Limbic Encephalitis Associated with COVID-19
Definition
:1. History of Limbic Encephalitis
2. Para-Infectious Variants of Limbic Encephalitis
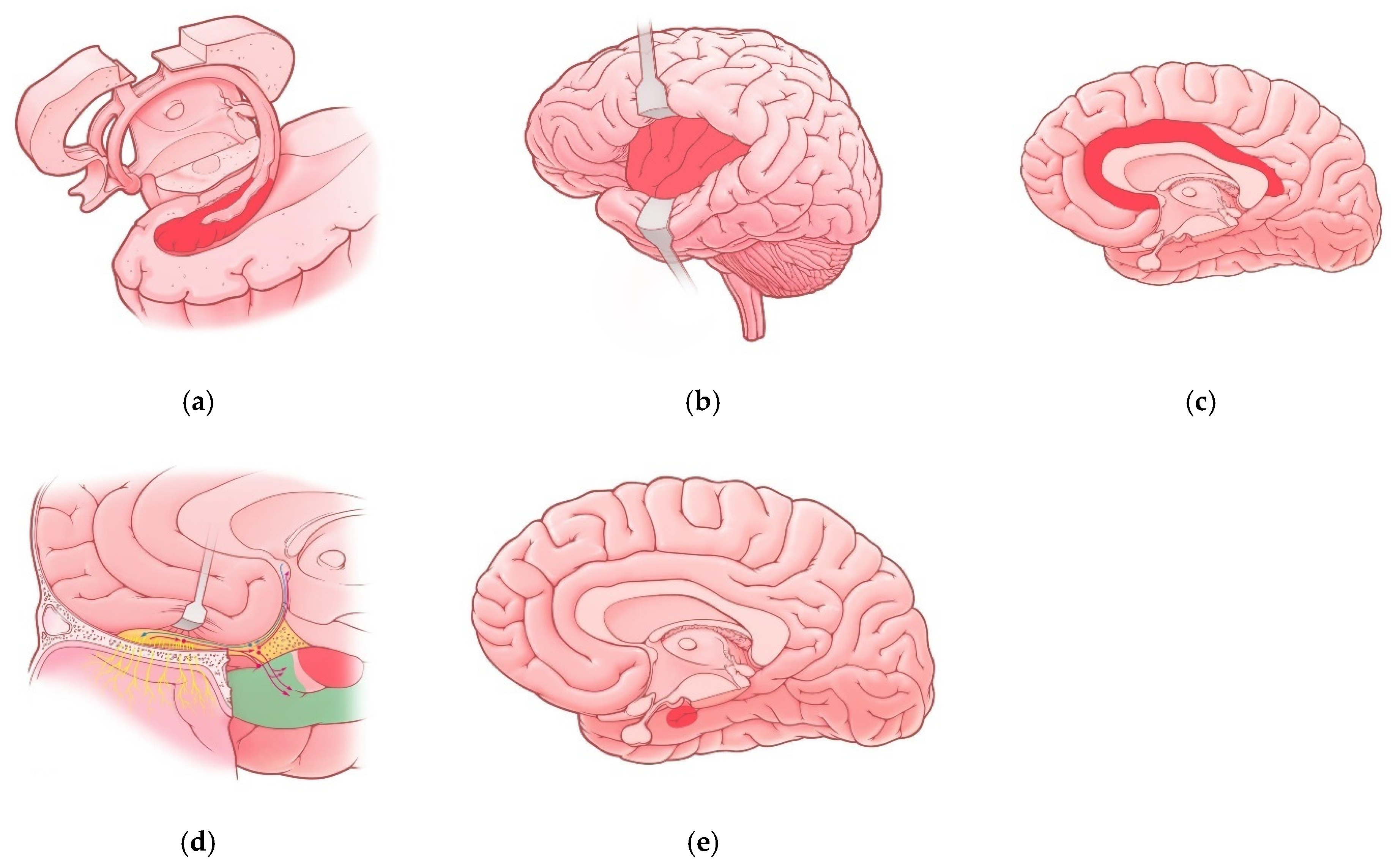
Neuroanatomy of Limbic System and COVID-19
| Region of Limbic System | Associated Manifestations in COVID-19 Patients | References |
|---|---|---|
| Hippocampus | Memory impairment Impaired Consciousness Seizures | [10,25] |
| Insula | Pain perception disturbances Interoception disturbances | [32] |
| Cingulate gyrus | Anxiety disorders Depressive disorders Cognitive impairments (brain fog) Impaired episodic memory Attention disturbances | [23] |
| Olfactory cortex | Olfactory dysfunction (hyposmia, anosmia) | [10,41] |
| Amygdala | Vulnerability to depressive disorders | [29] |
3. Discussion
- (1)
- Subacute onset (rapid progression of less than 3 months) of working memory deficits (short-term memory loss), altered mental status or psychiatric symptoms associated with limbic system involvement;
- (2)
- At least one of the following:
- -
- New focal CNS findings, seizures not explained by a previously known seizure disorder;
- -
- CSF pleocytosis (white blood cell counts of more than five cells per mm3);
- -
- MRI features suggestive of encephalitis;
- (3)
- Reasonable exclusion of alternative causes.
4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Entry Link on the Encyclopedia Platform
References
- Brierley, J.B.; Corsellis, J.A.N.; Hierons, R. Subacute encephalitis of later adult life. Mainly affecting the limbic areas. Brain 1960, 83, 357–368. [Google Scholar] [CrossRef]
- Corsellis, J.A.; Goldgerg, G.J.; Norton, A.R. “Limbic encephalitis” and its association with carcinoma. Brain 1968, 91, 481–496. [Google Scholar] [CrossRef] [PubMed]
- Bakhelt, A.M.; Kennedy, P.G.; Behan, P.O. Paraneoplastic limbic encephalitis: Clinico-pathological correletions. J. Neurol. Neurosurg. Psychiatry 1990, 53, 1084–1088. [Google Scholar] [CrossRef] [PubMed]
- Gultekin, S.H.; Rosenfeld, M.R.; Voltz, R.; Eichen, J.; Posner, J.B.; Dalmau, J. Paraneoplastic limbic encephalitis: Neurological symptoms, immunological findings and tumor association in 50 patients. Brain 2000, 123, 1481–1494. [Google Scholar] [CrossRef]
- Vitaliani, R.; Mason, W.; Ances, B.; Zwerdling, T.; Jiang, Z.; Dalmau, J. Paraneoplastic encephalitis, psychiatric symptoms, and hypoventilation in ovarian teratoma. Ann. Neurol. 2005, 58, 594–604. [Google Scholar] [CrossRef]
- Tüzün, E.; Dalmau, J. Limbic encephalitis and variants: Classification, diagnosis and treatment. Neurologist 2007, 13, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.; Witt, J.A.; Helmstaedter, C.; Malter, M.P.; Weber, B.; Elger, C.E. Automated volumetry of the mesiotemporal structures in antibody-associated limbic encephalitis. J. Neurol. Neurosurg. Psychiatry 2014, 86, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Shnayder, N.A.; Dmitrenko, D.V.; Dykno, Y.A.; Ezhikova, V.V. Paraneoplastic limbic encephalitis in neurological and oncological practice. Russ. J. Oncol. 2013, 1, 49–57. [Google Scholar]
- Shnayder, N.A.; Panina, Y.S.; Dmitrenko, D.V.; Krjzhanovskaya, S.V.; Molgachev, A.A. Parainfectious limbic encephalitis associated with herpes viridae viruses. Probl. Women Health 2014, 9, 58–69. [Google Scholar]
- Lu, Y.; Li, X.; Geng, D.; Mei, N.; Wu, P.Y.; Huang, C.C.; Jia, T.; Zhao, Y.; Wang, D.; Xiao, A.; et al. Cerebral Micro-Structural Changes in COVID-19 Patients–An MRI-based 3-month Follow-up Study. EClinicalMedicine 2020, 25, 100484. [Google Scholar] [CrossRef] [PubMed]
- Bridwell, R.; Long, B.; Gottlieb, M. Neurologic complications of COVID-19. Am. J. Emerg. Med. 2020, 38, 1549.e3–1549.e7. [Google Scholar] [CrossRef]
- Paterson, R.W.; Brown, R.L.; Benjamin, L.; Nortley, R.; Wiethoff, S.; Bharucha, T.; Jayaseelan, D.L.; Kumar, G.; Raftopoulos, R.E.; Zambreanu, L.; et al. The emerging spectrum of COVID-19 neurology: Clinical, radiological and laboratory findings. Brain 2020, 143, 3104–3120. [Google Scholar] [CrossRef] [PubMed]
- Kurd, M.; Hashavya, S.; Benenson, S.; Gilboa, T. Seizures as the main presenting manifestation of acute SARS-CoV-2 infection in children. Seizure 2021, 92, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Sohal, S.; Mansur, M. COVID-19 Presenting with Seizures. IDCases 2020, 20, e00782. [Google Scholar] [CrossRef] [PubMed]
- Santos de Lima, F.; Issa, N.; Seibert, K.; Davis, J.; Wlodarski, R.; Klein, S.; El Ammar, F.; Wu, S.; Rose, S.; Warnke, P.; et al. Epileptiform activity and seizures in patients with COVID-19. J. Neurol. Neurosurg. Psychiatry 2021, 92, 565–566. [Google Scholar] [CrossRef]
- Machhi, J.; Herskovitz, J.; Senan, A.M.; Dutta, D.; Nath, B.; Oleynikov, M.D.; Blomberg, W.R.; Meigs, D.D.; Hasan, M.; Patel, M.; et al. The Natural History, Pathobiology, and Clinical Manifestations of SARS-CoV-2 Infections. J. Neuroimmune Pharmacol. 2020, 15, 359–386. [Google Scholar] [CrossRef]
- Troyer, E.A.; Kohn, J.N.; Hong, S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav. Immun. 2020, 87, 34–39. [Google Scholar] [CrossRef]
- Carroll, E.; Neumann, H.; Aguero-Rosenfeld, M.E.; Lighter, J.; Czeisler, B.M.; Melmed, K.; Lewis, A. Post-COVID-19 inflammatory syndrome manifesting as refractory status epilepticus. Epilepsia 2020, 61, e135–e139. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, X.; Chen, Z.; Duan, J.; Hashimoto, K.; Yang, L.; Liu, C.; Yang, C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav. Immun. 2020, 87, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Zambreanu, L.; Lightbody, S.; Bhandari, M.; Hoskote, C.; Kandil, H.; Houlihan, C.F.; Lunn, M.P. A case of limbic encephalitis associated with asymptomatic COVID-19 infection. J. Neurol. Neurosurg. Psychiatry 2020, 91, 1229–1230. [Google Scholar] [CrossRef]
- Pizzanelli, C.; Milano, C.; Canovetti, S.; Tagliaferri, E.; Turco, F.; Verdenelli, S.; Nesti, L.; Franchi, M.; Bonanni, E.; Menichetti, F.; et al. Autoimmune limbic encephalitis related to SARS-CoV-2 infection: Case-report and review of the literature. Brain Behav. Immun. Health 2021, 12, 100–210. [Google Scholar] [CrossRef] [PubMed]
- Dinakaran, D.; Manjunatha, N.; Naveen Kumar, C.; Suresh, B.M. Neuropsychiatric aspects of COVID-19 pandemic: A selective review. Asian J. Psychiatry 2020, 53, 102–188. [Google Scholar] [CrossRef] [PubMed]
- Hugon, J.; Msika, E.F.; Queneau, M.; Farid, K.; Paquet, C. Long COVID: Cognitive complaints (brain fog) and dysfunction of the cingulate cortex. J. Neurol. 2021, 18, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Anand, K.S.; Dhikav, V. Hippocampus in health and disease: An overview. Ann. Indian Acad. Neurol. 2012, 15, 239–246. [Google Scholar]
- Moriguchi, T.; Harii, N.; Goto, J.; Harada, D.; Sugawara, H.; Takamino, J.; Ueno, M.; Sakata, H.; Kondo, K.; Myose, N.; et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int. J. Infect. Dis. 2020, 94, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Adolphs, R.; Tranel, D.; Hamann, S.; Young, A.W.; Calder, A.J.; Phelps, E.A.; Anderson, A.; Lee, G.P.; Damasio, A.R. Recognition of facial emotion in nine individuals with bilateral amygdala damage. Neuropsychologia 1999, 37, 1111–1117. [Google Scholar] [CrossRef]
- McGaugh, J.L. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu. Rev. Neurosci. 2004, 27, 1–28. [Google Scholar] [CrossRef]
- Patin, A.; Pause, B.M. Human amygdala activations during nasal chemoreception. Neuropsychologia 2015, 78, 171–194. [Google Scholar] [CrossRef]
- Zhang, S.; Cui, J.; Zhang, Z.; Wang, Y.; Liu, R.; Chen, X.; Feng, Y.; Zhou, J.; Zhou, Y.; Wang, G. Functional connectivity of amygdala subregions predicts vulnerability to depression following the COVID-19 pandemic. J. Affect. Disord. 2021, 297, 421–429. [Google Scholar] [CrossRef]
- Uddin, L.Q.; Nomi, J.S.; Hébert-Seropian, B.; Ghaziri, J.; Boucher, O. Structure and Function of the Human Insula. J. Clin. Neurophysiol. 2017, 34, 300–306. [Google Scholar] [CrossRef]
- Di Stefano, V.; De Angelis, M.V.; Montemitro, C.; Russo, M.; Carrarini, C.; di Giannantonio, M.; Brighina, F.; Onofrj, M.; Werring, D.J.; Simister, R. Clinical presentation of strokes confined to the insula: A systematic review of literature. Neurol. Sci. 2021, 42, 1697–1704. [Google Scholar] [CrossRef]
- Coen, M.; Kaiser, C.; Naimi, R.; Uginet, M.; Hentsch, L.; Serratrice, J.; Allali, G. Beyond silent hypoxemia: Does COVID-19 can blunt pain perception? Comment on The neuroinvasive potential of SARS CoV2 may play a role in the respiratory failure of COVID 19 patients. J. Med. Virol. 2021, 93, 1915–1916. [Google Scholar] [CrossRef] [PubMed]
- Leech, R.; Sharp, D.J. The role of the posterior cingulate cortex in cognition and disease. Brain A J. Neurol. 2014, 137, 12–32. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Lane, G.; Cooper, S.L.; Kahnt, T.; Zelano, C. Characterizing functional pathways of the human olfactory system. Elife 2019, 24, e47177. [Google Scholar] [CrossRef]
- Takehara-Nishiuchi, K. Entorhinal cortex and consolidated memory. Neurosci. Res. 2014, 84, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, D.N.; Jackson, G.D. The piriform cortex and human focal epilepsy. Front. Neurol. 2014, 5, 259. [Google Scholar] [CrossRef] [PubMed]
- Guedj, E.; Campion, J.Y.; Dudouet, P.; Kaphan, E.; Bregeon, F.; Tissot-Dupont, H.; Guis, S.; Barthelemy, F.; Habert, P.; Ceccaldi, M.; et al. 18F-FDG brain PET hypometabolism in patients with long COVID. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2823–2833. [Google Scholar] [CrossRef]
- Yousefi-Koma, A.; Haseli, S.; Bakhshayeshkaram, M.; Raad, N.; Karimi-Galougahi, M. Multimodality Imaging With PET/CT and MRI Reveals Hypometabolism in Tertiary Olfactory Cortex in Parosmia of COVID-19. Acad. Radiol. 2021, 28, 749–751. [Google Scholar] [CrossRef]
- Xydakis, M.S.; Albers, M.W.; Holbrook, E.H.; Lyon, D.M.; Shih, R.Y.; Frasnelli, J.A.; Pagenstecher, A.; Kupke, A.; Enquist, L.W.; Perlman, S. Post-viral effects of COVID-19 in the olfactory system and their implications. Lancet Neurol. 2021, 20, 753–761. [Google Scholar] [CrossRef]
- Makaronidis, J.; Firman, C.; Magee, C.G.; Mok, J.; Balogun, N.; Lechner, M.; Carnemolla, A.; Batterham, R.L. Distorted chemosensory perception and female sex associate with persistent smell and/or taste loss in people with SARS-CoV-2 antibodies: A community based cohort study investigating clinical course and resolution of acute smell and/or taste loss in people. BMC Infect. Dis. 2021, 21, 221. [Google Scholar] [CrossRef]
- Araújo, L.; Arata, V.; Figueiredo, R.G. Olfactory Disorders in Post-Acute COVID-19 Syndrome. Sinusitis 2021, 5, 116–122. [Google Scholar] [CrossRef]
- Mehraeen, E.; Behnezhad, F.; Salehi, M.A.; Noori, T.; Harandi, H.; SeyedAlinaghi, S. Olfactory and gustatory dysfunctions due to the coronavirus disease (COVID-19): A review of current evidence. Eur. Arch. Otorhinolaryngol. 2021, 278, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Borsetto, D.; Hopkins, C.; Philips, V.; Obholzer, R.; Tirelli, G.; Polesel, J.; Boscolo-Rizzo, P. Self-reported alteration of sense of smell or taste in patients with COVID-19: A systematic review and meta-analysis on 3563 patients. Rhinology 2020, 58, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Larco, R.M.; Altez-Fernandez, C. Anosmia and dysgeusia in COVID-19: A systematic review. Wellcome Open Res. 2020, 13, 594. [Google Scholar] [CrossRef] [PubMed]
- Costa, K.V.T.D.; Carnaúba, A.T.L.; Rocha, K.W.; Andrade, K.C.L.; Ferreira, S.M.S.; Menezes, P.L. Olfactory and taste disorders in COVID-19: A systematic review. Braz. J. Otorhinolaryngol. 2020, 86, 781–792. [Google Scholar] [CrossRef]
- Hur, K.; Choi, J.S.; Zheng, M.; Shen, J.; Wrobel, B. Association of alterations in smell and taste with depression in older adults. Laryngoscope Investig. Otolaryngol. 2018, 3, 94–99. [Google Scholar] [CrossRef]
- Kohli, P.; Soler, Z.M.; Nguyen, S.A.; Muus, J.S.; Schlosser, R.J. The Association Between Olfaction and Depression: A Systematic Review. Chem. Senses 2016, 41, 479–486. [Google Scholar] [CrossRef]
- Croy, I.; Nordin, S.; Hummel, T. Olfactory disorders and quality of life–an updated review. Chem. Senses 2014, 39, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Valsamidis, K.; Printza, A.; Constantinidis, J.; Triaridis, S. The Impact of Olfactory Dysfunction on the Psychological Status and Quality of Life of Patients with Nasal Obstruction and Septal Deviation. Int. Arch. Otorhinolaryngol. 2020, 24, e237–e246. [Google Scholar] [CrossRef]
- Schablitzky, S.; Pause, B.M. Sadness might isolate you in a non-smelling world: Olfactory perception and depression. Front. Psychol. 2014, 5, 45. [Google Scholar] [CrossRef]
- Nordin, S.; Brämerson, A. Complaints of olfactory disorders: Epidemiology, assessment and clinical implications. Curr. Opin. Allergy Clin. Immunol. 2008, 8, 10–15. [Google Scholar] [CrossRef]
- Ahmedy, F.; Mazlan, M.; Danaee, M.; Abu Bakar, M.Z. Post-traumatic brain injury olfactory dysfunction: Factors influencing quality of life. Eur. Arch. Oto-Rhino-Laryngol. 2020, 277, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Pirker-Kees, A.; Platho-Elwischger, K.; Hafner, S.; Redlich, K.; Baumgartner, C. Hyposmia Is Associated with Reduced Cognitive Function in COVID-19: First Preliminary Results. Dement. Geriatr. Cogn. Disord. 2021, 50, 68–73. [Google Scholar] [CrossRef]
- Dintica, C.S.; Marseglia, A.; Rizzuto, D.; Wang, R.; Seubert, J.; Arfanakis, K.; Bennett, D.A.; Xu, W. Impaired olfaction is associated with cognitive decline and neurodegeneration in the brain. Neurology 2019, 92, e700–e709. [Google Scholar] [CrossRef] [PubMed]
- Graus, F.; Titulaer, M.J.; Balu, R.; Benseler, S.; Bien, C.G.; Cellucci, T.; Cortese, I.; Dale, R.C.; Gelfand, J.M.; Geschwind, M.; et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016, 15, 391–404. [Google Scholar] [CrossRef]
- Solomatova, E.S.; Shnayder, N.A.; Molgachev, A.A.; Dmitrenko, D.V.; Strotskaya, I.G. Magnetic resonance spectroscopy of the brain in the diagnosis of temporal lobe epilepsy. Neurol. Neuropsychiatry Psychosom. 2018, 10, 51–55. [Google Scholar] [CrossRef]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. HLH Across Speciality Collaboration UK. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Perrin, P.; Collongues, N.; Baloglu, S.; Bedo, D.; Bassand, X.; Lavaux, T.; Gautier-Vargas, G.; Keller, N.; Kremer, S.; Fafi-Kremer, S.; et al. Cytokine release syndrome-associated encephalopathy in patients with COVID-19. Eur. J. Neurol. 2021, 28, 248–258. [Google Scholar] [CrossRef]
- Popova, T.E.; Shnayder, N.A.; Petrova, M.M.; Nikolaeva, T.Y.; Kantimirova, E.A.; Isaeva, N.V.; Shnayder, V.A.; Panina, Y.S.; Dyuzhakova, A.V.; Dyuzhakov, S.K. Herpesvirus-associated central and peripheral nervous system involvement: Two clinical cases. Neurol. Neuropsychiatry Psychosom. 2015, 7, 28–34. [Google Scholar] [CrossRef]
- Shnayder, N.A.; Panina, Y.S.; Popova, T.E. A clinical case of pseudotumorous chronic parainfectious limbic encephalitis. Neurol. Neuropsychiatry Psychosom. 2014, 6, 49–54. [Google Scholar] [CrossRef]
- Shnayder, N.A.; Kamzalakova, N.I.; Krijanovskaya, S.V.; Panina, Y.S. Perceived ways of diagnostic help optimisation to patients with symptomatic epilepsy on chronic herpesvirus encephalitis. Epilepsy Paroxysmal Cond. 2015, 7, 6–17. [Google Scholar] [CrossRef]
- Lorigados Pedre, L.; Morales Chacón, L.M.; Pavón Fuentes, N.; Robinson Agramonte, M.L.A.; Serrano Sánchez, T.; Cruz-Xenes, R.M.; Díaz Hung, M.L.; Estupiñán Díaz, B.; Báez Martín, M.M.; Orozco-Suárez, S. Follow-Up of Peripheral IL-1β and IL-6 and Relation with Apoptotic Death in Drug-Resistant Temporal Lobe Epilepsy Patients Submitted to Surgery. Behav. Sci. 2018, 8, 21. [Google Scholar] [CrossRef]
- Na, K.S.; Jung, H.Y.; Kim, Y.K. The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 48, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Hansen, N. Long-term memory dysfunction in limbic encephalitis. Front. Neurol. 2019, 10, 330. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.; Tam, W.W.; Zhang, M.W.; Ho, C.S.; Husain, S.F.; McIntyre, R.S.; Ho, R.C. IL-1β, IL-6, TNF- α and CRP in Elderly Patients with Depression or Alzheimer’s disease: Systematic Review and Meta-Analysis. Sci. Rep. 2018, 8, 12050. [Google Scholar] [CrossRef] [PubMed]
- Panina, Y.S.; Dmitrenko, D.V.; Shnayder, N.A.; Egorova, E.V.; Usoltseva, A.A. Association of carriers of single nucleotide polymorphisms rs1143634 and rs16944 in gene Il-1B and rs6265 in gene BDNF with temporal lobe epilepsy. Neurol. Neuropsychiatry Psychosom. 2019, 11, 46–51. [Google Scholar] [CrossRef]
- Sanchez, C.V.; Theel, E.; Binnicker, M.; Toledano, M.; McKeon, A. Autoimmune encephalitis after SARS-CoV-2 infection: Case frequency, findings, and outcomes. Neurology 2021, 97, e2262–e2268. [Google Scholar] [CrossRef]
| Metabolite | Commentary |
|---|---|
| N-acetylaspartate (NAA) Chemical Shift = 2.02 | It is a derivative of amino acids synthesized in neurons and further transported along axons. A reliable marker of the viability of neurons, axons and dendrites. |
| Choline (Cho) Chemical Shift = 3.22 | It is part of cell membranes and cholinergic synaptic endings of neurons and takes part in lipid metabolism. |
| Creatine (Cr) Chemical shift = 3.02; 3.94 | The main marker of energy processes in astrocytes and neurons. It is formed during the conversion of the high-energy compound Adenosine triphosphate to Adenosine diphosphate. In the brain tissue, the signal intensity in many cases remains constant, even with pathological changes. |
| Lactate (Lac) Chemical Shift = 1.33 | It is not detected in normal brain tissue. It is contained in the cerebrospinal fluid (0.9 mmol/L). Indicator of anaerobic glycolysis. |
| Glutamate-Glutamine Complex (Glx) Chemical shift = 2.1–2.5 | Astrocyte marker and neurotoxin, respectively. Glutamine (2-aminopentanamide-5-ovic acid) is the 1 of the 20 standard amino acids that make up the protein. Excitatory neurotransmitter. |
| Myoinositol (MI) Chemical Shift = 3.56 | Myelin degradation product. The concentration rises in multiple sclerosis and decreases in tumors. It rises in the center of the epileptogenic focus and decreases in the tissues adjacent to it. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shnayder, N.A.; Sirbiladze, T.K.; Demko, I.V.; Petrova, M.M.; Nasyrova, R.F. Limbic Encephalitis Associated with COVID-19. Encyclopedia 2022, 2, 26-35. https://doi.org/10.3390/encyclopedia2010003
Shnayder NA, Sirbiladze TK, Demko IV, Petrova MM, Nasyrova RF. Limbic Encephalitis Associated with COVID-19. Encyclopedia. 2022; 2(1):26-35. https://doi.org/10.3390/encyclopedia2010003
Chicago/Turabian StyleShnayder, Natalia A., Timur K. Sirbiladze, Irina V. Demko, Marina M. Petrova, and Regina F. Nasyrova. 2022. "Limbic Encephalitis Associated with COVID-19" Encyclopedia 2, no. 1: 26-35. https://doi.org/10.3390/encyclopedia2010003
APA StyleShnayder, N. A., Sirbiladze, T. K., Demko, I. V., Petrova, M. M., & Nasyrova, R. F. (2022). Limbic Encephalitis Associated with COVID-19. Encyclopedia, 2(1), 26-35. https://doi.org/10.3390/encyclopedia2010003







