Navigating the Dynamic Landscape of SARS-CoV-2: The Dual Role of Neutralizing Antibodies, Variability in Responses, and Strategies for Adaptive Pandemic Control
Abstract
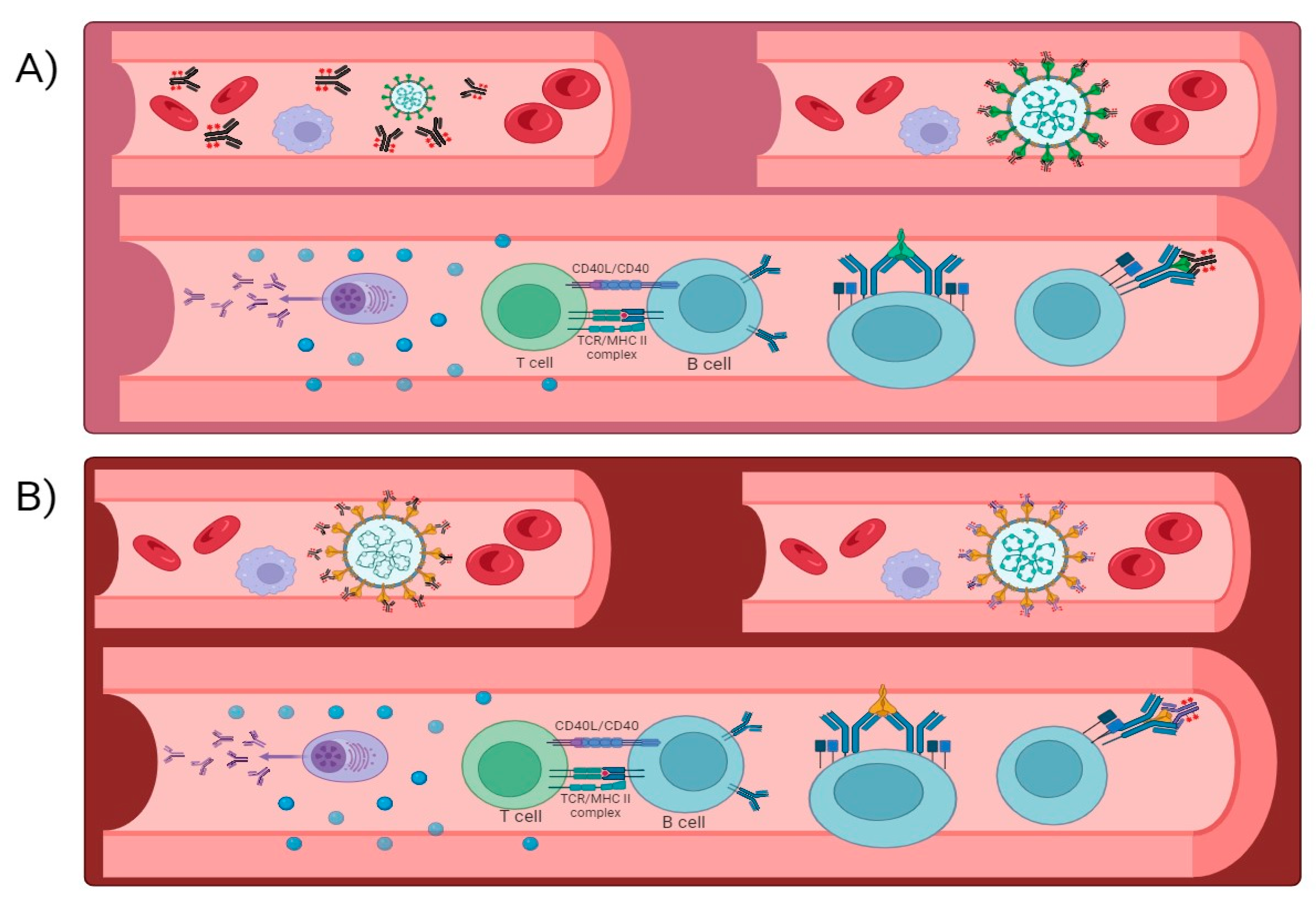
:1. Introduction
2. Materials and Methods
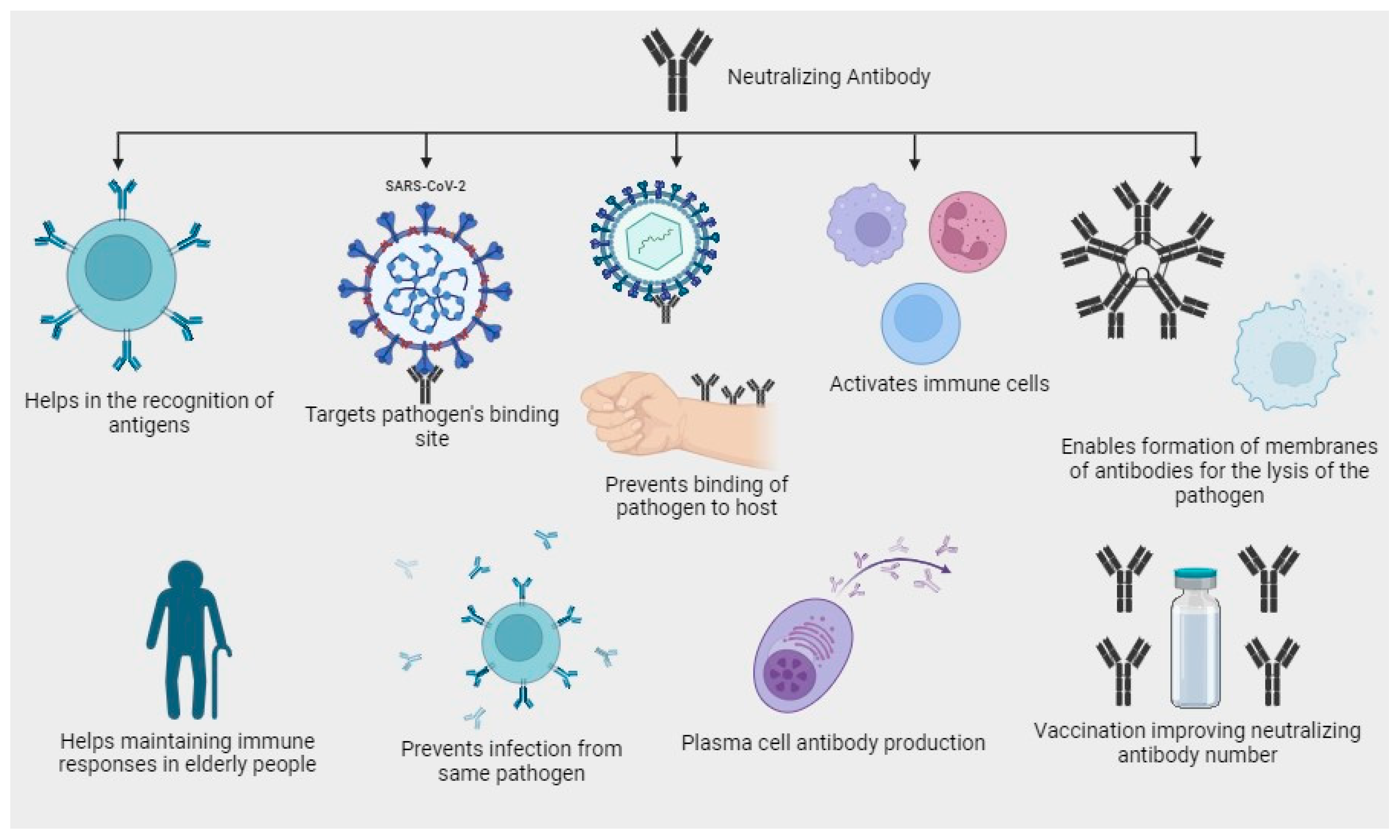
3. Role of Neutralizing Antibodies in Immune Response
3.1. Prevalence and Persistence of Neutralizing Antibodies
3.2. Effectiveness of Vaccination on Neutralizing Antibodies
4. Omicron Variant and Immune Escape
5. Therapeutic Monoclonal Antibodies and Neutralization
6. Strategies for Adaptive Pandemic Control
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vianello, C.; Strozzi, F.; Mocellin, P.; Cimetta, E.; Fabiano, B.; Manenti, F.; Pozzi, R.; Maschio, G. A perspective on early detection systems models for COVID-19 spreading. Biochem. Biophys. Res. Commun. 2021, 538, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, A.; Dahiya, P. COVID-19 Pandemic: Assessment of current strategies and socio-economic Impact. J. Health Manag. 2022, 24, 466–477. [Google Scholar] [CrossRef]
- Li, D.; Wu, M. Pattern recognition receptors in health and diseases. Signal Transduct. Target. Ther. 2021, 6, 291. [Google Scholar] [CrossRef] [PubMed]
- Raskov, H.; Orhan, A.; Salanti, A.; Gaggar, S.; Gögenur, I. Natural killer cells in cancer and cancer immunotherapy. Cancer Lett. 2021, 520, 233–242. [Google Scholar] [CrossRef]
- Furukawa, K.; Tjan, L.H.; Sutandhio, S.; Kurahashi, Y.; Iwata, S.; Tohma, Y.; Sano, S.; Nakamura, S.; Nishimura, M.; Arii, J.; et al. Cross-neutralizing activity against SARS-CoV-2 variants in COVID-19 patients: Comparison of 4 waves of the pandemic in Japan. InOpen Forum Infect. Dis. 2021, 8, 10. [Google Scholar] [CrossRef]
- Sun, L.; Su, Y.; Jiao, A.; Wang, X.; Zhang, B. T cells in health and disease. Signal Transduct. Target. Ther. 2023, 8, 235. [Google Scholar] [CrossRef]
- Cañete, P.F.; Vinuesa, C.G. COVID-19 makes B cells forget, but T cells remember. Cell 2020, 183, 13–15. [Google Scholar] [CrossRef]
- Laidlaw, B.J.; Cyster, J.G. Transcriptional regulation of memory B cell differentiation. Nat. Rev. Immunol. 2021, 21, 209–220. [Google Scholar] [CrossRef]
- Yang, S.; Yu, Y.; Xu, Y.; Jian, F.; Song, W.; Yisimayi, A.; Wang, P.; Wang, J.; Liu, J.; Yu, L.; et al. Fast evolution of SARS-CoV-2 BA. 2· 86 to JN. 1 under heavy immune pressure. Lancet Infect. Dis. 2024, 24, e70–e72. [Google Scholar] [CrossRef]
- Kaku, Y.; Okumura, K.; Padilla-Blanco, M.; Kosugi, Y.; Uriu, K.; Hinay, A.A., Jr.; Chen, L.; Plianchaisuk, A.; Kobiyama, K.; Ishii, K.J. Genotype to phenotype Japan (G2P-Japan) consortium. Virological characteristics of the SARS-CoV-2 JN.1 variant. bioRxiv 2023. [Google Scholar] [CrossRef]
- Yang, L.; Xie, X.; Tu, Z.; Fu, J.; Xu, D.; Zhou, Y. The signal pathways and treatment of cytokine storm in COVID-19. Signal Transduct. Target. Ther. 2021, 6, 255. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health. (n.d.). Long COVID. In NIH COVID-19 Research. Available online: https://covid19.nih.gov/covid-19-topics/long-covid (accessed on 1 September 2024).
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Al-Haidari, A.; Sun, J.; Kazi, J.U. T cell receptor (TCR) signaling in health and disease. Signal Transduct. Target. Ther. 2021, 6, 412. [Google Scholar] [CrossRef] [PubMed]
- Logue, J.K.; Franko, N.M.; McCulloch, D.J.; McDonald, D.; Magedson, A.; Wolf, C.R.; Chu, H.Y. Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw. Open 2021, 4, e210830. [Google Scholar] [CrossRef]
- Raveendran, A.V.; Jayadevan, R.; Sashidharan, S. Long COVID: An overview. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 869–875. [Google Scholar] [CrossRef]
- Callard, F.; Perego, E. How and why patients made Long Covid. Soc. Sci. Med. 2021, 268, 113426. [Google Scholar] [CrossRef]
- Garg, M.; Maralakunte, M.; Garg, S.; Dhooria, S.; Sehgal, I.; Bhalla, A.S.; Vijayvergiya, R.; Grover, S.; Bhatia, V.; Jagia, P.; et al. The conundrum of ‘long-COVID-19: A narrative review. Int. J. Gen. Med. 2021, 14, 2491–2506. [Google Scholar] [CrossRef]
- Mohandas, S.; Jagannathan, P.; Henrich, T.J.; Sherif, Z.A.; Bime, C.; Quinlan, E.; Portman, M.A.; Gennaro, M.; Rehman, J. Recover Mechanistic Pathways Task Force. Immune mechanisms underlying COVID-19 pathology and post-acute sequelae of SARS-CoV-2 infection (PASC). Elife 2023, 12, e86014. [Google Scholar] [CrossRef]
- Files, J.K.; Boppana, S.; Perez, M.D.; Sarkar, S.; Lowman, K.E.; Qin, K.; Sterrett, S.; Carlin, E.; Bansal, A.; Sabbaj, S.; et al. Sustained cellular immune dysregulation in individuals recovering from SARS-CoV-2 infection. J. Clin. Investig. 2021, 131, e140491. [Google Scholar] [CrossRef]
- Dai, W.; Zhang, B.; Jiang, X.M.; Su, H.; Li, J.; Zhao, Y.; Xie, X.; Jin, Z.; Peng, J.; Liu, F.; et al. Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease. Science 2020, 368, 1331–1335. [Google Scholar] [CrossRef]
- Crook, H.; Raza, S.; Nowell, J.; Young, M.; Edison, P. Long covid-mechanisms, risk factors, and management. BMJ (Clin. Res. Ed.) 2021, 374, n1648. [Google Scholar] [CrossRef] [PubMed]
- Altmann, D.M.; Boyton, R.J. SARS-CoV-2 T cell immunity: Specificity, function, durability, and role in protection. Sci. Immunol. 2021, 6, eabg6347. [Google Scholar] [CrossRef] [PubMed]
- Baig, A.M. Chronic COVID syndrome: Need for an appropriate medical terminology for long-COVID and COVID long-haulers. J. Med. Virol. 2021, 93, 2555–2556. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 Long-term effects of COVID-19: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Bliddal, S.; Banasik, K.; Pedersen, O.B.; Nissen, J.; Cantwell, L.; Schwinn, M.; Tulstrup, M.; Westergaard, D.; Ullum, H.; Brunak, S.; et al. Acute and persistent symptoms in non-hospitalized PCR-confirmed COVID-19 patients. Sci. Rep. 2021, 11, 19765. [Google Scholar] [CrossRef]
- Nabavi, N. Long COVID: How to define it and how to manage it. Clevel. Clin. J. Med. 2021. [Google Scholar] [CrossRef]
- Dweck, M.R.; Bularga, A.; Hahn, R.T.; Bing, R.; Lee, K.K.; Chapman, A.R.; White, A.; Salvo, G.D.; Sade, L.E.; Pearce, K.; et al. Global evaluation of echocardiography in patients with COVID-19. Eur. Heart J.—Cardiovasc. Imaging 2021, 22, 116–126. [Google Scholar] [CrossRef]
- Dennis, A.; Wamil, M.; Kapur, S.; Alberts, J.; Badley, A.D.; Decker, G.A.; Rizza, S.A.; Banerjee, R.; Banerjee, A. Multi-organ impairment in low-risk individuals with long COVID. bioRxiv 2020. [Google Scholar] [CrossRef]
- Huang, L.; Yao, Q.; Gu, X.; Wang, Q.; Ren, L.; Wang, Y.; Hu, P.; Guo, L.; Liu, M.; Xu, J.; et al. 1-year outcomes in hospital survivors with COVID-19: A longitudinal cohort study. Lancet 2021, 398, 747–758. [Google Scholar] [CrossRef]
- Paces, J.; Strizova, Z.; Daniel, S.M.R.Z.; Cerny, J. COVID-19 and the immune system. Physiol. Res. 2021, 69, 379–388. [Google Scholar] [CrossRef]
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; et al. Attributes and predictors of long COVID. Nat. Med. 2021, 27, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Buonsenso, D.; Munblit, D.; De Rose, C.; Sinatti, D.; Ricchiuto, A.; Carfi, A.; Valentini, P. Preliminary evidence on long COVID in children. Acta Paediatr. 2021, 110, 2208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Guo, R.; Lei, L.; Liu, H.; Wang, Y.; Wang, Y.; Qian, H.; Dai, T.; Zhang, T.; Lai, Y.; et al. COVID-19 infection induces readily detectable morphological and inflammation-related phenotypic changes in peripheral blood monocytes. J. Leukoc. Biol. 2020, 109, 13–22. [Google Scholar] [CrossRef]
- Lancet, T. Facing up to long COVID. Lancet 2020, 396, 1861. [Google Scholar] [CrossRef] [PubMed]
- Alwan, N.A. The road to addressing Long Covid. Science 2021, 373, 491–493. [Google Scholar] [CrossRef]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell 2020, 181, 1489–1501.e15. [Google Scholar] [CrossRef]
- Long, Q.-X.; Tang, X.-J.; Shi, Q.-L.; Li, Q.; Deng, H.-J.; Yuan, J.; Hu, J.-L.; Xu, W.; Zhang, Y.; Lv, F.-J.; et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020, 26, 1200–1204. [Google Scholar] [CrossRef]
- Gallais, F.; Velay, A.; Wendling, M.-J.; Nazon, C.; Partisani, M.; Sibilia, J.; Candon, S.; Fafi-Kremer, S. Intrafamilial exposure to SARS-CoV-2 induces cellular immune response without seroconversion. bioRxiv 2020. [Google Scholar] [CrossRef]
- Varnaitė, R.; García, M.; Glans, H.; Maleki, K.T.; Sandberg, J.T.; Tynell, J.; Christ, W.; Lagerqvist, N.; Asgeirsson, H.; Ljunggren, H.G.; et al. Expansion of SARS-CoV-2-specific antibody-secreting cells and generation of neutralizing antibodies in hospitalized COVID-19 patients. J. Immunol. 2021, 206, 2017–2026. [Google Scholar] [CrossRef]
- Perego, E.; Callard, F.; Stras, L.; Melville-Jóhannesson, B.; Pope, R.; Alwan, N.A. Why the patient-made term ‘long Covid’ is needed. Wellcome Open Res. 2020, 5, 224. [Google Scholar] [CrossRef]
- Halpin, S.; O’Connor, R.; Sivan, M. Long COVID and chronic COVID syndromes. J. Med. Virol. 2021, 93, 1242–1243. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.Y.; Mao, T.; Klein, J.; Dai, Y.; Huck, J.D.; Jaycox, J.R.; Liu, F.; Zhou, T.; Israelow, B.; Wong, P.; et al. Diverse functional autoantibodies in patients with COVID-19. Nature 2021, 595, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, P. NICE guideline on long COVID. Lancet Respir. Med. 2021, 9, 129. [Google Scholar] [CrossRef] [PubMed]
- Avendaño-Solá, C.; Ramos-Martínez, A.; Muñez-Rubio, E.; Ruiz-Antorán, B.; Malo de Molina, R.; Torres, F.; Fernández-Cruz, A.; Calderón-Parra, J.; Payares-Herrera, C.; Díaz de Santiago, A.; et al. ConPlas-19 Study Group A multicenter randomized open-label clinical trial for convalescent plasma in patients hospitalized with COVID-19 pneumonia. J. Clin. Investig. 2021, 131, e152740. [Google Scholar] [CrossRef]
- Xia, H.; Cao, Z.; Xie, X.; Zhang, X.; Chen, J.Y.; Wang, H.; Menachery, V.D.; Rajsbaum, R.; Shi, P.Y. Evasion of Type I Interferon by SARS-CoV-2. Cell Rep. 2020, 33, 108234. [Google Scholar] [CrossRef]
- Gaebler, C.; Wang, Z.; Lorenzi, J.C.C.; Muecksch, F.; Finkin, S.; Tokuyama, M.; Cho, A.; Jankovic, M.; Schaefer-Babajew, D.; Oliveira, T.Y.; et al. Evolution of antibody immunity to SARS-CoV-2. Nature 2021, 591, 639–644. [Google Scholar] [CrossRef]
- Pellegrini, D.; Kawakami, R.; Guagliumi, G.; Sakamoto, A.; Kawai, K.; Gianatti, A.; Nasr, A.; Kutys, R.; Guo, L.; Cornelissen, A.; et al. Microthrombi as a Major Cause of Cardiac Injury in COVID-19: A Pathologic Study. Circulation 2021, 143, 1031–1042. [Google Scholar] [CrossRef]
- Michelen, M.; Manoharan, L.; Elkheir, N.; Cheng, V.; Dagens, A.; Hastie, C.; O’Hara, M.; Suett, J.; Dahmash, D.; Bugaeva, P.; et al. Characterising long COVID: A living systematic review. BMJ Glob. Health 2021, 6, e005427. [Google Scholar] [CrossRef]
- Faraj, S.S.; Jalal, P.J. IL1β, IL-6, and TNF-α cytokines cooperate to modulate a complicated medical condition among COVID-19 patients: Case-control study. Ann. Med. Surg. 2023, 85, 2291–2297. [Google Scholar] [CrossRef]
- Sun, Y.; Zou, Y.; Wang, H.; Cui, G.; Yu, Z.; Ren, Z. Immune response induced by novel coronavirus infection. Front. Cell. Infect. Microbiol. 2022, 12, 988604. [Google Scholar] [CrossRef]
- Zuo, J.; Dowell, A.C.; Pearce, H.; Verma, K.; Long, H.M.; Begum, J.; Aiano, F.; Amin-Chowdhury, Z.; Hoschler, K.; Brooks, T.; et al. Robust SARS-CoV-2-specific T cell immunity is maintained at 6 months following primary infection. Nat. Immunol. 2021, 22, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.; Choudhary, M.C.; Regan, J.; Sparks, J.A.; Padera, R.F.; Qiu, X.; Solomon, I.H.; Kuo, H.H.; Boucau, J.; Bowman, K.; et al. Persistence and Evolution of SARS-CoV-2 in an Immunocompromised Host. N. Engl. J. Med. 2020, 383, 2291–2293. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Ayuzo Del Valle, N.C.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. Long-COVID in children and adolescents: A systematic review and meta-analyses. Sci. Rep. 2022, 12, 9950. [Google Scholar] [CrossRef] [PubMed]
- Rodda, L.B.; Netland, J.; Shehata, L.; Pruner, K.B.; Morawski, P.A.; Thouvenel, C.D.; Takehara, K.K.; Eggenberger, J.; Hemann, E.A.; Waterman, H.R.; et al. Functional SARS-CoV-2-Specific Immune Memory Persists after Mild COVID-19. Cell 2021, 184, 169–183.e17. [Google Scholar] [CrossRef]
- Goel, R.R.; Apostolidis, S.A.; Painter, M.M.; Mathew, D.; Pattekar, A.; Kuthuru, O.; Gouma, S.; Hicks, P.; Meng, W.; Rosenfeld, A.M.; et al. Distinct antibody and memory B cell responses in SARS-CoV-2 naïve and recovered individuals following mRNA vaccination. Sci. Immunol. 2021, 6, eabi6950. [Google Scholar] [CrossRef]
- Yong, S.J. Long COVID or post-COVID-19 syndrome: Putative pathophysiology, risk factors, and treatments. Infect. Dis. 2021, 53, 737–754. [Google Scholar] [CrossRef]
- Mateus, J.; Grifoni, A.; Tarke, A.; Sidney, J.; Ramirez, S.I.; Dan, J.M.; Burger, Z.C.; Rawlings, S.A.; Smith, D.M.; Phillips, E.; et al. Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Science 2020, 370, 89–94. [Google Scholar] [CrossRef]
- Thompson, M.G.; Burgess, J.L.; Naleway, A.L.; Tyner, H.; Yoon, S.K.; Meece, J.; Olsho, L.E.W.; Caban-Martinez, A.J.; Fowlkes, A.L.; Lutrick, K.; et al. Prevention and Attenuation of COVID-19 with the BNT162b2 and mRNA-1273 Vaccines. N. Engl. J. Med. 2021, 385, 320–329. [Google Scholar] [CrossRef]
- Asadi-Pooya, A.A.; Nemati, H.; Shahisavandi, M.; Akbari, A.; Emami, A.; Lotfi, M.; Rostamihosseinkhani, M.; Barzegar, Z.; Kabiri, M.; Zeraatpisheh, Z.; et al. Long COVID in children and adolescents. World J. Pediatr. 2021, 17, 495–499. [Google Scholar] [CrossRef]
- Feng, Z.; Diao, B.; Wang, R.; Wang, G.; Wang, C.; Tan, Y.; Liu, L.; Wang, C.; Liu, Y.; Liu, Y.; et al. The novel severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) directly decimates human spleens and lymph nodes. medRxiv 2020, 2020-03. [Google Scholar] [CrossRef]
- Mostafa, M.; Barhoum, A.; Sehit, E.; Gewaid, H.; Mostafa, E.; Omran, M.M.; Abdalla, M.S.; Abdel-Haleem, F.M.; Altintas, Z.; Forster, R.J. Current trends in COVID-19 diagnosis and its new variants in physiological fluids: Surface antigens, antibodies, nucleic acids, and RNA sequencing. Trends Anal. Chem. 2022, 157, 116750. [Google Scholar] [CrossRef]
- Rogers, T.F.; Zhao, F.; Huang, D.; Beutler, N.; Burns, A.; He, W.T.; Limbo, O.; Smith, C.; Song, G.; Woehl, J.; et al. Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model. Science 2020, 369, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Fact Sheet for Healthcare Providers: Emergency Use Authorization for EvusheldTM (Tixagevimab Co-Packaged with Cilgavimab) Highlights of Emergency Use Authorization (EUA) These Highlights of the EUA Do not Include All the Information Needed to Use EvusheldTM under the EUA. See the Full Fact Sheet for Healthcare Providers for Evusheld. (n.d.). Available online: https://www.fda.gov/media/154701/ (accessed on 22 May 2023).
- Liu, S.; Galat, V.; Galat, Y.; Lee, Y.K.A.; Wainwright, D.; Wu, J. NK cell-based cancer immunotherapy: From basic biology to clinical development. J. Hematol. Oncol. 2021, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Introna, M.; Franceschetti, M.; Ciocca, A.; Borleri, G.; Conti, E.; Golay, J.; Rambaldi, A. Rapid and massive expansion of cord blood-derived cytokine-induced killer cells: An innovative proposal for the treatment of leukemia relapse after cord blood transplantation. Bone Marrow Transplant. 2006, 38, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zeng, S.; Gong, Z.; Yan, Y. Exosome-based immunotherapy: A promising approach for cancer treatment. Mol. Cancer 2020, 19, 160. [Google Scholar] [CrossRef]
- Morse, M.A.; Nair, S.K.; Mosca, P.J.; Hobeika, A.C.; Clay, T.M.; Deng, Y.; Boczkowski, D.; Proia, A.; Neidzwiecki, D.; Clavien, P.A.; et al. Immunotherapy with autologous, human dendritic cells transfected with carcinoembryonic antigen mRNA. Cancer Investig. 2003, 21, 341–349. [Google Scholar] [CrossRef]
- RECOVERY Collaborative Group; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef]
- Salama, C.; Han, J.; Yau, L.; Reiss, W.G.; Kramer, B.; Neidhart, J.D.; Criner, G.J.; Kaplan-Lewis, E.; Baden, R.; Pandit, L.; et al. Tocilizumab in patients hospitalized with COVID-19 pneumonia. N. Engl. J. Med. 2021, 384, 20–30. [Google Scholar] [CrossRef]
- Liu, Y.; Sawalha, A.H.; Lu, Q. COVID-19 and autoimmune diseases. Curr. Opin. Rheumatol. 2021, 33, 155–162. [Google Scholar] [CrossRef]
- Tan, C.K.; Wong, Y.J.; Wang, L.M.; Ang, T.L.; Kumar, R. Autoimmune hepatitis following COVID-19 vaccination: True causality or mere association? J. Hepatol. 2021, 75, 1250–1252. [Google Scholar] [CrossRef]
- Zuo, Y.; Yalavarthi, S.; Shi, H.; Gockman, K.; Zuo, M.; Madison, J.A.; Blair, C.; Weber, A.; Barnes, B.J.; Egeblad, M.; et al. Neutrophil extracellular traps in COVID-19. JCI Insight 2020, 5, e138999. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Estes, S.K.; Ali, R.A.; Gandhi, A.A.; Yalavarthi, S.; Shi, H.; Sule, G.; Gockman, K.; Madison, J.A.; Zuo, M.; et al. Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Sci. Transl. Med. 2020, 12, eabd3876. [Google Scholar] [CrossRef] [PubMed]
- Abu-Rumeileh, S.; Abdelhak, A.; Foschi, M.; Tumani, H.; Otto, M. Guillain-Barré syndrome spectrum associated with COVID-19: An up-to-date systematic review of 73 cases. J. Neurol. 2021, 268, 1133–1170. [Google Scholar] [CrossRef] [PubMed]
- Marjot, T.; Buescher, G.; Sebode, M.; Barnes, E.; Barritt, A.S.; Armstrong, M.J., 4th; Baldelli, L.; Kennedy, J.; Mercer, C.; Ozga, A.-K.; et al. SARS-CoV-2 infection in patients with autoimmune hepatitis. J. Hepatol. 2021, 74, 1335–1343. [Google Scholar] [CrossRef]
- Woodruff, M.C.; Ramonell, R.P.; Nguyen, D.C.; Cashman, K.S.; Saini, A.S.; Haddad, N.S.; Ley, A.M.; Kyu, S.; Howell, J.C.; Ozturk, T.; et al. Extrafollicular B cell responses correlate with neutralizing antibodies and morbidity in COVID-19. Nat. Immunol. 2020, 21, 1506–1516. [Google Scholar] [CrossRef]
- Bastard, P.; Rosen, L.B.; Zhang, Q.; Michailidis, E.; Hoffmann, H.H.; Zhang, Y.; Dorgham, K.; Philippot, Q.; Rosain, J.; Béziat, V.; et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020, 370, eabd4585. [Google Scholar] [CrossRef]
- McMahon, D.E.; Amerson, E.; Rosenbach, M.; Lipoff, J.B.; Moustafa, D.; Tyagi, A.; Desai, S.R.; French, L.E.; Lim, H.W.; Thiers, B.H.; et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: A registry-based study of 414 cases. J. Am. Acad. Dermatol. 2021, 85, 46–55. [Google Scholar] [CrossRef]
- Cunningham, M.W. Molecular Mimicry, Autoimmunity, and Infection: The Cross-Reactive Antigens of Group A Streptococci and their Sequelae. Microbiol. Spectr. 2019, 7, 10-1128. [Google Scholar] [CrossRef]
- Del Valle, D.M.; Kim-Schulze, S.; Huang, H.H.; Beckmann, N.D.; Nirenberg, S.; Wang, B.; Lavin, Y.; Swartz, T.H.; Madduri, D.; Stock, A.; et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020, 26, 1636–1643. [Google Scholar] [CrossRef]
- Dotan, A.; Muller, S.; Kanduc, D.; David, P.; Halpert, G.; Shoenfeld, Y. The SARS-CoV-2 as an instrumental trigger of autoimmunity. Autoimmun. Rev. 2021, 20, 102792. [Google Scholar] [CrossRef]
- Morad, G.; Helmink, B.A.; Sharma, P.; Wargo, J.A. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell 2021, 184, 5309–5337. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Han, T.; Chen, J.; Hou, C.; Hua, L.; He, S.; Guo, Y.; Zhang, S.; Wang, Y.; Yuan, J.; et al. Clinical and Autoimmune Characteristics of Severe and Critical Cases of COVID-19. Clin. Transl. Sci. 2020, 13, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Groff, D.; Sun, A.; Ssentongo, A.E.; Ba, D.M.; Parsons, N.; Poudel, G.R.; Lekoubou, A.; Oh, J.S.; Ericson, J.E.; Ssentongo, P.; et al. Short-term and Long-term Rates of Postacute Sequelae of SARS-CoV-2 Infection: A Systematic Review. JAMA Netw. Open 2021, 4, e2128568. [Google Scholar] [CrossRef]
- WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group; Sterne, J.A.C.; Murthy, S.; Diaz, J.V.; Slutsky, A.S.; Villar, J.; Angus, D.C.; Annane, D.; Azevedo, L.C.P.; Berwanger, O.; et al. Association Between Administration of Systemic Corticosteroids and Mortality among Critically Ill Patients With COVID-19: A Meta-analysis. JAMA 2020, 324, 1330–1341. [Google Scholar] [CrossRef]
- Bartone, P.T.; McDonald, K.; Hansma, B.J.; Solomon, J. Hardiness moderates the effects of COVID-19 stress on anxiety and depression. J. Affect. Disord. 2022, 317, 236–244. [Google Scholar] [CrossRef]
- Polastri, M.; Nava, S.; Clini, E.; Vitacca, M.; Gosselink, R. COVID-19 and pulmonary rehabilitation: Preparing for phase three. Eur. Respir. J. 2020, 55, 2001822. [Google Scholar] [CrossRef]
- Spruit, M.A.; Singh, S.J.; Garvey, C.; ZuWallack, R.; Nici, L.; Rochester, C.; Hill, K.; Holland, A.E.; Lareau, S.C.; Man, W.D.; et al. ATS/ERS Task Force on Pulmonary Rehabilitation An official American Thoracic Society/European Respiratory Society statement: Key concepts and advances in pulmonary rehabilitation. Am. J. Respir. Crit. Care Med. 2013, 188, e13–e64. [Google Scholar] [CrossRef]
- Holland, A.E.; Spruit, M.A.; Troosters, T.; Puhan, M.A.; Pepin, V.; Saey, D.; McCormack, M.C.; Carlin, B.W.; Sciurba, F.C.; Pitta, F.; et al. An official European Respiratory Society/American Thoracic Society technical standard: Field walking tests in chronic respiratory disease. Eur. Respir. J. 2014, 44, 1428–1446. [Google Scholar] [CrossRef]
- Larson, J.L.; Covey, M.K.; Wirtz, S.E.; Berry, J.K.; Alex, C.G.; Langbein, W.E.; Edwards, L. Cycle ergometer and inspiratory muscle training in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1999, 160, 500–507. [Google Scholar] [CrossRef]
- Nici, L.; Donner, C.; Wouters, E.; Zuwallack, R.; Ambrosino, N.; Bourbeau, J.; Carone, M.; Celli, B.; Engelen, M.; Fahy, B.; et al. ATS/ERS Pulmonary Rehabilitation Writing Committee American Thoracic Society/European Respiratory Society statement on pulmonary rehabilitation. Am. J. Respir. Crit. Care Med. 2006, 173, 1390–1413. [Google Scholar] [CrossRef]
- Mazza, M.G.; De Lorenzo, R.; Conte, C.; Poletti, S.; Vai, B.; Bollettini, I.; Melloni, E.M.T.; Furlan, R.; Ciceri, F.; Rovere-Querini, P.; et al. Anxiety and depression in COVID-19 survivors: Role of inflammatory and clinical predictors. Brain Behav. Immun. 2020, 89, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.P.; Chesney, E.; Oliver, D.; Pollak, T.A.; McGuire, P.; Fusar-Poli, P.; Zandi, M.S.; Lewis, G.; David, A.S. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: A systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry 2020, 7, 611–627. [Google Scholar] [CrossRef]
- Hampshire, A.; Trender, W.; Chamberlain, S.R.; Jolly, A.E.; Grant, J.E.; Patrick, F.; Mazibuko, N.; Williams, S.C.; Barnby, J.M.; Hellyer, P.; et al. Cognitive deficits in people who have recovered from COVID-19. EClinicalMedicine 2021, 39, 101044. [Google Scholar] [CrossRef]
- Zheng, K.I.; Feng, G.; Liu, W.Y.; Targher, G.; Byrne, C.D.; Zheng, M.H. Extrapulmonary complications of COVID-19: A multisystem disease? J. Med. Virol. 2021, 93, 323–335. [Google Scholar] [CrossRef]
- Barker-Davies, R.M.; O’Sullivan, O.; Senaratne, K.P.P.; Baker, P.; Cranley, M.; Dharm-Datta, S.; Ellis, H.; Goodall, D.; Gough, M.; Lewis, S.; et al. The Stanford Hall consensus statement for post-COVID-19 rehabilitation. Br. J. Sports Med. 2020, 54, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Andrenelli, E.; Negrini, F.; de Sire, A.; Arienti, C.; Patrini, M.; Negrini, S.; Ceravolo, M.G. International Multiprofessional Steering Committee of Cochrane Rehabilitation REH-COVER action Systematic rapid living review on rehabilitation needs due to COVID-19: Update to May 31st, 2020. Eur. J. Phys. Rehabil. Med. 2020, 56, 508–514. [Google Scholar] [CrossRef]
- Lee, M.J.; Sayers, A.E.; Drake, T.M.; Singh, P.; Bradburn, M.; Wilson, T.R.; Murugananthan, A.; Walsh, C.J.; Fearnhead, N.S.; NASBO Steering Group and NASBO Collaborators. Malnutrition, nutritional interventions and clinical outcomes of patients with acute small bowel obstruction: Results from a national, multicentre, prospective audit. BMJ Open 2019, 9, e029235. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Bhuiyan, F.R.; Emon, T.H.; Hasan, M. Prospects of nutritional interventions in the care of COVID-19 patients. Heliyon 2021, 7, e06285. [Google Scholar] [CrossRef]
- Calder, P.C.; Carr, A.C.; Gombart, A.F.; Eggersdorfer, M. Optimal Nutritional Status for a Well-Functioning Immune System Is an Important Factor to Protect against Viral Infections. Nutrients 2020, 12, 1181. [Google Scholar] [CrossRef]
- World Health Organization. Water, Sanitation, Hygiene, and Waste Management for SARS-CoV-2, the Virus That Causes COVID-19. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-IPC-WASH-2020.4 (accessed on 1 September 2024).
- Shah, K.; Saxena, D.; Mavalankar, D. Secondary attack rate of COVID-19 in household contacts: A systematic review. QJM Mon. J. Assoc. Physicians 2020, 113, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Ceban, F.; Ling, S.; Lui, L.M.W.; Lee, Y.; Gill, H.; Teopiz, K.M.; Rodrigues, N.B.; Subramaniapillai, M.; Di Vincenzo, J.D.; Cao, B.; et al. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain Behav. Immun. 2022, 101, 93–135. [Google Scholar] [CrossRef] [PubMed]
- Naliboff, B.D.; Wu, S.M.; Schieffer, B.; Bolus, R.; Pham, Q.; Baria, A.; Aragaki, D.; Van Vort, W.; Davis, F.; Shekelle, P. A randomized trial of 2 prescription strategies for opioid treatment of chronic nonmalignant pain. J. Pain 2011, 12, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Katon, W.; Ciechanowski, P. Impact of major depression on chronic medical illness. J. Psychosom. Res. 2002, 53, 859–863. [Google Scholar] [CrossRef]
- Alsubaie, M.; Abbott, R.; Dunn, B.; Dickens, C.; Keil, T.F.; Henley, W.; Kuyken, W. Mechanisms of action in mindfulness-based cognitive therapy (MBCT) and mindfulness-based stress reduction (MBSR) in people with physical and/or psychological conditions: A systematic review. Clin. Psychol. Rev. 2017, 55, 74–91. [Google Scholar] [CrossRef]
- Dennis, C.-L. Peer support within a health care context: A concept analysis. Int. J. Nurs. Stud. 2003, 40, 321–332. [Google Scholar] [CrossRef]
- Carfì, A.; Bernabei, R.; Landi, F.; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent Symptoms in Patients After Acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef]
- Greenhalgh, T.; Jimenez, J.L.; Prather, K.A.; Tufekci, Z.; Fisman, D.; Schooley, R. Ten scientific reasons in support of airborne transmission of SARS-CoV-2. Lancet 2021, 397, 1603–1605. [Google Scholar] [CrossRef]
- Mazza, M.G.; Palladini, M.; De Lorenzo, R.; Magnaghi, C.; Poletti, S.; Furlan, R.; Ciceri, F.; COVID-19 BioB Outpatient Clinic Study Group; Rovere-Querini, P.; Benedetti, F. Persistent psychopathology and neurocognitive impairment in COVID-19 survivors: Effect of inflammatory biomarkers at three-month follow-up. Brain Behav. Immun. 2021, 94, 138–147. [Google Scholar] [CrossRef]
- Menni, C.; Valdes, A.M.; Freidin, M.B.; Sudre, C.H.; Nguyen, L.H.; Drew, D.A.; Ganesh, S.; Varsavsky, T.; Cardoso, M.J.; El-Sayed Moustafa, J.S.; et al. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat. Med. 2020, 26, 1037–1040. [Google Scholar] [CrossRef]
- Halpin, S.J.; McIvor, C.; Whyatt, G.; Adams, A.; Harvey, O.; McLean, L.; Walshaw, C.; Kemp, S.; Corrado, J.; Singh, R.; et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: A cross-sectional evaluation. J. Med. Virol. 2021, 93, 1013–1022. [Google Scholar] [CrossRef]
- Cajanding, R.J.M. Comprehensive Review of Cardiovascular Involvement in COVID-19. AACN Adv. Crit. Care 2021, 32, 169–187. [Google Scholar] [CrossRef] [PubMed]
- Meckawy, R.; Stuckler, D.; Mehta, A.; Al-Ahdal, T.; Doebbeling, B.N. Effectiveness of early warning systems in the detection of infectious diseases outbreaks: A systematic review. BMC Public Health 2022, 22, 2216. [Google Scholar] [CrossRef] [PubMed]
- Haldane, V.; Jung, A.S.; De Foo, C.; Bonk, M.; Jamieson, M.; Wu, S.; Verma, M.; Abdalla, S.M.; Singh, S.; Nordström, A.; et al. Strengthening the basics: Public health responses to prevent the next pandemic. BMJ 2021, 375, e067510. [Google Scholar] [CrossRef] [PubMed]
- Excler, J.L.; Saville, M.; Berkley, S.; Kim, J.H. Vaccine development for emerging infectious diseases. Nat. Med. 2021, 27, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Varkey, B. Principles of Clinical Ethics and Their Application to Practice. Med. Princ. Pract. Int. J. Kuwait Univ. Health Sci. Cent. 2021, 30, 17–28. [Google Scholar] [CrossRef]
- Dai, H.; Saccardo, S.; Han, M.A.; Roh, L.; Raja, N.; Vangala, S.; Modi, H.; Pandya, S.; Sloyan, M.; Croymans, D.M. Behavioural nudges increase COVID-19 vaccinations. Nature 2021, 597, 404–409. [Google Scholar] [CrossRef]
- Kinder, K.; Bazemore, A.; Taylor, M.; Mannie, C.; Strydom, S.; George, J.; Goodyear-Smith, F. Integrating primary care and public health to enhance response to a pandemic. Prim. Health Care Res. Dev. 2021, 22, e27. [Google Scholar] [CrossRef]
- Mumtaz, H.; Riaz, M.H.; Wajid, H.; Saqib, M.; Zeeshan, M.H.; Khan, S.E.; Chauhan, Y.R.; Sohail, H.; Vohra, L.I. Current challenges and potential solutions to the use of digital health technologies in evidence generation: A narrative review. Front. Digit. Health 2023, 5, 1203945. [Google Scholar] [CrossRef]
- WHO. Launches New Initiative to Improve Pandemic Preparedness. Available online: https://www.who.int/news/item/26-04-2023-who-launches-new-initiative-to-improve-pandemic-preparedness (accessed on 1 September 2024).
| Factors Responsible for the Role of Neutralizing Antibodies | Type of Neutralizing Agents | Region of Action | Site of Origin | References |
|---|---|---|---|---|
| Targeting the Pathogen | Monoclonal antibodies targeting the spike protein of SARS-CoV-2 | Throughout the body | Produced by B cells in lymphoid tissues and bone marrow | [19] |
| Virus Neutralization | Convalescent plasma containing neutralizing antibodies against SARS-CoV-2 | Throughout the body | Produced by plasma cells derived from B cells | [20] |
| Immune Cell Recruitment | Antibodies engaging in antibody-dependent cellular cytotoxicity (ADCC) | Localized to infected tissue | Produced by plasma cells derived from B cells | [21] |
| Herd Immunity | Vaccines inducing neutralizing antibodies in a population | Population-wide | Not Applicable | [22] |
| Complement Activation | Antibodies triggering the complement system to lyse virus particles | Throughout the body | Produced by plasma cells derived from B cells | [23] |
| Memory B Cells and Long-Term Immunity | Vaccines inducing memory B cells to produce neutralizing antibodies | Throughout the body | Produced by memory B cells derived from B cells | [24] |
| Vaccination Aspect | Data for Two-Dose mRNA Vaccination | Data for Booster Vaccination | Data for Bivalent Booster Strategies and Omicron Variant | References |
|---|---|---|---|---|
| Dosage | Two doses of mRNA-based COVID-19 vaccine | The additional dose administered after the primary vaccination series | Two different COVID-19 vaccines administered in sequence or simultaneously | [33,34,35,36] |
| Examples | Pfizer-BioNTech (Comirnaty), Moderna | Pfizer-BioNTech or Moderna as a booster dose | Examples: Pfizer-BioNTech (mRNA) + AstraZeneca (viral vector) | [33,37,38,39] |
| Drawbacks | Requires ultra-cold storage (Moderna) | Potential rare adverse effects with booster | May increase logistical challenges and vaccine hesitancy | [40,41,42,43] |
| Site of action | Produces immunity in lymph nodes and tissues near the injection site | Enhances immunity in lymphoid tissues and generates a systemic response | Both vaccines may elicit distinct immune responses in different tissues | [43,44,45,46] |
| Effect on different age groups | Efficacious across a wide age range with varying immune response | Reinforces protection in all age groups, especially older individuals | Limited data on bivalent strategies’ effect on different age groups | [47,48,49,50] |
| Side effects | Common side effects: Pain at the injection site, fatigue, mild fever | Side effects similar to the primary series but generally milder | Side effects may vary depending on the combination of vaccines | [51,52,53,54] |
| Effect on immunocompromised patients | May have reduced immune response, may benefit from booster dose | Immunocompromised patients may gain additional protection from booster | Limited data on the effect of bivalent strategies in immunocompromised patient | [55,56,57,58] |
| Effectiveness against virus strain | High efficacy against the original virus and some variants | Enhances protection against variants, providing broader coverage | Effectiveness against Omicron and other emerging variants may vary depending on the combination and vaccine efficacy | [59,60,61,62,63] |
| Types of Omicron Variant | Mechanism of Immune Escape | Possible Challenges | Immune Response | Site of Action | Drawbacks | References |
|---|---|---|---|---|---|---|
| B.1.1.529 | Multiple spike protein mutations, especially in the RBD and N-terminal domain (NTD). These mutations may alter critical epitopes, reducing recognition by neutralizing antibodies. | 1. Reduced efficacy of existing vaccines in preventing infection and transmission. 2. Increased risk of breakthrough infections in previously infected and vaccinated individuals. 3. Challenges in developing effective treatments targeting Omicron’s evading mechanisms. | -Reduced neutralizing antibody response against the Omicron variant. -T cell response may still provide some level of protection. | -Spike protein’s RBD and NTD regions. | -Potential for vaccine breakthrough infections. -Uncertainty about long-term immunity. | [69,70,71] |
| AY.4.2 | Contains additional spike protein mutations, distinct from the original Omicron variant (B.1.1.529). These mutations may contribute to enhanced immune evasion and infectivity. | 1. Challenges in developing variant-specific vaccines due to unique mutations in AY.4.2. 2. Potential for more severe infections and increased transmission, requiring heightened public health measures. | -Impact on neutralizing antibodies and T cell response is yet to be fully understood. | -Spike protein’s RBD and NTD regions. | -Potential for global vaccine ineffectiveness. -Challenges in controlling spread. | [72,73,74] |
| Other Sub-Lineages | Different sub-lineages of the Omicron variant may arise due to continuous viral evolution. Each sub-lineage may possess distinct mutations affecting immune escape mechanisms. | 1. Difficulties in tracking and understanding the potential impact of evolving sub-lineages on immune escape and vaccine efficacy. 2. Need for ongoing surveillance and research to identify emerging sub-lineages and their characteristics. | -Immune response to different sub-lineages may vary. | -Spike protein’s RBD and NTD regions. | -Challenges in predicting immune responses to emerging sub-lineages. | [43,75,76] |
| New Mutations and Variants | The Omicron variant continues to undergo genetic changes, leading to the emergence of novel mutations and variants. These genetic variations may further enhance immune escape mechanisms. | 1. Challenges in predicting the evolution of Omicron and its potential impact on global health. 2. Urgent need for real-time monitoring and research to respond effectively to emerging variants. | -Immune response may need to be constantly updated with evolving variants. | -Spike protein’s RBD and NTD regions. | -Continuous adaptation of vaccines and therapeutics. | [77,78,79] |
| Unknown Implications | The full extent of the Omicron variant’s immune escape mechanisms is still being studied. Discoveries and insights into viral evolution may reveal further challenges for neutralization strategies. | 1. Uncertainties regarding the long-term impact of the Omicron variant on global pandemic control. 2. Need for international collaboration and data-sharing to address emerging concerns. | -Ongoing research is required to understand immune response against new variants. | -Spike protein’s RBD and NTD regions. | -Difficulties in predicting future immune escape mechanisms. | [43,80] |
| Therapeutic Monoclonal Antibody | Mechanism of Action | Immune Response | Dosage | Possible Challenges | Advantages | References |
|---|---|---|---|---|---|---|
| Casirivimab and Imdevimab | Neutralization of SARS-CoV-2 by binding to the spike protein’s RBD, blocking viral entry into host cells. | Elicits an immune response by targeting the virus and enhancing natural immune defenses. | Administered together via IV infusion. | 1. Variants with mutations in the RBD may reduce binding efficacy. 2. Potential for viral escape from antibody-mediated immunity. | Effective early in mild to moderate COVID-19 cases to prevent disease progression. | [87,88,89,90] |
| Sotrovimab | Binds to a conserved epitope in the spike protein’s RBD, preventing viral attachment and entry into host cells. | Stimulates an immune response that aids in viral clearance and limits viral replication. | Administered via IV infusion. | 1. Potential for reduced efficacy against certain variants. 2. Viral escape from antibody-mediated immunity. | Effective against certain variants, useful for early COVID-19 treatment. | [91,92,93,94] |
| REGN-COV2 (Casirivimab + Imdevimab) | Targets two non-overlapping regions of the spike protein’s RBD, reducing the likelihood of escape mutants. | Triggers an immune response by targeting the virus and engaging natural immune defenses. | Administered together via IV infusion. | 1. Challenges in treating variants with mutations outside the targeted regions. 2. Possibility of emerging resistant variants. | Effective early in mild to moderate COVID-19 cases to prevent disease progression. | [95,96,97,98] |
| Bamlanivimab and Etesevimab | Neutralization of SARS-CoV-2 by binding to the spike protein, inhibiting viral attachment and entry into host cells. | Boosts the immune response, aiding in viral clearance and reducing viral load. | IV infusion with loading and maintenance doses. | 1. Reduced efficacy against certain variants with mutations in the RBD. 2. Potential for viral escape from antibody-mediated immunity. | Used for early treatment in individuals with mild to moderate COVID-19 and risk factors. | [99,100,101,102] |
| Tixagevimab and Cilgavimab | Targets non-overlapping epitopes in the spike protein’s RBD, reducing the risk of escape mutants. | Triggers an immune response by targeting the virus and engaging natural immune defenses. | IV infusion at regular intervals. | 1. Possibility of reduced efficacy against certain RBD variants. 2. Risk of viral escape from antibody-mediated immunity. | Effective against certain variants, used for early COVID-19 treatment. | [103,104,105,106] |
| Regdanvimab | Blocks viral attachment and entry by binding to the spike protein’s RBD and inhibiting its interaction with ACE2 receptors. | Enhances natural immune responses and viral clearance. | In subcutaneous injection, the dosing frequency varies based on the indication. | 1. Reduced efficacy against certain viral variants with RBD mutations. 2. Potential for viral escape from antibody-mediated immunity. | Offers an option for early treatment of COVID-19 in high-risk individuals. | [32,107,108,109] |
| Etesevimab | Binds to the spike protein’s RBD, inhibiting viral attachment and entry into host cells. | Stimulates an immune response that aids in viral clearance and reduces viral replication. | Administered via IV infusion. | 1. Potential for reduced efficacy against certain variants. 2. Viral escape from antibody-mediated immunity. | Used in combination therapy for early COVID-19 treatment. | [110,111,112,113] |
| Regkirona (Sotrovimab) | Binds to the spike protein’s RBD, preventing viral attachment and entry into host cells. | Stimulates an immune response that aids in viral clearance and reduces viral replication. | Variable dosing based on indication, usually given as an IV infusion. | 1. Potential for reduced efficacy against certain variants. 2. Risk of viral escape from antibody-mediated immunity. | Useful for early COVID-19 treatment, effective against certain variants. | [114,115,116] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iyer, V.A.; Mohan, A.; Kumar, D.; Dahiya, P. Navigating the Dynamic Landscape of SARS-CoV-2: The Dual Role of Neutralizing Antibodies, Variability in Responses, and Strategies for Adaptive Pandemic Control. COVID 2024, 4, 1395-1412. https://doi.org/10.3390/covid4090100
Iyer VA, Mohan A, Kumar D, Dahiya P. Navigating the Dynamic Landscape of SARS-CoV-2: The Dual Role of Neutralizing Antibodies, Variability in Responses, and Strategies for Adaptive Pandemic Control. COVID. 2024; 4(9):1395-1412. https://doi.org/10.3390/covid4090100
Chicago/Turabian StyleIyer, Venkatesh Anand, Aditi Mohan, Dharmender Kumar, and Praveen Dahiya. 2024. "Navigating the Dynamic Landscape of SARS-CoV-2: The Dual Role of Neutralizing Antibodies, Variability in Responses, and Strategies for Adaptive Pandemic Control" COVID 4, no. 9: 1395-1412. https://doi.org/10.3390/covid4090100
APA StyleIyer, V. A., Mohan, A., Kumar, D., & Dahiya, P. (2024). Navigating the Dynamic Landscape of SARS-CoV-2: The Dual Role of Neutralizing Antibodies, Variability in Responses, and Strategies for Adaptive Pandemic Control. COVID, 4(9), 1395-1412. https://doi.org/10.3390/covid4090100








