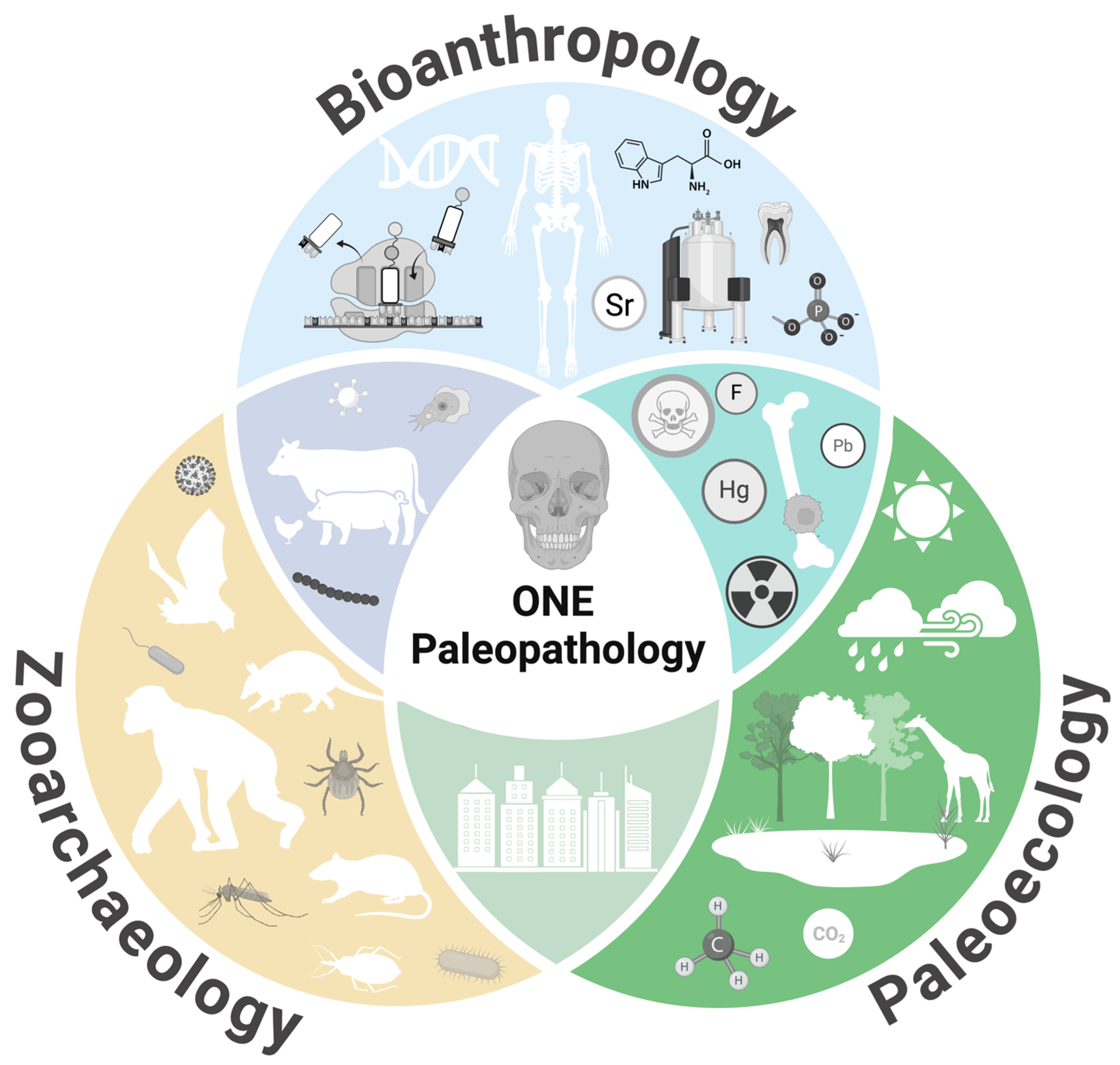
From ONE Health to ONE Paleopathology: Deep-Time Perspectives on Health in the Face of Climate and Environmental Change
Definition
1. Introduction
2. ONE Paleopathology Approaches to Key Health Topics
2.1. Animals as Sentinels
2.2. Climate Change, Zoonoses, and ILK
2.3. Ancient Syndemics, Urbanism, and Pollution
3. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Conrad, L.I. The Western Medical Tradition: 800 BC to AD 1800; Cambridge University Press: Cambridge, UK, 1995; Volume 1. [Google Scholar]
- Zinsstag, J.; Schelling, E.; Waltner-Toews, D.; Tanner, M. From “ONE Medicine” to “ONE Health” and Systemic Approaches to Health and Well-Being. Prev. Vet. Med. 2011, 101, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Brenna, C.T. Bygone Theatres of Events: A History of Human Anatomy and Dissection. Anat. Rec. 2022, 305, 788–802. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.K. Human Cadaveric Dissection: A Historical Account from Ancient Greece to the Modern Era. Anat. Cell Biol. 2015, 48, 153–169. [Google Scholar] [CrossRef] [PubMed]
- Schwabe, C.W. Veterinary Medicine and Human Health; Williams & Wilkins: Baltimore, MD, USA, 1984. [Google Scholar]
- Gyles, C. One Medicine, One Health, One World. Can. Vet. J. 2016, 57, 345. [Google Scholar] [PubMed]
- Zhou, X.; Zheng, J. Building a Transdisciplinary Science of One Health with a Global Vision. Glob. Health J. 2024, 8, 99–102. [Google Scholar] [CrossRef]
- Whitmee, S.; Haines, A.; Beyrer, C.; Boltz, F.; Capon, A.G.; De Souza Dias, B.F.; Ezeh, A.; Frumkin, H.; Gong, P.; Head, P.; et al. Safeguarding Human Health in the Anthropocene Epoch: Report of the Rockefeller Foundation–Lancet Commission on Planetary Health. Lancet 2015, 386, 1973–2028. [Google Scholar] [CrossRef] [PubMed]
- Bird-David, N. “Animism” Revisited: Personhood, Environment, and Relational Epistemology. Curr. Anthropol. 1999, 40, S67–S91. [Google Scholar] [CrossRef]
- Hernandez, J. Fresh Banana Leaves: Healing Indigenous Landscapes Through Indigenous Science; North Atlantic Books: Berkeley, CA, USA, 2022. [Google Scholar]
- Kimmerer, R. Braiding Sweetgrass: Indigenous Wisdom, Scientific Knowledge and the Teachings of Plants; Milkweed Editions: Minneapolis, MN, USA, 2013. [Google Scholar]
- Bartosiewicz, L.; Mansouri, K. Zooarchaeology and the Paleopathological Record. In The Routledge Handbook of Paleopathology; Routledge: London, UK, 2022; pp. 557–575. [Google Scholar]
- Gluckman, P.D.; Low, F.M.; Hanson, M.A. Anthropocene-Related Disease: The Inevitable Outcome of Progressive Niche Modification? Evol. Med. Pub. Health 2020, 2020, 304–310. [Google Scholar] [CrossRef]
- Bendrey, R.; Fournié, G. Zoonotic Brucellosis from the Long View: Can the Past Contribute to the Present? Infect. Control Hosp. Epidemiol. 2021, 42, 505–506. [Google Scholar] [CrossRef] [PubMed]
- Bendrey, R.; Cassidy, J.P.; Fournié, G.; Merrett, D.C.; Oakes, R.H.; Taylor, G.M. Approaching Ancient Disease from a One Health Perspective: Interdisciplinary Review for the Investigation of Zoonotic Brucellosis. Int. J. Osteoarchaeol. 2020, 30, 99–108. [Google Scholar] [CrossRef]
- Buikstra, J.E.; Uhl, E.W. 21st Century Paleopathology: Integrating Theoretical Models with Biomedical Advances. Asian J. Paleopathol. 2023, 5, 1–7. [Google Scholar] [CrossRef]
- Fournié, G.; Pfeiffer, D.U.; Bendrey, R. Early Animal Farming and Zoonotic Disease Dynamics: Modelling Brucellosis Transmission in Neolithic Goat Populations. R. Soc. Open Sci. 2017, 4, 160943. [Google Scholar] [CrossRef] [PubMed]
- Littleton, J.; Karstens, S.; Busse, M.; Malone, N. Human-Animal interactions and infectious Disease: A View for Bioarchaeology. Bioarchaeol. Int. 2022, 6, 133–148. [Google Scholar] [CrossRef]
- Mitchell, P.D. Using Paleopathology to Provide a Deep-Time Perspective that Improves our Understanding of One Health Challenges: Exploring Urbanization. Res. Dir. One Health 2024, 2, E5. [Google Scholar] [CrossRef]
- Rayfield, K.M.; Mychajliw, A.M.; Singleton, R.R.; Sholts, S.B.; Hofman, C.A. Uncovering the Holocene Roots of Contemporary Disease-Scapes: Bringing Archaeology into One Health. Proc. R. Soc. B 2023, 290, 20230525. [Google Scholar] [CrossRef] [PubMed]
- Robbins Schug, G.; Buikstra, J.E.; Dewitte, S.N.; Baker, B.J.; Berger, E.; Buzon, M.R.; Davies-Barrett, A.M.; Goldstein, L.; Grauer, A.L.; Gregoricka, L.A.; et al. Climate Change, Human Health, and Resilience in the Holocene. Proc. Nat. Acad. Sci. USA 2023, 120, E2209472120. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R. Nonhuman Animal Paleopathology—Are We So Different? In Ortner’s Identification of Pathological Conditions in Human Skeletal Remains; Academic Press: New York, NY, USA, 2019; pp. 809–822. [Google Scholar]
- Uhl, E.W.; Kelderhouse, C.; Buikstra, J.; Blick, J.P.; Bolon, B.; Hogan, R.J. New World Origin of Canine Distemper: Interdisciplinary Insights. Int. J. Paleopathol. 2019, 24, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Van Langevelde, F.; Mondoza, H.R.R.; Matson, K.D.; Esser, H.J.; De Boer, W.F.; Schindler, S. The Link Between Biodiversity Loss and the Increasing Spread of Zoonotic Diseases; European Parliament: Luxembourg, 2020. [Google Scholar]
- Schilling, A.K.; Avanzi, C.; Ulrich, R.G.; Busso, P.; Pisanu, B.; Ferrari, N.; Romeo, C.; Mazzamuto, M.V.; McLuckie, J.; Shuttleworth, C.M.; et al. British red squirrels remain the only known wild rodent host for leprosy bacilli. Front. Vet. Sci. 2019, 6, 8. [Google Scholar] [CrossRef]
- Stone, A.C.; Wilbur, A.K.; Buikstra, J.E.; Roberts, C.A. Tuberculosis and Leprosy in Perspective. Am. J. Phys. Anthropol. 2009, 140, 66–94. [Google Scholar] [CrossRef]
- Urban, C.; Blom, A.A.; Pfrengle, S.; Walker-Meikle, K.; Stone, A.C.; Inskip, S.A.; Schuenemann, V.J. One Health Approaches to Trace Mycobacterium Leprae’s Zoonotic Potential Through Time. Front. Microbiol. 2021, 12, 762263. [Google Scholar] [CrossRef]
- Urban, C.; Blom, A.A.; Avanzi, C.; Walker-Meikle, K.; Warren, A.K.; White-Iribhogbe, K.; Turle, R.; Marter, P.; Dawson-Hobbis, H.; Roffey, S.; et al. Ancient Mycobacterium Leprae Genome Reveals Medieval English Red Squirrels as Animal Leprosy Host. Curr. Biol. 2024, 34, 2221–2230. [Google Scholar] [CrossRef]
- Brosch, R.; Gordon, S.V.; Marmiesse, M.; Brodin, P.; Buchrieser, C.; Eiglmeier, K.; Garnier, T.; Gutierrez, C.; Hewinson, G.; Kremer, K.; et al. A New Evolutionary Scenario for the Mycobacterium tuberculosis Complex. Proc. Nat. Acad. Sci. USA 2002, 99, 3684–3689. [Google Scholar] [CrossRef] [PubMed]
- Gagneux, S.; DeRiemer, K.; Van, T.; Kato-Maeda, M.; de Jong, B.C.; Narayanan, S.; Nicol, M.; Niemann, S.; Kremer, K.; Gutierrez, M.C.; et al. Variable Host-Pathogen Compatibility in Mycobacterium tuberculosis. Proc. Nat. Acad. Sci. USA 2006, 103, 2869–2873. [Google Scholar] [CrossRef] [PubMed]
- Hershberg, R.; Lipatov, M.; Small, P.M.; Sheffer, H.; Niemann, S.; Homolka, S.; Roach, J.C.; Kremer, K.; A Petrov, D.; Feldman, M.W.; et al. High Functional Diversity in Mycobacterium tuberculosis Driven by Genetic Drift and Human Demography. PLoS Biol. 2008, 6, E311. [Google Scholar] [CrossRef] [PubMed]
- Bos, K.I.; Harkins, K.M.; Herbig, A.; Coscolla, M.; Weber, N.; Comas, I.; Krause, J. Pre-Columbian Mycobacterial Genomes Reveal Seals as a Source of New World Human Tuberculosis. Nature 2014, 514, 494–497. [Google Scholar] [CrossRef]
- Köster, P.C.; Lapuente, J.; Cruz, I.; Carmena, D.; Ponce-Gordo, F. Human-Borne Pathogens: Are They Threatening Wild Great Ape Populations? Vet. Sci. 2022, 9, 356. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, M.; Mendoza, P.; Ghersi, B.M.; Wilbur, A.K.; Perez-Brumer, A.; Yong, N.C.; Kasper, M.R.; Montano, S.; Zunt, J.R.; Jones-Engel, L. Detection of Mycobacterium tuberculosis Complex in New World Monkeys in Peru. Ecohealth 2015, 12, 288–297. [Google Scholar] [CrossRef]
- Benjak, A.; Avanzi, C.; Singh, P.; Loiseau, C.; Girma, S.; Busso, P.; Fontes, A.N.B.; Miyamoto, Y.; Namisato, M.; Bobosha, K.; et al. Phylogenomics and Antimicrobial Resistance of the Leprosy Bacillus Mycobacterium leprae. Nature Comm. 2018, 9, 352. [Google Scholar] [CrossRef]
- Blevins, K.E.; Crane, A.E.; Lum, C.; Furuta, K.; Fox, K.; Stone, A.C. Evolutionary History of Mycobacterium leprae in the Pacific Islands. Philo. Trans. R. Soc. B 2020, 375, 20190582. [Google Scholar] [CrossRef] [PubMed]
- Pfrengle, S.; Neukamm, J.; Guellil, M.; Keller, M.; Molak, M.; Avanzi, C.; Kushniarevich, A.; Montes, N.; Neumann, G.U.; Reiter, E.; et al. Mycobacterium leprae Diversity and Population Dynamics in Medieval Europe from Novel Ancient Genomes. BMC Biol. 2021, 19, 220–237. [Google Scholar] [CrossRef]
- Schuenemann, V.J.; Avanzi, C.; Krause-Kyora, B.; Seitz, A.; Herbig, A.; Inskip, S.; Bonazzi, M.; Reiter, E.; Urban, C.; Pedersen, D.D.; et al. Ancient Genomes Reveal a High Diversity of Mycobacterium leprae in Medieval Europe. PLoS Pathog. 2018, 14, E1006997. [Google Scholar] [CrossRef] [PubMed]
- Robbins, G.; Tripathy, V.M.; Misra, V.N.; Mohanty, R.K.; Shinde, V.S.; Gray, K.M.; Schug, M.D. Ancient Skeletal Evidence for Leprosy in India (2000 BC). PLoS ONE 2009, 4, E5669. [Google Scholar] [CrossRef]
- Ploemacher, T.; Faber, W.R.; Menke, H.; Rutten, V.; Pieters, T. Reservoirs and Transmission Routes of Leprosy; A Systematic Review. PLoS Neg. Trop. Dis. 2020, 14, E0008276. [Google Scholar] [CrossRef]
- Patz, J.A.; Githeko, A.K.; Mccarty, J.P.; Hussein, S.; Confalonieri, U.; De Wet, N. Climate Change and infectious Diseases. Clim. Change Hum. Health Risks Resp. 2003, 2, 103–132. [Google Scholar]
- McMichael, A.J.; Woodruff, R.E. Climate Change and Infectious Diseases. In The Social Ecology of Infectious Diseases; Academic Press: Cambridge, MA, USA, 2008; pp. 378–407. [Google Scholar]
- Bos, K.I.; Stevens, P.; Nieselt, K.; Poinar, H.N.; Dewitte, S.N.; Krause, J. Yersinia pestis: New Evidence for an Old Infection. PLoS ONE 2012, 7, E49803. [Google Scholar] [CrossRef]
- Plowright, R.K.; Reaser, J.K.; Locke, H.; Woodley, S.J.; A Patz, J.; Becker, D.J.; Oppler, G.; Hudson, P.J.; Tabor, G.M. Land Use-Induced Spillover: A Call to Action to Safeguard Environmental, Animal, and Human Health. Lancet Planet. Health 2021, 5, E237–E245. [Google Scholar] [CrossRef] [PubMed]
- Ellwanger, J.H.; Fearnside, P.M.; Ziliotto, M.; Valverde-Villegas, J.M.; Veiga, A.B.G.D.; Vieira, G.F.; Bach, E.; Cardoso, J.C.; Müller, N.F.D.; Lopes, G.; et al. Synthesizing the Connections Between Environmental Disturbances and Zoonotic Spillover. An. Acad. Bras. Ciências 2022, 94 (Suppl. S3), E20211530. [Google Scholar] [CrossRef] [PubMed]
- Faust, C.L.; McCallum, H.I.; Bloomfield, L.S.; Gottdenker, N.L.; Gillespie, T.R.; Torney, C.J.; Dobson, A.P.; Plowright, R.K. Pathogen Spillover During Land Conversion. Ecol. Lett. 2018, 21, 471–483. [Google Scholar] [CrossRef]
- Boivin, N.L.; Zeder, M.A.; Fuller, D.Q.; Crowther, A.; Larson, G.; Erlandson, J.M.; Denham, T.; Petraglia, M.D. Ecological Consequences of Human Niche Construction: Examining Long-Term Anthropogenic Shaping of Global Species Distributions. Proc. Nat. Acad. Sci. USA 2016, 113, 6388–6396. [Google Scholar] [CrossRef] [PubMed]
- Gibb, R.; Redding, D.W.; Chin, K.Q.; Donnelly, C.A.; Blackburn, T.M.; Newbold, T.; Jones, K.E. Zoonotic Host Diversity Increases in Human-Dominated Ecosystems. Nature 2020, 584, 398–402. [Google Scholar] [CrossRef]
- Peterson, A.T.; Sánchez-Cordero, V.; Beard, C.B.; Ramsey, J.M. Ecologic Niche Modeling and Potential Reservoirs for Chagas Disease, Mexico. Emerg. Inf. Dis. 2002, 8, 662. [Google Scholar] [CrossRef]
- Barbera, A.R.; Brooks, D.R.; Fink, T.M.; Reinhardt, K.J. Exploring the Evolution of Trypanosoma cruzi and the Emergence of Chagas Disease in the Context of Environmental Change. Bioarchaeol. Int. 2023. early view. Available online: https://journals.upress.ufl.edu/bioarchaeology/article/view/2665 (accessed on 12 October 2024).
- Araújo, A.; Reinhard, K.; Leles, D.; Sianto, L.; Iñiguez, A.; Fugassa, M.; Arriaza, B.; Orellana, N.; Ferreira, L.F. Paleoepidemiologia De Parasitos Intestinales y Piojos En Sudamerica Precolombina. Chungará 2011, 43, 303–313. [Google Scholar] [CrossRef]
- King, C.L.; Halcrow, S.E.; Millard, A.R.; Gröcke, D.R.; Standen, V.G.; Portilla, M.; Arriaza, B.T. Let’s Talk About Stress, Baby! Infant-Feeding Practices and Stress in the Ancient Atacama Desert, Northern Chile. Am. J. Phys. Anthropol. 2018, 166, 139–155. [Google Scholar] [CrossRef]
- Snoddy, A.M.E.; King, C.L.; Halcrow, S.E.; Millard, A.R.; Buckley, H.R.; Standen, V.G.; Arriaza, B.T. Living on the Edge: Climate-induced Micronutrient Famines in the Ancient Atacama Desert? In The Routledge Handbook of the Bioarchaeology of Climate and Environmental Change; Routledge: Abingdon, UK, 2020; pp. 60–82. [Google Scholar]
- Standen, V.G.; Santoro, C.M.; Arriaza, B.; Verano, J.; Monsalve, S.; Coleman, D.; Valenzuela, D.; Marquet, P.A. Violence Among the First Horticulturists in the Atacama Desert (1000 BCE–600 CE). J. Anthropol. Archaeol. 2021, 63, 101324. [Google Scholar] [CrossRef]
- Standen, V.G.; Santoro, C.M.; Valenzuela, D.; Arriaza, B.; Verano, J.; Monsalve, S.; Coleman, D.; Marquet, P.A. Violence in Fishing, Hunting, and Gathering Societies of the Atacama Desert Coast: A Long-Term Perspective (10,000 BP–AD 1450). PLoS ONE 2023, 18, E0290690. [Google Scholar] [CrossRef]
- Sandweiss, D.; Maasch, K.; Chai, F.; Andrus, C.; Reitz, E. Geoarchaeological Evidence for Multidecadal Natural Climatic Variability and Ancient Peruvian Fisheries. Quat. Res. 2004, 61, 330–334. [Google Scholar] [CrossRef]
- Sandweiss, D.H.; Solís, R.S.; Moseley, M.E.; Keefer, D.K.; Ortloff, C.R. Environmental Change and Economic Development in Coastal Peru Between 5800 and 3600 Years Ago. Proc. Nat. Acad. Sci. USA 2009, 106, 1359–1363. [Google Scholar] [CrossRef]
- Klaus, H.D.; Shimada, I. Bodies and Blood: Middle Sicán Human Sacrifice in the Lambayeque Valley Complex (A.D. 900–1100). In Ritual Violence in the Ancient Andes: Reconstructing Sacrifice on the North Coast of Peru; University of Texas Press: Austin, TX, USA, 2016; pp. 120–149. [Google Scholar]
- Snyder, T.J.; Haas, R. Climate Change Intensified Violence in the South-Central Andean Highlands from 1.5 to 0.5 KA. Quat. Res. 2023, 115, 109–119. [Google Scholar] [CrossRef]
- Caminade, C.; McIntyre, K.M.; Jones, A.E. Impact of Recent and Future Climate Change on Vector-Borne Diseases. Ann. N. Y. Acad. Sci. 2019, 1436, 157–173. [Google Scholar] [CrossRef]
- Karypidou, M.C.; Almpanidou, V.; Tompkins, A.M.; Mazaris, A.D.; Gewehr, S.; Mourelatos, S.; Katragkou, E. Projected Shifts in the Distribution of Malaria Vectors Due to Climate Change. Clim. Change 2020, 163, 2117–2133. [Google Scholar] [CrossRef]
- Ryan, S.J.; Lippi, C.A.; Zermoglio, M.F. Shifting Transmission Risk for Malaria in Africa with Climate Change: A Framework for Planning and Intervention. Malar. J. 2020, 19, 170. [Google Scholar] [CrossRef]
- Samarasekera, U. Climate Change and Malaria: Predictions Becoming Reality. Lancet 2023, 402, 361–362. [Google Scholar] [CrossRef] [PubMed]
- Bianucci, R.; Mattutino, G.; Lallo, R.; Charlier, P.; Jouin-Spriet, H.; Peluso, A. Immunological Evidence of Plasmodium falciparum Infection in An Egyptian Child Mummy from the Early Dynastic Period. J. Archaeol. Sci. 2008, 35, 1880–1885. [Google Scholar] [CrossRef]
- Gowland, R.L.; Western, A.G. Morbidity in the Marshes: Using Spatial Epidemiology to Investigate Skeletal Evidence for Malaria in Anglo-Saxon England (AD 410–1050). Am. J. Phys. Anthropol. 2011, 147, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Lalremruata, A.; Ball, M.; Bianucci, R.; Welte, B.; Nerlich, A.G.; Kun, J.F.; Pusch, C.M. Molecular Identification of Falciparum Malaria and Human Tuberculosis Co-infections in Mummies from the Fayum Depression (Lower Egypt). PLoS ONE 2013, 8, E60307. [Google Scholar] [CrossRef] [PubMed]
- Loufouma Mbouaka, A.; Gamble, M.; Wurst, C.; Jäger, H.Y.; Maixner, F.; Zink, A.; Noedl, H.; Binder, M. The Elusive Parasite: Comparing Macroscopic, Immunological, and Genomic Approaches to Identifying Malaria in Human Skeletal Remains from Sayala, Egypt (Third to Sixth Centuries AD). Archaeol. Anthropol. Sci. 2021, 13, 115. [Google Scholar] [CrossRef] [PubMed]
- Marciniak, S.; Herring, D.A.; Sperduti, A.; Poinar, H.N.; Prowse, T.L. A Multi-Faceted Anthropological and Genomic Approach to Framing Plasmodium Falciparum Malaria in Imperial Period Central-Southern Italy (1st–4th C. CE). J. Anthropol. Archaeol. 2018, 49, 210–224. [Google Scholar] [CrossRef]
- Nerlich, A.G. Paleopathology and Paleomicrobiology of Malaria. In Paleomicrobiology of Humans; ASM Press: Washington, DC, USA, 2016; pp. 155–160. [Google Scholar]
- Nerlich, A.G.; Schraut, B.; Dittrich, S.; Jelinek, T.; Zink, A.R. Plasmodium Falciparum in Ancient Egypt. Emerg. Infect. Dis. 2008, 14, 1317–1319. [Google Scholar] [CrossRef]
- Schats, R. Developing an Archaeology of Malaria. A Critical Review of Current Approaches and a Discussion on Ways Forward. Int. J. Paleopathol. 2023, 41, 32–42. [Google Scholar] [CrossRef]
- Shin, D.H.; Seo, M.; Hong, J.H.; Lee, E. Paleopathological Considerations on Malaria Infection in Korea Before the 20th Century. Biomed. Res. Int. 2018, 2018, 8516785. [Google Scholar] [CrossRef]
- Smith-Guzmán, N.E. The Skeletal Manifestation of Malaria: An Epidemiological Approach Using Documented Skeletal Collections. Am. J. Phys. Anthropol. 2015, 158, 624–635. [Google Scholar] [CrossRef]
- Perry, M.A.; Gowland, R.L. Compounding Vulnerabilities: Syndemics and the Social Determinants of Disease in the Past. Int. J. Paleopathol. 2022, 39, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Zink, A.R.; Reischl, U.; Wolf, H.; Nerlich, A.G. Molecular Analysis of Ancient Microbial Infections. FEMS Microbiol. Lett. 2002, 213, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Larsen, C.S. The Past 12,000 Years of Behavior, Adaptation, Population, and Evolution Shaped Who We Are Today. Proc. Natl. Acad. Sci. USA 2023, 120, E2209613120. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.J.; Coban, C. Unforeseen Pathologies Caused by Malaria. Int. Immunol. 2018, 30, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Tishkoff, S.A.; Williams, S.M. Genetic Analysis of African Populations: Human Evolution and Complex Disease. Nat. Rev. Genet. 2002, 3, 611–621. [Google Scholar] [CrossRef]
- Antinori, S.; Bonazzetti, C.; Giacomelli, A.; Corbellino, M.; Galli, M.; Parravicini, C.; Ridolfo, A.L. Non-Human Primate and Human Malaria: Past, Present, and Future. J. Travel Med. 2021, 28, Taab036. [Google Scholar] [CrossRef]
- Conway, D.J.; Cavanagh, D.R.; Tanabe, K.; Roper, C.R.; Mikes, Z.S.; Sakihama, N.; Bojang, K.A.; Oduola, A.M.J.; Kremsner, P.G.; Arnot, D.E. A Principal Target of Human Immunity to Malaria Identified by Molecular Population Genetic and Immunological Analyses. Nat. Med. 2000, 6, 689–692. [Google Scholar] [CrossRef]
- Escalante, A.A.; Cornejo, O.E.; Freeland, D.E.; Poe, A.C.; Durrego, E.; Collins, W.E.; Lal, A.A. A Monkey’s Tale: The Origin of Plasmodium vivax as a Human Malaria Parasite. Proc. Natl. Acad. Sci. USA 2005, 102, 1980–1985. [Google Scholar] [CrossRef] [PubMed]
- Loy, D.E.; Plenderleith, L.J.; Sundararaman, S.A.; Liu, W.; Gruszczyk, J.; Chen, Y.J.; Trimboli, S.; Learn, G.H.; MacLean, O.A.; Morgan, A.L.K.; et al. Evolutionary History of Human Plasmodium vivax Revealed by Genome-Wide Analyses of Related Ape Parasites. Proc. Natl. Acad. Sci. USA 2018, 115, E1810053115. [Google Scholar] [CrossRef]
- Beard, M. SPQR: History of Ancient Rome; Profile Books Ltd.: London, UK, 2015. [Google Scholar]
- Llanos-Lizcano, A.; Haemmerle, M.; Sperduri, A.; Sawyer, S.; Zagorc, B.; Ozdogan, K.T.; Guellil, M.; Cheronet, O.; Kuhlwilm, M.; Pinhasi, R.; et al. A Complete Mitochondrial Genome of a Roman-Era Plasmodium falciparum. biorXiv 2024. [Google Scholar] [CrossRef]
- Newfield, T.P. Mysterious and Mortiferous Clouds: The Climate Cooling and Disease Burden of Late Antiquity. Late Antiq. Archaeol. 2016, 12, 89–115. [Google Scholar] [CrossRef]
- Wilson, J.; Pickel, D.G.; Newfield, T.; Malis, S. Nested Environments: A Biocultural Examination of Malaria, Disease Stress, and Mother-infant Health in a Rural Community in Late Antique Umbria. Env. Archaeol. 2023, 1–16. [Google Scholar] [CrossRef]
- Robb, J.; Cessford, C.; Dittmar, J.; Inskip, S.A.; Mitchell, P.D. The Greatest Health Problem of the Middle Ages? Estimating the Burden of Disease in Medieval England. Int. J. Paleopathol. 2021, 34, 101–112. [Google Scholar] [CrossRef]
- Ostfeld, R.S.; Keesing, F. Planetary Health and Infectious Disease. In Planetary Health: Protecting Nature to Protect Ourselves; Island Press: Washington, DC, USA, 2020; pp. 141–164. [Google Scholar]
- Fletcher, I.K. Assessing the Impact of Global Environmental Change on Mosquito-Borne Disease: A Planetary Health Approach. Ph.D. Thesis, London School of Hygiene & Tropical Medicine, London, UK, 2022. Available online: https://researchonline.lshtm.ac.uk/id/eprint/4668987/ (accessed on 12 October 2024).
- Von Der Heydt, A.S. Can The Miocene Climate Inform the Future? Science 2022, 377, 26–27. [Google Scholar] [CrossRef] [PubMed]
- Naserrudin, N.A.; Monroe, A.; Culleton, R.; Hod, R.; Jeffree, M.S.; Ahmed, K.; Hassan, M.R. Reimagining Zoonotic Malaria Control in Communities Exposed to Plasmodium knowlesi Infection. J. Physiol. Anthropol. 2022, 41, 14. [Google Scholar] [CrossRef] [PubMed]
- Crawford, M.J. The Andean Wonder Drug: Cinchona Bark and Imperial Science in the Spanish Atlantic, 1630–1800; University of Pittsburgh Press: Pittsburgh, PA, USA, 2016. [Google Scholar]
- Tu, Y. From Artemisia annua L. to Artemisinins: The Discovery and Development of Artemisinins and Antimalarial Agents; Academic Press: New York, NY, USA, 2017. [Google Scholar]
- Friant, S.; Bonwitt, J.; Ayambem, J.; Ifebueme, N.M.; Alobi, O.O.; Otukpa, A.O.; Bennett, A.J. Zootherapy as a Potential Pathway for Zoonotic Spillover: A Mixed-Methods Study of the Use of Animal Products in Medicinal and Cultural Practices in Nigeria. One Health Outlook 2022, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Munajat, M.B.; Rahim, M.A.F.A.; Wahid, W.; Seri Rakna, M.I.M.; Divis, P.C.S.; Chuangchaiya, S.; Lubis, I.N.D.; Osman, E.; Kasri, M.R.M.; Idris, Z.M. Perceptions and Prevention Practices on Malaria Among the Indigenous Orang Asli Community in Kelantan, Peninsular Malaysia. Malar. J. 2021, 20, 202. [Google Scholar] [CrossRef]
- Macherera, M.; Chimbari, M.J.; Mukaratirwa, S. Indigenous Environmental Indicators for Malaria: A District Study in Zimbabwe. Acta Trop. 2017, 175, 50–59. [Google Scholar] [CrossRef]
- Al-Adhroey, A.H.; Zurainee, M.N.; Hesham, M.; Al-Mekhlafi, R. Opportunities and Obstacles to the Elimination of Malaria from Peninsular Malaysia: Knowledge, Attitudes, and Practices on Malaria Among Aboriginal and Rural Communities. Malar. J. 2010, 9, 137–142. [Google Scholar] [CrossRef]
- Joshi, A.B.; Banjara, M.R. Malaria Related Knowledge, Practices and Behaviour of People in Nepal. J. Vector Borne Dis. 2008, 45, 44–50. [Google Scholar] [PubMed]
- Tabuti, J.R.S.; Obakiro, S.B.; Nabatanzi, A.; Anywar, G.; Nambejja, C.; Mutyaba, M.R.; Omara, T.; Waako, P. Medicinal Plants Used for Treatment of Malaria by Indigenous Communities of Tororo District, Eastern Uganda. Trop. Med. Health 2023, 51, 34. [Google Scholar] [CrossRef] [PubMed]
- Agozino, B. Indigenous Knowledge Systems and Innovations in Malaria Control in Nigeria. J. Immuno Virol. 2017, 2, 555–582. [Google Scholar] [CrossRef]
- Cáceres, L.; Calzada, J.E.; Gabster, A.; Young, R.; Márquez, R.; Torres, R.; Griffith, M. Social Representations of Malaria in the Guna Indigenous Population of Comarca Guna De Madungandi, Panama. Malar. J. 2017, 16, 256. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.M. Remarkable Solutions to Impossible Problems: Lessons for Malaria from the Eradication of Smallpox. Malar. J. 2019, 18, 323. [Google Scholar] [CrossRef] [PubMed]
- Kader, M.; Fatimah, S.; Rashid, A.; Ahmad, N.I.; Zahari, S.N.A.; Hamat, R.A. Sero-Prevalence of Malaria and the Knowledge, Attitudes, and Practices Relating to the Prevention of Malaria Among Indigenous People Living in the Central Forest Spine in Peninsular Malaysia: A Mixed-Methods Study. Malar. J. 2022, 21, 281. [Google Scholar] [CrossRef]
- Munguti, K.J. Indigenous Knowledge in the Management of Malaria and Visceral Leishmaniasis Among the Tugen of Kenya. Ph.D. Thesis, University of Nairobi, Nairobi, Kenya, 1997. Available online: http://Erepository.Uonbi.Ac.Ke/Bitstream/Handle/11295/39556/Munguti_Indigenous%20Knowledge%20in%20the%20Management%20of%20Malaria%20and%20Visceral%20Leishmaniasis%20among%20the%20Tugen%20of%20KenyA.Pdf?Sequence=2 (accessed on 12 October 2024).
- Odonne, G.; Musset, L.; Cropet, C.; Philogene, B.; Gaillet, M.; Tareau, M.-A.; Douine, M.; Michaud, C.; Davy, D.; Epelboin, L.; et al. When Local Phytotherapies Meet Biomedicine. Cross-Sectional Study of Knowledge and Intercultural Practices Against Malaria in Eastern French Guiana. J. Ethnopharmacol. 2021, 279, 114384. [Google Scholar] [CrossRef]
- Phoobane, P.; Masinde, M. Investigating the Adoption of Indigenous Knowledge in Mitigating Climate-Linked Challenges: A Case Study of Vhembe District in South Africa. Int. J. Res. Bus. Soc. Sci. 2023, 12, 394–404. [Google Scholar] [CrossRef]
- Zakariya, A.M.; Adamu, A.; Nuhu, A.; Kiri, I.Z. Assessment of Indigenous Knowledge on Medicinal Plants Used in the Management of Malaria in Kafin Hausa, North-Western Nigeria. Ethnobot. Res. Appl. 2021, 22, 1–18. [Google Scholar] [CrossRef]
- Garnett, S.T.; Burgess, N.D.; Fa, J.E.; Fernández-Llamazares, Á.; Molnár, Z.; Robinson, C.J. A Spatial Overview of the Global Importance of Indigenous Lands for Conservation. Nat. Sustain. 2018, 1, 369–374. [Google Scholar] [CrossRef]
- Dawson, N.M.; Coolsaet, B.; Sterling, E.J.; Loveridge, R.; Gross-Camp, N.D.; Wongbusarakum, S.; Sangha, K.K.; Scherl, L.M.; Phan, H.P.; Zafra-Calvo, N. The Role of Indigenous Peoples and Local Communities in Effective and Equitable Conservation. Ecol. Soc. 2021, 26, 19–58. [Google Scholar] [CrossRef]
- Heller, N.E.; McManus, K.C.; Chauvin, D.S.; Skybrook, D.; Barnosky, A.D. Including Stewardship in Ecosystem Health Assessment. Nat. Sustain. 2023, 6, 731–741. [Google Scholar] [CrossRef]
- Bijoy, C.R.; Chakma, A.; Guillao, J.A.; Hien, B.H.; Indrarto, G.B.; Lim, T.; Min, N.E.; Rai, T.B.; Smith, O.A.; Rattanakrajangsri, K. Nationally Determined Contributions in Asia: Are Governments Recognizing the Rights, Roles, and Contributions of Indigenous Peoples? Available online: https://weadapt.org/knowledge-base/adaptation-decision-making/nationally-determined-contributions-in-asia-are-governments-recognizing-the-rights-roles-and-contributions-of-indigenous-peoples/ (accessed on 12 October 2024).
- Davies-Barrett, A.M.; Antoine, D.; Roberts, C.A. Respiratory Disease in the Middle Nile Valley: The Impact of Environment and Aridification. In The Routledge Handbook of the Bioarchaeology of Climate and Environmental Change; Routledge: London, UK, 2020; pp. 122–140. [Google Scholar]
- Davies-Barrett, A.; Antoine, D.; Roberts, C. Desert Dust, and City Smoke: Investigating the Impact of Urbanization and Aridification on the Prevalence of Pulmonary/Pleural Inflammation in the Middle Nile Valley (2500 BC to 1500 AD). Bioarchaeol. Int. 2023. early view. [Google Scholar] [CrossRef]
- Roberts, C.A. A Bioarcheological Study of Maxillary Sinusitis. Am. J. Phys. Anthropol. 2007, 133, 792–807. [Google Scholar] [CrossRef]
- Roberts, C.A. Exploring the Third “Epidemiological Transition”: Paleopathology’s Contribution to Understanding Health and Well-Being Today and For the Future. In The Routledge Handbook of the Bioarchaeology of Climate and Environmental Change; Routledge: London, UK, 2020; pp. 19–42. [Google Scholar]
- Austin, R.M.; Zuckerman, M.; Honap, T.P.; Lee, H.; Ward, G.K.; Warinner, C.; Hofman, C.A. Remembering St. Louis individual—Structural Violence and Acute Bacterial infections in a Historical Anatomical Collection. Commun. Biol. 2022, 5, 1050. [Google Scholar]
- Miller, M.; Robbins Schug, G.; Pagani, L.; Carrara, N. A Bioarchaeology of Madness: Modernity, Pellagra, and the Rise of the Manicomio System in the Veneto Region of Italy. In The Routledge Handbook of the Bioarchaeology of Climate and Environmental Change; Routledge: London, UK, 2020; pp. 255–276. [Google Scholar]
- Nystrom, K.C.; Robbins Schug, G. A Bioarchaeology of Social inequality and Environmental Change. In The Routledge Handbook of the Bioarchaeology of Climate and Environmental Change; Routledge: London, UK, 2020; pp. 159–188. [Google Scholar]
- Betsinger, T.K.; DeWitte, S.N. The Bioarchaeology of Urbanization. In Demographic and Social Consequences of Living in Cities; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Roberts, C.A. Old World Tuberculosis: Evidence from Human Remains with a Review of Current Research and Future Prospects. Tuberculosis 2015, 95, S117–S121. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Roberts, C.A.; Buikstra, J.E. The Bioarchaeology of Tuberculosis: A Global View on a Re-Emerging Disease; University Press of Florida: Gainesville, FL, USA, 2003. [Google Scholar]
- Roberts, C.A. Palaeopathology and Its Relevance to Understanding Health and Disease Today: The Impact of the Environment on Health, Past and Present. Anthropol. Rev. 2016, 79, 1–20. [Google Scholar] [CrossRef]
- Van Doren, T.P. Biocultural Perspectives of Infectious Diseases and Demographic Evolution: Tuberculosis and Its Comorbidities Through History. Evol. Anthropol. 2023, 32, 100–117. [Google Scholar] [CrossRef] [PubMed]
- Nerlich, A.G.; Haas, C.J.; Zink, A.; Szeimies, U.; Hagedorn, H.G. Molecular Evidence for Tuberculosis in An Ancient Egyptian Mummy. Lancet 1997, 350, 1404. [Google Scholar] [CrossRef]
- Rubini, M.; Zaio, P.; Roberts, C. Tuberculosis and Leprosy in Italy. New Skeletal Evidence. Homo 2014, 65, 13–32. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Millard, A.; Montgomery, J.; Trickett, M.; Beaumont, J.; Evans, J.; Chenery, S. Childhood Lead Exposure in the British Isles During the Industrial Revolution. In Modern Environments and Human Health: Revisiting the Second Epidemiologic Transition; Wiley: New York, NY, USA, 2014; pp. 279–299. [Google Scholar]
- Montgomery, J.; Evans, J.A.; Chenery, S.R.; Pashley, V.; Killgrove, K. “Gleaming, White and Deadly”: Using Lead to Track Human Exposure and Geographic Origins in the Roman Period in Britain. J. Roman Archaeol. 2010, 78, 199–226. [Google Scholar]
- Moore, J.; Filipek, K.; Kalenderian, V.; Gowland, R.; Hamilton, E.; Evans, J.; Montgomery, J. Death Metal: Evidence for the Impact of Lead Poisoning on Childhood Health Within the Roman Empire. Int. J. Osteoarchaeol. 2021, 31, 846–856. [Google Scholar] [CrossRef]
- Arriaza, B.; Amarasiriwardena, D.; Cornejo, L.; Standen, V.; Byrne, S.; Bartkus, L.; Bandak, B. Exploring Chronic Arsenic Poisoning in Pre-Columbian Chilean Mummies. J. Archaeol. Sci. 2010, 37, 1274–1278. [Google Scholar] [CrossRef]
- Emslie, S.D.; Brasso, R.; Patterson, W.P.; Carlos Valera, A.; Mckenzie, A.; Maria Silva, A.; Blum, J.D. Chronic Mercury Exposure in Late Neolithic/Chalcolithic Populations in Portugal from the Cultural Use of Cinnabar. Sci. Rep. 2015, 5, 14679. [Google Scholar] [CrossRef] [PubMed]
- Proctor, T.K. Mercury, Mitayos, and the Violence of the Everyday: The Bioarchaeology of the Santa Bárbara Mercury Mines in Huancavelica, Peru (16th–19th Centuries CE). Ph.D. Thesis, Vanderbilt University, Nashville, TN, USA, 2021. Available online: https://www.Proquest.Com/Docview/2598668802?Pq-Origsite=Gscholar&Fromopenview=True&Sourcetype=Dissertations%20&%20Theses (accessed on 12 October 2024).
- Robins, N.A.; Hagan, N.A. Mercury Production and Use in Colonial Andean Silver Production: Emissions and Health Implications. Environ. Health Perspect. 2012, 120, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Meneses, H.D.N.D.M.; Oliveira-Da-Costa, M.; Basta, P.C.; Morais, C.G.; Pereira, R.J.B.; De Souza, S.M.S.; Hacon, S.D.S. Mercury Contamination: A Growing Threat to Riverine and Urban Communities in the Brazilian Amazon. Int. J. Environ. Res. Pub. Health 2022, 19, 2816. [Google Scholar] [CrossRef]
- Campanacho, V. Phosphorus Necrosis of the Jaw: A Review of a 19th and Early 20th Century Occupational Disease. Cad. GEEvH 2020, 9, 1–13. [Google Scholar]
- Qualls, C.; Bianucci, R.; Legeros, R.; Bromage, T.; Lanzirotti, A.; Giuffra, V.; Ferroglio, E.; Fornaciari, G.; Appenzeller, O. Neurotoxins During the Renaissance. Bioarcheology of Ferrante II of Aragon (1469–1496) and Isabella of Aragon (1470–1524). J. Archaeol. Sci. Rep. 2016, 5, 542–546. [Google Scholar] [CrossRef]
- Roberts, C.A.; Caffell, A.; Filipek-Ogden, K.L.; Gowland, R.; Jakob, T. ‘Til Poison Phosphorous Brought Them Death’: A Potentially Occupationally Related Disease in a Post-Medieval Skeleton from North-East England. Int. J. Paleopathol. 2016, 13, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Walser III, J.W.; Kristjánsdóttir, S.; Gowland, R.; Desnica, N. Volcanoes, Medicine, and Monasticism: Investigating Mercury Exposure in Medieval Iceland. Int. J. Osteoarchaeol. 2019, 29, 48–61. [Google Scholar] [CrossRef]
- Marques, C. Cancer: Lessons to Learn from the Past. In Bone Sarcomas and Bone Metastases-from Bench to Bedside; Academic Press: New York, NY, USA, 2022; pp. 5–15. [Google Scholar]
- Marques, C.; Compton, Z.; Boddy, A.M. Connecting Palaeopathology and Evolutionary Medicine to Cancer Research: Past and Present. Palaeopathology Evol. Med. Integr. Approach 2022, 239, 239–260. [Google Scholar]
- Whitley, C.B.; Boyer, J.L. Assessing Cancer Risk Factors Faced by An Ancestral Puebloan Population in the North American Southwest. Int. J. Paleopathol. 2018, 21, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.H.; Lin, C.Y.; Su, S.B.; Chen, K.T. Leprosy: A Review of Epidemiology, Clinical Diagnosis, and Management. J. Trop. Med. 2022, 2022, 8652062. [Google Scholar] [CrossRef]
- Eichelmann, K.; González, S.G.; Salas-Alanis, J.C.; Ocampo-Candiani, J. Leprosy. An Update: Definition, Pathogenesis, Classification, Diagnosis, and Treatment. Actas Dermo-Sifiliográficas (Engl. Ed.) 2013, 104, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Dogra, S. Leprosy: A Disease with Diagnostic and Management Challenges! Indian J. Dermatol. Venereol. Leprol. 2009, 75, 111. [Google Scholar] [CrossRef]
- Naaz, F.; Mohanty, P.S.; Bansal, A.K.; Kumar, D.; Gupta, U.D. Challenges Beyond Elimination in Leprosy. Int. J. Mycobacteriol. 2017, 6, 222–228. [Google Scholar]
- Rao, P.N. Leprosy: The Challenges Ahead for India. J. Ski. Sex. Transm. Dis. 2021, 3, 106–110. [Google Scholar] [CrossRef]
- Santos, V.S.; De Mendonça Neto, P.T.; Raposo, O.F.F.; Fakhouri, R.; Reis, F.P.; Feitosa, V.L.C. Evaluation of Agreement Between Clinical and Histopathological Data for Classifying Leprosy. Int. J. Infect. Dis. 2013, 17, E189–E192. [Google Scholar] [CrossRef]
- Do Espírito Santo, R.B.; Gonçalves, D.V.C.; Serafim, R.A.; Loureiro, R.M.; Sumi, D.V.; De Mello, R.A.F.; Collin, S.M.; Deps, P. Evaluation of Proposed Cranial and Maxillary Bone Alteration Parameters in Persons Affected by Hansen’s Disease. PLoS Neglected Trop. Dis. 2021, 15, E0009694. [Google Scholar] [CrossRef] [PubMed]
- Do Espírito Santo, R.B.; Serafim, R.A.; Loureiro, R.M.; Gonçalves, D.V.C.; Sumi, D.V.; De Mello, R.A.F.; Collin, S.M.; Deps, P.D. Clinical and Radiological Evaluation of Maxillofacial and Otorhinolaryngological Manifestations of Hansen’s Disease. Sci. Rep. 2022, 12, 14912. [Google Scholar] [CrossRef] [PubMed]
- Kasai, N.; Kondo, O.; Suzuki, K.; Aoki, Y.; Ishii, N.; Goto, M. Quantitative Evaluation of Maxillary Bone Deformation by Computed Tomography in Patients with Leprosy. PLoS Neglected Trop. Dis. 2018, 12, E0006341. [Google Scholar] [CrossRef]
- Loureiro, R.M.; Do Espirito Santo, R.B.; Deps, P.D. Diagnostic Imaging in Hansen’s Disease: Conventional Radiography, Computed Tomography, Magnetic Resonance Imaging, and Dual-Energy X-Ray Absorptiometry. In Hansen’s Disease: A Complete Clinical Guide; Springer International Publishing: Cham, Switzerland, 2023; pp. 221–228. [Google Scholar]
- Bowland, G.B.; Weyrich, L.S. The Oral-Microbiome-Brain Axis and Neuropsychiatric Disorders: An Anthropological Perspective. Front. Psychiatr. 2022, 13, 810008. [Google Scholar] [CrossRef]
- Guzzo, G.L.; Mittinty, M.N.; Llamas, B.; Andrews, J.M.; Weyrich, L.S. Individuals with inflammatory Bowel Disease Have an Altered Gut Microbiome Composition of Fungi and Protozoa. Microorganisms 2022, 10, 1910. [Google Scholar] [CrossRef] [PubMed]
- Weyrich, L.S.; Nath, S.; Jamieson, L. Commercializing Equitable, Accessible Oral Microbiome Transplantation Therapy. Commun. Dent. Health 2024, 41, 83–88. [Google Scholar]
- Battles, H.; Gilmour, R. Beyond Mortality: Survivors of Epidemic Infections and the Bioarchaeology of Impairment and Disability. Bioarchaeol. Int. 2022, 6, 23–40. [Google Scholar] [CrossRef]
- DeWitte, S.; Wissler, A. Demographic and Evolutionary Consequences of Pandemic Diseases. Bioarchaeol. Int. 2021, 6, 108–132. [Google Scholar] [CrossRef]
- Dutour, O. Paleopathology of Human infections: Old Bones, Antique Books, Ancient and Modern Molecules. Microbiol. Spectr. 2016, 4, 10–1128. [Google Scholar] [CrossRef]
- Yaussy, S. Intersectionality and the interpretation of Past Pandemics. Bioarchaeol. Int. 2022, 6, 58–76. [Google Scholar] [CrossRef]
- Zuckerman, M.K.; Taylor, E.; Degaglia, C.M.; Gibson, L.B. Institutionalization Within the Context of Pandemic Infectious Disease: Examining Social Vulnerability to the 1918 Influenza Pandemic Among Individuals Institutionalized in the Mississippi State Asylum. Bioarchaeol. Int. 2022, 6, 41–57. [Google Scholar] [CrossRef]
- Rasmussen, S.; Allentoft, M.E.; Nielsen, K.; Orlando, L.; Sikora, M.; Sjögren, K.G.; Pedersen, A.G.; Schubert, M.; Van Dam, A.; Kapel, C.M.O.; et al. Early Divergent Strains of Yersinia Pestis in Eurasia 5000 Years Ago. Cell 2015, 163, 571–582. [Google Scholar] [CrossRef]
- Vogler, A.J.; Andrianaivoarimanana, V.; Telfer, S.; Hall, C.M.; Sahl, J.W.; Hepp, C.M.; Centner, H.; Andersen, G.; Birdsell, D.N.; Rahalison, L.; et al. Temporal Phylogeography of Yersinia Pestis in Madagascar: Insights into the Long-Term Maintenance of Plague. PLoS Neglected Trop. Dis. 2017, 11, E0005887. [Google Scholar] [CrossRef] [PubMed]
- Aufderheide, A.C.; Rodríguez-Martin, C. The Cambridge Encyclopedia of Human Paleopathology; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Shin, D.H.; Bianucci, R. The Handbook of Mummy Studies; Springer: Singapore, 2021. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robbins Schug, G.; Buikstra, J.E. From ONE Health to ONE Paleopathology: Deep-Time Perspectives on Health in the Face of Climate and Environmental Change. Encyclopedia 2025, 5, 13. https://doi.org/10.3390/encyclopedia5010013
Robbins Schug G, Buikstra JE. From ONE Health to ONE Paleopathology: Deep-Time Perspectives on Health in the Face of Climate and Environmental Change. Encyclopedia. 2025; 5(1):13. https://doi.org/10.3390/encyclopedia5010013
Chicago/Turabian StyleRobbins Schug, Gwen, and Jane E. Buikstra. 2025. "From ONE Health to ONE Paleopathology: Deep-Time Perspectives on Health in the Face of Climate and Environmental Change" Encyclopedia 5, no. 1: 13. https://doi.org/10.3390/encyclopedia5010013
APA StyleRobbins Schug, G., & Buikstra, J. E. (2025). From ONE Health to ONE Paleopathology: Deep-Time Perspectives on Health in the Face of Climate and Environmental Change. Encyclopedia, 5(1), 13. https://doi.org/10.3390/encyclopedia5010013







