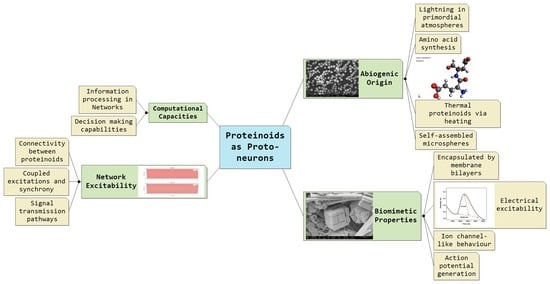
Proto-Neurons from Abiotic Polypeptides
Abstract
1. Introduction
1.1. Thermodynamic Perspectives on Life’s Origins
1.2. Requirements for Early Biomolecular Systems
2. Proteinoids as Primitive Biopolymers
2.1. Thermal Polycondensation of Amino Acids
2.2. Fox’s Proteinoids: Characteristics and Capabilities
2.2.1. Catalytic Activity
2.2.2. Microspheres Formation
2.2.3. Information Transfer
2.2.4. Evolutionary Potential
3. Proteinoids and Cellular Emergence
3.1. From Amino Acids to Protocells: Self-Organisation of Proteinoids into Models of Primordial Life
3.2. Membrane Assembly and Composition
3.3. Homeostasis Mechanisms
3.3.1. Structural Heterogeneity in Proteinoids
3.3.2. Permeability Regulation
4. Proteinoids as Molecular Assemblies
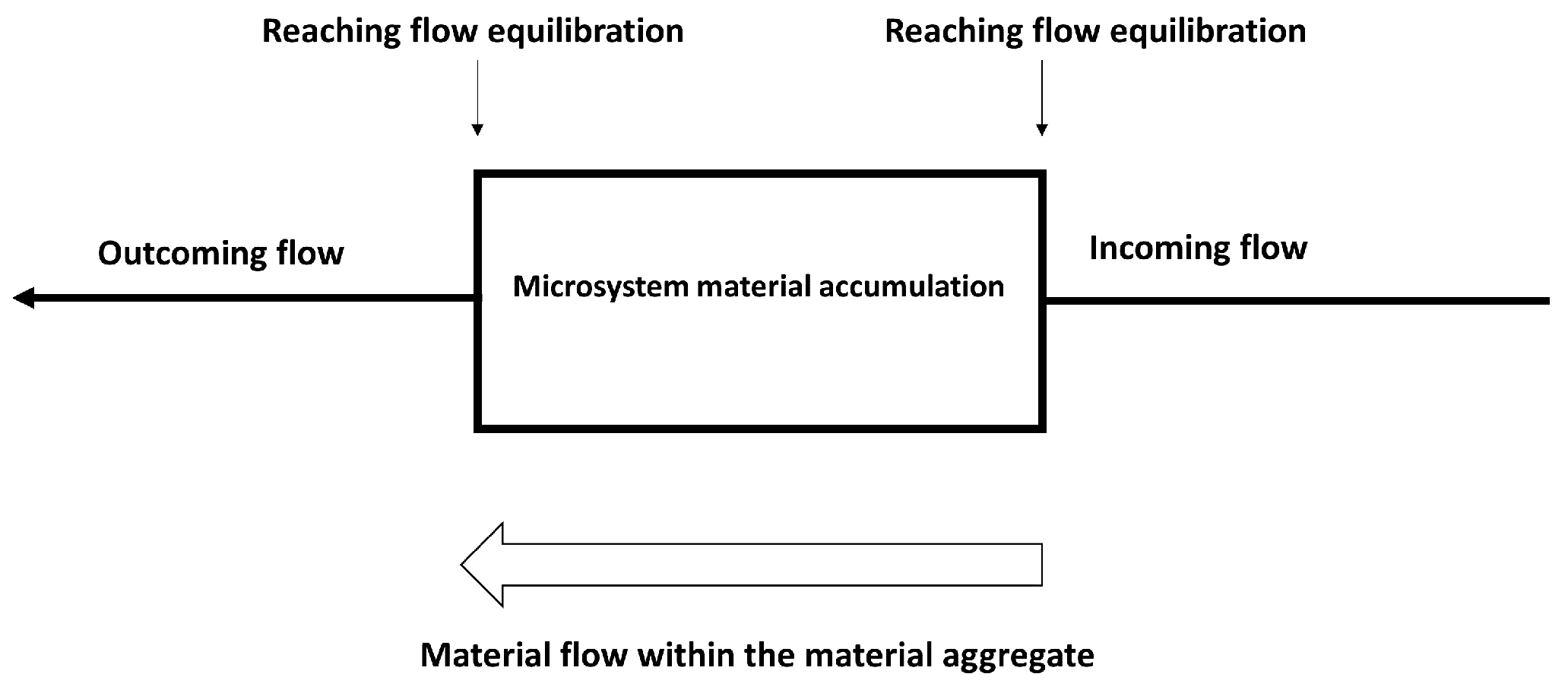
4.1. Aggregation States and Dynamics
4.2. Environmental Interactions
Mineral Templating
4.3. Emergent Cognitive Properties in Proteinoids
Adsorption Phenomena
4.4. Self-Organisation Tendencies
5. Implications of Proteinoids for Thermodynamic Inversion
5.1. Dissipative Proteinoid Systems
5.2. Proteinoid-Mediated Energy Flow
5.3. Drivers of Complexity in Proteinoid Networks
6. Unconventional Computing with Proteinoids
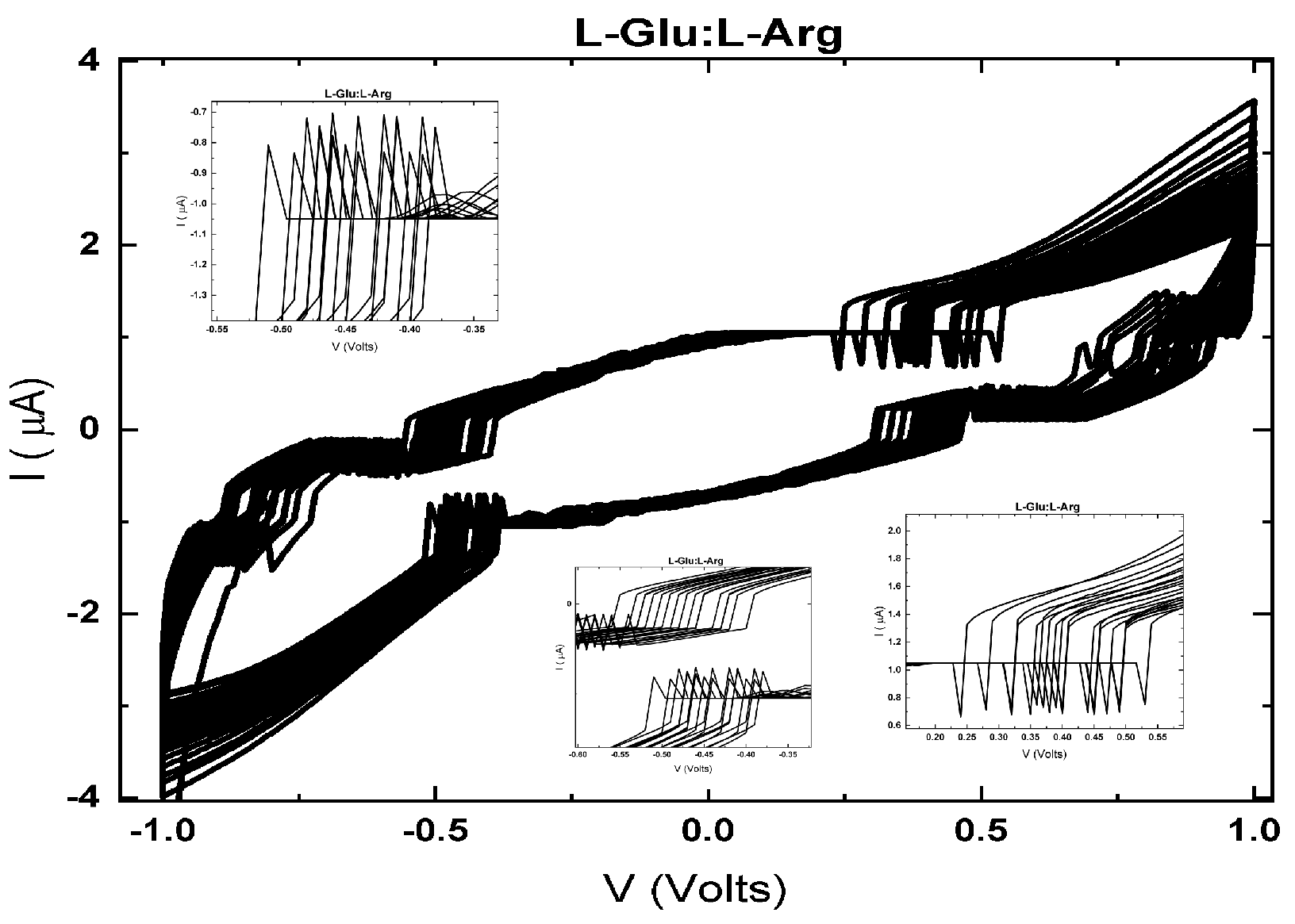
6.1. Memristive and Memcapacitive Behaviors
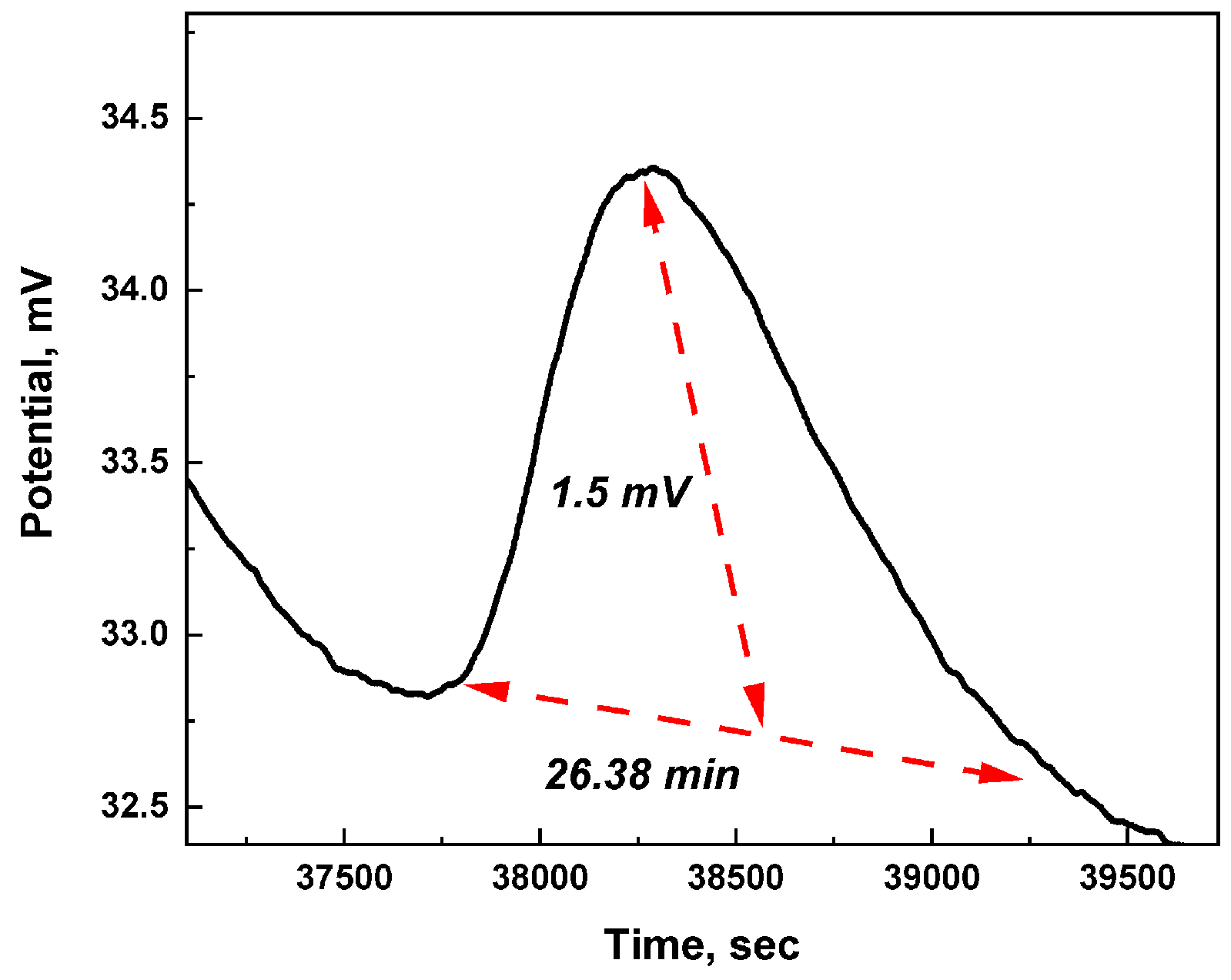
6.1.1. Electrical Excitability
6.1.2. Ionic and Protonic Conduction
6.2. Configurable Analog Logic Operations
6.3. Spiking Neural Network Dynamics
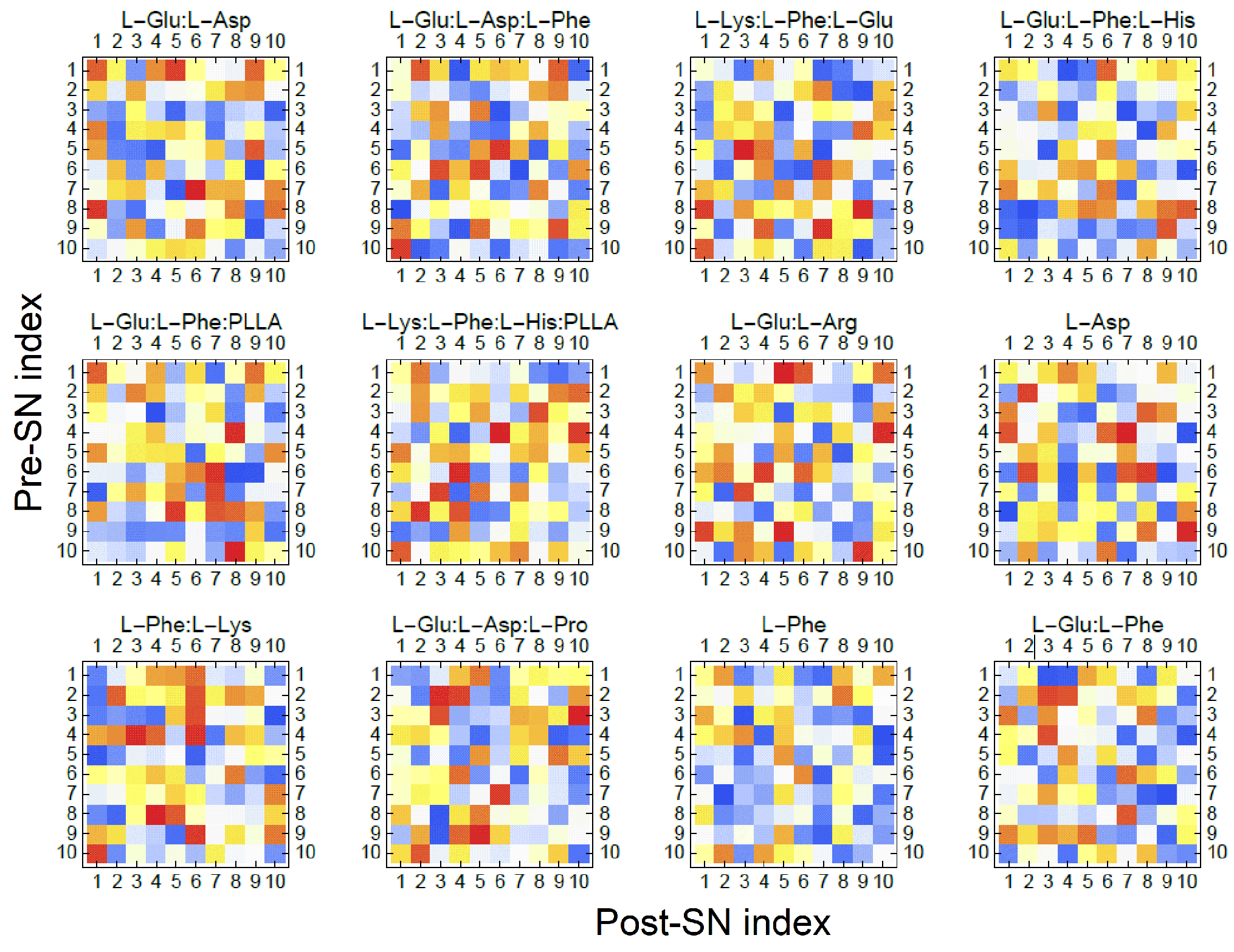
6.4. Evolutionary Learning Capacities
6.5. Hybrid Proteinoid–Inorganic Systems
6.6. Potential for Low-Power, Adaptive Biocomputing Devices
6.6.1. Memory and Switching Applications
6.6.2. Neuromorphic Circuits
6.6.3. Elucidating Synaptic-Like Linkages in Proteinoid Systems
6.6.4. Biosensing and Bioelectronics
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Pascal, R.; Pross, A.; Sutherland, J.D. Towards an evolutionary theory of the origin of life based on kinetics and thermodynamics. Open Biol. 2013, 3, 130156. [Google Scholar] [CrossRef]
- Bartlett, S.J.; Beckett, P. Probing complexity: Thermodynamics and computational mechanics approaches to origins studies. Interface Focus 2019, 9, 20190058. [Google Scholar] [CrossRef]
- Liang, S.; De Los Rios, P.; Busiello, D.M. Non-Equilibrium Chemical Reactions Under Non-Isothermal Conditions: Kinetic Stabilization, Selection and Thermophoresis. Master’s Thesis, Section of Physics, École Polytechnique Fédérale de Lausanne, Lausanne, Switzerland, 2020. [Google Scholar]
- Kleidon, A.; Lorenz, R.D. Non-Equilibrium Thermodynamics and the Production of Entropy: Life, Earth, and Beyond; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Fox, S.W. Thermodynamic perspectives and the origin of life. In Quantum Statistical Mechanics in the Natural Sciences: A Volume Dedicated to Lars Onsager on the Occasion of his Seventieth Birthday; Springer: Boston, MA, USA, 1974; pp. 119–142. [Google Scholar]
- Michaelian, K. Non-equilibrium thermodynamic foundations of the origin of life. Foundations 2022, 2, 308–337. [Google Scholar] [CrossRef]
- Ruiz-Mirazo, K.; Briones, C.; de la Escosura, A. Prebiotic systems chemistry: New perspectives for the origins of life. Chem. Rev. 2014, 114, 285–366. [Google Scholar] [CrossRef]
- Ruiz-Mirazo, K.; Briones, C.; de la Escosura, A. Chemical roots of biological evolution: The origins of life as a process of development of autonomous functional systems. Open Biol. 2017, 7, 170050. [Google Scholar] [CrossRef]
- Frenkel-Pinter, M.; Samanta, M.; Ashkenasy, G.; Leman, L.J. Prebiotic peptides: Molecular hubs in the origin of life. Chem. Rev. 2020, 120, 4707–4765. [Google Scholar] [CrossRef] [PubMed]
- Mann, S. Systems of creation: The emergence of life from nonliving matter. Accounts Chem. Res. 2012, 45, 2131–2141. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, H.S. The RNA world hypothesis: The worst theory of the early evolution of life (except for all the others). Biol. Direct 2012, 7, 1–10. [Google Scholar] [CrossRef]
- Robertson, M.P.; Joyce, G.F. The origins of the RNA world. Cold Spring Harb. Perspect. Biol. 2012, 4, a003608. [Google Scholar] [CrossRef] [PubMed]
- Joyce, G.F.; Orgel, L.E. Prospects for understanding the origin of the RNA world. Cold Spring Harb. Monogr. Ser. 1993, 24, 1–25. [Google Scholar]
- Orgel, L.E. Some consequences of the RNA world hypothesis. Orig. Life Evol. Biosph. 2003, 33, 211–218. [Google Scholar] [CrossRef]
- Sacerdote, M.; Szostak, J. Semipermeable lipid bilayers exhibit diastereoselectivity favoring ribose. Proc. Natl. Acad. Sci. USA 2005, 102, 6004–6008. [Google Scholar] [CrossRef] [PubMed]
- Pross, A.; Pascal, R. The origin of life: What we know, what we can know and what we will never know. Open Biol. 2013, 3, 120190. [Google Scholar] [CrossRef] [PubMed]
- Deamer, D. The role of lipid membranes in life’s origin. Life 2017, 7, 5. [Google Scholar] [CrossRef]
- Barge, L.; Branscomb, E.; Brucato, J.; Cardoso, S.; Cartwright, J.; Danielache, S.; Galante, D.; Kee, T.; Miguel, Y.; Mojzsis, S.; et al. Thermodynamics, disequilibrium, evolution: Far-from-equilibrium geological and chemical considerations for origin-of-life research. Orig. Life Evol. Biosph. 2017, 47, 39–56. [Google Scholar] [CrossRef]
- Ferris, J.P.; Hill, A.R., Jr.; Liu, R.; Orgel, L.E. Synthesis of long prebiotic oligomers on mineral surfaces. Nature 1996, 381, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.S.; Mishra, V.; Mukherjee, M.D.; Saini, P.; Ranjan, K.R. Amino acid derived biopolymers: Recent advances and biomedical applications. Int. J. Biol. Macromol. 2021, 188, 542–567. [Google Scholar] [CrossRef]
- Harada, K.; Matsuyama, M. Polycondensation of thermal precursors of amino acids and characterization of constituent amino acids. BioSystems 1979, 11, 47–53. [Google Scholar] [CrossRef]
- Paecht-Horowitz, M.; Berger, J.; Katchalsky, A. Prebiotic synthesis of polypeptides by heterogeneous polycondensation of amino-acid adenylates. Nature 1970, 228, 636–639. [Google Scholar] [CrossRef]
- Fox, S.W.; Harada, K. The thermal copolymerization of amino acids common to protein. J. Am. Chem. Soc. 1960, 82, 3745–3751. [Google Scholar] [CrossRef]
- Fox, S.W. Thermal proteins in the first life and in the “mind-body” problem. In Evolution of Information Processing Systems; Cerda-Olmedo, H.A., Eduardo, E.C., Guillermo, N., Erwin, T., Eds.; Springer: Berlin/Heidelberg, Germany, 1992; pp. 203–228. [Google Scholar]
- Przybylski, A.T.; Fox, S.W. Excitable artificial cells of proteinoid. Appl. Biochem. Biotechnol. 1984, 10, 301–307. [Google Scholar] [CrossRef]
- Wolman, Y.; Haverland, W.J.; Miller, S.L. Nonprotein amino acids from spark discharges and their comparison with the Murchison meteorite amino acids. Proc. Natl. Acad. Sci. USA 1972, 69, 809–811. [Google Scholar] [CrossRef]
- Przybylski, A.T.; Stratten, W.P.; Syren, R.M.; Fox, S.W. Membrane, action, and oscillatory potentials in simulated protocells. Naturwissenschaften 1982, 69, 561–563. [Google Scholar] [CrossRef]
- Egel, R. Origins and emergent evolution of life: The colloid microsphere hypothesis revisited. Orig. Life Evol. Biosph. 2014, 44, 87–110. [Google Scholar] [CrossRef]
- Nakashima, T. Metabolism of proteinoid microspheres. In Organic Geo-and Cosmochemistry; Springer: Berlin/Heidelberg, Germany, 2005; pp. 57–81. [Google Scholar]
- Schwartz, A.W.; Visscher, J.; Bakker, C.; Niessen, J. Nucleic acid-like structures II. Polynucleotide analogues as possible primitive precursors of nucleic acids. Orig. Life Evol. Biosph. 1987, 17, 351–357. [Google Scholar] [CrossRef]
- Hua, Z. On the origin of life: A possible way from Fox’s microspheres into primitive life. SOJ Biochem. 2018, 4, 1–7. [Google Scholar] [CrossRef]
- Kokufuta, E.; Sakai, H.; Harada, K. Factors controlling the size of proteinoid microspheres. BioSystems 1983, 16, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.W.; Bahn, P.R.; Dose, K.; Harada, K.; Hsu, L.; Ishima, Y.; Jungck, J.; Kendrick, J.; Krampitz, G.; Lacey, J.C., Jr.; et al. Experimental retracement of the origins of a protocell: It was also a protoneuron. J. Biol. Phys. 1995, 20, 17–36. [Google Scholar] [CrossRef]
- Ignatov, I.; Mosin, O. The Formation of Thermal Proteinoids in Hot Water. J. Med. Physiol. Biophys. 2016, 26, 15–27. [Google Scholar]
- Ignatov, I.; Mosin, O.S. Miller’s Experiments in Modelling of Non-Equilibrium Conditions with Gas Electric Discharge Simulating Primary Atmosphere. J. Med. Physiol. Biophys. 2015, 15, 61–76. [Google Scholar]
- Steinman, G.; Cole, M.N. Synthesis of biologically pertinent peptides under possible primordial conditions. Proc. Natl. Acad. Sci. USA 1967, 58, 735–742. [Google Scholar] [CrossRef]
- Fox, S.W. A theory of macromolecular and cellular origins. Nature 1965, 205, 328–340. [Google Scholar] [CrossRef]
- Fox, S.W.; Harada, K.; Kendrick, J. Production of spherules from synthetic proteinoid and hot water. Science 1959, 129, 1221–1223. [Google Scholar] [CrossRef]
- Rohlfing, D.L. Coacervate-like microspheres from lysine-rich proteinoid. Orig. Life 1975, 6, 203–209. [Google Scholar] [CrossRef]
- Fox, S.W.; Dose, K. Molecular Evolution and the Origin of Life; M. Dekker: New York, NY, USA, 1977. [Google Scholar]
- Fox, S.W. Metabolic microspheres: Origins and evolution. Naturwissenschaften 1980, 67, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Villanueva, D.; Ciaccia, M.; Iadevaia, G.; Sanna, E.; Hunter, C.A. Sequence information transfer using covalent template-directed synthesis. Chem. Sci. 2019, 10, 5258–5266. [Google Scholar] [CrossRef] [PubMed]
- Hansen, B.N.; Larsen, K.S.; Merkle, D.; Mihalchuk, A. DNA-templated synthesis optimization. Nat. Comput. 2018, 17, 693–707. [Google Scholar] [CrossRef]
- Sharma, S.; Mougoyannis, P.; Tarabella, G.; Adamatzky, A. A review on the protocols for the synthesis of proteinoids. arXiv 2022, arXiv:2212.02261. [Google Scholar]
- Matsuno, K. Molecular Evolution and Protobiology; Springer Science & Business Media: New York, NY, USA, 2012. [Google Scholar]
- Fox, S.W. Physical principles and proteinoid experiments in the emergence of life. In Information Processing in Biological Systems; Springer: Boston, MA, USA, 1985; pp. 69–91. [Google Scholar]
- Adamala, K.; Szostak, J.W. Nonenzymatic template-directed RNA synthesis inside model protocells. Science 2013, 342, 1098–1100. [Google Scholar] [CrossRef] [PubMed]
- Ertem, G.; Ferris, J.P. Synthesis of RNA oligomers on heterogeneous templates. Nature 1996, 379, 238–240. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.W. Origins of the protein synthesis cycle. Int. J. Quantum Chem. 1981, 20, 441–454. [Google Scholar] [CrossRef]
- Ratner, V.A.; Zharkikh, A.A.; Kolchanov, N.; Rodin, S.N.; Solovyov, V.V.; Antonov, A.S.; Ratner, V.A.; Zharkikh, A.A.; Kolchanov, N.; Rodin, S.N.; et al. The Origin and Evolution of the Genetic Coding-System. Molecular Evolution; Springer: Berlin/Heidelberg, Germany, 1996; pp. 39–70. [Google Scholar]
- Fox, S.W. Molecular evolution of the first cells. Pure Appl. Chem. 1973, 34, 641–670. [Google Scholar] [CrossRef]
- Snyder, W.; Fox, S.W. A model for the origin of stable protocells in a primitive alkaline ocean. BioSystems 1975, 7, 222–229. [Google Scholar] [CrossRef]
- Fox, S.W.; Jungck, J.R.; Nakashima, T. From proteinoid microsphere to contemporary cell: Formation of internucleotide and peptide bonds by proteinoid particles. Orig. Life 1974, 5, 227–237. [Google Scholar] [CrossRef]
- Bion, I.J. Electromagnetic origin of life. Electro-Magnetobiology 1998, 17, 401–413. [Google Scholar] [CrossRef]
- Deamer, D.W. Prebiotic amphiphilic compounds: Self-assembly and properties of early membrane structures. In Origins: Genesis, Evolution and Diversity of Life; Springer: Dordrecht, The Netherlands, 2004; pp. 75–89. [Google Scholar]
- Fox, S.W.; Harada, K. Thermal copolymerization of amino acids to a product resembling protein. Science 1958, 128, 1214. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.W. Bioorganic Chemistry and the Emergence of the First Cell; NASA: Washington, DC, USA, 1977.
- Adamatzky, A. Towards proteinoid computers. arXiv 2021, arXiv:2106.00883. [Google Scholar]
- Itzhaki, E.; Elias, Y.; Moskovits, N.; Stemmer, S.M.; Margel, S. Proteinoid Polymers and Nanocapsules for Cancer Diagnostics, Therapy and Theranostics: In Vitro and In Vivo Studies. J. Funct. Biomater. 2023, 14, 215. [Google Scholar] [CrossRef] [PubMed]
- Kolitz-Domb, M.; Margel, S. Engineering of novel proteinoids and PLLA-proteinoid polymers of narrow size distribution and uniform nano/micro-hollow particles for biomedical applications. In Advances in Bioengineering; IntechOpen: London, UK, 2015. [Google Scholar]
- Hsu, L.L.; Brooke, S.; Fox, S.W. Conjugation of proteinoid microspheres: A model of primordial communication. Biosystems 1971, 4, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Grimes, P.J.; Galanti, A.; Gobbo, P. Bioinspired networks of communicating synthetic protocells. Front. Mol. Biosci. 2021, 8, 804717. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.W. Coacervate droplets, proteinoid microspheres, and the genetic apparatus. In The Origin of Life and Evolutionary Biochemistry; Springer: Boston, MA, USA, 1974; pp. 119–132. [Google Scholar]
- Cannelli, S.M.; Gupta, R.; Nguyen, T.; Poddar, A.; Sharma, S.; Vithole, P.V.; Jia, T.Z. A compositional view comparing modern biological condensates and primitive phase-separated compartment. Pept. Sci. 2023, 115, e24331. [Google Scholar] [CrossRef]
- Cannelli, S.M.; Gupta, R.; Nguyen, T.; Poddar, A.; Sharma, S.; Vithole, P.V.; Jia, T.Z. Bridging the Gap between Primitive and Modern Phase Separation. ChemRxiv 2023. [Google Scholar] [CrossRef]
- Ikehara, K. How Did Life Emerge in Chemically Complex Messy Environments? Life 2022, 12, 1319. [Google Scholar] [CrossRef]
- Matveev, V.V. Cell theory, intrinsically disordered proteins, and the physics of the origin of life. Prog. Biophys. Mol. Biol. 2019, 149, 114–130. [Google Scholar] [CrossRef]
- Dalai, P.; Sahai, N. Protocell emergence and evolution. In Handbook of Astrobiology; CRC Press: Boca Raton, FL, USA, 2018; pp. 491–520. [Google Scholar]
- Ayoubi-Joshaghani, M.H.; Dianat-Moghadam, H.; Seidi, K.; Jahanban-Esfahalan, A.; Zare, P.; Jahanban-Esfahlan, R. Cell-free protein synthesis: The transition from batch reactions to minimal cells and microfluidic devices. Biotechnol. Bioeng. 2020, 117, 1204–1229. [Google Scholar] [CrossRef]
- Ishima, Y.; Przybylski, A.T.; Fox, S.W. Electrical membrane phenomena in spherules from proteinoid and lecithin. BioSystems 1981, 13, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.D.; Yang, S.R.; Cho, Y.W.; Park, K. 18 Fast Responsive Nanoparticles of Hydrophobically Modified Poly (Amino Acid) s and Proteinoids. In Reflexive Polymers and Hydrogels: Understanding and Designing Fast Responsive Polymeric Systems; CRC Press: Boca Raton, FL, USA, 2004; p. 373. [Google Scholar]
- Lugasi, L.; Grinberg, I.; Rudnick-Glick, S.; Okun, E.; Einat, H.; Margel, S. Designed proteinoid polymers and nanoparticles encapsulating risperidone for enhanced antipsychotic activity. J. Nanobiotechnol. 2020, 18, 149. [Google Scholar] [CrossRef] [PubMed]
- Bahn, P.R.; Fox, S.W. Models for protocellular photophosphorylation. BioSystems 1981, 14, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Hadad, E.; Rudnick-Glick, S.; Grinberg, I.; Kolitz-Domb, M.; Chill, J.H.; Margel, S. Synthesis and characterization of Poly (RGD) proteinoid polymers and NIR fluorescent nanoparticles of optimal D, L-configuration for drug-delivery applications—In vitro study. ACS Omega 2020, 5, 23568–23577. [Google Scholar] [CrossRef] [PubMed]
- Tallawi, M. Proteinoid/hydroxyapatite hybrid microsphere composites. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 96, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Santiago, N.; Chen, Y.S.; Chaudhary, K.; Milstein, S.J.; Baughman, R.A. Stability study of drug-loaded proteinoid microsphere formulations during freeze-drying. J. Drug Target. 1994, 2, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Quirk, S. Triggered release of small molecules from proteinoid microspheres. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2009, 91, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.M.; Rao, K.P. Preparation and characterization of pH-sensitive proteinoid microspheres for the oral delivery of methotrexate. Biomaterials 1998, 19, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Correa, P.N.; Correa, A.N. The PA & SAPA Bion Experiments and Proto-Prokaryotic Biopoiesis. J. Biophys. Hematol. Oncol. 2010, 1, 1–49. [Google Scholar]
- Monnard, P.A.; Deamer, D.W. Membrane self-assembly processes: Steps toward the first cellular life. Anat. Rec. Off. Publ. Am. Assoc. Anat. 2002, 268, 196–207. [Google Scholar] [CrossRef]
- Rasmussen, S.; Bailey, J.; Boncella, J.; Chen, L.; Collis, G.; Colgate, S.; DeClue, M.; Fellermann, H.; Goranovic, G.; Jiang, Y.; et al. Assembly of a minimal protocell. In Protocells: Bridging Nonliving and Living Matter; Rasmussen, S., Bedau, M., Chen, L., Deamer, D., Krakauer, D., Packard, N., Stadler, P., Eds.; Massachusetts Institute of Technology: Cambridge, MA, USA, 2008; pp. 125–156. [Google Scholar]
- Peterson, I. Proteinoids: Clues to Cellular Origins? BioScience 1985, 35, 74–76. [Google Scholar] [CrossRef]
- Ottenbrite, R.M.; Fadeeva, N. Polymer systems for biomedical applications: An overview. ACS Symp. Ser. 1994, 545, 1–14. [Google Scholar]
- Ling, G.N. The functions of polarized water and membrane lipids: A rebuttal. Physiol. Chem. Phys. 1977, 9, 301–311. [Google Scholar]
- Monnard, P.A.; Walde, P. Current ideas about prebiological compartmentalization. Life 2015, 5, 1239–1263. [Google Scholar] [CrossRef]
- Wicken, J.S. An organismic critique of molecular Darwinism. J. Theor. Biol. 1985, 117, 545–561. [Google Scholar] [CrossRef]
- Fox, S.W. Stereomolecular interactions and microsystems in experimental protobiogenesis. BioSystems 1975, 7, 22–36. [Google Scholar] [CrossRef]
- Bilotta, E.; Lafusa, A.; Pantano, P. Life-like self-reproducers. Complexity 2003, 9, 38–55. [Google Scholar] [CrossRef]
- Kauffman, S.A. Metabolic stability and epigenesis in randomly constructed genetic nets. J. Theor. Biol. 1969, 22, 437–467. [Google Scholar] [CrossRef] [PubMed]
- Bilotta, E.; Pantano, P. Biological traits in artificial self-reproducing systems. In Reflexing Interfaces: The Complex Coevolution of Information Technology Ecosystems; IGI Global: Hershey, PA, USA, 2008; pp. 109–123. [Google Scholar]
- Solé, R. Synthetic transitions: Towards a new synthesis. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150438. [Google Scholar] [CrossRef]
- Kauffman, S.A. Cellular homeostasis, epigenesis and replication in randomly aggregated macromolecular systems. J. Cybern. 1971, 1, 71–96. [Google Scholar] [CrossRef]
- Abbas, M.; Lipiński, W.P.; Wang, J.; Spruijt, E. Peptide-based coacervates as biomimetic protocells. Chem. Soc. Rev. 2021, 50, 3690–3705. [Google Scholar] [CrossRef]
- Wu, H.; Qiao, Y. Engineering coacervate droplets towards the building of multiplex biomimetic protocells. Supramol. Mater. 2022, 1, 100019. [Google Scholar] [CrossRef]
- Wei, M.; Lin, Y.; Qiao, Y. Engineered Colloidosomes as Biomimetic Cellular Models. Giant 2023, 13, 100143. [Google Scholar] [CrossRef]
- Elbourne, A.; Crawford, R.J.; Ivanova, E.P. Nano-structured antimicrobial surfaces: From nature to synthetic analogues. J. Colloid Interface Sci. 2017, 508, 603–616. [Google Scholar] [CrossRef]
- Laursen, J.B.; Nielsen, J. Phenazine natural products: Biosynthesis, synthetic analogues, and biological activity. Chem. Rev. 2004, 104, 1663–1686. [Google Scholar] [CrossRef]
- Kilgour, O. Irritability. In Mastering Biology; Springer: London, UK, 1987; pp. 270–303. [Google Scholar]
- Koppenhöfer, E. Electrical responses of isolated protoplasm from Nitella. Pflügers Arch. 1975, 358, 179–187. [Google Scholar] [CrossRef]
- Bentrup, F.W. Cell electrophysiology and membrane transport. In Progress in Botany; Springer: Berlin/Heidelberg, Germany, 1989; pp. 70–79. [Google Scholar]
- Dennis, R.G.; Kosnik, P.E.; Gilbert, M.E.; Faulkner, J.A. Excitability and contractility of skeletal muscle engineered from primary cultures and cell lines. Am. J. Physiol.-Cell Physiol. 2001, 280, C288–C295. [Google Scholar] [CrossRef]
- De Biase, L.M.; Nishiyama, A.; Bergles, D.E. Excitability and synaptic communication within the oligodendrocyte lineage. J. Neurosci. 2010, 30, 3600–3611. [Google Scholar] [CrossRef] [PubMed]
- Teorell, T. Excitability phenomena in artificial membranes. Biophys. J. 1962, 2, 25. [Google Scholar] [CrossRef] [PubMed]
- Matsuno, K. Proteinoid Microsphere. In Encyclopedia of Astrobiology; Gargaud, M., Irvine, W.M., Amils, R., Cleaves, H.J.J., Pinti, D.L., Quintanilla, J.C., Rouan, D., Spohn, T., Tirard, S., Viso, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 2033–2034. [Google Scholar] [CrossRef]
- Stratten, W.P. Protocell action potentials: A new perspective of bio-excitation. In Molecular Evolution and Protobiology; Springer: Boston, MA, USA, 1984; pp. 233–251. [Google Scholar]
- Przybylski, A.T.; Fox, S.W. Electrical phenomena in proteinoid cells. In Modern Bioelectrochemistry; Springer: Boston, MA, USA, 1986; pp. 377–396. [Google Scholar]
- Matsuno, K. Electrical excitability of proteinoid microspheres composed of basic and acidic proteinoids. BioSystems 1984, 17, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Takinoue, M. Creation of artificial cell-like structures promoted by microfluidics technologies. Micromachines 2019, 10, 216. [Google Scholar] [CrossRef] [PubMed]
- Dose, K. Chemical and catalytical properties of thermal polymers of amino acids (proteinoids). Orig. Life 1974, 5, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.W.; Nakashima, T. Fractionation and characterization of an amidated thermal 1: 1: 1-proteinoid. Biochim. Biophys. Acta (BBA) Protein Struct. 1967, 140, 155–167. [Google Scholar] [CrossRef]
- Fox, S.W. The Emergence of Life: Darwinian Evolution from the Inside; Basic Books: New York, NY, USA, 1988. [Google Scholar]
- Fox, S.W. The Origins of Prebiological Systems and of Their Molecular Matrices: Proceedings of a Conference Conducted at Wakulla Springs, Florida, on 27–30 October 1963 under the Auspices of the Institute for Space Biosciences, the Florida State University and the National Aeronautics and Space Administration; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Fox, S.W. Molecular selection and natural selection. Q. Rev. Biol. 1986, 61, 375–386. [Google Scholar] [CrossRef]
- Gish, D.T. Speculations and Experiments Related to Theories on the Origin of Life: A Critique; Institute for Creation Research: Dallas, TX, USA, 1972. [Google Scholar]
- Tkachenko, A.V.; Maslov, S. Spontaneous emergence of autocatalytic information-coding polymers. J. Chem. Phys. 2015, 143, 045102. [Google Scholar] [CrossRef]
- Black, R.A.; Blosser, M.C. A self-assembled aggregate composed of a fatty acid membrane and the building blocks of biological polymers provides a first step in the emergence of protocells. Life 2016, 6, 33. [Google Scholar] [CrossRef]
- Monnard, P.A.; Apel, C.L.; Kanavarioti, A.; Deamer, D.W. Influence of ionic inorganic solutes on self-assembly and polymerization processes related to early forms of life: Implications for a prebiotic aqueous medium. Astrobiology 2002, 2, 139–152. [Google Scholar] [CrossRef]
- Fox, S.W.; Nakashima, T. The assembly and properties of protobiological structures: The beginnings of cellular peptide synthesis. BioSystems 1980, 12, 155–166. [Google Scholar] [CrossRef]
- Ubrich, N.; Maincent, P. Recent advances in heparin delivery. Drugs Pharm. Sci. 2006, 158, 481. [Google Scholar]
- Xu, J.; Vanderlick, T.K.; LaVan, D.A. Energy conversion in protocells with natural nanoconductors. Int. J. Photoenergy 2011, 2012, 425735. [Google Scholar] [CrossRef]
- Bae, S.K.; Kim, J.D. Aggregation behaviors and their pH sensitivity of cholesterol-conjugated proteinoids composed of glutamic acid and aspartic acid matrix. J. Biomed. Mater. Res. Part A 2003, 64, 282–290. [Google Scholar] [CrossRef]
- Haratake, M.; Ottenbrite, R.M. Oligopeptides as an oral delivery system: I. Aggregation characteristics and drug encapsulation. J. Bioact. Compat. Polym. 1997, 12, 112–126. [Google Scholar] [CrossRef]
- Jia, T.Z.; Wang, P.H.; Niwa, T.; Mamajanov, I. Connecting primitive phase separation to biotechnology, synthetic biology, and engineering. J. Biosci. 2021, 46, 79. [Google Scholar] [CrossRef] [PubMed]
- Freire, M.Á. Nontemplate-driven polymers: Clues to a minimal form of organization closure at the early stages of living systems. Theory Biosci. 2015, 134, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Dose, K. Of thermal polymers of amino acids (proteinoids). In Cosmochemical Evolution and the Origins of Life: Proceedings of the Fourth International Conference on the Origin of Life and the First Meeting of the International Society for the Study of the Origin of Life, Barcelona, 25–28 June 1973, Volume I: Invited Papers and Volume II: Contributed Papers; Springer Science & Business Media: Dordrecht, The Netherlands, 2013; p. 239. [Google Scholar]
- Matsuno, K. A theoretical construction of protobiological synthesis: From amino acids to functional protocells. Int. J. Quantum Chem. 1982, 22, 181–193. [Google Scholar] [CrossRef]
- Brochier-Armanet, C. Minimal cell: The biologist’s point of view. Orig. Evol. Life: Astrobiol. Perspect. 2011, 6, 26. [Google Scholar]
- Korn, R.W. Biological organization—A new look at an old problem. BioScience 1999, 49, 51–57. [Google Scholar] [CrossRef]
- Silverstein, A.M. A History of Immunology; Academic Press: Cambridge, MA, USA, 2009. [Google Scholar]
- Shaw, G.H. Evidence and arguments for methane and ammonia in Earth’s earliest atmosphere and an organic compound-rich early ocean. In Earth’s Early Atmosphere and Surface Environment; Special Paper 504; Geological Society of America: Boulder, CO, USA, 2014; pp. 1–10. [Google Scholar]
- Miller, S.L.; Urey, H.C.; Oró, J. Origin of organic compounds on the primitive earth and in meteorites. J. Mol. Evol. 1976, 9, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Baross, J.A. The rocky road to biomolecules. Nature 2018, 564, 42–43. [Google Scholar] [CrossRef]
- Ferrazzano, L.; Catani, M.; Cavazzini, A.; Martelli, G.; Corbisiero, D.; Cantelmi, P.; Fantoni, T.; Mattellone, A.; De Luca, C.; Felletti, S.; et al. Sustainability in peptide chemistry: Current synthesis and purification technologies and future challenges. Green Chem. 2022, 24, 975–1020. [Google Scholar] [CrossRef]
- Jensen, K.J.; Shelton, P.T.; Pedersen, S.L. Peptide Synthesis and Applications; Springer: New York, NY, USA, 2013. [Google Scholar]
- Nagendrappa, G.; Chowreddy, R.R. Organic reactions using clay and clay-supported catalysts: A survey of recent literature. Catal. Surv. Asia 2021, 25, 231–278. [Google Scholar] [CrossRef]
- Mougkogiannis, P.; Adamatzky, A. Learning in ensembles of proteinoid microspheres. arXiv 2023, arXiv:2306.14362. [Google Scholar] [CrossRef]
- Manouras, T.; Vamvakaki, M. Field responsive materials: Photo-, electro-, magnetic-and ultrasound-sensitive polymers. Polym. Chem. 2017, 8, 74–96. [Google Scholar] [CrossRef]
- Manoj, K.M.; Tamagawa, H. Revisiting the mechanisms for cellular homeostasis and electrophysiological responses: Classical membrane theory, association-induction hypothesis and murburn concept. OSF Preprints 2020. [Google Scholar] [CrossRef]
- Kompanichenko, V.; Kotsyurbenko, O. Role of Stress in the Origin of Life. Life 2022, 12, 1930. [Google Scholar] [CrossRef] [PubMed]
- Woese, C.R. A proposal concerning the origin of life on the planet earth. J. Mol. Evol. 1979, 13, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Mougkogiannis, P.; Adamatzky, A. Proteinoid microspheres as protoneural networks. ACS Omega 2023, 8, 35417–35426. [Google Scholar] [CrossRef] [PubMed]
- Mougkogiannis, P.; Adamatzky, A. Logical gates in ensembles of proteinoid microspheres. PLoS ONE 2023, 18, e0289433. [Google Scholar] [CrossRef] [PubMed]
- Brack, A. From interstellar amino acids to prebiotic catalytic peptides: A review. Chem. Biodivers. 2007, 4, 665–679. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, Y.; Nakazawa, H.; Sekine, T.; Kobayashi, T.; Kakegawa, T. Nucleobase and amino acid formation through impacts of meteorites on the early ocean. Earth Planet. Sci. Lett. 2015, 429, 216–222. [Google Scholar] [CrossRef]
- Pasek, M.; Lauretta, D. Extraterrestrial flux of potentially prebiotic C, N, and P to the early Earth. Orig. Life Evol. Biosph. 2008, 38, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Sachar, M.; Anderson, K.E.; Ma, X. Protoporphyrin IX: The good, the bad, and the ugly. J. Pharmacol. Exp. Ther. 2016, 356, 267–275. [Google Scholar] [CrossRef]
- Suo, Z.; Avci, R.; Schweitzer, M.H.; Deliorman, M. Porphyrin as an ideal biomarker in the search for extraterrestrial life. Astrobiology 2007, 7, 605–615. [Google Scholar] [CrossRef]
- Lozovaya, G.; Masinovsky, Z.; Sivash, A. Protoporphyrin IX as a possible ancient photosensitizer: Spectral and photochemical studies. Orig. Life Evol. Biosph. 1990, 20, 321–330. [Google Scholar] [CrossRef]
- Matveev, V.V. Membraneless physiology of the living cell. The past and the present. 4open 2022, 5, 15. [Google Scholar] [CrossRef]
- Wilson, J.W. Virchow’s contribution to the cell theory. J. Hist. Med. Allied Sci. 1947, 2, 163–178. [Google Scholar] [CrossRef]
- DeWalt, D.A.; Pincus, T. The legacies of Rudolf Virchow: Cellular medicine in the 20th century and social medicine in the 21st century. IMAJ-RAMAT GAN 2003, 5, 395–397. [Google Scholar]
- Maulitz, R.C. Rudolf Virchow, Julius Cohnheim and the program of pathology. Bull. Hist. Med. 1978, 52, 162–182. [Google Scholar]
- Matveev, V. Comparison of fundamental physical properties of the model cells (protocells) and the living cells reveals the need in protophysiology. Int. J. Astrobiol. 2017, 16, 97–104. [Google Scholar] [CrossRef]
- Patten, B.C. Network orientors: Steps toward a cosmography of ecosystems: Orientors for directional development, self-organization, and autoevolution. In Eco Targets, Goal Functions, and Orientors; Springer: Berlin/Heidelberg, Germany, 1998; pp. 137–160. [Google Scholar]
- Weber, B. What does natural selection have to be like in order to work with self-organization? Cybern. Hum. Knowing 1998, 5, 18–31. [Google Scholar]
- Yockey, H.P. Self organization origin of life scenarios and information theory. J. Theor. Biol. 1981, 91, 13–31. [Google Scholar] [CrossRef]
- Abel, D.L. The Birth of Protocells. First Gene 2011, 504, 189–230. [Google Scholar]
- Mitleton-Kelly, E. Ten principles of complexity and enabling infrastructures. Complex Syst. Evol. Perspect. Organ. Appl. Complex. Theory Organ. 2003, 1, 23–50. [Google Scholar]
- Spitzer, J.; Pielak, G.J.; Poolman, B. Emergence of life: Physical chemistry changes the paradigm. Biol. Direct 2015, 10, 33. [Google Scholar] [CrossRef]
- Pross, A. On the emergence of biological complexity: Life as a kinetic state of matter. Orig. Life Evol. Biosph. 2005, 35, 151–166. [Google Scholar] [CrossRef]
- Trifonov, E.N. Tracing life back to elements. Phys. Life Rev. 2008, 5, 121–132. [Google Scholar] [CrossRef]
- Pino, S.; Trifonov, E.N.; Di Mauro, E. On the observable transition to living matter. Genom. Proteom. Bioinform. 2011, 9, 7–14. [Google Scholar] [CrossRef]
- Di Mauro, E.; Dunker, A.K.; Trifonov, E.N. Disorder to order, nonlife to life: In the beginning there was a mistake. In Genesis—In the Beginning: Precursors of Life, Chemical Models and Early Biological Evolution; Springer: Dordrecht, The Netherlands, 2012; pp. 415–435. [Google Scholar]
- Trifonov, E.N.; Gabdank, I.; Barash, D.; Sobolevsky, Y. Primordia vita. Deconvolution from modern sequences. Orig. Life Evol. Biosph. 2006, 36, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Kompanichenko, V. Thermodynamic inversion and self-reproduction with variations: Integrated view on the life-nonlife border. J. Biomol. Struct. Dyn. 2012, 29, 637–639. [Google Scholar] [CrossRef] [PubMed]
- Walde, P.; Boiteau, L. Prebiotic Chemistry: From Simple Amphiphiles to Protocell Models; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2005; Volume 259. [Google Scholar]
- Imai, M.; Sakuma, Y.; Kurisu, M.; Walde, P. From vesicles toward protocells and minimal cells. Soft Matter 2022, 18, 4823–4849. [Google Scholar] [CrossRef] [PubMed]
- Kahana, A.; Lancet, D. Self-reproducing catalytic micelles as nanoscopic protocell precursors. Nat. Rev. Chem. 2021, 5, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Kompanichenko, V. Origin of life by thermodynamic inversion: A universal process. In Genesis—In The Beginning: Precursors of Life, Chemical Models and Early Biological Evolution; Springer: Dordrecht, The Netherlands, 2012; pp. 305–320. [Google Scholar]
- Hua, Z. From Fox’s microspheres into primitive life: An inferencing hypothesis on the origin of life. Preprints 2018, 2018080547. [Google Scholar] [CrossRef]
- Berger, G. Deterministic hypotheses on the origin of life and of its reproduction. Med. Hypotheses 2003, 61, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Michaelian, K.; Simeonov, A. Fundamental molecules of life are pigments which arose and co-evolved as a response to the thermodynamic imperative of dissipating the prevailing solar spectrum. Biogeosciences 2015, 12, 4913–4937. [Google Scholar] [CrossRef]
- Willems, J.C. Dissipative dynamical systems part I: General theory. Arch. Ration. Mech. Anal. 1972, 45, 321–351. [Google Scholar] [CrossRef]
- Lyapunov, A.M. The general problem of the stability of motion. Int. J. Control 1992, 55, 531–534. [Google Scholar] [CrossRef]
- Kraft, K.S.; Freeman, J.J.; Serwinski, P.; Otto, V.P.; Kaur, P.N. Methods for the Synthesis of Activated Ethylfumarates and Their Use as Intermediates. U.S. Patent US 11,479,535 B2, 25 October 2022. [Google Scholar]
- Kritsky, M.; Telegina, T.; Buglak, A.; Kolesnikov, M.; Lyudnikova, T.; Vechtomova, Y.L. Modeling of abiotic ATP synthesis in the context of problems of early biosphere evolution. Geochem. Int. 2014, 52, 1227–1238. [Google Scholar] [CrossRef]
- Amaroli, A.; Ravera, S.; Zekiy, A.; Benedicenti, S.; Pasquale, C. A narrative review on oral and periodontal bacteria Microbiota photobiomodulation, through visible and near-infrared light: From the origins to modern therapies. Int. J. Mol. Sci. 2022, 23, 1372. [Google Scholar] [CrossRef] [PubMed]
- Egel, R. Life’s order, complexity, organization, and its thermodynamic-holistic imperatives. Life 2012, 2, 323–363. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.; Frankel, A.D. Structural variety of arginine-rich RNA-binding peptides. Proc. Natl. Acad. Sci. USA 1995, 92, 5282–5286. [Google Scholar] [CrossRef]
- García-García, C.; Draper, D.E. Electrostatic interactions in a peptide–RNA complex. J. Mol. Biol. 2003, 331, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Kikvidze, Z.; Callaway, R.M. Ecological facilitation may drive major evolutionary transitions. BioScience 2009, 59, 399–404. [Google Scholar] [CrossRef][Green Version]
- Abdelouahab, M.S.; Lozi, R.; Chua, L. Memfractance: A mathematical paradigm for circuit elements with memory. Int. J. Bifurc. Chaos 2014, 24, 1430023. [Google Scholar] [CrossRef]
- Przybylski, A.T. Excitable cell made of thermal proteinoids. BioSystems 1985, 17, 281–288. [Google Scholar] [CrossRef]
- Müller-Herold, U.; Nickel, G. The stability of proteinoid microspheres. Biosystems 1994, 33, 215–220. [Google Scholar] [CrossRef]
- Mougkogiannis, P.; Adamatzky, A. Low frequency electrical waves in ensembles of proteinoid microspheres. Sci. Rep. 2023, 13, 1992. [Google Scholar] [CrossRef] [PubMed]
- Mougkogiannis, P.; Adamatzky, A. Light induced spiking of proteinoids. arXiv 2023, arXiv:2303.17563. [Google Scholar] [CrossRef] [PubMed]
- Kolitz-Domb, M.; Margel, S. Recent advances of novel proteinoids and proteinoid nanoparticles and their applications in biomedicine and industrial uses. Isr. J. Chem. 2018, 58, 1277–1285. [Google Scholar] [CrossRef]
- Manoj, K.M.; Tamagawa, H. Critical analysis of explanations for cellular homeostasis and electrophysiology from murburn perspective. J. Cell. Physiol. 2022, 237, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Manoj, K.M.; Bazhin, N.; Tamagawa, H. The murburn precepts for cellular ionic homeostasis and electrophysiology. J. Cell. Physiol. 2022, 237, 804–814. [Google Scholar] [CrossRef]
- Andrade, E. The processing of information (analog/digital) is the causal factor of the emergence of natural hierarchies. Ludus Vitalis 2003, 11, 85–106. [Google Scholar]
- Browner, D.; Sareh, S.; Anderson, P. Additive manufacture of polymeric organometallic ferroelectric diodes (POMFeDs) for structural neuromorphic hardware. In Proceedings of the 2023 Annual Neuro-Inspired Computational Elements Conference, San Antonio, TX, USA, 11–13 April 2023; pp. 92–99. [Google Scholar]
- Dose, K. Self-instructed condensation of amino acids and the origin of biological information. Int. J. Quantum Chem. 1984, 26, 91–101. [Google Scholar] [CrossRef]
- Mamajanov, I.; Caudan, M.; Jia, T.Z. Protoenzymes: The case of hyperbranched polymer-scaffolded ZnS nanocrystals. Life 2020, 10, 150. [Google Scholar] [CrossRef]
- Kauffman, S.A. Autocatalytic sets of proteins. J. Theor. Biol. 1986, 119, 1–24. [Google Scholar] [CrossRef]
- Fox, S.W.; Nakashima, T.; Przybylski, A.; Syren, R.M. The updated experimental proteinoid model. Int. J. Quantum Chem. 1982, 22, 195–204. [Google Scholar] [CrossRef]
- Mann, S. Biomineralization: Principles and Concepts in Bioinorganic Materials Chemistry; Oxford University Press: New York, NY, USA, 2001. [Google Scholar]
- Babu, A.K.; Raja, M.M.M.; Zehravi, M.; Mohammad, B.D.; Anees, M.I.; Prasad, C.; Yahya, B.A.; Sultana, R.; Sharma, R.; Singh, J.; et al. An overview of polymer surface coated synthetic quantum dots as therapeutics and sensors applications. Prog. Biophys. Mol. Biol. 2023, 184, 1–12. [Google Scholar] [CrossRef]
- Kolitz-Domb, M.; Corem-Salkmon, E.; Grinberg, I.; Margel, S. Synthesis and characterization of bioactive conjugated near-infrared fluorescent proteinoid-poly (L-lactic acid) hollow nanoparticles for optical detection of colon cancer. Int. J. Nanomed. 2014, 9, 5041–5053. [Google Scholar]
- Basu, A.; Acharya, J.; Karnik, T.; Liu, H.; Li, H.; Seo, J.S.; Song, C. Low-power, adaptive neuromorphic systems: Recent progress and future directions. IEEE J. Emerg. Sel. Top. Circuits Syst. 2018, 8, 6–27. [Google Scholar] [CrossRef]
- ávan Doremaele, E.R.; de Burgt, Y. Towards organic neuromorphic devices for adaptive sensing and novel computing paradigms in bioelectronics. J. Mater. Chem. C 2019, 7, 12754–12760. [Google Scholar] [CrossRef]
- Abedin, M.I. RRAM Device Optimization and Circuit Design for Low Power Low Latency Domain Specific Edge Applications. Ph.D. Thesis, College of Nanoscale Science and Engineering, Albany, NY, USA, 2023. [Google Scholar]
- Liu, X.; Wang, F.; Su, J.; Zhou, Y.; Ramakrishna, S. Bio-Inspired 3D Artificial Neuromorphic Circuits. Adv. Funct. Mater. 2022, 32, 2113050. [Google Scholar] [CrossRef]
- Sorkin, A.; Von Zastrow, M. Endocytosis and signalling: Intertwining molecular networks. Nat. Rev. Mol. Cell Biol. 2009, 10, 609–622. [Google Scholar] [CrossRef]
- Marks, F.; Klingmüller, U.; Müller-Decker, K. Cellular Signal Processing: An Introduction to the Molecular Mechanisms of Signal Transduction; Garland Science: New York, NY, USA, 2017. [Google Scholar]
- Mu, Q.; Jiang, G.; Chen, L.; Zhou, H.; Fourches, D.; Tropsha, A.; Yan, B. Chemical basis of interactions between engineered nanoparticles and biological systems. Chem. Rev. 2014, 114, 7740–7781. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.W. The origins of behavior in macromolecules and protocells. Comp. Biochem. Physiol. Part B Comp. Biochem. 1980, 67, 423–436. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Huang, X. Bioinspired Protein-Based Assembling: Toward Advanced Life-Like Behaviors. Adv. Mater. 2020, 32, 2001436. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Singh, M.; Pareek, D.; Wasnik, K.; Gupta, P.S.; Paik, P. Advances in the Development of Biodegradable Polymeric Materials for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]


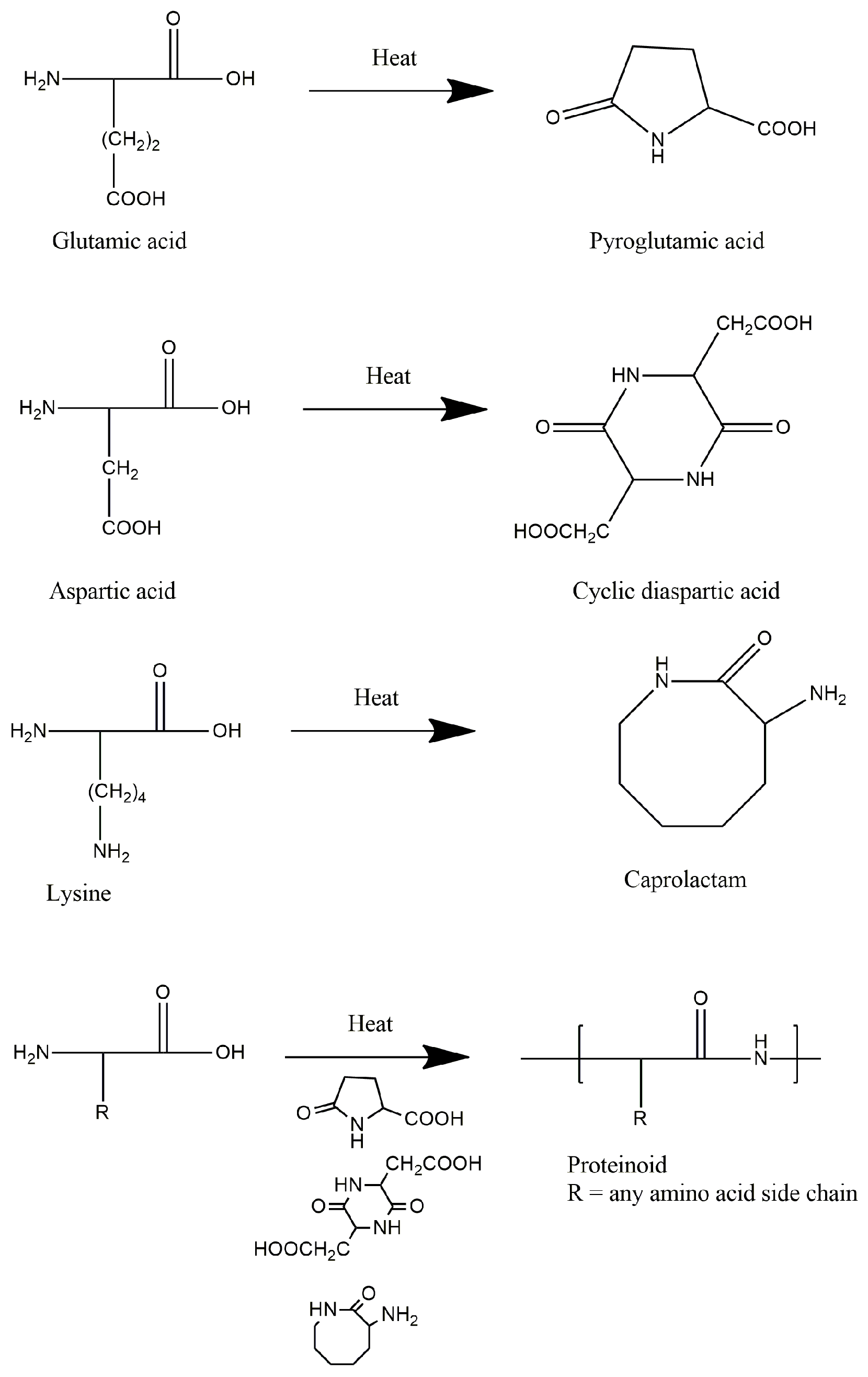


| Type of Proteinoid | Amino Acid Used | Uses |
|---|---|---|
| Dipeptide | Diphenylalanine, Glyoxylamide, Tryptophan | Scaffolds, carriers, tissue engineering |
| Tripeptide | Cysteine, Phenylalanine | Biosensing, biomedicine |
| Tetrapeptide | Glycine, Phenylalanine, Tyrosine, lysine | Ocular drug delivery |
| Pentapeptide | Histidine, Proline, Lysine, Valine | Self-repair, cell transplantation |
| Hexapeptide | Alanine, Valine, Glycine, Proline | pH measurement, metastasis suppression |
| Oligo-peptide | Phenylalanine, Tryptophan | VCatalysis, immunisation, biosensing |
| Polypeptide | Arginine, Glycine, Aspartic acid, Glutamic acid | Anti-inflammatory drug delivery, cell culture |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mougkogiannis, P.; Adamatzky, A. Proto-Neurons from Abiotic Polypeptides. Encyclopedia 2024, 4, 512-543. https://doi.org/10.3390/encyclopedia4010034
Mougkogiannis P, Adamatzky A. Proto-Neurons from Abiotic Polypeptides. Encyclopedia. 2024; 4(1):512-543. https://doi.org/10.3390/encyclopedia4010034
Chicago/Turabian StyleMougkogiannis, Panagiotis, and Andrew Adamatzky. 2024. "Proto-Neurons from Abiotic Polypeptides" Encyclopedia 4, no. 1: 512-543. https://doi.org/10.3390/encyclopedia4010034
APA StyleMougkogiannis, P., & Adamatzky, A. (2024). Proto-Neurons from Abiotic Polypeptides. Encyclopedia, 4(1), 512-543. https://doi.org/10.3390/encyclopedia4010034