Importance of High-Concentration Electrolytes for Lithium-Based Batteries
Abstract
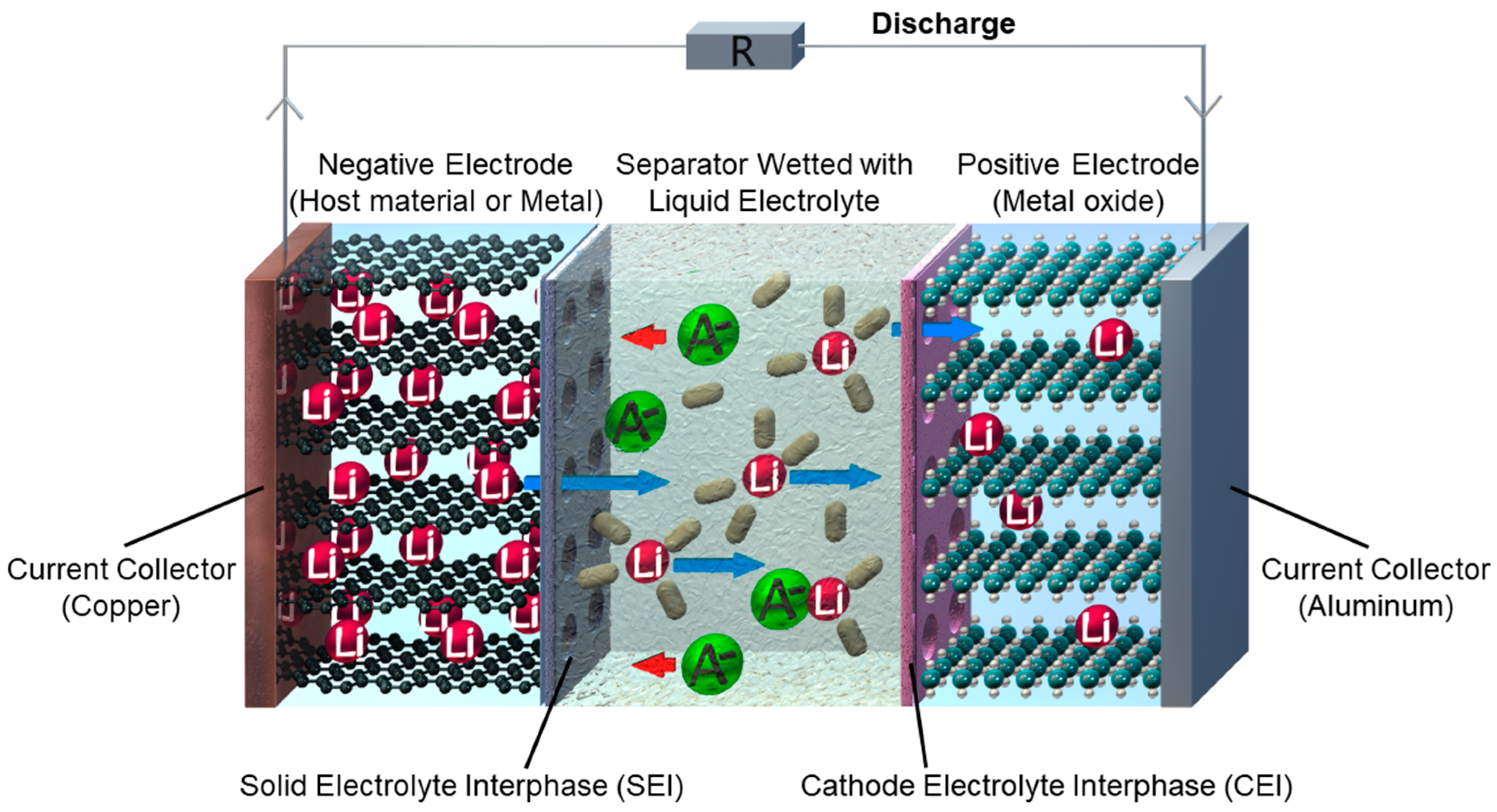
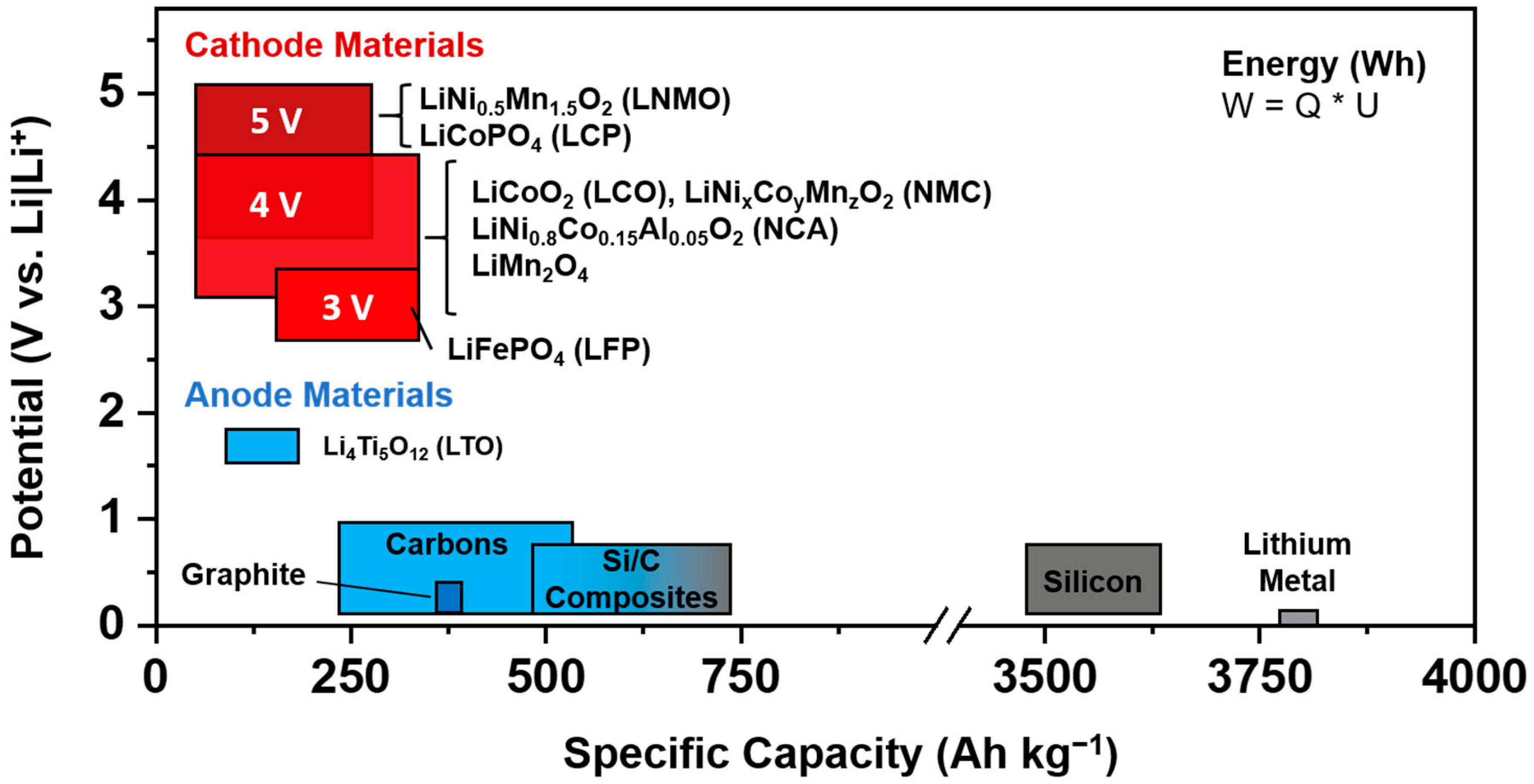
:1. Introduction
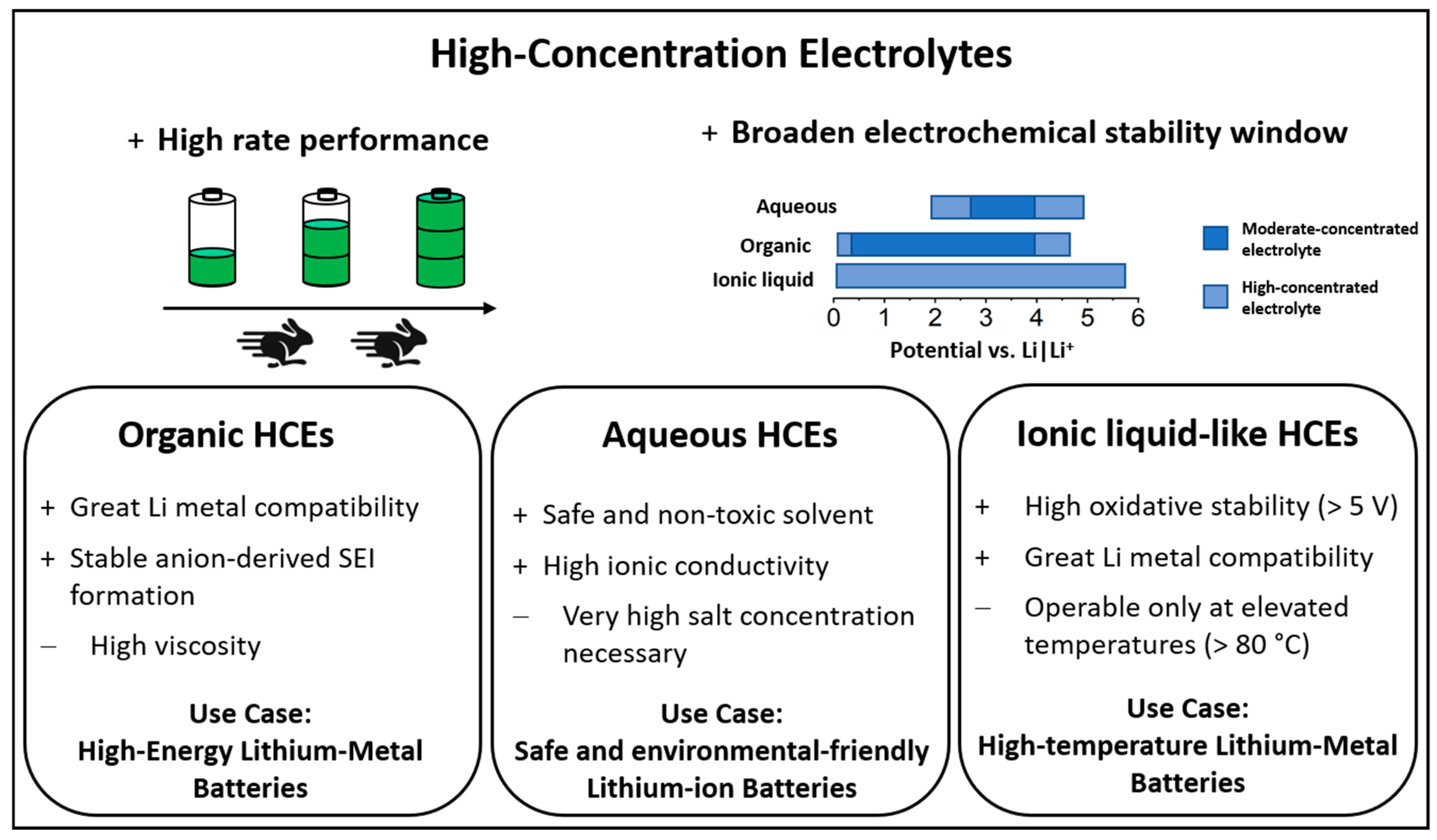
2. Chronology of High-Concentration Electrolytes
2.1. HCEs for Graphite-Based LIBs
2.2. Ionic-Liquid-like HCEs in LIBs and LMBs
2.3. HCEs for Lithium Metal Batteries
2.4. Aqueous-Based HCE in LIBs and LMBs
3. Impact of High Salt Concentration on Physicochemical and Electrochemical Properties
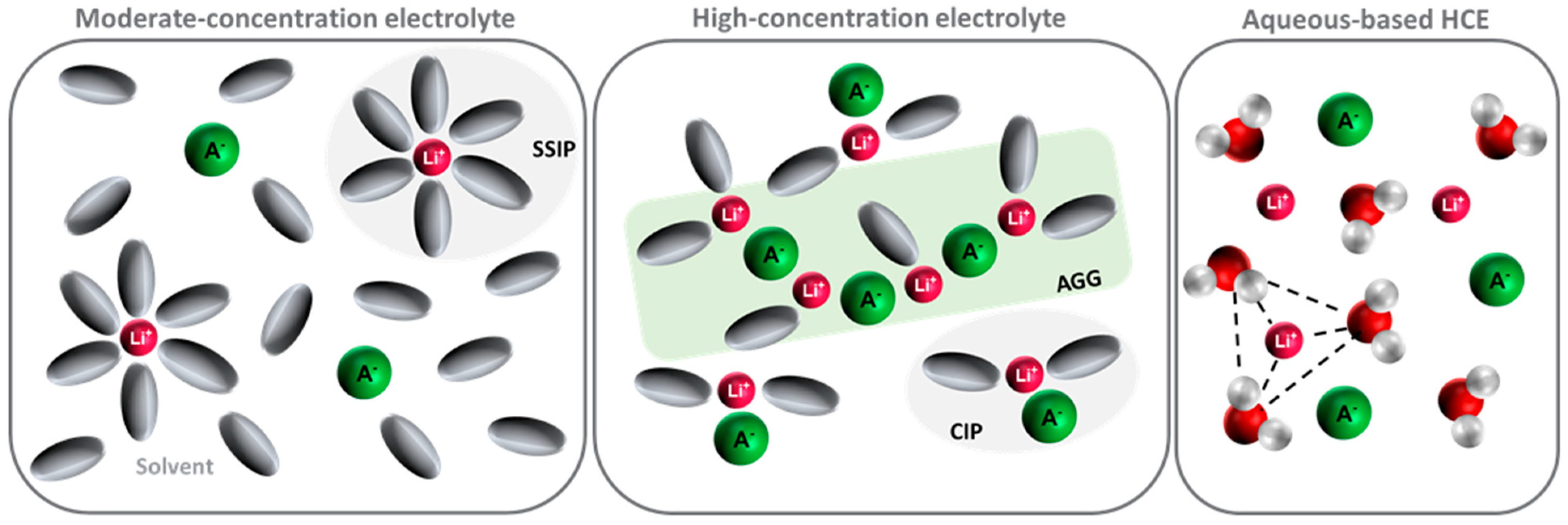
3.1. Solvation Structure
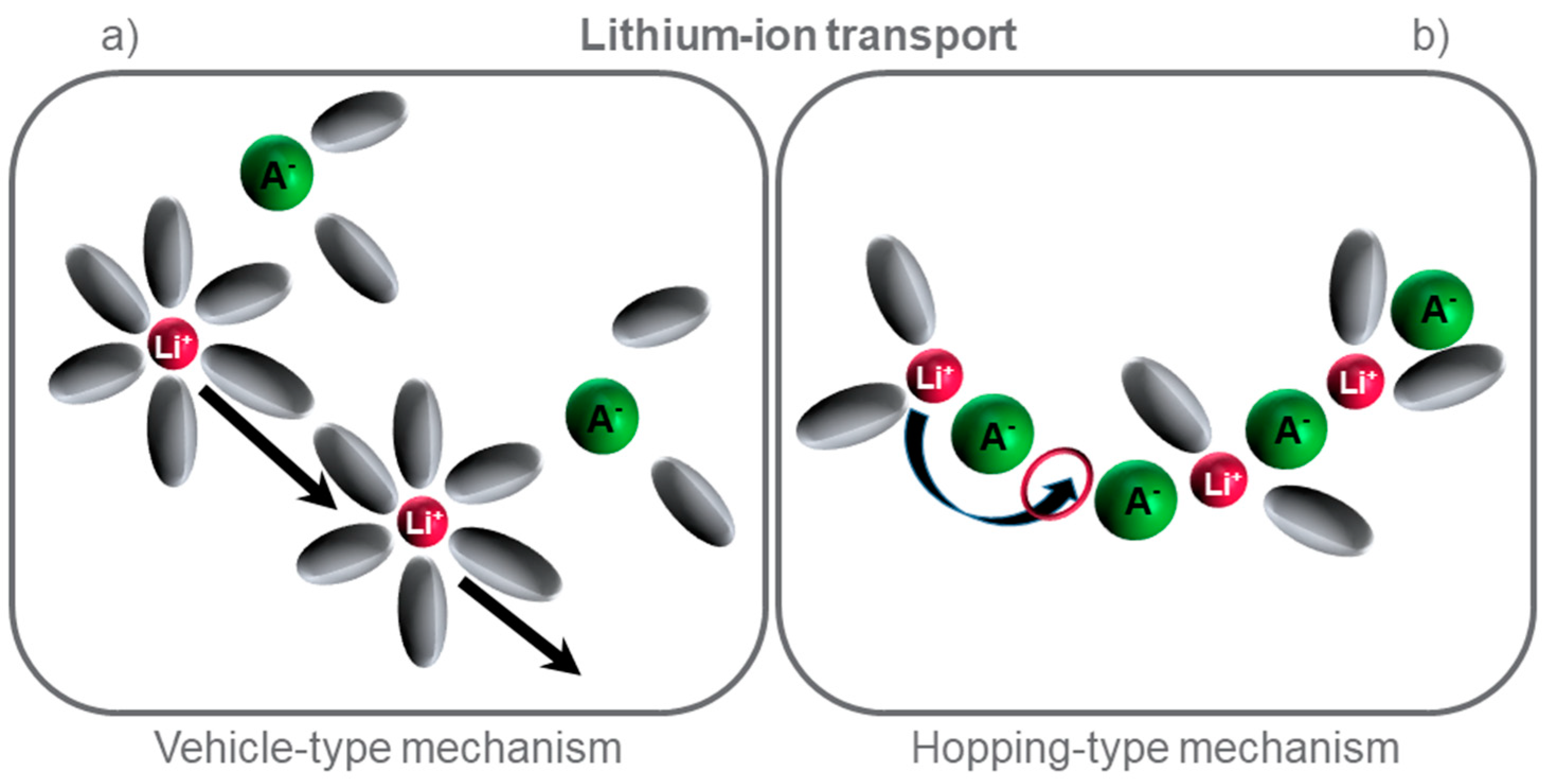
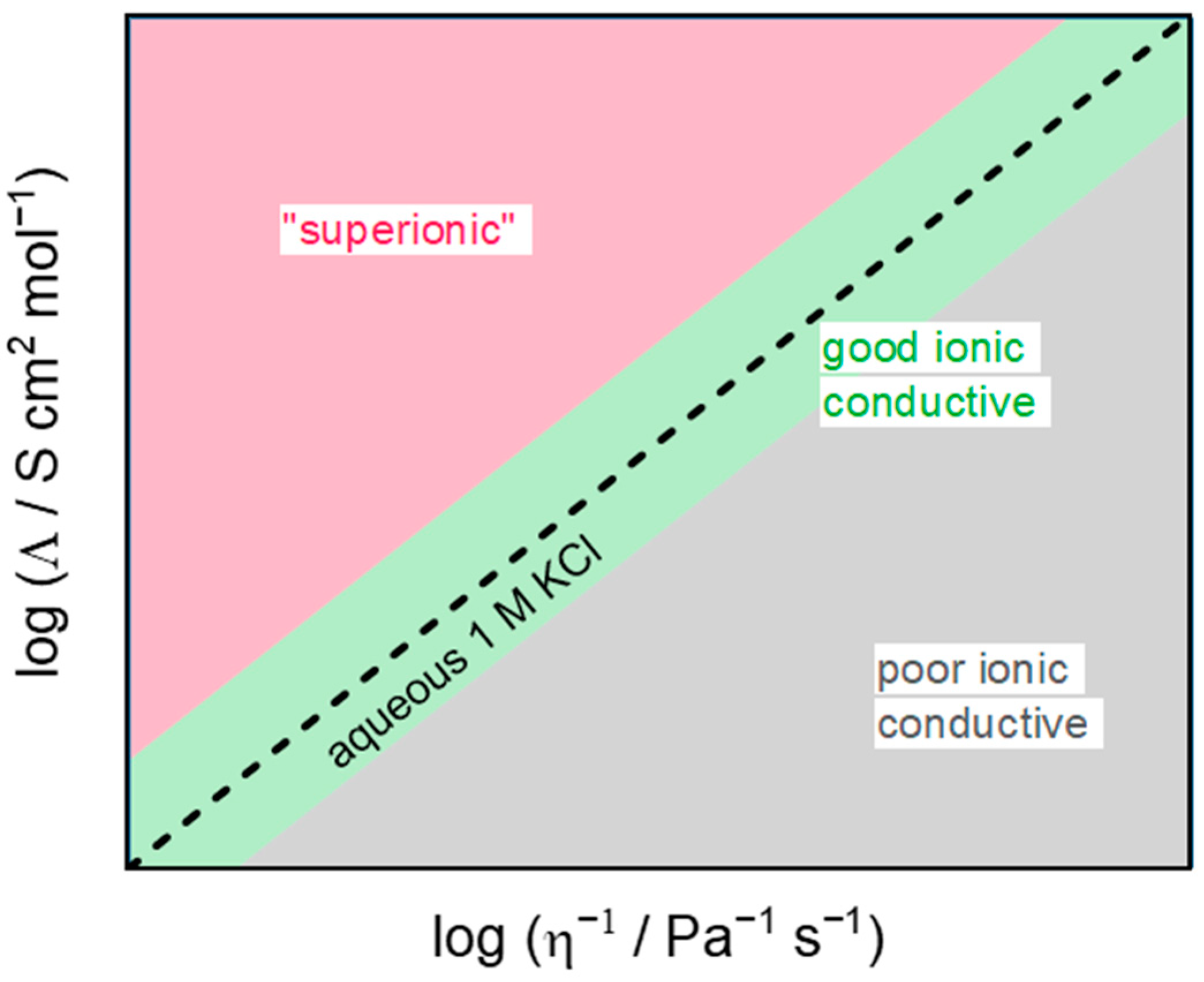
3.2. Ionic Conductivity
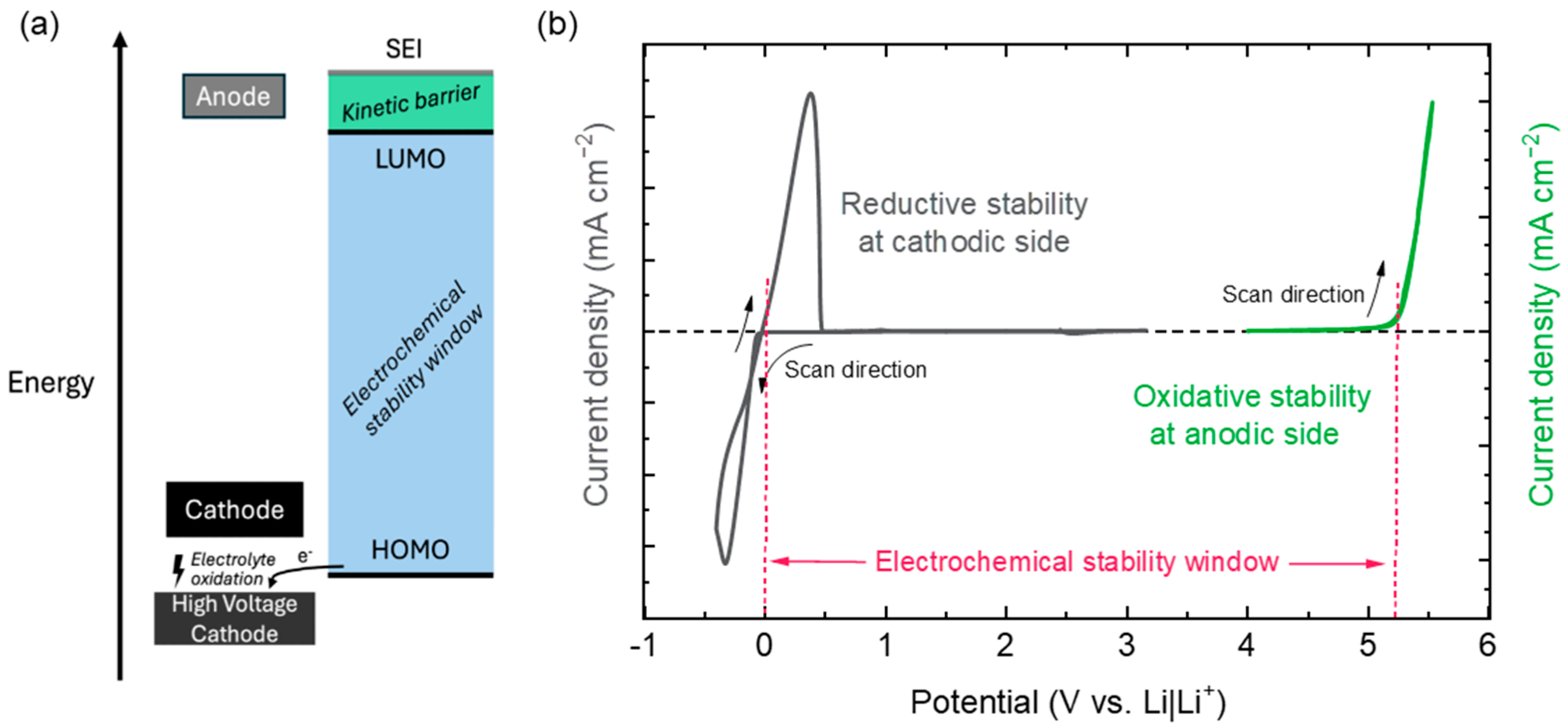
3.3. Electrochemical Stability Window
3.4. Li Stripping/Plating
4. Safety Aspects
5. Remaining Challenges of HCEs
6. Modifications of HCEs to Face Challenges
7. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Holze, R. Metal-Ion Batteries. Encyclopedia 2022, 2, 1611–1623. [Google Scholar] [CrossRef]
- Xu, K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 2004, 104, 4303–4417. [Google Scholar] [CrossRef]
- Cekic-Laskovic, I.; von Aspern, N.; Imholt, L.; Kaymaksiz, S.; Oldiges, K.; Rad, B.R.; Winter, M. Synergistic Effect of Blended Components in Nonaqueous Electrolytes for Lithium Ion Batteries. In Electrochemical Energy Storage; Eichel, R.-A., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–64. ISBN 978-3-030-26128-3. [Google Scholar]
- Tan, J.; Matz, J.; Dong, P.; Shen, J.; Ye, M. A Growing Appreciation for the Role of LiF in the Solid Electrolyte Interphase. Adv. Energy Mater. 2021, 11, 2100046. [Google Scholar] [CrossRef]
- Verma, P.; Maire, P.; Novák, P. A review of the features and analyses of the solid electrolyte interphase in Li-ion batteries. Electrochim. Acta 2010, 55, 6332–6341. [Google Scholar] [CrossRef]
- Winter, M. The Solid Electrolyte Interphase—The Most Important and the Least Understood Solid Electrolyte in Rechargeable Li Batteries. Z. Phys. Chem. 2009, 223, 1395–1406. [Google Scholar] [CrossRef]
- Bieker, G.; Winter, M.; Bieker, P. Electrochemical in situ investigations of SEI and dendrite formation on the lithium metal anode. Phys. Chem. Chem. Phys. 2015, 17, 8670–8679. [Google Scholar] [CrossRef]
- Suo, L.; Hu, Y.-S.; Li, H.; Armand, M.; Chen, L. A new class of Solvent-in-Salt electrolyte for high-energy rechargeable metallic lithium batteries. Nat. Commun. 2013, 4, 1481. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yao, X.; Misra, R.K.; Cai, Q.; Zhao, Y. Progress in electrolytes for beyond-lithium-ion batteries. J. Mater. Sci. Technol. 2020, 44, 237–257. [Google Scholar] [CrossRef]
- Fong, R.; von Sacken, U.; Dahn, J.R. Studies of Lithium Intercalation into Carbons Using Nonaqueous Electrochemical Cells. J. Electrochem. Soc. 1990, 137, 2009–2013. [Google Scholar] [CrossRef]
- Aurbach, D.; Ein-Eli, Y. The Study of Li-Graphite Intercalation Processes in Several Electrolyte Systems Using In Situ X-Ray Diffraction. J. Electrochem. Soc. 1995, 142, 1746–1752. [Google Scholar] [CrossRef]
- Chung, G.-C.; Kim, H.-J.; Yu, S.-I.; Jun, S.-H.; Choi, J.; Kim, M.-H. Origin of Graphite Exfoliation An Investigation of the Important Role of Solvent Cointercalation. J. Electrochem. Soc. 2000, 147, 4391. [Google Scholar] [CrossRef]
- Wagner, M.R.; Albering, J.H.; Moeller, K.-C.; Besenhard, J.O.; Winter, M. XRD evidence for the electrochemical formation of Li+(PC)yCn- in PC-based electrolytes. Electrochem. Commun. 2005, 7, 947–952. [Google Scholar] [CrossRef]
- Tasaki, K.; Goldberg, A.; Winter, M. On the difference in cycling behaviors of lithium-ion battery cell between the ethylene carbonate- and propylene carbonate-based electrolytes. Electrochim. Acta 2011, 56, 10424–10435. [Google Scholar] [CrossRef]
- McKinnon, W.R.; Dahn, J.R. How to Reduce the Cointercalation of Propylene Carbonate in Li x ZrS2 and Other Layered Compounds. J. Electrochem. Soc. 1985, 132, 364–366. [Google Scholar] [CrossRef]
- Yamada, Y.; Takazawa, Y.; Miyazaki, K.; Abe, T. Electrochemical Lithium Intercalation into Graphite in Dimethyl Sulfoxide-Based Electrolytes: Effect of Solvation Structure of Lithium Ion. J. Phys. Chem. C 2010, 114, 11680–11685. [Google Scholar] [CrossRef]
- Yamada, Y.; Yaegashi, M.; Abe, T.; Yamada, A. A superconcentrated ether electrolyte for fast-charging Li-ion batteries. Chem. Commun. 2013, 49, 11194–11196. [Google Scholar] [CrossRef]
- Yamada, Y.; Usui, K.; Chiang, C.H.; Kikuchi, K.; Furukawa, K.; Yamada, A. General observation of lithium intercalation into graphite in ethylene-carbonate-free superconcentrated electrolytes. ACS Appl. Mater. Interfaces 2014, 6, 10892–10899. [Google Scholar] [CrossRef]
- Jeong, S.-K.; Inaba, M.; Iriyama, Y.; Abe, T.; Ogumi, Z. Electrochemical Intercalation of Lithium Ion within Graphite from Propylene Carbonate Solutions. Electrochem. Solid-State Lett. 2003, 6, A13. [Google Scholar] [CrossRef]
- Jeong, S.-K.; Inaba, M.; Iriyama, Y.; Abe, T.; Ogumi, Z. Interfacial reactions between graphite electrodes and propylene carbonate-based solutions: Electrolyte-concentration dependence of electrochemical lithium intercalation reaction. J. Power Sources 2008, 175, 540–546. [Google Scholar] [CrossRef]
- Doi, T.; Masuhara, R.; Hashinokuchi, M.; Shimizu, Y.; Inaba, M. Concentrated LiPF6/PC electrolyte solutions for 5-V LiNi0.5Mn1.5O4 positive electrode in lithium-ion batteries. Electrochim. Acta 2016, 209, 219–224. [Google Scholar] [CrossRef]
- Alvarado, J.; Schroeder, M.A.; Zhang, M.; Borodin, O.; Gobrogge, E.; Olguin, M.; Ding, M.S.; Gobet, M.; Greenbaum, S.; Meng, Y.S.; et al. A carbonate-free, sulfone-based electrolyte for high-voltage Li-ion batteries. Mater. Today 2018, 21, 341–353. [Google Scholar] [CrossRef]
- Zheng, J.; Lochala, J.A.; Kwok, A.; Deng, Z.D.; Xiao, J. Research Progress towards Understanding the Unique Interfaces between Concentrated Electrolytes and Electrodes for Energy Storage Applications. Adv. Sci. 2017, 4, 1700032. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Yamada, A. Superconcentrated Electrolytes to Create New Interfacial Chemistry in Non-aqueous and Aqueous Rechargeable Batteries. Chem. Lett. 2017, 46, 1056–1064. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, Q.; Fang, M.; Ko, S.; Yamada, Y.; Yamada, A. Concentrated Electrolytes Widen the Operating Temperature Range of Lithium-Ion Batteries. Adv. Sci. 2021, 8, e2101646. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yamada, Y.; Sodeyama, K.; Chiang, C.H.; Tateyama, Y.; Yamada, A. Superconcentrated electrolytes for a high-voltage lithium-ion battery. Nat. Commun. 2016, 7, 12032. [Google Scholar] [CrossRef] [PubMed]
- Guidotti, R.A.; Masset, P. Thermally activated (“thermal”) battery technology. J. Power Sources 2006, 161, 1443–1449. [Google Scholar] [CrossRef]
- Masset, P.; Guidotti, R.A. Thermal activated (thermal) battery technology—Part II. Molten salt electrolytes. J. Power Sources 2007, 164, 397–414. [Google Scholar] [CrossRef]
- Brouillette, D.; Irish, D.E.; Taylor, N.J.; Perron, G.; Odziemkowski, M.; Desnoyers, J.E. Stable solvates in solution of lithium bis(trifluoromethylsulfone)imide in glymes and other aprotic solvents: Phase diagrams, crystallography and Raman spectroscopy. Phys. Chem. Chem. Phys. 2002, 4, 6063–6071. [Google Scholar] [CrossRef]
- Brouillette, D.; Perron, G.; Desnoyers, J.E. Apparent molar volume, heat capacity, and conductance of lithium bis(trifluoromethylsulfone) imide in glymes and other aprotic solvents. J. Solut. Chem. 1998, 27, 151–182. [Google Scholar] [CrossRef]
- Choquette, Y.; Brisard, G.; Parent, M.; Brouillette, D.; Perron, G.; Desnoyers, J.E.; Armand, M.; Gravel, D.; Slougui, N. Sulfamides and Glymes as Aprotic Solvents for Lithium Batteries. J. Electrochem. Soc. 1998, 145, 3500–3507. [Google Scholar] [CrossRef]
- Grondin, J.; Lassègues, J.-C.; Chami, M.; Servant, L.; Talaga, D.; Henderson, W.A. Raman study of tetraglyme–LiClO 4 solvate structures. Phys. Chem. Chem. Phys. 2004, 6, 4260–4267. [Google Scholar] [CrossRef]
- Grondin, J.; Talaga, D.; Lassègues, J.-C.; Henderson, W.A. Raman study of crystalline solvates between glymes CH3 (OCH2CH2)nOCH3 (n = 1, 2 and 3) and LiClO4. Phys. Chem. Chem. Phys. 2004, 6, 938–944. [Google Scholar] [CrossRef]
- Henderson, W.A. Glyme-lithium salt phase behavior. J. Phys. Chem. B 2006, 110, 13177–13183. [Google Scholar] [CrossRef]
- Henderson, W.A. Crystallization Kinetics of Glyme−LiX and PEO−LiX Polymer Electrolytes. Macromolecules 2007, 40, 4963–4971. [Google Scholar] [CrossRef]
- Henderson, W.A.; Brooks, N.R. Crystals from concentrated glyme mixtures. The single-crystal structure of LiClO4. Inorg. Chem. 2003, 42, 4522–4524. [Google Scholar] [CrossRef]
- Henderson, W.A.; Brooks, N.R.; Brennessel, W.W.; Young, V.G. LiClO4 Electrolyte Solvate Structures. J. Phys. Chem. A 2004, 108, 225–229. [Google Scholar] [CrossRef]
- Henderson, W.A.; Brooks, N.R.; Brennessel, W.W.; Young, V.G. Triglyme−Li + Cation Solvate Structures: Models for Amorphous Concentrated Liquid and Polymer Electrolytes (I). Chem. Mater. 2003, 15, 4679–4684. [Google Scholar] [CrossRef]
- Henderson, W.A.; Brooks, N.R.; Young, V.G. Tetraglyme−Li + Cation Solvate Structures: Models for Amorphous Concentrated Liquid and Polymer Electrolytes (II). Chem. Mater. 2003, 15, 4685–4690. [Google Scholar] [CrossRef]
- Henderson, W.A.; McKenna, F.; Khan, M.A.; Brooks, N.R.; Young, V.G.; Frech, R. Glyme−Lithium Bis(trifluoromethanesulfonyl)imide and Glyme−Lithium Bis(perfluoroethanesulfonyl)imide Phase Behavior and Solvate Structures. Chem. Mater. 2005, 17, 2284–2289. [Google Scholar] [CrossRef]
- Johansson, P. First principles modelling of amorphous polymer electrolytes: Li+–PEO, Li+–PEI, and Li+–PES complexes. Polymer 2001, 42, 4367–4373. [Google Scholar] [CrossRef]
- Pappenfus, T.M.; Henderson, W.A.; Owens, B.B.; Mann, K.R.; Smyrl, W.H. Complexes of Lithium Imide Salts with Tetraglyme and Their Polyelectrolyte Composite Materials. J. Electrochem. Soc. 2004, 151, A209. [Google Scholar] [CrossRef]
- Johansson, P.; Tegenfeldt, J.; Lindgren, J. Modelling amorphous lithium salt–PEO polymer electrolytes: Ab initio calculations of lithium ion–tetra-, penta- and hexaglyme complexes. Polymer 1999, 40, 4399–4406. [Google Scholar] [CrossRef]
- Tamura, T.; Hachida, T.; Yoshida, K.; Tachikawa, N.; Dokko, K.; Watanabe, M. New glyme–cyclic imide lithium salt complexes as thermally stable electrolytes for lithium batteries. J. Power Sources 2010, 195, 6095–6100. [Google Scholar] [CrossRef]
- Tamura, T.; Yoshida, K.; Hachida, T.; Tsuchiya, M.; Nakamura, M.; Kazue, Y.; Tachikawa, N.; Dokko, K.; Watanabe, M. Physicochemical Properties of Glyme–Li Salt Complexes as a New Family of Room-temperature Ionic Liquids. Chem. Lett. 2010, 39, 753–755. [Google Scholar] [CrossRef]
- Orita, A.; Kamijima, K.; Yoshida, M.; Dokko, K.; Watanabe, M. Favorable combination of positive and negative electrode materials with glyme–Li salt complex electrolytes in lithium ion batteries. J. Power Sources 2011, 196, 3874–3880. [Google Scholar] [CrossRef]
- Seki, S.; Takei, K.; Miyashiro, H.; Watanabe, M. Physicochemical and Electrochemical Properties of Glyme-LiN(SO2F)2 Complex for Safe Lithium-ion Secondary Battery Electrolyte. J. Electrochem. Soc. 2011, 158, A769. [Google Scholar] [CrossRef]
- Tachikawa, N.; Yamauchi, K.; Takashima, E.; Park, J.-W.; Dokko, K.; Watanabe, M. Reversibility of electrochemical reactions of sulfur supported on inverse opal carbon in glyme-Li salt molten complex electrolytes. Chem. Commun. 2011, 47, 8157–8159. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Nakamura, M.; Kazue, Y.; Tachikawa, N.; Tsuzuki, S.; Seki, S.; Dokko, K.; Watanabe, M. Oxidative-stability enhancement and charge transport mechanism in glyme-lithium salt equimolar complexes. J. Am. Chem. Soc. 2011, 133, 13121–13129. [Google Scholar] [CrossRef]
- Yoshida, K.; Tsuchiya, M.; Tachikawa, N.; Dokko, K.; Watanabe, M. Change from Glyme Solutions to Quasi-ionic Liquids for Binary Mixtures Consisting of Lithium Bis(trifluoromethanesulfonyl)amide and Glymes. J. Phys. Chem. C 2011, 115, 18384–18394. [Google Scholar] [CrossRef]
- Ueno, K.; Yoshida, K.; Tsuchiya, M.; Tachikawa, N.; Dokko, K.; Watanabe, M. Glyme-lithium salt equimolar molten mixtures: Concentrated solutions or solvate ionic liquids? J. Phys. Chem. B 2012, 116, 11323–11331. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Tsuchiya, M.; Tachikawa, N.; Dokko, K.; Watanabe, M. Correlation between Battery Performance and Lithium Ion Diffusion in Glyme–Lithium Bis(trifluoromethanesulfonyl)amide Equimolar Complexes. J. Electrochem. Soc. 2012, 159, A1005–A1012. [Google Scholar] [CrossRef]
- Dokko, K.; Tachikawa, N.; Yamauchi, K.; Tsuchiya, M.; Yamazaki, A.; Takashima, E.; Park, J.-W.; Ueno, K.; Seki, S.; Serizawa, N.; et al. Solvate Ionic Liquid Electrolyte for Li–S Batteries. J. Electrochem. Soc. 2013, 160, A1304–A1310. [Google Scholar] [CrossRef]
- Mandai, T.; Nozawa, R.; Tsuzuki, S.; Yoshida, K.; Ueno, K.; Dokko, K.; Watanabe, M. Phase diagrams and solvate structures of binary mixtures of glymes and Na salts. J. Phys. Chem. B 2013, 117, 15072–15085. [Google Scholar] [CrossRef] [PubMed]
- Seki, S.; Serizawa, N.; Takei, K.; Dokko, K.; Watanabe, M. Charge/discharge performances of glyme–lithium salt equimolar complex electrolyte for lithium secondary batteries. J. Power Sources 2013, 243, 323–327. [Google Scholar] [CrossRef]
- Serizawa, N.; Seki, S.; Takei, K.; Miyashiro, H.; Yoshida, K.; Ueno, K.; Tachikawa, N.; Dokko, K.; Katayama, Y.; Watanabe, M.; et al. EQCM Measurement of Deposition and Dissolution of Lithium in Glyme-Li Salt Molten Complex. J. Electrochem. Soc. 2013, 160, A1529–A1533. [Google Scholar] [CrossRef]
- Tatara, R.; Tachikawa, N.; Kwon, H.-M.; Ueno, K.; Dokko, K.; Watanabe, M. Solvate Ionic Liquid, [Li(triglyme)1] [NTf2], as Electrolyte for Rechargeable Li–Air Battery: Discharge Depth and Reversibility. Chem. Lett. 2013, 42, 1053–1055. [Google Scholar] [CrossRef]
- Tsuzuki, S.; Shinoda, W.; Seki, S.; Umebayashi, Y.; Yoshida, K.; Dokko, K.; Watanabe, M. Intermolecular interactions in Li+-glyme and Li+-glyme-TFSA- complexes: Relationship with physicochemical properties of Li(glyme)TFSA ionic liquids. Chemphyschem 2013, 14, 1993–2001. [Google Scholar] [CrossRef]
- Ueno, K.; Park, J.-W.; Yamazaki, A.; Mandai, T.; Tachikawa, N.; Dokko, K.; Watanabe, M. Anionic Effects on Solvate Ionic Liquid Electrolytes in Rechargeable Lithium–Sulfur Batteries. J. Phys. Chem. C 2013, 117, 20509–20516. [Google Scholar] [CrossRef]
- Mandai, T.; Yoshida, K.; Ueno, K.; Dokko, K.; Watanabe, M. Criteria for solvate ionic liquids. Phys. Chem. Chem. Phys. 2014, 16, 8761–8772. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.; Tatara, R.; Mandai, T.; Ueno, K.; Yoshida, K.; Tachikawa, N.; Yasuda, T.; Dokko, K.; Watanabe, M. Mechanism of Li Ion Desolvation at the Interface of Graphite Electrode and Glyme–Li Salt Solvate Ionic Liquids. J. Phys. Chem. C 2014, 118, 20246–20256. [Google Scholar] [CrossRef]
- Terada, S.; Mandai, T.; Nozawa, R.; Yoshida, K.; Ueno, K.; Tsuzuki, S.; Dokko, K.; Watanabe, M. Physicochemical properties of pentaglyme-sodium bis(trifluoromethanesulfonyl)amide solvate ionic liquid. Phys. Chem. Chem. Phys. 2014, 16, 11737–11746. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ueno, K.; Yamazaki, A.; Yoshida, K.; Moon, H.; Mandai, T.; Umebayashi, Y.; Dokko, K.; Watanabe, M. Chelate effects in glyme/lithium bis(trifluoromethanesulfonyl)amide solvate ionic liquids. I. Stability of solvate cations and correlation with electrolyte properties. J. Phys. Chem. B 2014, 118, 5144–5153. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.; Mandai, T.; Tatara, R.; Ueno, K.; Yamazaki, A.; Yoshida, K.; Seki, S.; Dokko, K.; Watanabe, M. Solvent Activity in Electrolyte Solutions Controls Electrochemical Reactions in Li-Ion and Li-Sulfur Batteries. J. Phys. Chem. C 2015, 119, 3957–3970. [Google Scholar] [CrossRef]
- Ueno, K.; Tatara, R.; Tsuzuki, S.; Saito, S.; Doi, H.; Yoshida, K.; Mandai, T.; Matsugami, M.; Umebayashi, Y.; Dokko, K.; et al. Li(+) solvation in glyme-Li salt solvate ionic liquids. Phys. Chem. Chem. Phys. 2015, 17, 8248–8257. [Google Scholar] [CrossRef]
- Tsuzuki, S.; Shinoda, W.; Matsugami, M.; Umebayashi, Y.; Ueno, K.; Mandai, T.; Seki, S.; Dokko, K.; Watanabe, M. Structures of Li(glyme)(+) complexes and their interactions with anions in equimolar mixtures of glymes and LiTFSA: Analysis by molecular dynamics simulations. Phys. Chem. Chem. Phys. 2015, 17, 126–129. [Google Scholar] [CrossRef]
- Mandai, T.; Yoshida, K.; Tsuzuki, S.; Nozawa, R.; Masu, H.; Ueno, K.; Dokko, K.; Watanabe, M. Effect of ionic size on solvate stability of glyme-based solvate ionic liquids. J. Phys. Chem. B 2015, 119, 1523–1534. [Google Scholar] [CrossRef] [PubMed]
- Mandai, T.; Tsuzuki, S.; Ueno, K.; Dokko, K.; Watanabe, M. Pentaglyme-K salt binary mixtures: Phase behavior, solvate structures, and physicochemical properties. Phys. Chem. Chem. Phys. 2015, 17, 2838–2849. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yamazaki, A.; Murai, J.; Park, J.-W.; Mandai, T.; Ueno, K.; Dokko, K.; Watanabe, M. Chelate Effects in Glyme/Lithium Bis(trifluoromethanesulfonyl)amide Solvate Ionic Liquids, Part 2: Importance of Solvate-Structure Stability for Electrolytes of Lithium Batteries. J. Phys. Chem. C 2014, 118, 17362–17373. [Google Scholar] [CrossRef]
- Liang, H.; Li, H.; Wang, Z.; Wu, F.; Chen, L.; Huang, X. New Binary Room-Temperature Molten Salt Electrolyte Based on Urea and LiTFSI. J. Phys. Chem. B 2001, 105, 9966–9969. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Z.; Li, H.; Huang, X.; Chen, L. Ionic Conductivity and Association Studies of Novel RTMS Electrolyte Based on LiTFSI and Acetamide. J. Electrochem. Soc. 2004, 151, A1424. [Google Scholar] [CrossRef]
- Chen, R.; Wu, F.; Li, L.; Xu, B.; Qiu, X.; Chen, S. Novel Binary Room-Temperature Complex System Based on LiTFSI and 2-Oxazolidinone and Its Characterization as Electrolyte. J. Phys. Chem. C 2007, 111, 5184–5194. [Google Scholar] [CrossRef]
- Chen, R.; Wu, F.; Liang, H.; Li, L.; Xu, B. Novel Binary Room-Temperature Complex Electrolytes Based on LiTFSI and Organic Compounds with Acylamino Group. J. Electrochem. Soc. 2005, 152, A1979. [Google Scholar] [CrossRef]
- Chen, R.; Wu, F.; Li, L.; Qiu, X.; Chen, L.; Chen, S. The structure–activity relationship studies of binary room temperature complex electrolytes based on LiTFSI and organic compounds with acylamino group. Vib. Spectrosc. 2007, 44, 297–307. [Google Scholar] [CrossRef]
- Kubota, K.; Matsumoto, H. Melting and Crystallization Behaviors of Alkali Metal (Fluorosulfonyl)(trifluoromethylsulfonyl)amides. Chem. Lett. 2011, 40, 1105–1106. [Google Scholar] [CrossRef]
- Kubota, K.; Matsumoto, H. Cation Mixtures of Alkali Metal (Fluorosulfonyl)(trifluoromethylsulfonyl)Amide as Electrolytes for Lithium Secondary Battery. J. Electrochem. Soc. 2014, 161, A902–A907. [Google Scholar] [CrossRef]
- Kubota, K.; Nohira, T.; Goto, T.; Hagiwara, R. Novel inorganic ionic liquids possessing low melting temperatures and wide electrochemical windows: Binary mixtures of alkali bis(fluorosulfonyl)amides. Electrochem. Commun. 2008, 10, 1886–1888. [Google Scholar] [CrossRef]
- Kubota, K.; Nohira, T.; Goto, T.; Hagiwara, R. Ternary phase diagrams of alkali bis(trifluoromethylsulfonyl)amides. J. Chem. Eng. Data 2008, 53, 2144–2147. [Google Scholar] [CrossRef]
- Kubota, K.; Nohira, T.; Hagiwara, R. Thermal Properties of Alkali Bis(fluorosulfonyl)amides and Their Binary Mixtures. J. Chem. Eng. Data 2010, 55, 3142–3146. [Google Scholar] [CrossRef]
- Kubota, K.; Nohira, T.; Hagiwara, R.; Matsumoto, H. Thermal Properties of Alkali (Fluorosulfonyl)(trifluoromethylsulfonyl)amides. Chem. Lett. 2010, 39, 1303–1304. [Google Scholar] [CrossRef]
- Watarai, A.; Kubota, K.; Yamagata, M.; Goto, T.; Nohira, T.; Hagiwara, R.; Ui, K.; Kumagai, N. A rechargeable lithium metal battery operating at intermediate temperatures using molten alkali bis(trifluoromethylsulfonyl)amide mixture as an electrolyte. J. Power Sources 2008, 183, 724–729. [Google Scholar] [CrossRef]
- Kubota, K.; Matsumoto, H. Investigation of an Intermediate Temperature Molten Lithium Salt Based on Fluorosulfonyl(trifluoromethylsulfonyl)amide as a Solvent-Free Lithium Battery Electrolyte. J. Phys. Chem. C 2013, 117, 18829–18836. [Google Scholar] [CrossRef]
- Jeong, S.-K.; Seo, H.-Y.; Kim, D.-H.; Han, H.-K.; Kim, J.-G.; Lee, Y.B.; Iriyama, Y.; Abe, T.; Ogumi, Z. Suppression of dendritic lithium formation by using concentrated electrolyte solutions. Electrochem. Commun. 2008, 10, 635–638. [Google Scholar] [CrossRef]
- Qian, J.; Henderson, W.A.; Xu, W.; Bhattacharya, P.; Engelhard, M.; Borodin, O.; Zhang, J.-G. High rate and stable cycling of lithium metal anode. Nat. Commun. 2015, 6, 6362. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, V.; Kotronia, A.; Lacey, M.; Edström, K.; Johansson, P. Highly Concentrated LiTFSI–EC Electrolytes for Lithium Metal Batteries. ACS Appl. Energy Mater. 2020, 3, 200–207. [Google Scholar] [CrossRef]
- Vu, T.T.; Kim, B.G.; Kim, J.H.; Moon, J. Suppression of dendritic lithium-metal growth through concentrated dual-salt electrolyte and its accurate prediction. J. Mater. Chem. A 2021, 9, 22833–22841. [Google Scholar] [CrossRef]
- Li, L.; Zhou, S.; Han, H.; Li, H.; Nie, J.; Armand, M.; Zhou, Z.; Huang, X. Transport and Electrochemical Properties and Spectral Features of Non-Aqueous Electrolytes Containing LiFSI in Linear Carbonate Solvents. J. Electrochem. Soc. 2011, 158, A74. [Google Scholar] [CrossRef]
- Hu, J.J.; Long, G.K.; Liu, S.; Li, G.R.; Gao, X.P. A LiFSI-LiTFSI binary-salt electrolyte to achieve high capacity and cycle stability for a Li-S battery. Chem. Commun. 2014, 50, 14647–14650. [Google Scholar] [CrossRef]
- Song, Z.; Wang, X.; Wu, H.; Feng, W.; Nie, J.; Yu, H.; Huang, X.; Armand, M.; Zhang, H.; Zhou, Z. Bis(fluorosulfonyl)imide-based electrolyte for rechargeable lithium batteries: A perspective. J. Power Sources Adv. 2022, 14, 100088. [Google Scholar] [CrossRef]
- Cuisinier, M.; Cabelguen, P.-E.; Adams, B.D.; Garsuch, A.; Balasubramanian, M.; Nazar, L.F. Unique behaviour of nonsolvents for polysulphides in lithium–sulphur batteries. Energy Environ. Sci. 2014, 7, 2697–2705. [Google Scholar] [CrossRef]
- Doi, T.; Shimizu, Y.; Hashinokuchi, M.; Inaba, M. Dilution of Highly Concentrated LiBF 4 /Propylene Carbonate Electrolyte Solution with Fluoroalkyl Ethers for 5-V LiNi0.5Mn1.5O4 Positive Electrodes. J. Electrochem. Soc. 2017, 164, A6412–A6416. [Google Scholar] [CrossRef]
- Ren, X.; Chen, S.; Lee, H.; Mei, D.; Engelhard, M.H.; Burton, S.D.; Zhao, W.; Zheng, J.; Li, Q.; Ding, M.S.; et al. Localized High-Concentration Sulfone Electrolytes for High-Efficiency Lithium-Metal Batteries. Chem 2018, 4, 1877–1892. [Google Scholar] [CrossRef]
- Li, W.; Dahn, J.R.; Wainwright, D.S. Rechargeable lithium batteries with aqueous electrolytes. Science 1994, 264, 1115–1118. [Google Scholar] [CrossRef] [PubMed]
- Suo, L.; Borodin, O.; Gao, T.; Olguin, M.; Ho, J.; Fan, X.; Luo, C.; Wang, C.; Xu, K. “Water-in-salt” electrolyte enables high-voltage aqueous lithium-ion chemistries. Science 2015, 350, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Suo, L.; Borodin, O.; Sun, W.; Fan, X.; Yang, C.; Wang, F.; Gao, T.; Ma, Z.; Schroeder, M.; von Cresce, A.; et al. Advanced High-Voltage Aqueous Lithium-Ion Battery Enabled by “Water-in-Bisalt” Electrolyte. Angew. Chem. 2016, 128, 7252–7257. [Google Scholar] [CrossRef]
- Droguet, L.; Grimaud, A.; Fontaine, O.; Tarascon, J.-M. Water-in-Salt Electrolyte (WiSE) for Aqueous Batteries: A Long Way to Practicality. Adv. Energy Mater. 2020, 10, 2002440. [Google Scholar] [CrossRef]
- Yamada, Y.; Usui, K.; Sodeyama, K.; Ko, S.; Tateyama, Y.; Yamada, A. Hydrate-melt electrolytes for high-energy-density aqueous batteries. Nat. Energy 2016, 1, 16129. [Google Scholar] [CrossRef]
- Ko, S.; Yamada, Y.; Miyazaki, K.; Shimada, T.; Watanabe, E.; Tateyama, Y.; Kamiya, T.; Honda, T.; Akikusa, J.; Yamada, A. Lithium-salt monohydrate melt: A stable electrolyte for aqueous lithium-ion batteries. Electrochem. Commun. 2019, 104, 106488. [Google Scholar] [CrossRef]
- Yang, C.; Chen, J.; Qing, T.; Fan, X.; Sun, W.; von Cresce, A.; Ding, M.S.; Borodin, O.; Vatamanu, J.; Schroeder, M.A.; et al. 4.0 V Aqueous Li-Ion Batteries. Joule 2017, 1, 122–132. [Google Scholar] [CrossRef]
- Yamada, Y.; Yamada, A. Review—Superconcentrated Electrolytes for Lithium Batteries. J. Electrochem. Soc. 2015, 162, A2406–A2423. [Google Scholar] [CrossRef]
- Ponnuchamy, V.; Mossa, S.; Skarmoutsos, I. Solvent and Salt Effect on Lithium Ion Solvation and Contact Ion Pair Formation in Organic Carbonates: A Quantum Chemical Perspective. J. Phys. Chem. C 2018, 122, 25930–25939. [Google Scholar] [CrossRef]
- Esch, T.E.H.; Smid, J. Studies of Contact and Solvent-Separated Ion Pairs of Carbanions. I. Effect of Temperature, Counterion, and Solvent. J. Am. Chem. Soc. 1966, 88, 307–318. [Google Scholar] [CrossRef]
- Yamada, Y.; Furukawa, K.; Sodeyama, K.; Kikuchi, K.; Yaegashi, M.; Tateyama, Y.; Yamada, A. Unusual stability of acetonitrile-based superconcentrated electrolytes for fast-charging lithium-ion batteries. J. Am. Chem. Soc. 2014, 136, 5039–5046. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Wang, J.; Ko, S.; Watanabe, E.; Yamada, A. Advances and issues in developing salt-concentrated battery electrolytes. Nat. Energy 2019, 4, 269–280. [Google Scholar] [CrossRef]
- Han, S.-D.; Borodin, O.; Seo, D.M.; Zhou, Z.-B.; Henderson, W.A. Electrolyte Solvation and Ionic Association. J. Electrochem. Soc. 2014, 161, A2042–A2053. [Google Scholar] [CrossRef]
- Yang, H.; Wu, N. Ionic conductivity and ion transport mechanisms of solid-state lithium-ion battery electrolytes: A review. Energy Sci. Eng. 2022, 10, 1643–1671. [Google Scholar] [CrossRef]
- Dokko, K.; Watanabe, D.; Ugata, Y.; Thomas, M.L.; Tsuzuki, S.; Shinoda, W.; Hashimoto, K.; Ueno, K.; Umebayashi, Y.; Watanabe, M. Direct Evidence for Li Ion Hopping Conduction in Highly Concentrated Sulfolane-Based Liquid Electrolytes. J. Phys. Chem. B 2018, 122, 10736–10745. [Google Scholar] [CrossRef] [PubMed]
- Diederichsen, K.M.; McShane, E.J.; McCloskey, B.D. Promising Routes to a High Li + Transference Number Electrolyte for Lithium Ion Batteries. ACS Energy Lett. 2017, 2, 2563–2575. [Google Scholar] [CrossRef]
- Dai, W.; Dong, N.; Xia, Y.; Chen, S.; Luo, H.; Liu, Y.; Liu, Z. Localized concentrated high-concentration electrolyte enhanced stability and safety for high voltage Li-ion batteries. Electrochim. Acta 2019, 320, 134633. [Google Scholar] [CrossRef]
- Guan, D.; Zeng, J.; Xue, Z.; Cao, Y.; Hu, G.; Peng, Z.; Du, K. Rational Design of the Li + -Solvation Structure Contributes to Constructing a Robust Cathode-Electrolyte Interphase for a 5 V High-Voltage LiNi0.5Mn1.5O4 Cathode. ACS Appl. Energy Mater. 2023, 6, 9568–9576. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, J.; Yang, Q.; Wang, S.; Wang, W.; Li, B. Stable Cycling of High-Voltage Lithium-Metal Batteries Enabled by High-Concentration FEC-Based Electrolyte. ACS Appl. Mater. Interfaces 2020, 12, 22901–22909. [Google Scholar] [CrossRef] [PubMed]
- Kuehnel, R.-S.; Luebke, M.; Winter, M.; Passerini, S.; Balducci, A. Suppression of aluminum current collector corrosion in ionic liquid containing electrolytes. J. Power Sources 2012, 214, 178–184. [Google Scholar] [CrossRef]
- Matsumoto, K.; Inoue, K.; Nakahara, K.; Yuge, R.; Noguchi, T.; Utsugi, K. Suppression of aluminum corrosion by using high concentration LiTFSI electrolyte. J. Power Sources 2013, 231, 234–238. [Google Scholar] [CrossRef]
- McOwen, D.W.; Seo, D.M.; Borodin, O.; Vatamanu, J.; Boyle, P.D.; Henderson, W.A. Concentrated electrolytes: Decrypting electrolyte properties and reassessing Al corrosion mechanisms. Energy Environ. Sci. 2014, 7, 416–426. [Google Scholar] [CrossRef]
- Yamada, Y.; Chiang, C.H.; Sodeyama, K.; Wang, J.; Tateyama, Y.; Yamada, A. Corrosion Prevention Mechanism of Aluminum Metal in Superconcentrated Electrolytes. ChemElectroChem 2015, 2, 1687–1694. [Google Scholar] [CrossRef]
- Liu, W.; Li, J.; Li, W.; Xu, H.; Zhang, C.; Qiu, X. Inhibition of transition metals dissolution in cobalt-free cathode with ultrathin robust interphase in concentrated electrolyte. Nat. Commun. 2020, 11, 3629. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Li, C.; Zhang, J.; Wang, P.; Zhao, D.; Quan, Y.; Sun, J.; Cui, X.; Li, S. Inhibition of transition-metal dissolution with an inert soluble product interface constructed by high-concentration electrolyte. iScience 2023, 26, 107052. [Google Scholar] [CrossRef]
- Bai, P.; Li, J.; Brushett, F.R.; Bazant, M.Z. Transition of lithium growth mechanisms in liquid electrolytes. Energy Environ. Sci. 2016, 9, 3221–3229. [Google Scholar] [CrossRef]
- Kim, H.; Wu, F.; Lee, J.T.; Nitta, N.; Lin, H.-T.; Oschatz, M.; Cho, W.I.; Kaskel, S.; Borodin, O.; Yushin, G. In Situ Formation of Protective Coatings on Sulfur Cathodes in Lithium Batteries with LiFSI-Based Organic Electrolytes. Adv. Energy Mater. 2015, 5, 1401792. [Google Scholar] [CrossRef]
- Park, M.S.; Ma, S.B.; Lee, D.J.; Im, D.; Doo, S.-G.; Yamamoto, O. A Highly Reversible Lithium Metal Anode. Sci. Rep. 2014, 4, 3815. [Google Scholar] [CrossRef] [PubMed]
- Perez Beltran, S.; Balbuena, P.B. SEI formation mechanisms and Li+ dissolution in lithium metal anodes: Impact of the electrolyte composition and the electrolyte-to-anode ratio. J. Power Sources 2022, 551, 232203. [Google Scholar] [CrossRef]
- Ren, X.; Gao, P.; Zou, L.; Jiao, S.; Cao, X.; Zhang, X.; Jia, H.; Engelhard, M.H.; Matthews, B.E.; Wu, H.; et al. Role of inner solvation sheath within salt-solvent complexes in tailoring electrode/electrolyte interphases for lithium metal batteries. Proc. Natl. Acad. Sci. USA 2020, 117, 28603–28613. [Google Scholar] [CrossRef]
- Vu, M.C.; Mirmira, P.; Gomes, R.J.; Ma, P.; Doyle, E.S.; Srinivasan, H.S.; Amanchukwu, C.V. Low melting alkali-based molten salt electrolytes for solvent-free lithium-metal batteries. Matter 2023, 6, 4357–4375. [Google Scholar] [CrossRef]
- Zeng, Z.; Murugesan, V.; Han, K.S.; Jiang, X.; Cao, Y.; Xiao, L.; Ai, X.; Yang, H.; Zhang, J.-G.; Sushko, M.L.; et al. Non-flammable electrolytes with high salt-to-solvent ratios for Li-ion and Li-metal batteries. Nat. Energy 2018, 3, 674–681. [Google Scholar] [CrossRef]
- Xu, Z.; Deng, K.; Zhou, S.; Mo, D. High-performance lithium metal batteries enabled by fluorinated aromatic diluent assisted nonflammable localized high-concentration electrolytes. J. Power Sources 2023, 559, 232631. [Google Scholar] [CrossRef]
- Shi, P.; Zheng, H.; Liang, X.; Sun, Y.; Cheng, S.; Chen, C.; Xiang, H. A highly concentrated phosphate-based electrolyte for high-safety rechargeable lithium batteries. Chem. Commun. 2018, 54, 4453–4456. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, N.; Li, C.; Zhang, Y.; Jia, M.; Wang, Y.; Zhao, Y.; Jiao, L.; Cheng, F.; Xu, J. Fire-Retardant Phosphate-Based Electrolytes for High-Performance Lithium Metal Batteries. ACS Appl. Energy Mater. 2019, 2, 2708–2716. [Google Scholar] [CrossRef]
- Davoodabadi, A.; Li, J.; Zhou, H.; Wood, D.L.; Singler, T.J.; Jin, C. Effect of calendering and temperature on electrolyte wetting in lithium-ion battery electrodes. J. Energy Storage 2019, 26, 101034. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, R.; Wang, J.; Wang, Y. Current and future lithium-ion battery manufacturing. iScience 2021, 24, 102332. [Google Scholar] [CrossRef]
- Cao, X.; Jia, H.; Xu, W.; Zhang, J.-G. Review—Localized High-Concentration Electrolytes for Lithium Batteries. J. Electrochem. Soc. 2021, 168, 10522. [Google Scholar] [CrossRef]
- Kim, S.C.; Wang, J.; Xu, R.; Zhang, P.; Chen, Y.; Huang, Z.; Yang, Y.; Yu, Z.; Oyakhire, S.T.; Zhang, W.; et al. High-entropy electrolytes for practical lithium metal batteries. Nat. Energy 2023, 8, 814–826. [Google Scholar] [CrossRef]
- Tyrrell, N.D. A Proposal That Would Ban Manufacture, Supply, and Use of All Fluoropolymers and Most Fluorinated Reagents within the Entire EU. Org. Process Res. Dev. 2023, 27, 1422–1426. [Google Scholar] [CrossRef]
- Sonne, C.; Jenssen, B.M.; Rinklebe, J.; Lam, S.S.; Hansen, M.; Bossi, R.; Gustavson, K.; Dietz, R. EU need to protect its environment from toxic per- and polyfluoroalkyl substances. Sci. Total Environ. 2023, 876, 162770. [Google Scholar] [CrossRef] [PubMed]
- Guelfo, J.L.; Ferguson, P.L.; Beck, J.; Chernick, M.; Doria-Manzur, A.; Faught, P.W.; Flug, T.; Gray, E.P.; Jayasundara, N.; Knappe, D.R.U.; et al. Lithium-ion battery components are at the nexus of sustainable energy and environmental release of per- and polyfluoroalkyl substances. Nat. Commun. 2024, 15, 5548. [Google Scholar] [CrossRef] [PubMed]
- Rensmo, A.; Savvidou, E.K.; Cousins, I.T.; Hu, X.; Schellenberger, S.; Benskin, J.P. Lithium-ion battery recycling: A source of per- and polyfluoroalkyl substances (PFAS) to the environment? Environ. Sci. Process. Impacts 2023, 25, 1015–1030. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Mariani, A.; Diemant, T.; Di Pietro, M.E.; Dong, X.; Su, P.-H.; Mele, A.; Passerini, S. PFAS-Free Locally Concentrated Ionic Liquid Electrolytes for Lithium Metal Batteries. ACS Energy Lett. 2024, 9, 3049–3057. [Google Scholar] [CrossRef]
- Hai, F.; Yi, Y.; Xiao, Z.; Guo, J.; Gao, X.; Chen, W.; Xue, W.; Hua, W.; Tang, W.; Li, M. A Low-Cost, Fluorine-Free Localized Highly Concentrated Electrolyte Toward Ultra-High Loading Lithium Metal Batteries. Adv. Energy Mater. 2024, 14, 2304253. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krämer, S.; Weintz, D.; Winter, M.; Cekic-Laskovic, I.; Grünebaum, M. Importance of High-Concentration Electrolytes for Lithium-Based Batteries. Encyclopedia 2025, 5, 20. https://doi.org/10.3390/encyclopedia5010020
Krämer S, Weintz D, Winter M, Cekic-Laskovic I, Grünebaum M. Importance of High-Concentration Electrolytes for Lithium-Based Batteries. Encyclopedia. 2025; 5(1):20. https://doi.org/10.3390/encyclopedia5010020
Chicago/Turabian StyleKrämer, Susanna, Dominik Weintz, Martin Winter, Isidora Cekic-Laskovic, and Mariano Grünebaum. 2025. "Importance of High-Concentration Electrolytes for Lithium-Based Batteries" Encyclopedia 5, no. 1: 20. https://doi.org/10.3390/encyclopedia5010020
APA StyleKrämer, S., Weintz, D., Winter, M., Cekic-Laskovic, I., & Grünebaum, M. (2025). Importance of High-Concentration Electrolytes for Lithium-Based Batteries. Encyclopedia, 5(1), 20. https://doi.org/10.3390/encyclopedia5010020






