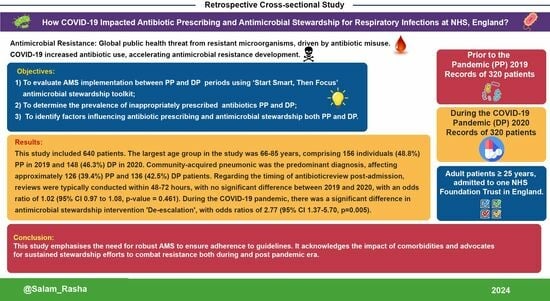
Impact of COVID-19 on ‘Start Smart, Then Focus’ Antimicrobial Stewardship at One NHS Foundation Trust in England Prior to and during the Pandemic
Abstract
:1. Introduction
- (1)
- To evaluate AMS implementation between PP and DP periods using the SSTF toolkit;
- (2)
- To determine the prevalence of inappropriately prescribed antibiotics PP and DP;
- (3)
- To identify factors influencing antibiotic prescribing and AMS implementation both PP and DP.
2. Materials and Methods
2.1. Study Design and Setting
2.2. Participants
2.3. Data Sources and Variables
2.4. Statistical Methods
3. Results
3.1. Clinical and Demographic Characteristics
3.2. Antibiotic Prescription ‘Start Smart’ Approach
3.3. Antibiotic Prescription: ‘Then Focus’ Approach
3.4. Antimicrobial Stewardship Implementation
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Tasak, N.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. Arch. Pharm. Pract. 2016, 7, 110. [Google Scholar] [CrossRef]
- Antimicrobial Stewardship Programmes in Health-Care Facilities in Low-and Middle-Income Countries. Available online: https://www.who.int/publications/i/item/9789241515481 (accessed on 5 November 2023).
- Department of Health and Social Care. UK 5-Year Action Plan for Antimicrobial Resistance 2019 to 2024. Available online: https://www.gov.uk/government/publications/uk-5-year-action-plan-for-antimicrobial-resistance-2019-to-2024 (accessed on 10 October 2023).
- Start Smart Then Focus: Antimicrobial Stewardship Toolkit for Inpatient Care Settings. 2023. Available online: https://www.gov.uk/government/publications/antimicrobial-stewardship-start-smart-then-focus/start-smart-then-focus-antimicrobial-stewardship-toolkit-for-inpatient-care-settings (accessed on 15 October 2023).
- Elshenawy, R.A.; Umaru, N.; Alharbi, A.B.; Aslanpour, Z. Antimicrobial Stewardship Implementation before and during the COVID-19 Pandemic in the Acute Care Settings: A Systematic Review. BMC Public Health 2023, 23, 309. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Stewardship-from Principles to Practice e-Book. The British Society for Antimicrobial Chemotherapy. 2018. Available online: https://bsac.org.uk/antimicrobial-stewardship-from-principles-to-practice-e-book/ (accessed on 10 November 2023).
- Phelan, A.L.; Katz, R.; Gostin, L.O. The Novel Coronavirus Originating in Wuhan, China. JAMA 2020, 323, 709–710. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard; World Health Organization: Geneva, Switzerland, 2023. Available online: https://covid19.who.int/ (accessed on 20 November 2023).
- Murgadella-Sancho, A.; Coloma-Conde, A.; Oriol-Bermúdez, I. Impact of the Strategies Implemented by an Antimicrobial Stewardship Program on the Antibiotic Consumption in the COVID-19 Pandemic. Infect. Control. Hosp. Epidemiol. 2021, 43, 1292–1293. [Google Scholar] [CrossRef]
- CDC. COVID-19: U.S. Impact on Antimicrobial Resistance, Special Report. 2022. Available online: https://stacks.cdc.gov/view/cdc/119025 (accessed on 25 October 2023).
- English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR) Report. 2023. Available online: https://assets.publishing.service.gov.uk/media/6555026e544aea000dfb2e19/ESPAUR-report-2022-to-2023.pdf (accessed on 25 October 2023).
- UK Government Web Report. 2021. Available online: https://webarchive.nationalarchives.gov.uk/ukgwa/20231002172235/https://www.gov.uk/government/publications/english-surveillance-programme-antimicrobial-utilisation-and-resistance-espaur-report (accessed on 20 September 2023).
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef] [PubMed]
- ISRCTN. Antibiotic Prescribing in an English Secondary Care Setting before and during the COVID-19 Pandemic. 2021. Available online: https://www.isrctn.com/ISRCTN14825813 (accessed on 14 October 2023).
- Octopus. Octopus.ac. 2022. Available online: https://www.octopus.ac/publications/372b-6747/versions/latest (accessed on 10 December 2023).
- Phillips, C.J.; Gilchrist, M.; Cooke, F.J.; Franklin, B.D.; Enoch, D.A.; Murphy, M.E.; Santos, R.; Brannigan, E.T.; Holmes, A.H. Adherence to Antibiotic Guidelines and Reported Penicillin Allergy: Pooled Cohort Data on Prescribing and Allergy Documentation from Two English National Health Service (NHS) Trusts. BMJ Open 2019, 9, e026624. [Google Scholar] [CrossRef]
- Ebell, M.H.; Bentivegna, M.; Cai, X.; Hulme, C.; Kearney, M. Accuracy of Biomarkers for the Diagnosis of Adult Community-Acquired Pneumonia: A Meta-Analysis. Acad. Emerg. Med. 2020, 27, 195–206. [Google Scholar] [CrossRef]
- Public Health England. Research Reveals Levels of Inappropriate Prescriptions in England. 2018. Available online: https://www.gov.uk/government/news/research-reveals-levels-of-inappropriate-prescriptions-in-england (accessed on 12 December 2023).
- Cartuliares, M.B.; Søgaard, S.N.; Rosenvinge, F.S.; Mogensen, C.B.; Hertz, M.A.; Skjøt-Arkil, H. Antibiotic Guideline Adherence at the Emergency Department: A Descriptive Study from a Country with a Restrictive Antibiotic Policy. Antibiotics 2023, 12, 1680. [Google Scholar] [CrossRef]
- SPSS Statistics 22.0 Available for Download. 2023. Available online: https://www.ibm.com/support/pages/spss-statistics-220-available-download (accessed on 22 November 2023).
- The Comprehensive R Archive Network. 2023. Available online: https://cran.r-project.org/ (accessed on 20 September 2023).
- Antimicrobial Stewardship. European Centre for Disease Prevention and Control (ECDC). 2022. Available online: https://www.ecdc.europa.eu/en/publications-data/directory-guidance-prevention-and-control/prudent-use-antibiotics/antimicrobial (accessed on 7 December 2023).
- Abdelsalam Elshenawy, R.; Umaru, N.; Aslanpour, Z. WHO AWaRe Classification for Antibiotic Stewardship: Tackling Antimicrobial Resistance—A Descriptive Study from an English NHS Foundation Trust prior to and during the COVID-19 Pandemic. Front. Microbiol. 2023, 14, 1298858. [Google Scholar] [CrossRef]
- Nowakowska, M.; van Staa, T.; Mölter, A.; Ashcroft, D.M.; Tsang, J.Y.; White, A.; Welfare, W.; Palin, V. Antibiotic Choice in UK General Practice: Rates and Drivers of Potentially Inappropriate Antibiotic Prescribing. J. Antimicrob. Chemother. 2019, 74, 3371–3378. [Google Scholar] [CrossRef] [PubMed]
- Hartman, E.A.R.; van de Pol, A.C.; Heltveit-Olsen, S.R.; Lindbæk, M.; Høye, S.; Lithén, S.S.; Sundvall, P.-D.; Sundvall, S.; Arnljots, E.S.; Gunnarsson, R.; et al. Effect of a Multifaceted Antibiotic Stewardship Intervention to Improve Antibiotic Prescribing for Suspected Urinary Tract Infections in Frail Older Adults (ImpresU): Pragmatic Cluster Randomised Controlled Trial in Four European Countries. BMJ 2023, 380, e072319. [Google Scholar] [CrossRef] [PubMed]
- Mölstad, S.; Lundborg, C.S.; Karlsson, A.-K.; Cars, O. Antibiotic prescription rates vary markedly between 13 European countries. Scand. J. Infect. Dis. 2002, 34, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Pate, A.; Yang, Y.-T.; Fahmi, A.; Ashcroft, D.M.; Goldacre, B.; MacKenna, B.; Mehrkar, A.; Bacon, S.; Massey, J.; et al. Impact of COVID-19 on Broad-Spectrum Antibiotic Prescribing for Common Infections in Primary Care in England: A Time-Series Analyses Using OpenSAFELY and Effects of Predictors Including Deprivation. Lancet Reg. Health 2023, 30, 100653. [Google Scholar] [CrossRef] [PubMed]
- Borek, A.J.; Maitland, K.; McLeod, M.; Campbell, A.; Hayhoe, B.; Butler, C.C.; Morrell, L.; Roope, L.S.J.; Holmes, A.; Walker, A.S.; et al. Impact of the COVID-19 Pandemic on Community Antibiotic Prescribing and Stewardship: A Qualitative Interview Study with General Practitioners in England. Antibiotics 2021, 10, 1531. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Vásquez, A.; Azañedo, D.; Vargas-Fernández, R.; Bendezu-Quispe, G. Association of Comorbidities with Pneumonia and Death among COVID-19 Patients in Mexico: A Nationwide Cross-Sectional Study. J. Prev. Med. Public Health 2020, 53, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Saez de Gordoa, E.; Portella, A.; Escudero-Fernández, J.M.; Andreu Soriano, J. Usefulness of Chest X-rays for Detecting COVID-19 Pneumonia during the SARS-CoV-2 Pandemic. Radiologia 2022, 64, 310–316. [Google Scholar] [CrossRef]
- Nikolaou, V.; Massaro, S.; Fakhimi, M.; Stergioulas, L.; Garn, W. COVID-19 Diagnosis from Chest X-rays: Developing a Simple, Fast, and Accurate Neural Network. Health Inf. Sci. Syst. 2021, 9, 36. [Google Scholar] [CrossRef]
- Francis, N.A.; Gillespie, D.; Wootton, M.; White, P.; Bates, J.; Richards, J.; Melbye, H.; Hood, K.; Butler, C.C. Clinical Features and C-Reactive Protein as Predictors of Bacterial Exacerbations of COPD. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 3147–3158. [Google Scholar] [CrossRef]
- Palin, V.; Welfare, W.; Ashcroft, D.M.; van Staa, T.P. Shorter and Longer Courses of Antibiotics for Common Infections and the Association with Reductions of Infection-Related Complications Including Hospital Admissions. Clin. Infect. Dis. 2021, 73, 1805–1812. [Google Scholar] [CrossRef]
- Braykov, N.P.; Morgan, D.J.; Schweizer, M.L.; Uslan, D.Z.; Kelesidis, T.; Weisenberg, S.A.; Johannsson, B.; Young, H.; Cantey, J.; Srinivasan, A.; et al. Assessment of Empirical Antibiotic Therapy Optimisation in Six Hospitals: An Observational Cohort Study. Lancet Infect. Dis. 2014, 14, 1220–1227. [Google Scholar] [CrossRef] [PubMed]
- NICE. Overview|Pneumonia (Community-Acquired): Antimicrobial Prescribing|Guidance|NICE. 2020. Available online: https://www.nice.org.uk/guidance/ng138 (accessed on 4 December 2023).
- Rodríguez-Baño, J.; Rossolini, G.M.; Schultsz, C.; Tacconelli, E.; Murthy, S.; Ohmagari, N.; Holmes, A.; Bachmann, T.; Goossens, H.; Canton, R.; et al. Key Considerations on the Potential Impacts of the COVID-19 Pandemic on Antimicrobial Resistance Research and Surveillance. Trans. R. Soc. Trop. Med. Hyg. 2021, 115, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Ashiru-Oredope, D.; Budd, E.L.; Bhattacharya, A.; Din, N.; McNulty, C.A.M.; Micallef, C.; Ladenheim, D.; Beech, E.; Murdan, S.; Hopkins, S. Implementation of Antimicrobial Stewardship Interventions Recommended by National Toolkits in Primary and Secondary Healthcare Sectors in England: TARGET and Start Smart Then Focus. J. Antimicrob. Chemother. 2016, 71, 1408–1414. [Google Scholar] [CrossRef] [PubMed]
- Llewelyn, M.J.; Budgell, E.P.; Laskawiec-Szkonter, M.; Cross, E.L.A.; Alexander, R.; Bond, S.; Coles, P.; Conlon-Bingham, G.; Dymond, S.; Evans, M.; et al. Antibiotic Review Kit for Hospitals (ARK-Hospital): A Stepped-Wedge Cluster-Randomised Controlled Trial. Lancet Infect. Dis. 2022, 23, 207–221. [Google Scholar] [CrossRef]
- Cunney, R.; Kirrane-Scott, M.; Rafferty, A.; Stapleton, P.; Okafor, I.; McNamara, R. “Start Smart”: Using Front-Line Ownership to Improve the Quality of Empiric Antibiotic Prescribing in a Paediatric Hospital. BMJ Open Qual. 2019, 8, e000445. [Google Scholar] [CrossRef]
- Antibiotic Stewardship: Start Smart, Then Focus & Surveillance. 2022. Available online: https://medicalaudits.co.uk/antimicrobial_stewardish.aspx (accessed on 11 November 2023).
| Patient Characteristics | Prior to the Pandemic—2019 n (%) | During the Pandemic—2020 n (%) | p-Value | |
|---|---|---|---|---|
| Age (Range = 25–99) | Mean (SD) | 74.3 (16.0) | 76.2 (15.5) | 0.127 |
| Gender | Female (%) | 158 (49.4) | 159 (49.7) | |
| Male (%) | 162 (50.6) | 161 (50.3) | ||
| Patient Outcome | Deceased (%) | 48 (15.0) | 50 (15.4) | 0.886 |
| Discharged (%) | 272 (85.0) | 270 (84.4) | ||
| LOS * (Range = 1–119) | Mean (SD) | 13.7 (13.7) | 13.1 (12.3) | 0.525 |
| Prior to Pandemic—2019 n (%) | During the Pandemic—2020 n (%) | Adjusted OR (95% CI) | ||
|---|---|---|---|---|
| Age | 25–45 | 22 (6.9) | 22 (6.9) | - |
| 46–65 | 52 (16.3) | 46 (14.4) | 1.13 (0.49–2.68, p = 0.775) | |
| 66–85 | 156 (48.8) | 148 (46.3) | 1.35 (0.62–3.04, p = 0.455) | |
| >85 | 90 (28.0) | 104 (32.4) | 1.75 (0.77–4.08, p = 0.186) | |
| Gender | Female | 158 (49.4) | 161 (50.3) | - |
| Male | 162 (50.6) | 159 (49.7) | 0.98 (0.67–1.42, p = 0.910) | |
| Allergy | Allergy | 18 (5.6) | 17 (5.3) | - |
| No Allergy | 254 (79.4) | 258 (80.6) | 1.00 (0.46–2.20, p = 1.000) | |
| Not Documented | 46 (14.4) | 29 (9.1) | 0.58 (0.23–1.45, p = 0.243) | |
| Side Effects | 2 (0.6) | 16 (5.0) | 7.23 (1.54–53.37, p = 0.023) * | |
| Indication | CAP | 126 (39.4) | 136 (42.5) | - |
| COPD | 30 (9.4) | 14 (4.4) | 0.42 (0.19–0.90, p = 0.029) * | |
| HAP | 67 (20.9) | 52 (16.2) | 0.74 (0.46–1.20, p = 0.221) | |
| VAP | 5 (1.5) | 1 (0.3) | 0.20 (0.01–1.38, p = 0.156) | |
| URTI | 6 (1.9) | 8 (2.5) | 1.61 (0.46–5.85, p = 0.455) | |
| LRTI | 30 (9.4) | 23 (7.2) | 0.77 (0.39–1.51, p = 0.452) | |
| Pneumonia | 56 (17.5) | 42 (13.1) | 0.92 (0.53–1.60, p = 0.769) | |
| COVID-19 Pneumonia | - | 44 (13.8) | 20.24 (5.82–128.19, p < 0.001) *** | |
| Comorbidities | Hypertension | 143 (44.7) | 148 (46.2) | 1.17 (0.80–1.72, p = 0.414) |
| Hypotension | 13 (4.0) | 14 (4.4) | 1.20 (0.49–2.91, p = 0.689) | |
| Atrial Fibrillation | 61 (19.0) | 64 (20.0) | 1.02 (0.64–1.63, p = 0.922) | |
| Heart Failure | 32 (10.0) | 63 (19.6) | 2.06 (1.23–3.52, p = 0.007) ** | |
| Hypercholesteremia | 40 (12.5) | 58 (18.1) | 1.90 (1.14–3.20, p = 0.014) * | |
| Diabetes Mellitus | 65 (20.3) | 54 (16.9) | 0.76 (0.47–1.22, p = 0.256) | |
| Hypothyroidism | 24 (7.5) | 20 (6.2) | 0.81 (0.40–1.63, p = 0.555) | |
| Kidney Diseases | 75 (23.4) | 46 (14.4) | 0.52 (0.32–0.84, p = 0.008) ** | |
| Liver Diseases | 8 (2.5) | 19 (5.9) | 3.55 (1.41–9.82, p = 0.010) * | |
| Malignancy | 50 (15.6) | 43 (13.4) | 0.95 (0.57–1.57, p = 0.850) | |
| Osteoarthritis | 31 (9.7) | 40 (12.5) | 1.06 (0.58–1.93, p = 0.843) | |
| Asthma | 35 (10.9) | 21 (6.5) | 0.50 (0.25–0.95, p = 0.038) * | |
| COPD | 42 (13.1) | 40 (12.5) | 1.38 (0.76–2.49, p = 0.289) | |
| Dementia | 25 (7.8) | 23 (7.2) | 0.81 (0.41–1.59, p = 0.538) | |
| Epilepsy | 10 (3.1) | 13 (4.1) | 1.32 (0.49–3.65, p = 0.580) | |
| Depression | 12 (3.7) | 20 (6.2) | 1.81 (0.77–4.39, p = 0.178) | |
| Duration | ≤3 Days (Shorter) | 168 (52.5) | 164 (51.3) | - |
| ≥6 Days (longer) | 152 (47.5) | 156 (48.7) | 1.16 (0.82–1.66, p = 0.400) | |
| Prior to Pandemic—2019 n (%) | During the Pandemic—2020 n (%) | Adjusted OR (95% CI) | ||
|---|---|---|---|---|
| WBCs | 12 (3.8) | 11 (3.4) | ||
| CRP | 82 (25.6) | 78 (24.4) | 1.00 (1.00–1.00, p = 0.595) | |
| Serum Creatinine | 126 (39.4) | 123 (38.4) | 1.00 (1.00–1.00, p = 0.860) | |
| Chest X-rays | Pneumonia % | 39 (12.2) | 54 (16.9) | 1.75 (1.04–2.97, p = 0.037) * |
| No Pneumonia % | 82 (25.6) | 65 (20.3) | - | |
| Not taken % | 199 (62.2) | 201 (62.8) | 1.26 (0.86–1.85, p = 0.231) | |
| Day of Antibiotic Review | Mean (SD) | 4.2 (2.8) | 4.4 (2.9) | 1.02 (0.97–1.08, p = 0.461) |
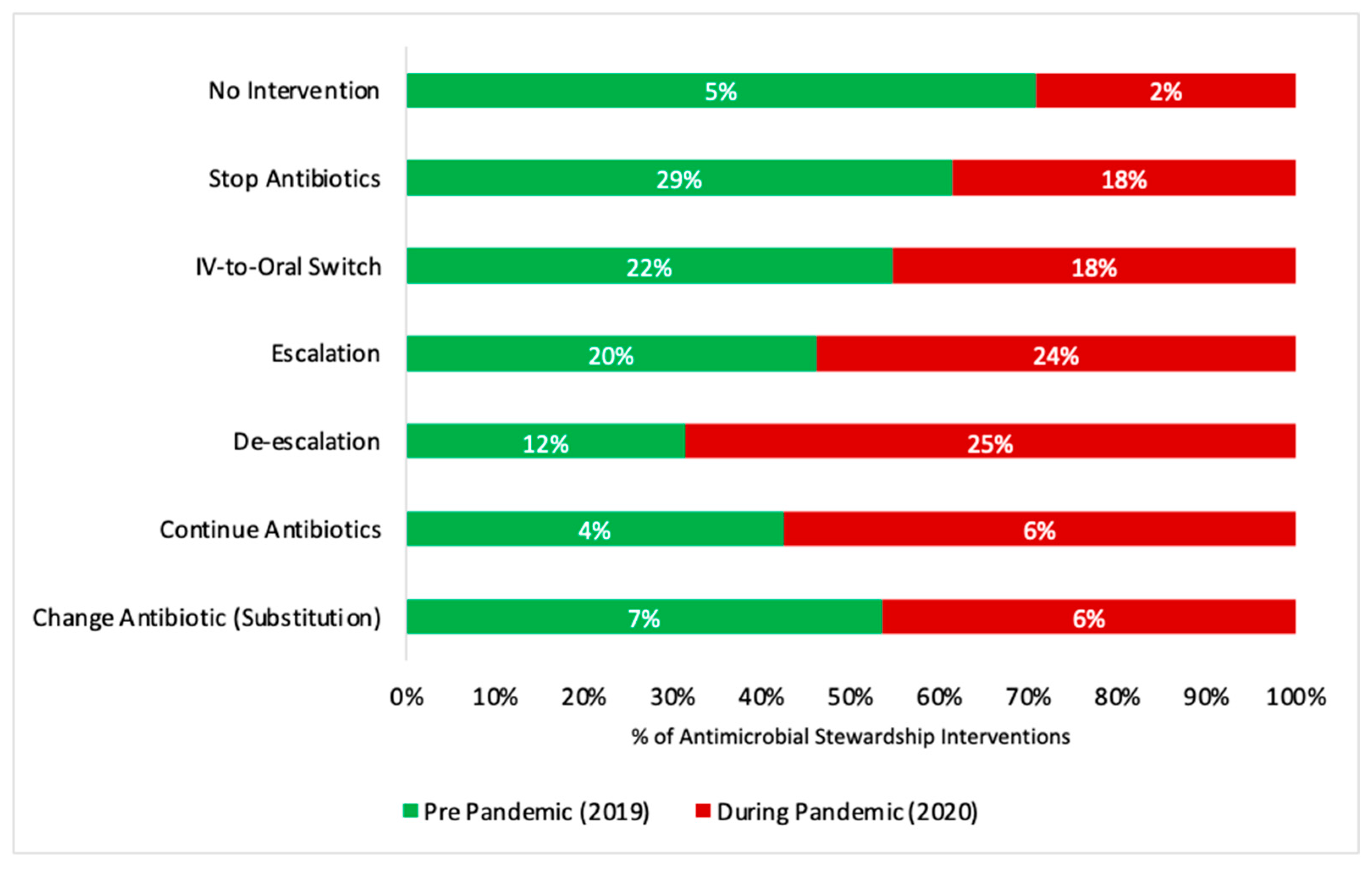
| Type of AMS Intervention | Change Antibiotics (Substitution) | 25 (7.8) | 20 (6.3) | - |
| Continue Antibiotics | 14 (4.4) | 19 (5.9) | 3.36 (1.30–9.25, p = 0.015) * | |
| De-escalation | 37 (11.6) | 81 (25.3) | 2.77 (1.37–5.70, p = 0.005) ** | |
| Escalation | 65 (20.3) | 76 (23.8) | 1.50 (0.76–2.99, p = 0.248) | |
| IV-to-Oral Switch | 70 (21.9) | 58 (18.1) | 0.97 (0.48–1.96, p = 0.928) | |
| Stop Antibiotics | 94 (29.4) | 59 (18.4) | 0.86 (0.44–1.71, p = 0.659) | |
| No Intervention | 15 (4.6) | 7 (2.2) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelsalam Elshenawy, R.; Umaru, N.; Aslanpour, Z. Impact of COVID-19 on ‘Start Smart, Then Focus’ Antimicrobial Stewardship at One NHS Foundation Trust in England Prior to and during the Pandemic. COVID 2024, 4, 102-116. https://doi.org/10.3390/covid4010010
Abdelsalam Elshenawy R, Umaru N, Aslanpour Z. Impact of COVID-19 on ‘Start Smart, Then Focus’ Antimicrobial Stewardship at One NHS Foundation Trust in England Prior to and during the Pandemic. COVID. 2024; 4(1):102-116. https://doi.org/10.3390/covid4010010
Chicago/Turabian StyleAbdelsalam Elshenawy, Rasha, Nkiruka Umaru, and Zoe Aslanpour. 2024. "Impact of COVID-19 on ‘Start Smart, Then Focus’ Antimicrobial Stewardship at One NHS Foundation Trust in England Prior to and during the Pandemic" COVID 4, no. 1: 102-116. https://doi.org/10.3390/covid4010010
APA StyleAbdelsalam Elshenawy, R., Umaru, N., & Aslanpour, Z. (2024). Impact of COVID-19 on ‘Start Smart, Then Focus’ Antimicrobial Stewardship at One NHS Foundation Trust in England Prior to and during the Pandemic. COVID, 4(1), 102-116. https://doi.org/10.3390/covid4010010