4. Discussion
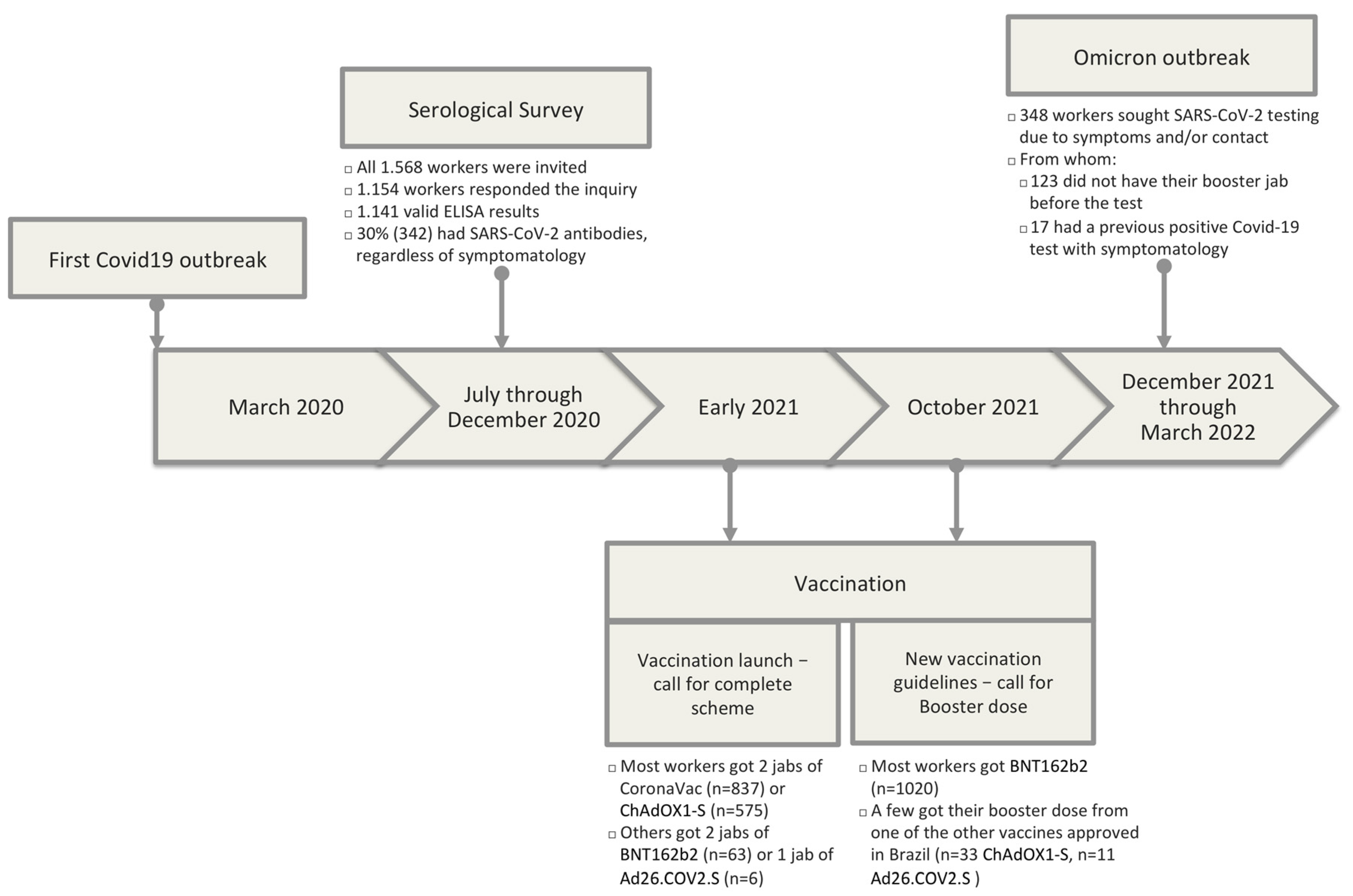
In 2020, we conducted a serological survey on 1154 IFF/Fiocruz workers to monitor SARS-CoV-2 infection in a public hospital environment. We observed a 30% prevalence of SARS-CoV-2 positivity, and the highest infection rates in non-white workers, with lower income and schooling and users of the mass transportation system. This group was mainly composed of hospital support workers and cleaning personnel. These data revealed, once more, Brazilian high inequality even amongst healthcare workers. The present study aimed to verify if the healthcare worker profile at risk was the same during the Omicron outbreak.
Before analyzing the results of the present study, it is worthy to establish the differences between our 2020 serological survey and the data presented herein. In the 2020 serological survey, all institutional workforce was invited to participate in the study, and we evaluated the presence of IgA and IgG in a blood sample from workers that accepted to participate, along with the data from a thorough socio-demographic questionnaire each participant answered. In the present study, we relied on the same cohort of workers, but only those individuals tested for SARS-CoV-2 during the Omicron outbreak, hence, those who either sought to test themselves in our employee testing facilities or reported a positive test result from a private laboratory, confirming the infection during the Omicron wave. In general, individuals were either tested through a PCR or with an antigen test. A positive antigen test was enough to consider the individual as infected, but a negative antigen test further required confirmation through PCR. This is important in light of the reduced sensitivity of antigen tests in comparison to PCR, especially upon the emergence of the Omicron variant [
16]. We also analyzed the data from the vaccination scheme and status.
Despite these differences, in the present study, the proportion of individuals in each socio-demographic characteristic was equivalent to the overall previous serology evaluation (
Table S3) [
8]. The sole feature with a noticeable difference was the occupation. The proportion of support workers in the present study is somewhat higher than in the serology study (43.4% versus 30.2%, respectively). The previous serology study accounted for all workers regardless of symptomatology, whereas the present study was directed to subjects who tested for SARS-CoV-2 due to symptoms. These data might suggest that support workers were slightly more prone to seek SARS-CoV-2 testing during the Omicron wave than willing to adhere to the previous serology inquiry during the first COVID-19 wave. Upon the original outbreak, non-essential workers (mainly support staff) worked from home in compliance with social distance mitigation strategies employed at that time. Upon reaching a high proportion of vaccinated individuals in Rio de Janeiro, most support workers returned to work on-site or in hybrid schemes, alternating on-site with home-office work. This increased proportion of support workers that sought SARS-CoV-2 during the Omicron wave might merely reflect the enhanced opportunity and availability of testing because testing was available at their working place. As mentioned above, our institution is not a referral center for COVID-19 treatment but a pediatric and maternal health facility.
Regarding their vaccination status, Rio de Janeiro called healthcare workers for their booster jab in September 2021 [
17]. By the time the Omicron wave started, most workers had already received their booster. Consistent with the reality in Brazil [
18], most healthcare workers in our institution received either CoronaVac or ChAdOX1-S as the first two vaccine doses. Later, booster doses were mainly of BNT162b2 or ChAdOX1-S, particularly due to the literature indicating more robust protection with either of these vaccines than with CoronaVac [
18,
19].
During the pandemic’s evolution, VOC began to arise. The Alpha variant did not circulate importantly in Brazil, but in December 2020, the Gamma variant (P.1) outbreak in the North region of Brazil began and rapidly spread throughout the country. During the first semester of 2021, Brazil went through the Gamma variant wave, followed by the Delta variant’s arrival in the second semester and the Omicron variant in late 2021. From December 2021, COVID-19 cases in Brazil were virtually entirely composed of the Omicron variant (
Figure S1) [
20].
From December 2021 through March 2022, Brazil, and more specifically, Rio de Janeiro, went through the Omicron wave, which again hit our workforce, with a high number of cases in a short time window. We then analyzed SARS-CoV-2 test results from the 351 healthcare workers from our previous cohort study, tested for SARS-CoV-2 during the Omicron wave. Notably, we observed a different profile of workers with a positive test compared with the initial outbreak in 2020 (
Figure 2). As opposed to the first cohort report, socio-demographic features such as race, income, schooling, and use of mass transportation were no longer associated with a positive test. Actually, the profile of a worker with a positive test during the Omicron outbreak was more associated with a healthcare professional working in high exposure (in-patient and out-patient sectors) than any other socio-demographic characteristic. These data suggest a decreased impact of transmission in commuting to work as well as an increased workplace transmission with patient-worker transmission as the most probable scenario.
During the Omicron wave, the professionals more prone to test positive were those working directly with patients, regardless of education, income, and type of commute to work. These data suggest that in the Omicron phase of the pandemic, workers were more exposed to SARS-CoV-2 in the workplace than in the first COVID-19 wave. These data also indicate that different categories of workers are more equally exposed in transportation or personal life.
In this regard, a few explanations are possible. Firstly, at the beginning of the pandemic, an unprecedented worldwide effort to mitigate transmission based on non-pharmacological measures was severely employed, such as extreme social isolation and mask-wearing whenever social isolation was not possible. However, after two years into those restrictions imposed by the pandemic and a few vaccine jabs later, individuals were no longer sustaining proper transmission control measures. Thus, one possible explanation is the relaxation of non-pharmacological measures, especially in Brazil. Here, the spread of fake news and the lack of effective government policies to control transmission contributed to generating a significant amount of fear in the general population and healthcare workers from public institutions. Moreover, Brazil also experienced a severe Gamma variant outbreak in late 2020/beginning of 2021, which contributed to maintaining high-stress levels in healthcare workers throughout this period.
Secondly, the advent of vaccination and the positive effect on reducing the severity of the disease, hospitalization, and death due to COVID-19 also caused a sensation of safety, therefore promoting the relaxation of individual efforts to mitigate transmission. In the same way, almost all official restrictions regarding transmission mitigation were lifted during the second semester of 2021 in Rio de Janeiro. The sole public measure still sustained at that time was the use of masks in closed environments and public transportation. Altogether, we believe this combination of fatigue and relaxation of transmission mitigation measures throughout the population, including healthcare workers, may have contributed to this pattern shift.
However, two other reasons may have been especially relevant and related to the fact that we are mainly a children’s hospital. First, after over one year of school closure in Brazil, most schools reopened mid-2021. Nevertheless, particularly during the Omicron wave, schools were closed for summer vacations (between December and February). This is consistent with the results of many studies from countries that chose to reopen schools as early as mid-2020, revealing higher infection rates among children during vacation periods than school periods [
21].
Furthermore, concerning our in-hospital reality, very few cases were detected during the first year of the pandemic, and this number increased by several folds during the Omicron outbreak. Moreover, the vaccination of children aged 5 to 11 years in Brazil only began by late January 2022, which was already during the Omicron outbreak [
22]. Additionally, vaccination for children under five years was not approved until mid-July 2022, when vaccination was authorized for children aged 3 to 4 [
23]. Together, these elements may have contributed to an unprecedented number of new COVID-19 cases in patients and their relatives, which in turn highly increased the exposure of children’s hospital workers to the virus. Furthermore, consistent with the increased transmissibility of the Omicron variant [
24], nearly 50% of tests performed in our testing facilities were positive during this period.
An important added contribution of the current study in comparison to our previous one is the evaluation of vaccination in our workforce. It is important to highlight that immune response to COVID-19 vaccination is dependent on several factors such as age, sex, serostatus, and pre-existing comorbidities [
25]. In addition, despite the chronological waning of humoral response post-SARS-CoV-2 vaccination [
26], there has been evidence showing that vaccination could confer protection against long COVID-19 development [
27]. Regarding the lack of association between the booster jab and positivity, our data should be interpreted cautiously because individuals who sought testing were mainly persons with mild symptoms. Therefore, it does not mean that the complete scheme or the booster jab did not protect against moderate and severe disease. In fact, none of the workers were hospitalized following SARS-CoV-2 positive testing during the Omicron wave, which argues for the effectiveness of vaccination.
Because CoronaVac was one of the first COVID-19 vaccines to be approved in Brazil, hundreds of thousands of healthcare workers received two CoronaVac jabs at the beginning of 2021. A few months later, the number of older adults who received two jabs from CoronaVac was rising among patients admitted to hospitals due to COVID-19 with moderate to severe disease [
18]. At the same time, the Delta variant was beginning to strike, and increased protection from a heterologous booster dose with RNA vaccines or ChAdOX1-S was verified [
28,
29]. As a result, Brazil’s first approved booster doses were BNT162b2 and ChAdOX1-S, and started to be offered in September 2021 [
17].
In the present study, we were unable to detect a different level of protection against SARS-CoV-2 symptomatic infection during the Omicron outbreak between healthcare workers who were vaccinated and had a booster dose when compared those who had their first two jabs with either CoronaVac to those who were vaccinated with the other vaccines in use in Brazil. These data are reassuring of the increased protection due to the heterologous vaccination schema previously reported [
19,
28,
29].
Several studies have demonstrated the higher prevalence of SARS-CoV-2 in healthcare workers compared to the general population [
30,
31,
32,
33]. Identifying the profile of infected individuals is important for protecting both the healthcare workforce and the patients; through better designing of safe workplaces, maintaining effective health services, reducing patient exposure to COVID-19, and driving public policies for the protection and vaccination of each group, especially given situations many countries still face of vaccine shortage [
34].
Our data highlight the fact that during the Omicron outbreak, in-patient and out-patient hospital workers were more exposed and, indeed, more infected. Furthermore, these data demonstrate the challenge of constantly monitoring disease-spread behavior to efficiently contribute to policy makers about vaccine prioritization and booster schedules in underprivileged countries. Accordingly, the findings reported here might be applied to healthcare facilities in general and highlight the importance of vaccination to mitigate socio-economical inequalities among healthcare professionals regarding SARS-CoV-2 infection.
One limitation of our analyses is that we did not confirm SARS-CoV-2 variant through virus genome sequencing. However, nearly all SARS-CoV-2 genomes sequenced in Brazil during the study period (21 December–22 March) were of the Omicron lineage. We highlight that our institution is part of the Brazilian Network of SARS-CoV-2 Genomic Surveillance, a group of over 20 academic, health, and research institutions in Brazil that monitor SARS-CoV-2 variants’ circulation in our country. Almost 200 thousand SARS-CoV-2 genomes have been sequenced from infected individuals all over the country since March 2020. Genomic Surveillance data is depicted in
Supplementary Table S2.
Another limitation is that the cohort of this study is very specific: health professionals who work in a maternal and infant institution, where we do not have emergency attendance and we do not receive patients with COVID-19 as a referral center. Thus, we cannot extrapolate our results to the general population. However, our data is valuable for the particular subset of healthcare workers in non-COVID-19 referral centers, which represent the vast majority of healthcare institutions.
Regarding vaccination, we have one of the largest National Immunization Program in the world and very low vaccine hesitancy in Brazil, especially amongst healthcare workers [
35,
36]. Nearly all health workers had a complete vaccination schedule by the time the Omicron wave hit us. So, we could not detect a statistically significant difference between the vaccinated and unvaccinated groups. Furthermore, our cohort was not large enough to detect a statistically significant difference between the boosted and non-boosted groups.
As for the strengths of our study, we highlight that we are observing this cohort since the beginning of the COVID-19 pandemic and we have information on almost every worker on a large amount of SARS-CoV-2 epidemiological- and laboratorial-relevant features, such as serological characteristics, habits, contamination by SARS-CoV-2, and its consequences. Because of that, we were able to compare the same population in two different time frames of the pandemics: the very beginning and the post-vaccination era.