Lichen as Multipartner Symbiotic Relationships
Definition
:1. General Context of Lichens
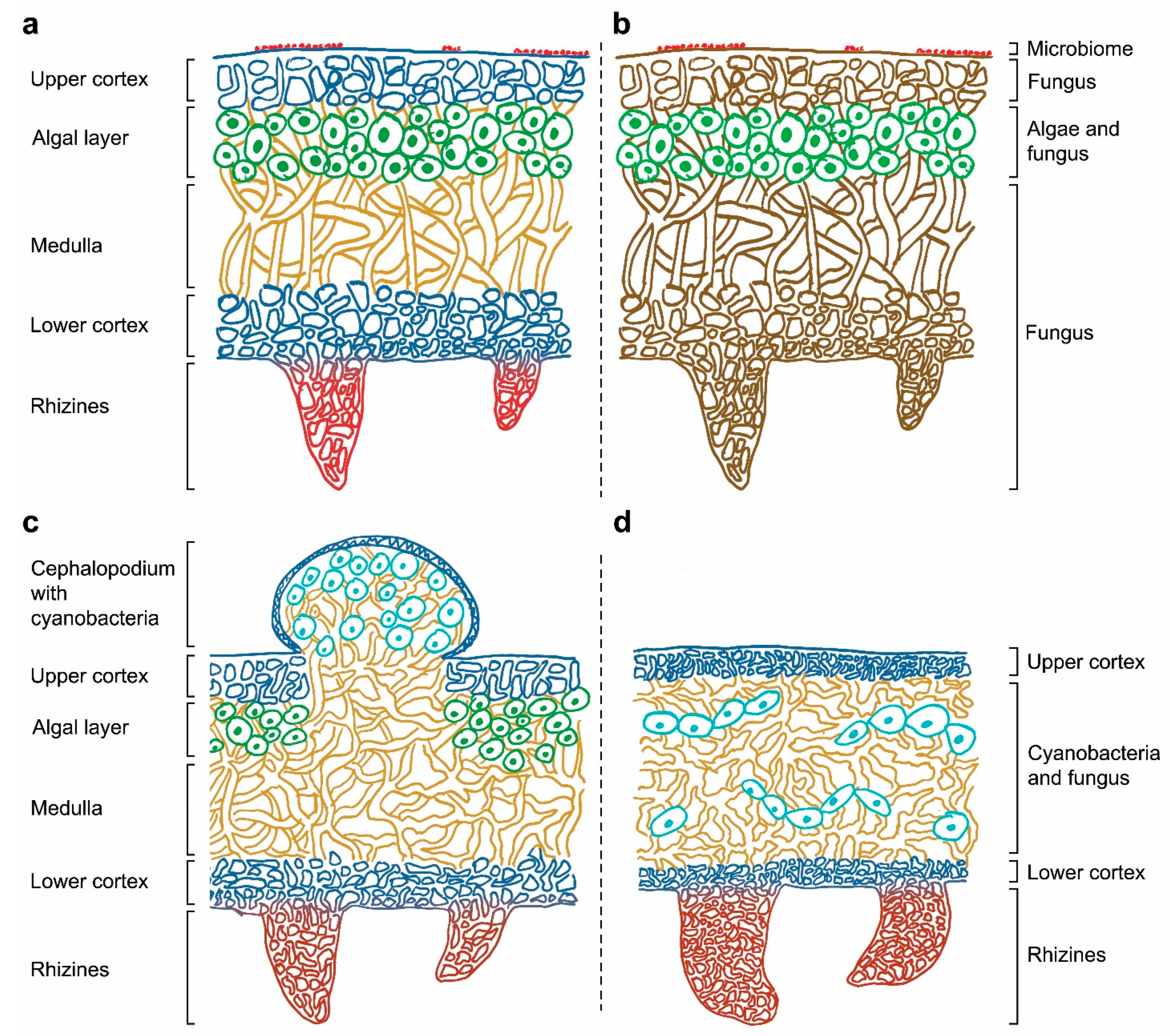
2. From a Dual Partnership to a Multi-Species Symbiotic Relationship
3. Potential Roles of Recently Discovered Partners
4. The Increasing Complexity Surrounding the Concept of Lichen
5. Mutualism or Parasitism?
6. Conclusions and Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Agerer, R. Characterization of Ectomycorrhiza. Methods Microbiol. 1991, 23, 25–73. [Google Scholar]
- Rasmussen, H.N. Recent developments in the study of orchid mycorrhiza. Plant Soil 2002, 244, 149–163. [Google Scholar] [CrossRef]
- Hawksworth, D.L. Observations on three algicolous microfungi. Notes R. Bot. Gard. Edinburgh 1987, 44, 549–560.ill. [Google Scholar]
- Hawksworth, D.L. The variety of fungal-algal symbioses, their evolutionary significance, and the nature of lichens. Bot. J. Linn. Soc. 1988, 96, 3–20. [Google Scholar] [CrossRef]
- Kohlmeyer, J.; Demoulin, D. Parasitic and Symbiotic Fungi on Marine Algae. Bot. Mar. 1981, 24, 9–18. [Google Scholar] [CrossRef]
- Sønstebø, J.H.; Rohrlack, T. Possible implications of Chytrid parasitism for population subdivision in freshwater cyanobacteria of the genus Planktothrix. Appl. Environ. Microbiol. 2011, 77, 1344–1351. [Google Scholar] [CrossRef]
- Kohlmeyer, J.; Kohlmeyer, E. Is Ascophyllum nodosum Lichenized? Bot. Mar. 1972, 15, 109–112. [Google Scholar] [CrossRef]
- Nash, T.H.I. Lichen Biology, 2nd ed.; Cambridge University Press: Cambridge, UK, 2008; ISBN 9780521871624. [Google Scholar]
- Schwendener, S. Die Algentypen der Flechtengonidien; Universitætsbuchdruckerei von C. Schultze: Basel, Switzerland, 1869. [Google Scholar]
- Oulhen, N.; Schulz, B.J.; Carrier, T.J. English translation of Heinrich Anton de Bary’s 1878 speech, ‘Die Erscheinung der Symbiose’ (‘De la symbiose’). Symbiosis 2016, 69, 131–139. [Google Scholar] [CrossRef]
- Rikkinen, J. Molecular studies on cyanobacterial diversity in lichen symbioses. MycoKeys 2013, 6, 3–32. [Google Scholar] [CrossRef]
- Margulis, L.; Fester, R. Symbiosis as a Source of Evolutionary Innovation: Speciation and Morphogenesis; Margulis, L., Fester, R., Eds.; MIT Press: Cambridge, MA, USA, 1991; ISBN 0262132699. [Google Scholar]
- Douglas, A.E.; Werren, J.H. Holes in the hologenome: Why host-microbe symbioses are not holobionts. mBio 2016, 7, e02099-15. [Google Scholar] [CrossRef]
- Rafferty, N.E.; Caradonna, P.J.; Bronstein, J.L. Phenological shifts and the fate of mutualisms. Oikos 2015, 124, 14–21. [Google Scholar] [CrossRef]
- Chomicki, G.; Renner, S.S. Partner abundance controls mutualism stability and the pace of morphological change over geologic time. Proc. Natl. Acad. Sci. USA 2017, 114, 3951–3956. [Google Scholar] [CrossRef]
- Büdel, B.; Scheidegger, C. Thallus morphology and anatomy. In Lichen Biology; Nash, T.H.I., Ed.; Cambridge University Press: Cambridge, UK, 2008; pp. 40–68. ISBN 9780521871624. [Google Scholar]
- Lücking, R.; Dal-Forno, M.; Sikaroodi, M.; Gillevet, P.M.; Bungartz, F.; Moncada, B.; Yánez-Ayabaca, A.; Chaves, J.L.; Coca, L.F.; Lawrey, J.D. A single macrolichen constitutes hundreds of unrecognized species. Proc. Natl. Acad. Sci. USA 2014, 111, 11091–11096. [Google Scholar] [CrossRef] [PubMed]
- Honegger, R. Lichen-Forming Fungi and Their Photobionts. In Plant Relationships; Deising, H.B., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 307–333. ISBN 978-3-540-87407-2. [Google Scholar]
- Henskens, F.L.; Green, T.G.A.; Wilkins, A. Cyanolichens can have both cyanobacteria and green algae in a common layer as major contributors to photosynthesis. Ann. Bot. 2012, 110, 555–563. [Google Scholar] [CrossRef]
- Piercey-Normore, M.D.; DePriest, P.T. Algal switching among lichen symbioses. Am. J. Bot. 2001, 88, 1490–1498. [Google Scholar] [CrossRef]
- Magain, N.; Miadlikowska, J.; Goffinet, B.; Serusiaux, E.; Lutzoni, F. Macroevolution of specificity in cyanolichens of the genus Peltigera section Polydactylon (Lecanoromycetes, Ascomycota). Syst. Biol. 2017, 66, 74–99. [Google Scholar] [CrossRef]
- Wirtz, N.; Lumbsch, H.T.; Green, T.G.A.; Türk, R.; Pintado, A.; Sancho, L.; Schroeter, B. Lichen fungi have low cyanobiont selectivity in maritime Antarctica. New Phytol. 2003, 160, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Guzow-Krzemińska, B. Photobiont flexibility in the lichen Protoparmeliopsis muralis as revealed by ITS rDNA analyses. Lichenol. 2006, 38, 469–476. [Google Scholar] [CrossRef]
- Muggia, L.; Vancurova, L.; Škaloud, P.; Peksa, O.; Wedin, M.; Grube, M. The symbiotic playground of lichen thalli—A highly flexible photobiont association in rock-inhabiting lichens. FEMS Microbiol. Ecol. 2013, 85, 313–323. [Google Scholar] [CrossRef]
- Blaha, J.; Baloch, E.; Grube, M. High photobiont diversity associated with the euryoecious lichen-forming ascomycete Lecanora rupicola (Lecanoraceae, Ascomycota). Biol. J. Linn. Soc. 2006, 88, 283–293. [Google Scholar] [CrossRef]
- Yahr, R.; Vilgalys, R.; DePriest, P.T. Geographic variation in algal partners of Cladonia subtenuis (Cladoniaceae) highlights the dynamic nature of a lichen symbiosis. New Phytol. 2006, 171, 847–860. [Google Scholar] [CrossRef]
- Fernández-Mendoza, F.; Domaschke, S.; García, M.A.; Jordan, P.; Martín, M.P.; Printzen, C. Population structure of mycobionts and photobionts of the widespread lichen Cetraria aculeata. Mol. Ecol. 2011, 20, 1208–1232. [Google Scholar] [CrossRef]
- Vargas Castillo, R.; Beck, A. Photobiont selectivity and specificity in Caloplaca species in a fog-induced community in the Atacama Desert, northern Chile. Fungal Biol. 2012, 116, 665–676. [Google Scholar] [CrossRef]
- Scheidegger, C. Systematische Studien zur Krustenflechte Anzina carneonivea (Trapeliaceae, Lecanorales). Nov. Hedwigia 1985, 41, 191–218. [Google Scholar]
- Hoz, C.J.P.-D.L.; Magain, N.; Lutzoni, F.; Goward, T.; Restrepo, S.; Miadlikowska, J. Contrasting Symbiotic Patterns in Two Closely Related Lineages of Trimembered Lichens of the Genus Peltigera. Front. Microbiol. 2018, 9, 2770. [Google Scholar] [CrossRef]
- del Campo, E.M.; Casano, L.M.; Gasulla, F.; Barreno, E. Suitability of chloroplast LSU rDNA and its diverse group I introns for species recognition and phylogenetic analyses of lichen-forming Trebouxia algae. Mol. Phylogenet. Evol. 2010, 54, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Moya, P.; Molins, A.; Chiva, S.; Bastida, J.; Barreno, E. Symbiotic microalgal diversity within lichenicolous lichens and crustose hosts on Iberian Peninsula gypsum biocrusts. Sci. Rep. 2020, 10, 14060. [Google Scholar] [CrossRef]
- Bačkor, M.; Peksa, O.; Škaloud, P.; Bačkorová, M. Photobiont diversity in lichens from metal-rich substrata based on ITS rDNA sequences. Ecotoxicol. Environ. Saf. 2010, 73, 603–612. [Google Scholar] [CrossRef]
- Casano, L.M.; Del Campo, E.M.; García-Breijo, F.J.; Reig-Armiñana, J.; Gasulla, F.; Del Hoyo, A.; Guéra, A.; Barreno, E. Two Trebouxia algae with different physiological performances are ever-present in lichen thalli of Ramalina farinacea. Coexistence versus Competition? Environ. Microbiol. 2011, 13, 806–818. [Google Scholar] [CrossRef]
- del Campo, E.M.; Catalá, S.; Gimeno, J.; del Hoyo, A.; Martínez-Alberola, F.; Casano, L.M.; Grube, M.; Barreno, E. The genetic structure of the cosmopolitan three-partner lichen Ramalina farinacea evidences the concerted diversification of symbionts. FEMS Microbiol. Ecol. 2013, 83, 310–323. [Google Scholar] [CrossRef]
- Uphof, J.C.T. Purple bacteria as symbionts of a lichen. Science 1925, 61, 67. [Google Scholar] [CrossRef] [PubMed]
- Henkel, P.A.; Yuzhakova, L.A. Nitrogen-fixing bacteria in lichens. Izv. Biol. Inst. Permsk. Gos. Univ. 1936, 10, 9–10. [Google Scholar]
- Iskina, R.Y. On nitrogen fixing bacteria in lichens. Isv. Biol. Inst. Permsk. 1938, 11, 133–139. [Google Scholar]
- González, I.; Ayuso-Sacido, A.; Anderson, A.; Genilloud, O. Actinomycetes isolated from lichens: Evaluation of their diversity and detection of biosynthetic gene sequences. FEMS Microbiol. Ecol. 2005, 54, 401–415. [Google Scholar] [CrossRef]
- Amann, R.I.; Ludwig, W.; Schleifer, K.-H. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Rev. 1995, 59, 143–169. [Google Scholar] [CrossRef]
- Rappé, M.S.; Giovannoni, S.J. The Uncultured Microbial Majority. Annu. Rev. Microbiol. 2003, 57, 369–394. [Google Scholar] [CrossRef]
- Almendras, K.; García, J.; Carú, M.; Orlando, J. Nitrogen-fixing bacteria associated with Peltigera cyanolichens and Cladonia chlorolichens. Molecules 2018, 23, 3077. [Google Scholar] [CrossRef]
- Zúñiga, C.; Leiva, D.; Carú, M.; Orlando, J. Substrates of Peltigera Lichens as a Potential Source of Cyanobionts. Microb. Ecol. 2017, 74, 561–569. [Google Scholar] [CrossRef]
- Graham, L.E.; Trest, M.T.; Will-Wolf, S.; Miicke, N.S.; Atonio, L.M.; Piotrowski, M.J.; Knack, J.J. Microscopic and metagenomic analyses of peltigera ponojensis (Peltigerales, ascomycota). Int. J. Plant Sci. 2018, 179, 241–255. [Google Scholar] [CrossRef]
- Grube, M.; Cardinale, M.; De Castro, J.V., Jr.; Müller, H.; Berg, G. Species-specific structural and functional diversity of bacterial communities in lichen symbioses. ISME J. 2009, 3, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Hodkinson, B.P.; Lutzoni, F. A microbiotic survey of lichen-associated bacteria reveals a new lineage from the Rhizobiales. Symbiosis 2009, 49, 163–180. [Google Scholar] [CrossRef]
- Bates, S.T.; Cropsey, G.W.G.; Caporaso, J.G.; Knight, R.; Fierer, N. Bacterial communities associated with the lichen symbiosis. Appl. Environ. Microbiol. 2011, 77, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- Krassowski, M.; Das, V.; Sahu, S.K.; Misra, B.B. State of the Field in Multi-Omics Research: From Computational Needs to Data Mining and Sharing. Front. Genet. 2020, 11, 610798. [Google Scholar] [CrossRef] [PubMed]
- Grube, M.; Cernava, T.; Soh, J.; Fuchs, S.; Aschenbrenner, I.; Lassek, C.; Wegner, U.; Becher, D.; Riedel, K.; Sensen, C.W.; et al. Exploring functional contexts of symbiotic sustain within lichen-associated bacteria by comparative omics. ISME J. 2015, 9, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Cernava, T.; Erlacher, A.; Aschenbrenner, I.A.; Krug, L.; Lassek, C.; Riedel, K.; Grube, M.; Berg, G. Deciphering functional diversification within the lichen microbiota by meta-omics. Microbiome 2017, 5, 82. [Google Scholar] [CrossRef]
- Lendemer, J.C.; Keepers, K.G.; Tripp, E.A.; Pogoda, C.S.; McCain, C.M.; Kane, N.C. A taxonomically broad metagenomic survey of 339 species spanning 57 families suggests cystobasidiomycete yeasts are not ubiquitous across all lichens. Am. J. Bot. 2019, 106, 1090–1095. [Google Scholar] [CrossRef]
- Kono, M.; Kon, Y.; Ohmura, Y.; Satta, Y.; Terai, Y. In vitro resynthesis of lichenization reveals the genetic background of symbiosis-specific fungal-algal interaction in Usnea hakonensis. BMC Genom. 2020, 21, 671. [Google Scholar] [CrossRef]
- Tuovinen, V.; Ekman, S.; Thor, G.; Vanderpool, D.; Spribille, T.; Johannesson, H. Two Basidiomycete Fungi in the Cortex of Wolf Lichens. Curr. Biol. 2019, 29, 476–483.e5. [Google Scholar] [CrossRef]
- Noh, H.-J.; Park, Y.; Hong, S.G.; Lee, Y.M. Diversity and Physiological Characteristics of Antarctic Lichens-Associated Bacteria. Microorganisms 2021, 9, 607. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, M.; Vieira De Castro Jr., J.; Müller, H.; Berg, G.; Grube, M. In situ analysis of the bacterial community associated with the reindeer lichen Cladonia arbuscula reveals predominance of Alphaproteobacteria. FEMS Microbiol. Ecol. 2008, 66, 63–71. [Google Scholar] [CrossRef]
- Aschenbrenner, I.A.; Cernava, T.; Berg, G.; Grube, M. Understanding microbial multi-species symbioses. Front. Microbiol. 2016, 7, 180. [Google Scholar] [CrossRef]
- Spribille, T.; Tagirdzhanova, G.; Goyette, S.; Tuovinen, V.; Case, R.; Zandberg, W.F. 3D biofilms: In search of the polysaccharides holding together lichen symbioses. FEMS Microbiol. Lett. 2020, 367, fnaa023. [Google Scholar] [CrossRef]
- Spribille, T.; Tuovinen, V.; Resl, P.; Vanderpool, D.; Wolinski, H.; Aime, M.C.; Schneider, K.; Stabentheiner, E.; Toome-Heller, M.; Thor, G.; et al. Basidiomycete yeasts in the cortex of ascomycete macrolichens. Science 2016, 353, 488–492. [Google Scholar] [CrossRef]
- Černajová, I.; Škaloud, P. The first survey of Cystobasidiomycete yeasts in the lichen genus Cladonia; with the description of Lichenozyma pisutiana gen. nov., sp. nov. Fungal Biol. 2019, 123, 625–637. [Google Scholar] [CrossRef]
- Millanes, A.M.; Diederich, P.; Wedin, M. Cyphobasidium gen. nov., a new lichen-inhabiting lineage in the Cystobasidiomycetes (Pucciniomycotina, Basidiomycota, Fungi). Fungal Biol. 2016, 120, 1468–1477. [Google Scholar] [CrossRef]
- Oberwinkler, F. Yeasts in Pucciniomycotina. Mycol. Prog. 2017, 16, 831–856. [Google Scholar] [CrossRef]
- Wilkinson, D.M.; Creevy, A.L.; Kalu, C.L.; Schwartzman, D.W. Are heterotrophic and silica-rich eukaryotic microbes an important part of the lichen symbiosis? Mycology 2015, 6, 4–7. [Google Scholar] [CrossRef]
- Petrzik, K.; Koloniuk, I.; Sehadová, H.; Sarkisova, T. Chrysoviruses inhabited symbiotic fungi of lichens. Viruses 2019, 11, 1120. [Google Scholar] [CrossRef]
- Simon, J.-C.; Marchesi, J.R.; Mougel, C.; Selosse, M.-A. Host-microbiota interactions: From holobiont theory to analysis. Microbiome 2019, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Hawksworth, D.L.; Grube, M. Lichens redefined as complex ecosystems. New Phytol. 2020, 227, 1281–1283. [Google Scholar] [CrossRef] [PubMed]
- Honegger, R. The symbiotic phenotype of lichen-forming ascomycetes and their endo- and epibionts. In Fungal Associations, 2nd ed.; Springer Berlin Heidelberg; Institute of Plant Biology, University of Zürich: Zürich, Switzerland, 2012; Volume 9, pp. 287–339. ISBN 9783642308260. [Google Scholar]
- Grimm, M.; Grube, M.; Schiefelbein, U.; Zühlke, D.; Bernhardt, J.; Riedel, K. The Lichens’ Microbiota, Still a Mystery? Front. Microbiol. 2021, 12, 714. [Google Scholar] [CrossRef] [PubMed]
- Ahmadjian, V.; Jacobs, J.B. Relationship between fungus and alga in the lichen Cladonia cristatella Tuck. Nature 1981, 289, 169–172. [Google Scholar] [CrossRef]
- Athukorala, S.N.P.; Huebner, E.; Piercey-Normore, M.D. Identification and comparison of the 3 early stages of resynthesis for the lichen Cladonia rangiferina. Can. J. Microbiol. 2014, 60, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Honegger, R. Morphogenesis. In Lichen Biology; Nash, T.H., III, Ed.; Cambridge University Press: Cambridge, UK, 2008; pp. 69–93. ISBN 9780521871624. [Google Scholar]
- Meeßen, J.; Ott, S. Recognition mechanisms during the pre-contact state of lichens: I. Mycobiont-photobiont interactions of the mycobiont of Fulgensia bracteata. Symbiosis 2013, 59, 121–130. [Google Scholar] [CrossRef]
- Joneson, S.; Armaleo, D.; Lutzoni, F. Fungal and algal gene expression in early developmental stages of lichen-symbiosis. Mycologia 2011, 103, 291–306. [Google Scholar] [CrossRef] [PubMed]
- Ahmadjian, V.; Jacobs, J.B.; Russell, L.A. Scanning Electron Microscope Study of Early Lichen Synthesis. Science 1978, 200, 1062–1064. [Google Scholar] [CrossRef] [PubMed]
- Galun, M. Lichenization. In CRC Handbook of Lichenology; Galun, M., Ed.; CRC Press: Boca Raton, FL, USA, 1988; Volume II, pp. 153–169. [Google Scholar]
- Armaleo, D. Experimental Microbiology of Lichens—Mycelia Fragmentation, a Novel Growth Chamber, and the Origins of Thallus Differentiation. Symbiosis 1991, 11, 163–177. [Google Scholar]
- Joneson, S.; Lutzoni, F. Compatibility and thigmotropism in the lichen symbiosis: A reappraisal. Symbiosis 2009, 47, 109–115. [Google Scholar] [CrossRef]
- Yoshimura, I.; Kurokawa, T.; Yamamoto, Y.; Kinoshita, Y. Development of Lichen Thalli in Vitro. Bryologist 1993, 96, 412–421. [Google Scholar] [CrossRef]
- Kon, Y.; Kashiwadani, H.; Masada, M.; Tamura, G. Artificial Syntheses of Mycobionts of Usnea confusa ssp. kitamiensis and Usnea orientalis with Their Natural and Non-Natural Phycobiont. J. Jpn. Bot. 1993, 68, 129–137. [Google Scholar]
- Stocker-Wörgötter, E. Experimental studies of the lichen symbiosis: DNA-analyses, differentiation and secondary chemistry of selected mycobionts, artificial resynthesis of two- and tripartite symbioses. Symbiosis 2001, 30, 207–227. [Google Scholar]
- Trembley, M.L.; Ringli, C.; Honegger, R. Morphological and molecular analysis of early stages in the resynthesis of the lichen Baeomyces rufus. Mycol. Res. 2002, 106, 768–776. [Google Scholar] [CrossRef]
- Guzow-Krzemińska, B.; Stocker-Wörgötter, E. In vitro culturing and resynthesis of the mycobiont Protoparmeliopsis muralis with algal bionts. Lichenologist 2013, 45, 65–76. [Google Scholar] [CrossRef]
- Ahmadjian, V.; Russell, L.A.; Hildreth, K.C. Artificial Reestablishment of Lichens. I. Morphological Interactions Between the Phycobionts of Different Lichens and the Mycobionts Cladonia Cristatella and Lecanora Chrysoleuca. Mycologia 1980, 72, 73–89. [Google Scholar] [CrossRef]
- Bubrick, P.; Galun, M. Spore to spore resynthesis of Xanthoria Parietina. Lichenologist 1986, 18, 47–49. [Google Scholar] [CrossRef]
- Culberson, C.F.; Culberson, W.L.; Johnson, A. Genetic and environmental effects of growth and production of secondary compounds in Cladonia cristatella. Biochem. Syst. Ecol. 1983, 11, 77–84. [Google Scholar] [CrossRef]
- Stocker-Wörgötter, E.; Türk, R. Artificial cultures of the cyanobacterial lichenPeltigera didactyla (Peltigeraceae) in the natural environment. Plant Syst. Evol. 1989, 165, 39–48. [Google Scholar] [CrossRef]
- Stocker-Wörgötter, E.; Türk, R. The resynthesis of thalli of Dermatocarpon miniatum under laboratory conditions. Symbiosis 1989, 7, 37–50. [Google Scholar]
- Athukorala, S.N.P.; Piercey-Normore, M.D. Recognition-and defense-related gene expression at 3 resynthesis stages in lichen symbionts. Can. J. Microbiol. 2014, 61, 1–12. [Google Scholar] [CrossRef]
- Insarova, I.D.; Blagoveshchenskaya, E.Y. Lichen symbiosis: Search and recognition of partners. Biol. Bull. 2016, 43, 408–418. [Google Scholar] [CrossRef]
- Gaßmann, A.; Ott, S. Growth strategy and the gradual symbiotic interactions of the lichen Ochrolechia frigida. Plant Biol. 2000, 2, 368–378. [Google Scholar] [CrossRef]
- Meeßen, J.; Eppenstein, S.; Ott, S. Recognition mechanisms during the pre-contact state of lichens: II. Influence of algal exudates and ribitol on the response of the mycobiont of Fulgensia bracteata. Symbiosis 2013, 59, 131–143. [Google Scholar] [CrossRef]
- Díaz, E.M.; Vicente-Manzanares, M.; Sacristan, M.; Vicente, C.; Legaz, M.-E. Fungal lectin of Peltigera canina induces chemotropism of compatible Nostoc cells by constriction-relaxation pulses of cyanobiont cytoskeleton. Plant Signal. Behav. 2011, 6, 1525–1536. [Google Scholar] [CrossRef] [PubMed]
- Munzi, S.; Gouveia, C.; Cruz, C.; Branquinho, C.; Coelho, A.V. Proteomic analysis contributes to unveil the mechanisms of nitrogen tolerance in the lichen Xanthoria parietina. Not. Della Soc. Lichenol. Ital. 2018, 31, 23. [Google Scholar]
- Crosino, A.; Moscato, E.; Blangetti, M.; Carotenuto, G.; Spina, F.; Bordignon, S.; Puech-Pagès, V.; Anfossi, L.; Volpe, V.; Prandi, C.; et al. Extraction of short chain chitooligosaccharides from fungal biomass and their use as promoters of arbuscular mycorrhizal symbiosis. Sci. Rep. 2021, 11, 3798. [Google Scholar] [CrossRef]
- Volpe, V.; Carotenuto, G.; Berzero, C.; Cagnina, L.; Puech-Pagès, V.; Genre, A. Short chain chito-oligosaccharides promote arbuscular mycorrhizal colonization in Medicago truncatula. Carbohydr. Polym. 2020, 229, 115505. [Google Scholar] [CrossRef]
- Feng, F.; Sun, J.; Radhakrishnan, G.V.; Lee, T.; Bozsóki, Z.; Fort, S.; Gavrin, A.; Gysel, K.; Thygesen, M.B.; Andersen, K.R.; et al. A combination of chitooligosaccharide and lipochitooligosaccharide recognition promotes arbuscular mycorrhizal associations in Medicago truncatula. Nat. Commun. 2019, 10, 5047. [Google Scholar] [CrossRef]
- Diédhiou, I.; Diouf, D. Transcription factors network in root endosymbiosis establishment and development. World J. Microbiol. Biotechnol. 2018, 34, 37. [Google Scholar] [CrossRef]
- Omoarelojie, L.O.; Van Staden, J. Plant-endophytic fungi interactions: A strigolactone perspective. S. Afr. J. Bot. 2020, 134, 280–284. [Google Scholar] [CrossRef]
- Peláez-Vico, M.A.; Bernabéu-Roda, L.; Kohlen, W.; Soto, M.J.; López-Ráez, J.A. Strigolactones in the Rhizobium-legume symbiosis: Stimulatory effect on bacterial surface motility and down-regulation of their levels in nodulated plants. Plant Sci. 2016, 245, 119–127. [Google Scholar] [CrossRef]
- Maclean, A.M.; Bravo, A.; Harrison, M.J. Plant signaling and metabolic pathways enabling arbuscular mycorrhizal symbiosis. Plant Cell 2017, 29, 2319–2335. [Google Scholar] [CrossRef] [PubMed]
- Kuga, Y.; Schläppi, K.; Reinhardt, D. From Imaging to Functional Traits in Interactions between Roots and Microbes. In Methods in Rhizosphere Biology Research; Reinhardt, D., Sharma, A.K., Eds.; Springer: Singapore, 2019; pp. 227–239. ISBN 978-981-13-5767-1. [Google Scholar]
- Liu, C.-W.; Breakspear, A.; Stacey, N.; Findlay, K.; Nakashima, J.; Ramakrishnan, K.; Liu, M.; Xie, F.; Endre, G.; de Carvalho-Niebel, F.; et al. A protein complex required for polar growth of rhizobial infection threads. Nat. Commun. 2019, 10, 2848. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, G.V.; Keller, J.; Rich, M.K.; Vernié, T.; Mbadinga Mbadinga, D.L.; Vigneron, N.; Cottret, L.; Clemente, H.S.; Libourel, C.; Cheema, J.; et al. An ancestral signalling pathway is conserved in intracellular symbioses-forming plant lineages. Nat. Plants 2020, 6, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Boschiero, C.; Dai, X.; Lundquist, P.K.; Roy, S.; de Bang, T.C.; Zhang, S.; Zhuang, Z.; Torres-Jerez, I.; Udvardi, M.K.; Scheible, W.-R.; et al. MtSSPDB: The Medicago truncatula Small Secreted Peptide Database. Plant Physiol. 2020, 183, 399–413. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morillas, L.; Roales, J.; Cruz, C.; Munzi, S. Lichen as Multipartner Symbiotic Relationships. Encyclopedia 2022, 2, 1421-1431. https://doi.org/10.3390/encyclopedia2030096
Morillas L, Roales J, Cruz C, Munzi S. Lichen as Multipartner Symbiotic Relationships. Encyclopedia. 2022; 2(3):1421-1431. https://doi.org/10.3390/encyclopedia2030096
Chicago/Turabian StyleMorillas, Lourdes, Javier Roales, Cristina Cruz, and Silvana Munzi. 2022. "Lichen as Multipartner Symbiotic Relationships" Encyclopedia 2, no. 3: 1421-1431. https://doi.org/10.3390/encyclopedia2030096
APA StyleMorillas, L., Roales, J., Cruz, C., & Munzi, S. (2022). Lichen as Multipartner Symbiotic Relationships. Encyclopedia, 2(3), 1421-1431. https://doi.org/10.3390/encyclopedia2030096








