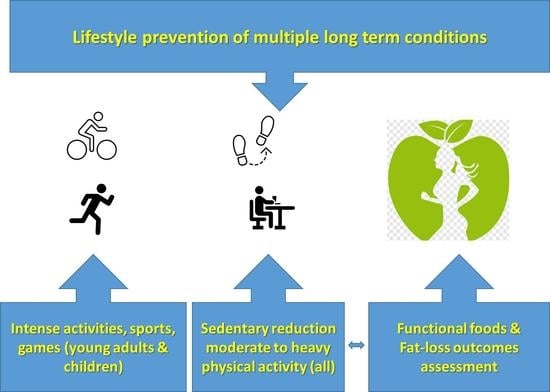
Optimizing Lifestyle Behaviors in Preventing Multiple Long-Term Conditions
Definition
1. Introduction
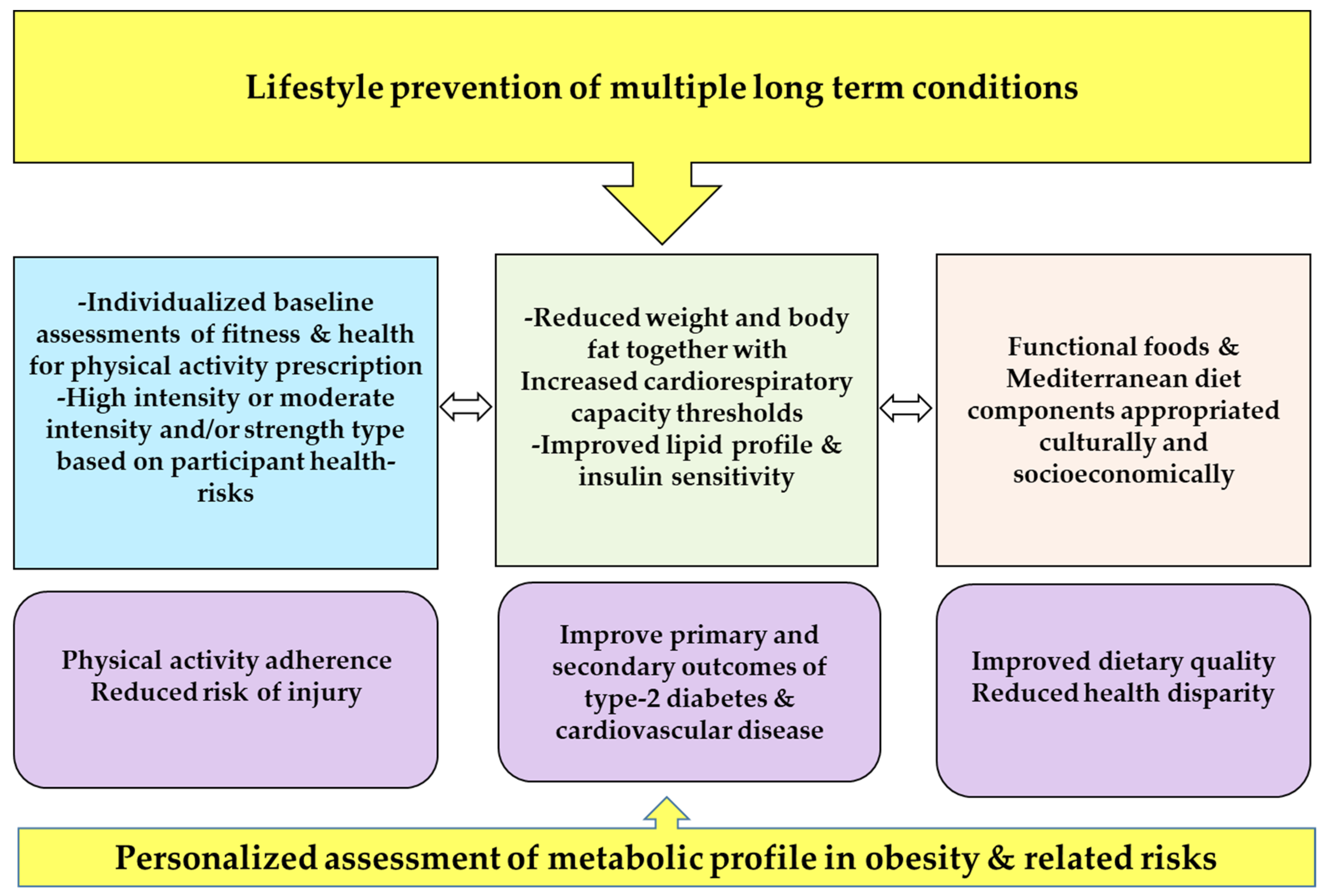
2. Optimization Components of Lifestyle Interventions for Multiple Long-Term Conditions
2.1. Simplifying Exercise Prescription for Multiple Long-Term Conditions
2.2. Addressing Gaps in Prescribing Physical Activity High-Intensity Training
2.3. Optimizing Prevention of Multiple Conditions through Targeting Sedentary Behavior
2.4. Addressing Weight and Fat Loss Prescription
3. Conclusions and Prospects
Funding
Data Availability Statement
Conflicts of Interest
References
- International Diabetes Federation. Diabetes Atlas, 7th ed.; 2015. Available online: http://www.diabetesatlas.org/ (accessed on 1 April 2019).
- Rokas, N.; Vesna-Kerstin, P.; Andrea, F.; Martin, S. Multimorbidity: What do we know? What should we do? J. Comorbidity 2016, 6, 4–11. [Google Scholar]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M.; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [PubMed]
- Tuomilehto, J.; Lindström, J.; Eriksson, J.G.; Valle, T.T.; Hämäläinen, H.; Ilanne-Parikka, P.; Keinänen-Kiukaanniemi, S.; Laakso, M.; Louheranta, A.; Rastas, M.; et al. Prevention of Type 2 Diabetes Mellitus by Changes in Lifestyle among Subjects with Impaired Glucose Tolerance. N. Engl. J. Med. 2001, 344, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, A.; Snehalatha, C.; Mary, S.; Mukesh, B.; Bhaskar, A.D.; Vijay, V.; Indian Diabetes Prevention Programme (IDPP). The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 2006, 49, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.-R.; Li, G.-W.; Hu, Y.-H.; Wang, J.-X.; Yang, W.-Y.; An, Z.-X.; Hu, Z.-X.; Lin, J.; Xiao, J.-Z.; Cao, H.-B.; et al. Effects of Diet and Exercise in Preventing NIDDM in People With Impaired Glucose Tolerance: The Da Qing IGT and Diabetes Study. Diabetes Care 1997, 20, 537–544. [Google Scholar] [CrossRef]
- Herman, W.H. The cost-effectiveness of diabetes prevention: Results from the Diabetes Prevention Program and the Diabetes Prevention Program Outcomes Study. Clin. Diabetes Endocrinol. 2015, 1, 9. [Google Scholar] [CrossRef]
- Alkhatib, A. Personalising Exercise and Nutrition Behaviours in Diabetes Lifestyle Prevention. Eur. Med. J. 2020, 5, 67–77. [Google Scholar] [CrossRef]
- Esposito, K.; Maiorino, M.I.; Petrizzo, M.; Bellastella, G.; Giugliano, D. The Effects of a Mediterranean Diet on the Need for Diabetes Drugs and Remission of Newly Diagnosed Type 2 Diabetes: Follow-up of a Randomized Trial. Diabetes Care 2014, 37, 1824–1830. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef]
- Salas-Salvadó, J.; Bulló, M.; Estruch, R.; Ros, E.; Covas, M.-I.; Ibarrola-Jurado, N.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; et al. Prevention of Diabetes With Mediterranean Diets. Ann. Intern. Med. 2014, 160, 1–10. [Google Scholar] [CrossRef]
- Tuomilehto, J.; Schwarz, P.E. Preventing Diabetes: Early Versus Late Preventive Interventions. Diabetes Care 2016, 39, S115–S120. [Google Scholar] [CrossRef] [PubMed]
- Alkhatib, A.; Nnyanzi, L.A.; Mujuni, B.; Amanya, G.; Ibingira, C. Preventing Multimorbidity with Lifestyle Interventions in Sub-Saharan Africa: A New Challenge for Public Health in Low and Middle-Income Countries. Int. J. Environ. Res. Public Health 2021, 18, 12449. [Google Scholar] [CrossRef] [PubMed]
- Obita, G.; Alkhatib, A. Disparities in the Prevalence of Childhood Obesity-Related Comorbidities: A Systematic Review. Front. Public Health 2022, 10, 923744. [Google Scholar] [CrossRef]
- Adu, M.D.; Malabu, U.H.; Malau-Aduli, A.E.O.; Malau-Aduli, B.S. Enablers and barriers to effective diabetes self-management: A multi-national investigation. PLoS ONE 2019, 14, e0217771. [Google Scholar] [CrossRef] [PubMed]
- Public Health England. A Systematic Review and Metaanalysis Assessing the Effectiveness of Pragmatic Lifestyle Interventions for the Prevention of type 2 Diabetes Mellitus in Routine Practice. 2015. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/733053/PHE_Evidence_Review_of_diabetes_prevention_programmes-_FINAL.pdf (accessed on 1 June 2019).
- Dunkley, A.J.; Bodicoat, D.H.; Greaves, C.J.; Russell, C.; Yates, T.; Davies, M.J.; Khunti, K. Diabetes Prevention in the Real World: Effectiveness of Pragmatic Lifestyle Interventions for the Prevention of Type 2 Diabetes and of the Impact of Adherence to Guideline Recommendations: A systematic review and meta-analysis. Diabetes Care 2014, 37, 922–933. [Google Scholar] [CrossRef] [PubMed]
- Slentz, C.A.; Bateman, L.A.; Willis, L.H.; Granville, E.O.; Piner, L.W.; Samsa, G.P.; Setji, T.L.; Muehlbauer, M.J.; Huffman, K.M.; Bales, C.W.; et al. Effects of exercise training alone vs a combined exercise and nutritional lifestyle intervention on glucose homeostasis in prediabetic individuals: A randomised controlled trial. Diabetologia 2016, 59, 2088–2098. [Google Scholar] [CrossRef] [PubMed]
- Zimmet, P.; Alberti, K.G.M.M.; Stern, N.; Bilu, C.; El-Osta, A.; Einat, H.; Kronfeld-Schor, N. The Circadian Syndrome: Is the Metabolic Syndrome and much more! J. Intern. Med. 2019, 286, 181–191. [Google Scholar] [CrossRef]
- Lamb, M.J.E.; Westgate, K.; Brage, S.; Ekelund, U.; Long, G.H.; Griffin, S.J.; Simmons, R.K.; Cooper, A.J.M.; ADDITION-Plus study team. Prospective associations between sedentary time, physical activity, fitness and cardiometabolic risk factors in people with type 2 diabetes. Diabetologia 2016, 59, 110–120. [Google Scholar] [CrossRef]
- The Look AHEAD Research Group; Wing, R.R.; Bolin, P.; Brancati, F.L.; Bray, G.A.; Clark, J.M.; Coday, M.; Crow, R.S.; Curtis, J.M.; Egan, C.M.; et al. Cardiovascular Effects of Intensive Lifestyle Intervention in Type 2 Diabetes. N. Engl. J. Med. 2013, 369, 145–154. [Google Scholar] [CrossRef]
- Bancks, M.P.; Chen, H.; Balasubramanyam, A.; Bertoni, A.G.; Espeland, M.A.; Kahn, S.E.; Pilla, S.; Vaughan, E.; Wagenknecht, L.E. The Look AHEAD Research Group Type 2 Diabetes Subgroups, Risk for Complications, and Differential Effects Due to an Intensive Lifestyle Intervention. Diabetes Care 2021, 44, 1203–1210. [Google Scholar] [CrossRef]
- Colberg, S.R.; Sigal, R.J.; Yardley, J.E.; Riddell, M.C.; Dunstan, D.W.; Dempsey, P.C.; Horton, E.S.; Castorino, K.; Tate, D.F. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care 2016, 39, 2065–2079. [Google Scholar] [CrossRef] [PubMed]
- World Health organization (WHO). Global Report on Physical Activity. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/physical-activity (accessed on 31 January 2023).
- Diabetes UK, Living with Diabetes, High-Intensity Interval Training. Available online: https://www.diabetes.co.uk/high-intensity-interval-training.html (accessed on 1 April 2019).
- Little, J.P.; Gillen, J.B.; Percival, M.E.; Safdar, A.; Tarnopolsky, M.A.; Punthakee, Z.; Jung, M.E.; Gibala, M.J. Low-volume high-intensity interval training reduces hyperglycemia and increases muscle mitochondrial capacity in patients with type 2 diabetes. J. Appl. Physiol. 2011, 111, 1554–1560. [Google Scholar] [CrossRef] [PubMed]
- Shaban, N.; Kenno, K.A.; Milne, K.J. The effects of a 2 week modified high intensity interval training program on the homeostatic model of insulin resistance (HOMA-IR) in adults with type 2 diabetes. J. Sports Med. Phys. Fit. 2014, 54, 203–209. [Google Scholar]
- Laursen, P.B.; Jenkins, D.G. The scientific basis for high-intensity interval training: Optimising training programmes and maximising performance in highly trained endurance athletes. Sports Med. 2002, 32, 53–73. [Google Scholar] [CrossRef]
- Daniels, J.; Scardina, N. Interval Training and Performance. Sports Med. 1984, 1, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Gibala, M.J.; McGee, S. Metabolic Adaptations to Short-term High-Intensity Interval Training: A little pain for a lot of gain? Exerc. Sport Sci. Rev. 2008, 36, 58–63. [Google Scholar] [CrossRef]
- Trapp, E.G.; Chisholm, D.J.; Freund, J.; Boutcher, S.H. The effects of high-intensity intermittent exercise training on fat loss and fasting insulin levels of young women. Int. J. Obes. 2008, 32, 684–691. [Google Scholar] [CrossRef]
- Francois, M.E.; Little, J.P. Effectiveness and Safety of High-Intensity Interval Training in Patients With Type 2 Diabetes. Diabetes Spectr. 2015, 28, 39–44. [Google Scholar] [CrossRef]
- Savikj, M.; Gabriel, B.M.; Alm, P.S.; Smith, J.; Caidahl, K.; Björnholm, M.; Fritz, T.; Krook, A.; Zierath, J.R.; Wallberg-Henriksson, H. Afternoon exercise is more efficacious than morning exercise at improving blood glucose levels in individuals with type 2 diabetes: A randomised crossover trial. Diabetologia 2019, 62, 233–237. [Google Scholar] [CrossRef]
- Gillen, J.B.; Percival, M.E.; Ludzki, A.; Tarnopolsky, M.A.; Gibala, M.J. Interval training in the fed or fasted state improves body composition and muscle oxidative capacity in overweight women. Obesity 2013, 21, 2249–2255. [Google Scholar] [CrossRef]
- Alkhatib, A.; Hsieh, M.-J.; Kuo, C.-H.; Hou, C.-W. Caffeine Optimizes HIIT Benefits on Obesity-associated Metabolic Adversity in Women. Med. Sci. Sports Exerc. 2020, 52, 1793–1800. [Google Scholar] [CrossRef]
- Biddle, S.J.; Batterham, A.M. High-intensity interval exercise training for public health: A big HIT or shall we HIT it on the head? Int. J. Behav. Nutr. Phys. Act. 2015, 12, 95. [Google Scholar] [CrossRef]
- Neto, M.G.; Durães, A.R.; Conceição, L.S.R.; Saquetto, M.B.; Ellingsen, Ø.; Carvalho, V. High intensity interval training versus moderate intensity continuous training on exercise capacity and quality of life in patients with heart failure with reduced ejection fraction: A systematic review and meta-analysis. Int. J. Cardiol. 2018, 261, 134–141. [Google Scholar] [CrossRef]
- Lunt, H.; Draper, N.; Marshall, H.C.; Logan, F.J.; Hamlin, M.J.; Shearman, J.P.; Cotter, J.D.; Kimber, N.E.; Blackwell, G.; Frampton, C.M.A. High Intensity Interval Training in a Real World Setting: A Randomized Controlled Feasibility Study in Overweight Inactive Adults, Measuring Change in Maximal Oxygen Uptake. PLoS ONE 2014, 9, e83256. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.M.; Dasari, S.; Konopka, A.R.; Johnson, M.L.; Manjunatha, S.; Esponda, R.R.; Carter, R.E.; Lanza, I.R.; Nair, K.S. Enhanced protein translation underlies improved metabolic and physical adaptations to different exercise training modes in young and old humans. Cell Metab. 2017, 25, 581–592. [Google Scholar] [CrossRef]
- Hill, A.V.; Furusawa, K.; Long, C.N.H.; Lupton, H. Muscular exercise, lactic acid and the supply and utilisation of oxygen. Parts VII–VIII. Proc. R. Soc. London. Ser. B Boil. Sci. 1924, 97, 155–176. [Google Scholar] [CrossRef]
- Sluik, D.; Buijsse, B.; Muckelbauer, R.; Kaaks, R.; Teucher, B.; Johnsen, N.F.; Tjønneland, A.; Overvad, K.; Østergaard, J.N.; Amiano, P.; et al. Physical Activity and Mortality in Individuals With Diabetes Mellitus: A Prospective Study and Meta-analysis. Arch Intern. Med. 2012, 172, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Patterson, R.; McNamara, E.; Tainio, M.; De Sá, T.H.; Smith, A.D.; Sharp, S.J.; Edwards, P.; Woodcock, J.; Brage, S.; Wijndaele, K. Sedentary behaviour and risk of all-cause, cardiovascular and cancer mortality, and incident type 2 diabetes: A systematic review and dose response meta-analysis. Eur. J. Epidemiol. 2018, 33, 811–829. [Google Scholar] [CrossRef]
- Alkhatib, A. Sedentary Risk Factors across Genders and Job Roles within a University Campus Workplace: Preliminary Study. J. Occup. Health 2013, 55, 218–224. [Google Scholar] [CrossRef]
- Alkhatib, A. High prevalence of sedentary risk factors amongst university employees and potential health benefits of campus workplace exercise intervention. Work 2015, 52, 589–595. [Google Scholar] [CrossRef]
- Kneffel, Z.; Goebel, R.; Alkhatib, A. Cardiovascular Risk Factors and their Responses to a 10 Weeks Training Program in Young Qatari Adults. Obes. Res. Open J. 2015, 2, 57–63. [Google Scholar] [CrossRef]
- Wang, P.-Y.; Fang, J.-C.; Gao, Z.-H.; Zhang, C.; Xie, S.-Y. Higher intake of fruits, vegetables or their fiber reduces the risk of type 2 diabetes: A meta-analysis. J. Diabetes Investig. 2016, 7, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Fritschi, C.; Park, H.; Richardson, A.; Park, C.; Collins, E.G.; Mermelstein, R.; Riesche, L.; Quinn, L. Association Between Daily Time Spent in Sedentary Behavior and Duration of Hyperglycemia in Type 2 Diabetes. Biol. Res. Nurs. 2016, 18, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Alkhatib, A.; Klonizakis, M. Effects of Exercise Training and Mediterranean Diet on Reducing Post-Menopausal Vascular Risk. Clin. Hemorheol. Microcirc. 2014, 57, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Alkhatib, A.; Tsang, C.; Tiss, A.; Bahorun, T.; Arefanian, H.; Barake, R.; Khadir, A.; Tuomilehto, J. Functional Foods and Lifestyle Approaches for Diabetes Prevention and Management. Nutrients 2017, 9, 1310. [Google Scholar] [CrossRef] [PubMed]
- Jeukendrup, A.E.; Wallis, G.A. Measurement of Substrate Oxidation During Exercise by Means of Gas Exchange Measurements. Int. J. Sports Med. 2005, 26, S28–S37. [Google Scholar] [CrossRef]
- Fernández-Verdejo, R.; Bajpeyi, S.; Ravussin, E.; Galgani, J.E. Metabolic flexibility to lipid availability during exercise is enhanced in individuals with high insulin sensitivity. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E715–E722. [Google Scholar] [CrossRef]
- Alkhatib, A. Maximal Fat Metabolism Explained by Lactate-Carbohydrate Model. Physiologia 2022, 2, 121–131. [Google Scholar] [CrossRef]
- Hargreaves, M.; Spriet, L.L. Skeletal muscle energy metabolism during exercise. Nat. Metab. 2020, 2, 817–828. [Google Scholar] [CrossRef]
| Study | Method | Outcomes | Practical Recommendations |
|---|---|---|---|
| Six-month randomized controlled trial involving participants with obesity and prediabetes [18] | 1-Moderate exercise low volume (walking ~16 km/wk at 50% ); 2-Moderate high volume (walking ~22 km/wk); 3-Vigorous run, high volume (67 km/wk at 75% ) 4-Caloric restriction with low volume exercise. | Diet and exercise induced better FBG. Only Moderate exercise high-volume group improved T2D outcomes(OGTT), better than all other groups (including high-intensity-exercise) with a better compliance. | Physical activity recommendation need not to be complex for T2D prevention and can be more effective than high-intensity exercise, with better compliance. |
| Four years intervention involving high CVD risk individuals diagnosed T2D patients [20] | Objective measurement of physical activity while measuring obesity, hypertension and CVD outcomes (waist circumference, blood pressure, CVD risk via cardiorespiratory fitness). | Simple intervention aimed at increasing physical activity using accelerometers and reducing sedentariness increased cardiorespiratory fitness and CVD risks. | CVD risk in those with T2D can be reduced by a single component intervention aimed at reducing sedentary behavior. |
| Intensive lifestyle intervention to prevent CVD mortality in Look AHED cohort of 5145 T2D individuals with overweight [21] | 1 year (within 5 years) with 15 years follow up Intensive calorie restriction weight-loss and increased PA vs. counselling. CVD mortality outcomes (MI/stroke, angina). | Improved weight loss (8.6 vs. 0.7% at yr1; 6.0% vs. 3.5% at end), initial fitness, HbA1c. did not reduce CVD mortality (403 vs. 418). | Intensive intervention that is good for reducing T2D risks may not be good for CVD mortality. Weight loss caloric restriction may not be a sufficient T2D prevention strategy |
| Intensive Lifestyle Intervention as above [21] but with categorization of subgroups [22] | Same as above, but sample was stratified as four subgroups related to older age at diabetes onset (42% of sample), poor glucose control (14%), severe obesity (24%) and younger age at diabetes onset (20%). | CVD risk was not reduced in the poor-glucose-control subgroup (hazard ratio >1.32) but was reduced in all remaining 3 subgroups. | T2D preventative interventions in poorly controlled glucose should carefully consider CVD risks. Nutritional intervention component in this group could focus on alternatives to caloric restriction. |
| Intervention | Whole Group | Men | Women | |||
|---|---|---|---|---|---|---|
| Assessment | Before | After | Before | After | Before | After |
| BMI | 27.8 ± 3.9 | 27.6 ± 3.6 | 28.6 ± 3.6 | 28.3 ± 3.3 | 27.2 ± 4.2 | 27.1 ± 3.9 |
| SBP (mmHg) | 125.9 ± 17.8 | 124.4 ± 10.6 | 129.1 ± 13.8 | 130.1 ± 8.4 | 124.1 ± 20.0 | 121.3 ± 10.7 |
| DBP (mmHg) | 78.6 ± 12.5 | 79.8 ± 7.2 | 83.7 ± 9.7 | 80.3 ± 9.7 | 75.8 ± 13.3 | 79.5 ± 7.2 |
| VT (ml.kg−1.min−1) | 12.0 ± 2.7 | 14.7 ± 2.9 ** | 4.7 ± 1.0 | 5.6 ± 0.8 ** | 4.4 ± 1.0 | 5.0 ± 0.8 ** |
| VT Velocity (km.h−1) | 4.5 ± 0.9 | 5.2 ± 0.8 ** | 12.7 ± 3.5 | 15.8 ± 2.5 ** | 11.54 ± 2.1 | 13.97 ± 3.0 * |
| (ml.kg−1.min−1) | 25.7 ± 6.6 | 29.3 ± 6.7 * | 29.4 ± 6.7 | 31.6 ± 7.3 | 23.2 ± 5.5 | 27.7 ± 6.0 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkhatib, A. Optimizing Lifestyle Behaviors in Preventing Multiple Long-Term Conditions. Encyclopedia 2023, 3, 468-477. https://doi.org/10.3390/encyclopedia3020032
Alkhatib A. Optimizing Lifestyle Behaviors in Preventing Multiple Long-Term Conditions. Encyclopedia. 2023; 3(2):468-477. https://doi.org/10.3390/encyclopedia3020032
Chicago/Turabian StyleAlkhatib, Ahmad. 2023. "Optimizing Lifestyle Behaviors in Preventing Multiple Long-Term Conditions" Encyclopedia 3, no. 2: 468-477. https://doi.org/10.3390/encyclopedia3020032
APA StyleAlkhatib, A. (2023). Optimizing Lifestyle Behaviors in Preventing Multiple Long-Term Conditions. Encyclopedia, 3(2), 468-477. https://doi.org/10.3390/encyclopedia3020032