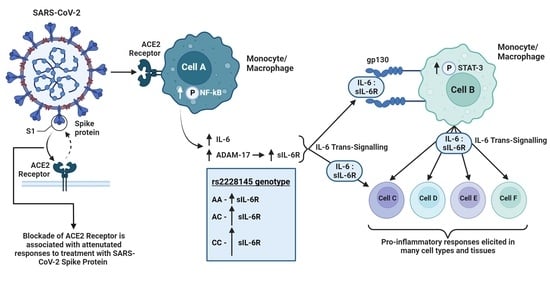
The rs2228145 Variant of the Interleukin-6 Receptor (IL-6R) Gene Impacts on In Vitro Cellular Responses to SARS-CoV-2 VOC B1.1.7 Recombinant Spike Protein
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Maintenance, Differentiation and Treatment of Cell Lines
2.3. Genotyping Assay
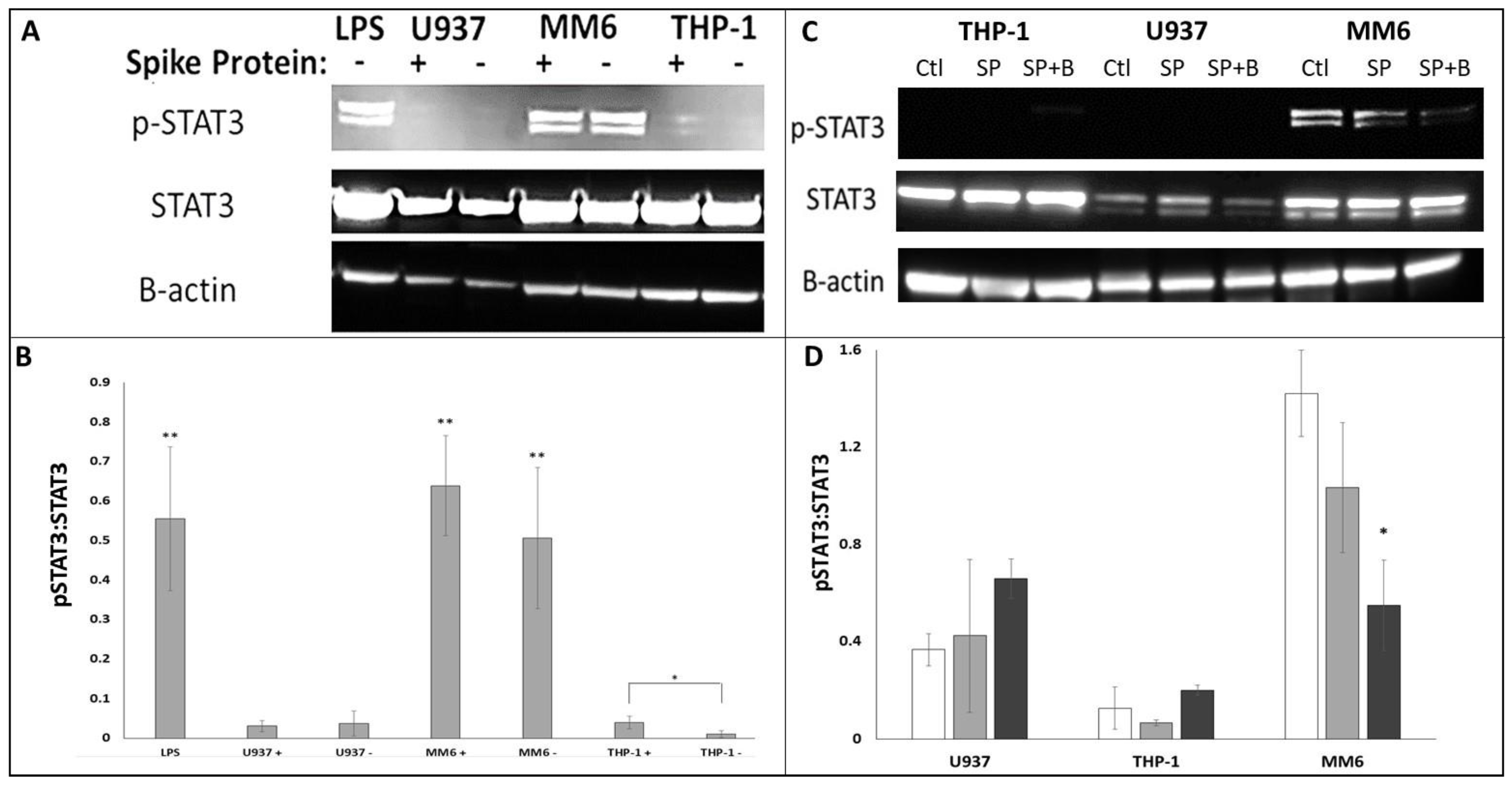
2.4. Western Blot
2.5. Flow Cytometry
2.6. Bioinformatics
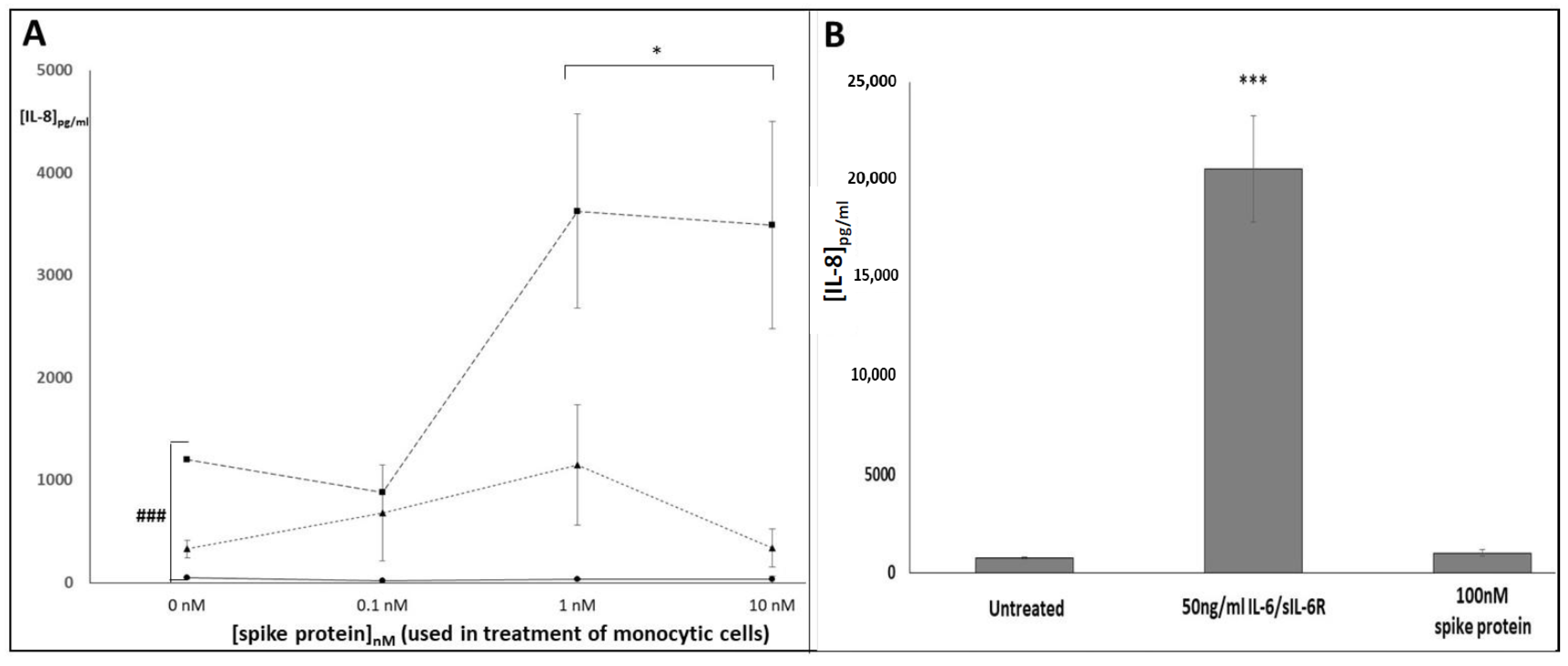
2.7. DuoSet ELISA: Human IL-6, Human sIL-6R, Human sgp130 and Human IL-8
2.8. Statistical Analysis
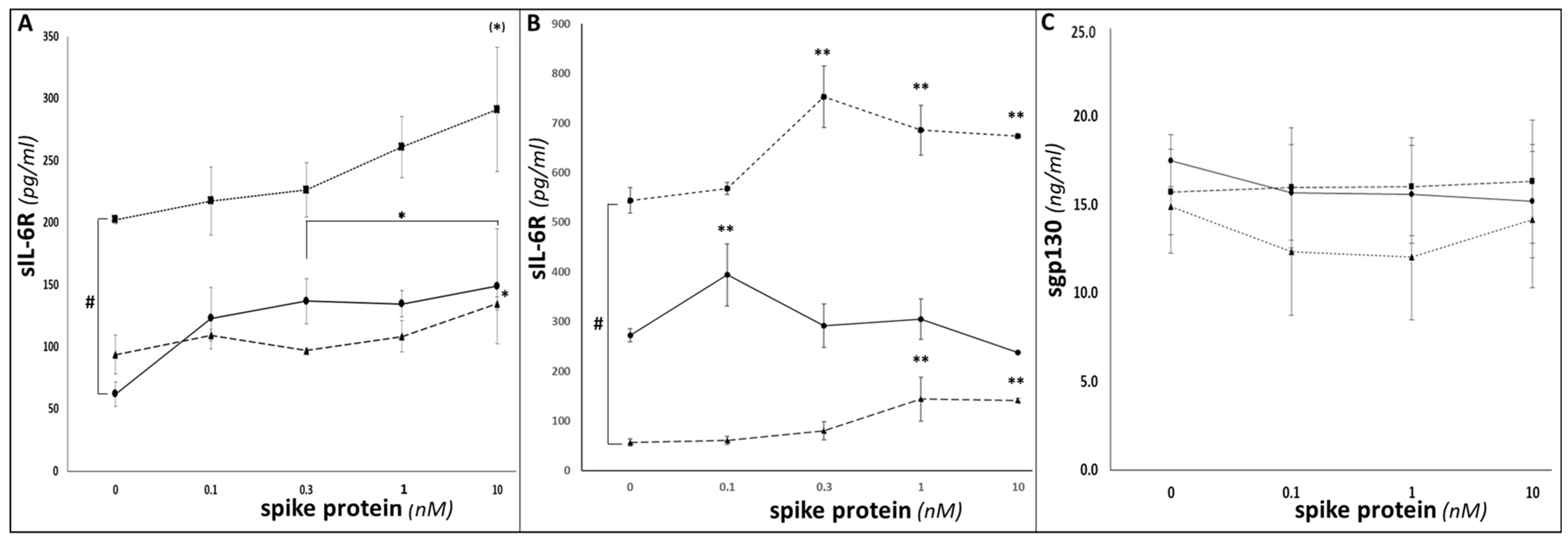
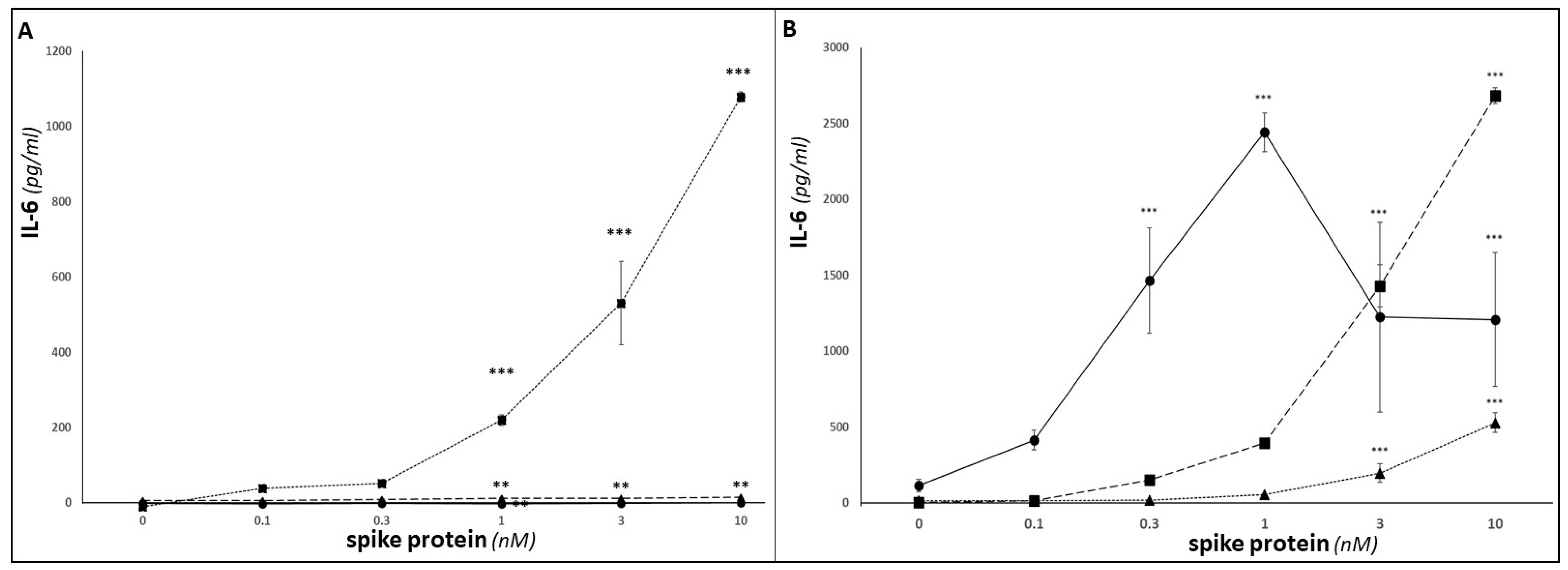
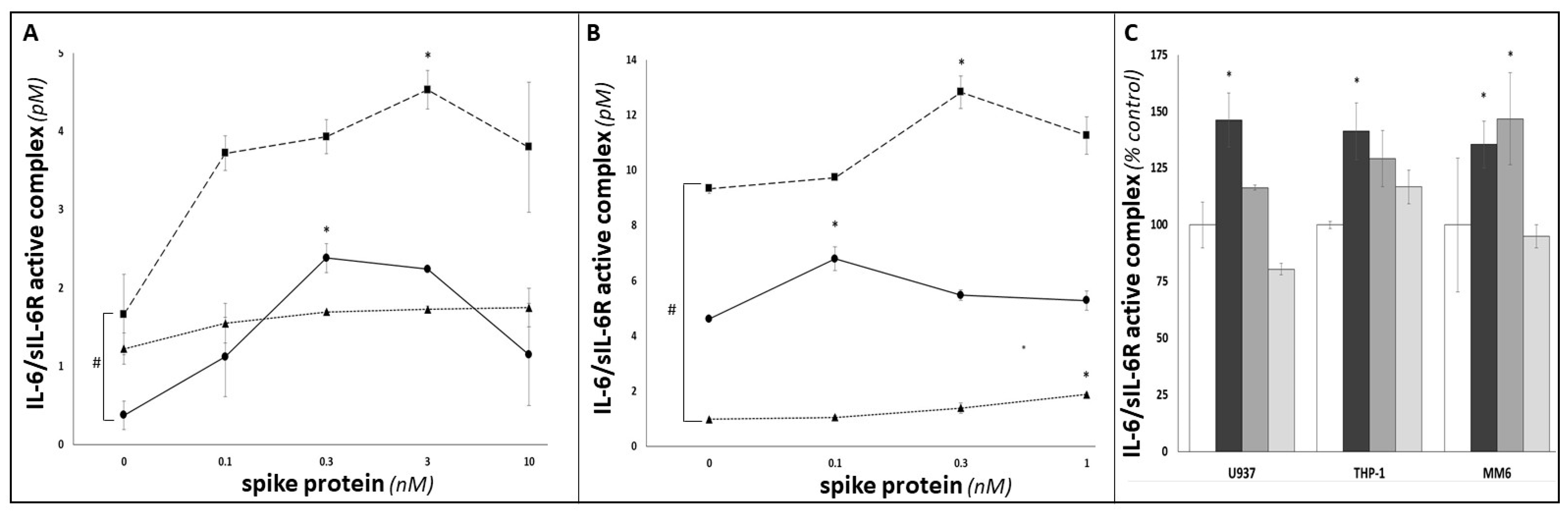
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pedersen, S.F.; Ho, Y.-C. SARS-CoV-2: A storm is raging. J. Clin. Investig. 2020, 130, 2202–2205. [Google Scholar] [CrossRef]
- Chen, C.; Haupert, S.R.; Zimmermann, L.; Shi, X.; Fritsche, L.G.; Mukherjee, B. Global Prevalence of Post-Coronavirus Disease 2019 (COVID-19) Condition or Long COVID: A Meta-Analysis and Systematic Review. J. Infect. Dis. 2022, 226, 1593–1607. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Klein, S.L.; Garibaldi, B.T.; Li, H.; Wu, C.; Osevala, N.M.; Li, T.; Margolick, J.B.; Pawelec, G.; Leng, S.X. Aging in COVID-19: Vulnerability, immunity and intervention. Ageing Res. Rev. 2021, 65, 101205. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Biggs, C.M.; Jamal, S.; Stukas, S.; Wellington, C.L.; Sekhon, M.S. Soluble interleukin-6 receptor in the COVID-19 cytokine storm syndrome. Cell Rep. Med. 2021, 2, 100269. [Google Scholar] [CrossRef]
- Elezkurtaj, S.; Greuel, S.; Ihlow, J.; Michaelis, E.G.; Bischoff, P.; Kunze, C.A.; Sinn, B.V.; Gerhold, M.; Hauptmann, K.; Ingold-Heppner, B.; et al. Causes of death and comorbidities in hospitalized patients with COVID-19. Sci. Rep. 2021, 11, 4263. [Google Scholar] [CrossRef] [PubMed]
- Merad, M.; Martin, J.C. Pathological inflammation in patients with COVID-19: A key role for monocytes and macrophages. Nat. Rev. Immunol. 2020, 20, 355–362. [Google Scholar] [CrossRef]
- McGonagle, D.; Sharif, K.; O’Regan, A.; Bridgewood, C. The Role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and Macrophage Activation Syndrome-Like Disease. Autoimmun. Rev. 2020, 19, 102537. [Google Scholar] [CrossRef]
- Merad, M.; Blish, C.A.; Sallusto, F.; Iwasaki, A. The immunology and immunopathology of COVID-19. Science 2022, 375, 1122–1127. [Google Scholar] [CrossRef]
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 23 June 2023).
- Sukocheva, O.A.; Maksoud, R.; Beeraka, N.M.; Madhunapantula, S.V.; Sinelnikov, M.; Nikolenko, V.N.; Neganova, M.E.; Klochkov, S.G.; Kamal, M.A.; Staines, D.R.; et al. Analysis of post COVID-19 condition and its overlap with myalgic encephalomyelitis/chronic fatigue syndrome. J. Adv. Res. 2022, 40, 179–196. [Google Scholar] [CrossRef]
- Kappelmann, N.; Dantzer, R.; Khandaker, G.M. Interleukin-6 as potential mediator of long-term neuropsychiatric symptoms of COVID-19. Psychoneuroendocrinology 2021, 131, 105295. [Google Scholar] [CrossRef]
- Shirato, K.; Kizaki, T. SARS-CoV-2 spike protein S1 subunit induces pro-inflammatory responses via toll-like recep-tor 4 signaling in murine and human macrophages. Heliyon 2021, 7, e06187. [Google Scholar] [CrossRef] [PubMed]
- Lokau, J.; Agthe, M.; Garbers, C. Generation of Soluble Interleukin-11 and Interleukin-6 Receptors: A Crucial Function for Proteases during Inflammation. Mediat. Inflamm. 2016, 2016, 1785021. [Google Scholar] [CrossRef] [PubMed]
- Patra, T.; Meyer, K.; Geerling, L.; Isbell, T.S.; Hoft, D.F.; Brien, J.; Pinto, A.K.; Ray, R.B.; Ray, R. SARS-CoV-2 spike protein promotes IL-6 trans-signaling by activation of angiotensin II receptor signaling in epithelial cells. PLOS Pathog. 2020, 16, e1009128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wu, Z.; Li, J.-W.; Zhao, H.; Wang, G.Q. Cytokine Release Syndrome in Severe COVID-19: Interleukin-6 Receptor Antagonist Tocilizumab may be the Key to Reduce Mortality. Int. J. Antimicrob. Agents 2020, 55, 105954. [Google Scholar] [CrossRef] [PubMed]
- Di Spigna, G.; Cernia, D.S.; Vargas, M.; Buonavolontà, L.; Servillo, G.; Postiglione, L. Drastically elevated levels of Interleukin-6 and its soluble receptor complex in COVID-19 patients with acute respiratory distress. Clin. Med. Investig. 2020, 5, 1–4. [Google Scholar] [CrossRef]
- Xu, X.; Han, M.; Li, T.; Sun, W.; Wang, D.; Fu, B.; Zhou, Y.; Zheng, X.; Yang, Y.; Li, X.; et al. Effective Treatment of Severe COVID-19 Patients with Tocilizumab. Proc. Natl. Acad. Sci. USA 2020, 117, 10970–10975. [Google Scholar] [CrossRef]
- Guaraldi, G.; Meschiari, M.; Cozzi-Lepri, A.; Milic, J.; Tonelli, R.; Menozzi, M.; Franceschini, E.; Cuomo, G.; Orlando, G.; Borghi, V.; et al. Tocilizumab in patients with severe COVID-19: A retrospective cohort study. Lancet Rheumatol. 2020, 2, e474–e484. [Google Scholar] [CrossRef]
- RECOVERY (Randomised Evaluation of COVID-19 Therapy) Trial. Available online: https://www.recoverytrial.net/ (accessed on 6 July 2023).
- REMAP-CAP (Randomised, Embedded, Multi-factorial, Adaptive Platform Trial for Community-Acquired Pneumonia) Trial. Available online: www.remapcap.org (accessed on 6 July 2023).
- Galicia, J.C.; Tai, H.; Komatsu, Y.; Shimada, Y.; Akazawa, K.; Yoshie, H. Polymorphisms in the IL-6 receptor (IL-6R) gene: Strong evidence that serum levels of soluble IL-6R are genetically influenced. Genes Immun. 2004, 5, 513–516. [Google Scholar] [CrossRef]
- van Dongen, J.; Jansen, R.; Smit, D.; Hottenga, J.J.; Mbarek, H.; Willemsen, G.; Kluft, C.; AAGC Collaborators; Penninx, B.W.; Ferreira, M.A.; et al. The contribution of the IL-6R polymorphism rs228145 to the heritability of plasma sIL-6R levels. Behav. Genet. 2014, 44, 368–382. [Google Scholar] [CrossRef]
- Ferreira, M.A.; Matheson, M.C.; Duffy, D.L.; Marks, G.B.; Hui, J.; Le Souëf, P.; Danoy, P.; Baltic, S.; Nyholt, D.R.; Jenkins, M.; et al. Identification of IL6R and chromosome 11q13.5 as risk loci for asthma. Lancet 2011, 378, 1006–1014. [Google Scholar] [CrossRef]
- Ferreira, R.C.; Freitag, D.F.; Cutler, A.J.; Howson, J.M.M.; Rainbow, D.B.; Smyth, D.J.; Kaptoge, S.; Clarke, P.; Boreham, C.; Coulson, R.M.; et al. Functional IL6R 358Ala Allele Impairs Classical IL-6 Receptor Signaling and Influences Risk of Diverse Inflammatory Diseases. PLoS Genet. 2013, 9, e1003444. [Google Scholar] [CrossRef] [PubMed]
- Tregoning, J.S.; Flight, K.E.; Higham, S.L.; Wang, Z.; Pierce, B.F. Progress of the COVID-19 vaccine effort: Viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat. Rev. Immunol. 2021, 21, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Grygiel-Górniak, B.; Puszczewicz, M. Fatigue and interleukin-6—A multi-faceted relationship. Rheumatology 2015, 53, 207–212. [Google Scholar] [CrossRef]
- Newton, T.; Fernandez-Botran, R.; Miller, J.J.; Burns, V.E. IL-6 and sIL-6R levels in PTSD: Associations with diagnostic status and psychological Context. Biol. Psychol. 2014, 99, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Vargas, N.; Marino, F. Neuroinflammation, cortical activity, and fatiguing behaviour during self-paced exercise. Pflug. Arch. Eur. J. Physiol. 2017, 470, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Quillen, D.; Hughes, T.M.; Craft, S.; Howard, T.; Register, T.; Suerken, C.; Hawkins, G.A.; Milligan, C. Levels of Soluble Interleukin 6 Receptor and Asp358Ala Are Associated with Cognitive Performance and Alzheimer Disease Biomarkers. Neurol.—Neuroimmunol. Neuroinflammation 2023, 10, e200095. [Google Scholar] [CrossRef]
- Barnett, M.L.; Sax, P.E. Long-term Follow-up After Critical COVID-19. JAMA 2023, 329, 25–27. [Google Scholar] [CrossRef]
- Florescu, S.; Stanciu, D.; Zaharia, M.; Kosa, A.; Codreanu, D.; Kidwai, A.; Masood, S.; Kaye, C.; Coutts, A.; MacKay, L.; et al. Long-term (180-Day) Outcomes in Critically Ill Patients With COVID-19 in the REMAP-CAP Randomized Clinical Trial. JAMA 2023, 329, 39–51. [Google Scholar] [CrossRef]
- Benedetti, F.; Mazza, M.; Cavalli, G.; Ciceri, F.; Dagna, L.; Rovere-Querini, P. Can Cytokine Blocking Prevent Depression in COVID-19 Survivors? J. Neuroimmune Pharmacol. 2021, 16, 1–3. [Google Scholar] [CrossRef]
- Kodosaki, E.; Daniels-Morgan, A.; Webb, R.; Morris, K.; Kelly, C. Development and characterisation of mgTHP-1, a novel in-vitro model for neural macrophages with microglial characteristics. Neurol. Res. 2023, in press. [Google Scholar]
- Shechter, R.; London, A.; Varol, C.; Raposo, C.; Cusimano, M.; Yovel, G.; Rolls, A.; Mack, M.; Pluchino, S.; Martino, G.; et al. Infiltrating Blood-Derived Macrophages Are Vital Cells Playing an Anti-inflammatory Role in Recovery from Spinal Cord Injury in Mice. PLoS Med. 2009, 6, e1000113. [Google Scholar] [CrossRef] [PubMed]
- Karwaciak, I.; Sałkowska, A.; Karaś, K.; Dastych, J.; Ratajewski, M. Nucleocapsid and Spike Proteins of the Coronavirus SARS-CoV-2 Induce IL6 in Monocytes and Macrophages—Potential Implications for Cytokine Storm Syndrome. Vaccines 2021, 9, 54. [Google Scholar] [CrossRef] [PubMed]
- Ziegler-Heitbrock, H.W.L.; Thiel, E.; Futterer, A.; Herzog, V.; Wirtz, A.; Riethmullar, G. Establishment of a Human Cell Line (Mono Mac 6) with Characteristics of Mature Monocytes. Int. J. Cancer 1988, 41, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, S.; Yamabe, M.; Yamaguchi, Y.; Kobayashi, Y.; Konno, T.; Tada, K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int. J. Cancer 1980, 26, 171–176. [Google Scholar] [CrossRef]
- Sundström, C.; Nilsson, K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int. J. Cancer 1976, 17, 565–577. [Google Scholar] [CrossRef]
- Bowdish, D.M.E. Maintenance & Culture OF THP-1 Cells. 2011. Available online: http://www.bowdish.ca/lab/wp-content/uploads/2011/07/THP-1-propagation-culture.pdf (accessed on 22 July 2023).
- Isa, S.A.; Ruffino, J.S.; Ahluwalia, M.; Thomas, A.W.; Morris, K.; Webb, R. M2 macrophages exhibit higher sensitivity to oxLDL-induced lipotoxicity than other monocyte/macrophage subtypes. Lipids Health Dis. 2011, 10, 229. [Google Scholar] [CrossRef]
- Barhoumi, T.; Alghanem, B.; Shaibah, H.; Mansour, F.A.; Alamri, H.S.; Akiel, M.A.; Alroqi, F.; Boudjelal, M. SARS-CoV-2 Coronavirus Spike Protein-Induced Apoptosis, Inflammatory, and Oxidative Stress Responses in THP-1-Like-Macrophages: Potential Role of Angiotensin-Converting Enzyme Inhibitor (Perindopril). Front. Immunol. 2021, 12, 728896. [Google Scholar] [CrossRef]
- Buzhdygan, T.P.; DeOre, B.J.; Baldwin-Leclair, A.; Bullock, T.A.; McGary, H.M.; Khan, J.A.; Razmpour, R.; Hale, J.F.; Galie, P.A.; Potula, R.; et al. The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in-vitro models of the human blood–brain barrier. Neurobiol. Dis. 2020, 146, 105131. [Google Scholar] [CrossRef]
- Webb, R.; Hughes, M.G.; Nash, D.; Early, A.; Scarlett, B.; Clark, J.; Doran, J.; Butcher, L. Provisional Designation of IL-6R as a Novel Exercise Marker Gene. Exerc. Med. 2021, 5, 1–6. [Google Scholar] [CrossRef]
- Davies, N.A.; Watkeys, L.; Butcher, L.; Potter, S.; Hughes, M.G.; Moir, H.; Morris, K.; Thomas, A.W.; Webb, R. The contributions of oxidative stress, oxidised lipoproteins and AMPK towards exercise-associated PPARγ signalling within human monocytic cells. Free. Radic. Res. 2014, 49, 45–56. [Google Scholar] [CrossRef]
- Garbers, C.; Thaiss, W.; Jones, G.W.; Waetzig, G.H.; Lorenzen, I.; Guilhot, F.; Lissilaa, R.; Ferlin, W.G.; Grötzinger, J.; Jones, S.A.; et al. Inhibition of classic signaling is a novel function of soluble glycoprotein 130 (sgp130), which is controlled by the ration of interleukin 6 and soluble interleukin 6 receptor. J. Biol. Chem. 2011, 286, 42959–42970. [Google Scholar] [CrossRef] [PubMed]
- Garbers, C.; Aparicio-Siegmund, S.; Rose-John, S. The IL-6/gp130/STAT3 signaling axis: Recent advances towards specific inhibition. Curr. Opin. Immunol. 2015, 34, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Aparicio-Siegmund, S.; Garbers, Y.; Flynn, C.M.; Waetzig, G.H.; Gouni-Berthold, I.; Krone, W.; Berthold, H.K.; Laudes, M.; Rose-John, S.; Garbers, C. The IL-6-neutralizing sIL-6R-sgp130 buffer system is disturbed in patients with type 2 diabetes. Am. J. Physiol. Metab. 2019, 317, E411–E420. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Mora, P.; Ander, B.P.; Jickling, G.C.; Dykstra-Aiello, C.; Zhan, X.; Ferino, E.; Hamade, F.; Amini, H.; Hull, H.; Sharp, F.R.; et al. Distinct peripheral blood monocyte and neutrophil transcriptional programs following intracerebral hemorrhage and different etiologies of ischemic stroke. J. Cereb. Blood Flow Metab. 2021, 41, 1398–1416. [Google Scholar] [CrossRef]
- Bosshart, H.; Heinzelmann, M. THP-1 cells as a model for human monocytes. Ann. Transl. Med. 2016, 4, 438. [Google Scholar] [CrossRef]
- Strefford, J.C.; Foot, N.J.; Chaplin, T.; Neat, M.J.; Oliver, R.T.D.; Young, B.D.; Jones, L.K. The characterisation of the lymphoma cell line U937, using comparative genomic hybridisation and multi-plex FISH. Cytogenet. Cell Genet. 2001, 94, 9–14. [Google Scholar] [CrossRef]
- Akula, S.; Lara, S.; Olsson, A.-K.; Hellman, L. Quantitative Analysis of the Transcriptome of Two Commonly Used Human Monocytic Cell Lines—THP-1 and Mono Mac 6—Reveals Their Arrest during Early Monocyte/Neutrophil Differentiation. Int. J. Mol. Sci. 2022, 23, 5818. [Google Scholar] [CrossRef]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS- CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef]
- Padberg, F.; Feneberg, W.; Schmidt, S.; Schwarz, M.; Körschenhausen, D.; Greenberg, B.; Nolde, T.; Müller, N.; Trapmann, H.; König, N.; et al. CSF and serum levels of soluble interleukin-6 receptors (sIL-6R and sgp130), but not of interleukin-6 are altered in multiple sclerosis. J. Neuroimmunol. 1999, 99, 218–223. [Google Scholar] [CrossRef]
- Michalopoulou, M.; Nikolaou, C.; Tavernarakis, A.; Alexandri, N.-M.; Rentzos, M.; Chatzipanagiotou, S.; Cambouri, C.; Vassilopoulos, D. Soluble interleukin-6 receptor (sIL-6R) in cerebrospinal fluid of patients with inflammatory and non inflammatory neurological diseases. Immunol. Lett. 2004, 94, 183–189. [Google Scholar] [CrossRef]
- Bovijn, J.; Lindgren, C.M.; Holmes, M.V. Genetic variants mimicking therapeutic inhibition of IL-6 receptor signaling and risk of COVID-19. Lancet Rheumatol. 2020, 2, e658–e659. [Google Scholar] [CrossRef] [PubMed]
- Nash, D. IL-6 signaling in acute exercise and chronic training: Potential consequences for health and athletic performance. Ph.D. Thesis, Cardiff Metropolitan University, Cardiff, UK, 2023. [Google Scholar]
- Vargas, V.; Bonatto, S.; Macagnan, F.; Feoli, A.; Alho, C.; Santos, N.; Schmitt, V. Influence of the 48867A>C (Asp358Ala) IL6R polymorphism on response to a lifestyle modification intervention in individuals with metabolic syndrome. Genet. Mol. Res. 2013, 12, 3983–3991. [Google Scholar] [CrossRef] [PubMed]
- Cullen, T.; Thomas, A.; Webb, R.; Phillips, T.; Hughes, M.G. sIL-6R is related to weekly training mileage and psychological wellbeing in athletes. Med. Sci. Sport Exerc. 2017, 49, 1176–1183. [Google Scholar] [CrossRef] [PubMed]
- Starkweather, A. Psychologic and Biologic Factors Associated with Fatigue in Patients with Persistent Radiculopathy. Pain Manag. Nurs. 2013, 14, 41–49. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarwar, S.; Aicheler, R.; Butcher, L.; Rees, K.; Potter, S.; Rowlands, R.; Webb, R. The rs2228145 Variant of the Interleukin-6 Receptor (IL-6R) Gene Impacts on In Vitro Cellular Responses to SARS-CoV-2 VOC B1.1.7 Recombinant Spike Protein. COVID 2023, 3, 1554-1570. https://doi.org/10.3390/covid3100106
Sarwar S, Aicheler R, Butcher L, Rees K, Potter S, Rowlands R, Webb R. The rs2228145 Variant of the Interleukin-6 Receptor (IL-6R) Gene Impacts on In Vitro Cellular Responses to SARS-CoV-2 VOC B1.1.7 Recombinant Spike Protein. COVID. 2023; 3(10):1554-1570. https://doi.org/10.3390/covid3100106
Chicago/Turabian StyleSarwar, Saira, Rebecca Aicheler, Lee Butcher, Katie Rees, Stephen Potter, Richard Rowlands, and Richard Webb. 2023. "The rs2228145 Variant of the Interleukin-6 Receptor (IL-6R) Gene Impacts on In Vitro Cellular Responses to SARS-CoV-2 VOC B1.1.7 Recombinant Spike Protein" COVID 3, no. 10: 1554-1570. https://doi.org/10.3390/covid3100106