Magnetite Nanoparticles for Biomedical Applications
Definition
:1. Introduction
2. Synthesis of Magnetic Nanoparticles
3. Toxicity of Magnetic Nanoparticles
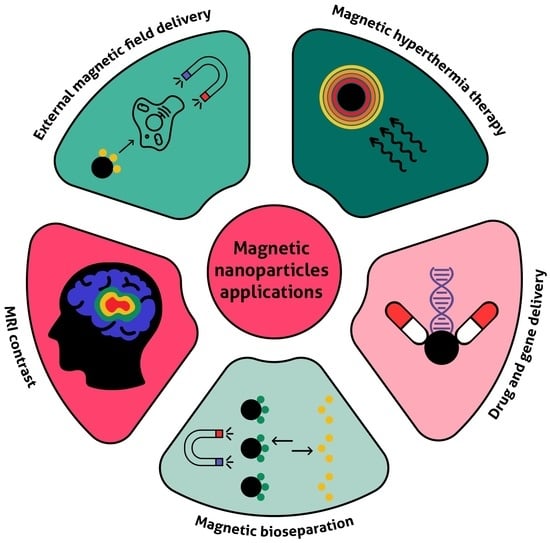
4. Biomedical Applications of Magnetic Nanoparticles
4.1. Therapy
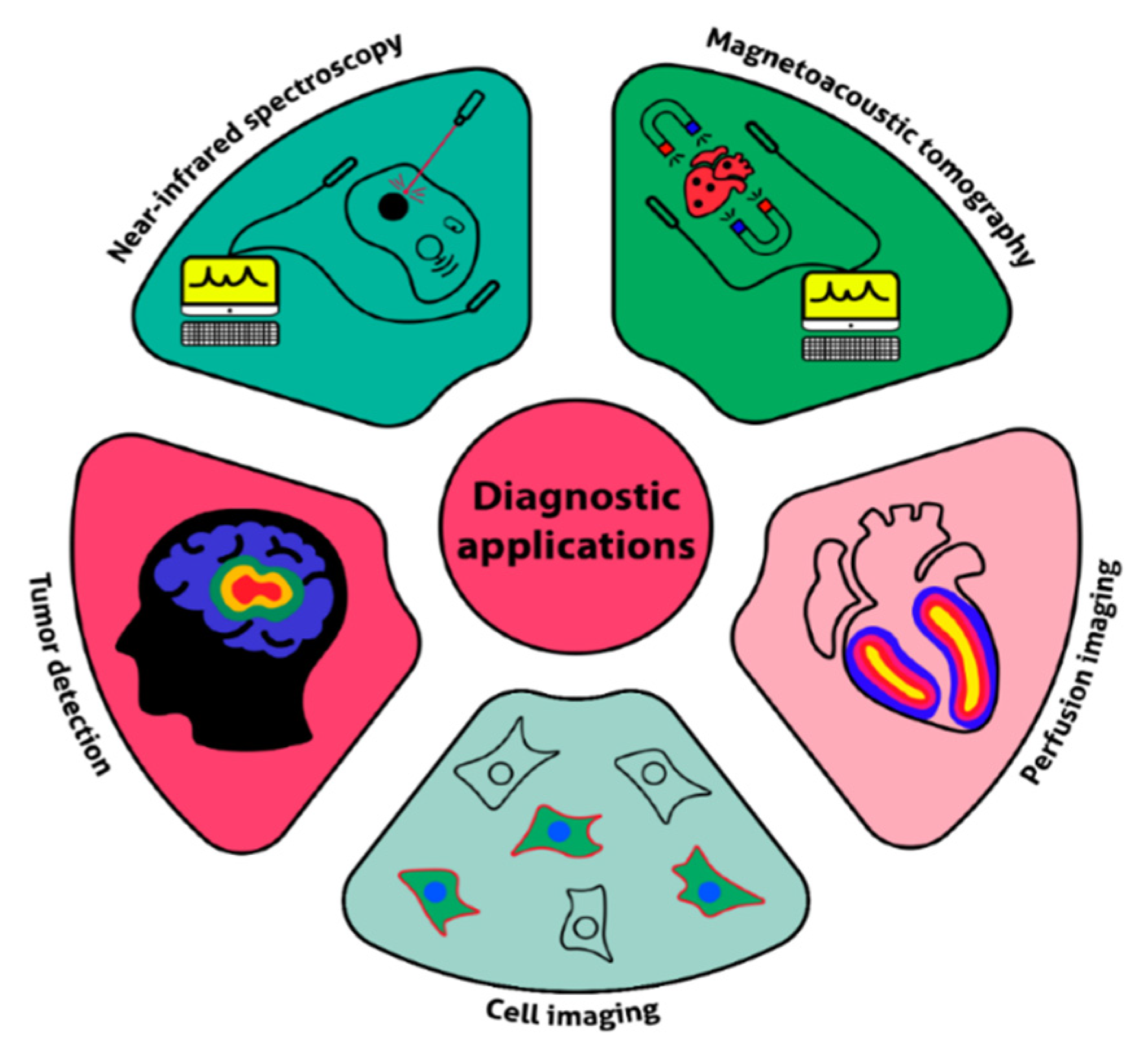
4.2. Diagnostics and Theranostics
4.3. Magnetic Separation, MNP-Based Biosensors, and Magnetic Microreactors
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ganapathe, L.S.; Mohamed, M.A.; Yunus, R.M.; Berhanuddin, D.D. Magnetite (Fe3O4) nanoparticles in biomedical application: From synthesis to surface functionalisation. Magnetochemistry 2020, 6, 68. [Google Scholar] [CrossRef]
- Anik, M.I.; Hossain, M.K.; Hossain, I.; Mahfuz, A.M.U.B.; Rahman, M.T.; Ahmed, I. Recent progress of magnetic nanoparticles in biomedical applications: A review. Nano Sel. 2021, 2, 1146–1186. [Google Scholar] [CrossRef]
- Shabatina, T.I.; Vernaya, O.I.; Shabatin, V.P.; Melnikov, M.Y. Magnetic nanoparticles for biomedical purposes: Modern trends and prospects. Magnetochemistry 2020, 6, 30. [Google Scholar] [CrossRef]
- Socoliuc, V.; Peddis, D.; Petrenko, V.I.; Avdeev, M.V.; Susan-Resiga, D.; Szabó, T.; Turcu, R.; Tombácz, E.; Vékás, L. Magnetic nanoparticle systems for nanomedicine—A materials science perspective. Magnetochemistry 2020, 6, 2. [Google Scholar] [CrossRef] [Green Version]
- Hepel, M. Magnetic nanoparticles for nanomedicine. Magnetochemistry 2020, 6, 3. [Google Scholar] [CrossRef] [Green Version]
- Frenea-Robin, M.; Marchalot, J. Basic Principles and Recent Advances in Magnetic Cell Separation. Magnetochemistry 2022, 8, 11. [Google Scholar] [CrossRef]
- Chubarov, A.S. Serum Albumin for Magnetic Nanoparticles Coating. Magnetochemistry 2022, 8, 13. [Google Scholar] [CrossRef]
- Mittal, A.; Roy, I.; Gandhi, S. Magnetic Nanoparticles: An Overview for Biomedical Applications. Magnetochemistry 2022, 8, 107. [Google Scholar] [CrossRef]
- Katz, E. Synthesis, properties and applications of magnetic nanoparticles and nanowires—A brief introduction. Magnetochemistry 2019, 5, 61. [Google Scholar] [CrossRef] [Green Version]
- Antone, A.J.; Sun, Z.; Bao, Y. Preparation and application of iron oxide nanoclusters. Magnetochemistry 2019, 5, 45. [Google Scholar] [CrossRef]
- Kudr, J.; Haddad, Y.; Richtera, L.; Heger, Z.; Cernak, M.; Adam, V.; Zitka, O. Magnetic nanoparticles: From design and synthesis to real world applications. Nanomaterials 2017, 7, 243. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.D.; Gwenin, V.V.; Gwenin, C.D. Magnetic Functionalized Nanoparticles for Biomedical, Drug Delivery and Imaging Applications. Nanoscale Res. Lett. 2019, 14, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamichhane, N.; Sharma, S.; Parul; Verma, A.K.; Roy, I.; Sen, T. Iron oxide-based magneto-optical nanocomposites for in vivo biomedical applications. Biomedicines 2021, 9, 288. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, R.S.; Horvat, M.; Ahmed, J.; Alhokbany, N.; Alshehri, S.M.; Gandhi, S. Magnetic nanoparticles—A multifunctional potential agent for diagnosis and therapy. Cancers 2021, 13, 2213. [Google Scholar] [CrossRef]
- Dulińska-Litewka, J.; Łazarczyk, A.; Hałubiec, P.; Szafrański, O.; Karnas, K.; Karewicz, A. Superparamagnetic iron oxide nanoparticles-current and prospective medical applications. Materials 2019, 12, 617. [Google Scholar] [CrossRef] [Green Version]
- Stueber, D.D.; Villanova, J.; Aponte, I.; Xiao, Z. Magnetic Nanoparticles in Biology and Medicine: Past, Present, and Future Trends. Pharmaceutics 2021, 13, 943. [Google Scholar] [CrossRef]
- Krishnan; Goud Magnetic Particle Bioconjugates: A Versatile Sensor Approach. Magnetochemistry 2019, 5, 64. [CrossRef] [Green Version]
- Sharma, B.; Pervushin, K. Magnetic nanoparticles as in vivo tracers for alzheimer’s disease. Magnetochemistry 2020, 6, 13. [Google Scholar] [CrossRef] [Green Version]
- Bruschi, M.L.; de Toledo, L.d.A.S. Pharmaceutical applications of iron-oxide magnetic nanoparticles. Magnetochemistry 2019, 5, 50. [Google Scholar] [CrossRef] [Green Version]
- Creţu, B.E.B.; Dodi, G.; Shavandi, A.; Gardikiotis, I.; Şerban, I.L.; Balan, V. Imaging constructs: The rise of iron oxide nanoparticles. Molecules 2021, 26, 3437. [Google Scholar] [CrossRef]
- Ulbrich, K.; Holá, K.; Šubr, V.; Bakandritsos, A.; Tuček, J.; Zbořil, R. Targeted Drug Delivery with Polymers and Magnetic Nanoparticles: Covalent and Noncovalent Approaches, Release Control, and Clinical Studies. Chem. Rev. 2016, 116, 5338–5431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bobrikova, E.; Chubarov, A.; Dmitrienko, E. The Effect of pH and Buffer on Oligonucleotide Affinity for Iron Oxide Nanoparticles. Magnetochemistry 2021, 7, 128. [Google Scholar] [CrossRef]
- Obaidat, I.M.; Narayanaswamy, V.; Alaabed, S.; Sambasivam, S.; Muralee Gopi, C.V.V. Principles of Magnetic Hyperthermia: A Focus on Using Multifunctional Hybrid Magnetic Nanoparticles. Magnetochemistry 2019, 5, 67. [Google Scholar] [CrossRef] [Green Version]
- Jiao, W.; Zhang, T.; Peng, M.; Yi, J.; He, Y.; Fan, H. Design of Magnetic Nanoplatforms for Cancer Theranostics. Biosensors 2022, 12, 38. [Google Scholar] [CrossRef]
- Schneider, M.G.M.; Martín, M.J.; Otarola, J.; Vakarelska, E.; Simeonov, V.; Lassalle, V.; Nedyalkova, M. Biomedical Applications of Iron Oxide Nanoparticles: Current Insights Progress and Perspectives. Pharmaceutics 2022, 14, 204. [Google Scholar] [CrossRef]
- Tran, H.; Ngo, N.M.; Medhi, R.; Srinoi, P.; Liu, T.; Rittikulsittichai, S.; Lee, T.R. Multifunctional Iron Oxide Magnetic Nanoparticles for Biomedical Applications: A Review. Materials 2022, 15, 503. [Google Scholar] [CrossRef]
- Caspani, S.; Magalhães, R.; Araújo, J.P.; Sousa, C.T. Magnetic nanomaterials as contrast agents for MRI. Materials 2020, 13, 2586. [Google Scholar] [CrossRef]
- Kostevšek, N. A review on the optimal design of magnetic nanoparticle-based t2 mri contrast agents. Magnetochemistry 2020, 6, 11. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Barahona, I.; Muñoz-Hernando, M.; Ruiz-Cabello, J.; Herranz, F.; Pellico, J. Iron oxide nanoparticles: An alternative for positive contrast in magnetic resonance imaging. Inorganics 2020, 8, 28. [Google Scholar] [CrossRef] [Green Version]
- Katz, E. Magnetic Nanoparticles. Magnetochemistry 2020, 6, 6. [Google Scholar] [CrossRef]
- Berensmeier, S. Magnetic particles for the separation and purification of nucleic acids. Appl. Microbiol. Biotechnol. 2006, 73, 495–504. [Google Scholar] [CrossRef]
- Li, P.; Li, M.; Yue, D.; Chen, H. Solid-phase extraction methods for nucleic acid separation. A review. J. Sep. Sci. 2022, 45, 172–184. [Google Scholar] [CrossRef]
- Tang, C.; He, Z.; Liu, H.; Xu, Y.; Huang, H.; Yang, G.; Xiao, Z.; Li, S.; Liu, H.; Deng, Y.; et al. Application of magnetic nanoparticles in nucleic acid detection. J. Nanobiotechnol. 2020, 18, 62. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Lee, T.R. Fe3O4 Nanoparticles: Structures, Synthesis, Magnetic Properties, Surface Functionalization, and Emerging Applications. Appl. Sci. 2021, 11, 11301. [Google Scholar]
- Hosu, O.; Tertis, M.; Cristea, C. Implication of magnetic nanoparticles in cancer detection, screening and treatment. Magnetochemistry 2019, 5, 55. [Google Scholar] [CrossRef] [Green Version]
- Akbarzadeh, A.; Samiei, M.; Davaran, S. Magnetic nanoparticles: Preparation, physical properties, and applications in biomedicine. Nanoscale Res. Lett. 2012, 7, 144. [Google Scholar] [CrossRef] [Green Version]
- Caizer, C. Nanoparticle Size Effect on Some Magnetic Properties. In Handbook of Nanoparticles; Springer: Cham, Switzerland, 2016; pp. 1–1426. ISBN 9783319153384. [Google Scholar]
- Li, Q.; Kartikowati, C.W.; Horie, S.; Ogi, T.; Iwaki, T.; Okuyama, K. Correlation between particle size/domain structure and magnetic properties of highly crystalline Fe3O4 nanoparticles. Sci. Rep. 2017, 7, 9894. [Google Scholar] [CrossRef]
- Kolhatkar, A.G.; Jamison, A.C.; Litvinov, D.; Willson, R.C.; Lee, T.R. Tuning the Magnetic Properties of Nanoparticles. Int. J. Mol. Sci. 2013, 14, 15977–16009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koksharov, Y.A. Magnetism of Nanoparticles: Effects of Size, Shape, and Interactions. In Magnetic Nanoparticles; Wiley-VCH: Hoboken, NJ, USA, 2009; pp. 197–254. ISBN 9783527407903. [Google Scholar]
- Lee, J.S.; Cha, J.M.; Yoon, H.Y.; Lee, J.K.; Kim, Y.K. Magnetic multi-granule nanoclusters: A model system that exhibits universal size effect of magnetic coercivity. Sci. Rep. 2015, 5, 12135. [Google Scholar] [CrossRef] [Green Version]
- Frenkel, J.; Doefman, J. Spontaneous and Induced Magnetisation in Ferromagnetic Bodies. Nature 1930, 126, 274–275. [Google Scholar] [CrossRef]
- Xu, H.; Li, S.; Liu, Y. Nanoparticles in the diagnosis and treatment of vascular aging and related diseases. Signal Transduct. Target. Ther. 2022, 7, 231. [Google Scholar] [CrossRef] [PubMed]
- Pucci, C.; Degl’Innocenti, A.; Belenli Gümüş, M.; Ciofani, G. Superparamagnetic iron oxide nanoparticles for magnetic hyperthermia: Recent advancements, molecular effects, and future directions in the omics era. Biomater. Sci. 2022, 10, 2103–2121. [Google Scholar] [CrossRef]
- Ramin, N.A.; Ramachandran, M.R.; Saleh, N.M.; Mat Ali, Z.M.; Asman, S. Magnetic Nanoparticles Molecularly Imprinted Polymers: A Review. Curr. Nanosci. 2022, 18, 1–29. [Google Scholar] [CrossRef]
- Harish, V.; Tewari, D.; Gaur, M.; Yadav, A.B.; Swaroop, S.; Bechelany, M.; Barhoum, A. Review on Nanoparticles and Nanostructured Materials: Bioimaging, Biosensing, Drug Delivery, Tissue Engineering, Antimicrobial, and Agro-Food Applications. Nanomaterials 2022, 12, 457. [Google Scholar] [CrossRef]
- Footer, C. Tuneable Magnetic Nanocomposites for Remote self-healing. Sci. Rep. 2022, 12, 10180. [Google Scholar]
- Koksharov, Y.A.; Gubin, S.P.; Taranov, I.V.; Khomutov, G.B.; Gulyaev, Y.V. Magnetic Nanoparticles in Medicine: Progress, Problems, and Advances. J. Commun. Technol. Electron. 2022, 67, 101–116. [Google Scholar] [CrossRef]
- Geppert, M.; Himly, M. Iron Oxide Nanoparticles in Bioimaging—An Immune Perspective. Front. Immunol. 2021, 12, 688927. [Google Scholar] [CrossRef]
- Malhotra, N.; Lee, J.S.; Liman, R.A.D.; Ruallo, J.M.S.; Villaflore, O.B.; Ger, T.R.; Hsiao, C. Der Potential toxicity of iron oxide magnetic nanoparticles: A review. Molecules 2020, 25, 3159. [Google Scholar] [CrossRef]
- Abakumov, M.A.; Semkina, A.S.; Skorikov, A.S.; Vishnevskiy, D.A.; Ivanova, A.V.; Mironova, E.; Davydova, G.A.; Majouga, A.G.; Chekhonin, V.P. Toxicity of iron oxide nanoparticles: Size and coating effects. J. Biochem. Mol. Toxicol. 2018, 32, e22225. [Google Scholar] [CrossRef]
- Kim, J.E.; Shin, J.Y.; Cho, M.H. Magnetic nanoparticles: An update of application for drug delivery and possible toxic effects. Arch. Toxicol. 2012, 86, 685–700. [Google Scholar] [CrossRef]
- Seeney, C.E. The emerging applications of magnetic nanovectors in nanomedicine. Pharm. Pat. Anal. 2015, 4, 285–304. [Google Scholar] [CrossRef] [PubMed]
- Chrishtop, V.V.; Mironov, V.A.; Prilepskii, A.Y.; Nikonorova, V.G.; Vinogradov, V.V. Organ-specific toxicity of magnetic iron oxide-based nanoparticles. Nanotoxicology 2021, 15, 167–204. [Google Scholar] [CrossRef] [PubMed]
- Batlle, X.; Moya, C.; Escoda-Torroella, M.; Iglesias, Ò.; Fraile Rodríguez, A.; Labarta, A. Magnetic nanoparticles: From the nanostructure to the physical properties. J. Magn. Magn. Mater. 2022, 543, 168594. [Google Scholar] [CrossRef]
- Chircov, C.; Vasile, B.S. New Approaches in Synthesis and Characterization Methods of Iron Oxide Nanoparticles. In Iron Oxide Nanoparticles; IntechOpen: London, UK, 2022. [Google Scholar]
- Ali, A.; Shah, T.; Ullah, R.; Zhou, P.; Guo, M.; Ovais, M.; Tan, Z.; Rui, Y.K. Review on Recent Progress in Magnetic Nanoparticles: Synthesis, Characterization, and Diverse Applications. Front. Chem. 2021, 9, 629054. [Google Scholar] [CrossRef] [PubMed]
- Vargas, G.; Cypriano, J.; Correa, T.; Leao, P.; Bazylinski, D.; Abreu, F. Applications of Magnetotactic Bacteria, Magnetosomes and Magnetosome Crystals in Biotechnology and Nanotechnology: Mini-Review. Molecules 2018, 23, 2438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canaparo, R.; Foglietta, F.; Limongi, T.; Serpe, L. Biomedical applications of reactive oxygen species generation by metal nanoparticles. Materials 2021, 14, 53. [Google Scholar] [CrossRef] [PubMed]
- Nelson, N.; Port, J.; Pandey, M. Use of Superparamagnetic Iron Oxide Nanoparticles (SPIONs) via Multiple Imaging Modalities and Modifications to Reduce Cytotoxicity: An Educational Review. J. Nanotheranostics 2020, 1, 105–135. [Google Scholar] [CrossRef]
- Zelepukin, I.V.; Yaremenko, A.V.; Ivanov, I.N.; Yuryev, M.V.; Cherkasov, V.R.; Deyev, S.M.; Nikitin, P.I.; Nikitin, M.P. Long-Term Fate of Magnetic Particles in Mice: A Comprehensive Study. ACS Nano 2021, 15, 11341–11357. [Google Scholar] [CrossRef]
- Khramtsov, P.; Barkina, I.; Kropaneva, M.; Bochkova, M.; Timganova, V.; Nechaev, A.; Byzov, I.; Zamorina, S.; Yermakov, A.; Rayev, M. Magnetic nanoclusters coated with albumin, casein, and gelatin: Size tuning, relaxivity, stability, protein corona, and application in nuclear magnetic resonance immunoassay. Nanomaterials 2019, 9, 1345. [Google Scholar] [CrossRef] [Green Version]
- Schwaminger, S.P.; Blank-Shim, S.A.; Scheifele, I.; Pipich, V.; Fraga-García, P.; Berensmeier, S. Design of Interactions Between Nanomaterials and Proteins: A Highly Affine Peptide Tag to Bare Iron Oxide Nanoparticles for Magnetic Protein Separation. Biotechnol. J. 2019, 14, 55. [Google Scholar] [CrossRef]
- Mylkie, K.; Nowak, P.; Rybczynski, P.; Ziegler-Borowska, M. Polymer-coated magnetite nanoparticles for protein immobilization. Materials 2021, 14, 248. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Li, X.; Li, Q.; Lyu, J.; Wang, W.; Zhuang, L.; Xu, Y. Magnetic drug-loaded osteoinductive Fe3O4/CaCO3 hybrid microspheres system: Efficient for sustained release of antibiotics. J. Phys. D. Appl. Phys. 2020, 53, 245401. [Google Scholar] [CrossRef]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric nanoparticles for drug delivery: Recent developments and future prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef] [PubMed]
- Turrina, C.; Oppelt, A.; Mitzkus, M.; Berensmeier, S.; Schwaminger, S.P. Silica-coated superparamagnetic iron oxide nanoparticles: New insights into the influence of coating thickness on the particle properties and lasioglossin binding. MRS Commun. 2022, 12, 632–639. [Google Scholar] [CrossRef]
- Schwaminger, S.P.; Blank-Shim, S.A.; Scheifele, I.; Fraga-García, P.; Berensmeier, S. Peptide binding to metal oxide nanoparticles. Faraday Discuss. 2017, 204, 233–250. [Google Scholar] [CrossRef]
- Tarkistani, M.A.M.; Komalla, V.; Kayser, V. Recent advances in the use of iron–gold hybrid nanoparticles for biomedical applications. Nanomaterials 2021, 11, 1227. [Google Scholar] [CrossRef]
- Schwaminger, S.P.; Bauer, D.; Fraga-García, P. Gold-iron oxide nanohybrids: Insights into colloidal stability and surface-enhanced Raman detection. Nanoscale Adv. 2021, 3, 6438–6445. [Google Scholar] [CrossRef]
- Zaloga, J.; Feoktystov, A.; Garamus, V.M.; Karawacka, W.; Ioffe, A.; Brückel, T.; Tietze, R.; Alexiou, C.; Lyer, S. Studies on the Adsorption and Desorption of Mitoxantrone to Lauric Acid/Albumin Coated Iron Oxide Nanoparticles. Colloids Surf. B Biointerfaces 2018, 161, 18–26. [Google Scholar] [CrossRef]
- Vismara, E.; Bongio, C.; Coletti, A.; Edelman, R.; Serafini, A.; Mauri, M.; Simonutti, R.; Bertini, S.; Urso, E.; Assaraf, Y.G.; et al. Albumin and hyaluronic acid-coated superparamagnetic iron oxide nanoparticles loaded with paclitaxel for biomedical applications. Molecules 2017, 22, 1030. [Google Scholar] [CrossRef] [Green Version]
- Zaloga, J.; Pöttler, M.; Leitinger, G.; Friedrich, R.P.; Almer, G.; Lyer, S.; Baum, E.; Tietze, R.; Heimke-Brinck, R.; Mangge, H.; et al. Pharmaceutical formulation of HSA hybrid coated iron oxide nanoparticles for magnetic drug targeting. Eur. J. Pharm. Biopharm. 2016, 101, 152–162. [Google Scholar] [CrossRef]
- Zaloga, J.; Stapf, M.; Nowak, J.; Pöttler, M.; Friedrich, R.P.; Tietze, R.; Lyer, S.; Lee, G.; Odenbach, S.; Hilger, I.; et al. Tangential flow ultrafiltration allows purification and concentration of lauric acid-/albumin-coated particles for improved magnetic treatment. Int. J. Mol. Sci. 2015, 16, 19291–19307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaloga, J.; Janko, C.; Nowak, J.; Matuszak, J.; Knaup, S.; Eberbeck, D.; Tietze, R.; Unterweger, H.; Friedrich, R.P.; Duerr, S.; et al. Development of a lauric acid/albumin hybrid iron oxide nanoparticle system with improved biocompatibility. Int. J. Nanomed. 2014, 9, 4847–4866. [Google Scholar] [CrossRef] [PubMed]
- Corem-Salkmon, E.; Ram, Z.; Daniels, D.; Perlstein, B.; Last, D.; Salomon, S.; Tamar, G.; Shneor, R.; Guez, D.; Margel, S.; et al. Convection-enhanced delivery of methotrexate-loaded maghemite nanoparticles. Int. J. Nanomed. 2011, 6, 1595–1602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; Ye, L.; Lu, Y. Flexible and Effective Preparation of Magnetic Nanoclusters via One-Step Flow Synthesis. Nanomaterials 2022, 12, 350. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Joshi, N.; Chattopadhyay, K.; De, G. A facile synthesis of PEG-coated magnetite (Fe3O4) nanoparticles and their prevention of the reduction of cytochrome C. ACS Appl. Mater. Interfaces 2012, 4, 142–149. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, B.; Xie, S.; Yang, B.; Xu, Q.; Tan, J. Superparamagnetic Iron Oxide Nanoparticles Modified with Tween 80 Pass through the Intact Blood-Brain Barrier in Rats under Magnetic Field. ACS Appl. Mater. Interfaces 2016, 8, 11336–11341. [Google Scholar] [CrossRef]
- Yoon, H.M.; Kang, M.S.; Choi, G.E.; Kim, Y.J.; Bae, C.H.; Yu, Y.B.; Jeong, Y. Il Stimuli-responsive drug delivery of doxorubicin using magnetic nanoparticle conjugated poly(Ethylene glycol)-g-chitosan copolymer. Int. J. Mol. Sci. 2021, 22, 13169. [Google Scholar] [CrossRef]
- Shete, P.B.; Patil, R.M.; Tiwale, B.M.; Pawar, S.H. Water dispersible oleic acid-coated Fe3O4 nanoparticles for biomedical applications. J. Magn. Magn. Mater. 2015, 377, 406–410. [Google Scholar] [CrossRef]
- Junejo, Y.; Baykal, A.; Sözeri, H. Simple hydrothermal synthesis of Fe3O4-PEG nanocomposite. Cent. Eur. J. Chem. 2013, 11, 1527–1532. [Google Scholar] [CrossRef]
- Snoderly, H.T.; Freshwater, K.A.; Martinez de la Torre, C.; Panchal, D.M.; Vito, J.N.; Bennewitz, M.F. PEGylation of Metal Oxide Nanoparticles Modulates Neutrophil Extracellular Trap Formation. Biosensors 2022, 12, 123. [Google Scholar] [CrossRef]
- Yallapu, M.M.; Foy, S.P.; Jain, T.K.; Labhasetwar, V. PEG-functionalized magnetic nanoparticles for drug delivery and magnetic resonance imaging applications. Pharm. Res. 2010, 27, 2283–2295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahdavi, M.; Ahmad, M.B.; Haron, M.J.; Namvar, F.; Nadi, B.; Ab Rahman, M.Z.; Amin, J. Synthesis, surface modification and characterisation of biocompatible magnetic iron oxide nanoparticles for biomedical applications. Molecules 2013, 18, 7533–7548. [Google Scholar] [CrossRef] [PubMed]
- Vavaev, E.S.; Novoselova, M.; Shchelkunov, N.M.; German, S.; Aleksei, S.; Mokrousov, M.D.; Zelepukin, I.V.; Burov, A.M.; Khlebtsov, B.N.; Lyubin, E.V.; et al. CaCO3 Nanoparticles Coated with Alternating Layers of Poly-L-Arginine Hydrochloride and Fe3O4 Nanoparticles as Navigable Drug Carriers and Hyperthermia Agents. ACS Appl. Nano Mater. 2022, 5, 2994–3006. [Google Scholar] [CrossRef]
- Zelepukin, I.V.; Shipunova, V.O.; Mirkasymov, A.B.; Nikitin, P.I.; Nikitin, M.P. Synthesis and Characterization of Hybrid Core-Shell Fe3O4/SiO2 Nanoparticles for Biomedical Applications. Acta Naturae 2017, 9, 58–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayub, A.; Wettig, S. An Overview of Nanotechnologies for Drug Delivery to the Brain. Pharmaceutics 2022, 14, 224. [Google Scholar] [CrossRef] [PubMed]
- Samanta, B.; Yan, H.; Fischer, N.O.; Shi, J.; Jerry, D.J.; Rotello, V.M. Protein-passivated Fe3O4 nanoparticles: Low toxicity and rapid heating for thermal therapy. J. Mater. Chem. 2008, 18, 1204–1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bychkova, A.V.; Sorokina, O.N.; Pronkin, P.G.; Tatikolov, A.S.; Kovarski, A.L.; Rosenfeld, M.A. Protein-Coated Magnetic Nanoparticles: Creation and Investigation. Proc. Int. Conf. Nanomater. Appl. Prop. 2013, 2, 1–5. [Google Scholar]
- Sakulkhu, U.; Mahmoudi, M.; Maurizi, L.; Salaklang, J.; Hofmann, H. Protein corona composition of superparamagnetic iron oxide nanoparticles with various physico-Chemical properties and coatings. Sci. Rep. 2014, 4, 5020. [Google Scholar] [CrossRef] [Green Version]
- Chuang, V.T.G.; Maruyama, T.; Otagiri, M. Human Serum Albumin in Blood Detoxification Treatment. In Albumin in Medicine; Springer: Singapore, 2016; pp. 209–225. [Google Scholar]
- Kragh-hansen, U. Human Serum Albumin: A Multifunctional Protein. In Albumine in Medicine; Springer: Singapore, 2016; pp. 1–24. ISBN 978-981-10-2115-2. [Google Scholar]
- Fanali, G.; di Masi, A.; Trezza, V.; Marino, M.; Fasano, M.; Ascenzi, P. Human serum albumin: From bench to bedside. Mol. Aspects Med. 2012, 33, 209–290. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Tang, Q.; Yin, D.; Tang, C.; He, E.; Zou, L.; Peng, Q. The Protein Corona and its Effects on Nanoparticle-Based Drug Delivery Systems. Acta Biomater. 2021, 129, 57–72. [Google Scholar] [CrossRef]
- Mazario, E.; Forget, A.; Belkahla, H.; Lomas, J.S.; Decorse, P.; Chevillot-Biraud, A.; Verbeke, P.; Wilhelm, C.; Ammar, S.; El Hage Chahine, J.M.; et al. Functionalization of Iron Oxide Nanoparticles With HSA Protein for Thermal Therapy. IEEE Trans. Magn. 2017, 53, 1–5. [Google Scholar] [CrossRef]
- Baki, A.; Remmo, A.; Löwa, N.; Wiekhorst, F.; Bleul, R. Albumin-coated single-core iron oxide nanoparticles for enhanced molecular magnetic imaging (Mri/mpi). Int. J. Mol. Sci. 2021, 22, 6235. [Google Scholar] [CrossRef] [PubMed]
- Rahdar, S.; Rahdar, A.; Ahmadi, S.; Trant, J.F. Adsorption of bovine serum albumin (BSA) by bare magnetite nanoparticles with surface oxidative impurities that prevent aggregation. Can. J. Chem. 2019, 97, 577–583. [Google Scholar] [CrossRef]
- Aires, A.; Ocampo, S.M.; Cabrera, D.; La Cueva, L.D.; Salas, G.; Teran, F.J.; Cortajarena, A.L. BSA-coated magnetic nanoparticles for improved therapeutic properties. J. Mater. Chem. B 2015, 3, 6239–6247. [Google Scholar] [CrossRef]
- Ziegler-Borowska, M. Magnetic nanoparticles coated with aminated starch for HSA immobilization- simple and fast polymer surface functionalization. Int. J. Biol. Macromol. 2019, 136, 106–114. [Google Scholar] [CrossRef]
- Yu, S.M.; Laromaine, A.; Roig, A. Enhanced stability of superparamagnetic iron oxide nanoparticles in biological media using a pH adjusted-BSA adsorption protocol. J. Nanoparticle Res. 2014, 16, 2484. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Moragas, L.; Yu, S.M.; Carenza, E.; Laromaine, A.; Roig, A. Protective Effects of Bovine Serum Albumin on Superparamagnetic Iron Oxide Nanoparticles Evaluated in the Nematode Caenorhabditis elegans. ACS Biomater. Sci. Eng. 2015, 1, 1129–1138. [Google Scholar] [CrossRef] [Green Version]
- Moya, C.; Escudero, R.; Malaspina, D.C.; De La Mata, M.; Hernández-Saz, J.; Faraudo, J.; Roig, A. Insights into Preformed Human Serum Albumin Corona on Iron Oxide Nanoparticles: Structure, Effect of Particle Size, Impact on MRI Efficiency, and Metabolization. ACS Appl. Bio Mater. 2019, 2, 3084–3094. [Google Scholar] [CrossRef]
- Mariam, J.; Sivakami, S.; Dongre, P.M. Albumin corona on nanoparticles–a strategic approach in drug delivery. Drug Deliv. 2016, 23, 2668–2676. [Google Scholar] [CrossRef] [Green Version]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Präbst, K.; Engelhardt, H.; Ringgeler, S.; Hübner, H. Basic Colorimetric Proliferation Assays: MTT, WST, and Resazurin. In Cell Viability Assays; Methods in Molecular Biology book series; Humana Press: New York, NY, USA, 2017; pp. 1–17. [Google Scholar]
- Bondarenko, O.; Mortimer, M.; Kahru, A.; Feliu, N.; Javed, I.; Kakinen, A.; Lin, S.; Xia, T.; Song, Y.; Davis, T.P.; et al. Nanotoxicology and nanomedicine: The Yin and Yang of nano-bio interactions for the new decade. Nano Today 2021, 39, 101184. [Google Scholar] [CrossRef]
- Lin, S.; Yu, T.; Yu, Z.; Hu, X.; Yin, D. Nanomaterials Safer-by-Design: An Environmental Safety Perspective. Adv. Mater. 2018, 30, 1705691. [Google Scholar] [CrossRef] [PubMed]
- Kenchegowda, M.; Rahamathulla, M.; Hani, U.; Begum, M.Y.; Guruswamy, S.; Osmani, R.A.M.; Gowrav, M.P.; Alshehri, S.; Ghoneim, M.M.; Alshlowi, A.; et al. Smart Nanocarriers as an Emerging Platform for Cancer Therapy: A Review. Molecules 2022, 27, 146. [Google Scholar] [CrossRef]
- Vangijzegem, T.; Stanicki, D.; Laurent, S.; Vangijzegem, T. Expert Opinion on Drug Delivery Magnetic iron oxide nanoparticles for drug delivery: Applications and characteristics Magnetic iron oxide nanoparticles for drug delivery: Applications and characteristics. Expert Opin. Drug Deliv. 2019, 16, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Leach, J.C.; Wang, A.; Ye, K.; Jin, S. A RNA-DNA hybrid aptamer for nanoparticle-based prostate tumor targeted drug delivery. Int. J. Mol. Sci. 2016, 17, 380. [Google Scholar] [CrossRef] [Green Version]
- Taghavi Pourianazar, N.; Gunduz, U. CpG oligodeoxynucleotide-loaded PAMAM dendrimer-coated magnetic nanoparticles promote apoptosis in breast cancer cells. Biomed. Pharmacother. 2016, 78, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Bassetto, M.; Sen, M.; Poulhes, F.; Arango-Gonzalez, B.; Bonvin, E.; Sapet, C.; Ueffing, M.; Zelphati, O. New Method for Efficient siRNA Delivery in Retina Explants: Reverse Magnetofection. Bioconjug. Chem. 2021, 32, 1078–1093. [Google Scholar] [CrossRef]
- Laurent, N.; Sapet, C.; Gourrierec, L.L.; Bertosio, E.; Zelphati, O. Nucleic acid delivery using magnetic nanoparticles: The Magnetofection TM technology. Ther. Deliv. 2011, 2, 471–482. [Google Scholar] [CrossRef]
- Gozuacik, D.; Akkoc, Y.; Kosar, A.; Dogan-ekici, A.I.; Ekici, S. Anticancer Use of Nanoparticles as Nucleic Acid Carriers. J. Biomed. Nanotechnol. 2014, 10, 1751–1783. [Google Scholar] [CrossRef]
- Cen, C.; Wu, J.; Zhang, Y.; Luo, C.; Xie, L.; Zhang, X.; Yang, X.; Li, M.; Bi, Y.; Li, T.; et al. Improving Magnetofection of Magnetic Polyethylenimine Nanoparticles into MG-63 Osteoblasts Using a Novel Uniform Magnetic Field. Nanoscale Res. Lett. 2019, 14, 90. [Google Scholar] [CrossRef]
- Zuvin, M.; Kuruoglu, E.; Kaya, V.O.; Unal, O.; Kutlu, O.; Yagci Acar, H.; Gozuacik, D.; Kosar, A. Magnetofection of green fluorescent protein encoding DNA-bearing polyethyleneimine-coated superparamagnetic iron oxide nanoparticles to human breast cancer cells. ACS Omega 2019, 4, 12366–12374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.; Hu, Y.; Zhao, N.; Xu, F.J. Well-Defined Peapod-like Magnetic Nanoparticles and Their Controlled Modification for Effective Imaging Guided Gene Therapy. ACS Appl. Mater. Interfaces 2016, 8, 11298–11308. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.Y.; Chiang, P.H.; Hsiao, W.C.; Chuang, C.C.; Chang, C.W. Redox-Sensitive Polymer/SPIO Nanocomplexes for Efficient Magnetofection and MR Imaging of Human Cancer Cells. Langmuir 2015, 31, 6523–6531. [Google Scholar] [CrossRef] [PubMed]
- Stephen, Z.R.; Dayringer, C.J.; Lim, J.J.; Revia, R.A.; Halbert, M.V.; Jeon, M.; Bakthavatsalam, A.; Ellenbogen, R.G.; Zhang, M. Approach to Rapid Synthesis and Functionalization of Iron Oxide Nanoparticles for High Gene Transfection. ACS Appl. Mater. Interfaces 2016, 8, 6320–6328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, Y.; Li, X.; Zeljic, K.; Shan, S.; Qiu, Z.; Wang, Z. Effect of PEGylated Magnetic PLGA-PEI Nanoparticles on Primary Hippocampal Neurons: Reduced Nanoneurotoxicity and Enhanced Transfection Efficiency with Magnetofection. ACS Appl. Mater. Interfaces 2019, 11, 38190–38204. [Google Scholar] [CrossRef]
- Kievit, F.M.; Veiseh, O.; Fang, C.; Bhattarai, N.; Lee, D.; Ellenbogen, R.G.; Zhang, M. Chlorotoxin labeled magnetic nanovectors for targeted gene delivery to glioma. ACS Nano 2010, 4, 4587–4594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo, Y.L.; Chou, H.L.; Liao, Z.X.; Huang, S.J.; Ke, J.H.; Liu, Y.S.; Chiu, C.C.; Wang, L.F. Chondroitin sulfate-polyethylenimine copolymer-coated superparamagnetic iron oxide nanoparticles as an efficient magneto-gene carrier for microRNA-encoding plasmid DNA delivery. Nanoscale 2015, 7, 8554–8565. [Google Scholar] [CrossRef]
- Xie, L.; Jiang, Q.; He, Y.; Nie, Y.; Yue, D.; Gu, Z. Insight into the efficient transfection activity of a designed low aggregated magnetic polyethyleneimine/DNA complex in serum-containing medium and the application in vivo. Biomater. Sci. 2015, 3, 446–456. [Google Scholar] [CrossRef]
- Tian, G.; Zhang, X.; Gu, Z.; Zhao, Y. Recent Advances in Upconversion Nanoparticles-Based Multifunctional Nanocomposites for Combined Cancer Therapy. Adv. Mater. 2015, 27, 7692–7712. [Google Scholar] [CrossRef]
- Sritharan, S.; Sivalingam, N. A comprehensive review on time-tested anticancer drug doxorubicin. Life Sci. 2021, 278, 119527. [Google Scholar] [CrossRef]
- Christidi, E.; Brunham, L.R. Regulated cell death pathways in doxorubicin-induced cardiotoxicity. Cell Death Dis. 2021, 12, 339. [Google Scholar] [CrossRef] [PubMed]
- Popescu, R.C.; Savu, D.; Dorobantu, I.; Vasile, B.S.; Hosser, H.; Boldeiu, A.; Temelie, M.; Straticiuc, M.; Iancu, D.A.; Andronescu, E.; et al. Efficient uptake and retention of iron oxide-based nanoparticles in HeLa cells leads to an effective intracellular delivery of doxorubicin. Sci. Rep. 2020, 10, 10530. [Google Scholar] [CrossRef] [PubMed]
- Popescu, R.C.; Savu, D.I.; Bierbaum, M.; Grbenicek, A.; Schneider, F.; Hosser, H.; Vasile, B.Ș.; Andronescu, E.; Wenz, F.; Giordano, F.A.; et al. Intracellular delivery of doxorubicin by iron oxide-based nano-constructs increases clonogenic inactivation of ionizing radiation in hela cells. Int. J. Mol. Sci. 2021, 22, 6778. [Google Scholar] [CrossRef] [PubMed]
- Piehler, S.; Dähring, H.; Grandke, J.; Göring, J.; Couleaud, P.; Aires, A.; Cortajarena, A.L.; Courty, J.; Latorre, A.; Somoza, Á.; et al. Iron oxide nanoparticles as carriers for DOX and magnetic hyperthermia after intratumoral application into breast cancer in mice: Impact and future perspectives. Nanomaterials 2020, 10, 1016. [Google Scholar] [CrossRef]
- Norouzi, M.; Yathindranath, V.; Thliveris, J.A.; Kopec, B.M.; Siahaan, T.J.; Miller, D.W. Doxorubicin-loaded iron oxide nanoparticles for glioblastoma therapy: A combinational approach for enhanced delivery of nanoparticles. Sci. Rep. 2020, 10, 11292. [Google Scholar] [CrossRef]
- Khaledian, M.; Nourbakhsh, M.S.; Saber, R.; Hashemzadeh, H.; Darvishi, M.H. Preparation and evaluation of doxorubicin-loaded pla–peg–fa copolymer containing superparamagnetic iron oxide nanoparticles (Spions) for cancer treatment: Combination therapy with hyperthermia and chemotherapy. Int. J. Nanomed. 2020, 15, 6167–6182. [Google Scholar] [CrossRef]
- Shen, C.; Wang, X.; Zheng, Z.; Gao, C.; Chen, X.; Zhao, S.; Dai, Z. Doxorubicin and indocyanine green loaded superparamagnetic iron oxide nanoparticles with PEGylated phospholipid coating for magnetic resonance with fluorescence imaging and chemotherapy of glioma. Int. J. Nanomed. 2019, 14, 101–117. [Google Scholar] [CrossRef] [Green Version]
- Eslami, P.; Albino, M.; Scavone, F.; Chiellini, F.; Morelli, A.; Baldi, G.; Cappiello, L.; Doumett, S.; Lorenzi, G.; Ravagli, C.; et al. Smart Magnetic Nanocarriers for Multi-Stimuli On-Demand Drug Delivery. Nanomaterials 2022, 12, 303. [Google Scholar] [CrossRef]
- Nieciecka, D.; Celej, J.; Żuk, M.; Majkowska-pilip, A.; Żelechowska-Matysiak, K.; Lis, A.; Osial, M. Hybrid system for local drug delivery and magnetic hyperthermia based on spions loaded with doxorubicin and epirubicin. Pharmaceutics 2021, 13, 480. [Google Scholar] [CrossRef]
- Nogueira, J.; Soares, S.F.; Amorim, C.O.; Amaral, J.S.; Silva, C.; Martel, F.; Trindade, T.; Daniel-Da-Silva, A.L. Magnetic driven nanocarriers for pH-responsive doxorubicin release in cancer therapy. Molecules 2020, 25, 333. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.; Sallem, F.; Mirjolet, C.; Nury, T.; Sahoo, S.K.; Millot, N.; Kumar, R. Polydopamine modified superparamagnetic iron oxide nanoparticles as multifunctional nanocarrier for targeted prostate cancer treatment. Nanomaterials 2019, 9, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovrigina, E.; Chubarov, A.; Dmitrienko, E. High Drug Capacity Doxorubicin-Loaded Iron Oxide Nanocomposites for Cancer Therapy. Magnetochemistry 2022, 8, 54. [Google Scholar] [CrossRef]
- Al-Musawi, S.; Albukhaty, S.; Al-Karagoly, H.; Almalki, F. Design and synthesis of multi-functional superparamagnetic core-gold shell coated with chitosan and folate nanoparticles for targeted antitumor therapy. Nanomaterials 2021, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Xue, J.; Wu, S.; Pei, Y.; Xu, L.; Wang, Y. Cell-Friendly Isolation and pH-Sensitive Controllable Release of Circulating Tumor Cells by Fe3O4@CaCO3 Nanoplatform. Adv. Mater. Interfaces 2021, 8, 2101191. [Google Scholar] [CrossRef]
- Piñeiro, Y.; Gómez, M.G.; Alves, L.d.C.; Prieto, A.A.; Acevedo, P.G.; Gudiña, R.S.; Puig, J.; Teijeiro, C.; Vilar, S.Y.; Rivas, J. Hybrid nanostructured magnetite nanoparticles: From bio-detection and theragnostics to regenerative medicine. Magnetochemistry 2020, 6, 4. [Google Scholar] [CrossRef] [Green Version]
- Hassanin, I.; Elzoghby, A. Albumin-based nanoparticles: A promising strategy to overcome cancer drug resistance. Cancer Drug Resist. 2020, 3, 930–946. [Google Scholar] [CrossRef]
- Lamichhane, S.; Lee, S. Albumin nanoscience: Homing nanotechnology enabling targeted drug delivery and therapy. Arch. Pharm. Res. 2020, 43, 118–133. [Google Scholar] [CrossRef]
- Kudarha, R.R.; Sawant, K.K. Albumin based versatile multifunctional nanocarriers for cancer therapy: Fabrication, surface modification, multimodal therapeutics and imaging approaches. Mater. Sci. Eng. C 2017, 81, 607–626. [Google Scholar] [CrossRef]
- Chubarov, A.S.; Shakirov, M.M.; Koptyug, I.V.; Sagdeev, R.Z.; Knorre, D.G.; Godovikova, T.S. Synthesis and characterization of fluorinated homocysteine derivatives as potential molecular probes for 19F magnetic resonance spectroscopy and imaging. Bioorg. Med. Chem. Lett. 2011, 21, 4050–4053. [Google Scholar] [CrossRef]
- Chubarov, A.S.; Zakharova, O.D.; Koval, O.A.; Romaschenko, A.V.; Akulov, A.E.; Zavjalov, E.L.; Razumov, I.A.; Koptyug, I.V.; Knorre, D.G.; Godovikova, T.S. Design of protein homocystamides with enhanced tumor uptake properties for 19F magnetic resonance imaging. Bioorg. Med. Chem. 2015, 23, 6943–6954. [Google Scholar] [CrossRef]
- Dobrynin, S.; Kutseikin, S.; Morozov, D.; Krumkacheva, O.; Spitsyna, A.; Gatilov, Y.; Silnikov, V.; Angelovski, G.; Bowman, M.K.; Kirilyuk, I.; et al. Human Serum Albumin Labelled with Sterically-Hindered Nitroxides as Potential MRI Contrast Agents. Molecules 2020, 25, 1709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lisitskiy, V.A.; Khan, H.; Popova, T.V.; Chubarov, A.S.; Zakharova, O.D.; Akulov, A.E.; Shevelev, O.B.; Zavjalov, E.L.; Koptyug, I.V.; Moshkin, M.P.; et al. Multifunctional human serum albumin-therapeutic nucleotide conjugate with redox and pH-sensitive drug release mechanism for cancer theranostics. Bioorganic Med. Chem. Lett. 2017, 27, 3925–3930. [Google Scholar] [CrossRef] [PubMed]
- Gavilán, H.; Simeonidis, K.; Myrovali, E.; Mazarío, E.; Chubykalo-Fesenko, O.; Chantrell, R.; Balcells, L.; Angelakeris, M.; Morales, M.P.; Serantes, D. How size, shape and assembly of magnetic nanoparticles give rise to different hyperthermia scenarios. Nanoscale 2021, 13, 15631–15646. [Google Scholar] [CrossRef] [PubMed]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.J.; Chuang, E.Y.; Cheng, Y.H.; Anilkumar, T.S.; Chen, H.A.; Chen, J.P. Thermosensitive magnetic liposomes for alternating magnetic field-inducible drug delivery in dual targeted brain tumor chemotherapy. Chem. Eng. J. 2019, 373, 720–733. [Google Scholar] [CrossRef]
- Le, T.A.; Bui, M.P.; Yoon, J. Theoretical analysis for wireless magnetothermal deep brain stimulation using commercial nanoparticles. Int. J. Mol. Sci. 2019, 20, 2873. [Google Scholar] [CrossRef] [Green Version]
- Munshi, R.; Qadri, S.M.; Zhang, Q.; Rubio, I.C.; del Pino, P.; Pralle, A. Magnetothermal genetic deep brain stimulation of motor behaviors in awake, freely moving mice. eLife 2017, 6, e27069. [Google Scholar] [CrossRef]
- Mleczko, J.; Defort, A.; Kozioł, J.J.; Nguyen, T.T.; Mirończyk, A.; Zapotoczny, B.; Nowak-Jary, J.; Gronczewska, E.; Marć, M.; Dudek, M.R. Limitation of tuning the antibody-antigen reaction by changing the value of pH and its consequence for hyperthermia. J. Biochem. 2016, 159, 421–427. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Liu, J.; Cui, X.; Wang, X.; Zhang, L.; Tang, P. Recent Advances on Magnetic Sensitive Hydrogels in Tissue Engineering. Front. Chem. 2020, 8, 124. [Google Scholar] [CrossRef]
- Peng, J.; Zhao, J.; Long, Y.; Xie, Y.; Nie, J.; Chen, L. Magnetic Materials in Promoting Bone Regeneration. Front. Mater. 2019, 6, 268. [Google Scholar] [CrossRef]
- Yun, H.M.; Ahn, S.J.; Park, K.R.; Kim, M.J.; Kim, J.J.; Jin, G.Z.; Kim, H.W.; Kim, E.C. Magnetic nanocomposite scaffolds combined with static magnetic field in the stimulation of osteoblastic differentiation and bone formation. Biomaterials 2016, 85, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.S.; Chu, I.M. Injectable polypeptide hydrogel/inorganic nanoparticle composites for bone tissue engineering. PLoS ONE 2019, 14, e0210285. [Google Scholar] [CrossRef] [PubMed]
- Shuai, C.; Yang, W.; He, C.; Peng, S.; Gao, C.; Yang, Y.; Qi, F.; Feng, P. A magnetic micro-environment in scaffolds for stimulating bone regeneration. Mater. Des. 2020, 185, 108275. [Google Scholar] [CrossRef]
- Pesqueira, T.; Costa-Almeida, R.; Mithieux, S.M.; Babo, P.S.; Franco, A.R.; Mendes, B.B.; Domingues, R.M.A.; Freitas, P.; Reis, R.L.; Gomes, M.E.; et al. Engineering magnetically responsive tropoelastin spongy-like hydrogels for soft tissue regeneration. J. Mater. Chem. B 2018, 6, 1066–1075. [Google Scholar] [CrossRef]
- Silva, E.D.; Babo, P.S.; Costa-Almeida, R.; Domingues, R.M.A.; Mendes, B.B.; Paz, E.; Freitas, P.; Rodrigues, M.T.; Granja, P.L.; Gomes, M.E. Multifunctional magnetic-responsive hydrogels to engineer tendon-to-bone interface. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2375–2385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, L. Magnetically Actuated Biomaterials and Prospects in Tendon Healing. Nanomedicine 2016, 11, 1107–1122. [Google Scholar] [CrossRef] [PubMed]
- Betsch, M.; Cristian, C.; Lin, Y.Y.; Blaeser, A.; Schöneberg, J.; Vogt, M.; Buhl, E.M.; Fischer, H.; Duarte Campos, D.F. Incorporating 4D into Bioprinting: Real-Time Magnetically Directed Collagen Fiber Alignment for Generating Complex Multilayered Tissues. Adv. Healthc. Mater. 2018, 7, 1800894. [Google Scholar] [CrossRef] [PubMed]
- Bonhome-Espinosa, A.B.; Campos, F.; Durand-Herrera, D.; Sánchez-López, J.D.; Schaub, S.; Durán, J.D.G.; Lopez-Lopez, M.T.; Carriel, V. In vitro characterization of a novel magnetic fibrin-agarose hydrogel for cartilage tissue engineering. J. Mech. Behav. Biomed. Mater. 2020, 104, 103619. [Google Scholar] [CrossRef]
- Bianchi, E.; Vigani, B.; Viseras, C.; Ferrari, F.; Rossi, S.; Sandri, G. Inorganic Nanomaterials in Tissue Engineering. Pharmaceutics 2022, 14, 1127. [Google Scholar] [CrossRef]
- Funnell, J.L.; Balouch, B.; Gilbert, R.J. Magnetic composite biomaterials for neural regeneration. Front. Bioeng. Biotechnol. 2019, 7, 179. [Google Scholar] [CrossRef]
- Johnson, C.D.L.; Ganguly, D.; Zuidema, J.M.; Cardinal, T.J.; Ziemba, A.M.; Kearns, K.R.; McCarthy, S.M.; Thompson, D.M.; Ramanath, G.; Borca-Tasciuc, D.A.; et al. Injectable, Magnetically Orienting Electrospun Fiber Conduits for Neuron Guidance. ACS Appl. Mater. Interfaces 2019, 11, 356–372. [Google Scholar] [CrossRef] [PubMed]
- Tay, A.; Sohrabi, A.; Poole, K.; Seidlits, S.; Di Carlo, D. A 3D Magnetic Hyaluronic Acid Hydrogel for Magnetomechanical Neuromodulation of Primary Dorsal Root Ganglion Neurons. Adv. Mater. 2018, 30, 1800927. [Google Scholar] [CrossRef]
- Rose, J.C.; Cámara-Torres, M.; Rahimi, K.; Köhler, J.; Möller, M.; De Laporte, L. Nerve Cells Decide to Orient inside an Injectable Hydrogel with Minimal Structural Guidance. Nano Lett. 2017, 17, 3782–3791. [Google Scholar] [CrossRef] [PubMed]
- Pavón, J.J.; Allain, J.P.; Verma, D.; Echeverry-Rendón, M.; Cooper, C.L.; Reece, L.M.; Shetty, A.R.; Tomar, V. In situ Study Unravels Bio-Nanomechanical Behavior in a Magnetic Bacterial Nano-cellulose (MBNC) Hydrogel for Neuro-Endovascular Reconstruction. Macromol. Biosci. 2019, 19, 1800225. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Wang, Y.; Qin, Y.X. Promoting neuroregeneration by applying dynamic magnetic fields to a novel nanomedicine: Superparamagnetic iron oxide (SPIO)-gold nanoparticles bounded with nerve growth factor (NGF). Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1337–1347. [Google Scholar] [CrossRef]
- Pal, A.; Kumar, S.; Jain, S.; Nag, T.C.; Mathur, R. Neuroregenerative Effects of Electromagnetic Field and Magnetic Nanoparticles on Spinal Cord Injury in Rats. J. Nanosci. Nanotechnol. 2018, 18, 6756–6764. [Google Scholar] [CrossRef]
- Liu, T.; Wang, Y.; Lu, L.; Liu, Y. SPIONs mediated magnetic actuation promotes nerve regeneration by inducing and maintaining repair-supportive phenotypes in Schwann cells. J. Nanobiotechnol. 2022, 20, 159. [Google Scholar] [CrossRef]
- Wallyn, J.; Anton, N.; Vandamme, T.F. Synthesis, principles, and properties of magnetite nanoparticles for in vivo imaging applications—A review. Pharmaceutics 2019, 11, 601. [Google Scholar] [CrossRef] [Green Version]
- Ellis, C.M.; Pellico, J.; Davis, J.J. Magnetic Nanoparticles Supporting Bio-responsive T1/T2 Magnetic Resonance Imaging. Materials 2019, 12, 4096. [Google Scholar] [CrossRef] [Green Version]
- Bruno, F.; Granata, V.; Bellisari, F.C.; Sgalambro, F.; Tommasino, E.; Palumbo, P.; Arrigoni, F.; Cozzi, D.; Grassi, F.; Brunese, M.C.; et al. Advanced Magnetic Resonance Imaging (MRI) Techniques: Technical Principles and Applications in Nanomedicine. Cancers 2022, 14, 1626. [Google Scholar] [CrossRef]
- Xie, J.; Chen, K.; Huang, J.; Lee, S.; Wang, J.; Gao, J.; Li, X.; Chen, X. PET/NIRF/MRI triple functional iron oxide nanoparticles. Biomaterials 2010, 31, 3016–3022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Tu, M.; Tian, B.; Yi, Y.; Wei, Z.Z.; Wei, F. Synthesis of tumor-targeted folate conjugated fluorescent magnetic albumin nanoparticles for enhanced intracellular dual-modal imaging into human brain tumor cells. Anal. Biochem. 2016, 512, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Bowers, A.N.; Trujillo-Rodríguez, M.J.; Farooq, M.Q.; Anderson, J.L. Extraction of DNA with magnetic ionic liquids using in situ dispersive liquid–liquid microextraction. Anal. Bioanal. Chem. 2019, 411, 7375–7385. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Chen, T.; Iqbal, M.Z.; Yang, F.; Hampp, N.; Wu, A.; Luo, L. Applications of magnetic materials separation in biological nanomedicine. Electrophoresis 2019, 40, 2011–2028. [Google Scholar] [CrossRef]
- Marengo, A.; Cagliero, C.; Sgorbini, B.; Anderson, J.L.; Emaus, M.N.; Bicchi, C.; Bertea, C.M.; Rubiolo, P. Development of an innovative and sustainable one-step method for rapid plant DNA isolation for targeted PCR using magnetic ionic liquids. Plant Methods 2019, 15, 23. [Google Scholar] [CrossRef]
- Wang, L.; He, K.; Sadak, O.; Wang, X.; Wang, Q.; Xu, X. Visual detection of in vitro nucleic acid replication by submicro- and nano-sized materials. Biosens. Bioelectron. 2020, 169, 112602. [Google Scholar] [CrossRef]
- Sosa-Acosta, J.R.; Iriarte-Mesa, C.; Ortega, G.A.; Díaz-García, A.M. DNA–Iron Oxide Nanoparticles Conjugates: Functional Magnetic Nanoplatforms in Biomedical Applications. Top. Curr. Chem. 2020, 378, 13. [Google Scholar] [CrossRef]
- Gessner, I.; Fries, J.W.U.; Brune, V.; Mathur, S. Magnetic nanoparticle-based amplification of microRNA detection in body fluids for early disease diagnosis. J. Mater. Chem. B 2021, 9, 9–22. [Google Scholar] [CrossRef]
- Sosa-Acosta, J.R.; Silva, J.A.; Fernández-Izquierdo, L.; Díaz-Castañón, S.; Ortiz, M.; Zuaznabar-Gardona, J.C.; Díaz-García, A.M. Iron Oxide Nanoparticles (IONPs) with potential applications in plasmid DNA isolation. Colloids Surf. A Physicochem. Eng. Asp. 2018, 545, 167–178. [Google Scholar] [CrossRef]
- Vanyorek, L.; Ilosvai, Á.M.; Szőri-Dorogházi, E.; Váradi, C.; Kristály, F.; Prekob, Á.; Fiser, B.; Varga, T.; Kónya, Z.; Viskolcz, B. Synthesis of iron oxide nanoparticles for DNA purification. J. Dispers. Sci. Technol. 2021, 42, 693–700. [Google Scholar] [CrossRef]
- Wang, J.; Ali, Z.; Si, J.; Wang, N.; He, N.; Li, Z. Simultaneous extraction of DNA and RNA from hepatocellular carcinoma (Hep G2) based on silica-coated magnetic nanoparticles. J. Nanosci. Nanotechnol. 2017, 17, 802–806. [Google Scholar] [CrossRef] [PubMed]
- Danthanarayana, A.N.; Manatunga, D.C.; De Silva, R.M.; Chandrasekharan, N.V.; De Silva, K.M.N. Magnetofection and isolation of DNA using polyethyleneimine functionalized magnetic iron oxide nanoparticles. R. Soc. Open Sci. 2018, 5, 181369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chacón-Torres, J.C.; Reinoso, C.; Navas-León, D.G.; Briceño, S.; González, G. Optimized and scalable synthesis of magnetic nanoparticles for RNA extraction in response to developing countries’ needs in the detection and control of SARS-CoV-2. Sci. Rep. 2020, 10, 19004. [Google Scholar] [CrossRef]
- Ali, T.H.; Mandal, A.M.; Heidelberg, T.; Hussen, R.S.D. Sugar based cationic magnetic core–shell silica nanoparticles for nucleic acid extraction. RSC Adv. 2022, 12, 13566–13579. [Google Scholar] [CrossRef]
- Bulgakova, A.; Chubarov, A.; Dmitrienko, E. Magnetic Nylon 6 Nanocomposites for the Microextraction of Nucleic Acids from Biological Samples. Magnetochemistry 2022, 8, 85. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Bahreinizad, H.; Amiri, Z.; Aliabadi, H.A.M.; Salimi-Bani, M.; Nakisa, A.; Davoodi, F.; Tahmasebi, B.; Ahmadpour, F.; Radinekiyan, F.; et al. Functionalized magnetic nanoparticles for the separation and purification of proteins and peptides. TrAC-Trends Anal. Chem. 2021, 141, 116291. [Google Scholar] [CrossRef]
- Damavandi, F.; Wang, W.; Shen, W.Z.; Cetinel, S.; Jordan, T.; Jovel, J.; Montemagno, C.; Wong, G.K.S. Enrichment of low abundance DNA/RNA by oligonucleotide-clicked iron oxide nanoparticles. Sci. Rep. 2021, 11, 13053. [Google Scholar] [CrossRef]
- Jiang, S.; Hua, L.; Guo, Z.; Sun, L. One-pot green synthesis of doxorubicin loaded-silica nanoparticles for in vivo cancer therapy. Mater. Sci. Eng. C 2018, 90, 257–263. [Google Scholar] [CrossRef]
- Pinchon, E.; Leon, F.; Temurok, N.; Morvan, F.; Vasseur, J.J.; Clot, M.; Foulongne, V.; Cantaloube, J.F.; Perre, P.V.; Daynès, A.; et al. Rapid and specific DNA detection by magnetic field-enhanced agglutination assay. Talanta 2020, 219, 121344. [Google Scholar] [CrossRef]
- Yildiz, I. Applications of magnetic nanoparticles in biomedical separation and purification. Nanotechnol. Rev. 2016, 5, 331–340. [Google Scholar] [CrossRef]
- Haun, J.B.; Yoon, T.J.; Lee, H.; Weissleder, R. Magnetic nanoparticle biosensors. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 291–304. [Google Scholar] [CrossRef]
- Koh, I.; Josephson, L. Magnetic Nanoparticle Sensors. Sensors 2009, 9, 8130–8145. [Google Scholar] [CrossRef]
- Rocha-Santos, T.A.P. Sensors and biosensors based on magnetic nanoparticles. TrAC-Trends Anal. Chem. 2014, 62, 28–36. [Google Scholar] [CrossRef]
- Sayad, A.; Skafidas, E.; Kwan, P. Magneto-impedance biosensor sensitivity: Effect and enhancement. Sensors 2020, 20, 5213. [Google Scholar] [CrossRef]
- Magnetic, B.E.; Selective, N.E.; Morpholin--yl, A.; Evelin, S. A Convenient U-Shape Microreactor for Continuous Flow. Catalyst 2022, 12, 1065. [Google Scholar]
- Ender, F.; Weiser, D.; Poppe, L. Microfluidic Multiple Chamber Chip Reactor Filled with Enzyme-Coated Magnetic Nanoparticles. In Lab-on-a-Chip Fabrication and Application; IntechOpen: London, UK, 2016. [Google Scholar]
- Digigow, R.G.; Dechézelles, J.F.; Kaufmann, J.; Vanhecke, D.; Knapp, H.; Lattuada, M.; Rothen-Rutishauser, B.; Petri-Fink, A. Magnetic microreactors for efficient and reliable magnetic nanoparticle surface functionalization. Lab Chip 2014, 14, 2276–2286. [Google Scholar] [CrossRef] [Green Version]
- Gkantzou, E.; Patila, M.; Stamatis, H. Magnetic microreactors with immobilized enzymes-From assemblage to contemporary applications. Catalysts 2018, 8, 282. [Google Scholar] [CrossRef] [Green Version]
- Peñaranda, P.A.; Noguera, M.J.; Florez, S.L.; Husserl, J.; Ornelas-Soto, N.; Cruz, J.C.; Osma, J.F. Treatment of Wastewater, Phenols and Dyes Using Novel Magnetic Torus Microreactors and Laccase Immobilized on Magnetite Nanoparticles. Nanomaterials 2022, 12, 1688. [Google Scholar] [CrossRef]
- Baki, A.; Wiekhorst, F.; Bleul, R. Advances in magnetic nanoparticles engineering for biomedical applications—A review. Bioengineering 2021, 8, 134. [Google Scholar] [CrossRef]
- Abedini-nassab, R.; Pouryosef Miandoab, M.; Şaşmaz, M. Microfluidic synthesis, control, and sensing of magnetic nanoparticles: A review. Micromachines 2021, 12, 768. [Google Scholar] [CrossRef]
- Mariño, M.A.; Fulaz, S.; Tasic, L. Magnetic nanomaterials as biocatalyst carriers for biomass processing: Immobilization strategies, reusability, and applications. Magnetochemistry 2021, 7, 133. [Google Scholar] [CrossRef]







| Methods | Procedure | Conditions | Temperature, Time | MNPs Size and Yield * | |
|---|---|---|---|---|---|
| Chemical | Co-precipitation | Very simple | Ambient | 20–150 °C, min | Relatively narrow, High |
| Hydro/Solvothermal | Simple | High pressure | 150–250 °C, h/day | Very narrow, High | |
| Sonochemichal | Very simple | Ambient | 20–50 °C, min | Narrow, Medium | |
| Emulsion | Complicated | Ambient | 20–80 °C, h | Narrow, Low | |
| Thermal decomposition | Very simple | High temperature | 250–400 °C, h | Very narrow, High | |
| Sol-hel | Simple | High temperature | 300–500 °C, 3–4 h | Very narrow, High | |
| Wet Reduction | Very simple | Ambient | 20–150 °C, min | Relatively narrow, High | |
| Electrochemical | Complicated | Ambient | 25 °C, min/h | Narrow, High | |
| Polyol Synthesis | Simple | High temperature | 200–350 °C, 7–10 h | Relatively narrow, High | |
| Physical | Gas-phase deposition | Simple | High temperature | 150–250 °C, h | Narrow, Medium |
| Ball milling | Very simple | Power ball/Ambient | 25 °C, h/day | Highly broad, Medium | |
| Spattering | Very simple | Ambient | 25 °C, min/h | Broad, High | |
| Laser ablation | Simple | Ambient | 25 °C, min/h | Broad, High | |
| Electron beam deposition | Simple | Ambient | 25 °C, min/h | Broad, Medium | |
| Aerosol spray pyrolysis | Simple | High temperature | 300–500 °C, h | Broad, Medium | |
| Biological | Microorganism and virus mediated | Complicated | Ambient | 25 °C, h/day | Broad, Medium |
| Template-mediated | Simple | Ambient | 25 °C, min/h | Relatively narrow, High | |
| Plant-mediated | Complicated | Ambient | 25 °C, h/day | Broad, Low | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrov, K.D.; Chubarov, A.S. Magnetite Nanoparticles for Biomedical Applications. Encyclopedia 2022, 2, 1811-1828. https://doi.org/10.3390/encyclopedia2040125
Petrov KD, Chubarov AS. Magnetite Nanoparticles for Biomedical Applications. Encyclopedia. 2022; 2(4):1811-1828. https://doi.org/10.3390/encyclopedia2040125
Chicago/Turabian StylePetrov, Kirill D., and Alexey S. Chubarov. 2022. "Magnetite Nanoparticles for Biomedical Applications" Encyclopedia 2, no. 4: 1811-1828. https://doi.org/10.3390/encyclopedia2040125
APA StylePetrov, K. D., & Chubarov, A. S. (2022). Magnetite Nanoparticles for Biomedical Applications. Encyclopedia, 2(4), 1811-1828. https://doi.org/10.3390/encyclopedia2040125