1. Introduction
Coronavirus disease 2019 (COVID-19) is a disease that led to a worldwide pandemic and is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Various clinical outcomes are observed in patients, ranging from asymptomatic, mild, or moderate disease to severe and progressive pneumonia, leading to acute respiratory distress syndrome and multisystem organ failure [
1,
2]. With the COVID-19 outbreak, in several patients, severe bilateral pneumonia was followed by worsening acute respiratory distress syndrome, leading to endotracheal intubation and mechanical ventilation (MV) and other forms of intensive care treatment [
2,
3]. Furthermore, an increased incidence of COVID-19 respiratory-related conditions, such as pneumomediastinum, pneumothorax, and subcutaneous emphysema, were reported in the literature recently [
4,
5].
Tracheomegaly (TM) is a rare and often underdiagnosed appearance that is radiologically defined as an excessive expansion of the tracheal diameter toward higher limits, i.e., 21 and 25 mm in the coronal plane and 23 and 27 mm in the sagittal plane in females and males, respectively [
6,
7,
8,
9,
10]. Though TM is usually congenital, possible causes in adulthood could be connective tissue diseases, such as Mounier–Kuhn syndrome and Ehlers Danlos syndrome, and inflammatory conditions, such as chronic bronchitis, pulmonary and cystic fibrosis, prolonged smoking, and mechanical ventilation with increased cuff pressure [
7,
8,
9,
10]. TM in patients undergoing prolonged mechanical ventilation could be one of the reasons for cuff leaks, leading to unsatisfactory patient ventilation [
8,
9,
10]. TM management is based on distal endotracheal tube repositioning, with the cuff placed outside the dilatation, as previously described [
3,
6,
8,
9,
10]. Till nowadays, only several case reports and a few case series regarding TM have been published [
6,
7,
8,
9,
10]. Since the COVID-19 pandemic occurred, several new published studies regarding the incidence and management of TM in COVID-19 patients were published [
3,
4,
5].
Thus, in our research, we investigated the TM incidence and measurements of tracheal diameters in patients admitted and treated in the intensive care unit (ICU) due to severe COVID-19 pneumonia and their associations with possible respiratory complications. Moreover, we wondered the following: could we consider TM as a potential predictive factor of severe tracheal damage in COVID-19 patients on prolonged MV?
3. Results
The trachea diameter in the first X-ray image in the group of patients treated with HFV was 19.20 ± 2.03 mm overall (19.64 ± 1.63 mm in female patients and 20.04 ± 1.70 mm in male patients), while in the group of patients treated with NC, it was 18.93 ± 1.31 mm overall (18.29 ± 1.66 mm in female patients and 19.58 ± 1.72 mm in male patients). Furthermore, the initial trachea diameter in the first X-ray image in the group of patients treated with MV was 20.44 ± 2.21 mm overall (19.37 ± 1.59 mm in female patients and 20.80 ± 2.28 mm in male patients). The tracheal diameter was analyzed between all included groups using the Kruskal–Wallis test with pairwise comparisons according to Conover; statistical significance was observed between patients treated with MV in regard to HFV and NC (DF = 2,
p < 0.000001). Additionally, the same significance was obtained for both female and male patients (DF = 2,
p < 0.000001) (
Figure 3).
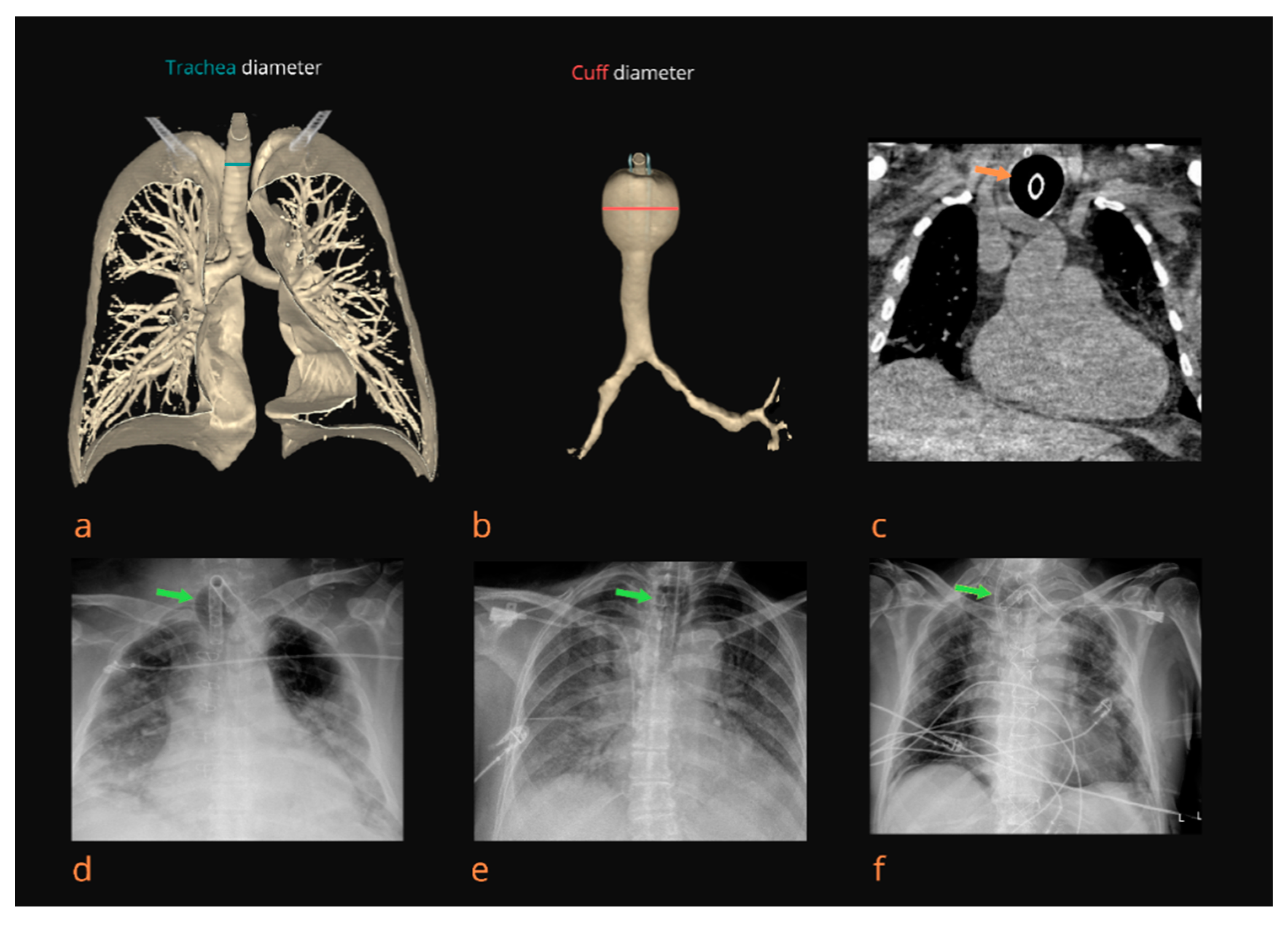
In the group of patients treated with MV, we detected TM in 71 patients (18.54%). The trachea diameter at the level of the cuff when TM was clearly visible was 24.51 ± 3.58 mm overall (23.80 ± 3.85 mm in female patients and 24.81 ± 3.42 mm in male patients). Maximal measured trachea diameter at the level of the cuff during continuous measurement was 25.57 ± 4.90 mm (24.89 ± 4.49 mm in female patients and 26.43 ± 5.00 in male patients). Furthermore, the C/T diameter ratio was, on average, 1.28 ± 1.29 overall (1.28 ± 1.05 in female patients and 1.27 ± 1.28 in male patients). The C/T diameter ratio was >1.5 in 48 patients (12.53%). On average, patients underwent MV for 7.14 ± 5.36 days; the average for female patients was 6.60 ± 4.99 days, while for male patients, it was 7.36 ± 5.51 days. Moreover, 32 patients (8.35%) underwent prolonged MV (≥14 days). In the group of patients with TM, the average time spent on MV was 11.02 ± 1.08 days. In addition, the proportion of TM in patients on prolonged MV was 61.29%.
Regression analysis showed statistical significance for the trachea diameter at the level of the cuff (
p = 0.018, r = 0.12, R
2 = 0.015), maximal trachea diameter (
p = 0.004, r = 0.15, R
2 = 0.02), and C/T diameter ratio (
p = 0.008, r = 0.14, R
2 = 0.02) with respect to days on MV in all analyzed patients (
Figure 4). Furthermore, the analysis was performed for the whole group of patients, as well as separately for females and males (
Table 2).
We observed a number of respiratory complications, namely, tracheoesophageal fistula, pneumomediastinum, pneumothorax, subcutaneous emphysema, and tracheomalacia, in 16.71% of patients overall (
Figure 2). The Spearman’s rank correlation between the C/T diameter ratio and described complications showed statistical significance in all analyzed patients (
p < 0.001, r = 0.27, R
2 = 0.072) (
Figure 3). Furthermore, statistical significance was found between each complication and C/T diameter ratio as follows: tracheoesophageal fistula (
p < 0.001, r = 0.38, R
2 = 0.146), pneumomediastinum (
p < 0.001, r = 0.25, R
2 = 0.062), pneumothorax (
p = 0.001, r = 0.16, R
2 = 0.026), subcutaneous emphysema (
p < 0.001, r = 0.21, R
2 = 0.045), and tracheomalacia (
p = 0.008, r = 0.14, R
2 = 0.018). In addition, we found an association between C/T diameter ratio and performed tracheotomies in 8.09% of our overall cohort (
p < 0.001, r = 0.25, R
2 = 0.061) (
Figure 5).
Additionally, the ROC curve analysis was performed to assess the ability of the C/T diameter ratio to distinguish potential complications. The C/T diameter ratio (area under curve, AUC = 0.66;
p < 0.0001; sensitivity, SE = 50.8%; specificity, SP = 67.0%) was a reliable predictor of complication onset, namely, tracheoesophageal fistula (AUC = 0.95,
p < 0.0001, SE = 100.0%, SP = 84.5%), pneumomediastinum (AUC = 0.65,
p = 0.04, SE = 70.0%, SP = 42.9%), pneumothorax (AUC = 0.61,
p = 0.02, SE = 71.7%, SP = 46.15%), and subcutaneous emphysema (AUC = 0.66,
p = 0.006, SE = 64.5%, SP = 61.8%) (
Figure 6).
4. Discussion
Due to the SARS-CoV-2 outbreak, the healthcare system was restructured; the Primary Respiratory Intensive Center of the Croatian National Covid Hospital was established and located in the Dubrava University Hospital, Zagreb, Croatia. Therefore, we encountered many critically ill COVID-19 ICU patients who required MV. ICU experience obtained during the COVID-19 pandemic with a large number of intubated patients who developed tracheoesophageal fistulas, pneumothorax, and subcutaneous emphysema prompted us to retrospectively analyze the chest X-rays of all ICU patients according to the hospital’s protocol. In the analyzed patients, we observed radiologically visible TM in the cuff area in 18.54%, while the proportion of TM in patients on prolonged MV was 90.6%.
It is known that numerous syndromes and conditions, such as Mounier–Kuhn syndrome, Ehlers Danlos syndrome, pulmonary fibrosis, and cystic fibrosis, as well as multifactorial contributory factors, such as previous smoking history, unregulated diabetes, corticosteroid therapy, airway infection (namely, chronic bronchitis), and multiple chondritis, rigid nasogastric tube, hemodynamic instability of patients, atherosclerotic disease, and an inflated cuff, can lead to the development of TM [
6,
7,
8,
9,
10]. However, iatrogenic TM in patients on prolonged MV was described in the literature only through case reports [
8,
10]. The reason for the descriptions of TM through case reports can be either because of its rarity or because it was not recognized at all. In addition, the incidence of TM in COVID-19 patients on MV is unknown. That was why we were led to believe that the clinical significance of TM in the development of tracheal damage and life-threatening complications has not been sufficiently recognized. Unrecognized and untreated TM leads to severe tracheal damage and life-threatening complications, such as tracheal stenosis, tracheoesophageal fistula, and tracheomalacia.
This rare occurrence in patients undergoing prolonged MV may be one of the causes of cuff leaks and unsatisfactory patient ventilation. To avoid cuff leaks, aspiration, and pneumonia, the cuff must be sufficiently inflated and, according to the recommendations, the cuff pressure and volume must not exceed 30 cm H
2O or 8 mL of air. According to the available literature, the cuff is often overinflated because the cuff pressure is not monitored with a manometer but rather estimated with the pilot balloon palpation or the “finger-pressure” method [
13]. In this case, the finger estimation method was used, and we opted for a more inflated cuff for the purpose of reducing infectious aerosol. This method of cuff pressure estimation was also used in other reported cases of TM [
8,
10]. Prolonged high intra-cuff pressure (more than 40 cm H
2O) leads to a decrease in local perfusion, which causes morphological changes and damage to the mucous membrane and cartilage of the trachea, leading to the development of TM [
6].
According to available data from the literature, the average time of occurrence of TM in non-COVID patients is after approximately 14 days of intubation, while in our cohort, TM was recorded earlier, with a mean appearance time on the ninth day. Fiacchini et al. suggested some of the possible causes of increased incidence of tracheal damage in COVID-19 patients on prolonged MV, such as the implementation of pronation maneuvers, prothrombotic and antifibrinolytic state of patients, high viral replication within the tracheal epithelium, high dose of systematic steroids, hypoxic damage to tracheal mucosa witnessed by a lower PaO
2/FiO
2 ratio, and accidental errors of emotionally and physically exhausted healthcare professionals [
4]. Our patients were also exposed to these factors with the difference being monitored cuff pressure with the pilot balloon palpation or “finger-pressure” method. All of these abovementioned risk factors were present in our patients with TM. According to hospital COVID protocols, all of our patients in the ICU with severe ARDS were treated with a high dose of systemic corticosteroid therapy (methylprednisolone 1–2 mg/kg) for up to 10 days. This is consistent with the national guidelines for COVID-19 treatment and based on a large, randomized trial of corticosteroid therapy’s impact on survival [
14]. It is known that corticosteroids have an impact on mucosal atrophy and alter the normal healing of tracheal wall micro-wounds. Husta et al. found that inhalational corticosteroid therapy decreases the size of the vascular component of the airway wall and may reduce the volume of blood distributed, potentially resulting in smooth muscle weakness, as well as muscle atrophy via catabolic effects on muscle tissue and decreasing protein synthesis [
15]. Immunomodulation caused by high-dose corticosteroid therapy can lead to a higher incidence of secondary bacterial infection, which was also present in all our patients with TM [
15]. All of our patients with TM were represented as severe ARDS, with a PaO
2/FiO
2 ratio below 100. Early implementation of pronation maneuvers (for 12–16 h daily) was reported as beneficial in COVID-19 patients [
16]. However, the prone position increases the cuff pressure on the tracheal wall, leading to its hypoperfusion.
Taking into consideration the rarity of TM, adequate therapy is still not defined. However, treatment can be conservative or surgical. In most described cases, due to the poor condition of patients and present comorbidities, conservative treatment was applied by repositioning the cuff distal to TM, and in cases of low TM, a double-lumen endotracheal tube was used. In patients with a demonstrated tracheoesophageal fistula, tracheomalacia, and cuff leak due to TM, repositioning of the tube distal to TM was performed, with the tracheostomy tube being replaced by an endotracheal tube in tracheotomized patients [
13,
17].
Previously, the correlation between prolonged MV and occurrence of TM and further complications were discussed [
5,
10]. In our cohort among patients in whom TM was observed, the average time spent on MV was 11.02 ± 1.08 days. Although further studies are needed, especially multicentric ones, the fair number of 71 patients in which we observed TM was related to fewer average days of MV than that described in the literature [
6]. Furthermore, pathologic studies of the tracheas of patients who underwent MV showed unique tracheal damage at the site of the cuff [
6,
18]. Lesions appeared within 48 h and progressed from inflammation, mucosa ulceration, cartilage fragmentation, and finally to tracheal wall replacement with scar tissue [
6,
16]. Moreover, tracheal lesions and damage of the tracheal wall, leading to related respiratory complications were shown to increase in COVID-19 patients treated using MV in ICU compared to non-COVID patients, as well as in several case series [
4,
19,
20].
In our cohort, proper pathohistological analysis of trachea wall tissue was not performed due to Croatian national guidelines during the pandemic outbreak. A few studies involving the autopsy of COVID-19 patients showed that SARS-CoV-2 infections cause multiorgan pathology; the most significant changes occur in the respiratory system, such as diffuse alveolar damage, microthrombi, bronchopneumonia, necrotizing bronchiolitis, and viral pneumonia [
21,
22]. Thus, we suggest a significant impact of SARS-CoV-2 on trachea tissue and accelerated mechanism of TM occurrence in COVID-19 patients and occurrence of related complications.
Based on the presented cohort, our clinical experience, and a review of recent literature, we propose recommendations for the prevention, diagnosis, and treatment of TM in patients on MV due to severe COVID-19. For the TM prevention, we recommend the use of soft nasogastric tubes for enteral nutrition, periodic and repeated control of cuff pressure with a manometer, use of endotracheal tubes and a tracheostomy tube with double cuff, careful dosing of corticosteroids, and periodic bronchoscopies for early detection of tracheal lesions in the cuff area. In order to detect damage to the trachea in the cuff area, it is necessary to perform bronchoscopy with the positioning of the tube more proximal, which is not easily feasible in all severe patients on MV. Since frequent radiological monitoring of patients’ lung status is a standard procedure, especially chest X-rays, it is necessary to determine the C/T diameter ratio, which, if greater than 1.5, speaks in favor of TM. A chest X-ray as a very simple radiological method can be extremely important in recognizing tracheomegaly. In the case of an unexplained and sudden appearance of a cuff leak, it is necessary to search for TM. The appearance of pneumomediastinum, pneumothorax, and subcutaneous emphysema may indicate the appearance of tracheal damage. Therefore, the appearance of the aforementioned respiratory complications could present indirect signs of tracheal damage. Furthermore, we encountered tracheomalacia in TM complications. Although our study was retrospective, in one patient, focal tracheomalacia was diagnosed using computerized tomography and bronchoscopy during work in the ICU.
Though we have learned a lot about SARS-CoV-2 infection since the beginning of the pandemic, there is still no consensus on the optimal timing for a tracheostomy, which was debatable even before the pandemic. It cannot be clearly stated whether an early tracheostomy yields better outcomes in critically ill patients [
23]. Our physicians, guided by previous experience with non-COVID patients with acute respiratory distress syndrome, opted for an early tracheostomy, on average, performed on day 9. A multidisciplinary team indicated tracheostomy, while the main contraindications were non-perspective patients and those who often required prone ventilation. As mentioned previously, a tracheostomy was performed in 8.09% of patients in our cohort (31/383). Nonetheless, a higher frequency of TM occurred in patients with a tracheostomy (19/31, 61,29%). The reasons for TM in patients with a tracheostomy may be additional damage done to the trachea at the site of insertion, hyperinflation of the cuff, and damage to the trachea by the tip of the tracheostomy tube. After the cuff is inflated, due to the curlineage of the tracheostomy tube, the tip of the tracheal tube often additionally damages the posterior wall of the trachea. The position of the tracheal part of the tracheostomy tube can also be negatively affected by the respiratory tubes. In patients with pre-existing TM and a C/T ratio greater than 1.5, the tracheostomy further worsened TM, thus, it can be concluded that in these patients it is necessary to postpone the tracheostomy, i.e., it is necessary to place the endotracheal tube more distal to the TM. If a tracheostomy is performed in patients who do not have TM, we recommend the use of a tracheostomy tube with a double cuff (cuffs should be alternately inflated). In patients with a tracheostomy tube that are diagnosed with TM, we recommend reintubation with an endotracheal tube that should be positioned distal to the TM.
Our study offers potential novel insights into COVID-19-related TM; however, the presented results should be considered with respect to several limitations. First of all, the retrospective nature of the study and the single-center experience; our study would benefit if several centers were included. Additionally, we did not include patients who underwent mechanical ventilation due to conditions other than COVID-19. Due to the high incidence of lethal outcomes of COVID-19 patients on MV treated in ICU, the proper conclusion of whether TM is related to a worse outcome was not established. Furthermore, proper pathohistological analysis of tissue was not performed due to Croatian national guidelines during the pandemic outbreak. Finally, we cannot exclude other factors that can lead to TM in COVID-19 patients.