The Antiviral Activity of GcMAF in the Treatment of Experimental Animals Infected with SARS-CoV-2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. Virus
2.3. GcMAF
2.4. Animals
2.5. Experimental Design
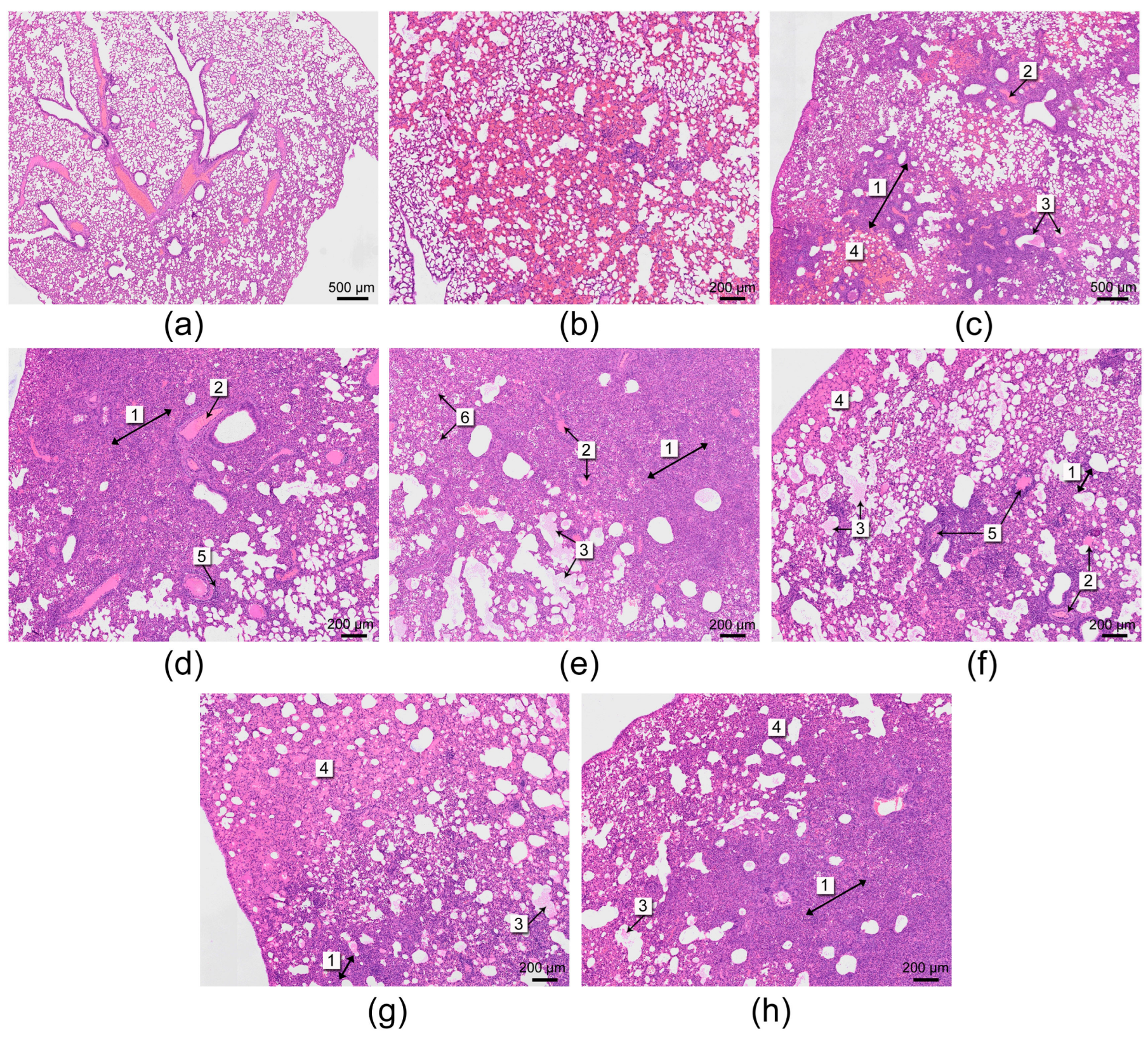
2.6. Histological Studies
2.7. RT-PCR Quantification of SARS-CoV-2 Viral RNA in Bodily Fluids
2.8. Virus Titration
2.9. Obtaining cDNA from Lung Tissue to Analyze the Synthesis of Pro- and Anti-Inflammatory Cytokine mRNA
2.10. Cytokine Real-Time PCR
2.11. Statistical Analysis
3. Results
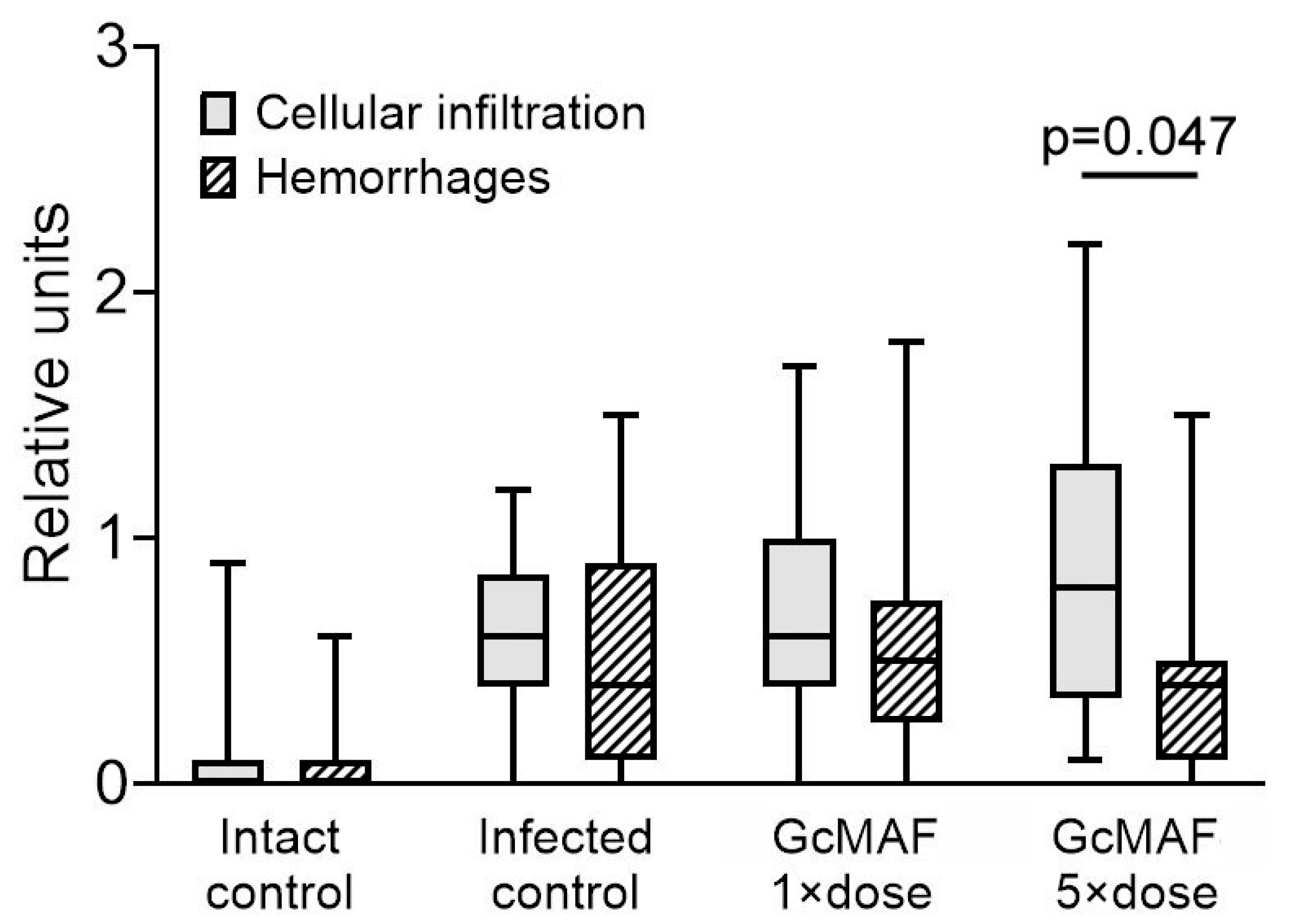
3.1. Histological Analysis
3.2. Quantification of the Viral Load (Infectious Titer) in the Nasal Cavity and Lungs of Experimental Animals
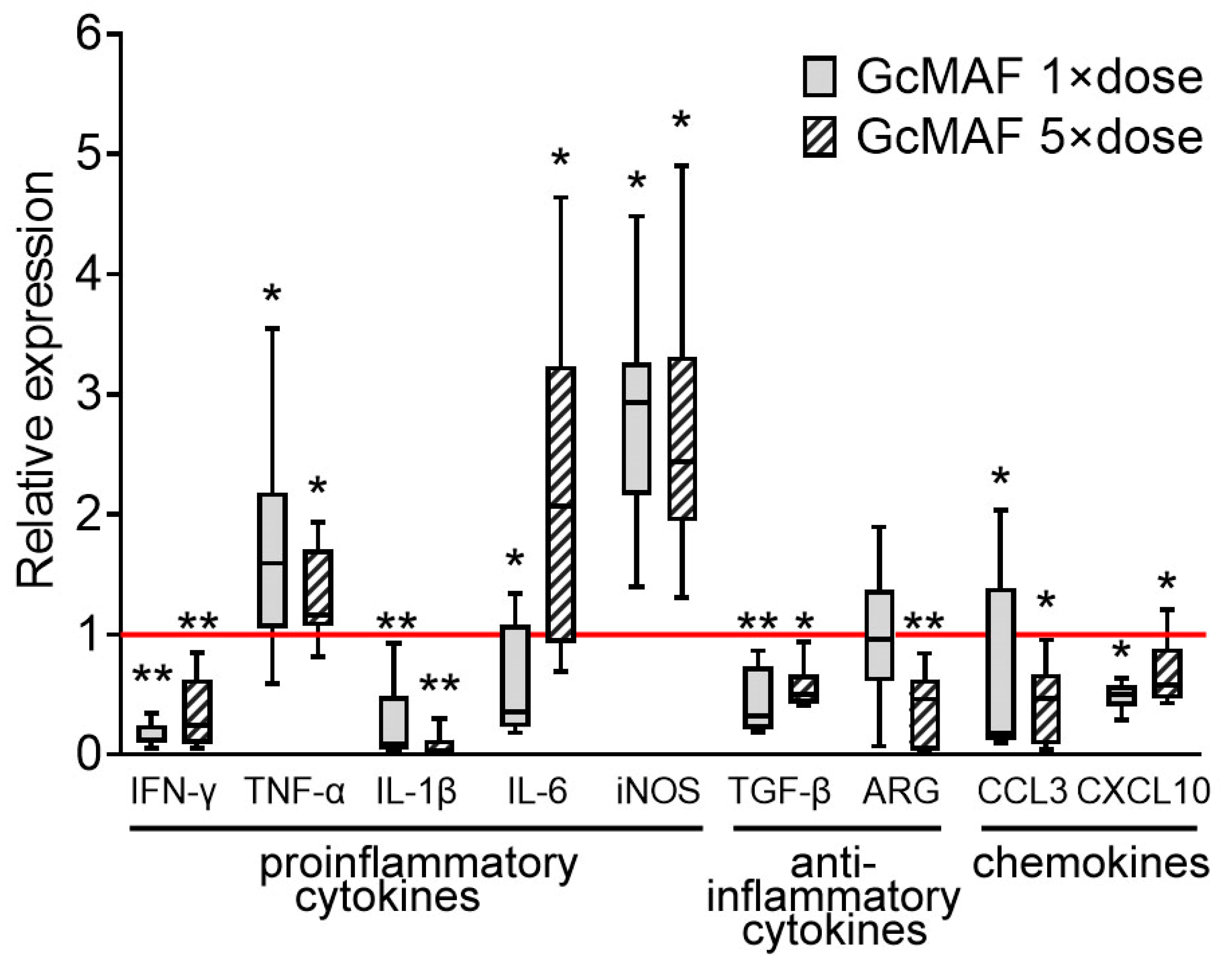
3.3. Analysis of the Synthesis of mRNA of Certain Pro- and Anti-Inflammatory Cytokines
4. Discussion
4.1. The Structure and Functions of the Macrophage-Activating Factor GcMAF
4.2. Factors Ensuring Cell Infection by the Virus
4.2.1. ACE2
4.2.2. C-Type Lectin Receptors: CLEC10A
4.3. The Putative Conceptual Events Occurring When Hamsters Are Infected with the SARS-CoV-2 Virus and Simultaneously Treated with GcMAF
4.3.1. Reduction in Viral Load in the Lungs of Infected Hamsters Treated with GcMAF
4.3.2. Hypothesized Mechanism of Massive Infiltration of the Lungs by Lymphocytes and the Acquisition of Non-Inflammatory Reactions by Lung Tissue at the Level of Cytokine mRNA Synthesis
4.3.3. A Mechanistic, Putative Mechanism for Relieving Hemorrhagic Manifestations in the Lungs of Hamsters Infected with SARS-CoV-2
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE2 | Angiotensin-converting enzyme 2 |
| ARG | Arginase |
| CLEC10A | C-Type lectin domain containing 10A |
| COVID-19 | Coronavirus disease 2019 |
| CRD | Carbohydrate recognition domain |
| DBP | Vitamin D-binding protein |
| GcMAF | Gc protein-derived macrophage-activating factor |
| iNOS | Inducible nitric acid synthase |
| SARS-CoV-2 | Severe acute respiratory syndrome-related coronavirus 2 |
References
- Acuti Martellucci, C.; Flacco, M.E.; Cappadona, R.; Bravi, F.; Mantovani, L.; Manzoli, L. SARS-CoV-2 Pandemic: An Overview. Adv. Biol. Regul. 2020, 77, 100736. [Google Scholar] [CrossRef]
- Adil, M.T.; Rahman, R.; Whitelaw, D.; Jain, V.; Al-Taan, O.; Rashid, F.; Munasinghe, A.; Jambulingam, P. SARS-CoV-2 and the Pandemic of COVID-19. Postgrad. Med. J. 2021, 97, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Hamming, I.; Timens, W.; Bulthuis, M.L.C.; Lely, A.T.; Navis, G.J.; van Goor, H. Tissue Distribution of ACE2 Protein, the Functional Receptor for SARS Coronavirus. A First Step in Understanding SARS Pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Matusewicz, L.; Golec, M.; Czogalla, A.; Kuliczkowski, K.; Konka, A.; Zembala-John, J.; Sikorski, A.F. COVID-19 Therapies: Do We See Substantial Progress? Cell. Mol. Biol. Lett. 2022, 27, 42. [Google Scholar] [CrossRef]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 Entry into Cells. Nat. Rev. Mol. Cell. Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef]
- Cantuti-Castelvetri, L.; Ojha, R.; Pedro, L.D.; Djannatian, M.; Franz, J.; Kuivanen, S.; van der Meer, F.; Kallio, K.; Kaya, T.; Anastasina, M.; et al. Neuropilin-1 Facilitates SARS-CoV-2 Cell Entry and Infectivity. Science 2020, 370, 856–860. [Google Scholar] [CrossRef]
- Zalpoor, H.; Akbari, A.; Samei, A.; Forghaniesfidvajani, R.; Kamali, M.; Afzalnia, A.; Manshouri, S.; Heidari, F.; Pornour, M.; Khoshmirsafa, M.; et al. The Roles of Eph Receptors, Neuropilin-1, P2X7, and CD147 in COVID-19-Associated Neurodegenerative Diseases: Inflammasome and JaK Inhibitors as Potential Promising Therapies. Cell Mol. Biol. Lett. 2022, 27, 10. [Google Scholar] [CrossRef] [PubMed]
- Daly, J.L.; Simonetti, B.; Klein, K.; Chen, K.E.; Williamson, M.K.; Antón-Plágaro, C.; Shoemark, D.K.; Simón-Gracia, L.; Bauer, M.; Hollandi, R.; et al. Neuropilin-1 Is a Host Factor for SARS-CoV-2 Infection. Science 2020, 370, 861–865. [Google Scholar] [CrossRef]
- Wang, S.; Qiu, Z.; Hou, Y.; Deng, X.; Xu, W.; Zheng, T.; Wu, P.; Xie, S.; Bian, W.; Zhang, C.; et al. AXL Is a Candidate Receptor for SARS-CoV-2 That Promotes Infection of Pulmonary and Bronchial Epithelial Cells. Cell Res. 2021, 31, 126–140. [Google Scholar] [CrossRef]
- Gu, Y.; Cao, J.; Zhang, X.; Gao, H.; Wang, Y.; Wang, J.; He, J.; Jiang, X.; Zhang, J.; Shen, G.; et al. Receptome Profiling Identifies KREMEN1 and ASGR1 as Alternative Functional Receptors of SARS-CoV-2. Cell Res. 2022, 32, 24–37. [Google Scholar] [CrossRef]
- Eslami, N.; Aghbash, P.S.; Shamekh, A.; Entezari-Maleki, T.; Nahand, J.S.; Sales, A.J.; Baghi, H.B. SARS-CoV-2: Receptor and Co-Receptor Tropism Probability. Curr. Microbiol. 2022, 79, 133. [Google Scholar] [CrossRef] [PubMed]
- Avdonin, P.P.; Rybakova, E.Y.; Trufanov, S.K.; Avdonin, P.V. SARS-CoV-2 Receptors and Their Involvement in Cell Infection. Biochem. (Mosc) Suppl. Ser. A Membr. Cell. Biol. 2023, 17, 1–11. [Google Scholar] [CrossRef]
- Rahimi, N. C-Type Lectin CD209L/L-SIGN and CD209/DC-SIGN: Cell Adhesion Molecules Turned to Pathogen Recognition Receptors. Biology 2020, 10, 1. [Google Scholar] [CrossRef]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; MacAry, P.A.; Ng, L.F.P. The Trinity of COVID-19: Immunity, Inflammation and Intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef]
- Cron, R.Q.; Schulert, G.S.; Tattersall, R.S. Defining the Scourge of COVID-19 Hyperinflammatory Syndrome. Lancet Rheumatol. 2020, 2, e727–e729. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, S.B.; Nagasawa, H.; Uto, Y.; Hori, H. Preparation of Gc Protein-Derived Macrophage Activating Factor (GcMAF) and Its Structural Characterization and Biological Activities. Anticancer Res. 2002, 22, 4297–4300. [Google Scholar] [PubMed]
- Naraparaju, V.R.; Yamamoto, N. Roles of β-Galactosidase of B Lymphocytes and Sialidase of T Lymphocytes in Inflammation-Primed Activation of Macrophages. Immunol. Lett. 1994, 43, 143–148. [Google Scholar] [CrossRef]
- Saburi, E.; Saburi, A.; Ghanei, M. Promising Role for Gc-MAF in Cancer Immunotherapy: From Bench to Bedside. Casp. J. Intern. Med. 2017, 8, 228–238. [Google Scholar]
- Ostanin, A.A.; Kirikovich, S.S.; Dolgova, E.V.; Proskurina, A.S.; Chernykh, E.R.; Bogachev, S.S. A Thorny Pathway of Macrophage Activating Factor (GcMAF): From Bench to Bedside. Vavilov J. Genet. Breed. 2019, 23, 624–631. [Google Scholar] [CrossRef]
- Kirikovich, S.S.; Levites, E.V.; Proskurina, A.S.; Ritter, G.S.; Peltek, S.E.; Vasilieva, A.R.; Ruzanova, V.S.; Dolgova, E.V.; Oshihmina, S.G.; Sysoev, A.V.; et al. The Molecular Aspects of Functional Activity of Macrophage-Activating Factor GcMAF. Int. J. Mol. Sci. 2023, 24, 17396. [Google Scholar] [CrossRef]
- Dolgova, E.V.; Kirikovich, S.S.; Levites, E.V.; Ruzanova, V.S.; Proskurina, A.S.; Ritter, G.S.; Taranov, O.S.; Varaksin, N.A.; Ryabicheva, T.G.; Leplina, O.Y.; et al. Analysis of the Biological Properties of Blood Plasma Protein with GcMAF Functional Activity. Int. J. Mol. Sci. 2022, 23, 8075. [Google Scholar] [CrossRef] [PubMed]
- Ruzanova, V.S.; Kirikovich, S.S.; Levites, E.V.; Proskurina, A.S.; Dolgova, E.V.; Ritter, G.S.; Efremov, Y.R.; Dubatolova, T.D.; Sysoev, A.V.; Koleno, D.I.; et al. The Macrophage Activator GcMAF-RF Enhances the Antitumor Effect of Karanahan Technology Through Induction of M2–M1 Macrophage Reprogramming. J. Immunol. Res. 2024, 2024, 7484490. [Google Scholar] [CrossRef] [PubMed]
- Kenneth Hoober, J. ASGR1 and Its Enigmatic Relative, CLEC10A. Int. J. Mol. Sci. 2020, 21, 4818. [Google Scholar] [CrossRef]
- Inui, T.; Kuchiike, D.; Kubo, K.; Mette, M.; Uto, Y.; Hori, H.; Sakamoto, N. Clinical Experience of Integrative Cancer Immunotherapy with GcMAF. Anticancer Res. 2013, 33, 2917–2920. [Google Scholar] [PubMed]
- Kisker, O.; Onizuka, S.; Becker, C.M.; Fannon, M.; Flynn, E.; D’Amato, R.; Zetter, B.; Folkman, J.; Ray, R.; Swamy, N.; et al. Vitamin D Binding Protein-Macrophage Activating Factor (DBP-Maf) Inhibits Angiogenesis and Tumor Growth in Mice. Neoplasia 2003, 5, 32–40. [Google Scholar] [CrossRef]
- Korbelik, M.; Naraparaju, V.R.; Yamamoto, N. Macrophage-Directed Immunotherapy as Adjuvant to Photodynamic Therapy of Cancer. Br. J. Cancer 1997, 75, 202–207. [Google Scholar] [CrossRef]
- Kuchiike, D.; Uto, Y.; Mukai, H.; Ishiyama, N.; Abe, C.; Tanaka, D.; Kawai, T.; Kubo, K.; Mette, M.; Inui, T.; et al. Degalactosylated/Desialylated Human Serum Containing GcMAF Induces Macrophage Phagocytic Activity and in Vivo Antitumor Activity. Anticancer Res. 2013, 33, 2881–2885. [Google Scholar]
- Pacini, S.; Morucci, G.; Punzi, T.; Gulisano, M.; Ruggiero, M.; Amato, M.; Aterini, S. Effect of Paricalcitol and GcMAF on Angiogenesis and Human Peripheral Blood Mononuclear Cell Proliferation and Signaling. J. Nephrol. 2012, 25, 577–581. [Google Scholar] [CrossRef]
- Thyer, L.; Ward, E.; Smith, R.; Branca, J.J.V.; Morucci, G.; Gulisano, M.; Noakes, D.; Eslinger, R.; Pacini, S. GC Protein-Derived Macrophage-Activating Factor Decreases α-N-Acetylgalactosaminidase Levels in Advanced Cancer Patients. Oncoimmunology 2013, 2, e25769. [Google Scholar] [CrossRef]
- Thyer, L.; Ward, E.; Smith, R.; Fiore, M.; Magherini, S.; Branca, J.; Morucci, G.; Gulisano, M.; Ruggiero, M.; Pacini, S. A Novel Role for a Major Component of the Vitamin D Axis: Vitamin D Binding Protein-Derived Macrophage Activating Factor Induces Human Breast Cancer Cell Apoptosis through Stimulation of Macrophages. Nutrients 2013, 5, 2577–2589. [Google Scholar] [CrossRef]
- Toyohara, Y.; Hashitani, S.; Kishimoto, H.; Noguchi, K.; Yamamoto, N.; Urade, M. Inhibitory Effect of Vitamin D-Binding Protein-Derived Macrophage Activating Factor on DMBA-Induced Hamster Cheek Pouch Carcinogenesis and Its Derived Carcinoma Cell Line. Oncol. Lett. 2011, 2, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Rehder, D.S.; Nelson, R.W.; Borges, C.R. Glycosylation Status of Vitamin D Binding Protein in Cancer Patients. Protein Sci. 2009, 18, 2036–2042. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A Simple Method of Estimating Fifty Percent Endpoints. Am. J. Hyg. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Kirikovich, S.S.; Levites, E.V.; Proskurina, A.S.; Ritter, G.S.; Dolgova, E.V.; Ruzanova, V.S.; Oshihmina, S.G.; Snegireva, J.S.; Gamaley, S.G.; Sysoeva, G.M.; et al. Production of GcMAF with anti-inflammatory properties and its effect on models of induced arthritis in mice and cystitis in rats. Curr. Issues Mol. Biol. 2024, 46, 10934–10959. [Google Scholar] [CrossRef] [PubMed]
- Matchett, C.A.; Marr, R.; Berard, F.M.; Cawthon, A.G.; Swing, S.P. The Laboratory Ferret; CRC Press: Boca Raton, FL, USA, 2012; 123p. [Google Scholar]
- Li, X.; Wang, Y.; Li, J.; Mei, X.; Liu, Y.; Huang, H. qPCRtools: An R package for qPCR data processing and visualization. Front. Genet. 2022, 13, 1002704. [Google Scholar] [CrossRef]
- Vernel-Pauillac, F.R.; Goarant, C. Differential Cytokine Gene Expression According to Outcome in a Hamster Model of Leptospirosis. PLoS Negl. Trop. Dis. 2010, 4, e582. [Google Scholar] [CrossRef]
- Osorio, Y.; Melby, P.C.; Pirmez, C.; Chandrasekar, B.; Guarín, N.; Travi, B.L. The Site of Cutaneous Infection Influences the Immunological Response and Clinical Outcome of Hamsters Infected with Leishmania Panamensis. Parasite Immunol. 2003, 25, 139–148. [Google Scholar] [CrossRef]
- Matsui, M.; Rouleau, V.; Bruyère-Ostells, L.; Goarant, C. Gene Expression Profiles of Immune Mediators and Histopathological Findings in Animal Models of Leptospirosis: Comparison between Susceptible Hamsters and Resistant Mice. Infect. Immun. 2011, 79, 4480–4492. [Google Scholar] [CrossRef]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef]
- Malik, S.; Fu, L.; Juras, D.J.; Karmali, M.; Wong, B.Y.L.; Gozdzik, A.; Cole, D.E.C. Common Variants of the Vitamin D Binding Protein Gene and Adverse Health Outcomes. Crit. Rev. Clin. Lab. Sci. 2013, 50, 1–22. [Google Scholar] [CrossRef]
- Otterbein, L.R.; Cosio, C.; Graceffa, P.; Dominguez, R. Crystal Structures of the Vitamin D-Binding Protein and Its Complex with Actin: Structural Basis of the Actin-Scavenger System. Proc. Natl. Acad. Sci. USA 2002, 99, 8003–8008. [Google Scholar] [CrossRef] [PubMed]
- Verboven, C.; Rabijns, A.; De Maeyer, M.; Van Baelen, H.; Bouillon, R.; De Ranter, C. A Structural Basis for the Unique Binding Features of the Human Vitamin D-Binding Protein. Nat. Struct. Biol. 2002, 9, 131–136. [Google Scholar] [CrossRef]
- Yamamoto, N.; Suyama, H.; Yamamoto, N. Immunotherapy for Prostate Cancer with Gc Protein-Derived Macrophage-Activating Factor, GcMAF. Transl. Oncol. 2008, 1, 65–72. [Google Scholar] [CrossRef]
- Yamamoto, N.; Kumashiro, R. Conversion of Vitamin D3 Binding Protein (Group-Specific Component) to a Macrophage Activating Factor by the Stepwise Action of Beta-Galactosidase of B Cells and Sialidase of T Cells. J. Immunol. 1993, 151, 2794–2802. [Google Scholar] [CrossRef]
- McCracken, I.R.; Saginc, G.; He, L.; Huseynov, A.; Daniels, A.; Fletcher, S.; Peghaire, C.; Kalna, V.; Andaloussi-Mäe, M.; Muhl, L.; et al. Lack of Evidence of Angiotensin-Converting Enzyme 2 Expression and Replicative Infection by SARS-CoV-2 in Human Endothelial Cells. Circulation 2021, 143, 865–868. [Google Scholar] [CrossRef]
- Muus, C.; Luecken, M.D.; Eraslan, G.; Sikkema, L.; Waghray, A.; Heimberg, G.; Kobayashi, Y.; Vaishnav, E.D.; Subramanian, A.; Smillie, C.; et al. Single-Cell Meta-Analysis of SARS-CoV-2 Entry Genes across Tissues and Demographics. Nat. Med. 2021, 27, 546–559. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Hu, W.; Yu, H.; Zhao, L.; Zhao, Y.; Zhao, X.; Xue, H.H.; Zhao, Y. Little to No Expression of Angiotensin-Converting Enzyme-2 on Most Human Peripheral Blood Immune Cells but Highly Expressed on Tissue Macrophages. Cytometry A 2020, 103, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Gerard, L.; Lecocq, M.; Bouzin, C.; Hoton, D.; Schmit, G.; Pereira, J.P.; Montiel, V.; Plante-Bordeneuve, T.; Laterre, P.F.; Pilette, C. Increased Angiotensin-Converting Enzyme 2 and Loss of Alveolar Type II Cells in COVID-19-Related Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2021, 204, 1024–1034. [Google Scholar] [CrossRef]
- Sharif-Askari, F.S.; Sharif-Askari, N.S.; Goel, S.; Mahboub, B.; Ansari, A.W.; Temsah, M.H.; Zakri, A.M.; Ratemi, E.; Hamoudi, R.; Hamid, Q.; et al. Upregulation of Interleukin-19 in Severe Asthma: A Potential Saliva Biomarker for Asthma Severity. ERJ Open Res. 2021, 7, 00984–2020. [Google Scholar] [CrossRef]
- Qu, L.; Chen, C.; Yin, T.; Fang, Q.; Hong, Z.; Zhou, R.; Tang, H.; Dong, H. ACE2 and Innate Immunity in the Regulation of SARS-CoV-2-Induced Acute Lung Injury: A Review. Int. J. Mol. Sci. 2021, 22, 11483. [Google Scholar] [CrossRef]
- Raes, G.; Brys, L.; Dahal, B.K.; Brandt, J.; Grooten, J.; Brombacher, F.; Vanham, G.; Noël, W.; Bogaert, P.; Boonefaes, T.; et al. Macrophage Galactose-Type C-Type Lectins as Novel Markers for Alternatively Activated Macrophages Elicited by Parasitic Infections and Allergic Airway Inflammation. J. Leukoc. Biol. 2005, 77, 321–327. [Google Scholar] [CrossRef] [PubMed]
- van Vliet, S.J.; Saeland, E.; van Kooyk, Y. Sweet Preferences of MGL: Carbohydrate Specificity and Function. Trends Immunol. 2008, 29, 83–90. [Google Scholar] [CrossRef]
- van Kooyk, Y.; Ilarregui, J.M.; van Vliet, S.J. Novel Insights into the Immunomodulatory Role of the Dendritic Cell and Macrophage-Expressed C-Type Lectin MGL. Immunobiology 2015, 220, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Santana, M.F.; Frank, C.H.M.; Almeida, T.V.R.; Jeronimo, C.M.P.; de Araújo Pinto, R.A.; Martins, Y.F.; de Farias, M.E.L.; Dutra, B.G.; Brito-Sousa, J.D.; Baía-Da-Silva, D.C.; et al. Hemorrhagic and Thrombotic Manifestations in the Central Nervous System in COVID-19: A Large Observational Study in the Brazilian Amazon with a Complete Autopsy Series. PLoS ONE 2021, 16, e0255950. [Google Scholar] [CrossRef] [PubMed]
- Lodigiani, C.; Iapichino, G.; Carenzo, L.; Cecconi, M.; Ferrazzi, P.; Sebastian, T.; Kucher, N.; Studt, J.D.; Sacco, C.; Alexia, B.; et al. Venous and Arterial Thromboembolic Complications in COVID-19 Patients Admitted to an Academic Hospital in Milan, Italy. Thromb. Res. 2020, 191, 9–14. [Google Scholar] [CrossRef]
- Tan, B.K.; Mainbourg, S.; Friggeri, A.; Bertoletti, L.; Douplat, M.; Dargaud, Y.; Grange, C.; Lobbes, H.; Provencher, S.; Lega, J.C. Arterial and Venous Thromboembolism in COVID-19: A Study-Level Meta-Analysis. Thorax 2021, 76, 970–979. [Google Scholar] [CrossRef]
- Sreedharan, R.; Factora, F.; Trombetta, C.; Khanna, S. Hypercoagulability Resulting in Adrenal Hemorrhage in COVID-19. Colomb. J. Anesthesiol. 2022, 50, e992. [Google Scholar] [CrossRef]

| Primer | Oligonucleotide Sequence |
|---|---|
| IFN-γ for | 5′-GACAACCAGGCCATCC-3′ |
| IFN-γ rev | 5′-CAAAACAGCACCGACT-3′ |
| TNF-α for | 5′-AACGGCATGTCTCTCAA-3′ |
| TNF-α rev | 5′-AGTCGGTCACCTTTCT-3′ |
| IL-1β for | 5′-ATCTTCTGTGACTCCTGG-3′ |
| IL-1β rev | 5′-GGTTTATGTTCTGTCCGT-3′ |
| IL-6 for | 5′-AGACAAAGCCAGAGTCATT-3′ |
| IL-6 rev | 5′-TCGGTATGCTAAGGCACAG-3′ |
| iNOS for | 5′-TGAGCCACTGAGTTCTCCTAAGG-3′ |
| iNOS rev | 5′-TCCTATTTCAACTCCAAGATGTTCTG-3′ |
| TGF-β for | 5′-ACGGAGAAGAACTGCT-3′ |
| TGF-β rev | 5′-ACGTAGTACACGATGGG-3′ |
| ARG for | 5′-ACCTATGTGTCATTTGGGTGGA-3′ |
| ARG rev | 5′-GCAGATATGCAGGGAGTCACC-3′ |
| CCL3 for | 5′-CTCCTGCTGCTTCTTCTA-3′ |
| CCL3 rev | 5′-TGGGTTCCTCACTGACTC-3′ |
| CXCL10 for | 5′-CTCTACTAAGAGCTGGTCC-3′ |
| CXCL10 rev | 5′-CTAACACACTTTAAGGTGGG-3′ |
| GAPDH for | 5′-GCAGTTCAAAGGCACAGTCA-3′ |
| GAPDH rev | 5′-TGGTGGTGAAGATGCCAGTA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Proskurina, A.S.; Taranov, O.S.; Kirikovich, S.S.; Aidagulova, S.V.; Ivleva, E.K.; Shipovalov, A.V.; Kudrov, G.A.; Bodnev, S.A.; Ovchinnikova, A.S.; Zaykovskaya, A.V.; et al. The Antiviral Activity of GcMAF in the Treatment of Experimental Animals Infected with SARS-CoV-2. COVID 2025, 5, 36. https://doi.org/10.3390/covid5030036
Proskurina AS, Taranov OS, Kirikovich SS, Aidagulova SV, Ivleva EK, Shipovalov AV, Kudrov GA, Bodnev SA, Ovchinnikova AS, Zaykovskaya AV, et al. The Antiviral Activity of GcMAF in the Treatment of Experimental Animals Infected with SARS-CoV-2. COVID. 2025; 5(3):36. https://doi.org/10.3390/covid5030036
Chicago/Turabian StyleProskurina, Anastasia S., Oleg S. Taranov, Svetlana S. Kirikovich, Svetlana V. Aidagulova, Elena K. Ivleva, Andrey V. Shipovalov, Gleb A. Kudrov, Sergei A. Bodnev, Alena S. Ovchinnikova, Anna V. Zaykovskaya, and et al. 2025. "The Antiviral Activity of GcMAF in the Treatment of Experimental Animals Infected with SARS-CoV-2" COVID 5, no. 3: 36. https://doi.org/10.3390/covid5030036
APA StyleProskurina, A. S., Taranov, O. S., Kirikovich, S. S., Aidagulova, S. V., Ivleva, E. K., Shipovalov, A. V., Kudrov, G. A., Bodnev, S. A., Ovchinnikova, A. S., Zaykovskaya, A. V., Pyankov, O. V., Levites, E. V., Ritter, G. S., Ruzanova, V. S., Oshikhmina, S. G., Dolgova, E. V., Zavjalov, E. L., Ostanin, A. A., Chernykh, E. R., ... Bogachev, S. S. (2025). The Antiviral Activity of GcMAF in the Treatment of Experimental Animals Infected with SARS-CoV-2. COVID, 5(3), 36. https://doi.org/10.3390/covid5030036







