Effects of High-Temperature Milk Processing
Definition
:1. Introduction
2. Bacteriological Effects
3. Chemical Effects
3.1. Effect on Caseins
3.2. Effect on Whey Proteins
3.3. Inactivation of Enzymes
3.4. Practical Consequences of the Effect on Milk Proteins
- An effect of the release of κ-casein from the micelle by heat is that it removes a proportion of the negative charge from the micelles and makes them more susceptible to coalescence. This has been proposed as an important factor in sedimentation and age gelation in UHT milk [31].
- The standard heating conditions for yogurt manufacture are 90–95 °C for ~5 min. This results in 70–80% denaturation of whey proteins (~99% of β-Lg). The denaturation and the concomitant interaction of β-Lg with κ-casein increases the viscosity of the milk and helps to give yogurt a stable body [33].
- The heating of milk causes an increase in reactive or free sulfhydryl groups due to their unmasking during the unfolding of β-Lg. The level of reactive sulfhydryls increases with the severity of heating and has been used as a measure of the severity of heat treatments, provided the milk is analyzed soon after processing; the level decreases during storage due to oxidation, particularly at room temperature [34].
- Heating milk at >90 °C such as in UHT processing, also results in a cooked flavor which is attributable to volatile sulfur compounds produced by the degradation of whey proteins, chiefly β-Lg, and the proteins of the milk fat globule membrane. The compounds formed include hydrogen sulfide, which disappears during storage of UHT milk for about a week, hydrogen sulfide, methanethiol, dimethyl sulfide, dimethyl sulfoxide, carbon disulfide and dimethyl disulfide [35].
- In a study of the effects of various heat treatments on the production of cooked flavor, Gaafar [36] found that it was first detected by panellists when the milk was heated at 94 °C for 20 s. This corresponded to ~60% denaturation of β-Lg. This fact, together with bacteriological considerations, was used in determining the optimum conditions for ESL processing [9]; heating at 134 °C for 4 s is sufficient for the inactivation of psychrotrophic spores and causes ~56% denaturation of β-Lg, ensuring minimal cooked flavor.
- The extent of whey protein denaturation is the basis of the Whey Protein Nitrogen Index (WPNI) used for distinguishing low-heat-, medium-heat-, and high-heat skim milk powders. The WPNI, the amount of undenatured whey protein, for these powders is, respectively, ≥6.00, 1.51–5.99 and ≤1.50 mg WPN·g−1 powder. Typical processing conditions for these powders are 72–80 °C for 15–30 s, 90 °C for 30 s and 90 °C for 5 min. The classification of the powders determines their most suitable application. For example, medium-heat powders are used in confectionary, bakery products and recombined milk while high-heat powders are ideal for use in recombined evaporated and sweetened condensed milk.
- Whole milk powder is produced from milk heated at 90–95 °C for 30–60 s. These conditions are designed to denature whey proteins and produce antioxidant sulfhydryls to protect the fat in the powder from oxidation during storage.
- Whey protein denaturation has a major role in fouling of UHT heat exchangers. Fouling deposits in the UHT plant where temperatures are between 95 and 110 °C (known as Type A deposits) are largely composed of whey proteins. Deposits at higher temperatures are predominantly mineral with some casein. It has been hypothesized that maximum fouling occurs when β-Lg is in the denatured, non-aggregated molten-state form when it is “sticky” and readily attaches to the stainless-steel wall and other deposits. Fouling can be minimized when preheat conditions are severe enough to minimise the time the whey proteins spend in this “sticky” state [37].
- Heating milk at ~90 °C for 30 min with calcium ions (and sometimes acid) to denature the whey proteins and complex them with casein, causes the formation of the product, co-precipitate. Co-precipitate contains almost all of the proteins in milk [38] (pp. 476–477).
- Pre-heating, or fore-warming, is an integral part of most sterilizing procedures for concentrated milk. This prolongs the storage life of the product by retarding the development of structure leading to viscosity increase and gelation. Various temperature–time combinations (e.g., 100 °C for 17 min [39]; 120 °C for 3 min [40]; 117 °C for 2 min [41]; and 135 °C for 15 s [42]) have been used but all are designed to denature most of the whey proteins.
- High-temperature heat treatment has an adverse effect on rennet coagulation and hence cheese is not produced from high-heat-treated milk. Milk heated at 140 °C forms either a very weak coagulum or none at all with rennet. The effect has been attributed to the denaturation of whey proteins and their interaction with the casein micelle, inhibiting aggregation of the micelles. The impairment of rennet coagulation occurs when more than 60% of the whey proteins are denatured. The first stage of rennet action, the proteolysis stage is not affected but the second stage, the aggregation stage is affected. The casein-derived peptide (CMP) resulting from proteolysis of κ-casein differs after high heat treatment; it contains less glycosylated form as the denatured whey proteins influence the release of the glycosylated more than the non-glycosylated form of CMP.
- Another effect of high heat treatment which can affect rennet coagulation is the deposition of calcium phosphate on the micelle. This occurs because the solubility of calcium phosphate decreases with heating at high temperatures.
3.5. The Maillard Reaction
Practical Consequences of the Maillard Reaction in Milk
- The first major stable product of the Maillard reaction in milk is the (protein-bound) Amadori product, lactulosyl-lysine. This product has assumed significance because when it is acid digested it forms furosine which is commonly used as a chemical heat index for freshly processed UHT milk.
- The Maillard reaction causes a loss of available lysine as it becomes nutritionally unavailable when combined with lactose in, for example, lactulosyl-lysine. The percentage of lysine that is blocked depends on the heat treatment applied (and storage temperature and time); for example, the percentage for UHT milk is up to 10% and for in-container sterilized milk it is up to 15%. Infant formulae with a high percentage of whey protein (usually ~60% of the protein) have relatively high levels, 25–30%. However, the nutritive value of these products is not substantially diminished as the amount of available lysine is still high [43].
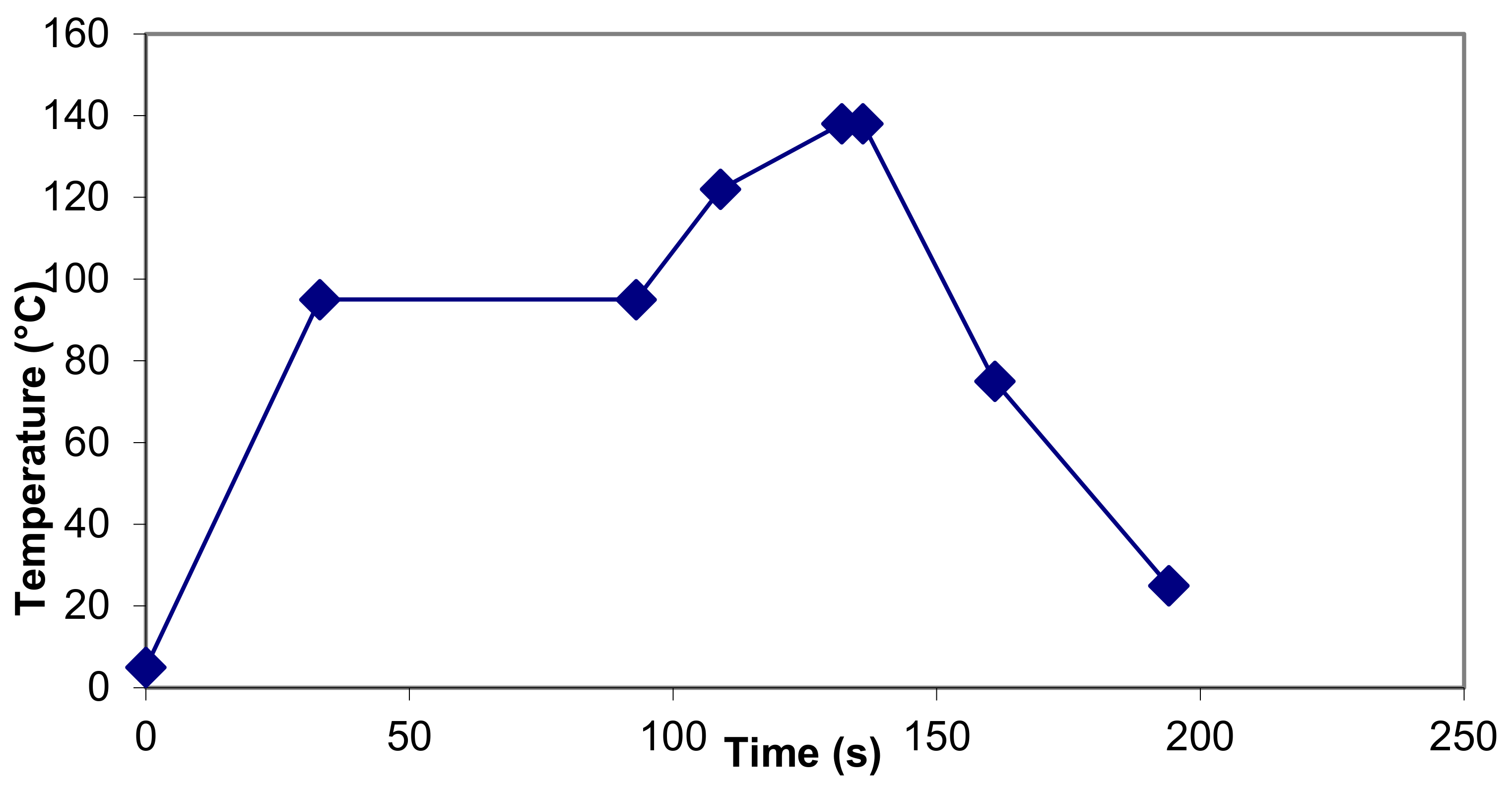
- It has been reported that heating unmodified milk at 121 °C for 400 s is required before browning can be detected by eye [44]. In the illustration of a commercial UHT process in Figure 1 and Table 2, the browning value has been estimated to reach the equivalent of only 107.6 s at 121 °C, well below the reported threshold value. However, in Table 1, the estimated browning for an in-container sterilization process is equivalent to 555 s at 121 °C, which exceeds the published threshold.
- Dulce de leche is a golden brown, viscous, sweet milk product. It has been traditionally produced by concentrating milk, with added sucrose (and sometimes with added glucose), in heated open kettles over several hours until the solids content is around 70%. Its color, which is one of its defining features, is due to Maillard browning, chiefly through lactose but also glucose when added [45].
- Sweetened condensed milk has a creamy yellow color. A brown color in this product is considered a defect. It can occur if the sucrose used contains some invert sugar (hydrolysed to glucose and fructose) which is more Maillard-reactive than sucrose [46].
- Protein cross-linking can occur via Maillard reaction products such as glyoxal and methyl glyoxal. Such cross-linking may contribute to the loss of solubility of milk powders such as milk protein concentrate in which the Maillard reaction is known to proceed during storage of the powder [47].
- Some advanced Maillard reaction products are also known (especially in medical fields) as advanced glycation end-products (AGEs). A major one formed in milk is carboxymethyl-lysine (CML) which is sometimes used as a marker of AGEs. It is formed by cleavage of lactulosyl-lysine. AGEs such as CML are significant medically as they are ingested with food and absorbed along with endogenous AGEs which occur naturally in the body of healthy people. They have been implicated in the progression of diseases such as diabetes but the exact significance of dietary AGEs is still unclear [43,48].
3.6. Effect on Carbohydrates
Practical Consequences of the Effect on Carbohydrates
- Lactulose is not present in raw milk and is present in heated milk. Its level in milk increases with the severity of heat treatment. Furthermore, lactulose increases very little during storage of UHT milk. These facts mean that it is an ideal chemical heat index.
- Lactulose is a laxative and is used as for treating constipation. However, the levels in heated milk are unlikely to have a laxative effect.
3.7. Effect on Vitamins
Practical Consequence of the Effect on Vitamins
- The destruction of water-soluble vitamins reduces the nutritive value of the product. This is significant for the most intense heating only.
- The destruction of thiamine (vitamin B1) is the basis of the chemical index C* proposed by Kessler and Horak [13] as a measure of the severity of heat treatment. C* can be determined by using the reference temperature of 135 °C, and the z-value is 31.4 °C. The formula for C* (at constant temperature) is 10 (T−135)/31.4) × t/30.5. C* of 1 is equivalent to a 3% destruction in thiamine, which relates to heating at 135 °C for 30.5 s, or equivalent conditions such as 140 °C for 21 s; this is the recommended maximum severity for a UHT process [14] although many commercial UHT plants operate at C* of >1 [14]. Table 2 shows a commercial UHT plant with a C* of 1.2, 90% of which was attributable to sections at > 122 °C, Direct UHT plants generally have C* of <1 while the C* for in-container sterilization is much higher; the plant depicted in Table 1 has a C* of 6.55.
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Entry Link on the Encyclopedia Platform
References
- Deeth, H. Heat treatment of milk: Extended shelf life (ESL) and ultra-high-temperature (UHT) treatments. In Encyclopedia of Dairy Sciences, 3rd ed.; McNamara, J., McSweeney, P., Eds.; Academic Press: London, UK, 2021; Volume 4, pp. 618–631. [Google Scholar]
- Deeth, H.C.; Lewis, M.J. High Temperature Processing of Milk and Milk Products; Wiley Blackwell: Oxford, UK, 2017; ISBN 978-1-118-46050-4. [Google Scholar]
- Abd-el-Malek, Y.; Gibson, T. Studies in the bacteriology of milk. 3. The Corynebacteria of milk. J. Dairy Res. 1952, 19, 153–159. [Google Scholar] [CrossRef]
- Smelt, J.P.P.M.; Brul, S. Thermal inactivation of microorganisms. Crit. Rev. Food Sci. Nutr. 2014, 54, 1371–1385. [Google Scholar] [CrossRef] [PubMed]
- McGuiggan, J.T.M.; McCleery, D.R.; Hannan, A.; Gilmour, A. Aerobic spore-forming bacteria in bulk raw milk: Factors influencing the numbers of psychrotrophic, mesophilic and thermophilic Bacillus spores. Int. J. Dairy Technol. 2002, 55, 100–107. [Google Scholar] [CrossRef]
- Deeth, H.C.; Smithers, G. Heat Treatment of Milk—Overview. IDF Factsheet 2018, 001/2018/02. Available online: https://fil-idf.org/wp-content/uploads/2018/02/Factsheet-001_Heat-treatment-1-1.pdf (accessed on 10 November 2021).
- Mayr, R.; Gutser, K.; Busse, M.; Seiler, H. Indigenous aerobic sporeformers in high heat treated (127 °C, 5 s) German ESL (extended shelf life) milk. Milchwiss. Milk Sci. Int. 2004, 59, 143–146. [Google Scholar]
- Deeth, H.C. Extended shelf-life milk thermal processing. Encyclopedia 2021. Available online: https://encyclopedia.pub/13160 (accessed on 10 November 2021).
- Huemer, I.A.; Klijn, N.; Vogelsang, H.W.J.; Langeveld, L.P.M. Thermal death kinetics of spores of Bacillus sporothermodurans isolated from UHT milk. Int. Dairy J. 1998, 8, 851–855. [Google Scholar] [CrossRef]
- Scheldeman, P.; Herman, L.; Foster, S.; Heyndrickx, M. Bacillus sporothermodurans and other highly heat-resistant spore formers in milk. J. Appl. Microbiol. 2006, 101, 542–555. [Google Scholar] [CrossRef]
- Hammer, P.; Lembke, F.; Suhren, G.; Heesschen, W. Characterization of a heat-resistant mesophilic Bacillus species affecting the quality of UHT milk. In Heat Treatments and Alternative Methods; IDF Document 9602; International Dairy Federation: Brussels, Belgium, 1996; pp. 9–16. [Google Scholar]
- Stumbo, C.R.; Murphy, J.R.; Cochran, J. Nature of thermal death time curves for PA-3679 and Clostridium botulinum. Food Technol. 1950, 4, 321–326. [Google Scholar]
- Kessler, H.G.; Horak, P. [Objective assessment of UHT treatment of milk by standardization of bacteriological and chemical effects.] Objektive Beurteilung der UHT-Milcherhitzung durch Normierung bakteriologischer und chemischer Effekte. Milchwiss. Milk Sci. Int. 1981, 36, 129–133. [Google Scholar]
- Tran, H.; Datta, N.; Lewis, M.J.; Deeth, H.C. Predictions of some product parameters based on the processing conditions of ultra-high-temperature milk plants. Int. Dairy J. 2008, 18, 939–944. [Google Scholar] [CrossRef]
- Lyster, R.L.J. The denaturation of a-lactalbumin and b-lactoglobulin in heated milk. J. Dairy Res. 1970, 37, 233–243. [Google Scholar] [CrossRef]
- Horak, F.P.; Kessler, H.G. [Colour measurement as an indicator of heat-treatment of foods, as exemplified by milk products.] Die Farbmessung als Indikator hitzebehandelter Lebensmittel am Beispiel von Milchprodukten. Z. Lebensm. Technol. Verfahr. 1981, 32, 180–184. [Google Scholar]
- Rombaut, R.; Dewettinck, K.; De Mangelaere, G.; Huyghebaert, A. Inactivation of heat resistant spores in bovine milk and lactulose formation. Milchwiss. Milk Sci. Int. 2002, 57, 432–436. [Google Scholar]
- Claeys, W.L.; Ludikhuyze, L.R.; Hendrickx, M.E. Formation kinetics of hydroxymethylfurfural, lactulose and furosine in milk heated under isothermal and non-isothermal conditions. J. Dairy Res. 2001, 68, 287–301. [Google Scholar] [CrossRef]
- Anema, S.G. On heating milk, the dissociation of kappa-casein from the casein micelles can precede interactions with the denatured whey proteins. J. Dairy Res. 2008, 75, 415–421. [Google Scholar] [CrossRef]
- Fritsch, R.J.; Hoffmann, H.; Klostermeyer, H. Formation of lysinoalanine during heat treatment of milk. Z. Lebensm. Unters. Forsch. 1983, 176, 341–345. [Google Scholar]
- Friedman, M. Chemistry, biochemistry, nutrition, and microbiology of lysinoalanine, lanthionine, and histidinoalanine in food and other proteins. J. Agric. Food Chem. 1999, 47, 1295–1319. [Google Scholar] [CrossRef]
- Al-Saadi, J.M.S.; Deeth, H.C. Cross-linking of proteins and other changes in UHT milk during storage at different temperatures. Aust. J. Dairy Technol. 2008, 63, 93–99. [Google Scholar]
- Al-Saadi, J.M.S.; Easa, A.M.; Deeth, H.C. Effect of lactose on cross-linking of milk proteins during heat treatments. Int. J. Dairy Technol. 2013, 66, 1–6. [Google Scholar] [CrossRef]
- Wijayanti, H.B.; Bansal, N.; Deeth, H.C. Stability of whey proteins during thermal processing: A review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1235–1251. [Google Scholar] [CrossRef]
- Deeth, H.C. Heat-induced inactivation of enzymes in milk and dairy products. A review. Int. Dairy J. 2021, 121, 105104. [Google Scholar] [CrossRef]
- Saint Denis, T.; Humbert, G.; Gaillard, J.L. Heat inactivation of native plasmin, plasminogen and plasminogen activators in bovine milk: A revisited study. Lait 2001, 81, 715–729. [Google Scholar] [CrossRef]
- Newstead, D.F.; Paterson, G.; Anema, S.G.; Coker, C.J.; Wewala, A.R. Plasmin activity in direct-steam-injection UHT-processed reconstituted milk: Effects of preheat treatment. Int. Dairy J. 2006, 16, 573–579. [Google Scholar] [CrossRef]
- Rauh, V.M.; Johansen, L.B.; Ipsen, R.; Paulsson, M.; Larsen, L.B.; Hammershoj, M. Plasmin activity in UHT milk: Relationship between proteolysis, age gelation, and bitterness. J. Agric. Food Chem. 2014, 62, 6852–6860. [Google Scholar] [CrossRef]
- Kroll, S.; Klostermeyer, H. Heat inactivation of exogenous proteinases from Pseudomonas fluorescens. I. Possibility of inactivation in milk. Z. Lebensm. Unters. Forsch. 1984, 179, 288–295. [Google Scholar] [CrossRef]
- Christen, G.L.; Wang, W.C.; Ren, T.J. Comparison of the heat resistance of bacterial lipases and proteases and the effect on ultra-high temperature milk quality. J. Dairy Sci. 1986, 69, 2769–2778. [Google Scholar] [CrossRef]
- Anema, S.G. Age gelation, sedimentation, and creaming in UHT milk: A review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 140–166. [Google Scholar] [CrossRef]
- Kelly, A.L.; Foley, J. Proteolysis and storage stability of UHT milk as influenced by milk plasmin activity, plasmin/beta-lactoglobulin complexation, plasminogen activation and somatic cell count. Int. Dairy J. 1997, 7, 411–420. [Google Scholar] [CrossRef]
- Tetra Pak. Dairy Processing Handbook; Tetra Pak Processing Systems: Lund, Sweden, 2003; p. 247. [Google Scholar]
- Patrick, P.S.; Swaisgood, H.E. Sulfhydryl and disulfide groups in skim milk as affected by direct ultra-high-temperature heating and subsequent storage. J. Dairy Sci. 1976, 59, 594–600. [Google Scholar] [CrossRef]
- Al-Attabi, Z.; D’Arcy, B.R.; Deeth, H.C. Volatile sulfur compounds in pasteurised and UHT milk during storage. Dairy Sci. Technol. 2014, 94, 241–253. [Google Scholar] [CrossRef]
- Gaafar, A.M.M. Investigation into the Cooked Flavor in Heat-Treated Milk. Ph.D. Thesis, University of Reading, Reading, UK, 1987. [Google Scholar]
- Grijspeerdt, K.; Mortier, L.; De Block, J.; Van Renterghem, R. Applications of modelling to optimise ultra-high temperature milk heat exchangers with respect to fouling. Food Control 2004, 15, 117–130. [Google Scholar] [CrossRef]
- Walstra, P.; Geurts, T.J.; Noomen, A.; Jellema, A.; van Boekel, M.A.J.S. Dairy Technology. Principles of Milk Properties and Processes; Marcel Dekker: New York, NY, USA, 1999. [Google Scholar]
- Leviton, A.; Pallansch, J. High temperature-short time sterilized evaporated milk. IV. The retardaion of gelation with condensed phosphates, manganous ions, polyhydric compounds, and phosphatides. J. Dairy Sci. 1962, 45, 1045–1056. [Google Scholar] [CrossRef]
- De Koning, P.J.; Kaper, J.; Rollema, H.S.; Driessen, F.M. Age-thinning and gelation in unconcentrated and concentrated UHT-sterilized skim milk. Effect of native milk proteinase. Neth. Milk Dairy J. 1985, 39, 71–87. [Google Scholar]
- Schmidt, D.G. Electron-microscopic studies on gelation of UHTST sterilized concentrated skim milk. Neth. Milk Dairy J. 1968, 22, 40–49. [Google Scholar]
- Nieuwenhuijse, J.A. Changes in heat-treated milk products during storage. In Heat-Induced Changes in Milk, 2nd ed.; Fox, P.F., Ed.; International Dairy Federation: Brussels, Belgium, 1995; pp. 231–255. [Google Scholar]
- Mehta, B.M.; Deeth, H.C. Blocked lysine in dairy products: Formation, occurrence, analysis, and nutritional implications. Compr. Rev. Food Sci. Food Saf. 2016, 15, 206–218. [Google Scholar] [CrossRef]
- Fink, R.; Kessler, H.G. Comparison of methods for distinguishing UHT treatment and sterilization of milk. Milchwiss. Milk Sci. Int. 1988, 43, 275–280. [Google Scholar]
- Vargas, M.O.; Prestes, A.A.; Miotto, M.; Prudencio, E.S. Dulce de leche: Product types, production processes, quality aspects and innovations. Int. J. Dairy Technol. 2021, 74, 262–276. [Google Scholar] [CrossRef]
- Nieuwenhujse, J.A. Sweetened condensed milk. In Encyclopedia of Dairy Sciences, 2nd ed.; Fuquay, J.W., Fox, P.F., McSweeney, P.L.H., Eds.; Academic Press: London, UK, 2011; Volume 1, pp. 869–873. [Google Scholar]
- Le, T.T.; Holland, J.W.; Bhandari, B.; Alewood, P.F.; Deeth, H.C. Direct evidence for the role of Maillard reaction products in protein cross-linking in milk powder during storage. Int. Dairy J. 2013, 31, 83–91. [Google Scholar] [CrossRef]
- Barbosa, J.H.P.; Oliveira, S.L.; Seara, L.T.E. The role of advanced glycation end-products (AGEs) in the development of vascular diabetic complications. Arq. Bras. Endocrinol. Metabol. 2008, 52, 940–950. [Google Scholar] [CrossRef]
- Andrews, G.R.; Morant, S.V. Lactulose content, colour and the organoleptic assessment of ultra-heat treated and sterilized milks. J. Dairy Res. 1987, 54, 493–507. [Google Scholar] [CrossRef]
- Olano, A.; Calvo, M.M.; Corzo, N. Changes in the carbohydrate fraction of milk during heating processes. Food Chem. 1989, 31, 259–265. [Google Scholar] [CrossRef]
- Troyano, E.; Villamiel, M.; Olano, A.; Sanz, J.; MartinezCastro, I. Monosaccharides and myo-inositol in commercial milks. J. Agric. Food Chem. 1996, 44, 815–817. [Google Scholar] [CrossRef]
- Burton, H. Ultra High Temperature Processing of Milk and Milk Products; Elsevier Applied Science Publishers: New York, NY, USA, 1988. [Google Scholar]
| Temp. (°C) | B* | F0 | C* | β-Lg Denaturation 1 (Cumulative) | α-La Denaturation 1 (Cumulative) | Browning 2 (Equivalent Time [s] at 121 °C) | Lactulose 3 (mg/kg Milk) | Furosine 4 (mg/100 g Protein) |
|---|---|---|---|---|---|---|---|---|
| 10 s holding | ||||||||
| 90 | 0 | 0 | 0.01 | 29.1 | 1.8 | 0.66 | 0.4 | 0.49 |
| 100 | 0 | 0 | 0.03 | 40.2 | 3.2 | 1.6 | 1.5 | 1.02 |
| 110 | 0 | 0.1 | 0.05 | 51.7 | 5.6 | 3.8 | 4.6 | 2.06 |
| 120 | 0.04 | 0.13 | 0.11 | 62.3 | 9.5 | 9.3 | 13.3 | 4.0 |
| 130 | 0.33 | 1.29 | 0.23 | 71.4 | 15.7 | 22.3 | 37.1 | 7.5 |
| 140 | 2.96 | 12.94 | 0.47 | 78.8 | 24.2 | 53.6 | 98 | 13.8 |
| 150 | 26.7 | 129.4 | 0.98 | 84.4 | 35.9 | 129.2 | 247 | 24.9 |
| 10 min holding | ||||||||
| 120 | 2.21 | 7.76 | 6.55 | 99.98 | 99.73 | 555 | 80 | 236 |
| Temperature (°C) | Time in Section (s) | B* | F0 | C* | β-Lg Denat’n 1 | α-La Denat’n 1 | Browning 2 (Equivalent Time [s] at 121 °C) | Lactulose 3 (mg/kg Milk) | Furosine 4 (mg/100 g Protein) | |
|---|---|---|---|---|---|---|---|---|---|---|
| In | Out | |||||||||
| 5 | 95 | 33.0 | 0.00 | 0.00 | 0.01 | 12.11 | 0.44 | 0.3 | ||
| 95 | 95 | 60.0 | 0.00 | 0.00 | 0.10 | 76.54 | 6.17 | 4.9 | 5.24 | |
| 95 | 122 | 16.0 | 0.02 | 0.05 | 0.09 | 83.13 | 8.76 | 6.77 | 8.7 | 8.55 |
| 122 | 138 | 23.0 | 1.22 | 4.97 | 0.55 | 91.45 | 38.42 | 55.65 | 94.7 | 34.55 |
| 138 | 138 | 4.0 | 0.76 | 3.27 | 0.16 | 92.33 | 44.23 | 18.00 | 32.4 | 39.37 |
| 138 | 75 | 25.0 | 0.35 | 1.41 | 0.22 | 93.69 | 53.26 | 20.60 | 30.9 | 49.91 |
| 75 | 25 | 33.0 | 0.00 | 0.00 | 0.00 | 93.69 | 53.26 | 0.00 | 0.00 | 49.91 |
| 25 | 25 | 0.0 | 0.00 | 0.00 | 0.00 | 93.69 | 53.26 | 0.00 | 0.00 | 49.91 |
| 194 | 2.34 | 9.70 | 1.14 | 93.69 | 53.26 | 107.63 | 171.9 | 49.91 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deeth, H.C. Effects of High-Temperature Milk Processing. Encyclopedia 2021, 1, 1312-1321. https://doi.org/10.3390/encyclopedia1040098
Deeth HC. Effects of High-Temperature Milk Processing. Encyclopedia. 2021; 1(4):1312-1321. https://doi.org/10.3390/encyclopedia1040098
Chicago/Turabian StyleDeeth, Hilton C. 2021. "Effects of High-Temperature Milk Processing" Encyclopedia 1, no. 4: 1312-1321. https://doi.org/10.3390/encyclopedia1040098
APA StyleDeeth, H. C. (2021). Effects of High-Temperature Milk Processing. Encyclopedia, 1(4), 1312-1321. https://doi.org/10.3390/encyclopedia1040098





