COVID-19: Evidenced Health Disparity
Definition
1. Introduction
2. Defining Health Disparity
| Organization, Country | Definition |
|---|---|
| National Institute of Minority Health Disparities [14] | A health difference, determined on the basis of one or more health outcomes that adversely affect disadvantaged populations. |
| Healthy People 2020 [16] | A particular type of health difference closely linked with social, economic, and environmental disadvantages. |
| Centers for Disease Control and Prevention [17] | Preventable differences in the burden of disease, injury, violence, or opportunities to achieve optimal health experienced by socially disadvantaged racial, ethnic, and other population groups and communities. |
| Institute of Medicine [18] | A health service disparity between population groups is determined as differences in treatment or access not justified by differences in the health status or preferences of the groups. |
| National Health Service [15] | Unfair and avoidable differences in health across the population and between different groups within society. |
3. Intersectionality Framework and Health Disparity
3.1. COVID-19 Disparity
3.2. COVID-19 and Environmental Injustice
3.3. COVID-19 and Incidence of Violence
4. Determinants of Health Disparities
4.1. Social and Structural Determinants of Health
4.2. Structural Racism
4.3. Age and Gender Disparity
4.4. Case Study of COVID-19
5. Implication for Achieving Global and Sustainable Health

6. Measuring Health Disparity
7. The Role of Geospatial and Machine Learning Techniques in Health Disparity
7.1. GIS and Health Data Linkage
7.2. Disease Epidemiology, Machine Learning, and Artificial Intelligence
8. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Entry Link on the Encyclopedia Platform
References
- Iyanda, A.E.; Adeleke, R.; Lu, Y.; Osayomi, T.; Adaralegbe, A.; Lasode, M.; Chima-Adaralegbe, N.J.; Osundina, A.M. A retrospective cross-national examination of COVID-19 outbreak in 175 countries: A multiscale geographically weighted regression analysis (January 11–June 28, 2020). J. Infect. Public Health 2020, 13, 1438–1445. [Google Scholar] [CrossRef]
- John Hopkins Coronavirus Research Center. Available online: https://coronavirus.jhu.edu/map.html (accessed on 23 June 2021).
- Kandi, V.; Thungaturthi, S.; Vadakedath, S.; Gundu, R.; Mohapatra, R.K. Mortality Rates of Coronavirus Disease 2019 (COVID-19) Caused by the Novel Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2). Cureus 2021, 13, e14081. [Google Scholar]
- Onder, G.; Rezza, G.; Brusaferro, S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA 2020, 323, 1775–1776. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, R.; Dranitsaris, G.; Mubashir, T.; Bartoszko, J.; Riazi, S. A country level analysis measuring the impact of government actions, country preparedness and socioeconomic factors on COVID-19 mortality and related health outcomes. EClinicalMedicine 2020, 25, 100464. [Google Scholar] [CrossRef]
- Christie, A. Decreases in COVID-19 Cases, Emergency Department Visits, Hospital Admissions, and Deaths Among Older Adults Following the Introduction of COVID-19 Vaccine—United States, September 6, 2020–May 1, 2021. Morb. Mortal. Wkly. Rep. 2021, 70, 858–864. Available online: https://www.cdc.gov/mmwr/volumes/70/wr/mm7023e2.htm (accessed on 16 July 2021). [CrossRef] [PubMed]
- Moghadas, S.M.; Vilches, T.N.; Zhang, K.; Wells, C.R.; Shoukat, A.; Singer, B.H.; Meyers, L.A.; Neuzil, K.M.; Langley, J.M.; Fitzpatrick, M.C.; et al. The impact of vaccination on COVID-19 outbreaks in the United States. Clin. Infect. Dis. 2021, ciab079. [Google Scholar] [CrossRef] [PubMed]
- Reuters. UK Coronavirus Vaccines Have Weakened Link between Infections and Death, Says Scientist. 2021. Available online: https://www.reuters.com/world/uk/uk-coronavirus-vaccines-have-weakened-link-between-infections-death-says-2021-06-27/ (accessed on 16 July 2021).
- World Health Organization. Vaccine Efficacy, Effectiveness and Protection. 2021. Available online: https://www.who.int/news-room/feature-stories/detail/vaccine-efficacy-effectiveness-and-protection (accessed on 16 July 2021).
- Roser, M.; Ritchie, H.; Ortiz-Ospina, E.; Hasell, J. Coronavirus Pandemic (COVID-19). Available online: https://ourworldindata.org/coronavirus (accessed on 10 August 2020).
- Braveman, P.A.; Kumanyika, S.; Fielding, J.; LaVeist, T.; Borrell, L.; Manderscheid, R.; Troutman, A. Health Disparities and Health Equity: The Issue Is Justice. Am. J. Public Health 2011, 101, S149–S155. [Google Scholar] [CrossRef]
- Whitehead, M. The concepts and principles of equity and health. Health Promot. Int. 1991, 6, 217–228. [Google Scholar] [CrossRef]
- Braveman, P.; Gruskin, S. Defining equity in health. J. Epidemiol. Community Health 2003, 57, 254–258. [Google Scholar] [CrossRef]
- National Institute of Minority Health and Health Disparity. National Minority Health and Health Disparities Research Framework. NIMHD. 2021. Available online: https://www.nimhd.nih.gov/about/overview/ (accessed on 16 July 2021).
- The National Health Service. Health Inequalities. 2021. Available online: https://www.nhs.uk/ (accessed on 15 July 2021).
- Office of Disease Prevention and Health Promotion. Determinants of Health: Healthy People 2020. 2020. Available online: https://www.healthypeople.gov/2020/about/foundation-health-measures/Determinants-of-Health#policymaking (accessed on 28 February 2021).
- Center for Disease Control and Prevention. Health Disparities. 2019. Available online: https://www.cdc.gov/aging/disparities/index.htm (accessed on 16 July 2021).
- Nelson, A. Unequal treatment: Confronting racial and ethnic disparities in health care. J. Natl. Med. Assoc. 2002, 94, 666–668. [Google Scholar]
- Rubin, I.L.; Geller, R.J.; Martinuzzi, K.; Gitterman, B.A.; Wells, L.; Garfinkel, W.; Coles, C.D.; Merrick, J. Children’s environmental health disparities: The costs and benefits of breaking the cycle. Int. J. Child Health Hum. Dev. 2016, 9, 419–430. [Google Scholar]
- Carter-Pokras, O.; Baquet, C. What is a “health disparity”? Public Health Rep. 2002, 117, 426–434. [Google Scholar] [CrossRef]
- American Medical Association. Reducing Disparities in Health Care. 2019. Available online: https://www.ama-assn.org/delivering-care/patient-support-advocacy/reducing-disparities-health-care (accessed on 16 June 2021).
- Dy, A.M.; Jayawarna, D. Bios, mythoi and women entrepreneurs: A Wynterian analysis of the intersectional impacts of the COVID-19 pandemic on self-employed women and women-owned businesses. Int. Small Bus. J. Res. Entrep. 2020, 38, 391–403. [Google Scholar] [CrossRef]
- Eaves, L.; Al-Hindi, K.F. Intersectional geographies and COVID-19. Dialogues Hum. Geogr. 2020, 10, 132–136. [Google Scholar] [CrossRef]
- Sekalala, S.; Perehudoff, K.; Parker, M.; Forman, L.; Rawson, B.; Smith, M. An intersectional human rights approach to prioritising access to COVID-19 vaccines. BMJ Glob. Health 2021, 6, e004462. [Google Scholar] [CrossRef] [PubMed]
- Bowleg, L. We’re Not All in This Together: On COVID-19, Intersectionality, and Structural Inequality. Am. J. Public Health 2020, 110, 917. [Google Scholar] [CrossRef] [PubMed]
- Crenshaw, K. Demarginalizing the intersection of race and sex: A Black feminist critique of antidiscrimination doctrine, feminist theory, and antiracist politics. In University of Chicago Legal Forum; Routledge: London, UK, 1989. [Google Scholar]
- Mackey, K.; Ayers, C.K.; Kondo, K.K.; Saha, S.; Advani, S.M.; Young, S.; Spencer, H.; Rusek, M.; Anderson, J.; Veazie, S.; et al. Racial and ethnic disparities in COVID-19–related infections, hospitalizations, and deaths: A systematic review. Ann. Intern. Med. 2021, 174, 362–373. [Google Scholar] [CrossRef]
- Iyanda, A.E.; Boakye, K.A.; Lu, Y.; Oppong, J.R. Racial/Ethnic Heterogeneity and Rural-Urban Disparity of COVID-19 Case Fatality Ratio in the USA: A Negative Binomial and GIS-Based Analysis. J. Racial Ethn. Health Disparities 2021, 1–14. [Google Scholar] [CrossRef]
- Moore, J.T.; Ricaldi, J.N.; Rose, C.E.; Fuld, J.; Parise, M.; Kang, G.J.; Driscoll, A.K.; Norris, T.; Wilson, N.; Rainisch, G.; et al. Disparities in Incidence of COVID-19 Among Underrepresented Racial/Ethnic Groups in Counties Identified as Hotspots During June 5–18, 2020—22 States, February–June 2020. Morb. Mortal Wkly. Rep. 2020, 69, 1122–1126. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7439982/ (accessed on 14 July 2021). [CrossRef]
- Elias, A.; Ben, J.; Mansouri, F.; Paradies, Y. Racism and nationalism during and beyond the COVID-19 pandemic. Ethn. Racial Stud. 2021, 44, 783–793. [Google Scholar] [CrossRef]
- Patel, J.; Nielsen, F.; Badiani, A.; Assi, S.; Unadkat, V.; Patel, B.; Ravindrane, R.; Wardle, H. Poverty, inequality and COVID-19: The forgotten vulnerable. Public Health 2020, 183, 110–111. [Google Scholar] [CrossRef]
- Patel, P.; Hiam, L.; Sowemimo, A.; Devakumar, D.; McKee, M. Ethnicity and COVID-19. BMJ 2020, 369, m2282. Available online: https://www.bmj.com/content/369/bmj.m2282 (accessed on 13 July 2021). [CrossRef]
- Gravlee, C.C. Systemic racism, chronic health inequities, and COVID-19: A syndemic in the making? Am. J. Hum. Biol. 2020, 32, e23482. [Google Scholar] [CrossRef]
- Louis-Jean, J.; Cenat, K.; Njoku, C.V.; Angelo, J.; Sanon, D. Coronavirus (COVID-19) and Racial Disparities: A Perspective Analysis. J. Racial Ethn. Health Disparities 2020, 7, 1039–1045. [Google Scholar] [CrossRef]
- Abedi, V.; Olulana, O.; Avula, V.; Chaudhary, D.; Khan, A.; Shahjouei, S.; Li, J.; Zand, R. Racial, Economic, and Health Inequality and COVID-19 Infection in the United States. J. Racial Ethn. Health Disparities 2020, 8, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, K.; Chakrabarti, A.; Lima, J.M.; Chandan, J.S.; Bandyopadhyay, S. The interaction of ethnicity and deprivation on COVID-19 mortality risk: A retrospective ecological study. Sci. Rep. 2021, 11, 11555. [Google Scholar] [CrossRef]
- Wilson, S.M.; Bullard, R.; Patterson, J.; Thomas, S.B. Environmental Justice Roundtable on COVID-19. Environ. Justice 2020, 13, 56–64. [Google Scholar] [CrossRef]
- Zhanga, R.; Lib, H.; Khannaa, N. The Environmental Injustice of the COVID-19 Pandemic: Evidence from New York State. 2020. Available online: https://www.researchgate.net/publication/343441430_The_Environmental_Injustice_of_the_COVID-19_Pandemic_Evidence_from_New_York_State (accessed on 16 July 2021).
- Bashir, M.F.; Jiang, B.; Komal, B.; Bashir, M.A.; Farooq, T.H.; Iqbal, N.; Bashir, M. Correlation between environmental pollution indicators and COVID-19 pandemic: A brief study in Californian context. Environ. Res. 2020, 187, 109652. [Google Scholar] [CrossRef] [PubMed]
- Bashir, M.F.; Benghoul, M.; Numan, U.; Shakoor, A.; Komal, B.; Bashir, M.A.; Bashir, M.; Tan, D. Environmental pollution and COVID-19 outbreak: Insights from Germany. Air Qual. Atmos. Health 2020, 13, 1385–1394. [Google Scholar]
- Mollalo, A.; Sadeghian, A.; Israel, G.D.; Rashidi, P.; Sofizadeh, A.; Glass, G.E. Machine learning approaches in GIS-based ecological modeling of the sand fly Phlebotomus papatasi, a vector of zoonotic cutaneous leishmaniasis in Golestan province, Iran. Acta Trop. 2018, 188, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Fattorini, D.; Regoli, F. Role of the chronic air pollution levels in the COVID-19 outbreak risk in Italy. Environ. Pollut. 2020, 264, 114732. [Google Scholar] [CrossRef]
- Arimiyaw, A.W.; Abass, K.; Morgan, A.K. Minimizing the long-term impact of COVID-19 on environmental pollution in Sub-Saharan Africa. Sustain. Sci. Pract. Policy 2021, 17, 82–85. [Google Scholar] [CrossRef]
- Travaglio, M.; Yu, Y.; Popovic, R.; Selley, L.; Leal, N.S.; Martins, L.M. Links between air pollution and COVID-19 in England. Environ. Pollut. 2021, 268, 115859. [Google Scholar] [CrossRef] [PubMed]
- Berman, J.D.; Ebisu, K. Changes in U.S. air pollution during the COVID-19 pandemic. Sci. Total Environ. 2020, 739, 139864. [Google Scholar] [CrossRef]
- Lippi, G.; Sanchis-Gomar, F.; Henry, B.M. Association between environmental pollution and prevalence of coronavirus disease 2019 (COVID-19) in Italy. MedRxiv 2020, preprint. [Google Scholar] [CrossRef]
- Brandt, E.B.; Beck, A.F.; Mersha, T.B. Air pollution, racial disparities, and COVID-19 mortality. J. Allergy Clin. Immunol. 2020, 146, 61–63. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7204717/ (accessed on 14 July 2021). [CrossRef]
- Adams, M.D. Air pollution in Ontario, Canada during the COVID-19 State of Emergency. Sci. Total Environ. 2020, 742, 140516. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wang, M.; Huang, C.; Kinney, P.L.; Anastas, P.T. Air pollution reduction and mortality benefit during the COVID-19 outbreak in China. Lancet Planet. Health 2020, 4, e210–e212. [Google Scholar] [CrossRef]
- Comunian, S.; Dongo, D.; Milani, C.; Palestini, P. Air Pollution and COVID-19: The Role of Particulate Matter in the Spread and Increase of COVID-19’s Morbidity and Mortality. Int. J. Environ. Res. Public Health 2020, 17, 4487. [Google Scholar] [CrossRef] [PubMed]
- Copat, C.; Cristaldi, A.; Fiore, M.; Grasso, A.; Zuccarello, P.; Signorelli, S.S.; Conti, G.O.; Ferrante, M. The role of air pollution (PM and NO2) in COVID-19 spread and lethality: A systematic review. Environ. Res. 2020, 191, 110129. [Google Scholar] [CrossRef]
- Dey, T.; Dominici, F. COVID-19, Air Pollution, and Racial Inequity: Connecting the Dots. Chem. Res. Toxicol. 2020, 34, 669–671. [Google Scholar] [CrossRef]
- Allen, O.; Brown, A.; Wang, E. Socioeconomic Disparities in the Effects of Pollution on Spread of COVID-19: Evidence from US Counties. J. Econ. Behav. Stud. 2021, 12, 33–42. [Google Scholar] [CrossRef]
- Chakraborty, J. Convergence of COVID-19 and chronic air pollution risks: Racial/ethnic and socioeconomic inequities in the U.S. Environ. Res. 2021, 193, 110586. [Google Scholar] [CrossRef] [PubMed]
- Millett, G.A.; Jones, A.T.; Benkeser, D.; Baral, S.; Mercer, L.; Beyrer, C.; Honermann, B.; Lankiewicz, E.; Mena, L.; Crowley, J.S.; et al. Assessing differential impacts of COVID-19 on black communities. Ann. Epidemiol. 2020, 47, 37–44. [Google Scholar] [CrossRef]
- Terrell, K.A.; James, W. Racial Disparities in Air Pollution Burden and COVID-19 Deaths in Louisiana, USA, in the Context of Long-Term Changes in Fine Particulate Pollution. Environ. Justice 2020. [Google Scholar] [CrossRef]
- The Atlantic. Racial Data Dashboard. The COVID Tracking Project. Available online: https://covidtracking.com/race/dashboard (accessed on 10 June 2021).
- Cheng, K.J.G.; Sun, Y.; Monnat, S.M. COVID-19 Death Rates Are Higher in Rural Counties with Larger Shares of Blacks and Hispanics. J. Rural Health 2020, 36, 602–608. [Google Scholar] [CrossRef]
- Godsil, R.D.; Tropp, L.R.; Goff, P.A.; Powell, J.A. Addressing implicit bias, racial anxiety, and stereotype threat in education and health care. Sci. Equal. 2014, 1, 14. [Google Scholar]
- Sonu, S.; Marvin, D.; Moore, C. The Intersection and Dynamics between COVID-19, Health Disparities, and Adverse Childhood Experiences. J. Child Adolesc. Trauma 2021, 1–10. [Google Scholar] [CrossRef]
- Reicher, S.; Stott, C. On order and disorder during the COVID-19 pandemic. Br. J. Soc. Psychol. 2020, 59, 694–702. [Google Scholar] [CrossRef]
- Corpuz, J.C.G. ‘Pandemic within a pandemic’: A call to end police brutality. J. Public Health 2021. [Google Scholar] [CrossRef]
- Njoku, A.; Ahmed, Y.; Bolaji, B. Police brutality against Blacks in the United States and ensuing protests: Implications for social distancing and Black health during COVID-19. J. Hum. Behav. Soc. Environ. 2020, 31, 262–270. [Google Scholar] [CrossRef]
- Coyne, C.J.; Yatsyshina, Y. Pandemic Police States. Peace Econ. Peace Sci. Public Policy 2020, 26. Available online: https://www.degruyter.com/document/doi/10.1515/peps-2020-0021/html (accessed on 12 July 2021). [CrossRef]
- Bailey, J.; Flynn, A.; Henry, N. Pandemics and systemic discrimination: Technology-facilitated violence and abuse in an era of COVID-19 and antiracist protest. In The Emerald International Handbook of Technology Facilitated Violence and Abuse; Emerald Publishing Limited: Bingley, UK, 2021; pp. 787–797. [Google Scholar] [CrossRef]
- Gibson, A.N.; Chancellor, R.L.; Cooke, N.A.; Dahlen, S.P.; Patin, B.; Shorish, Y.L. Struggling to breathe: COVID-19, protest and the LIS response. Equal. Divers. Incl. Int. J. 2020, 40, 74–82. [Google Scholar] [CrossRef]
- Biana, H.T. The Matter of Class: COVID-19 in the Philippines. Soc. Ethics Soc. J. Appl. Philos. 2020, 6, 17–36. [Google Scholar]
- Naja, F.; Hamadeh, R. Nutrition amid the COVID-19 pandemic: A multi-level framework for action. Eur. J. Clin. Nutr. 2020, 74, 1117–1121. [Google Scholar] [CrossRef]
- Gautam, S. The Influence of COVID-19 on Air Quality in India: A Boon or Inutile. Bull. Environ. Contam. Toxicol. 2020, 104, 724–726. [Google Scholar] [CrossRef]
- Ming, W.; Zhou, Z.; Ai, H.; Bi, H.; Zhong, Y. COVID-19 and Air Quality: Evidence from China. Emerg. Mark. Financ. Trade 2020, 56, 2422–2442. [Google Scholar] [CrossRef]
- Zangari, S.; Hill, D.; Charette, A.T.; Mirowsky, J.E. Air quality changes in New York City during the COVID-19 pandemic. Sci. Total Environ. 2020, 742, 140496. [Google Scholar] [CrossRef]
- World Health Organization. Commission on Social Determinants of Health—CSDH; WHO: Geneva, Switzerland, 2008. [Google Scholar]
- Bin Tan, S.; Desouza, P.; Raifman, M. Structural Racism and COVID-19 in the USA: A County-Level Empirical Analysis. J. Racial Ethn. Health Disparities 2021, 1–11. [Google Scholar] [CrossRef]
- Egede, L.E.; Walker, R.J. Structural racism, social risk factors, and COVID-19—A dangerous convergence for Black Americans. N. Engl. J. Med. 2020, 383, e77. [Google Scholar] [CrossRef]
- Zalla, L.C.; Martin, C.L.; Edwards, J.K.; Gartner, D.R.; Noppert, G.A. A Geography of Risk: Structural Racism and COVID-19 Mortality in the United States. Am. J. Epidemiol. 2021, kwab059. [Google Scholar] [CrossRef]
- Yearby, R.; Mohapatra, S. Law, structural racism, and the COVID-19 pandemic. J. Law Biosci. 2020, 7, lsaa036. [Google Scholar] [CrossRef] [PubMed]
- Krieger, N. Enough: COVID-19, structural racism, police brutality, plutocracy, climate change—And time for health justice, democratic governance, and an equitable, sustainable future. Am. J. Public Health 2020, 110, 1620–1623. [Google Scholar] [CrossRef]
- Rothstein, R. The Color of Law: A Forgotten History of How Our Government Segregated America; Liveright Publishing: New York, NY, USA, 2017. [Google Scholar]
- Nardone, A.; Casey, J.A.; Morello-Frosch, R.; Mujahid, M.; Balmes, J.R.; Thakur, N. Associations between historical residential redlining and current age-adjusted rates of emergency department visits due to asthma across eight cities in California: An ecological study. Lancet Planet. Health 2020, 4, e24–e31. [Google Scholar] [CrossRef]
- Nardone, A.L.; Casey, J.A.; Rudolph, K.E.; Karasek, D.; Mujahid, M.; Morello-Frosch, R. Associations between historical redlining and birth outcomes from 2006 through 2015 in California. PLoS ONE 2020, 15, e0237241. [Google Scholar] [CrossRef]
- Huggins, J.C. A cartographic perspective on the correlation between redlining and public health in Austin, Texas–1951. Cityscape 2017, 19, 267–280. [Google Scholar]
- Beyer, K.M.; Zhou, Y.; Matthews, K.; Bemanian, A.; Laud, P.W.; Nattinger, A. New spatially continuous indices of redlining and racial bias in mortgage lending: Links to survival after breast cancer diagnosis and implications for health disparities research. Health Place 2016, 40, 34–43. [Google Scholar] [CrossRef]
- Krieger, N.; Wright, E.; Chen, J.T.; Waterman, P.D.; Huntley, E.R.; Arcaya, M. Cancer Stage at Diagnosis, Historical Redlining, and Current Neighborhood Characteristics: Breast, Cervical, Lung, and Colorectal Cancers, Massachusetts, 2001–2015. Am. J. Epidemiol. 2020, 189, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Collin, L.J.; Gaglioti, A.H.; Beyer, K.M.M.; Zhou, Y.; Moore, M.A.; Nash, R.; Switchenko, J.M.; Miller-Kleinhenz, J.M.; Ward, K.C.; McCullough, L.E. Neighborhood-Level Redlining and Lending Bias Are Associated with Breast Cancer Mortality in a Large and Diverse Metropolitan Area. Cancer Epidemiol. Biomark. Prev. 2020, 30, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Price-Haywood, E.G.; Burton, J.; Fort, D.; Seoane, L. Hospitalization and Mortality among Black Patients and White Patients with COVID-19. N. Engl. J. Med. 2020, 382, 2534–2543. [Google Scholar] [CrossRef] [PubMed]
- Abuelgasim, E.; Saw, L.J.; Shirke, M.; Zeinah, M.; Harky, A. COVID-19: Unique public health issues facing Black, Asian and minority ethnic communities. Curr. Probl. Cardiol. 2020, 45, 100621. [Google Scholar] [CrossRef]
- Mamluk, L.; Jones, T. The impact of COVID-19 on black, Asian and minority ethnic communities. Natl. Inst. Health. Res. NHR Rep. 2020, 20, 1–10. [Google Scholar]
- Aldridge, R.W.; Lewer, D.; Katikireddi, S.V.; Mathur, R.; Pathak, N.; Burns, R.; Fragaszy, E.B.; Johnson, A.M.; Devakumar, D.; Abubakar, I.; et al. Black, Asian and Minority Ethnic groups in England are at increased risk of death from COVID-19: Indirect standardisation of NHS mortality data. Wellcome Open Res. 2020, 5, 88. [Google Scholar] [CrossRef] [PubMed]
- Phiri, P.; Delanerolle, G.; Al-Sudani, A.; Rathod, S. COVID-19 and Black, Asian, and Minority Ethnic Communities: A Complex Relationship Without Just Cause. JMIR Public Health Surveill. 2021, 7, e22581. [Google Scholar] [CrossRef] [PubMed]
- Power, T.; Wilson, D.; Best, O.; Brockie, T.; Bearskin, L.B.; Millender, E.; Lowe, J. COVID-19 and Indigenous Peoples: An imperative for action. J. Clin. Nurs. 2020, 29, 2737–2741. [Google Scholar] [CrossRef] [PubMed]
- Ibarra-Nava, I.; Flores-Rodriguez, K.G.; Ruiz-Herrera, V.; Ochoa-Bayona, H.C.; Salinas-Zertuche, A.; Padilla-Orozco, M.; Salazar-Montalvo, R.G. Ethnic disparities in COVID-19 mortality in Mexico: A cross-sectional study based on national data. PLoS ONE 2021, 16, e0239168. [Google Scholar] [CrossRef]
- Palamim, C.V.C.; Ortega, M.M.; Marson, F.A.L. COVID-19 in the Indigenous Population of Brazil. J. Racial Ethn. Health Disparities 2020, 7, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Argoty-Pantoja, A.; Robles-Rivera, K.; Rivera-Paredez, B.; Salmerón, J. COVID-19 fatality in Mexico’s indigenous populations. Public Health 2021, 193, 69–75. [Google Scholar] [CrossRef]
- Wurth, E. The health of the “forgotten” of Washington, DC: An analysis of gentrification, concentrated poverty and health. In Donald T. Campbell Social Science Research Prize; Paper 22; Lehigh University: New York, NY, USA, 2004. [Google Scholar]
- Madsen, T.E.; Bourjeily, G.; Hasnain, M.; Jenkins, M.; Morrison, M.F.; Sandberg, K.; Tong, I.L.; Trott, J.; Werbinski, J.L.; McGregor, A. Sex-and gender-based medicine: The need for precise terminology. Gend. Genome 2017, 1, 122–128. [Google Scholar] [CrossRef]
- Hughes, M.M. County-Level COVID-19 Vaccination Coverage and Social Vulnerability—United States, December 14, 2020–March 1, 2021. Morb. Mortal Wkly. Rep. 2021, 70, 431–436. Available online: https://www.cdc.gov/mmwr/volumes/70/wr/mm7012e1.htm (accessed on 29 April 2021). [CrossRef]
- Lebovic, S. Why is America the world’s police? Boston Review. 19 October 2020. Available online: http://bostonreview.net/politics/sam-lebovic-stephen-wertheim-tomorrow-the-world (accessed on 16 June 2021).
- The New York Times. Covid World Vaccination Tracker. 2021. Available online: https://www.nytimes.com/interactive/2021/world/covid-vaccinations-tracker.html (accessed on 16 July 2021).
- Medicaid and CHIP Payment and Access Commission. Comments on the U.S. Department of Health and Human Services Report to Congress on Improving the Identification of Health Care Disparities in Medicaid and CHIP [Internet]. MACPAC. 2015. Available online: https://www.macpac.gov/publication/macpac-comments-on-nov-2015-hhs-disparities-data-report/ (accessed on 16 July 2021).
- US Department of Health and Human Services: USDHHS—Google Scholar. Available online: https://scholar.google.com/scholar_lookup?title=With+understanding+and+improving+health+and+objectives+for+improving+health&publication_year=2000& (accessed on 18 May 2021).
- Moonesinghe, R.; Beckles, G.L. Measuring health disparities: A comparison of absolute and relative disparities. PeerJ 2015, 3, e1438. [Google Scholar] [CrossRef]
- Keppel, K.; Pamuk, E.; Lynch, J.; Carter-Pokras, O.; Mays, V.; Pearcy, J.; Schoenbach, V.; Weissman, J.S.; Kim, I. Methodological issues in measuring health disparities. Vital Health Stat. 2005, 2, 1–16. [Google Scholar]
- Penman-Aguilar, A.; Talih, M.; Huang, D.; Moonesinghe, R.; Bouye, K.; Beckles, G. Measurement of Health Disparities, Health Inequities, and Social Determinants of Health to Support the Advancement of Health Equity. J. Public Health Manag. Pract. 2016, 22 (Suppl. 1), S33–S42. [Google Scholar] [CrossRef]
- Talih, M. Examining socioeconomic health disparities using a rank-dependent Rényi index. Ann. Appl. Stat. 2015, 9, 992–1023. [Google Scholar] [CrossRef] [PubMed]
- Talih, M. A reference-invariant health disparity index based on Rényi divergence. Ann. Appl. Stat. 2013, 7, 1217–1243. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mele, M.; Magazzino, C. Pollution, economic growth, and COVID-19 deaths in India: A machine learning evidence. Environ. Sci. Pollut. Res. 2020, 28, 2669–2677. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-P.; Fu, J.S. Evaluating the impact of mobility on COVID-19 pandemic with machine learning hybrid predictions. Sci. Total Environ. 2021, 758, 144151. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Z.; Cao, W.; Liu, Y.; Du, B.; Chen, C.; Liu, Q.; Uddin, N.; Jiang, S.; Chen, C.; et al. Identifying novel factors associated with COVID-19 transmission and fatality using the machine learning approach. Sci. Total. Environ. 2021, 764, 142810. [Google Scholar] [CrossRef]
- Punn, N.S.; Sonbhadra, S.K.; Agarwal, S. COVID-19 epidemic analysis using machine learning and deep learning algorithms. MedRxiv 2020, preprint. [Google Scholar] [CrossRef]
- Pinter, G.; Felde, I.; Mosavi, A.; Ghamisi, P.; Gloaguen, R. COVID-19 Pandemic Prediction for Hungary; A Hybrid Machine Learning Approach. Mathematics 2020, 8, 890. [Google Scholar] [CrossRef]
- Kushwaha, S.; Bahl, S.; Bagha, A.K.; Parmar, K.S.; Javaid, M.; Haleem, A.; Singh, R.P. Significant Applications of Machine Learning for COVID-19 Pandemic. J. Ind. Integr. Manag. 2020, 5, 453–479. [Google Scholar] [CrossRef]
- Carvajal, T.M.; Viacrusis, K.M.; Hernandez, L.F.T.; Ho, H.T.; Amalin, D.M.; Watanabe, K. Machine learning methods reveal the temporal pattern of dengue incidence using meteorological factors in metropolitan Manila, Philippines. BMC Infect Dis. 2018, 18, 183. [Google Scholar] [CrossRef] [PubMed]
- Alimadadi, A.; Aryal, S.; Manandhar, I.; Munroe, P.B.; Joe, B.; Cheng, X. Artificial intelligence and machine learning to fight COVID-19. Physiol. Genom. 2020, 52, 200–202. [Google Scholar] [CrossRef] [PubMed]
- Bachtiger, P.; Peters, N.S.; Walsh, S.L. Machine learning for COVID-19—Asking the right questions. Lancet Digit. Health 2020, 2, e391–e392. [Google Scholar] [CrossRef]
- Krzysztofowicz, S.; Osińska-Skotak, K. The Use of GIS Technology to Optimize COVID-19 Vaccine Distribution: A Case Study of the City of Warsaw, Poland. Int. J. Environ. Res. Public Health 2021, 18, 5636. [Google Scholar] [CrossRef] [PubMed]


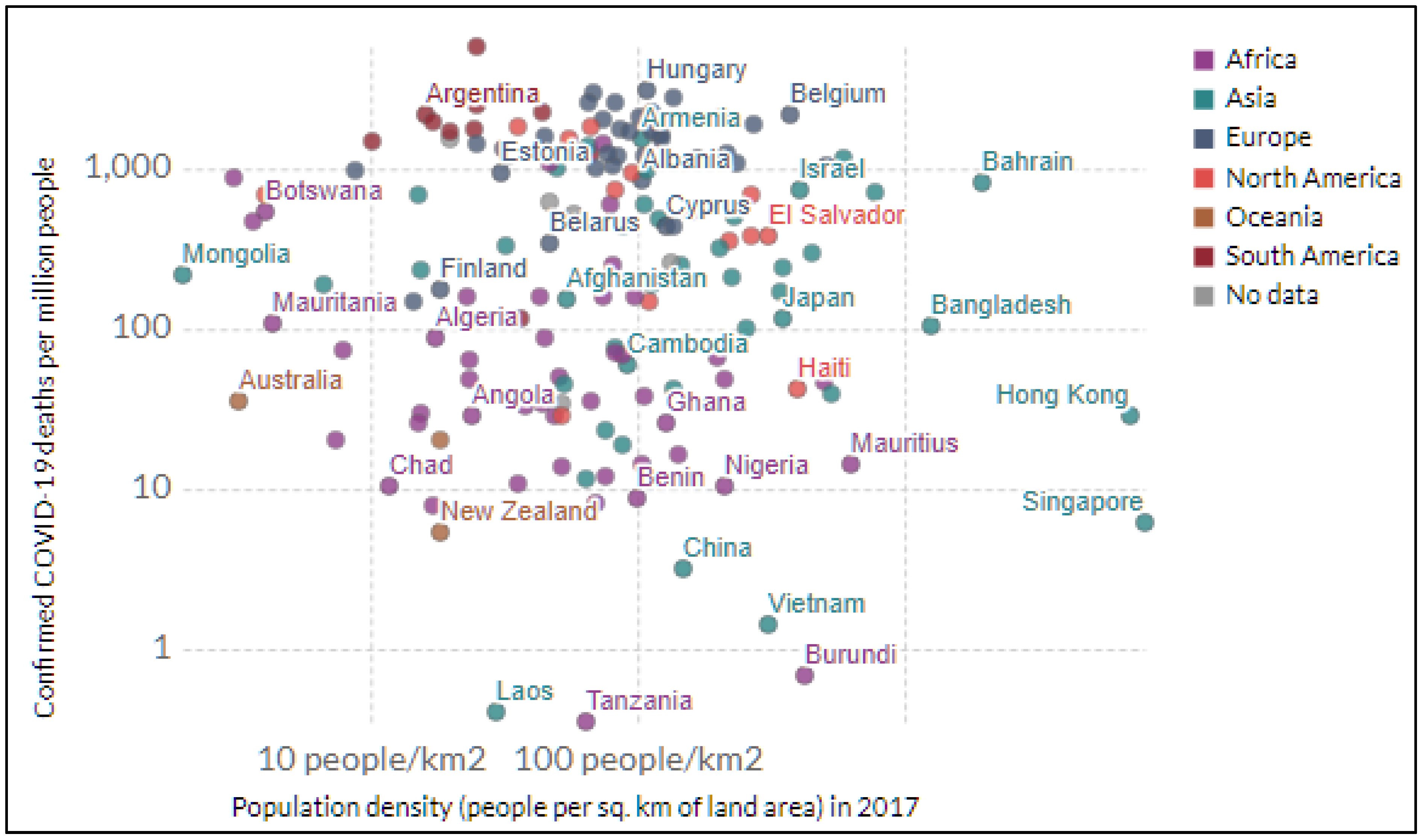
| Study | Location | Health Outcomes | Intersectionality Framework | Common Variables of Interest | Data Analytical Techniques | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Violence | Health Care | Public Policies | Mobility | Racial/Ethnic Heterogeneity | Sociodemographic | Environmental Injustice | Health Disparity | Geographic/Temporal Disparity | GIS/Spatial Statistics | AI, Machine, and Deep Learning | Aspatial | ||||
| Chaudhuri et al., 2021 | UK | Age-adjusted COVID-19 morality | x | x | x | x | SAR | OLS | |||||||
| Iyanda et al., 2020 | Global | COVID-19 outbreak | x | x | x | x | x | MGWR | OLS | ||||||
| Iyanda et al., 2021 | USA | COVID-19 case fatal ratio | x | x | x | x | GWR | Poisson | |||||||
| Louis-Jean et al., 2020 | USA | COVID-19 | x | x | |||||||||||
| Allen Et al., 2020 | USA | COVID-19 confirmed cases, deaths | x | x | x | x | x | Thematic mapping | OLS | ||||||
| Abedi et al., 2020 | USA | COVID-19 infection rate | x | x | x | x | x | Map overlay | OLS, Pearson correlation, Forest Plot | ||||||
| Chen et al., 2020 | China | COVID-19 Mortality rate | x | Remote sensing | Difference-in-Difference | ||||||||||
| Adams, 2020 | Canada | COVID-19 | x | x | x | x | Polynomial regression | ||||||||
| Lippi et al., 2020 | Italy | COVID-19 infection | x | x | Pearson’s correlation | ||||||||||
| Berman & Ebisu, 2020 | USA | COVID-19 infection | x | x | x | t-test | |||||||||
| Travaglio et al., 2021 | UK/England | COVID-19 mortality | x | x | x | Heatmap | GLM, BLR | ||||||||
| Arimiyaw et al., 2020 | SSA region | COVID-19 | X | x | |||||||||||
| Fattorini & Regoli, 2020 | Italy | COVID-19 | x | x | x | Thematic mapping | Pearson’s correlation | ||||||||
| Bashir et al., 2020 | Germany | COVID-19 confirmed cases, deaths, recoveries | x | x | wavelet transform coherence; correlation | ||||||||||
| Bashir et al., 2020 | California, USA | COVID-19 confirmed cases, deaths | x | x | x | Thematic mapping | Spearman’s/Kendall correlation | ||||||||
| Chakraborty, 2021 | USA | COVID-19 incidence rate | x | x | x | x | x | LISA | OLS, GEE | ||||||
| Terrel & James, 2020 | Louisiana, USA | COVID-19 deaths | x | x | x | x | x | Spearman correlation; Shapiro-Wilks’s test | |||||||
| Martinez Dy & Jayawarna, 2020 | UK | COVID-19 impacts | x | x | |||||||||||
| Krzysztofowicz & Osińska-Skotak, 2021 | Poland | COVID-19 Vaccination | Thiessen Polygon | ||||||||||||
| Bachtiger et al., 2020 | Generalized | COVID-19 | x | x | |||||||||||
| Punn et al., 2020 | Global | COVID-19 confirmed cases, death, recovery | x | ||||||||||||
| Cavaljal et al., 2018 | Philippines | Dengue | x | ||||||||||||
| Alimadadi et al., 2020 | Generalized | COVID-19 | x | ||||||||||||
| Kushwaha et al., 2020 | COVID-19 | x | x | ||||||||||||
| Pinter et al., 2020 | Hungary | COVID-19 | x | ||||||||||||
| Li et al., 2021 | Multi-country | x | x | x | x | ||||||||||
| Kuo & Fu, 2021 | USA | COVID-19 infection | x | ||||||||||||
| Mollalo et al., 2018 | Iran | Sandfly; Cutaneous leishmaniasis | x | x | Pearson’s correlation | ||||||||||
| Mele & Magazzino, 2020 | India | COVID-19 | x | ||||||||||||
| Biana, 2020 | Philippines | COVID-19 | x | x | x | x | x | ||||||||
| Sonu et al., 2021 | USA | COVID-19 | x | x | x | ||||||||||
| Wilson et al., 2020 | USA | COVID-19 | x | x | x | x | |||||||||
| Reicher & Stott, 2020 | UK, USA, France | COVID-19 | x | x | |||||||||||
| Corpuz 2021 | Philippines | COVID-19 | x | x | x | ||||||||||
| Njoku et al., 2021 | USA | COVID-19 | x | x | x | x | |||||||||
| Joseph-Salisbury et al., 2021 | UK | COVID-19 | x | x | x | ||||||||||
| Gibson et al., 2021 | USA | COVID-19 | x | x | x | ||||||||||
| Coyne & Yatsyshina, 2020 | Generalized | COVID-19 | x | x | |||||||||||
| Bailey et al., 2020 | Generalized | COVID-19 | x | x | x | x | |||||||||
| Elias et al., 2021 | Generalized | ||||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iyanda, A.; Boakye, K.; Lu, Y. COVID-19: Evidenced Health Disparity. Encyclopedia 2021, 1, 744-763. https://doi.org/10.3390/encyclopedia1030057
Iyanda A, Boakye K, Lu Y. COVID-19: Evidenced Health Disparity. Encyclopedia. 2021; 1(3):744-763. https://doi.org/10.3390/encyclopedia1030057
Chicago/Turabian StyleIyanda, Ayodeji, Kwadwo Boakye, and Yongmei Lu. 2021. "COVID-19: Evidenced Health Disparity" Encyclopedia 1, no. 3: 744-763. https://doi.org/10.3390/encyclopedia1030057
APA StyleIyanda, A., Boakye, K., & Lu, Y. (2021). COVID-19: Evidenced Health Disparity. Encyclopedia, 1(3), 744-763. https://doi.org/10.3390/encyclopedia1030057







