Follow-up of Interleukin 6 and Other Blood Markers during the Hospitalization of COVID-19 Patients: A Single-Center Study
Abstract
:1. Introduction
2. Materials and Methods
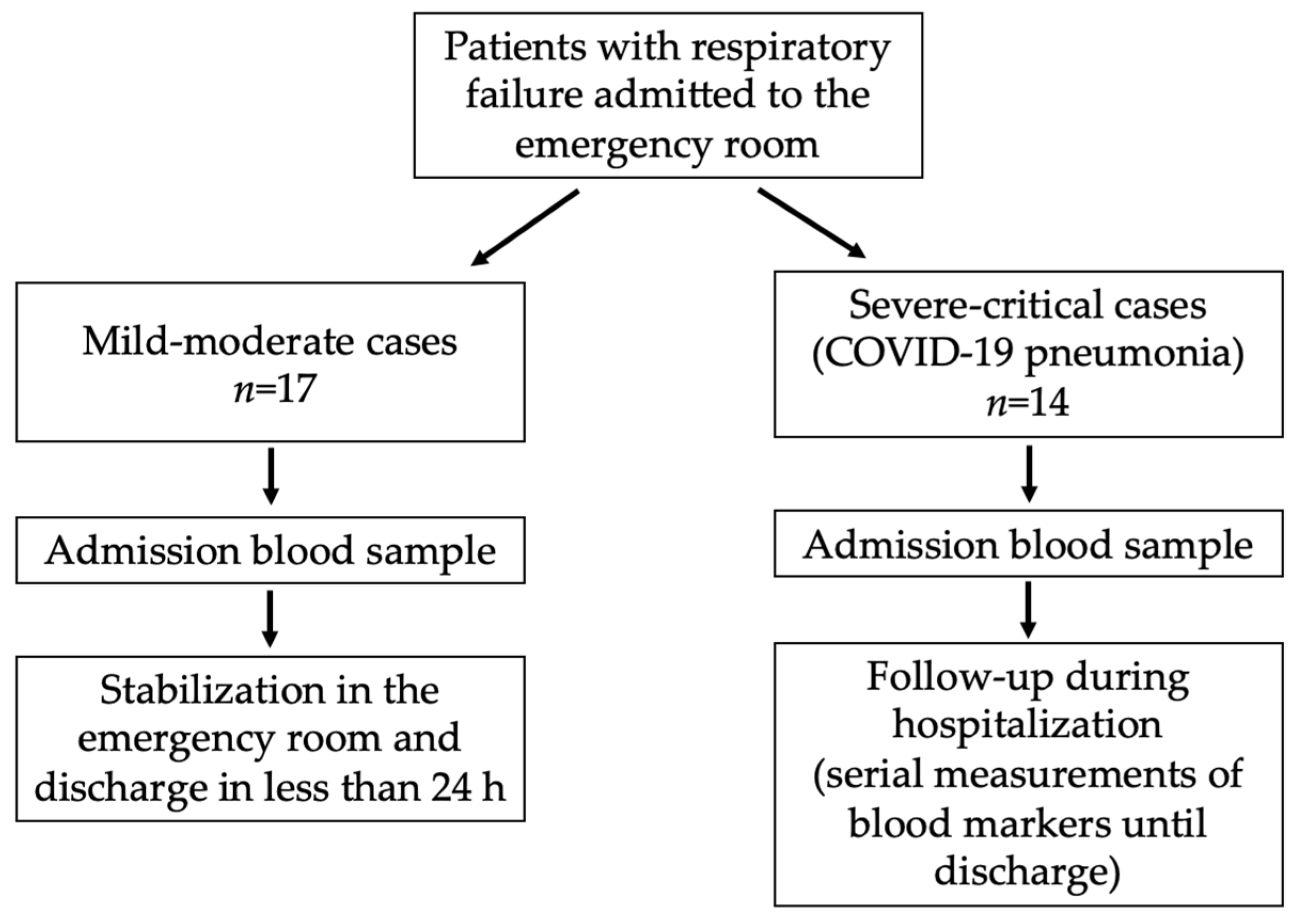
2.1. Patients and Samples
2.2. Negative and Positive Events
2.3. Blood Measurements
2.4. Detection of Viremia
2.5. Ethics Issues
2.6. Statistical Analysis
3. Results and Discussion
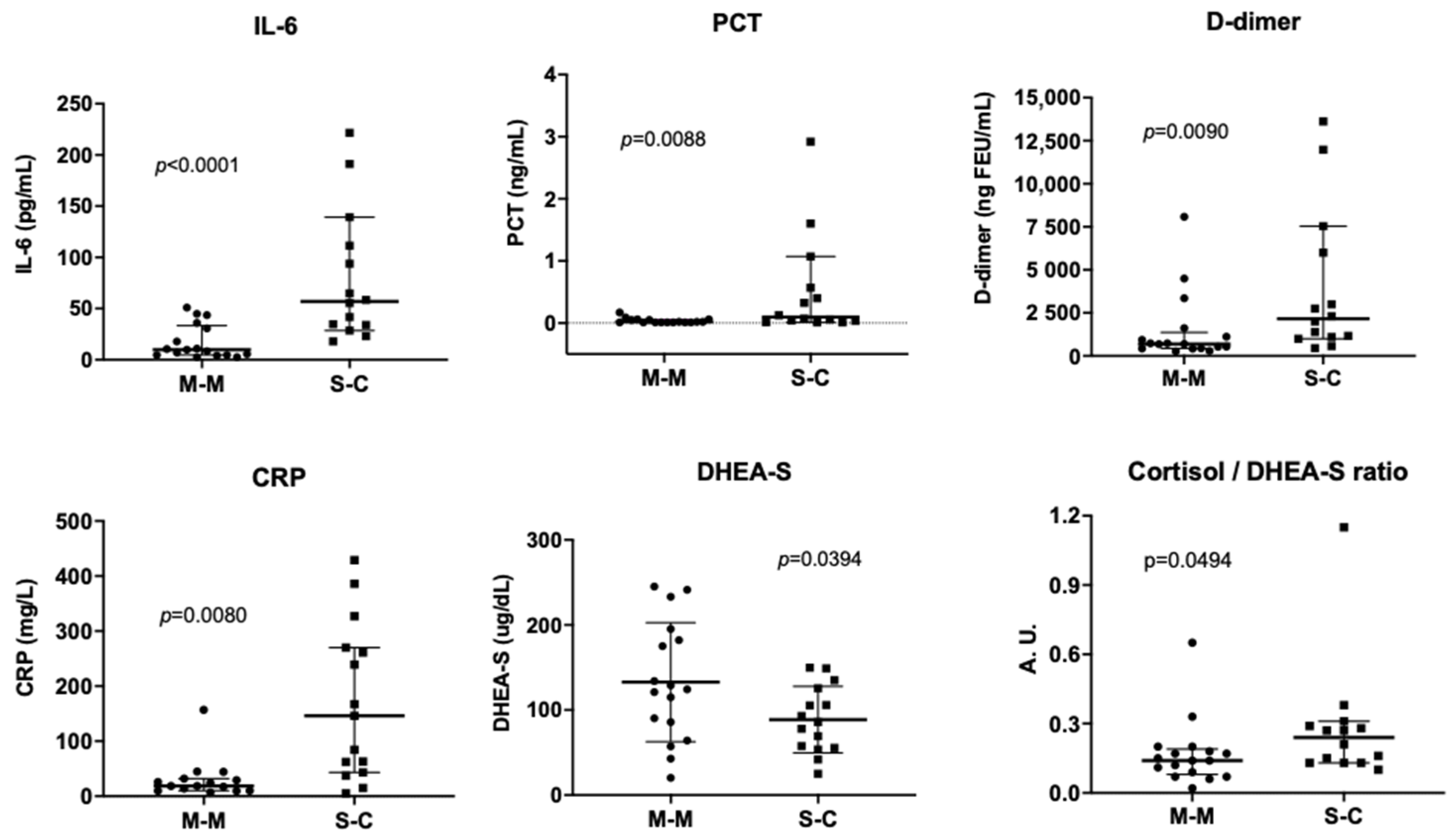
3.1. Levels of Blood Markers in COVID-19 Patients Requiring and Not Requiring Hospitalization
3.2. Time Patterns of IL-6 and Other Blood Markers during COVID-19 Course in Severe–Critical Patients
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Menachemi, N.; Dixon, B.E.; Wools-Kaloustian, K.K.; Yiannoutsos, C.T.; Halverson, P.K. How Many SARS-CoV-2-Infected People Require Hospitalization? Using Random Sample Testing to Better Inform Preparedness Efforts. J. Public Health Manag. Pract. 2021, 27, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Nakamichi, K.; Shen, J.Z.; Lee, C.S.; Lee, A.; Roberts, E.A.; Simonson, P.D.; Roychoudhury, P.; Andriesen, J.; Randhawa, A.K.; Mathias, P.C.; et al. Hospitalization and mortality associated with SARS-CoV-2 viral clades in COVID-19. Sci. Rep. 2021, 11, 4802. [Google Scholar] [CrossRef] [PubMed]
- Santa Cruz, A.; Mendes-Frias, A.; Oliveira, A.I.; Dias, L.; Matos, A.R.; Carvalho, A.; Capela, C.; Pedrosa, J.; Castro, A.G.; Silvestre, R. Interleukin-6 Is a Biomarker for the Development of Fatal Severe Acute Respiratory Syndrome Coronavirus 2 Pneumonia. Front. Immunol. 2021, 12, 613422. [Google Scholar] [CrossRef]
- Sabaka, P.; Koscalova, A.; Straka, I.; Hodosy, J.; Liptak, R.; Kmotorkova, B.; Kachlikova, M.; Kusnirova, A. Role of interleukin 6 as a predictive factor for a severe course of COVID-19: Retrospective data analysis of patients from a long-term care facility during COVID-19 outbreak. BMC Infect. Dis. 2021, 21, 308. [Google Scholar] [CrossRef]
- Liu, X.; Wang, H.; Shi, S.; Xiao, J. Association between IL-6 and severe disease and mortality in COVID-19 disease: A systematic review and meta-analysis. Postgrad. Med. J. 2021, 98, 871–879. [Google Scholar] [CrossRef]
- Hu, R.; Han, C.; Pei, S.; Yin, M.; Chen, X. Procalcitonin levels in COVID-19 patients. Int. J. Antimicrob. Agents 2020, 56, 106051. [Google Scholar] [CrossRef]
- Heidari-Beni, F.; Vahedian-Azimi, A.; Shojaei, S.; Rahimi-Bashar, F.; Shahriary, A.; Johnston, T.P.; Sahebkar, A. The Level of Procalcitonin in Severe COVID-19 Patients: A Systematic Review and Meta-Analysis. Adv. Exp. Med. Biol. 2021, 1321, 277–286. [Google Scholar] [CrossRef]
- Shen, Y.; Cheng, C.; Zheng, X.; Jin, Y.; Duan, G.; Chen, M.; Chen, S. Elevated Procalcitonin Is Positively Associated with the Severity of COVID-19: A Meta-Analysis Based on 10 Cohort Studies. Medicina 2021, 57, 594. [Google Scholar] [CrossRef]
- Yao, Y.; Cao, J.; Wang, Q.; Shi, Q.; Liu, K.; Luo, Z.; Chen, X.; Chen, S.; Yu, K.; Huang, Z.; et al. D-dimer as a biomarker for disease severity and mortality in COVID-19 patients: A case control study. J. Intensiv. Care 2020, 8, 49. [Google Scholar] [CrossRef]
- Poudel, A.; Poudel, Y.; Adhikari, A.; Aryal, B.B.; Dangol, D.; Bajracharya, T.; Maharjan, A.; Gautam, R. D-dimer as a biomarker for assessment of COVID-19 prognosis: D-dimer levels on admission and its role in predicting disease outcome in hospitalized patients with COVID-19. PLoS ONE 2021, 16, e0256744. [Google Scholar] [CrossRef] [PubMed]
- Paliogiannis, P.; Mangoni, A.A.; Dettori, P.; Nasrallah, G.K.; Pintus, G.; Zinellu, A. D-Dimer Concentrations and COVID-19 Severity: A Systematic Review and Meta-Analysis. Front. Public Health 2020, 8, 432. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zheng, K.I.; Liu, S.; Yan, Z.; Xu, C.; Qiao, Z. Plasma CRP level is positively associated with the severity of COVID-19. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 18. [Google Scholar] [CrossRef] [PubMed]
- Sharifpour, M.; Rangaraju, S.; Liu, M.; Alabyad, D.; Nahab, F.B.; Creel-Bulos, C.M.; Jabaley, C.S.; Emory, C.-Q.; Clinical Research, C. C-Reactive protein as a prognostic indicator in hospitalized patients with COVID-19. PLoS ONE 2020, 15, e0242400. [Google Scholar] [CrossRef]
- Ali, N. Elevated level of C-reactive protein may be an early marker to predict risk for severity of COVID-19. J. Med. Virol. 2020, 92, 2409–2411. [Google Scholar] [CrossRef]
- Jacobs, J.L.; Bain, W.; Naqvi, A.; Staines, B.; Castanha, P.M.S.; Yang, H.; Boltz, V.F.; Barratt-Boyes, S.; Marques, E.T.A.; Mitchell, S.L.; et al. SARS-CoV-2 Viremia is Associated with COVID-19 Severity and Predicts Clinical Outcomes. Clin. Infect. Dis. 2021, 74, 1525–1533. [Google Scholar] [CrossRef]
- Jarhult, J.D.; Hultstrom, M.; Bergqvist, A.; Frithiof, R.; Lipcsey, M. The impact of viremia on organ failure, biomarkers and mortality in a Swedish cohort of critically ill COVID-19 patients. Sci. Rep. 2021, 11, 7163. [Google Scholar] [CrossRef]
- Li, Y.; Schneider, A.M.; Mehta, A.; Sade-Feldman, M.; Kays, K.R.; Gentili, M.; Charland, N.C.; Gonye, A.L.K.; Gushterova, I.; Khanna, H.K.; et al. SARS-CoV-2 Viremia is Associated with Distinct Proteomic Pathways and Predicts COVID-19 Outcomes. J. Clin. Investig. 2021, 131, e148635. [Google Scholar] [CrossRef]
- Campi, I.; Gennari, L.; Merlotti, D.; Mingiano, C.; Frosali, A.; Giovanelli, L.; Torlasco, C.; Pengo, M.F.; Heilbron, F.; Soranna, D.; et al. Vitamin D and COVID-19 severity and related mortality: A prospective study in Italy. BMC Infect. Dis. 2021, 21, 566. [Google Scholar] [CrossRef]
- Vasheghani, M.; Jannati, N.; Baghaei, P.; Rezaei, M.; Aliyari, R.; Marjani, M. The relationship between serum 25-hydroxyvitamin D levels and the severity of COVID-19 disease and its mortality. Sci. Rep. 2021, 11, 17594. [Google Scholar] [CrossRef]
- Fajnzylber, J.; Regan, J.; Coxen, K.; Corry, H.; Wong, C.; Rosenthal, A.; Worrall, D.; Giguel, F.; Piechocka-Trocha, A.; Atyeo, C.; et al. SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat. Commun. 2020, 11, 5493. [Google Scholar] [CrossRef]
- Wheatland, R. Molecular mimicry of ACTH in SARS—Implications for corticosteroid treatment and prophylaxis. Med. Hypotheses 2004, 63, 855–862. [Google Scholar] [CrossRef]
- Siejka, A.; Barabutis, N. Adrenal insufficiency in the COVID-19 era. Am. J. Physiol. Metab. 2021, 320, E784–E785. [Google Scholar] [CrossRef] [PubMed]
- Freiburg, U.O. U-Net: Convolutional Networks for Biomedical Image Segmentation. Available online: https://lmb.informatik.uni-freiburg.de/people/ronneber/u-net/ (accessed on 18 January 2022).
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Salluh, J.I.; Verdeal, J.C.; Mello, G.W.; Araujo, L.V.; Martins, G.A.; de Sousa Santino, M.; Soares, M. Cortisol levels in patients with severe community-acquired pneumonia. Intensiv. Care Med. 2006, 32, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Korteweg, C. Pathology and pathogenesis of severe acute respiratory syndrome. Am. J. Pathol. 2007, 170, 1136–1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, Y.; Xu, B.; Guan, W.; Xu, D.; Li, F.; Ren, R.; Zhu, X.; Gao, Y.; Jiang, L. The Adrenal Cortex, an Underestimated Site of SARS-CoV-2 Infection. Front. Endocrinol. 2020, 11, 593179. [Google Scholar] [CrossRef] [PubMed]
- Radujkovic, A.; Hippchen, T.; Tiwari-Heckler, S.; Dreher, S.; Boxberger, M.; Merle, U. Vitamin D Deficiency and Outcome of COVID-19 Patients. Nutrients 2020, 12, 2757. [Google Scholar] [CrossRef] [PubMed]
- Chiodini, I.; Gatti, D.; Soranna, D.; Merlotti, D.; Mingiano, C.; Fassio, A.; Adami, G.; Falchetti, A.; Eller-Vainicher, C.; Rossini, M.; et al. Vitamin D Status and SARS-CoV2 Clinical Outcomes: A Systematic Review and Meta-Analysis. Lancet 2021, preprint. [Google Scholar] [CrossRef]
- Baktash, V.; Hosack, T.; Patel, N.; Shah, S.; Kandiah, P.; Van den Abbeele, K.; Mandal, A.K.J.; Missouris, C.G. Vitamin D status and outcomes for hospitalised older patients with COVID-19. Postgrad. Med. J. 2021, 97, 442–447. [Google Scholar] [CrossRef]
- Lakkireddy, M.; Gadiga, S.G.; Malathi, R.D.; Karra, M.L.; Raju, I.; Ragini; Chinapaka, S.; Baba, K.; Kandakatla, M. Impact of daily high dose oral vitamin D therapy on the inflammatory markers in patients with COVID-19 disease. Sci. Rep. 2021, 11, 10641. [Google Scholar] [CrossRef] [PubMed]
- Puig-Domingo, M.; Marazuela, M.; Yildiz, B.O.; Giustina, A. COVID-19 and endocrine and metabolic diseases. An updated statement from the European Society of Endocrinology. Endocrine 2021, 72, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Velazquez-Salinas, L.; Verdugo-Rodriguez, A.; Rodriguez, L.L.; Borca, M.V. The Role of Interleukin 6 during Viral Infections. Front. Microbiol. 2019, 10, 1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masia, M.; Fernandez-Gonzalez, M.; Padilla, S.; Ortega, P.; Garcia, J.A.; Agullo, V.; Garcia-Abellan, J.; Telenti, G.; Guillen, L.; Gutierrez, F. Impact of interleukin-6 blockade with tocilizumab on SARS-CoV-2 viral kinetics and antibody responses in patients with COVID-19: A prospective cohort study. eBioMedicine 2020, 60, 102999. [Google Scholar] [CrossRef] [PubMed]
- Galvan-Roman, J.M.; Rodriguez-Garcia, S.C.; Roy-Vallejo, E.; Marcos-Jimenez, A.; Sanchez-Alonso, S.; Fernandez-Diaz, C.; Alcaraz-Serna, A.; Mateu-Albero, T.; Rodriguez-Cortes, P.; Sanchez-Cerrillo, I.; et al. IL-6 serum levels predict severity and response to tocilizumab in COVID-19: An observational study. J. Allergy Clin. Immunol. 2021, 147, 72–80.e78. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, B.; Qu, Y.; Chen, Y.; Xiong, J.; Feng, Y.; Men, D.; Huang, Q.; Liu, Y.; Yang, B.; et al. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely associated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. medRxiv 2020, preprint. [Google Scholar] [CrossRef]
- Myhre, P.L.; Prebensen, C.; Jonassen, C.M.; Berdal, J.E.; Omland, T. SARS-CoV-2 Viremia is Associated With Inflammatory, But Not Cardiovascular Biomarkers, in Patients Hospitalized for COVID-19. J. Am. Heart Assoc. 2021, 10, e019756. [Google Scholar] [CrossRef]
- Coelho, L.; Povoa, P.; Almeida, E.; Fernandes, A.; Mealha, R.; Moreira, P.; Sabino, H. Usefulness of C-reactive protein in monitoring the severe community-acquired pneumonia clinical course. Crit. Care 2007, 11, R92. [Google Scholar] [CrossRef]


| Blood Markers Kits | Measuring Range of Test | Reference Values (Healthy Population) | Units |
|---|---|---|---|
| Maglumi IL-6 | 0.5–5000 | <7.0 | pg/mL |
| Maglumi PCT | 0.01–100 | <0.05 | ng/mL |
| Maglumi D-dimer | 100–10,000 | <500 | ng FEU/mL |
| Elecsys Anti-SARS-CoV-2 S | 0.40–250 | <0.80 | U/mL |
| Elecsys Cortisol II | 0.05–63.4 | 5.0–25 * | ug/dL |
| Elecsys DHEA-S | 0.1–1000 | 20–44 y: 60.9–340 (F)/88.9–492 (M) ** 45–74 y: 35.4–256 (F)/33.6–331 (M) ** >75 y: 12.0–154 (F)/16.2–123 (M) ** | ug/dL |
| Elecsys 25-OH Vitamin D III | 3.0–120 | >20 (sufficient) >30 (optimum) | ng/mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garrido, M.P.; Vaswani, V.; Contreras, K.; Barberán, M.; Valenzuela-Valderrama, M.; Klajn, D.; Romero, C.; Vial Covarrubias, M.J.; Cornejo, R.A. Follow-up of Interleukin 6 and Other Blood Markers during the Hospitalization of COVID-19 Patients: A Single-Center Study. COVID 2022, 2, 1584-1593. https://doi.org/10.3390/covid2110114
Garrido MP, Vaswani V, Contreras K, Barberán M, Valenzuela-Valderrama M, Klajn D, Romero C, Vial Covarrubias MJ, Cornejo RA. Follow-up of Interleukin 6 and Other Blood Markers during the Hospitalization of COVID-19 Patients: A Single-Center Study. COVID. 2022; 2(11):1584-1593. https://doi.org/10.3390/covid2110114
Chicago/Turabian StyleGarrido, Maritza P., Varsha Vaswani, Katherinne Contreras, Marcela Barberán, Manuel Valenzuela-Valderrama, Diana Klajn, Carmen Romero, María Jesús Vial Covarrubias, and Rodrigo Alfredo Cornejo. 2022. "Follow-up of Interleukin 6 and Other Blood Markers during the Hospitalization of COVID-19 Patients: A Single-Center Study" COVID 2, no. 11: 1584-1593. https://doi.org/10.3390/covid2110114
APA StyleGarrido, M. P., Vaswani, V., Contreras, K., Barberán, M., Valenzuela-Valderrama, M., Klajn, D., Romero, C., Vial Covarrubias, M. J., & Cornejo, R. A. (2022). Follow-up of Interleukin 6 and Other Blood Markers during the Hospitalization of COVID-19 Patients: A Single-Center Study. COVID, 2(11), 1584-1593. https://doi.org/10.3390/covid2110114









