Evaluation of CoronaVac and CoviShield Vaccines on SARS-CoV-2 Infection in Healthcare Workers in Salvador, Brazil
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Faria, N.R.; Mellan, T.A.; Whittaker, C.; Claro, I.M.; da Candido, D.S.; Mishra, S.; Crispim, M.A.E.; Sales, F.C.S.; Hawryluk, I.; McCrone, J.T.; et al. Genomics and Epidemiology of the P.1 SARS-CoV-2 Lineage in Manaus, Brazil. Science 2021, 372, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Campos, K.R.; Sacchi, C.T.; Abbud, A.; Caterino-De-Araujo, A. SARS-CoV-2 Variants in Severely Symptomatic and Deceased Persons Who Had Been Vaccinated against COVID-19 in São Paulo, Brazil. Rev. Panam. De Salud Publica 2021, 45, e126. [Google Scholar] [CrossRef] [PubMed]
- Alcantara, L.C.J.; Nogueira, E.; Shuab, G.; Tosta, S.; Fristch, H.; Pimentel, V.; Souza-Neto, J.A.; Coutinho, L.L.; Fukumasu, H.; Sampaio, S.C.; et al. SARS-CoV-2 Epidemic in Brazil: How the Displacement of Variants Has Driven Distinct Epidemic Waves. Virus Res. 2022, 315, 198785. [Google Scholar] [CrossRef]
- Freitas, A.R.R.; Beckedorff, O.A.; de Cavalcanti, L.P.G.; Siqueira, A.M.; de Castro, D.B.; da Costa, C.F.; Lemos, D.R.Q.; Barros, E.N.C. The Emergence of Novel SARS-CoV-2 Variant P.1 in Amazonas (Brazil) Was Temporally Associated with a Change in the Age and Sex Profile of COVID-19 Mortality: A Population Based Ecological Study. Lancet Reg. Health–Am. 2021, 1, 100021. [Google Scholar] [CrossRef]
- Giovanetti, M.; Slavov, S.N.; Fonseca, V.; Wilkinson, E.; Tegally, H.; Patané, J.S.L.; Viala, V.L.; San, E.J.; Rodrigues, E.S.; Santos, E.V.; et al. Genomic Epidemiology of the SARS-CoV-2 Epidemic in Brazil. Nat. Microbiol. 2022, 7, 1490–1500. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.F.; Esteves, R.J.; Siza, C.; Soares, E.P.; Ramos, T.C.; Campelo, E.C.; da Costa, C.F.; de Alencar, L.C.; Cavalcante, R.P.; Florêncio, C.R.; et al. Cluster of SARS-CoV-2 Gamma Variant Infections, Parintins, Brazil, March 2021. Emerg. Infect. Dis. 2022, 28, 262–264. [Google Scholar] [CrossRef]
- Naveca, F.G.; Nascimento, V.; de Souza, V.C.; de Corado, A.L.; Nascimento, F.; Silva, G.; Costa, Á.; Duarte, D.; Pessoa, K.; Mejía, M.; et al. COVID-19 in Amazonas, Brazil, Was Driven by the Persistence of Endemic Lineages and P.1 Emergence. Nat. Med. 2021, 27, 1230–1238. [Google Scholar] [CrossRef] [PubMed]
- Brasil, Agência Nacional de Vigilância Sanitária (ANVISA). Parecer No 2/2021 /SEI/GGFIS/DIRE4/ANVISA. Parecer Técnico Público—Uso Emergencial Instituto Butantan; Brazil. 2021; pp. 1–3. Available online: https://www.gov.br/anvisa/pt-br/assuntos/noticias-anvisa/2021/confira-materiais-da-reuniao-extraordinaria-da-dicol/parecer-no-2_2021_sei_ggfis_dire4_anvisa_butantan.pdf (accessed on 13 July 2023).
- Brasil, Agência Nacional de Vigilância Sanitária (ANVISA). Parecer No 3/2021 /SEI/GIMED/GGFIS/DIRE4/ANVISA. Parecer Técnico Público—Uso Emergencial FIOCRUZ; Brazil. 2021; pp. 1–5. Available online: https://www.gov.br/anvisa/pt-br/assuntos/noticias-anvisa/2021/confira-materiais-da-reuniao-extraordinaria-da-dicol/parecer-no-3_2021_sei_gimed_ggfis_dire4_anvisa_fiocruz.pdf (accessed on 13 July 2023).
- Ministerio da Saúde; Secretaria de Vigilância em Saúde; Departamento de Imunização e Doenças Transmissiveis. Plano Nacional de Operacionalização da Vacinação a COVID-19. Available online: https://sbim.org.br/images/files/notas-tecnicas/1-edicao-plano-operacionalizacao-vacinacao-covid19.pdf (accessed on 26 October 2022).
- Fundação Oswaldo Cruz; VigiVac Avaliação Digital Da Campanha de Vacinação Contra COVID-19 No Brasil, Brazil. 2021, pp. 1–13. Available online: https://www.arca.fiocruz.br/bitstream/handle/icict/50370/RelatorioEfetividade9dez2021.pdf?sequence=2&isAllowed=y (accessed on 13 July 2023).
- Hu, J.; Peng, P.; Cao, X.; Wu, K.; Chen, J.; Wang, K.; Tang, N.; Huang, A. Increased Immune Escape of the New SARS-CoV-2 Variant of Concern Omicron. Cell. Mol. Immunol. 2022, 19, 293–295. [Google Scholar] [CrossRef] [PubMed]
- Puhach, O.; Adea, K.; Hulo, N.; Sattonnet, P.; Genecand, C.; Iten, A.; Jacquérioz, F.; Kaiser, L.; Vetter, P.; Eckerle, I.; et al. Infectious Viral Load in Unvaccinated and Vaccinated Individuals Infected with Ancestral, Delta or Omicron SARS-CoV-2. Nat. Med. 2022, 28, 1491–1500. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Tracking SARS-CoV-2 Variants. Available online: https://www.who.int/activities/tracking-SARS-CoV-2-variants (accessed on 26 September 2023).
- Singanayagam, A.; Hakki, S.; Dunning, J.; Madon, K.J.; Crone, M.A.; Koycheva, A.; Derqui-Fernandez, N.; Barnett, J.L.; Whitfield, M.G.; Varro, R.; et al. Community Transmission and Viral Load Kinetics of the SARS-CoV-2 Delta (B.1.617.2) Variant in Vaccinated and Unvaccinated Individuals in the UK: A Prospective, Longitudinal, Cohort Study. Lancet Infect. Dis. 2022, 22, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, Z.S.; Salem, H.A.; Veiga, S.P.; de Lima, F.E.B.; da Gonçalves, C.R.S.; dos Santos, E.C.; Brandão, A.R.J.; Couceiro, K.N.; de Guerra, J.A.O.; das Guerra, M.G.V.B. Immunogenicity Characterization of COVID-19 Vaccines: A Systematic Review and Meta-Analysis. Rev. Soc. Bras. Med. Trop. 2023, 56, e0660-2021. [Google Scholar] [CrossRef] [PubMed]
- Brasil, Agência Nacional de Vigilância Sanitária (ANVISA). NOTA TÉCNICA GVIMS/GGTES/ANVISA No 04/2020. ORIENTAÇÕES PARA SERVIÇOS DE SAÚDE: MEDIDAS DE PREVENÇÃO E CONTROLE QUE DEVEM SER ADOTADAS DURANTE A ASSISTÊNCIA AOS CASOS SUSPEITOS OU CONFIRMADOS DE COVID-19: Atualizada Em 31/03/2023 e Revisada Em 02/05/2023, Brazil. 2023; pp. 1–193. Available online: https://www.gov.br/anvisa/pt-br/centraisdeconteudo/publicacoes/servicosdesaude/notas-tecnicas/notas-tecnicas-vigentes/NT042020covid31.03.2023.pdf (accessed on 13 July 2023).
- Governo do Estado da Bahia. Secretaria de Saúde Plano de Vacinação Contra COVID-19 No Estado Da Bahia, Brazil. 2021; pp. 1–46. Available online: https://www.saude.ba.gov.br/wp-content/uploads/2021/01/Plano-de-Vacinacao-Covid-19.pdf (accessed on 13 July 2023).
- Varadhan, H.; Ahuja, V.; Pitman, C.; Dwyer, D.E. Weak Positive SARS-CoV-2 N2 Gene Results Using the Xpress Xpert Assay: The Need for an Alternate Interpretative Criteria in a Low Prevalence Setting. Pathology 2022, 54, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Luna-Muschi, A.; Borges, I.C.; de Faria, E.; Barboza, A.S.; Maia, F.L.; Leme, M.D.; Guedes, A.R.; Mendes-Correa, M.C.; Kallas, E.G.; Segurado, A.C.; et al. Clinical Features of COVID-19 by SARS-CoV-2 Gamma Variant: A Prospective Cohort Study of Vaccinated and Unvaccinated Healthcare Workers. J. Infect. 2022, 84, 248–288. [Google Scholar] [CrossRef] [PubMed]
- Promlek, T.; Hansirisathit, T.; Kunno, J.; Thanunchai, M. The Effects of CoronaVac and ChAdOx1 NCoV-19 in Reducing Severe Illness in Thailand: A Retrospective Cohort Study. Trop. Med. Infect. Dis. 2023, 8, 95. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Mao, N.; Yi, C.; Simayi, A.; Feng, J.; Feng, Y.; He, M.; Ding, S.; Wang, Y.; Wang, Y.; et al. Impact of Vaccination on Kinetics of Neutralizing Antibodies against SARS-CoV-2 by Serum Live Neutralization Test Based on a Prospective Cohort. Emerg. Microbes Infect. 2023, 12, 1–73. [Google Scholar] [CrossRef]
- Hulme, W.J.; Williamson, E.J.; Green, A.C.A.; Bhaskaran, K.; McDonald, H.I.; Rentsch, C.T.; Schultze, A.; Tazare, J.; Curtis, H.J.; Walker, A.J.; et al. Comparative Effectiveness of ChAdOx1 versus BNT162b2 COVID-19 Vaccines in Health and Social Care Workers in England: Cohort Study Using OpenSAFELY. BMJ 2022, 378, 1–8. [Google Scholar] [CrossRef]
- Pramod, S.; Govindan, D.; Ramasubramani, P.; Kar, S.S.; Aggarwal, R.; Manoharan, N.; Chinnakali, P.; Thulasingam, M.; Sarkar, S.; Thabah, M.M. Effectiveness of Covishield Vaccine in Preventing COVID-19—A Test-Negative Case-Control Study. Vaccine 2022, 40, 3294–3297. [Google Scholar] [CrossRef]
- Dadras, O.; Afsahi, A.M.; Pashaei, Z.; Mojdeganlou, H.; Karimi, A.; Habibi, P.; Barzegary, A.; Fakhfouri, A.; Mirzapour, P.; Janfaza, N.; et al. The Relationship between COVID-19 Viral Load and Disease Severity: A Systematic Review. Immun. Inflamm. Dis. 2022, 10, e580. [Google Scholar] [CrossRef]
- Marra, A.R.; Miraglia, J.L.; Malheiros, D.T.; Guozhang, Y.; Teich, V.D.; Da Silva Victor, E.; Pinho, J.R.R.; Cypriano, A.; Vieira, L.W.; Polonio, M.; et al. Effectiveness of Two COVID-19 Vaccines (Viral Vector and Inactivated Viral Vaccine) against SARS-CoV-2 Infection in a Cohort of Healthcare Workers. Infect. Control Hosp. Epidemiol. 2022, 2019, 1–7. [Google Scholar] [CrossRef]
- Pawar, R.D.; Balaji, L.; Mehta, S.; Cole, A.; Liu, X.; Peradze, N.; Grossestreuer, A.V.; Issa, M.S.; Patel, P.; Kirby, J.E.; et al. Viral Load and Disease Severity in COVID-19. Intern. Emerg. Med. 2022, 17, 359–367. [Google Scholar] [CrossRef]
- Levine-Tiefenbrun, M.; Yelin, I.; Alapi, H.; Herzel, E.; Kuint, J.; Chodick, G.; Gazit, S.; Patalon, T.; Kishony, R. Waning of SARS-CoV-2 Booster Viral-Load Reduction Effectiveness. Nat. Commun. 2022, 13, 1237. [Google Scholar] [CrossRef] [PubMed]
- Salo, J.; Hägg, M.; Kortelainen, M.; Leino, T.; Saxell, T.; Siikanen, M.; Sääksvuori, L. The Indirect Effect of MRNA-Based COVID-19 Vaccination on Healthcare Workers’ Unvaccinated Household Members. Nat. Commun. 2022, 13, 1162. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Shankar, S.; Chatterjee, K.; Chatterjee, K.; Yadav, A.K.; Pandya, K.; Suryam, V.; Agrawal, S.; Ray, S.; Phutane, V.; et al. COVISHIELD (AZD1222) VaccINe Effectiveness among Healthcare and Frontline Workers of INdian Armed Forces: Interim Results of VIN-WIN Cohort Study. Med. J. Armed Forces India 2021, 77, S264–S270. [Google Scholar] [CrossRef] [PubMed]
- Mongin, D.; Bürgisser, N.; Laurie, G.; Schimmel, G.; Vu, D.-L.; Cullati, S.; Da Silva Mora, L.; Després, L.; Dudouit, R.; Hirsch, B.; et al. Effect of SARS-CoV-2 Prior Infection and MRNA Vaccination on Contagiousness and Susceptibility to Infection. Nat. Commun. 2023, 14, 5452. [Google Scholar] [CrossRef]
- Zaidi, A.; Harris, R.; Hall, J.; Woodhall, S.; Andrews, N.; Dunbar, K.; Lopez-Bernal, J.; Dabrera, G. Effects of Second Dose of SARS-CoV-2 Vaccination on Household Transmission, England. Emerg. Infect. Dis. 2023, 29, 127–132. [Google Scholar] [CrossRef]
- Azevedo, P.O.; Hojo-Souza, N.S.; Faustino, L.P.; Fumagalli, M.J.; Hirako, I.C.; Oliveira, E.R.; Figueiredo, M.M.; Carvalho, A.F.; Doro, D.; Benevides, L.; et al. Differential Requirement of Neutralizing Antibodies and T Cells on Protective Immunity to SARS-CoV-2 Variants of Concern. npj Vaccines 2023, 8, 15. [Google Scholar] [CrossRef]
- Qassim, S.H.; Hasan, M.R.; Tang, P.; Chemaitelly, H.; Ayoub, H.H.; Yassine, H.M.; Al-Khatib, H.A.; Smatti, M.K.; Abdul-Rahim, H.F.; Nasrallah, G.K.; et al. Effects of SARS-CoV-2 Alpha, Beta, and Delta Variants, Age, Vaccination, and Prior Infection on Infectiousness of SARS-CoV-2 Infections. Front. Immunol. 2022, 13, 984784. [Google Scholar] [CrossRef]
- Acharya, C.B.; Schrom, J.; Mitchell, A.M.; Coil, D.A.; Marquez, C.; Rojas, S.; Wang, C.Y.; Liu, J.; Pilarowski, G.; Solis, L.; et al. Viral Load Among Vaccinated and Unvaccinated, Asymptomatic and Symptomatic Persons Infected With the SARS-CoV-2 Delta Variant. In Open Forum Infectious Diseases; Oxford University Press: Oxford, UK, 2022; Volume 9, p. ofac135. [Google Scholar] [CrossRef]
- Pouwels, K.B.; Pritchard, E.; Matthews, P.C.; Stoesser, N.; Eyre, D.W.; Vihta, K.-D.; House, T.; Hay, J.; Bell, J.I.; Newton, J.N.; et al. Effect of Delta Variant on Viral Burden and Vaccine Effectiveness against New SARS-CoV-2 Infections in the UK. Nat. Med. 2021, 27, 2127–2135. [Google Scholar] [CrossRef]
- Riemersma, K.K.; Haddock, L.A.; Wilson, N.A.; Minor, N.; Eickhoff, J.; Grogan, B.E.; Kita-Yarbro, A.; Halfmann, P.J.; Segaloff, H.E.; Kocharian, A.; et al. Shedding of Infectious SARS-CoV-2 despite Vaccination. PLoS Pathog. 2022, 18, e1010876. [Google Scholar] [CrossRef]
- Kandel, C.E.; Banete, A.; Taylor, M.; Llanes, A.; McCready, J.; Crowl, G.; Young, M.; Li, A.X.; Chien, E.; Yim, W.; et al. Similar Duration of Viral Shedding of the Severe Acute Respiratory Coronavirus Virus 2 (SARS-CoV-2) Delta Variant between Vaccinated and Incompletely Vaccinated Individuals. Infect. Control Hosp. Epidemiol. 2023, 44, 1002–1004. [Google Scholar] [CrossRef]
- Eyre, D.W.; Taylor, D.; Purver, M.; Chapman, D.; Fowler, T.; Pouwels, K.B.; Walker, A.S.; Peto, T.E.A. Effect of COVID-19 Vaccination on Transmission of Alpha and Delta Variants. N. Engl. J. Med. 2022, 386, 744–756. [Google Scholar] [CrossRef] [PubMed]
- Leon, C.; Tokarev, A.; Bouchnita, A.; Volpert, V. Modelling of the Innate and Adaptive Immune Response to SARS Viral Infection, Cytokine Storm and Vaccination. Vaccines 2023, 11, 127. [Google Scholar] [CrossRef] [PubMed]
- Mohr, N.M.; Plumb, I.D.; Harland, K.K.; Pilishvili, T.; Fleming-Dutra, K.E.; Krishnadasan, A.; Hoth, K.F.; Saydah, S.H.; Mankoff, Z.; Haran, J.P.; et al. Presence of Symptoms 6 Weeks after COVID-19 among Vaccinated and Unvaccinated US Healthcare Personnel: A Prospective Cohort Study. BMJ Open 2023, 13, e063141. [Google Scholar] [CrossRef]
- Costa, P.R.; Correia, C.A.; Marmorato, M.P.; de Dias, J.Z.C.; Thomazella, M.V.; Cabral da Silva, A.; de Oliveira, A.C.S.; Gusmão, A.F.; Ferrari, L.; Freitas, A.C.; et al. Humoral and Cellular Immune Responses to CoronaVac up to One Year after Vaccination. Front. Immunol. 2022, 13, 1032411. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Verma, A.; Tiwari, P.; Katiyar, H.; Aggarwal, A.; Khetan, D.; Mayank; Kishore, R.V.K.; Kumar, P.; Singh, T.P.; et al. Serological Immune Response Following ChAdOx1 NCoV-19 Vaccine (Covishield®) in Patients with Liver Cirrhosis. Vaccines 2022, 10, 1837. [Google Scholar] [CrossRef]
- Turner, J.S.; O’Halloran, J.A.; Kalaidina, E.; Kim, W.; Schmitz, A.J.; Zhou, J.Q.; Lei, T.; Thapa, M.; Chen, R.E.; Case, J.B.; et al. SARS-CoV-2 MRNA Vaccines Induce Persistent Human Germinal Centre Responses. Nature 2021, 596, 109–113. [Google Scholar] [CrossRef]
- Ribas Freitas, A.R.; Giovanetti, M.; Alcantara, L.C.J. Variantes Emergentes Do SARS-CoV-2 e Suas Implicações Na Saúde Coletiva. Interam. J. Med. Heal. 2021, 4, 1–8. [Google Scholar] [CrossRef]
- Watson, O.J.; Barnsley, G.; Toor, J.; Hogan, A.B.; Winskill, P.; Ghani, A.C. Global Impact of the First Year of COVID-19 Vaccination: A Mathematical Modelling Study. Lancet Infect. Dis. 2022, 22, 1293–1302. [Google Scholar] [CrossRef]
| Characteristics | Total (n = 63, 100%) | Unvaccinated (n = 12; 19.1%) | CoronaVac (n = 23; 36.5%) | CoviShield (n = 28; 44.4%) | p * |
|---|---|---|---|---|---|
| Female | 46 (73.0%) | 6 (50.0%) | 17 (73.9%) | 23 (82.1%) | 0.11 |
| Age | 42.2 (35.7–50.9) | 34.4 (20.3–48.6) | 45.8 (36.8–52.1) | 41.9 (37.4–47.3) | 0.21 |
| Staff category | |||||
| Healthcare | 32 (51.0%) | 3 (25.0%) | 14 (60.9%) | 15 (53.6%) | 0.12 |
| Administrative | 31 (49%) | 9 (75.0%) | 9 (39.1%) | 13 (46.4%) | |
| Comorbidities | 21 (33.3%) | 5 (41.7%) | 6 (26.1%) | 10 (35.7%) | 0.61 |
| Number of doses | |||||
| 1 dose | 28 (44.5%) | NA | 9 (39.1%) | 19 (67.9%) | 0.04 † |
| 2 doses | 23 (36.5%) | NA | 14 (60.9%) | 9 (32.1%) | |
| Days to diagnosis after the last vaccine dose | 41.0 (19.0–75.0) | NA | 75.0 (21.0–93.0) | 32.0 (18.0–55.5) | 0.01 † |
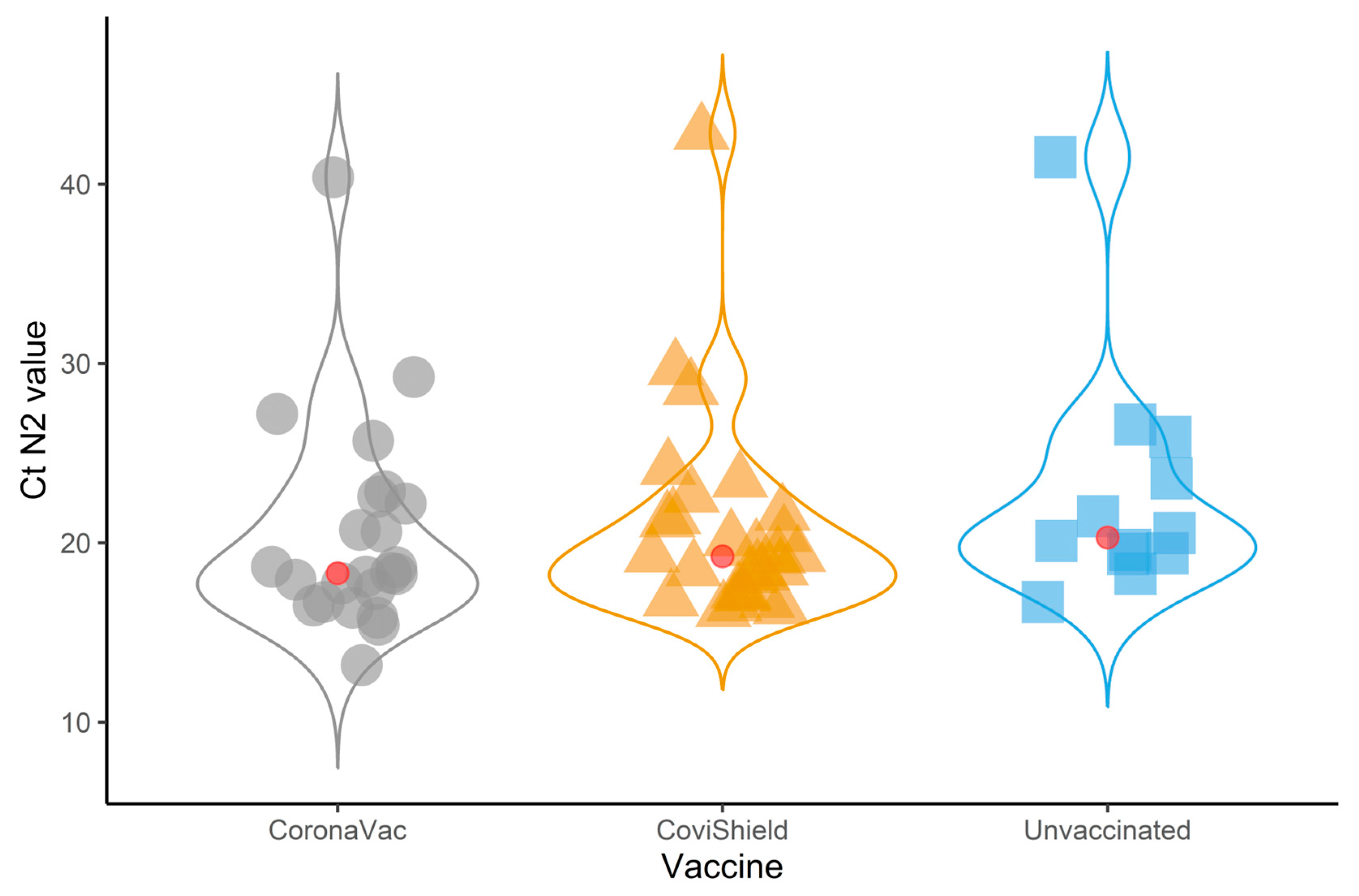
| N2 target Ct value | 19.3 (17.4–22.6) | 20.3 (19.4–24.8) | 18.3 (16.7–22.6) | 19.3 (17.2–21.5) | 0.21 |
| Asymptomatic | 9 (14.3%) | 2 (16.7%) | 5 (21.7%) | 2 (7.1%) | 0.32 |
| Intensity of symptoms | |||||
| Mild to moderate | 19 (35.2%) | 5 (50%) | 4 (22.2%) | 10 (38.5%) | 0.30 |
| Severe | 35 (64.6%) | 5 (50%) | 14 (77.5%) | 16 (61.5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patiño Escarcina, J.E.; Souza, F.d.J.; Da Silva, A.K.C.V.; Da Silva, K.K.A.; Souza, R.B.; Santos, S.R.N.; Netto, E.M. Evaluation of CoronaVac and CoviShield Vaccines on SARS-CoV-2 Infection in Healthcare Workers in Salvador, Brazil. COVID 2023, 3, 1639-1647. https://doi.org/10.3390/covid3110112
Patiño Escarcina JE, Souza FdJ, Da Silva AKCV, Da Silva KKA, Souza RB, Santos SRN, Netto EM. Evaluation of CoronaVac and CoviShield Vaccines on SARS-CoV-2 Infection in Healthcare Workers in Salvador, Brazil. COVID. 2023; 3(11):1639-1647. https://doi.org/10.3390/covid3110112
Chicago/Turabian StylePatiño Escarcina, Jesús Enrique, Felipe de Jesus Souza, Ana Keila Carvalho Vieira Da Silva, Keile Kemyly Assis Da Silva, Ruan Barbosa Souza, Saulo Reis Nery Santos, and Eduardo M. Netto. 2023. "Evaluation of CoronaVac and CoviShield Vaccines on SARS-CoV-2 Infection in Healthcare Workers in Salvador, Brazil" COVID 3, no. 11: 1639-1647. https://doi.org/10.3390/covid3110112
APA StylePatiño Escarcina, J. E., Souza, F. d. J., Da Silva, A. K. C. V., Da Silva, K. K. A., Souza, R. B., Santos, S. R. N., & Netto, E. M. (2023). Evaluation of CoronaVac and CoviShield Vaccines on SARS-CoV-2 Infection in Healthcare Workers in Salvador, Brazil. COVID, 3(11), 1639-1647. https://doi.org/10.3390/covid3110112






