Haematological Profile and ACE2 Levels of COVID-19 Patients in a Metropolis in Ghana
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites and Design
2.2. Study Population and Data Collection
2.3. Laboratory Investigations
2.4. Data Analysis
3. Results
3.1. General Description of Study Participants
3.2. Relationship between COVID-19, Participant Demographics and Selected Laboratory Parameters
3.3. Complete Blood Count Parameters, Plasma ACE2 Levels and COVID-19 Status
3.4. Levels of Full Blood Count Parameters with Severity of SARS-CoV-2 Infection
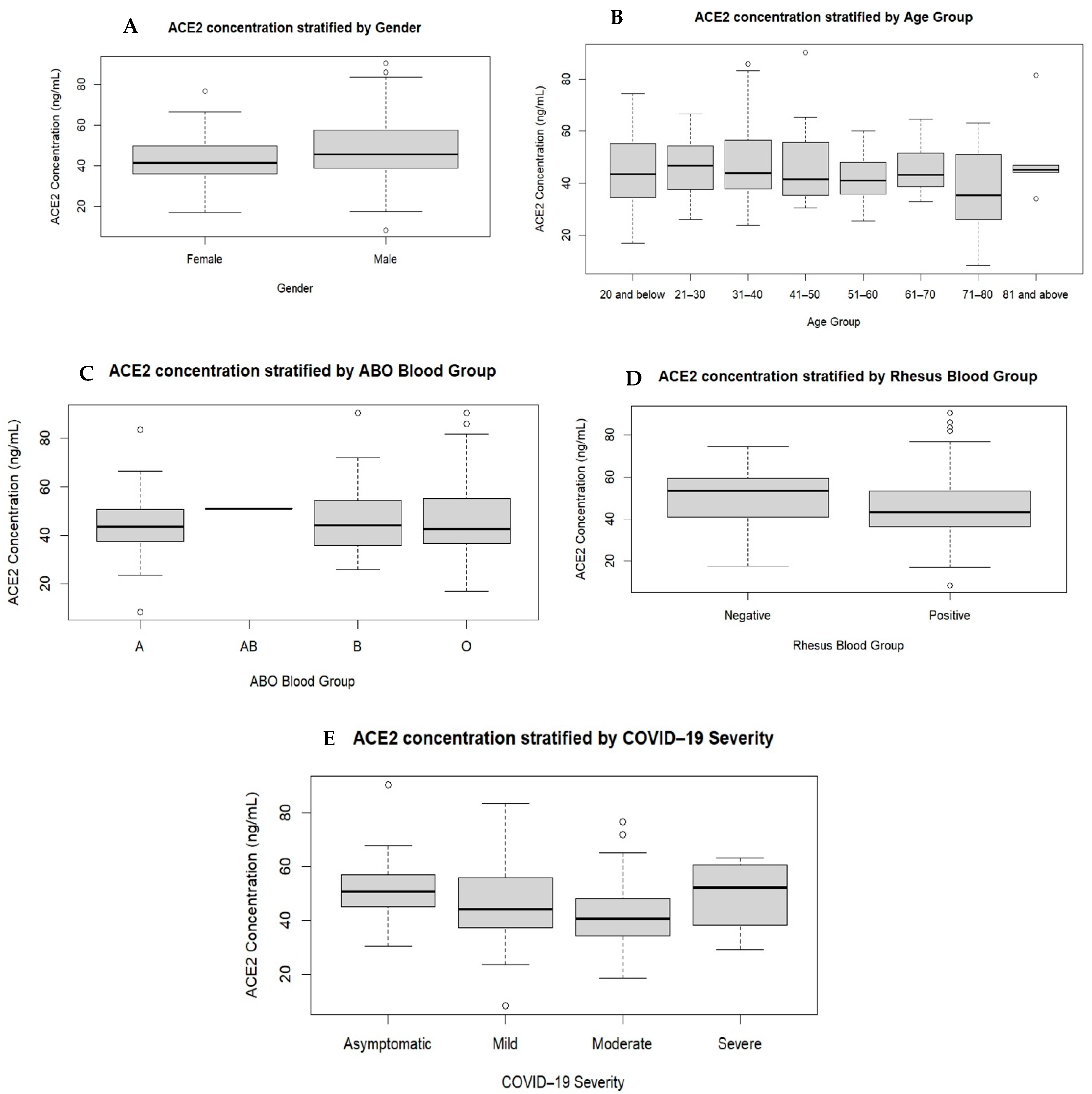
3.5. Plasma ACE2 Levels by Gender, Age, Blood Type and COVID-19 Infection Severity Level
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- WHO. World Health Organization Coronavirus Disease (COVID-19) Dashboard for Ghana. Available online: https://covid19.who.int/ (accessed on 12 June 2023).
- GHS. Ghana Health Service—COVID-19 Dashboard. Available online: https://www.ghs.gov.gh/covid19/archive.php# (accessed on 16 June 2023).
- Carabelli, A.M.; Peacock, T.P.; Thorne, L.G.; Harvey, W.T.; Hughes, J.; COVID-19 Genomics UK Consortium; Peacock, S.J.; Barclay, W.S.; de Silva, T.I.; Towers, G.J. SARS-CoV-2 variant biology: Immune escape, transmission and fitness. Nat. Rev. Microbiol. 2023, 21, 162–177. [Google Scholar] [CrossRef] [PubMed]
- Morang’a, C.M.; Ngoi, J.M.; Gyamfi, J.; Amuzu, D.S.; Nuertey, B.D.; Soglo, P.M.; Appiah, V.; Asante, I.A.; Owusu-Oduro, P.; Armoo, S. Genetic diversity of SARS-CoV-2 infections in Ghana from 2020–2021. Nat. Commun. 2022, 13, 2494. [Google Scholar] [CrossRef] [PubMed]
- WHO. Statement on the Fifteenth Meeting of the IHR (2005) Emergency Committee on the COVID-19 Pandemic. Available online: https://www.who.int/news/item/05-05-2023-statement-on-the-fifteenth-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-coronavirus-disease-(covid-19)-pandemic (accessed on 12 June 2023).
- Chen, J.; Jiang, Q.; Xia, X.; Liu, K.; Yu, Z.; Tao, W.; Gong, W.; Han, J.D.J. Individual variation of the SARS-CoV-2 receptor ACE2 gene expression and regulation. Aging Cell 2020, 19, e13168. [Google Scholar] [CrossRef] [PubMed]
- Xudong, X.; Junzhu, C.; Xingxiang, W.; Furong, Z.; Yanrong, L. Age-and gender-related difference of ACE2 expression in rat lung. Life Sci. 2006, 78, 2166–2171. [Google Scholar] [CrossRef] [PubMed]
- Atlas, S.A. The renin-angiotensin aldosterone system: Pathophysiological role and pharmacologic inhibition. J. Manag. Care Pharm. 2007, 13, 9–20. [Google Scholar] [CrossRef]
- Flack, J.M.; Atlas, S.A.; Pool, J.L.; White, W.B. Renin-angiotensin aldosterone system and hypertension: Current approaches and future directions. J. Manag. Care Pharm. 2007, 13, 1–39. [Google Scholar] [CrossRef]
- Patel, S.K.; Juno, J.A.; Lee, W.S.; Wragg, K.M.; Hogarth, P.M.; Kent, S.J.; Burrell, L.M. Plasma ACE2 activity is persistently elevated following SARS-CoV-2 infection: Implications for COVID-19 pathogenesis and consequences. Eur. Respir. J. 2021, 57, 2003730. [Google Scholar] [CrossRef]
- van Lier, D.; Kox, M.; Santos, K.; van der Hoeven, H.; Pillay, J.; Pickkers, P. Increased blood angiotensin converting enzyme 2 activity in critically ill COVID-19 patients. ERJ Open Res. 2021, 7, 00848–2020. [Google Scholar] [CrossRef]
- Emilsson, V.; Gudmundsson, E.F.; Aspelund, T.; Jonsson, B.G.; Gudjonsson, A.; Launer, L.J.; Lamb, J.R.; Gudmundsdottir, V.; Jennings, L.L.; Gudnason, V. ACE2 levels are altered in comorbidities linked to severe outcome in COVID-19. MedRxiv 2020. [Google Scholar] [CrossRef]
- Emilsson, V.; Gudmundsson, E.F.; Aspelund, T.; Jonsson, B.G.; Gudjonsson, A.; Launer, L.J.; Lamb, J.R.; Gudmundsdottir, V.; Jennings, L.L.; Gudnason, V. Serum levels of ACE2 are higher in patients with obesity and diabetes. Obes. Sci. Pract. 2021, 7, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Fagyas, M.; Kertész, A.; Siket, I.M.; Bánhegyi, V.; Kracskó, B.; Szegedi, A.; Szokol, M.; Vajda, G.; Rácz, I.; Gulyás, H. Level of the SARS-CoV-2 receptor ACE2 activity is highly elevated in old-aged patients with aortic stenosis: Implications for ACE2 as a biomarker for the severity of COVID-19. Geroscience 2021, 43, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Narula, S.; Yusuf, S.; Chong, M.; Ramasundarahettige, C.; Rangarajan, S.; Bangdiwala, S.I.; van Eikels, M.; Leineweber, K.; Wu, A.; Pigeyre, M. Plasma ACE2 and risk of death or cardiometabolic diseases: A case-cohort analysis. Lancet 2020, 396, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Sama, I.E.; Ravera, A.; Santema, B.T.; Van Goor, H.; Ter Maaten, J.M.; Cleland, J.G.; Rienstra, M.; Friedrich, A.W.; Samani, N.J.; Ng, L.L. Circulating plasma concentrations of angiotensin-converting enzyme 2 in men and women with heart failure and effects of renin–angiotensin–aldosterone inhibitors. Eur. Heart J. 2020, 41, 1810–1817. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Zhang, T.; Ma, L.; Zhang, H.; Wang, H.; Wei, W.; Pei, H.; Li, H. The impact of ABO blood group on COVID-19 infection risk and mortality: A systematic review and meta-analysis. Blood Rev. 2021, 48, 100785. [Google Scholar] [CrossRef]
- Jing, W.; Zhao, S.; Liu, J.; Liu, M. ABO blood groups and hepatitis B virus infection: A systematic review and meta-analysis. BMJ Open 2020, 10, e034114. [Google Scholar] [CrossRef]
- Lindesmith, L.; Moe, C.; Marionneau, S.; Ruvoen, N.; Jiang, X.; Lindblad, L.; Stewart, P.; LePendu, J.; Baric, R. Human susceptibility and resistance to Norwalk virus infection. Nat. Med. 2003, 9, 548–553. [Google Scholar] [CrossRef]
- Boren, T.; Falk, P.; Roth, K.A.; Larson, G.; Normark, S. Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science 1993, 262, 1892–1895. [Google Scholar] [CrossRef]
- Wang, D.S.; Chen, D.L.; Ren, C.; Wang, Z.Q.; Qiu, M.Z.; Luo, H.Y.; Zhang, D.S.; Wang, F.H.; Li, Y.H.; Xu, R.H. ABO blood group, hepatitis B viral infection and risk of pancreatic cancer. Int. J. Cancer 2012, 131, 461–468. [Google Scholar] [CrossRef]
- Cheng, Y.; Cheng, G.; Chui, C.; Lau, F.; Chan, P.K.; Ng, M.H.; Sung, J.J.; Wong, R.S. ABO blood group and susceptibility to severe acute respiratory syndrome. JAMA 2005, 293, 1447–1451. [Google Scholar] [CrossRef]
- Wu, Y.; Feng, Z.; Li, P.; Yu, Q. Relationship between ABO blood group distribution and clinical characteristics in patients with COVID-19. Clin. Chim. Acta 2020, 509, 220–223. [Google Scholar] [CrossRef]
- Chakkour, M.; Salami, A.; Olleik, D.; Kamal, I.; Noureddine, F.Y.; El Roz, A.; Ghssein, G. Risk Markers of COVID-19, a Study from South-Lebanon. Covid 2022, 2, 867–876. [Google Scholar] [CrossRef]
- Abdullah, I.; Cornelissen, H.M.; Musekwa, E.; Zemlin, A.; Jalavu, T.; Mashigo, N.; Chetty, C.; Nkosi, N.; Chapanduka, Z.C. Hematological findings in adult patients with SARS CoV-2 infection at Tygerberg Hospital Cape Town South Africa. Health Sci. Rep. 2022, 5, e550. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Ntanasis-Stathopoulos, I.; Elalamy, I.; Kastritis, E.; Sergentanis, T.N.; Politou, M.; Psaltopoulou, T.; Gerotziafas, G.; Dimopoulos, M.A. Hematological findings and complications of COVID-19. Am. J. Hematol. 2020, 95, 834–847. [Google Scholar] [CrossRef] [PubMed]
- Lovato, A.; de Filippis, C. Clinical presentation of COVID-19: A systematic review focusing on upper airway symptoms. Ear Nose Throat J. 2020, 99, 569–576. [Google Scholar] [CrossRef]
- Lansbury, L.; Lim, B.; Baskaran, V.; Lim, W.S. Co-infections in people with COVID-19: A systematic review and meta-analysis. J. Infect. 2020, 81, 266–275. [Google Scholar] [CrossRef]
- Bamgboye, E.L.; Omiye, J.A.; Afolaranmi, O.J.; Davids, M.R.; Tannor, E.K.; Wadee, S.; Niang, A.; Were, A.; Naicker, S. COVID-19 pandemic: Is Africa different? J. Natl. Med. Assoc. 2021, 113, 324–335. [Google Scholar] [CrossRef]
- Adams, J.; MacKenzie, M.J.; Amegah, A.K.; Ezeh, A.; Gadanya, M.A.; Omigbodun, A.; Sarki, A.M.; Thistle, P.; Ziraba, A.K.; Stranges, S. The conundrum of low COVID-19 mortality burden in sub-Saharan Africa: Myth or reality? Glob. Health Sci. Pract. 2021, 9, 433–443. [Google Scholar] [CrossRef]
- Doku, G.N.; Agbozo, W.K.; Annor, R.A.; Kisseh, G.D.; Owusu, M.A. Frequency of ABO/Rhesus (D) blood groupings and ethnic distribution in the Greater-Accra region of Ghana, towards effective blood bank inventory. Int. J. Immunogenet. 2019, 46, 67–73. [Google Scholar] [CrossRef]
- Apecu, R.O.; Mulogo, E.M.; Bagenda, F.; Byamungu, A. ABO and Rhesus (D) blood group distribution among blood donors in rural south western Uganda: A retrospective study. BMC Res. Notes 2016, 9, 513. [Google Scholar] [CrossRef]
- GSS. 2021 Population and Housing Census—Press Release on Provisional Results. Available online: https://statsghana.gov.gh/gssmain/fileUpload/pressrelease/2021%20PHC%20Provisional%20Results%20Press%20Release.pdf (accessed on 22 May 2023).
- WHO. Clinical Management of COVID-19: Living Guideline. Available online: https://app.magicapp.org/#/guideline/j1WBYn (accessed on 12 June 2023).
- Goel, R.; Bloch, E.M.; Pirenne, F.; Al-Riyami, A.Z.; Crowe, E.; Dau, L.; Land, K.; Townsend, M.; Jecko, T.; Rahimi-Levene, N. ABO blood group and COVID-19: A review on behalf of the ISBT COVID-19 working group. Vox Sang. 2021, 116, 849–861. [Google Scholar] [CrossRef] [PubMed]
- Latz, C.A.; DeCarlo, C.; Boitano, L.; Png, C.M.; Patell, R.; Conrad, M.F.; Eagleton, M.; Dua, A. Blood type and outcomes in patients with COVID-19. Ann. Hematol. 2020, 99, 2113–2118. [Google Scholar] [CrossRef] [PubMed]
- Nalbant, A.; Aydın, A.; Yaylacı, S.; Kaya, T.; Wermeulen, C.L.; Cinemre, H. Association of ABO blood group and age with COVID-19 positive test. Rev. Assoc. Médica Bras. 2021, 67, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Udoakang, A.; Oboh, M.; Henry-Ajala, A.; Anyigba, C.; Omoleke, S.; Amambua-Ngwa, A.; Paemka, L.; Awandare, G.; Quashie, P. Low COVID-19 impact in Africa: The multifactorial Nexus. AAS Open Res. 2021, 4, 47. [Google Scholar] [CrossRef]
- Gebhard, C.; Regitz-Zagrosek, V.; Neuhauser, H.K.; Morgan, R.; Klein, S.L. Impact of sex and gender on COVID-19 outcomes in Europe. Biol. Sex Differ. 2020, 11, 29. [Google Scholar] [CrossRef]
- Ali, H.N.; Ali, K.M.; Rostam, H.M.; Ali, A.M.; Tawfeeq, H.M.; Fatah, M.H.; Figueredo, G.P. Clinical laboratory parameters and comorbidities associated with severity of coronavirus disease 2019 (COVID-19) in Kurdistan Region of Iraq. Pract. Lab. Med. 2022, 31, e00294. [Google Scholar] [CrossRef]
- Dai, X. ABO blood group predisposes to COVID-19 severity and cardiovascular diseases. Eur. J. Prev. Cardiol. 2020, 27, 1436–1437. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Zietz, M.; Zucker, J.; Tatonetti, N.P. Associations between blood type and COVID-19 infection, intubation, and death. Nat. Commun. 2020, 11, 5761. [Google Scholar] [CrossRef]
- Wang, L.; Duan, Y.; Zhang, W.; Liang, J.; Xu, J.; Zhang, Y.; Wu, C.; Xu, Y.; Li, H. Epidemiologic and clinical characteristics of 26 cases of COVID-19 arising from patient-to-patient transmission in Liaocheng, China. Clin. Epidemiol. 2020, 12, 387–391. [Google Scholar] [CrossRef]
- Cen, Y.; Chen, X.; Shen, Y.; Zhang, X.-H.; Lei, Y.; Xu, C.; Jiang, W.-R.; Xu, H.-T.; Chen, Y.; Zhu, J. Risk factors for disease progression in patients with mild to moderate coronavirus disease 2019—A multi-centre observational study. Clin. Microbiol. Infect. 2020, 26, 1242–1247. [Google Scholar] [CrossRef] [PubMed]
- Diao, B.; Wang, C.; Tan, Y.; Chen, X.; Liu, Y.; Ning, L.; Chen, L.; Li, M.; Liu, Y.; Wang, G. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19). Front. Immunol. 2020, 11, 827. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Berube, J.; McNamara, M.; Saksena, S.; Hartman, M.; Arshad, T.; Bornheimer, S.J.; O’Gorman, M. Lymphocyte subset counts in COVID-19 patients: A meta-analysis. Cytom. Part A 2020, 97, 772–776. [Google Scholar] [CrossRef] [PubMed]
- Huang, I.; Pranata, R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): Systematic review and meta-analysis. J. Intensive Care 2020, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China; clinical infectious diseases; Oxford academic. Clin. Infect. Dis. 2020, 71, 762–768. [Google Scholar] [CrossRef]
- Chen, J.; Pan, Y.; Li, G.; Xu, W.; Zhang, L.; Yuan, S.; Xia, Y.; Lu, P.; Zhang, J. Distinguishing between COVID-19 and influenza during the early stages by measurement of peripheral blood parameters. J. Med. Virol. 2021, 93, 1029–1037. [Google Scholar] [CrossRef]
- Palladino, M. Complete blood count alterations in COVID-19 patients: A narrative review. Biochem. Medica 2021, 31, 030501. [Google Scholar] [CrossRef]
- Reindl-Schwaighofer, R.; Hödlmoser, S.; Eskandary, F.; Poglitsch, M.; Bonderman, D.; Strassl, R.; Aberle, J.H.; Oberbauer, R.; Zoufaly, A.; Hecking, M. ACE2 elevation in severe COVID-19. Am. J. Respir. Crit. Care Med. 2021, 203, 1191–1196. [Google Scholar] [CrossRef]
- Swärd, P.; Edsfeldt, A.; Reepalu, A.; Jehpsson, L.; Rosengren, B.E.; Karlsson, M.K. Age and sex differences in soluble ACE2 may give insights for COVID-19. Crit. Care 2020, 24, 221. [Google Scholar] [CrossRef]
- Viveiros, A.; Gheblawi, M.; Aujla, P.K.; Sosnowski, D.K.; Seubert, J.M.; Kassiri, Z.; Oudit, G.Y. Sex-and age-specific regulation of ACE2: Insights into severe COVID-19 susceptibility. J. Mol. Cell. Cardiol. 2022, 164, 13–16. [Google Scholar] [CrossRef]
- Viveiros, A.; Rasmuson, J.; Vu, J.; Mulvagh, S.L.; Yip, C.Y.; Norris, C.M.; Oudit, G.Y. Sex differences in COVID-19: Candidate pathways, genetics of ACE2, and sex hormones. Am. J. Physiol.-Heart Circ. Physiol. 2021, 320, H296–H304. [Google Scholar] [CrossRef] [PubMed]
| Variable | Frequency | Percentage (%) | Total | |
|---|---|---|---|---|
| Gender | Male | 288 | 55.9 | 515 |
| Female | 227 | 44.1 | ||
| Age (years) | Median (IQR) = 37 (26–53) | 497 * | ||
| 20 and below | 73 | 14.7 | ||
| 21–30 | 93 | 18.7 | ||
| 31–40 | 117 | 23.5 | ||
| 41–50 | 75 | 15.1 | ||
| 51–60 | 49 | 9.9 | ||
| 61–70 | 43 | 8.7 | ||
| 71–80 | 30 | 6.0 | ||
| 81 and above | 17 | 3.4 | ||
| Recruiting Hospital | University Hospital | 38 | 7.4 | 515 |
| KATH | 190 | 36.9 | ||
| KSH | 23 | 4.5 | ||
| MCHH | 7 | 1.4 | ||
| SDA Hospital | 142 | 27.6 | ||
| SGH | 115 | 22.3 | ||
| ABO Blood Group | A | 111 | 22.7 | 490 * |
| B | 106 | 21.6 | ||
| AB | 13 | 2.7 | ||
| O | 260 | 53.1 | ||
| Rhesus Blood Group | Positive | 437 | 89.2 | 490 * |
| Negative | 53 | 10.8 | ||
| SARS-CoV-2 Status | Positive | 259 | 50.3 | 515 |
| Negative | 256 | 49.7 | ||
| COVID-19 Severity | Asymptomatic | 68 | 26.3 | 259 |
| Mild | 110 | 42.5 | ||
| Moderate | 77 | 29.7 | ||
| Severe | 4 | 1.5 | ||
| Variable | SARS-CoV-2 Negative | SARS-CoV-2 Positive | p-Value | ||
|---|---|---|---|---|---|
| Frequency | Percentage (%) | Frequency | Percentage (%) | ||
| Age (in years) | N = 245 | N = 252 | |||
| Median (IQR) | 36 (24–50) | 39 (29–57) | |||
| 20 and below | 52 | 21.2 | 21 | 8.3 | 0.002 |
| 21–30 | 41 | 16.7 | 52 | 20.6 | |
| 31–40 | 55 | 22.4 | 62 | 24.6 | |
| 41–50 | 39 | 15.9 | 36 | 14.3 | |
| 51–60 | 21 | 8.6 | 28 | 11.1 | |
| 61–70 | 21 | 8.6 | 22 | 8.7 | |
| 71–80 | 11 | 4.5 | 19 | 7.5 | |
| 81 and above | 5 | 2.0 | 12 | 4.8 | |
| Gender | N = 256 | N = 259 | |||
| Male | 147 | 57.4 | 141 | 54.4 | 0.496 |
| Female | 109 | 42.6 | 118 | 45.6 | |
| ABO Blood Group | N = 239 | N = 251 | |||
| A | 55 | 23.0 | 56 | 22.3 | 0.403 |
| B | 56 | 23.4 | 50 | 19.9 | |
| AB | 7 | 2.9 | 6 | 2.4 | |
| O | 121 | 50.6 | 139 | 55.4 | |
| Rh Status | N = 239 | N = 251 | |||
| Positive | 214 | 89.5 | 223 | 88.8 | 0.805 |
| Negative | 25 | 10.5 | 28 | 11.2 | |
| Variable | Total | SARS-CoV-2 Positive | SARS-CoV-2 Negative | p-Value | |||
|---|---|---|---|---|---|---|---|
| N | Median (IQR) | N | Median (IQR) | N | Median (IQR) | ||
| Red Cell Count and Red Cell Indices | |||||||
| RBC (×106/μL) | 515 | 4.54 (4.04–5.09) | 259 | 4.59 (4.00–5.10) | 256 | 4.50 (4.07–5.07) | 0.929 |
| HGB (g/dL) | 515 | 12.60 (11.05–14.10) | 259 | 12.60 (11.10–13.90) | 256 | 12.60 (10.83–14.20) | 0.630 |
| HCT (%) | 515 | 38.80 (32.80–43.55) | 259 | 38.30 (32.70–42.40) | 256 | 39.35 (32.75–45.38) | 0.023 |
| MCV (fL) | 515 | 84.50 (78.90–90.00) | 259 | 83.40 (78.90–89.00) | 256 | 86.50 (78.95–91.90) | <0.001 |
| MCH (pg) | 515 | 27.80 (26.10–29.40) | 259 | 27.80 (26.10–29.10) | 256 | 27.80 (26.10–29.60) | 0.457 |
| MCHC (g/dL) | 515 | 32.80 (31.00–34.40) | 259 | 33.10 (31.80–34.40) | 256 | 32.10 (30.30–34.30) | <0.001 |
| Platelet Count and Platelet Indices | |||||||
| PLT (×103/μL) | 515 | 213.00 (156.50–291.00) | 259 | 230.00 (159.00–305.00) | 256 | 208.00 (153.25–270.25) | 0.046 |
| MPV (fL) | 462 | 11.20 (10.40–12.00) | 231 | 11.00 (10.30–11.60) | 231 | 11.60 (10.60–12.30) | <0.001 |
| PCT (%) | 462 | 0.25 (0.19–0.32) | 231 | 0.26 (0.20–0.33) | 231 | 0.24 (0.19–0.31) | 0.128 |
| WBC Count and DIFF | |||||||
| WBC (×103/μL) | 515 | 5.94 (4.13–9.31) | 259 | 6.36 (4.44–10.09) | 256 | 5.655 (3.78–8.38) | 0.006 |
| NEUT (×103/μL) | 515 | 3.09 (1.71–6.31) | 259 | 3.54 (1.87–7.43) | 256 | 2.93 (1.25–5.71) | 0.002 |
| LYMPH (×103/μL) | 515 | 1.80 (1.31–2.37) | 259 | 1.80 (1.25–2.37) | 256 | 1.81 (1.36–2.38) | 0.488 |
| MONO (×103/μL) | 515 | 0.48 (0.35–0.72) | 259 | 0.51 (0.36–0.72) | 256 | 0.45 (0.33–0.73) | 0.210 |
| EO (×103/μL) | 515 | 0.05 (0.02–0.13) | 259 | 0.05 (0.01–0.12) | 256 | 0.06 (0.02–0.14) | 0.208 |
| BASO (×103/μL) | 515 | 0.04 (0.02–0.06) | 259 | 0.03 (0.02–0.05) | 256 | 0.05 (0.03–0.09) | <0.001 |
| ELISA Test | |||||||
| Plasma ACE2 (ng/mL) | 168 | 43.65 (36.79–54.57) | 112 | 45.37 (37.68–55.88) | 56 | 41.55 (34.97–51.63) | 0.079 |
| Variable | Asymptomatic | Mild | Moderate | Severe | p-Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| N | Median (IQR) | N | Median (IQR) | N | Median (IQR) | N | Median (IQR) | ||
| Demographics | |||||||||
| Age (years) | 61 | 35.00 (28.50–42.50) | 110 | 35.50 (26.00–50.25) | 77 | 55.00 (38.50–69.50) | 4 | 70.00 (35.25–80.00) | <0.001 |
| Red Cell Count and Red Cell Indices | |||||||||
| RBC (×106/μL) | 68 | 4.82 (4.25–5.23) | 110 | 4.60 (3.97–5.03) | 77 | 4.31 (3.58–5.08) | 4 | 3.75 (2.64–4.79) | 0.014 |
| HGB (g/dL) | 68 | 13.10 (11.93–14.48) | 110 | 12.65 (11.05–13.90) | 77 | 12.20 (9.65–13.60) | 4 | 9.55 (7.85–11.93) | 0.003 |
| HCT (%) | 68 | 39.25 (36.20–43.73) | 110 | 38.85 (32.45–43.13) | 77 | 35.80 (29.80–40.45) | 4 | 28.55 (24.28–37.93) | 0.001 |
| MCV (fL) | 68 | 83.70 (80.03–87.13) | 110 | 83.85 (80.60–90.23) | 77 | 81.90 (76.90–88.50) | 4 | 80.70 (74.70–93.08) | 0.149 |
| MCH (pg) | 68 | 27.95 (26.50–29.10) | 110 | 27.80 (26.18–29.33) | 77 | 27.70 (25.55–29.10) | 4 | 27.25 (23.40–29.90) | 0.933 |
| MCHC (g/dL) | 68 | 33.30 (32.45–34.20) | 110 | 32.60 (31.38–34.33) | 77 | 33.70 (31.95–35.10) | 4 | 31.25 (31.10–34.93) | 0.082 |
| Platelet Count and Platelet Indices | |||||||||
| PLT (×103/μL) | 68 | 260.00 (197.25–307.50) | 110 | 227.00 (157.50–304.25) | 77 | 190.00 (134.50–296.00) | 4 | 207.00 (183.50–355.75) | 0.023 |
| MPV (fL) | 63 | 10.70 (10.20–11.40) | 97 | 11.10 (10.30–11.80) | 67 | 11.00 (10.40–11.80) | 4 | 10.00 (9.23–11.15) | 0.118 |
| PCT (%) | 63 | 0.29 (0.23–0.34) | 97 | 0.26 (0.20–0.33) | 67 | 0.23 (0.16–0.31) | 4 | 0.21 (0.20–0.35) | 0.042 |
| WBC Count and DIFF | |||||||||
| WBC (×103/μL) | 68 | 5.23 (4.15–6.53) | 110 | 6.06 (4.06–8.68) | 77 | 8.49 (6.15–12.64) | 4 | 15.87 (6.68–19.80) | <0.001 |
| NEUT (×103/μL) | 68 | 2.33 (1.70–3.39) | 110 | 2.91 (1.34–6.10) | 77 | 6.06 (3.63–10.61) | 4 | 14.69 (5.70–18.96) | <0.001 |
| LYMPH (×103/μL) | 68 | 2.17 (1.70–2.56) | 110 | 1.83 (1.23–2.26) | 77 | 1.41 (1.08–2.17) | 4 | 0.47 (0.22–0.66) | <0.001 |
| MONO (×103/μL) | 68 | 0.455 (0.35–0.62) | 110 | 0.52 (0.36–0.69) | 77 | 0.55 (0.39–0.84) | 4 | 0.35 (0.29–0.94) | 0.161 |
| EO (×103/μL) | 68 | 0.08 (0.03–0.15) | 110 | 0.05 (0.02–0.12) | 77 | 0.03 (0.00–0.10) | 4 | 0.00 (0.00–0.03) | 0.001 |
| BASO (×103/μL) | 68 | 0.03 (0.02–0.05) | 110 | 0.03 (0.02–0.04) | 77 | 0.02 (0.01–0.05) | 4 | 0.015 (0.00–0.05) | 0.343 |
| ELISA Test | |||||||||
| Plasma ACE2 (ng/mL) | 29 | 50.61 (43.90–58.61) | 39 | 44.19 (36.75–55.88) | 40 | 40.68 (34.09–48.10) | 4 | 52.34 (33.59–61.79) | 0.024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ackah, E.B.; Owusu, M.; Sackey, B.; Boamah, J.K.; Kamasah, J.S.; Aduboffour, A.A.; Akortia, D.; Nkrumah, G.; Amaniampong, A.; Klevor, N.; et al. Haematological Profile and ACE2 Levels of COVID-19 Patients in a Metropolis in Ghana. COVID 2024, 4, 117-129. https://doi.org/10.3390/covid4020011
Ackah EB, Owusu M, Sackey B, Boamah JK, Kamasah JS, Aduboffour AA, Akortia D, Nkrumah G, Amaniampong A, Klevor N, et al. Haematological Profile and ACE2 Levels of COVID-19 Patients in a Metropolis in Ghana. COVID. 2024; 4(2):117-129. https://doi.org/10.3390/covid4020011
Chicago/Turabian StyleAckah, Ezekiel B., Michael Owusu, Benedict Sackey, Justice K. Boamah, Japhet S. Kamasah, Albert A. Aduboffour, Debora Akortia, Gifty Nkrumah, Andrews Amaniampong, Nicholas Klevor, and et al. 2024. "Haematological Profile and ACE2 Levels of COVID-19 Patients in a Metropolis in Ghana" COVID 4, no. 2: 117-129. https://doi.org/10.3390/covid4020011
APA StyleAckah, E. B., Owusu, M., Sackey, B., Boamah, J. K., Kamasah, J. S., Aduboffour, A. A., Akortia, D., Nkrumah, G., Amaniampong, A., Klevor, N., Agyemang, L. D., Ayisi-Boateng, N. K., Sylverken, A., Phillips, R. O., & Owusu-Dabo, E. (2024). Haematological Profile and ACE2 Levels of COVID-19 Patients in a Metropolis in Ghana. COVID, 4(2), 117-129. https://doi.org/10.3390/covid4020011







