Intrinsically Conducting Polymer Binders for Battery Electrodes
Definition
:1. Introduction
2. Methods of Preparation of Composite Electrodes with ICPs
2.1. Mechanical Mixing and Blending Methods
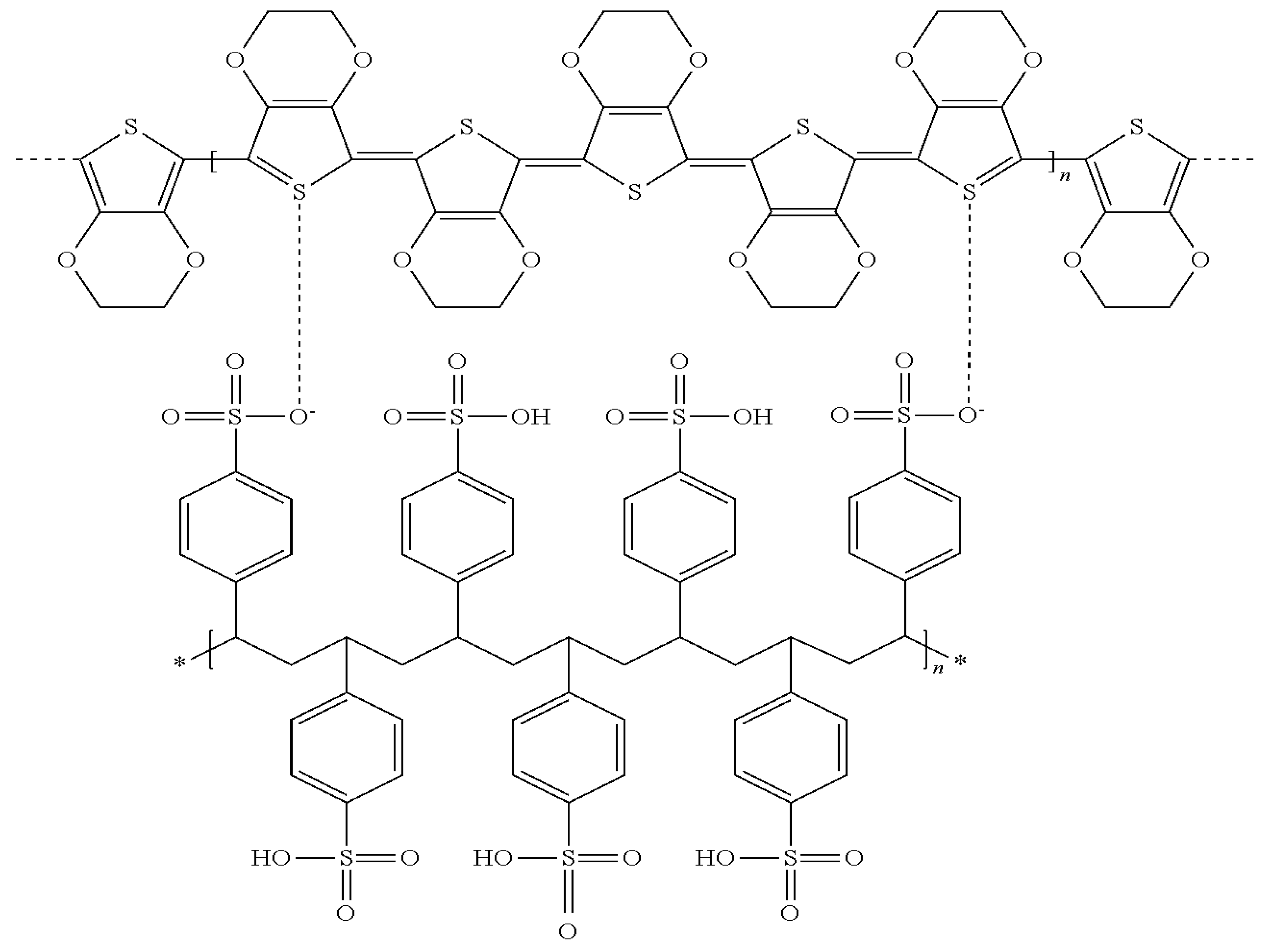
2.2. Conductive Binder Based on ICP Dispersions
2.3. Surface Modification of Active Materials
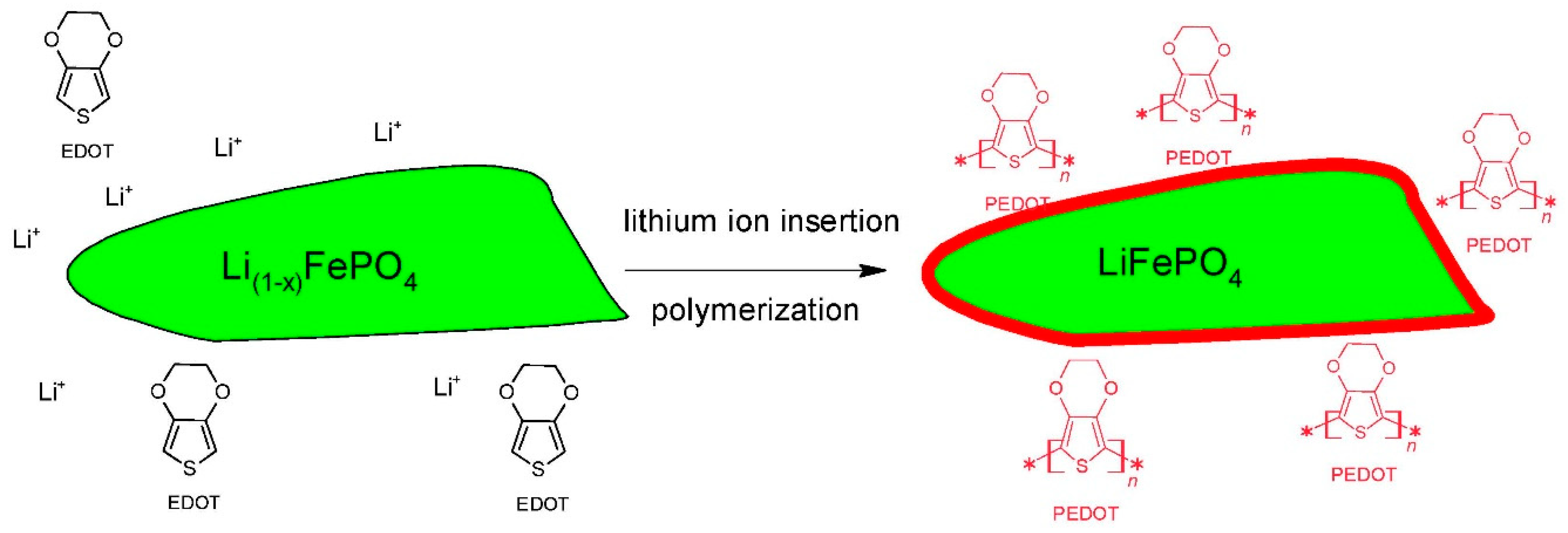
2.4. Chemical or Electrochemical Deposition on Electrodes
2.5. Mechanisms of Electrochemical Performance Improvement of Electrode Materials by ICPs
- (i)
- The electronic and ionic conductivities of the composite electrodes are to a great extent enhanced by the use of conductive binders. The binders control the distribution of conductive additive and active material in the composite electrode and strongly influence the electron transport in the electrodes.
- (ii)
- The use of small amounts of ICP dispersion leading to thin-layer coatings of active grains and short distances between them has been proven to be more effective for the improvement of the specific capacity and C-rate performance of electrodes.
- (iii)
- The conducting polymers protect transition metal oxides from undesired redox reactions with the electrolyte components and from dissolution [62].
- (iv)
- The polymer coating stabilizes the surface crystal structure and suppresses the phase transformation of the electrode material during charge–discharge cycles.
- (v)
- The conductive polymer binder provides mechanical flexibility and can buffer volume changes during intercalation processes into inorganic solid components. It supports more reliable electrical contacts between inorganic active and carbon particles.
- (vi)
- The conductive polymer improves the kinetics of interfacial charge transfer and bulk Li+-ion transport, thus decreasing polarization.
3. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holze, R. Composites and Copolymers Containing Redox-Active Molecules and Intrinsically Conducting Polymers as Active Masses for Supercapacitor Electrodes-An Introduction. Polymers 2020, 12, 1835. [Google Scholar] [CrossRef] [PubMed]
- Kondratiev, V.V.; Holze, R. Intrinsically conducting polymers and their combinations with redox-active molecules for rechargeable battery electrodes: An update. Chem. Pap. 2021, 75, 4981–5007. [Google Scholar] [CrossRef]
- Holze, R.; Wu, Y.P. Intrinsically conducting polymers in electrochemical energy technology: Trends and progress. Electrochim. Acta 2014, 122, 93–107. [Google Scholar] [CrossRef]
- Holze, R. Conjugated Molecules and Polymers in Secondary Batteries: A Perspective. Molecules 2022, 27, 546. [Google Scholar] [CrossRef]
- Nguyen, V.A.; Kuss, C. Review-Conducting Polymer-Based Binders for Lithium-Ion Batteries and Beyond. J. Electrochem. Soc. 2020, 167, 065501. [Google Scholar] [CrossRef]
- Eliseeva, S.N.; Kamenskii, M.A.; Tolstopyatova, E.G.; Kondratiev, V.V. Effect of combined conductive polymer binder on the electrochemical performance of electrode materials for lithium-ion batteries. Energies 2020, 13, 2163. [Google Scholar] [CrossRef]
- Fu, L.; Qu, Q.; Holze, R.; Kondratiev, V.V.; Wu, Y. Composites of metal oxides and intrinsically conducting polymers as supercapacitor electrode materials: The best of both worlds? J. Mater. Chem. A 2019, 7, 14937–14970. [Google Scholar] [CrossRef]
- Ma, M.; Ma, J.; Cui, G. Small things make big deal: Powerful binders of lithium batteries and post-lithium batteries. Energy Storage Mater. 2019, 20, 146–175. [Google Scholar] [CrossRef]
- Eliseeva, S.N.; Shkreba, E.V.; Kamenskii, M.A.; Tolstopjatova, E.G.; Holze, R.; Kondratiev, V.V. Effects of conductive binder on the electrochemical performance of lithium titanate anodes. Solid State Ionics 2019, 333, 18–29. [Google Scholar] [CrossRef]
- Li, S.; Liu, Y.-M.; Zhang, Y.C.; Song, Y.; Wang, G.K.; Liu, Y.X.; Wu, Z.G.; Zhong, B.H.; Zhong, Y.J.; Guo, X.D. A review of rational design and investigation of binders applied in silicon-based anodes for lithium-ion batteries. J. Power Sources 2021, 485, 229331. [Google Scholar] [CrossRef]
- Shao, D.; Zhong, H.; Zhang, L. Water-soluble conductive composite binder containing PEDOT: PSS as conduction promoting agent for Si anode of lithium-ion batteries. ChemElectroChem 2014, 1, 1679–1687. [Google Scholar] [CrossRef]
- Huang, H.; Park, K.S.; Goodenough, J.B. Improving lithium batteries by tethering carbon-coated LiFePO4 to polypyrrole. J. Electrochem. Soc. 2006, 153, A2282–A2286. [Google Scholar] [CrossRef]
- Park, K.S.; Schougaard, S.B.; Goodenough, J.B. Conducting-polymer/iron-redox couple composite cathodes for lithium secondary batteries. Adv. Mater. 2007, 19, 848–851. [Google Scholar] [CrossRef]
- Das, P.R.; Komsiyska, L.; Osters, O.; Wittstock, G. PEDOT:PSS as a functional binder for cathodes in lithium ion batteries. J. Electrochem. Soc. 2015, 162, A674–A678. [Google Scholar] [CrossRef]
- Huang, Y.H.; Goodenough, J.B. High-rate LiFePO4 lithium rechargeable battery promoted by electrochemically active polymers. Chem. Mater. 2008, 20, 7237–7241. [Google Scholar] [CrossRef]
- Lepage, D.; Michot, C.; Liang, G.; Gauthier, N.; Schougaard, S.B. A soft chemistry approach to coating of LiFePO4 with a conducting polymer. Angew. Chem. Int. Ed. 2011, 50, 6884–6887. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.M.M.; Park, H.S.S.; Park, J.H.; Kim, T.H.; Song, H.K.; Lee, S.Y. Conducting polymer-skinned electroactive materials of lithium-ion batteries: Ready for monocomponent electrodes without additional binders and conductive agents. ACS Appl. Mater. Interfaces 2014, 6, 12789–12797. [Google Scholar] [CrossRef]
- Tamura, T.; Aoki, Y.; Ohsawa, T.; Dokko, K. Polyaniline as a Functional Binder for LiFePO4 Cathodes in Lithium Batteries. Chem. Lett. 2011, 40, 828–830. [Google Scholar] [CrossRef]
- Das, P.R.; Komsiyska, L.; Osters, O.; Wittstock, G. Effect of solid loading on the processing and behavior of PEDOT:PSS binder based composite cathodes for lithium ion batteries. Synth. Met. 2016, 215, 86–94. [Google Scholar] [CrossRef]
- Kwon, Y.; Lee, Y.; Kim, S.-O.; Kim, H.-S.; Kim, K.J.; Byun, D.; Choi, W. Conducting Polymer Coating on High-voltage Cathode Based on Soft Chemistry Approach Towards Improving Battery Performance. ACS Appl. Mater. Interfaces 2018, 10, 29457–29466. [Google Scholar] [CrossRef]
- Cao, J.; Hu, G.; Peng, Z.; Du, K.; Cao, Y. Polypyrrole-coated LiCoO2 nanocomposite with enhanced electrochemical properties at high voltage for lithium-ion batteries. J. Power Sources 2015, 281, 49–55. [Google Scholar] [CrossRef]
- Her, L.J.; Hong, J.L.; Chang, C.C. Preparation and electrochemical characterizations of poly(3,4-dioxyethylenethiophene)/LiCoO2 composite cathode in lithium-ion battery. J. Power Sources 2006, 157, 457–463. [Google Scholar] [CrossRef]
- Volkov, F.S.; Tolstopjatova, E.G.; Eliseeva, S.N.; Kamenskii, M.A.; Vypritskaia, A.I.; Volkov, A.I.; Kondratiev, V.V. Vanadium(V) oxide coated by poly(3,4-ethylenedioxythiophene) as cathode for aqueous zinc-ion batteries with improved electrochemical performance. Mater. Lett. 2022, 308, 131210. [Google Scholar] [CrossRef]
- Syrový, T.; Kazda, T.; Syrová, L.; Vondrák, J.; Kubáč, L.; Sedlaříková, M. Cathode material for lithium ion accumulators prepared by screen printing for Smart Textile applications. J. Power Sources 2016, 309, 192–201. [Google Scholar] [CrossRef]
- Sengodu, P.; Deshmukh, A.D. Conducting polymers and their inorganic composites for advanced Li-ion batteries: A review. RSC Adv. 2015, 5, 42109–42130. [Google Scholar] [CrossRef]
- Wang, G.X.; Yang, L.; Chen, Y.; Wang, J.Z.; Bewlay, D.S.; Liu, H.K. An investigation of polypyrrole-LiFePO4 composite cathode materials for lithium-ion batteries. Electrochim. Acta 2005, 50, 4649–4654. [Google Scholar] [CrossRef]
- Sehrawat, R.; Sil, A. Effect of solvents on electrochemical performance of polypyrrole coated LiFePO4/C cathode materials for Li-ion battery. J. Mater. Sci. Mater. Electron. 2015, 26, 5175–5185. [Google Scholar] [CrossRef]
- Feng, S.; Shen, W.; Guo, S. Effects of polypyrrole and chemically reduced graphene oxide on electrochemical properties of lithium iron (II) phosphate. J. Solid State Electrochem. 2017, 21, 3021–3028. [Google Scholar] [CrossRef]
- Gong, Q.; He, Y.S.; Yang, Y.; Liao, X.Z.; Ma, Z.F. Synthesis and electrochemical characterization of LiFePO4/C-polypyrrole composite prepared by a simple chemical vapor deposition method. J. Solid State Electrochem. 2012, 16, 1383–1388. [Google Scholar] [CrossRef] [Green Version]
- Tian, F.; Liu, L.; Yang, Z.; Wang, X.; Chen, Q.; Wang, X. Electrochemical characterization of a LiV3O8- polypyrrole composite as a cathode material for lithium ion batteries. Mater. Chem. Phys. 2011, 127, 151–155. [Google Scholar] [CrossRef]
- Chew, S.Y.; Feng, C.; Ng, S.H.; Wang, J.; Guo, Z.; Liu, H. Low-Temperature Synthesis of Polypyrrole-Coated LiV3O8 Composite with Enhanced Electrochemical Properties. J. Electrochem. Soc. 2007, 154, A633–A637. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Wang, W.; Zhu, D.; Huang, L.; Chen, Y. Improvement of the overall performances of LiMn2O4 via surface-modification by polypyrrole. Mater. Res. Bull. 2015, 71, 91–97. [Google Scholar] [CrossRef]
- Kuwabata, S.; Masui, S.; Yoneyama, H. Charge–discharge properties of composites of LiMn2O4 and polypyrrole as positive electrode materials for 4 V class of rechargeable Li batteries. Electrochim. Acta 1999, 44, 593–4600. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, L.; Ren, X.; Yuan, Q.; Liu, J.; Zhang, Q. Preparation and electrochemical properties of LiNi1/3Co1/3Mn1/3O2-PPy composites cathode materials for lithium-ion battery. Synth. Met. 2011, 161, 1092–1097. [Google Scholar] [CrossRef]
- Wu, C.; Fang, X.; Guo, X.; Mao, Y.; Ma, J.; Zhao, C.; Wang, Z.; Chen, L. Surface modification of Li 1.2 Mn0.54Co0.13Ni0.13O2 with conducting polypyrrole. J. Power Sources 2013, 231, 44–49. [Google Scholar] [CrossRef]
- Gao, X.W.; Deng, Y.F.; Wexler, D.; Chen, G.H.; Chou, S.L.; Liu, H.K.; Shi, Z.C.; Wang, J.Z. Improving the electrochemical performance of the LiNi0.5Mn1.5O2 spinel by polypyrrole coating as a cathode material for the lithium-ion battery. J. Mater. Chem. A 2015, 3, 404–411. [Google Scholar] [CrossRef]
- Chen, S.; He, T.; Su, Y.; Lu, Y.; Bao, L.; Chen, L.; Zhang, Q.; Wang, J.; Chen, R.; Wu, F. Ni-Rich LiNi0.8Mn0.1Co0.1O2 Oxide Coated by Dual-Conductive Layers as High Performance Cathode Material for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 29732–29743. [Google Scholar] [CrossRef]
- Wu, H.; Li, H.; Yang, P.; Xing, Y.; Zhang, S. Surface Modification of Li1.2Ni0.2Mn0.6O2 with Electronic Conducting Polypyrrole. Int. J. Electrochem. Sci. 2018, 13, 6930–6939. [Google Scholar] [CrossRef]
- Shi, J.Y.; Yi, C.W.; Kim, K. An Investigation of LiFePO4/Poly(3,4-ethylenedioxythiophene) Composite Cathode Materials for Lithium-Ion Batteries. Bull. Korean Chem. Soc. 2010, 31, 2698–2700. [Google Scholar] [CrossRef] [Green Version]
- Bai, Y.; Qiu, P.; Wen, Z.; Han, S. Improvement of electrochemical performances of LiFePO4 cathode materials by coating of polythiophene. J. Alloys Compd. 2010, 508, 1–4. [Google Scholar] [CrossRef]
- Arbizzani, C.; Balducci, A.; Mastragostino, M.; Rossi, M.; Soavi, F. Li1.01Mn1.97O4 surface modification by poly(3,4-ethylenedioxythiophene). J. Power Sources 2003, 119–121, 695–700. [Google Scholar] [CrossRef]
- Arbizzani, C.; Mastragostino, M.; Rossi, M. Preparation and electrochemical characterization of a polymer Li1.03Mn1.97O4/PEDOT composite electrode. Electrochem. Commun. 2002, 4, 545–549. [Google Scholar] [CrossRef]
- Su, L.; Smith, P.M.; Anand, M.; Reeja-Jayan, B. Surface Engineering of a LiMn2O4 Electrode Using Nanoscale Polymer Thin Films via Chemical Vapor Deposition Polymerization. ACS Appl. Mater. Interfaces 2018, 10, 27063–27073. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Su, Z.; Sarfraz, S.; Xi, K.; Lai, C. General synthesis and electrochemical performance of TiO2-based microspheres with core-shell structure. Mater. Lett. 2012, 84, 143–146. [Google Scholar] [CrossRef]
- Guo, H.; Liu, L.; Shu, H.; Yang, X.; Yang, Z.; Zhou, M. Synthesis and electrochemical performance of LiV3O8/polythiophene composite as cathode materials for lithium ion batteries. J. Power Sources 2014, 247, 117–126. [Google Scholar] [CrossRef]
- Wang, X.; Shen, L.; Li, H.; Wang, J.; Dou, H.; Zhang, X. PEDOT coated Li4Ti5O12 nanorods: Soft chemistry approach synthesis and their lithium storage properties. Electrochim. Acta 2014, 129, 283–289. [Google Scholar] [CrossRef]
- Xu, D.; Wang, P.; Yang, R. Conducting polythiophene-wrapped Li4Ti5O12 spinel anode material for ultralong cycle-life Li-ion batteries. Ceram. Int. 2017, 43, 4712–4715. [Google Scholar] [CrossRef]
- Xu, D.; Wang, P.; Yang, R. Enhanced electrochemical performance of core-shell Li4Ti5O12/PTh as advanced anode for rechargeable lithium-ion batteries. Ceram. Int. 2017, 43, 7600–7606. [Google Scholar] [CrossRef]
- Yan, H.; Wu, X.; Li, Y. Preparation and characterization of conducting polyaniline-coated LiVPO4F nanocrystals with core-shell structure and its application in lithium-ion batteries. Electrochim. Acta 2015, 182, 437–444. [Google Scholar] [CrossRef]
- He, Z.; Xiong, L.; Chen, S.; Wu, X.; Liu, W.; Huang, K. In situ polymerization preparation and characterization of Li4Ti5O12-polyaniline anode material. Trans. Nonferrous Met. Soc. China 2010, 20, s262–s266. [Google Scholar] [CrossRef]
- Chen, W.M.; Qie, L.; Yuan, L.X.; Xia, S.A.; Hu, X.L.; Zhang, W.X.; Huang, Y.H. Insight into the improvement of rate capability and cyclability in LiFePO4/polyaniline composite cathode. Electrochim. Acta 2011, 56, 2689–2695. [Google Scholar] [CrossRef]
- Gong, C.; Deng, F.; Tsui, C.P.; Xue, Z.; Ye, Y.S.; Tang, C.Y.; Zhou, X.; Xie, X. PANI–PEG copolymer modified LiFePO4 as a cathode material for high-performance lithium ion batteries. J. Mater. Chem A 2014, 2, 19315–19323. [Google Scholar] [CrossRef]
- Shen, W.; Wang, Y.; Yan, J.; Wu, H.; Guo, S. Enhanced electrochemical performance of lithium iron(II) phosphate modified cooperatively via chemically reduced graphene oxide and polyaniline. Electrochim. Acta 2015, 173, 310–315. [Google Scholar] [CrossRef]
- Gao, X.W.; Wang, J.Z.; Chou, S.L.; Liu, H.K. Synthesis and electrochemical performance of LiV3O8/polyaniline as cathode material for the lithium battery. J. Power Sources 2012, 220, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Xie, L.L.; Cao, X.Y.; Zhang, L.X.; Dai, Z.X.; Qu, L.B. Synthesis and electrochemical properties of LiV3O8/PAn composite as a cathode material for lithium secondary batteries, Electron. Mater. Lett. 2013, 9, 183–186. [Google Scholar]
- Guo, H.; Liu, L.; Wei, Q.; Shu, H.; Yang, X.; Yang, Z.; Zhou, M.; Tan, J.; Yan, Z.; Wang, X. Electrochemical characterization of polyaniline-LiV3O8 nanocomposite cathode material for lithium ion batteries. Electrochim. Acta 2013, 94, 113–123. [Google Scholar] [CrossRef]
- Yan, H.; Chen, W.; Wu, X.; Li, Y. Conducting polyaniline-wrapped lithium vanadium phosphate nanocomposite as high-rate and cycling stability cathode for lithium-ion batteries. Electrochim. Acta 2014, 146, 295–300. [Google Scholar] [CrossRef]
- Xue, Q.; Li, J.; Xu, G.; Zhou, H.; Wang, X.; Kang, F. In situ polyaniline modified cathode material Li1.2Ni0.13Co0.13Mn0.54O2 with high rate capacity for lithium ion batteries. J. Mater. Chem. A 2014, 2, 18613–18623. [Google Scholar] [CrossRef]
- Ju, S.H.; Kang, J.; Lee, Y.; Shin, W.K.; Kim, S.; Shin, K.; Kim, D.W. Improvement of the Cycling Performance of LiNi0.6Co0.2Mn0.2 O2 Cathode Active Materials by a Dual-Conductive Polymer Coating. ACS Appl. Mater. Interfaces 2014, 6, 2546–2552. [Google Scholar] [CrossRef]
- Song, L.; Tang, F.; Xiao, X.; Cao, Z.; Zhu, H.; Li, A. Enhanced Electrochemical Properties of Polyaniline-Coated LiNi0.8Co0.1Mn0.1O2 Cathode Material for Lithium-Ion Batteries. J. Electron. Mater. 2018, 47, 5896–5904. [Google Scholar] [CrossRef]
- Van Hoang, H.; Holze, R. Electrochemical synthesis of polyaniline/montmorillonite nanocomposites and their characterization. Chem. Mater. 2006, 18, 1976–1980. [Google Scholar] [CrossRef]
- Li, R.; Xing, F.; Li, T.; Zhang, H.; Yan, J.; Zheng, H.; Li, X. Intercalated polyaniline in V2O5 as a unique vanadium oxide bronze cathode for highly stable aqueous zinc ion battery. Energy Storage Mater. 2021, 38, 590–598. [Google Scholar] [CrossRef]
- Bresser, D.; Buchholz, D.; Moretti, A.; Varzi, A.; Passerini, S. Alternative binders for sustainable electrochemical energy storage—The transition to aqueous electrode processing and bio-derived polymers. Energy Environ. Sci. 2018, 11, 3096–3127. [Google Scholar] [CrossRef] [Green Version]
- Chou, S.L.; Pan, Y.; Wang, J.Z.; Liu, H.K.; Dou, S.X. Small things make a big difference: Binder effects on the performance of Li and Na batteries. Phys. Chem. Chem. Phys. 2014, 16, 20347–20359. [Google Scholar] [CrossRef]
- Liu, X.; Xu, G.; Zhang, G.; Huang, S.; Li, L.; Wei, X.; Cao, J.; Yang, L.; Chu, P.K. Ultrathin hybrid nanobelts of single-crystalline VO2 and Poly(3,4-ethylenedioxythiophene) as cathode materials for aqueous zinc ion batteries with large capacity and high-rate capability. J. Power Sources 2020, 463, 228223. [Google Scholar] [CrossRef]
- Gauthier, M.; Carney, T.J.; Grimaud, A.; Giordano, L.; Pour, N.; Chang, H.H.; Fenning, D.P.; Lux, S.F.; Paschos, O.; Bauer, C.; et al. Electrode-Electrolyte Interface in Li-Ion Batteries: Current Understanding and New Insights. J. Phys. Chem. Lett. 2015, 6, 4653–4672. [Google Scholar] [CrossRef]
- Lee, J.; Choi, W. Surface Modification of Over-Lithiated Layered Oxides with PEDOT:PSS Conducting Polymer in Lithium-Ion Batteries. J. Electrochem. Soc. 2015, 162, A743–A748. [Google Scholar] [CrossRef]
- Cao, Y.; Qi, X.; Hu, K.; Wang, Y.; Gan, Z.; Li, Y.; Hu, G.; Peng, Z.; Du, K. Conductive Polymers Encapsulation to Enhance Electrochemical Performance of Ni-Rich Cathode Materials for Li-Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 18270–18280. [Google Scholar] [CrossRef]
- Wu, F.; Liu, J.; Li, L.; Zhang, X.; Luo, R.; Ye, Y.; Chen, R. Surface Modification of Li-rich Cathode Materials for Lithium-ion Batteries with PEDOT:PSS Conducting Polymer. ACS Appl. Mater. Interfaces 2016, 8, 23095–23104. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kondratiev, V.; Holze, R. Intrinsically Conducting Polymer Binders for Battery Electrodes. Encyclopedia 2022, 2, 1753-1762. https://doi.org/10.3390/encyclopedia2040120
Kondratiev V, Holze R. Intrinsically Conducting Polymer Binders for Battery Electrodes. Encyclopedia. 2022; 2(4):1753-1762. https://doi.org/10.3390/encyclopedia2040120
Chicago/Turabian StyleKondratiev, Veniamin, and Rudolf Holze. 2022. "Intrinsically Conducting Polymer Binders for Battery Electrodes" Encyclopedia 2, no. 4: 1753-1762. https://doi.org/10.3390/encyclopedia2040120
APA StyleKondratiev, V., & Holze, R. (2022). Intrinsically Conducting Polymer Binders for Battery Electrodes. Encyclopedia, 2(4), 1753-1762. https://doi.org/10.3390/encyclopedia2040120







