Mismatch Repair Deficiency and Microsatellite Instability
Definition
:1. Introduction
2. History
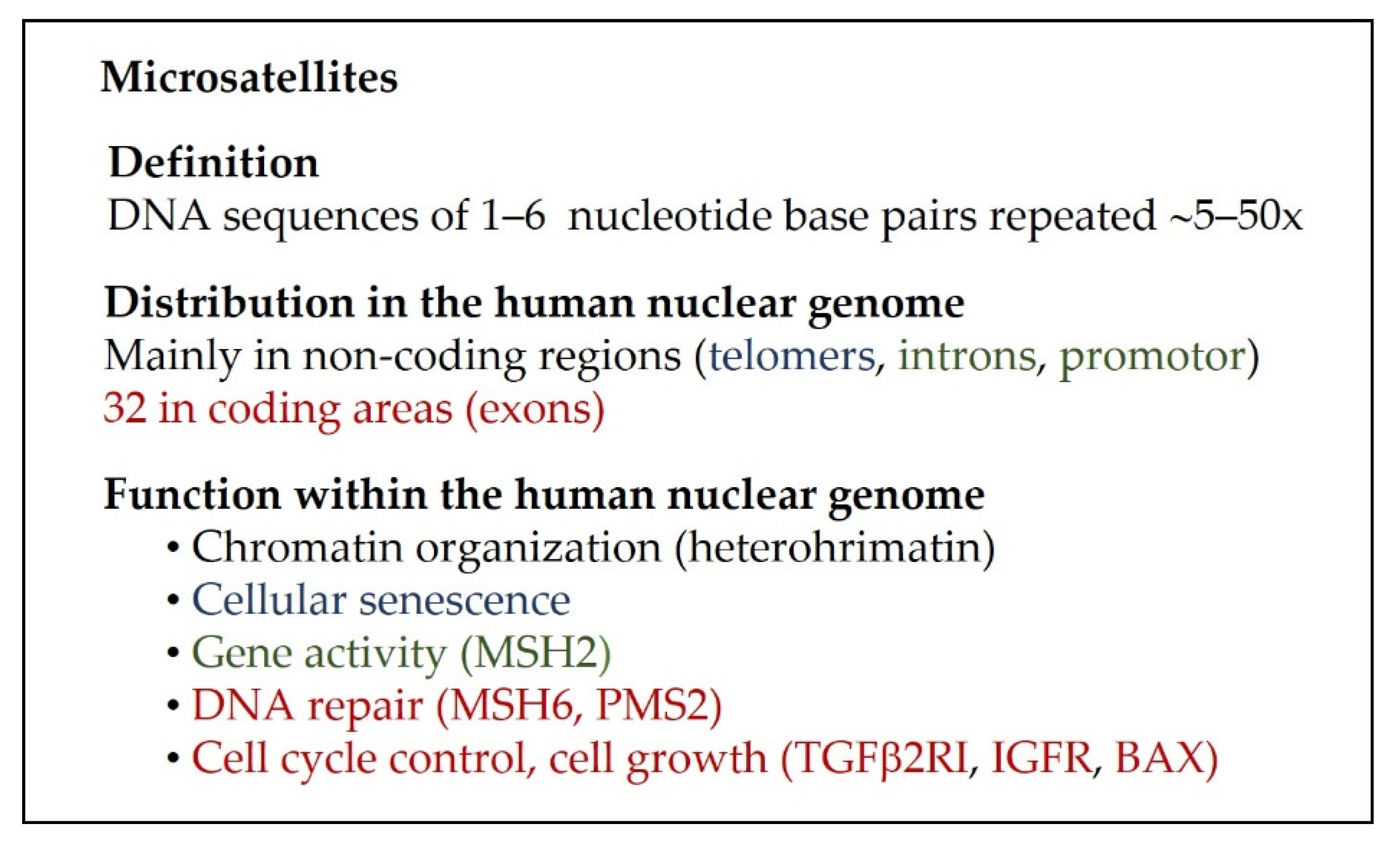
3. The Organization of the Human Nuclear Genome and Repetitive DNA Sequences
4. The Human Mismatch Repair System
5. Mismatch Repair Deficiency
6. The Diagnosis of Mismatch Repair Deficiency
6.1. Immunohistochemical Features of Mismatch Repair Proficiency and Deficiency
6.2. Molecular Mechanisms of Mismatch Repair Deficiency
7. Microsatellite Instability
8. Diagnosis of Microsatellite Instability
9. Mismatch Repair Deficiency and Microsatellite Instability as Predictive Biomarkers
10. Concordance and Disconcordance of Test Results
11. Testing Algorithm Applicable across Cancer Types
12. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Warthin, A.S. Heredity with reference to carcinoma as shown by the study of the cases examined in the Pathological Laboratory of the University of Michigan, 1895–1912. Arch. Intern. Med. 1913, 12, 546–555. [Google Scholar] [CrossRef]
- Warthin, A.S. The further study of a cancer family. J. Cancer Res. 1925, 9, 279–286. [Google Scholar] [CrossRef]
- Lynch, H.T.; Krush, A.J.; Larsen, A.L. Heredity and multiple primary malignant neoplasms: Six cancer families. Am. J. Med. Sci. 1967, 254, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Lynch, H.T.; Krush, A.J. Cancer family “G” revisited: 1895–1970. Cancer 1971, 27, 1505–1511. [Google Scholar] [CrossRef]
- Boland, C.R.; Troncale, F.J. Familial colonic cancer without antecedent polyposis. Ann. Intern. Med. 1984, 100, 700–701. [Google Scholar] [CrossRef] [PubMed]
- Lynch, H.T.; Lynch, P.M.; Pester, J.; Fusaro, R.M. The cancer family syndrome. Rare cutaneous phenotypic linkage of Torre’s syndrome. Arch. Intern. Med. 1981, 141, 607–611. [Google Scholar] [CrossRef]
- Muir, E.G.; Bell, A.J.; Barlow, K.A. Multiple primary carcinomata of the colon, duodenum, and larynx associated with kerato-acanthomata of the face. Br. J. Surg. 1967, 54, 191–195. [Google Scholar] [CrossRef]
- Torre, D. Multiple sebaceous tumors. Arch. Dermatol. 1968, 98, 549–551. [Google Scholar] [CrossRef]
- Lynch, H.T.; Kimberling, W.; Albano, W.A.; Lynch, J.F.; Biscone, K.; Schuelke, G.S.; Sandberg, A.A.; Lipkin, M.; Deschner, E.E.; Mikol, Y.B.; et al. Hereditary nonpolyposis colorectal cancer (Lynch syndromes I and II). I. Clinical description of resource. Cancer 1985, 56, 934–938. [Google Scholar] [CrossRef]
- Ionov, Y.; Peinado, M.A.; Malkhosyan, S.; Shibata, D.; Perucho, M. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature 1993, 363, 558–561. [Google Scholar] [CrossRef]
- Thibodeau, S.N.; Bren, G.; Schaid, D. Microsatellite instability in cancer of the proximal colon. Science 1993, 260, 816–819. [Google Scholar] [CrossRef] [PubMed]
- Aaltonen, L.A.; Peltomäki, P.; Leach, F.S.; Sistonen, P.; Pylkkänen, L.; Mecklin, J.P.; Järvinen, H.; Powell, S.M.; Jen, J.; Hamilton, S.R.; et al. Clues to the pathogenesis of familial colorectal cancer. Science 1993, 260, 812–816. [Google Scholar] [CrossRef]
- Peltomäki, P.; Aaltonen, L.A.; Sistonen, P.; Pylkkänen, L.; Mecklin, J.P.; Järvinen, H.; Green, J.S.; Jass, J.R.; Weber, J.L.; Leach, F.S.; et al. Genetic mapping of a locus predisposing to human colorectal cancer. Science 1993, 260, 810–812. [Google Scholar] [CrossRef]
- Modrich, P. DNA mismatch correction. Ann. Rev. Biochem. 1987, 56, 435–466. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.H.; Modrich, P. Human strand-specific mismatch repair occurs by a bidirectional mechanism similar to that of the bacterial reaction. J. Biol. Chem. 1993, 268, 11838–11844. [Google Scholar] [CrossRef]
- Fishel, R.; Lescoe, M.K.; Rao, M.R.; Copeland, N.-G.; Jenkins, N.A.; Garbeer, J.; Kane, M.; Kolodner, R. The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer. Cell 1993, 75, 1027–1038. [Google Scholar] [CrossRef]
- Leach, F.S.; Nicolaides, N.C.; Papadopoulos, N.; Liu, B.; Jen, J.; Parsons, R.; Peltomäki, P.; Sistonen, P.; Aaltonen, L.A.; Nyström-Lahti, M.; et al. Mutations of a mutS homolog in hereditary nonpolyposis colorectal cancer. Cell 1993, 75, 1215–1225. [Google Scholar] [CrossRef]
- Douglas, J.A.; Gruber, S.B.; Meister, K.A.; Bonner, J.; Watson, P.; Krush, A.J.; Lynch, H.T. History and molecular genetics of Lynch syndrome in family G: A century later. J. Am. Med. Assoc. 2005, 294, 2195–2202. [Google Scholar] [CrossRef]
- Bronner, C.; Baker, S.; Morrison, P.; Warren, G.; Smith, L.G.; Lescoe, M.K.; Kane, M.; Earabino, C.; Lipford, J.; Lindblom, A.; et al. Mutation in the DNA mismatch repair gene homologue hMLH 1 is associated with hereditary non-polyposis colon cancer. Nature 1994, 368, 258–261. [Google Scholar] [CrossRef]
- Papadopoulos, N.; Nicolaides, N.C.; Wei, Y.F.; Ruben, S.M.; Carter, K.C.; Rosen, C.A.; Haseltine, W.A.; Fleischmann, R.D.; Fraser, C.M.; Adams, M.D.; et al. Mutation of a mutL homolog in hereditary colon cancer. Science 1994, 263, 1625–1629. [Google Scholar] [CrossRef]
- Nicolaides, N.C.; Papadopoulos, N.; Liu, B.; Wei, Y.F.; Carter, K.C.; Ruben, S.M.; Rosen, C.A.; Haseltine, W.A.; Fleischmann, R.D.; Fraser, C.; et al. Mutations of two PMS homologues in hereditary nonpolyposis colon cancer. Nature 1994, 371, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Miyaki, M.; Konishi, M.; Tanaka, K.; Kikuchi-Yanoshita, R.; Muraoka, M.; Yasuno, M.; Igari, T.; Koike, M.; Chiba, M.; Mori, T. Germline mutation of MSH6 as the cause of hereditary nonpolyposis colorectal cancer. Nat. Genet. 1997, 17, 271–272. [Google Scholar] [CrossRef] [PubMed]
- Ligtenberg, M.J.; Kuiper, R.P.; Chan, T.L.; Goossens, M.; Hebeda, K.M.; Voorendt, M.; Lee, T.Y.; Bodmer, D.; Hoenselaar, E.; Hendriks-Cornelissen, S.J.; et al. Heritable somatic methylation and inactivation of MSH2 in families with Lynch syndrome due to deletion of the 3’ exons of TACSTD1. Nat. Genet. 2009, 41, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Porkka, N.; Lahtinen, L.; Ahtiainen, M.; Böhm, J.P.; Kuopio, T.; Eldfors, S.; Mecklin, J.-P.; Seppälä, T.; Peltomäki, P. Epidemiological, clinical and molecular characterization of Lynch-like syndrome: A population-based study. Int. J. Cancer 2019, 145, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Bateman, A.C. DNA mismatch repair proteins: Scientific update and practical guide. J. Clin. Pathol. 2021, 74, 264–268. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef]
- Le, D.T.; Kim, T.W.; Van Cutsem, E.; Geva, R.; Jäger, D.; Hara, H.; Burge, M.; O’Neil, B.; Kavan, P.; Yoshino, T.; et al. Phase II open-label study of pembrolizumab in treatment-refractory, microsatellite instability-high/ mismatch repair-deficient metastatic colorectal cancer: Keynote-164. J. Clin. Oncol. 2020, 38, 11–19. [Google Scholar] [CrossRef]
- Pollard, T.D.; Earnshaw, W.C.; Lippincott-Schwartz, J.; Johnson, G. Chromosome organisation. Chapter 7. In Cell Biology, 3rd ed.; Pollard, T.D., Earnshaw, W.C., Lippincott-Schwartz, J., Johnson, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 107–122. [Google Scholar]
- Nishibuchi, G.; Déjardin, J. The molecular basis of the organization of repetitive DNA-containing constitutive heterochromatin in mammals. Chromosome Res. 2017, 25, 77–87. [Google Scholar] [CrossRef]
- Thakur, J.; Packiaraj, J.; Henikoff, S. Sequence, chromatin, and evolution of satellite DNA. Int. J. Mol. Sci. 2021, 22, 4309. [Google Scholar] [CrossRef]
- Dumbovic, G.; Forcales, S.-V.; Perucho, M. Emerging roles of microsatellite repeats in genome organization and disease development. Epigenetics 2017, 12, 515–526. [Google Scholar] [CrossRef]
- Cooney, C.A.; Matthews, H.R. The isolation of satellite DNA by density gradient centrifugation. Methods Mol. Biol. 1985, 2, 21–29. [Google Scholar] [CrossRef]
- Schlötterer, C. Genome evolution: Are microsatellites really simple sequences? Curr. Biol. 1998, 8, R132–R134. [Google Scholar] [CrossRef]
- Shia, J. The diversity of tumours with microsatellite instability: Molecular mechanisms and impact upon microsatellite instability testing and mismatch repair protein immunohistochemistry. Histopathology 2021, 78, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Boland, C.R.; Koi, M.; Chang, D.K.; Carethers, J.M. The biochemical basis of microsatellite instability and abnormal immunohistochemistry and clinical behavior in Lynch syndrome: From bench to bedside. Fam. Cancer 2008, 7, 41–52. [Google Scholar] [CrossRef]
- Pećina-Šlaus, N.; Kafka, A.; Salamon, I.; Bukovac, A. Mismatch repair pathway, genome stability and cancer. Front. Mol. Biosci. 2020, 7, 122. [Google Scholar] [CrossRef] [PubMed]
- Dietmaier, W.; Wallinger, S.; Bocker, T.; Kullmann, F.; Fishel, R.; Rüschoff, J. Diagnostic microsatellite instability: Definition and correlation with mismatch repair protein expression. Cancer Res. 1997, 57, 4749–4756. [Google Scholar]
- Guanti, G.; Resta, N.; Simone, C.; Cariola, F.; Demma, I.; Fiorente, P.; Gentile, M. Involvement of PTEN mutations in the genetic pathways of colorectal cancerogenesis. Hum. Mol. Genet. 2000, 9, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Kim, K.S.; Lee, H.J.; Cho, Y.-M.; Park, K.-S.; Shin, D.-J.; Jang, Y.; Jung, J.; Kim, H.-L.; Oh, B.; et al. Allelic frequencies and heterozygosities of microsatellite markers covering the whole genome in the Korean. J. Hum. Genet. 2008, 53, 254–266. [Google Scholar] [CrossRef]
- Jun, S.H.; Kim, T.G.; Ban, C. DNA mismatch repair system. Classical and fresh roles. FEBS J. 2006, 273, 1609–1619. [Google Scholar] [CrossRef]
- Jiricny, J. The multifaceted mismatch-repair system. Nat. Rev. Mol. Cell Biol. 2006, 7, 335–346. [Google Scholar] [CrossRef]
- Modrich, P. Mechanisms in eukaryotic mismatch repair. J. Biol. Chem. 2006, 281, 30305–30309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gradia, S.; Subramanian, D.; Wilson, T.; Acharya, S.; Makhov, A.; Griffith, J.; Fishel, R. hMSH2-hMSH6 forms a hydrolysis-independent sliding clamp on mismatched DNA. Mol. Cell 1999, 3, 255–261. [Google Scholar] [CrossRef]
- Imai, K.; Yamamoto, H. Carcinogenesis, and microsatellite instability: The interrelationship between genetics and epigenetics. Carcinogenesis 2008, 29, 673–680. [Google Scholar] [CrossRef]
- Win, A.K.; Jenkins, M.A.; Dowty, J.G.; Antoniou, A.C.; Lee, A.; Giles, G.G.; Buchanan, D.D.; Clendenning, M.; Rosty, C.; Ahnen, D.J.; et al. Prevalence and penetrance of major genes and polygenes for colorectal cancer. Cancer Epidemiol. Biomarkers Prev. 2017, 26, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Peltomäki, P. Update on Lynch syndrome genomics. Fam. Cancer 2016, 15, 385–393. [Google Scholar] [CrossRef]
- Dominguez-Valentin, M.; Sampson, J.R.; Seppälä, T.T.; Ten Broeke, S.W.; Plazzer, J.P.; Nakken, S.; Engel, C.; Aretz, S.; Jenkins, M.A.; Sunde, L.; et al. Cancer risks by gene, age, and gender in 6350 carriers of pathogenic mismatch repair variants: Findings from the prospective Lynch syndrome database. Genet. Med. 2020, 22, 15–25. [Google Scholar] [CrossRef]
- Kurnit, K.C.; Westin, S.N.; Coleman, R.L. Microsatellite instability in endometrial cancer: New purpose for an old test. Cancer 2019, 125, 2154–2163. [Google Scholar] [CrossRef] [PubMed]
- Bonneville, R.; Krook, M.A.; Kautto, E.A.; Miya, J.; Wing, M.R.; Chen, H.-Z.; Reeser, J.W.; Yu, L.; Roychowdhury, S. Landscape of microsatellite instability across 39 cancer types. JCO Precis. Oncol. 2017, 1, 1–15. [Google Scholar] [CrossRef]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef]
- Stelloo, E.; Jansen, A.M.L.; Osse, E.M.; Nout, R.A.; Creutzberg, C.L.; Ruano, D.; Church, D.N.; Morreau, H.; Smit, V.T.H.B.M.; van Wezel, T.; et al. Practical guidance for mismatch repair-deficiency testing in endometrial cancer. Ann. Oncol. 2017, 28, 96–102. [Google Scholar] [CrossRef]
- Umar, A.; Boland, C.R.; Terdiman, J.P.; Syngal, S.; de la Chapelle, A.; Rüschoff, J.; Fishel, R.; Lindor, N.M.; Burgart, L.J.; Hamelin, R.; et al. Revised bethesda guidelines for hereditary nonpolyposis colorectal cancer (Lynch Syndrome) and microsatellite instability. J. Natl. Cancer Inst. 2004, 96, 261–268. [Google Scholar] [CrossRef]
- Russell, H.; Kedzierska, K.; Buchanan, D.D.; Thomas, R.; Tham, E.; Mints, M.; Keränen, A.; Giles, G.G.; Southey, M.C.; Milne, R.L.; et al. The MLH1 polymorphism rs1800734 and risk of endometrial cancer with microsatellite instability. Clin. Epigenetics 2020, 12, 102. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Ou, J.; Hutchinson, L.; Green, M.R. The BRAF oncoprotein functions through the transcriptional repressor MAFG to mediate the CpG Island Methylator phenotype. Mol. Cell 2014, 55, 904–915. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.C.; Li, A.F.; Lin, P.C.; Lin, C.C.; Lin, H.H.; Huang, S.C.; Lin, C.-H.; Liang, W.-Y.; Chen, W.-S.; Jiang, J.-K.; et al. Clinicopathological and molecular profiles of sporadic microsatellite unstable colorectal cancer with or without the CpG island methylator phenotype (CIMP). Cancers 2020, 12, 3487. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Peng, E.; Gum, J.; Terdiman, J.; Sleisenger, M.; Kim, Y.S. Methylation of hMLH1 promoter correlates with the gene silencing with a region-specific manner in colorectal cancer. Br. J. Cancer. 2002, 86, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Strazzullo, M.; Cossu, A.; Baldinu, P.; Colombino, M.; Satta, M.P.; Tanda, F.; De Bonis, M.L.; Cerase, A.; D’Urso, M.; D’Esposito, M.; et al. High-resolution methylation analysis of the hMLH1 promoter in sporadic endometrial and colorectal carcinomas. Cancer 2003, 98, 1540–1546. [Google Scholar] [CrossRef]
- Helmle, K.E.; Otto, C.J.; Constantinescu, G.; Honore, L.H.; Andrew, S.E. Variable MLH1 promoter methylation patterns in endometrial carcinomas of endometrioid subtype lacking DNA mismatch repair. Int. J. Gynecol. Cancer 2005, 15, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Varley, K.E.; Mutch, D.G.; Edmonston, T.B.; Goodfellow, P.J.; Mitra, R.D. Intra-tumor heterogeneity of MLH1 promoter methylation revealed by deep single molecule bisulfite sequencing. Nucleic Acids Res. 2009, 37, 4603–4612. [Google Scholar] [CrossRef]
- Watkins, J.C.; Nucci, M.R.; Ritterhouse, L.L.; Howitt, B.E.; Sholl, L.M. Unusual mismatch repair immunohistochemical patterns in endometrial carcinoma. Am. J. Surg. Pathol. 2016, 40, 909–916. [Google Scholar] [CrossRef]
- Kanaya, T.; Kyo, S.; Maida, Y.; Yatabe, N.; Tanaka, M.; Nakamura, M.; Inoue, M. Frequent hypermethylation of MLH1 promoter in normal endometrium of patients with endometrial cancers. Oncogene 2003, 22, 2352–2360. [Google Scholar] [CrossRef]
- Fadhil, W.; Ilyas, M. Immunostaining for mismatch repair (MMR) protein expression in colorectal cancer is better and easier to interpret when performed on diagnostic biopsies. Histopathology 2012, 60, 653–674. [Google Scholar] [CrossRef]
- Rüschoff, J.; Baretton, G.; Bläker, H.; Dietmaier, W.; Dietel, M.; Hartmann, A.; Horn, L.-C.; Jöhrens, K.; Kirchner, T.; Knüchel, R.; et al. MSI-Testung: Was ist neu? Was ist zu beachten? Pathologe 2021, 42 (Suppl. S1), 110–118. (In German) [Google Scholar] [CrossRef] [PubMed]
- Niu, B.T.; Hammond, R.F.L.; Leen, S.L.S.; Faruqi, A.Z.; Trevisan, G.; Gilks, C.B.; Singh, N. Artefactual punctate MLH1 staining can lead to erroneous reporting of isolated PMS2 loss. Histopathology 2018, 73, 703–705. [Google Scholar] [CrossRef] [PubMed]
- Loughrey, M.B.; Dunne, P.D.; Coleman, H.G.; McQuaid, S.; James, J.A. Punctate MLH1 mismatch repair immunostaining in colorectal cancer. Histopathology 2019, 74, 795–797. [Google Scholar] [CrossRef]
- Zhang, Q.; Young, G.Q.; Yang, Z. Pure discrete punctate nuclear staining pattern for MLH1 protein does not represent intact nuclear expression. Int. J. Surg. Pathol. 2020, 28, 146–152. [Google Scholar] [CrossRef]
- Edelbrock, M.A.; Kaliyaperumal, S.; Williams, K.J. Structural, molecular, and cellular functions of MSH2 and MSH6 during DNA mismatch repair, damage signaling and other noncanonical activities. Mutat. Res. 2013, 743–744, 53–66. [Google Scholar] [CrossRef]
- Kunkel, T. Slippery DNA, and diseases. Nature 1993, 365, 207–208. [Google Scholar] [CrossRef]
- Siemanowski, J.; Schömig-Markiefka, B.; Buhl, T.; Haak, A.; Siebolts, U.; Dietmaier, W.; Arens, N.; Pauly, N.; Ataseven, B.; Büttner, R.; et al. Difficulties of microsatellite instability testing in endometrial cancer-limitations and advantages of four different PCR-based approaches. Cancers 2021, 13, 1268. [Google Scholar] [CrossRef] [PubMed]
- Boland, C.R.; Thibodeau, S.N.; Hamilton, S.R.; Sidransky, D.; Eshleman, J.R.; Burt, R.W.; Meltzer, S.J.; Rodriguez-Bigas, M.A.; Fodde, R.; Ranzani, G.N.; et al. A National Cancer Institute Workshop on microsatellite instability for cancer detection and familial predisposition: Development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998, 58, 5248–5257. [Google Scholar] [PubMed]
- Carethers, J.M.; Koi, M.; Tseng-Rogenski, S.S. EMAST is a form of microsatellite instability that is initiated by inflammation and modulates colorectal cancer progression. Genes 2015, 6, 185–205. [Google Scholar] [CrossRef] [PubMed]
- Tseng-Rogenski, S.S.; Hamaya, Y.; Choi, D.Y.; Carethers, J.M. Interleukin 6 alters localization of hMSH3, leading to DNA mismatch repair defects in colorectal cancer cells. Gastroenterology 2015, 148, 579–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suraweera, N.; Duval, A.; Reperant, M.; Vaury, C.; Furlan, D.; Leroy, K.; Seruca, R.; Iacopetta, B.; Hamelin, R. Evaluation of tumor microsatellite instability using five quasimonomorphic mononucleotide repeats and pentaplex PCR. Gastroenterology 2002, 123, 1804–1811. [Google Scholar] [CrossRef] [PubMed]
- Velasco, A.; Tokat, F.; Bonde, J.; Trim, N.; Bauer, E.; Meeney, A.; de Leng, W.; Chong, G.; Dalstein, V.; Kis, L.L.; et al. Multi-center real-world comparison of the fully automated Idylla™ microsatellite instability assay with routine molecular methods and immunohistochemistry on formalin-fixed paraffin-embedded tissue of colorectal cancer. Virchows Arch. 2021, 478, 851–863. [Google Scholar] [CrossRef]
- Mas-Ponte, D.; McCullough, M.; Supek, F. Spectrum of DNA mismatch repair failures viewed through the lens of cancer genomics and implications for therapy. Clin. Sci. 2022, 136, 383–404. [Google Scholar] [CrossRef]
- Seo, M.-K.; Kang, H.; Kim, S. Tumor microenvironment-aware, single-transcriptome prediction of microsatellite instability in colorectal cancer using meta-analysis. Sci. Rep. 2022, 12, 6283. [Google Scholar] [CrossRef] [PubMed]
- Kather, J.N.; Pearson, A.T.; Halama, N.; Jäger, D.; Krause, J.; Loosen, S.H.; Marx, A.; Boor, P.; Tacke, F.; Neumann, U.P.; et al. Deep learning can predict microsatellite instability directly from histology in gastrointestinal cancer. Nat. Med. 2019, 25, 1054–1056. [Google Scholar] [CrossRef]
- Flinner, N.; Gretser, S.; Quaas, A.; Bankov, K.; Stoll, A.; Heckmann, L.E.; Mayer, R.S.; Doering, C.; Demes, M.C.; Buettner, R.; et al. Deep learning based on hematoxylin-eosin staining outperforms immunohistochemistry in predicting molecular subtypes of gastric adenocarcinoma. J. Pathol. 2022, 257, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Ballhausen, A.; Przybilla, M.J.; Jendrusch, M.; Haupt, S.; Pfaffendorf, E.; Seidler, F.; Witt, J.; Sanchez, A.H.; Urban, K.; Draxlbauer, M.; et al. The shared frameshift mutation landscape of microsatellite-unstable cancers suggests immunoediting during tumor evolution. Nat. Commun. 2020, 11, 4740. [Google Scholar] [CrossRef]
- Yamashita, H.; Nakayama, K.; Ishikawa, M.; Nakamura, K.; Ishibashi, T.; Sanuki, K.; Ono, R.; Sasamori, H.; Minamoto, T.; Iida, K.; et al. Microsatellite instability is a biomarker for immune checkpoint inhibitors in endometrial cancer. Oncotarget 2017, 9, 5652–5664. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Irving, B.A.; Hodi, F.S. Molecular pathways: Next-generation immunotherapy—Inhibiting programmed death-ligand 1 and programmed death-1. Clin. Cancer Res. 2012, 18, 6580–6587. [Google Scholar] [CrossRef] [PubMed]
- Galon, J.; Bruni, D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Rev. Drug Discov. 2019, 18, 197–218. [Google Scholar] [CrossRef]
- Wang, Q.; Bardhan, K.; Boussiotis, V.A.; Patsoukis, N. The PD-1 interactome. Adv. Biol. 2021, 5, e2100758. [Google Scholar] [CrossRef]
- Liu, S.; Gӧnen, M.; Stadler, Z.K.; Weiser, M.R.; Hechtman, J.F.; Vakiani, E.; Wang, T.; Vyas, M.; Joneja, U.; Al-Bayati, M.; et al. Cellular localization of PD-L1 expression in mismatch-repair-deficient and proficient colorectal carcinomas. Mod. Pathol. 2019, 32, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Marcus, L.; Lemery, S.J.; Keegan, P.; Pazdur, R. FDA approval summary: Pembrolizumab for the treatment of microsatellite instability-high solid tumors. Clin. Cancer Res. 2019, 25, 3753–3758. [Google Scholar] [CrossRef]
- Ma, X.; Dong, L.; Liu, X.; Ou, K.; Yang, L. POLE/POLD1 mutation and tumor immunotherapy. J. Exp. Clin. Cancer Res. 2022, 41, 216. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhao, Q.; Wang, Y.N.; Jin, Y.; He, M.M.; Liu, Z.X.; Xu, R.H. Evaluation of POLE and POLD1 mutations as biomarkers for immunotherapy Outcomes across multiple cancer types. JAMA Oncol. 2019, 5, 1504–1506. [Google Scholar] [CrossRef]
- Goodman, A.M.; Sokol, E.S.; Frampton, G.M.; Lippman, S.M.; Kurzrock, R. Microsatellite-stable tumors with high mutational burden benefit from immunotherapy. Cancer Immunol. Res. 2019, 7, 1570–1573. [Google Scholar] [CrossRef] [PubMed]
- Samstein, R.M.; Lee, C.H.; Shoushtari, A.N.; Hellmann, M.D.; Shen, R.; Janjigian, Y.Y.; Barron, D.A.; Zehir, A.; Jordan, E.J.; Omuro, A.; et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat. Genet. 2019, 51, 202–206. [Google Scholar] [CrossRef]
- Marabelle, A.; Fakih, M.; Lopez, J.; Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.A.; Miller, W.H., Jr.; et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: Prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020, 21, 1353–1365. [Google Scholar] [CrossRef]
- Marcus, L.; Fashoyin-Aje, L.A.; Donoghue, M.; Yuan, M.; Rodriguez, L.; Gallagher, P.S.; Philip, R.; Ghosh, S.; Theoret, M.R.; Beaver, J.A.; et al. FDA approval summary: Pembrolizumab for the treatment of tumor mutational burden-high solid tumors. Clin. Cancer Res. 2021, 27, 4685–4689. [Google Scholar] [CrossRef]
- Pai, R.K.; Plesec, T.P.; Abdul-Karim, F.W.; Yang, B.; Marquard, J.; Shadrach, B.; Roma, A.R. Abrupt loss of MLH1 and PMS2 expression in endometrial carcinoma: Molecular and morphologic analysis of 6 cases. Am. J. Surg. Pathol. 2015, 39, 993–999. [Google Scholar] [CrossRef]
- Jaffrelot, M.; Farés, N.; Brunac, A.C.; Laurenty, A.P.; Danjoux, M.; Grand, D.; Icher, S.; Meilleroux, J.; Mery, E.; Buscail, E.; et al. An unusual phenotype occurs in 15% of mismatch repair-deficient tumors and is associated with non-colorectal cancers and genetic syndromes. Mod. Pathol. 2022, 35, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Evrard, C.; Tachon, G.; Randrian, V.; Karayan-Tapon, L.; Tougeron, D. Microsatellite instability: Diagnosis, heterogeneity, discordance, and clinical impact in colorectal cancer. Cancers 2019, 11, 1567. [Google Scholar] [CrossRef] [PubMed]
- Kumarasinghe, A.P.; de Boer, B.; Bateman, A.C.; Kumarasinghe, M.P. DNA mismatch repair enzyme immunohistochemistry in colorectal cancer: A comparison of biopsy and resection material. Pathology 2010, 42, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Jöhrens, K.; Dietmaier, W.; Utpatel, K.; Dietel, M.; Rüschoff, J.; Fischer, J. Quality assurance in dMMR and MSI diagnostics. Pathologe 2021, 42, 405–413. (In German) [Google Scholar] [CrossRef] [PubMed]
- Luchini, C.; Bibeau, F.; Ligtenberg, M.J.L.; Singh, N.; Nottegar, A.; Bosse, T.; Miller, R.; Riaz, N.; Douillard, J.-Y.; Andre, F.; et al. ESMO recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD-1/PD-L1 expression and tumour mutational burden: A systematic review-based approach. Ann. Oncol. 2019, 30, 1232–1243. [Google Scholar] [CrossRef]
- Ramos, M.F.K.P.K.; Pereira, M.A.; Amorim, L.C.; de Mello, E.S.; Faraj, S.F.; Ribeiro, U.; Hoff, P.M.G.; Cecconello, I.; de Castria, T.B. Gastric cancer molecular classification and adjuvant therapy: Is there a different benefit according to the subtype? J. Surg. Oncol. 2020, 121, 804–813. [Google Scholar] [CrossRef]






| A. MSS * or MSI-L * despite MMRd # |
| • MLH1 promoter hypermethylation a |
| • Subclonal loss of a MMR protein a,b |
| • Low amount of tumor cells a,c,d |
| • Low proliferation rate of tumor cells e |
| • Loss of MSH6 after neoadjuvant treatment a |
| • Non-colorectal tumor c–g |
| B. MMRp # despite MSI-H * |
| • (Non-truncating) missense mutations h |
| • In a few cases of POLE mutated tumors a |
| • Misinterpretation of IHC (e.g., punctate stain of MLH1) i–k |
| Immunostaining Patterns That Require Follow-Up by PCR or NGS Testing |
|---|
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schöniger, S.; Rüschoff, J. Mismatch Repair Deficiency and Microsatellite Instability. Encyclopedia 2022, 2, 1559-1576. https://doi.org/10.3390/encyclopedia2030106
Schöniger S, Rüschoff J. Mismatch Repair Deficiency and Microsatellite Instability. Encyclopedia. 2022; 2(3):1559-1576. https://doi.org/10.3390/encyclopedia2030106
Chicago/Turabian StyleSchöniger, Sandra, and Josef Rüschoff. 2022. "Mismatch Repair Deficiency and Microsatellite Instability" Encyclopedia 2, no. 3: 1559-1576. https://doi.org/10.3390/encyclopedia2030106
APA StyleSchöniger, S., & Rüschoff, J. (2022). Mismatch Repair Deficiency and Microsatellite Instability. Encyclopedia, 2(3), 1559-1576. https://doi.org/10.3390/encyclopedia2030106







