s and Parkinson’s Diseases Predict Different COVID-19 Outcomes: A UK Biobank Study. Geriatrics 2021, 6, 10. [Google Scholar] [CrossRef]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors Associated with COVID-19-Related Death Using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef]
- Lumley, T.; Diehr, P.; Emerson, S.; Chen, L. The Importance of the Normality Assumption in Large Public Health Data Sets. Annu. Rev. Public Health 2002, 23, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Team, R. Development Core A Language and Environment for Statistical Computing. R Found. Stat. Comput. 2018, 2. Available online: https://www.R-project.org (accessed on 1 February 2021).
- Kluyver, T.; Ragan-Kelley, B.; Pérez, F.; Granger, B.; Bussonnier, M.; Frederic, J.; Kelley, K.; Hamrick, J.; Grout, J.; Corlay, S.; et al. Jupyter Notebooks—A Publishing Format for Reproducible Computational Workflows. In Proceedings of the Positioning and Power in Academic Publishing: Players, Agents and Agendas—Proceedings of the 20th International Conference on Electronic Publishing; ELPUB: Amsterdam, The Netherlands, 2016; pp. 87–90. [Google Scholar]
- Bycroft, C.; Freeman, C.; Petkova, D.; Band, G.; Elliott, L.T.; Sharp, K.; Motyer, A.; Vukcevic, D.; Delaneau, O.; O’Connell, J.; et al. The UK Biobank Resource with Deep Phenotyping and Genomic Data. Nature 2018, 562, 203–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- François, P.; Desrumaux, A.; Cans, C.; Pin, I.; Pavese, P.; Labarère, J. Prevalence and Risk Factors of Suppurative Complications in Children with Pneumonia. Acta Paediatr. 2010, 99, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Krenke, K.; Krawiec, M.; Kraj, G.; Peradzynska, J.; Krauze, A.; Kulus, M. Risk Factors for Local Complications in Children with Community-Acquired Pneumonia. Clin. Respir. J. 2018, 12, 253–261. [Google Scholar] [CrossRef]
- Martins-Filho, P.R.; do Nascimento-Júnior, E.M.; Santos, V.S. No Current Evidence Supporting Risk of Using Ibuprofen in Patients with COVID-19. Int. J. Clin. Pract. 2020, 74, e13576. [Google Scholar] [CrossRef]
- Herichová, I.; Šoltésová, D.; Szántóová, K.; Mravec, B.; Neupauerová, D.; Veselá, A.; Zeman, M. Effect of Angiotensin II on Rhythmic Per2 Expression in the Suprachiasmatic Nucleus and Heart and Daily Rhythm of Activity in Wistar Rats. Regul. Pept. 2013, 186, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Lund, L.C.; Kristensen, K.B.; Reilev, M.; Christensen, S.; Thomsen, R.W.; Christiansen, C.F.; Støvring, H.; Johansen, N.B.; Brun, N.C.; Hallas, J.; et al. Adverse Outcomes and Mortality in Users of Non-Steroidal Anti-Inflammatory Drugs Who Tested Positive for SARS-CoV-2: A Danish Nationwide Cohort Study. PLoS Med. 2020, 17, e1003308. [Google Scholar] [CrossRef] [PubMed]
- Gervin, K.; Nordeng, H.; Ystrom, E.; Reichborn-Kjennerud, T.; Lyle, R. Long-Term Prenatal Exposure to Paracetamol Is Associated with DNA Methylation Differences in Children Diagnosed with ADHD. Clin. Epigenetics 2017, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Wojnarová, L.; Kutinová Canová, N.; Farghali, H.; Kučera, T. Sirtuin 1 Modulation in Rat Model of Acetaminophen-Induced Hepatotoxicity. Physiol. Res. 2015, 64, S477–S487. [Google Scholar] [CrossRef]
- Clarke, N.E.; Belyaev, N.D.; Lambert, D.W.; Turner, A.J. Epigenetic Regulation of Angiotensin-Converting Enzyme 2 (ACE2) by SIRT1 under Conditions of Cell Energy Stress. Clin. Sci. 2014, 126, 507–516. [Google Scholar] [CrossRef]
- Grippo, F.; Navarra, S.; Orsi, C.; Manno, V.; Grande, E.; Crialesi, R.; Frova, L.; Marchetti, S.; Pappagallo, M.; Simeoni, S.; et al. The Role of COVID-19 in the Death of SARS-CoV-2–Positive Patients: A Study Based on Death Certificates. J. Clin. Med. 2020, 9, 3459. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leal, N.S.; Yu, Y.; Chen, Y.; Fedele, G.; Martins, L.M. Paracetamol Is Associated with a Lower Risk of COVID-19 Infection and Decreased ACE2 Protein Expression: A Retrospective Analysis. COVID 2021, 1, 218-229. https://doi.org/10.3390/covid1010018
Leal NS, Yu Y, Chen Y, Fedele G, Martins LM. Paracetamol Is Associated with a Lower Risk of COVID-19 Infection and Decreased ACE2 Protein Expression: A Retrospective Analysis. COVID. 2021; 1(1):218-229. https://doi.org/10.3390/covid1010018
Chicago/Turabian StyleLeal, Nuno Santos, Yizhou Yu, Yuwen Chen, Giorgio Fedele, and Luís Miguel Martins. 2021. "Paracetamol Is Associated with a Lower Risk of COVID-19 Infection and Decreased ACE2 Protein Expression: A Retrospective Analysis" COVID 1, no. 1: 218-229. https://doi.org/10.3390/covid1010018
APA StyleLeal, N. S., Yu, Y., Chen, Y., Fedele, G., & Martins, L. M. (2021). Paracetamol Is Associated with a Lower Risk of COVID-19 Infection and Decreased ACE2 Protein Expression: A Retrospective Analysis. COVID, 1(1), 218-229. https://doi.org/10.3390/covid1010018





