Primary Chondroprogenitors: Standardized & Versatile Allogeneic Cytotherapeutics
Definition
1. Introduction
2. Primary Chondroprogenitors for Novel Allogeneic Tissue Engineering Applications: High International Focus & Published Translational Studies
3. Starting Biological Material Procurement & Clinical-Grade FE002 Primary Chondroprogenitor Cell Source Establishment Methodology
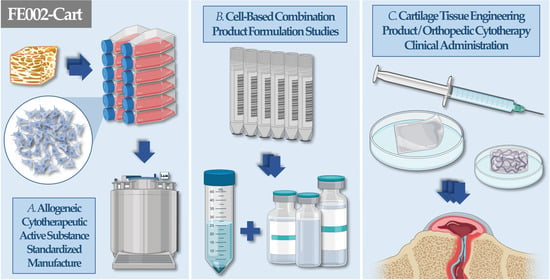
4. Optimized & Standardized FE002 Primary Chondroprogenitor Cell Banking & Biotechnological Manufacturing Processes
5. FE002 Primary Chondroprogenitor In Vitro Characterization & Qualification Data: Robust Fibroblastic Cells with Conserved Chondrogenic Functions
6. Therapeutic Formulation Options for FE002 Primary Chondroprogenitors: High Versatility in Potential Cell-Assisted Orthopedic Applications
7. FE002 Primary Chondroprogenitor Preclinical Safety Evidence: Consistency of Product Innocuity in Various Animal Models
8. Regulatory-Oriented Considerations for Allogeneic Tissue Engineering Products Containing Viable FE002 Primary Chondroprogenitors
9. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACAN | aggrecan |
| ACI | autologous chondrocyte implantation |
| ATMP | advanced therapy medicinal product |
| CAM | chorioallantoic membrane model |
| cATMP | combined advanced therapy medicinal product |
| Col | collagen |
| CPP | critical process parameter |
| CRIS | compression released-induced suction |
| CRP | C reactive protein |
| DMSO | dimethyl sulfoxide |
| ECM | extracellular matrix |
| EGDMA | ethylene glycol dimethacrylate |
| EOPCB | end of production cell bank |
| EU | European Union |
| FBS | fetal bovine serum |
| FDA | US Food and Drug Administration |
| GAG | glycosaminoglycan |
| GelB | gelatin norbornene |
| GLP | good laboratory practices |
| GMP | good manufacturing practices |
| HA | hyaluronic acid |
| HEMA | 2-hydroxyethyl methacrylate |
| Hep | heparin |
| KPP | key process parameter |
| IL | interleukin |
| IPC | in-process control |
| MCB | master cell bank |
| PCB | parental cell bank |
| PEGdiSH | poly(ethylene glycol)dithiol |
| Ph. Eur. | European pharmacopoeia |
| PPC | post-process control |
| SAA | serum amyloid A protein |
| SAP | serum amyloid P component |
| TG | transglutaminase |
| TGF | transforming growth factor |
| TNF | tumor necrosis factor |
| TRPV4 | transient receptor potential channels 4 |
| TrSt | standardized transplant product |
| USA | United States of America |
| WCB | working cell bank |
References
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N. Engl. J. Med. 1994, 331, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Bedi, A.; Feeley, B.T.; Williams, R.J. Management of articular cartilage defects of the knee. J. Bone Jt. Surg. Am. 2010, 92, 994–1009. [Google Scholar] [CrossRef] [PubMed]
- Urlic, I.; Ivkovic, A. Cell sources for cartilage repair-biological and clinical perspective. Cells 2021, 10, 2496. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.; Laurent, A.; Applegate, L.A.; Philippe, V. Grands défects chondraux et ostéochondraux du genou: Traitement par greffe chondrocytaire autologue. Rev. Med. Suisse 2022, 18, 2384–2390. [Google Scholar] [CrossRef] [PubMed]
- Makris, E.A.; Gomoll, A.H.; Malizos, K.N.; Hu, J.C.; Athanasiou, K.A. Repair and tissue engineering techniques for articular cartilage. Nat. Rev. Rheumatol. 2015, 11, 21–34. [Google Scholar] [CrossRef]
- Cherian, J.J.; Parvizi, J.; Bramlet, D.; Lee, K.H.; Romness, D.W.; Mont, M.A. Preliminary results of a phase II randomized study to determine the efficacy and safety of genetically engineered allogeneic human chondrocytes expressing TGF-B1 in patients with grade 3 chronic degenerative joint disease of the knee. Osteoarthr. Cartil. 2015, 23, 2109–2118. [Google Scholar] [CrossRef]
- Teo, A.Q.A.; Wong, K.L.; Shen, L.; Lim, J.Y.; Wei, S.T.; Lee, H.; Hui, J.H.P. Equivalent 10-year outcomes after implantation of autologous bone marrow-derived mesenchymal stem cells versus autologous chondrocyte implantation for chondral defects of the knee. Am. J. Sport. Med. 2019, 47, 2881–2887. [Google Scholar] [CrossRef]
- Asnaghi, M.A.; Power, L.; Barbero, A.; Haug, M.; Köppl, R.; Wendt, D.; Martin, I. Biomarker signatures of quality for engineering nasal chondrocyte-derived cartilage. Front. Bioeng. Biotechnol. 2020, 8, 283. [Google Scholar] [CrossRef]
- Ehlers, E.M.; Fuss, M.; Rohwedel, J.; Russlies, M.; Kühnel, W.; Behrens, P. Development of a biocomposite to fill out articular cartilage lesions. Light, scanning and transmission electron microscopy of sheep chondrocytes cultured on a collagen I/III sponge. Ann. Anat. 1999, 181, 513–518. [Google Scholar] [CrossRef]
- Marcacci, M.; Berruto, M.; Brocchetta, D.; Delcogliano, A.; Ghinelli, D.; Gobbi, A.; Kon, E.; Pederzini, L.; Rosa, D.; Sacchetti, G.L.; et al. Articular cartilage engineering with Hyalograft C: 3-year clinical results. Clin. Orthop. Rel. Res. 2005, 435, 96–105. [Google Scholar] [CrossRef]
- Dhollander, A.A.; Verdonk, P.C.; Lambrecht, S.; Verdonk, R.; Elewaut, D.; Verbruggen, G.; Almqvist, K.F. Midterm results of the treatment of cartilage defects in the knee using alginate beads containing human mature allogenic chondrocytes. Am. J. Sport. Med. 2012, 40, 75–82. [Google Scholar] [CrossRef]
- Marlovits, S.; Aldrian, S.; Wondrasch, B.; Zak, L.; Albrecht, C.; Welsch, G.; Trattnig, S. Clinical and radiological outcomes 5 years after matrix-induced autologous chondrocyte implantation in patients with symptomatic, traumatic chondral defects. Am. J. Sport. Med. 2012, 40, 2273–2280. [Google Scholar] [CrossRef]
- Brix, M.O.; Stelzeneder, D.; Chiari, C.; Koller, U.; Nehrer, S.; Dorotka, R.; Windhager, R.; Domayer, S.E. Treatment of full-thickness chondral defects with Hyalograft C in the knee: Long-term results. Am. J. Sport. Med. 2014, 42, 1426–1432. [Google Scholar] [CrossRef]
- Kon, E.; Roffi, A.; Filardo, G.; Tesei, G.; Marcacci, M. Scaffold-based cartilage treatments: With or without cells? A systematic review of preclinical and clinical evidence. Arthroscopy 2015, 31, 767–775. [Google Scholar] [CrossRef]
- Brittberg, M.; Gomoll, A.H.; Canseco, J.A.; Far, J.; Lind, M.; Hui, J. Cartilage repair in the degenerative ageing knee. Acta Orthop. 2016, 87, 26–38. [Google Scholar] [CrossRef]
- Kon, E.; Filardo, G.; Brittberg, M.; Busacca, M.; Condello, V.; Engebretsen, L.; Marlovits, S.; Niemeyer, P.; Platzer, P.; Posthumus, M.; et al. A multilayer biomaterial for osteochondral regeneration shows superiority vs. microfractures for the treatment of osteochondral lesions in a multicentre randomized trial at 2 years. Knee Surg. Sport. Traumatol. Arthrosc. 2018, 26, 2704–2715. [Google Scholar] [CrossRef]
- Binder, H.; Hoffman, L.; Zak, L.; Tiefenboeck, T.; Aldrian, S.; Albrecht, C. Clinical evaluation after matrix-associated autologous chondrocyte transplantation: A comparison of four different graft types. Bone Jt. Res. 2021, 10, 370–379. [Google Scholar] [CrossRef]
- Porcello, A.; Laurent, A.; Hirt-Burri, N.; Abdel-Sayed, P.; de Buys Roessingh, A.; Raffoul, W.; Jordan, O.; Allémann, E.; Applegate, L.A. Hyaluronan-based hydrogels as functional vectors for standardized therapeutics in tissue engineering and regenerative medicine. In Nanopharmaceuticals in Regenerative Medicine; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar] [CrossRef]
- Peterson, L.; Minas, T.; Brittberg, M.; Nilsson, A.; Sjögren-Jansson, E.; Lindahl, A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin. Orthop. Rel. Res. 2000, 374, 212–234. [Google Scholar] [CrossRef]
- Harris, J.D.; Siston, R.A.; Pan, X.; Flanigan, D.C. Autologous chondrocyte implantation: A systematic review. J. Bone Jt. Surg. 2010, 92, 2220–2233. [Google Scholar] [CrossRef]
- Peterson, L.; Vasiliadis, H.S.; Brittberg, M.; Lindahl, A. Autologous chondrocyte implantation: A long-term follow-up. Am. J. Sport. Med. 2010, 38, 1117–1124. [Google Scholar] [CrossRef]
- Bentley, G.; Biant, L.C.; Vijayan, S.; Macmull, S.; Skinner, J.A.; Carrington, R.W. Minimum ten-year results of a prospective randomised study of autologous chondrocyte implantation versus mosaicplasty for symptomatic articular cartilage lesions of the knee. J. Bone Jt. Surg. 2012, 94, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Biant, L.C.; Bentley, G.; Vijayan, S.; Skinner, J.A.; Carrington, R.W. Long-term results of autologous chondrocyte implantation in the knee for chronic chondral and osteochondral defects. Am. J. Sport. Med. 2014, 42, 2178–2183. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, S.Z.; Bentley, G.; Briggs, T.W.; Carrington, R.W.; Skinner, J.A.; Gallagher, K.R.; Dhinsa, B.S. Autologous chondrocyte implantation in the knee: Mid-term to long-term results. J. Bone Jt. Surg. 2014, 96, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Oussedik, S.; Tsitskaris, K.; Parker, D. Treatment of articular cartilage lesions of the knee by microfracture or autologous chondrocyte implantation: A systematic review. Arthroscopy 2015, 31, 732–744. [Google Scholar] [CrossRef]
- Mumme, M.; Barbero, A.; Miot, S.; Wixmerten, A.; Feliciano, S.; Wolf, F.; Asnaghi, A.M.; Baumhoer, D.; Bieri, O.; Kretzcchmar, M.; et al. Nasal chondrocyte-based engineered autologous cartilage tissue for repair of articular cartilage defects: An observational first-in-human trial. Lancet 2016, 388, 1985–1994. [Google Scholar] [CrossRef]
- Davies, R.L.; Kuiper, N.J. Regenerative medicine: A review of the evolution of autologous chondrocyte implantation (ACI) therapy. Bioengineering 2019, 6, 22. [Google Scholar] [CrossRef]
- Philippe, V.; Laurent, A.; Hirt-Burri, N.; Abdel-Sayed, P.; Scaletta, C.; Schneebeli, V.; Michetti, M.; Brunet, J.-F.; Applegate, L.A.; Martin, R. Retrospective analysis of autologous chondrocyte-based cytotherapy production for clinical use: GMP process-based manufacturing optimization in a Swiss university hospital. Cells 2022, 11, 1016. [Google Scholar] [CrossRef]
- Abelow, S.P.; Guillen, P.; Ramos, T. Arthroscopic technique for matrix-induced autologous chondrocyte implantation for the treatment of large chondral defects in the knee and ankle. Op. Tech. Orthop. 2006, 16, 257–261. [Google Scholar] [CrossRef]
- Brittberg, M. Cell carriers as the next generation of cell therapy for cartilage repair: A review of the matrix-induced autologous chondrocyte implantation procedure. Am. J. Sport. Med. 2010, 38, 1259–1271. [Google Scholar] [CrossRef]
- Albrecht, C.; Tichy, B.; Nürnberger, S.; Hosiner, S.; Zak, L.; Aldrian, S.; Marlovits, S. Gene expression and cell differentiation in matrix-associated chondrocyte transplantation grafts: A comparative study. Osteoarthr. Cart. 2011, 19, 1219–1227. [Google Scholar] [CrossRef]
- Flohé, S.; Betsch, M.; Ruße, K.; Wild, M.; Windolf, J.; Schulz, M. Comparison of two different matrix-based autologous chondrocyte transplantation systems: 1 year follow-up results. Eur. J. Trauma Emerg. Surg. 2011, 37, 397–403. [Google Scholar] [CrossRef]
- Cortese, F.; McNicholas, M.; Janes, G.; Gillogly, S.; Abelow, S.P.; Gigante, A.; Coletti, N. Arthroscopic delivery of matrix-induced autologous chondrocyte implant: International experience and technique recommendations. Cartilage 2012, 3, 156–164. [Google Scholar] [CrossRef]
- Crawford, D.C.; DeBerardino, T.M.; Williams, R.J., 3rd. NeoCart, an autologous cartilage tissue implant, compared with microfracture for treatment of distal femoral cartilage lesions: An FDA phase-II prospective, randomized clinical trial after two years. J. Bone Jt. Surg. 2012, 94, 979–989. [Google Scholar] [CrossRef]
- Vijayan, S.; Bartlett, W.; Bentley, G.; Carrington, R.W.; Skinner, J.A.; Pollock, R.C.; Alorjani, M.; Briggs, T.W. Autologous chondrocyte implantation for osteochondral lesions in the knee using a bilayer collagen membrane and bone graft: A two- to eight-year follow-up study. J. Bone Jt. Surg. 2012, 94, 488–492. [Google Scholar] [CrossRef]
- McCarthy, H.S.; Roberts, S. A histological comparison of the repair tissue formed when using either Chondrogide(®) or periosteum during autologous chondrocyte implantation. Osteoarthr. Cart. 2013, 21, 2048–2057. [Google Scholar] [CrossRef]
- Li, X.; Li, S.; Qian, J.; Chen, Y.; Zhou, Y.; Fu, P. Early efficacy of type I collagen-based matrix-assisted autologous chondrocyte transplantation for the treatment of articular cartilage lesions. Front. Bioeng. Biotechnol. 2021, 9, 760179. [Google Scholar] [CrossRef]
- Darwiche, S.E.; Scaletta, C.; Raffoul, W.; Pioletti, D.P.; Applegate, L.A. Epiphyseal chondroprogenitors provide a stable cell source for cartilage cell therapy. Cell Med. 2012, 4, 23–32. [Google Scholar] [CrossRef]
- Pelttari, K.; Pippenger, B.; Mumme, M.; Feliciano, S.; Scotti, C.; Mainil-Varlet, P.; Procino, A.; von Rechenberg, B.; Schwamborn, T.; Jakob, M.; et al. Adult human neural crest-derived cells for articular cartilage repair. Sci. Transl. Med. 2014, 6, 251ra119. [Google Scholar] [CrossRef]
- Huang, B.J.; Hu, J.C.; Athanasiou, K.A. Cell-based tissue engineering strategies used in the clinical repair of articular cartilage. Biomaterials 2016, 98, 1–22. [Google Scholar] [CrossRef]
- Mortazavi, F.; Shafaei, H.; Soleimani Rad, J.; Rushangar, L.; Montaceri, A.; Jamshidi, M. High quality of infant chondrocytes in comparison with adult chondrocytes for cartilage tissue engineering. World J. Plast. Surg. 2017, 6, 183–189. [Google Scholar]
- Hoburg, A.; Niemeyer, P.; Laute, V.; Zinser, W.; Becher, C.; Kolombe, T.; Fay, J.; Pietsch, S.; Kuźma, T.; Widuchowski, W.; et al. Sustained superiority in KOOS subscores after matrix-associated chondrocyte implantation using spheroids compared to microfracture. Knee Surg. Sport. Traumatol. Arthrosc. 2023, 31, 2482–2493. [Google Scholar] [CrossRef] [PubMed]
- Ha, C.-W.; Noh, M.J.; Choi, K.B.; Lee, K.H. Initial phase I safety of retrovirally transduced human chondrocytes expressing transforming growth factor-beta-1 in degenerative arthritis patients. Cytotherapy 2012, 14, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Tritschler, H.; Fischer, K.; Seissler, J.; Fiedler, J.; Halbgebauer, R.; Huber-Lang, M.; Schnieke, A.; Brenner, R.E. New insights into xenotransplantation for cartilage repair: Porcine multi-genetically modified chondrocytes as a promising cell source. Cells 2021, 10, 2152. [Google Scholar] [CrossRef]
- Evans, C.H.; Ghivizzani, S.C.; Robbins, P.D. Orthopaedic gene therapy: Twenty-five years on. JBJS Rev. 2021, 9, e20. [Google Scholar] [CrossRef] [PubMed]
- Manning, W.K.; Bonner, W.M., Jr. Isolation and culture of chondrocytes from human adult articular cartilage. Arthritis Rheum. 1967, 10, 235–239. [Google Scholar] [CrossRef]
- Tallheden, T.; Karlsson, C.; Brunner, A.; Van Der Lee, J.; Hagg, R.; Tommasini, R.; Lindahl, A. Gene expression during redifferentiation of human articular chondrocytes. Osteoarthr. Cart. 2004, 12, 525–535. [Google Scholar] [CrossRef]
- Kang, S.W.; Yoo, S.P.; Kim, B.S. Effect of chondrocyte passage number on histological aspects of tissue-engineered cartilage. Bio-Med. Mat. Eng. 2007, 17, 269–276. [Google Scholar] [PubMed]
- Martinez, I.; Elvenes, J.; Olsen, R.; Bertheussen, K.; Johansen, O. Redifferentiation of in vitro expanded adult articular chondrocytes by combining the hanging-drop cultivation method with hypoxic environment. Cell Transplant. 2008, 17, 987–996. [Google Scholar] [CrossRef]
- Lin, Z.; Fitzgerald, J.B.; Xu, J.; Willers, C.; Wood, D.; Grodzinsky, A.J.; Zheng, M.H. Gene expression profiles of human chondrocytes during passaged monolayer cultivation. J. Orthop. Res. 2008, 26, 1230–1237. [Google Scholar] [CrossRef]
- Enochson, L.; Brittberg, M.; Lindahl, A. Optimization of a chondrogenic medium through the use of factorial design of experiments. BioRes Open Access 2012, 1, 306–313. [Google Scholar] [CrossRef]
- Oseni, A.O.; Butler, P.E.; Seifalian, A.M. Optimization of chondrocyte isolation and characterization for large-scale cartilage tissue engineering. J. Surg. Res. 2013, 181, 41–48. [Google Scholar] [CrossRef]
- Chijimatsu, R.; Kobayashi, M.; Ebina, K.; Iwahashi, T.; Okuno, Y.; Hirao, M.; Fukuhara, A.; Nakamura, N.; Yoshikawa, H. Impact of dexamethasone concentration on cartilage tissue formation from human synovial derived stem cells in vitro. Cytotechnology 2018, 70, 819–829. [Google Scholar] [CrossRef]
- Kisiday, J.D. Expansion of chondrocytes for cartilage tissue engineering: A review of chondrocyte dedifferentiation and redifferentiation as a function of growth in expansion culture. Regen. Med. Front. 2020, 2, e200002. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, Y.; Wen, Y.; Chen, J.; Lin, J.; Sheng, Z.; Zhou, W.; Sun, H.; An, C.; Chen, J.; et al. A high-resolution route map reveals distinct stages of chondrocyte dedifferentiation for cartilage regeneration. Bone Res. 2022, 10, 38. [Google Scholar] [CrossRef]
- Mandl, E.W.; van der Veen, S.W.; Verhaar, J.A.; van Osch, G.J. Serum-free medium supplemented with high-concentration FGF2 for cell expansion culture of human ear chondrocytes promotes redifferentiation capacity. Tissue Eng. 2002, 8, 573–580. [Google Scholar] [CrossRef]
- Gaissmaier, C.; Fritz, J.; Krackhardt, T.; Flesch, I.; Aicher, W.K.; Ashammakhi, N. Effect of human platelet supernatant on proliferation and matrix synthesis of human articular chondrocytes in monolayer and three-dimensional alginate cultures. Biomaterials 2005, 26, 1953–1960. [Google Scholar] [CrossRef]
- Hildner, F.; Eder, M.J.; Hofer, K.; Aberl, J.; Redl, H.; van Griensven, M.; Gabriel, C.; Peterbauer-Scherb, A. Human platelet lysate successfully promotes proliferation and subsequent chondrogenic differentiation of adipose-derived stem cells: A comparison with articular chondrocytes. J. Tissue Eng. Regen. Med. 2015, 9, 808–818. [Google Scholar] [CrossRef]
- Sykes, J.G.; Kuiper, J.H.; Richardson, J.B.; Roberts, S.; Wright, K.T.; Kuiper, N.J. Impact of human platelet lysate on the expansion and chondrogenic capacity of cultured human chondrocytes for cartilage cell therapy. Eur. Cell Mater. 2018, 35, 255–267. [Google Scholar] [CrossRef]
- Rikkers, M.; Levato, R.; Malda, J.; Vonk, L.A. Importance of timing of platelet lysate-supplementation in expanding or redifferentiating human chondrocytes for chondrogenesis. Front. Bioeng. Biotechnol. 2020, 8, 804. [Google Scholar] [CrossRef]
- Philippe, V.; Laurent, A.; Abdel-Sayed, P.; Hirt-Burri, N.; Applegate, L.A.; Martin, R. Human platelet lysate as an alternative to autologous serum for human chondrocyte clinical use. Cartilage 2021, 13, 509S–518S. [Google Scholar] [CrossRef]
- Kachroo, U.; Zachariah, S.M.; Thambaiah, A.; Tabasum, A.; Livingston, A.; Rebekah, G.; Srivastava, A.; Vinod, E. Comparison of human platelet lysate versus fetal bovine serum for expansion of human articular cartilage-derived chondroprogenitors. Cartilage 2021, 13, 107S–116S. [Google Scholar] [CrossRef]
- Liau, L.L.; Hassan, M.N.F.b.; Tang, Y.L.; Ng, M.H.; Law, J.X. Feasibility of human platelet lysate as an alternative to fetal bovine serum for in vitro expansion of chondrocytes. Int. J. Mol. Sci. 2021, 22, 1269. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.C.; Bertram, T.A.; Tawil, B.; Hellman, K.B. Hurdles in tissue engineering/regenerative medicine product commercialization: A survey of North American academia and industry. Tissue Eng. Part A 2011, 17, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.D.; Siston, R.A.; Brophy, R.H.; Lattermann, C.; Carey, J.L.; Flanigan, D.C. Failures, re-operations, and complications after autologous chondrocyte implantation--A systematic review. Osteoarthr. Cart. 2011, 19, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Pearce, K.F.; Hildebrandt, M.; Greinix, H.; Scheding, S.; Koehl, U.; Worel, N.; Apperley, J.; Edinger, M.; Hauser, A.; Mischak-Weissinger, E.; et al. Regulation of advanced therapy medicinal products in Europe and the role of academia. Cytotherapy 2014, 16, 289–297. [Google Scholar] [CrossRef]
- Ikawa, T.; Yano, K.; Watanabe, N.; Masamune, K.; Yamato, M. Non-clinical assessment design of autologous chondrocyte implantation products. Regen. Ther. 2015, 1, 98–108. [Google Scholar] [CrossRef]
- Ramezankhani, R.; Torabi, S.; Minaei, N.; Madani, H.; Rezaeiani, S.; Hassani, S.N.; Gee, A.P.; Dominici, M.; Silva, D.N.; Baharvand, H.; et al. Two decades of global progress in authorized advanced therapy medicinal products: An emerging revolution in therapeutic strategies. Front. Cell Develop. Biol. 2020, 8, 547653. [Google Scholar] [CrossRef]
- Niethammer, T.R.; Gallik, D.; Chevalier, Y.; Holzgruber, M.; Baur-Melnyk, A.; Müller, P.E.; Pietschmann, M.F. Effect of the defect localization and size on the success of third-generation autologous chondrocyte implantation in the knee joint. Internat. Orthop. 2021, 45, 1483–1491. [Google Scholar] [CrossRef]
- Nordberg, R.C.; Otarola, G.A.; Wang, D.; Hu, J.C.; Athanasiou, K.A. Navigating regulatory pathways for translation of biologic cartilage repair products. Sci. Transl. Med. 2022, 14, eabp8163. [Google Scholar] [CrossRef]
- Laurent, A.; Hirt-Burri, N.; Scaletta, C.; Michetti, M.; de Buys Roessingh, A.S.; Raffoul, W.; Applegate, L.A. Holistic approach of Swiss fetal progenitor cell banking: Optimizing safe and sustainable substrates for regenerative medicine and biotechnology. Front. Bioeng. Biotechnol. 2020, 8, 557758. [Google Scholar] [CrossRef]
- Laurent, A.; Abdel-Sayed, P.; Ducrot, A.; Hirt-Burri, N.; Scaletta, C.; Jaccoud, S.; Nuss, K.; de Buys Roessingh, A.S.; Raffoul, W.; Pioletti, D.P.; et al. Development of standardized fetal progenitor cell therapy for cartilage regenerative medicine: Industrial transposition and preliminary safety in xenogeneic transplantation. Biomolecules 2021, 11, 250. [Google Scholar] [CrossRef]
- Abdel-Sayed, P.; Darwiche, S.E.; Kettenberger, U.; Pioletti, D.P. The role of energy dissipation of polymeric scaffolds in the mechanobiological modulation of chondrogenic expression. Biomaterials 2014, 35, 1890–1897. [Google Scholar] [CrossRef]
- Nasrollahzadeh, N.; Applegate, L.A.; Pioletti, D.P. Development of an effective cell seeding technique: Simulation, implementation, and analysis of contributing factors. Tissue Eng. Part C 2017, 23, 485–496. [Google Scholar] [CrossRef]
- Nasrollahzadeh, N.; Karami, P.; Wang, J.; Bagheri, L.; Guo, Y.; Abdel-Sayed, P.; Laurent-Applegate, L.; Pioletti, D.P. Temperature evolution following joint loading promotes chondrogenesis by synergistic cues via calcium signaling. eLife 2022, 11, e72068. [Google Scholar] [CrossRef]
- Studer, D.; Cavalli, E.; Formica, F.A.; Kuhn, G.A.; Salzmann, G.; Mumme, M.; Steinwachs, M.R.; Applegate, L.A.; Maniura-Weber, K.; Zenobi-Wong, M. Human chondroprogenitors in alginate-collagen hybrid scaffolds produce stable cartilage in vivo. J. Tissue Eng. Regen. Med. 2017, 11, 3014–3026. [Google Scholar] [CrossRef]
- Cavalli, E.; Fisch, P.; Formica, F.A.; Gareus, R.; Linder, T.; Applegate, L.A.; Zenobi-Wong, M. A comparative study of cartilage engineered constructs in immunocompromised, humanized and immunocompetent mice. J. Immunol. Regen. Med. 2018, 2, 36–46. [Google Scholar] [CrossRef]
- Levinson, C.; Lee, M.; Applegate, L.A.; Zenobi-Wong, M. An injectable heparin-conjugated hyaluronan scaffold for local delivery of transforming growth factor β1 promotes successful chondrogenesis. Acta Biomater. 2019, 99, 168–180. [Google Scholar] [CrossRef]
- Li, F.; Levinson, C.; Truong, V.X.; Laurent-Applegate, L.A.; Maniura-Weber, K.; Thissen, H.; Forsythe, J.F.; Zenobi-Wong, M.; Frith, J.E. Microencapsulation improves chondrogenesis in vitro and cartilaginous matrix stability in vivo compared to bulk encapsulation. Biomater. Sci. 2020, 8, 1711–1725. [Google Scholar] [CrossRef]
- Tosoratti, E.; Fisch, P.; Taylor, S.; Laurent-Applegate, L.A.; Zenobi-Wong, M. 3D-printed reinforcement scaffolds with targeted biodegradation properties for the tissue engineering of articular cartilage. Adv. Healthc. Mat. 2021, 10, e2101094. [Google Scholar] [CrossRef]
- Lee, S.J.; Oh, H.J.; Truong, M.D.; Lee, K.B.; Kim, J.; Kim, Y.J.; Park, S.R.; Min, B.H. Therapeutic possibility of human fetal cartilage-derived progenitor cells in rat arthritis model. Tissue Eng. Regen. Med. 2015, 12, 147–154. [Google Scholar] [CrossRef]
- Choi, W.H.; Kim, H.R.; Lee, S.J.; Jeong, N.; Park, S.R.; Choi, B.H.; Min, B.H. Fetal cartilage-derived cells have stem cell properties and are a highly potent cell source for cartilage regeneration. Cell Transplant. 2016, 25, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, J.; Park, S.R.; Park, D.Y.; Kim, Y.J.; Choi, B.H.; Min, B.H. Comparison of fetal cartilage-derived progenitor cells isolated at different developmental stages in a rat model. Dev. Growth Diff. 2016, 58, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Ribitsch, I.; Mayer, R.L.; Egerbacher, M.; Gabner, S.; Kańduła, M.M.; Rosser, J.; Haltmayer, E.; Auer, U.; Gültekin, S.; Huber, J.; et al. Fetal articular cartilage regeneration versus adult fibrocartilaginous repair: Secretome proteomics unravels molecular mechanisms in an ovine model. Dis. Mod. Mech. 2018, 11, dmm033092. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Kim, J.; Park, S.R.; Min, B.H.; Choi, B.H. Characterization of human fetal cartilage progenitor cells during long-term expansion in a xeno-free medium. Tissue Eng. Regen. Med. 2018, 15, 649–659. [Google Scholar] [CrossRef]
- Dasargyri, A.; Reichmann, E.; Moehrlen, U. Bio-engineering of fetal cartilage for in utero spina bifida repair. Ped. Surg. Int. 2020, 36, 25–31. [Google Scholar] [CrossRef]
- Park, D.Y.; Min, B.H.; Park, S.R.; Oh, H.J.; Truong, M.D.; Kim, M.; Choi, J.Y.; Park, I.S.; Choi, B.H. Engineered cartilage utilizing fetal cartilage-derived progenitor cells for cartilage repair. Sci. Rep. 2020, 10, 5722. [Google Scholar] [CrossRef]
- Park, I.S.; Kim, B.K.; Truong, M.D.; Yang, H.S.; Park, S.H.; Park, H.S.; Choi, B.H.; Won, B.H.; Min, B.H. Corneal repair with adhesive cell sheets of fetal cartilage-derived stem cells. Tissue Eng. Regen. Med. 2021, 18, 187–198. [Google Scholar] [CrossRef]
- Rikkers, M.; Korpershoek, J.V.; Levato, R.; Malda, J.; Vonk, L.A. The clinical potential of articular cartilage-derived progenitor cells: A systematic review. NPJ Regen. Med. 2022, 7, 2. [Google Scholar] [CrossRef]
- Kim, J.; Tran, A.N.; Lee, J.Y.; Park, S.H.; Park, S.R.; Min, B.H.; Choi, B.H. Human fetal cartilage-derived progenitor cells exhibit anti-inflammatory effect on IL-1β-mediated osteoarthritis phenotypes in vitro. Tissue Eng. Regen. Med. 2022, 19, 1237–1250. [Google Scholar] [CrossRef]
- Lee, K.H.; Song, S.U.; Hwang, T.S.; Yi, Y.; Oh, I.S.; Lee, J.Y.; Choi, K.B.; Choi, M.S.; Kim, S.J. Regeneration of hyaline cartilage by cell-mediated gene therapy using transforming growth factor beta 1-producing fibroblasts. Hum. Gene Ther. 2001, 12, 1805–1813. [Google Scholar] [CrossRef]
- Song, S.U.; Cha, Y.D.; Han, J.U.; Oh, I.S.; Choi, K.B.; Yi, Y.; Hyun, J.P.; Lee, H.Y.; Chi, G.F.; Lim, C.L.; et al. Hyaline cartilage regeneration using mixed human chondrocytes and transforming growth factor-beta1- producing chondrocytes. Tissue Eng. 2005, 11, 1516–1526. [Google Scholar] [CrossRef]
- Yi, Y.; Choi, K.B.; Lim, C.L.; Hyun, J.P.; Lee, H.Y.; Lee, K.B.; Yun, L.; Ayverdi, A.; Hwang, S.; Yip, V.; et al. Irradiated human chondrocytes expressing bone morphogenetic protein 2 promote healing of osteoporotic bone fracture in rats. Tissue Eng. Part A 2009, 15, 2853–2863. [Google Scholar] [CrossRef]
- Noh, M.J.; Copeland, R.O.; Yi, Y.; Choi, K.B.; Meschter, C.; Hwang, S.; Lim, C.L.; Yip, V.; Hyun, J.P.; Lee, H.Y.; et al. Pre-clinical studies of retrovirally transduced human chondrocytes expressing transforming growth factor-beta-1 (TG-C). Cytotherapy 2010, 12, 384–393. [Google Scholar] [CrossRef]
- Lee, B. INVOSSA, a first-in-class of cell and gene therapy for osteoarthritis treatment: The phase III trial. Osteoarthr. Cart. 2018, 26, S43–S44. [Google Scholar] [CrossRef]
- Evans, C.H. The vicissitudes of gene therapy. Bone Jt. Res. 2019, 8, 469–471. [Google Scholar] [CrossRef]
- Evans, C.H.; Ghivizzani, S.C.; Robbins, P.D. Getting arthritis gene therapy into the clinic. Nat. Rev. Rheumatol. 2011, 7, 244–249. [Google Scholar] [CrossRef]
- Kim, M.K.; Ha, C.W.; In, Y.; Cho, S.D.; Choi, E.S.; Ha, J.K.; Lee, J.H.; Yoo, J.D.; Bin, S.I.; Choi, C.H.; et al. A multicenter, double-blind, phase III clinical trial to evaluate the efficacy and safety of a cell and gene therapy in knee osteoarthritis patients. Hum. Gene Ther. 2018, 29, 48–59. [Google Scholar] [CrossRef]
- European Directorate for the Quality of Medicines & Healthcare. European Committee (Partial Agreement) on organ transplantation (CD-P-TO). In Guide to the Quality and Safety of Tissues and Cells for Human Application, 4th ed.; EDQM: Strasbourg, France, 2019; ISBN 978-92-871-8945-5. [Google Scholar]
- European Medicines Agency. ICH Topic Q 5 A (R1) Quality of Biotechnological Products: Viral Safety Evaluation of Biotechnology Products Derived from Cell Lines of Human or Animal Origin; CPMP/ICH/295/95; EMEA: London, UK, 1997. [Google Scholar]
- European Directorate for the Quality of Medicines & Healthcare. General Chapter 5.2.3. Cell substrates for the production of vaccines for human use. In European Pharmacopoeia 11.0; EDQM: Strasbourg, France, 2023. [Google Scholar]
- Broguiere, N.; Cavalli, E.; Salzmann, G.M.; Applegate, L.A.; Zenobi-Wong, M. Factor XIII cross-linked hyaluronan hydrogels for cartilage tissue engineering. ACS Biomat. Sci. Eng. 2016, 2, 2176–2184. [Google Scholar] [CrossRef]
- European Medicines Agency. EPAR Summary for the Public–MACI Matrix Applied Characterised Autologous Cultured Chondrocytes; EMA/282918/2013; EMEA: London, UK, 2013. [Google Scholar]
- Vonk, L.A.; Roël, G.; Hernigou, J.; Kaps, C.; Hernigou, P. Role of matrix-associated autologous chondrocyte implantation with spheroids in the treatment of large chondral defects in the knee: A systematic review. Int. J. Mol. Sci. 2021, 22, 7149. [Google Scholar] [CrossRef]
- Cottle, C.; Porter, A.P.; Lipat, A.; Turner-Lyles, C.; Nguyen, J.; Moll, G.; Chinnadurai, R. Impact of cryopreservation and freeze-thawing on therapeutic properties of mesenchymal stromal/stem cells and other common cellular therapeutics. Curr. Stem Cell Rep. 2022, 8, 72–92. [Google Scholar] [CrossRef]
- Ferruzzi, A.; Buda, R.; Faldini, C.; Vannini, F.; Di Caprio, F.; Luciani, D.; Giannini, S. Autologous chondrocyte implantation in the knee joint: Open compared with arthroscopic technique. Comparison at a minimum follow-up of five years. J. Bone Jt. Surg. 2008, 90, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Brun, P.; Dickinson, S.C.; Zavan, B.; Cortivo, R.; Hollander, A.P.; Abatangelo, G. Characteristics of repair tissue in second-look and third-look biopsies from patients treated with engineered cartilage: Relationship to symptomatology and time after implantation. Arthritis Res. Ther. 2008, 10, R132. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, A.; Kon, E.; Berruto, M.; Filardo, G.; Delcogliano, M.; Boldrini, L.; Bathan, L.; Marcacci, M. Patellofemoral full-thickness chondral defects treated with second-generation autologous chondrocyte implantation: Results at 5 years’ follow-up. Am. J. Sport. Med. 2009, 37, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Di Martino, A.; Filardo, G.; Tetta, C.; Busacca, M.; Iacono, F.; Delcogliano, M.; Albisinni, U.; Marcacci, M. Second-generation autologous chondrocyte transplantation: MRI findings and clinical correlations at a minimum 5-year follow-up. Eur. J. Radiol. 2011, 79, 382–388. [Google Scholar] [CrossRef]
- Aldrian, S.; Zak, L.; Wondrasch, B.; Albrecht, C.; Stelzeneder, B.; Binder, H.; Kovar, F.; Trattnig, S.; Marlovits, S. Clinical and radiological long-term outcomes after matrix-induced autologous chondrocyte transplantation: A prospective follow-up at a minimum of 10 years. Am. J. Sport. Med. 2014, 42, 2680–2688. [Google Scholar] [CrossRef]
- Wondrasch, B.; Risberg, M.A.; Zak, L.; Marlovits, S.; Aldrian, S. Effect of accelerated weightbearing after matrix-associated autologous chondrocyte implantation on the femoral condyle: A prospective, randomized controlled study presenting MRI-based and clinical outcomes after 5 years. Am. J. Sport. Med. 2015, 43, 146–153. [Google Scholar] [CrossRef]
- Ebert, J.R.; Fallon, M.; Wood, D.J.; Janes, G.C. A prospective clinical and radiological evaluation at 5 years after arthroscopic matrix-induced autologous chondrocyte implantation. Am. J. Sport. Med. 2017, 45, 59–69. [Google Scholar] [CrossRef]
- Brittberg, M.; Recker, D.; Ilgenfritz, J.; Saris, D.B.F.; SUMMIT Extension Study Group. Matrix-applied characterized autologous cultured chondrocytes versus microfracture: Five-year follow-up of a prospective randomized trial. Am. J. Sport. Med. 2018, 46, 1343–1351. [Google Scholar] [CrossRef]
- Ibarra, C.; Villalobos, E.; Madrazo-Ibarra, A.; Velasquillo, C.; Martinez-Lopez, V.; Izaguirre, A.; Olivos-Meza, A.; Cortes-Gonzalez, S.; Perez-Jimenez, F.J.; Vargas-Ramirez, A.; et al. Arthroscopic matrix-assisted autologous chondrocyte transplantation versus microfracture: A 6-year follow-up of a prospective randomized trial. Am. J. Sport. Med. 2021, 49, 2165–2176. [Google Scholar] [CrossRef]




| Study Subject/Domain | Scope of the Study/Investigated Parameters/Main Data | References |
|---|---|---|
| 1. Progenitor Cell Source Establishment | Biological starting material procurement (i.e., controlled organ donation within the Swiss progenitor cell transplantation program) and establishment of FE002 primary progenitor cell sources in a cryogenically preserved multi-tiered cell bank system. | [71] |
| 2. In Vitro Cell Type Characterization | Characterization of progenitor cell type key and critical attributes (e.g., cellular proliferative behavior in culture, cellular lot homogeneity and purity, cell genetic and phenotypic stability, proteomics, chondrogenic potential, in vitro safety parameters). | [38,72] |
| 3. Characterization of In Vitro Mechanobiological Cellular Behavior | Study of the influence of physical (i.e., mechanical) parameters on cellular biology and functional attributes 1. Optimization of physical processing workflows for cytotherapeutic material lots. | [73,74,75] |
| 4. In Vitro Cell Banking & Biotechnological Manufacturing | Optimization and standardization of in vitro progenitor cell manufacturing workflows (i.e., industrial-scale cellular lots). Confirmation of progenitor cell source sustainability at passage levels for clinical use 2. | [72] |
| 5. Formulation Studies for Functional Cytotherapeutic Products | Formulation and translational characterization/qualification of hydrogel-based (e.g., modified HA-based gels) standardized transplants and polymeric scaffold-based tissue engineering products yielding viable/functional progenitor cells. | [76,77,78,79,80] |
| 6. In Vivo Preclinical Safety Assessments | Study of progenitor cellular material or cytotherapeutic combination product safety in ovo (i.e., standardized CAM model) and in vivo (e.g., subcutaneous rodent implantation models, GLP study of knee chondral defect management in goats). | [72,76,77,79] |
| Testing Type/Assay Type | Testing Class/Testing Purpose | Testing Tiers 1 |
|---|---|---|
| 1. Morphology & proliferative behavior | Identification/general qualification | PCB; MCB; WCB; EOPCB 2 |
| 2. Cell type identification & fingerprinting | Identification/general qualification | PCB; MCB; WCB; EOPCB |
| 3. Cell type karyotype | Identification/general qualification | PCB; MCB; EOPCB |
| 4. Cell type in vitro lifespan | Identification/general qualification | MCB; WCB |
| 5. Testing for bacterial & fungal agents | Extraneous agent detection/microbiological qualification | MCB; WCB |
| 6. Testing for mycobacteria and mycoplasmas | Extraneous agent detection/microbiological qualification | MCB; WCB |
| 7. Testing for viruses and for retroviruses | Extraneous agent detection/microbiological qualification | EOPCB |
| 8. Electron microscopy characterization | Identification/general qualification/extraneous agent detection/microbiological qualification | EOPCB |
| 9. Safety/toxicity testing in ovo or in small animals | Microbiological qualification/safety qualification | EOPCB |
| 10. Tumorigenicity assays | Safety qualification | EOPCB |
| Cell Scaffolds/Cytotherapeutic Product Formulation Options | Summary of the Investigated Endpoints (Technical, Functional)/Experimental Data | References |
|---|---|---|
| 1. HA-TG | Good cellular viability in gels; good chondrogenic potential assessed by ACAN and COL2 gene expression; reported COL2 deposition and increase in compressive modulus. Gel attributes impact cell morphology, proliferation, and chondrogenic potential. | [77,102] |
| 2. Optimaix 3D ± alginate | Homogeneous cellular distribution throughout the scaffold; good in vitro chondrogenic potential assessed by gene expression, GAGs quantification, and immunohistology. Reported absence of hypertrophy markers. Increase in compressive modulus over time. Samples were tested in vivo in mouse subcutaneous implantation. | [76] |
| 3. Novocart Basic | Good cellular distribution throughout the scaffold. | [76] |
| 4. pHEMA scaffold crosslinked EGDMA-fibronectin | Good cellular adhesion and viability. Impact of the gel’s level of dissipation on the cellular differentiation potential. Cells resist to seeding protocols using CRIS method (i.e., compression released-induced suction). | [73,74] |
| 5. HA-TG/hep ± TGF-β1 | Good cellular viability and proliferation potential maintenance. TGF-β1 concentration and sustained release influence the proliferation and chondrogenic potentials. Compression modulus increases over time in a TGF-β1 dose-dependent manner. | [78] |
| 6. GelNB-PEGdiSH | Good cellular viability maintenance after microencapsulation protocol. Good cell migration potential in the gel. Higher chondrogenic gene induction in microencapsulation versus bulk encapsulation. Higher GAGs deposition in bulk encapsulation, but matrix quality is better with microencapsulation. Samples were tested in vivo in mouse subcutaneous implantation. | [79] |
| 7. Lactoprene combined to HA-TG | Good cellular viability and proliferation potential in the scaffold. Good chondrogenic potential with COL2 deposition. Increase in compressive modulus during cellular differentiation. Samples were tested in vivo in mouse subcutaneous implantation. | [80] |
| 8. pHEMA functionalized with RGD peptides | Cellular adhesion and proliferation potentials are preserved at 32.5 °C and 37.0 °C. Chondrogenic potential is directly influenced by external environmental stimuli (i.e., loading and temperature). Chondrogenic gene expression is increased by loading and self-induced heating (i.e., 32.5 °C–39.0 °C). TRPV4 channel expression is increased by mechanical loading and self-heating. Calcium signalling is involved in chondrogenic genes ACAN, COL2, and SOX9 induction. | [75] |
| 9. Chondro-Gide | Specific cellular distribution in the scaffold. Cryopreserved cells may be thawed and seeded on the matrix extemporaneously before implantation. Samples were tested in vivo in a GLP model of goat full thickness chondral defects. | [72,76] |
| In Vivo Model & Study Type | Summary of the Investigated Endpoints (Safety, Biocompatibility)/Experimental Data | References |
|---|---|---|
| 1. CAM model | No observed embryotoxicity, no observed angiotoxicity of non-viable cellular materials. | [72] |
| 2. NU/NU nude mice | Cell-seeded scaffolds implanted subcutaneously for 8 weeks. Scaffolds retained ECM. No observed scaffold mineralization or vessel infiltration. | [76] |
| 3. NSG, nude/hu-NSG, C57/BL/6 mice | Cell-seeded scaffolds implanted subcutaneously for 4 weeks. No observed adverse events (e.g., necrosis, oedema, hyperemia). Fibrous capsule formation (i.e., thicker in C57/BL/6 model). No increase in CRP levels. Reduction in SAA and SAP levels compared to empty scaffolds (i.e., except in C57/BL/6 model). Macrophages and T cell recruitment around the scaffolds in C57/BL/6 model, but absent or low in other mouse models. No observed induction of IL-1β, IL-4, IL-6, IL-10, and TNF-α. | [77] |
| 4. NU/NU nude mice | Cell-seeded scaffolds implanted subcutaneously for up to 5 weeks. No observed toxicity. Fibrous capsule formation around the scaffolds. Resistance to vascularization with microencapsulated cells. Regenerated matrix quality documented as being better with microencapsulated cells. | [79] |
| 5. Nude mice | Cell-seeded scaffolds implanted subcutaneously for 6 weeks. Small fibrous capsule formation around the scaffolds. No vascularization within the samples and biodegradation of lactoprene. | [80] |
| 6. Goat model, GLP study | Full thickness chondral defect of the knee. GLP study over 3 months. No test-item related mortality. No observed significant adverse reactions (i.e., local or systemic) in vivo. No changes in monitored clinical signs (i.e., lameness, body weight, neurological). A portion of the human cells were found to have engrafted locally in the host. | [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laurent, A.; Jeannerat, A.; Peneveyre, C.; Scaletta, C.; Philippe, V.; Abdel-Sayed, P.; Raffoul, W.; Martin, R.; Hirt-Burri, N.; Applegate, L.A. Primary Chondroprogenitors: Standardized & Versatile Allogeneic Cytotherapeutics. Encyclopedia 2023, 3, 622-641. https://doi.org/10.3390/encyclopedia3020045
Laurent A, Jeannerat A, Peneveyre C, Scaletta C, Philippe V, Abdel-Sayed P, Raffoul W, Martin R, Hirt-Burri N, Applegate LA. Primary Chondroprogenitors: Standardized & Versatile Allogeneic Cytotherapeutics. Encyclopedia. 2023; 3(2):622-641. https://doi.org/10.3390/encyclopedia3020045
Chicago/Turabian StyleLaurent, Alexis, Annick Jeannerat, Cédric Peneveyre, Corinne Scaletta, Virginie Philippe, Philippe Abdel-Sayed, Wassim Raffoul, Robin Martin, Nathalie Hirt-Burri, and Lee Ann Applegate. 2023. "Primary Chondroprogenitors: Standardized & Versatile Allogeneic Cytotherapeutics" Encyclopedia 3, no. 2: 622-641. https://doi.org/10.3390/encyclopedia3020045
APA StyleLaurent, A., Jeannerat, A., Peneveyre, C., Scaletta, C., Philippe, V., Abdel-Sayed, P., Raffoul, W., Martin, R., Hirt-Burri, N., & Applegate, L. A. (2023). Primary Chondroprogenitors: Standardized & Versatile Allogeneic Cytotherapeutics. Encyclopedia, 3(2), 622-641. https://doi.org/10.3390/encyclopedia3020045