Early Multi-Target Treatment of Mild-to-Moderate COVID-19, Particularly in Terms of Non-Steroidal Anti-Inflammatory Drugs and Indomethacin
Abstract
:1. Introduction
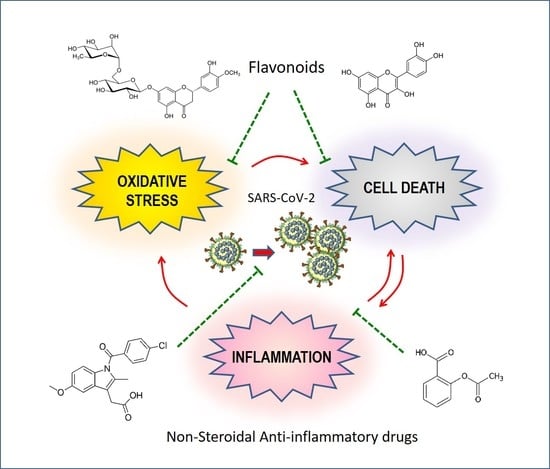
2. Non-Steroidal Anti-Inflammatory Drugs in the Fight against COVID-19
3. Potential of Indomethacin in the Treatment of COVID-19
3.1. Cyclooxygenases Inhibition
3.2. BCL2-Associated Agonist of Cell Death
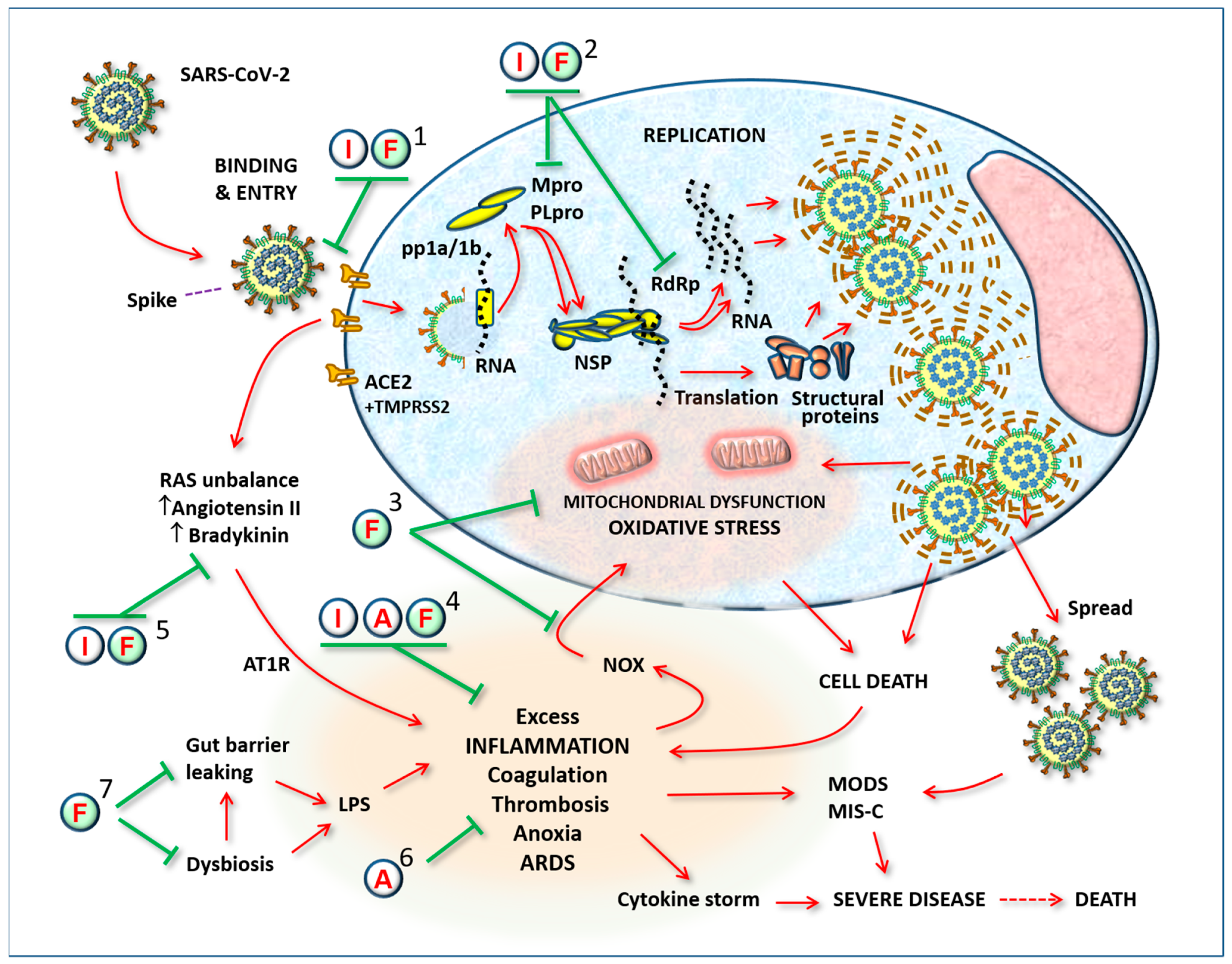
3.3. Renin–Angiotensin System
3.4. Bradykinin
3.5. Antiviral Action of Indomethacin
4. The Logic of a Target Synergistic Therapy
4.1. Antioxidant and Antiviral Potentials of Flavonoids
4.2. Vitamin C
4.3. Low-Dose Aspirin
4.4. A Gastric Protector with Possible Antiviral Action: Omeprazole
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Evidence-Based Medicine Working Group. Evidence-Based medicine. A new approach to teaching the practice of medicine. JAMA 1992, 268, 2420–2425. [Google Scholar] [CrossRef] [PubMed]
- Straus, S.E.; Glasziou, P.; Richardson, W.S.; Haynes, R.B. Evidence-Based Medicine: How to Practice and Teach It, 4th ed.; Churchill Livingstone: London, UK; Elsevier: Edimburg, UK, 2011. [Google Scholar]
- Adil, T.; Rahman, R.; Whitelaw, D.; Jain, V.; Al-Taan, O.; Rashid, F.; Munasinghe, A.; Jambulingam, P. SARS-CoV-2 and the pandemic of COVID-19. Postgrad. Med. J. 2021, 97, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Scotto Di Vetta, M.; Morrone, M.; Fazio, S. COVID-19: Off-label therapies based on mechanism of action while waiting for evidence-based medicine recommendations. World J. Meta Anal. 2020, 8, 173–177. [Google Scholar] [CrossRef]
- Fazio, S.; Cosentino, M.; Marino, F.; Pandolfi, S.; Zanolin, E.; Bellavite, P. The Problem of Home Therapy during COVID-19 Pandemic in Italy: Government Guidelines versus Freedom of Cure? J. Pharm. Pharmacol. Res. 2022, 6, 100–114. [Google Scholar] [CrossRef]
- Franco-Paredes, C. Transmissibility of SARS-CoV-2 among fully vaccinated individuals. Lancet Infect. Dis. 2022, 22, 16. [Google Scholar] [CrossRef] [PubMed]
- Bonaventura, A.; Vecchié, A.; Dagna, L.; Martinod, K.; Dixon, D.L.; Van Tassell, B.W.; Dentali, F.; Montecucco, F.; Massberg, S.; Levi, M.; et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat. Rev. Immunol. 2021, 21, 319–329. [Google Scholar] [CrossRef]
- Chen, R.; Lan, Z.; Ye, J.; Pang, L.; Liu, Y.; Wu, W.; Qin, X.; Guo, Y.; Zhang, P. Cytokine Storm: The Primary Determinant for the Pathophysiological Evolution of COVID-19 Deterioration. Front. Immunol. 2021, 12, 589095. [Google Scholar] [CrossRef]
- Day, M. COVID-19: Ibuprofen should not be used for managing symptoms, say doctors and scientists. BMJ 2020, 368, m1086. [Google Scholar] [CrossRef] [Green Version]
- Rezza, G.; Urbani, A. Circolare Recante “Gestione Domiciliare dei Pazienti con Infezione da SARS-CoV-2”; Ditrezioni Generali Programmazione Sanitaria e Prevenzione Sanitaria; Ministero della Salute: Roma, Italy, 2020. [Google Scholar]
- Suter, F.; Consolaro, E.; Pedroni, S.; Moroni, C.; Pastò, E.; Paganini, M.V.; Pravettoni, G.; Cantarelli, U.; Rubis, N.; Perico, N.; et al. A simple, home-therapy algorithm to prevent hospitalisation for COVID-19 patients: A retrospective observational matched-cohort study. EClinicalMedicine 2021, 37, 100941. [Google Scholar] [CrossRef]
- Fazio, S.; Bellavite, P.; Zanolin, E.; McCullough, P.A.; Pandolfi, S.; Affuso, F. Retrospective Study of Outcomes and Hospitalization Rates of Patients in Italy with a Confirmed Diagnosis of Early COVID-19 and Treated at Home within 3 Days or after 3 Days of Symptom Onset with Prescribed and Non-Prescribed Treatments between November 2020 and August 2021. Med. Sci. Monit. 2021, 27, e935379. [Google Scholar] [CrossRef]
- Consolaro, E.; Suter, F.; Rubis, N.; Pedroni, S.; Moroni, C.; Pasto, E.; Paganini, M.V.; Pravettoni, G.; Cantarelli, U.; Perico, N.; et al. A Home-Treatment Algorithm Based on Anti-inflammatory Drugs to Prevent Hospitalization of Patients with Early COVID-19: A Matched-Cohort Study (COVER 2). Front. Med. 2022, 9, 785785. [Google Scholar] [CrossRef]
- Cosentino, M.; Vernocchi, V.; Martini, S.; Marino, F.; Allasino, B.; Bàlzola, M.A.; Burigana, F.; Dallari, A.; Pagano, C.S.F.; Palma, A.; et al. Early Outpatient Treatment of COVID-19: A Retrospective Analysis of 392 Cases in Italy. J. Clin. Med. 2022, 11, 6138. [Google Scholar] [CrossRef] [PubMed]
- Fazio, S.; Grimaldi, S.; D’Emilio, M.; Mangiagalli, A.; Affuso, F. COVID-19 early treatment with non-steroidal anti-inflammatory drugs reduces hospitalizations and symptom duration. Am. J. Biomed. Sci. Res. 2022, 16, 99–101. [Google Scholar] [CrossRef]
- Guzman-Esquivel, J.; Galvan-Salazar, H.R.; Guzman-Solorzano, H.P.; Cuevas-Velazquez, A.C.; Guzman-Solorzano, J.A.; Mokay-Ramirez, K.A.; Paz-Michel, B.A.; Murillo-Zamora, E.; Delgado-Enciso, J.; Melnikov, V.; et al. Efficacy of the use of mefenamic acid combined with standard medical care vs. standard medical care alone for the treatment of COVID-19: A randomized double-blind placebo-controlled trial. Int. J. Mol. Med. 2022, 49, 29. [Google Scholar] [CrossRef]
- Kelleni, M.T. Early use of non-steroidal anti-inflammatory drugs in COVID-19 might reverse pathogenesis, prevent complications and improve clinical outcomes. Biomed. Pharmacother. 2021, 133, 110982. [Google Scholar] [CrossRef]
- Perico, N.; Cortinovis, M.; Suter, F.; Remuzzi, G. Home as the new frontier for the treatment of COVID-19: The case for anti-inflammatory agents. Lancet Infect. Dis. 2023, 23, e22–e33. [Google Scholar] [CrossRef] [PubMed]
- Maisch, B.; Seferović, P.M.; Ristić, A.D.; Erbel, R.; Rienmüller, R.; Adler, Y.; Tomkowski, W.Z.; Thiene, G.; Yacoub, M.H.; Priori, S.G.; et al. Guidelines on the diagnosis and management of pericardial diseases executive summary; The Task force on the diagnosis and management of pericardial diseases of the European society of cardiology. Eur. Heart J. 2004, 25, 587–610. [Google Scholar] [CrossRef]
- Baerts, W.; van Bel, F.; Thewissen, L.; Derks, J.B.; Lemmers, P.M. Tocolytic indomethacin: Effects on neonatal haemodynamics and cerebral autoregulation in the preterm newborn. Arch. Dis. Child. Fetal Neonatal Ed. 2013, 98, F419–F423. [Google Scholar] [CrossRef]
- Aminpour, M.; Delgado, W.E.M.; Wacker, S.; Noskov, S.; Houghton, M.; Tyrrell, D.L.J.; Tuszynski, J.A. Computational determination of toxicity risks associated with a selection of approved drugs having demonstrated activity against COVID-19. BMC Pharmacol. Toxicol. 2021, 22, 61. [Google Scholar] [CrossRef]
- Desantis, J.; Mercorelli, B.; Celegato, M.; Croci, F.; Bazzacco, A.; Baroni, M.; Siragusa, L.; Cruciani, G.; Loregian, A.; Goracci, L. Indomethacin-based PROTACs as pan-coronavirus antiviral agents. Eur. J. Med. Chem. 2021, 226, 113814. [Google Scholar] [CrossRef]
- Gomeni, R.; Xu, T.; Gao, X.; Bressolle-Gomeni, F. Model based approach for estimating the dosage regimen of indomethacin a potential antiviral treatment of patients infected with SARS-CoV-2. J. Pharmacokinet. Pharmacodyn. 2020, 47, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Krymchantowski, A.V.; Silva-Néto, R.P.; Jevoux, C.; Krymchantowski, A.G. Indomethacin for refractory COVID or post-COVID headache: A retrospective study. Acta Neurol. Belg. 2021, 122, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, R.; Mohan, S.K.; Sukumaran, S.K.; Kamaraj, D.; Daivasuga, S.S.; Ravi, S.O.A.S.; Vijayaraghavalu, S.; Kumar, R.K. An open label randomized clinical trial of Indomethacin for mild and moderate hospitalised COVID-19 patients. Sci. Rep. 2022, 12, 6413. [Google Scholar] [CrossRef] [PubMed]
- Prasher, P.; Sharma, M.; Gunupuru, R. Targeting cyclooxygenase enzyme for the adjuvant COVID-19 therapy. Drug Dev. Res. 2021, 82, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.K.; Adnan, M.; Cho, D.H. Network pharmacology approach to decipher signaling pathways associated with target proteins of NSAIDs against COVID-19. Sci. Rep. 2021, 11, 9606. [Google Scholar] [CrossRef]
- Bellavite, P. Renin-Angiotensin System, SARS-CoV-2 and Hypotheses about Adverse Effects Following Vaccination. EC Pharmacol. Toxicol. 2021, 9, 1–10. [Google Scholar]
- Garvin, M.R.; Alvarez, C.; Miller, J.I.; Prates, E.T.; Walker, A.M.; Amos, B.K.; Mast, A.E.; Justice, A.; Aronow, B.; Jacobson, D. A mechanistic model and therapeutic interventions for COVID-19 involving a RAS-mediated bradykinin storm. eLife 2020, 9, e59177. [Google Scholar]
- Haybar, H.; Maniati, M.; Saki, N.; Zayeri, Z.D. COVID-19: Imbalance of multiple systems during infection and importance of therapeutic choice and dosing of cardiac and anti-coagulant therapies. Mol. Biol. Rep. 2021, 48, 2917–2928. [Google Scholar] [CrossRef]
- Karamyan, V.T. Between two storms, vasoactive peptides or bradykinin underlie severity of COVID-19? Physiol. Rep. 2021, 9, e14796. [Google Scholar] [CrossRef]
- McCarthy, C.G.; Wilczynski, S.; Wenceslau, C.F.; Webb, R.C. A new storm on the horizon in COVID-19: Bradykinin-induced vascular complications. Vasc. Pharmacol. 2020, 137, 106826. [Google Scholar] [CrossRef]
- Rodriguez-Portales, J.A.; Lopez-Moreno, J.M.; Mahana, D. Inhibition of the kallikrein-kinin system and vascular reactivity in Bartter’s syndrome. Hypertension 1985, 7, 1017–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkotaji, M.; Al-Zidan, R.N. Indomethacin: Can It Counteract Bradykinin Effects in COVID-19 Patients? Curr. Pharmacol. Rep. 2021, 7, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.E.; Enquist, L.W. Biological interactions between herpesviruses and cyclooxygenase enzymes. Rev. Med. Virol. 2006, 16, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Schroer, J.; Shenk, T. Inhibition of cyclooxygenase activity blocks cell-to-cell spread of human cytomegalovirus. Proc. Natl. Acad. Sci. USA 2008, 105, 19468–19473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahrami, H.; Daryani, N.E.; Haghpanah, B.; Moayyeri, A.; Moghadam, K.F.; Mirmomen, S.; Kamangar, F. Effects of indomethacin on viral replication markers in asymptomatic carriers of hepatitis B: A randomized, placebo-controlled trial. Am. J. Gastroenterol. 2005, 100, 856–861. [Google Scholar] [CrossRef] [PubMed]
- Amici, C.; Di, C.A.; Ciucci, A.; Chiappa, L.; Castilletti, C.; Martella, V.; Decaro, N.; Buonavoglia, C.; Capobianchi, M.R.; Santoro, M.G. Indomethacin has a potent antiviral activity against SARS coronavirus. Antivir. Ther. 2006, 11, 1021–1030. [Google Scholar] [CrossRef]
- Kiani, P.; Scholey, A.; Dahl, T.; McMann, L.; Iversen, J.; Verster, J. In Vitro Assessment of the Antiviral Activity of Ketotifen, Indomethacin and Naproxen, Alone and in Combination, against SARS-CoV-2. Viruses 2021, 13, 558. [Google Scholar] [CrossRef]
- Xu, T.; Gao, X.; Wu, Z.; Selinger, D.W.; Zhou, Z. Indomethacin has a potent antiviral activity against SARS-CoV-2 in vitro and canine coronavirus in vivo. BioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Napolitano, F.; Gennaro, G.; Carrella, D.; Gao, X.; di Bernardo, D. Computational Drug Repositioning and Elucidation of Mechanism of Action of Compounds against SARSCoV-2. arXiv 2020, arXiv:2004.07697. [Google Scholar]
- Abo Elmaaty, A.; Hamed, M.; Ismail, M.; Elkaeed, E.B.; Abulkhair, H.S.; Khattab, M.; Al-Karmalawy, A. Computational Insights on the Potential of Some NSAIDs for Treating COVID-19: Priority Set and Lead Optimization. Molecules 2021, 26, 3772. [Google Scholar] [CrossRef]
- Mortezaei, Z.; Mohammadian, A.; Tavallaei, M. Variations of SARS-CoV-2 in the Iranian population and candidate putative drug-like compounds to inhibit the mutated proteins. Heliyon 2022, 8, e09910. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Song, X.; Ma, T.; Pan, X.; Zhou, Y.; Hou, Y.; Zhang, Z.; Li, K.; Karypis, G.; Cheng, F. Repurpose Open Data to Discover Therapeutics for COVID-19 Using Deep Learning. J. Proteome Res. 2020, 19, 4624–4636. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, R.; Bhattacharje, G.; Barak, J.; Manna, B.; Mullick, J.; Mathapati, B.S.; Abraham, P.; Madhumathi, J.; Hasiga, Y.; Ghosh, A.; et al. In-silico screening and in-vitro assay show the antiviral effect of indomethacin against SARS-CoV-2. Comput. Biol. Med. 2022, 147, 105788. [Google Scholar] [CrossRef]
- Shekhar, N.; Kaur, H.; Sarma, P.; Prakash, A.; Medhi, B. Indomethacin: An exploratory study of antiviral mechanism and host-pathogen interaction in COVID-19. Expert Rev. Anti Infect. Ther. 2022, 20, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Fazio, S.; Affuso, F.; Bellavite, P. A Review of the Potential Roles of Antioxidant and Anti-Inflammatory Pharmacological Approaches for the Management of Mild-to-Moderate Symptomatic COVID-19. Med. Sci. Monit. 2022, 28, e936292. [Google Scholar] [CrossRef]
- Divani, A.A.; Andalib, S.; Di, N.M.; Lattanzi, S.; Hussain, M.S.; Biller, J.; McCullough, L.D.; Azarpazhooh, M.R.; Seletska, A.; Mayer, S.A.; et al. Coronavirus Disease 2019 and Stroke: Clinical Manifestations and Pathophysiological Insights. J. Stroke Cerebrovasc. Dis. 2020, 29, 104941. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y.; Wang, X.; Yang, L.; Li, H.; Wang, Y.; Liu, M.; Zhao, X.; Xie, Y.; Yang, Y.; et al. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J. Hematol. Oncol. 2020, 13, 120. [Google Scholar] [CrossRef]
- Liu, P.P.; Blet, A.; Smyth, D.; Li, H. The Science Underlying COVID-19: Implications for the Cardiovascular System. Circulation 2020, 142, 68–78. [Google Scholar] [CrossRef] [Green Version]
- McFadyen, J.D.; Stevens, H.; Peter, K. The Emerging Threat of (Micro)Thrombosis in COVID-19 and Its Therapeutic Implications. Circ. Res 2020, 127, 571–587. [Google Scholar] [CrossRef]
- Zhu, H.; Cong, J.P.; Yu, D.; Bresnahan, W.A.; Shenk, T.E. Inhibition of cyclooxygenase 2 blocks human cytomegalovirus replication. Proc. Natl. Acad. Sci. USA 2002, 99, 3932–3937. [Google Scholar] [CrossRef] [Green Version]
- Homolak, J.; Kodvanj, I. Widely available lysosome targeting agents should be considered as potential therapy for COVID-19. Int. J. Antimicrob. Agents 2020, 56, 106044. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Brindisi, M.; Shahabi, D.; Chapman, M.; Mesecar, A.D. Drug Development and Medicinal Chemistry Efforts toward SARS-Coronavirus and COVID-19 Therapeutics. ChemMedChem 2020, 15, 907–932. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Sk, M.F.; Sonawane, A.; Kar, P.; Sadhukhan, S. Plant-derived natural polyphenols as potential antiviral drugs against SARS-CoV-2 via RNA-dependent RNA polymerase (RdRp) inhibition: An in-silico analysis. J. Biomol. Struct. Dyn. 2020, 39, 1796810. [Google Scholar] [CrossRef]
- Russo, M.; Moccia, S.; Spagnuolo, C.; Tedesco, I.; Russo, G.L. Roles of flavonoids against coronavirus infection. Chem. Biol. Interact. 2020, 328, 109211. [Google Scholar] [CrossRef] [PubMed]
- Narkhede, R.R.; Pise, A.V.; Cheke, R.S.; Shinde, S.D. Recognition of Natural Products as Potential Inhibitors of COVID-19 Main Protease (Mpro): In-Silico Evidences. Nat. Prod. Bioprospect. 2020, 10, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.; Marasini, B.P.; Rayamajhee, B.; Bhattarai, B.R.; Lamichhane, G.; Khadayat, K.; Adhikari, A.; Khanal, S.; Parajuli, N. Potential roles of medicinal plants for the treatment of viral diseases focusing on COVID-19: A review. Phytother. Res. 2020, 35, 1298–1312. [Google Scholar] [CrossRef]
- Di Pierro, F.; Derosa, G.; Maffioli, P.; Bertuccioli, A.; Togni, S.; Riva, A.; Allegrini, P.; Khan, A.; Khan, S.; Khan, B.A.; et al. Possible Therapeutic Effects of Adjuvant Quercetin Supplementation against Early-Stage COVID-19 Infection: A Prospective, Randomized, Controlled, and Open-Label Study. Int. J. Gen. Med. 2021, 14, 2359–2366. [Google Scholar] [CrossRef]
- Ho, P.; Zheng, J.Q.; Wu, C.C.; Hou, Y.C.; Liu, W.C.; Lu, C.L.; Zheng, C.M.; Lu, K.C.; Chao, Y.C. Perspective Adjunctive Therapies for COVID-19: Beyond Antiviral Therapy. Int. J. Med. Sci. 2021, 18, 314–324. [Google Scholar] [CrossRef]
- Brahmi, F.; Vejux, A.; Ghzaiel, I.; Ksila, M.; Zarrouk, A.; Ghrairi, T.; Essadek, S.; Mandard, S.; Leoni, V.; Poli, G.; et al. Role of Diet and Nutrients in SARS-CoV-2 Infection: Incidence on Oxidative Stress, Inflammatory Status and Viral Production. Nutrients 2022, 14, 2194. [Google Scholar] [CrossRef]
- Montenegro-Landívar, M.F.; Tapia-Quirós, P.; Vecino, X.; Reig, M.; Valderrama, C.; Granados, M.; Cortina, J.L.; Saurina, J. Polyphenols and their potential role to fight viral diseases: An overview. Sci. Total. Environ. 2021, 801, 149719. [Google Scholar] [CrossRef]
- Pawar, A.; Russo, M.; Rani, I.; Goswami, K.; Russo, G.L.; Pal, A. A critical evaluation of risk to reward ratio of quercetin supplementation for COVID-19 and associated comorbid conditions. Phytother. Res. 2022, 36, 2394–2415. [Google Scholar] [CrossRef] [PubMed]
- Shohan, M.; Nashibi, R.; Mahmoudian-Sani, M.R.; Abolnezhadian, F.; Ghafourian, M.; Alavi, S.M.; Sharhani, A.; Khodadadi, A. The therapeutic efficacy of quercetin in combination with antiviral drugs in hospitalized COVID-19 patients: A randomized controlled trial. Eur. J. Pharmacol. 2022, 914, 174615. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Iqtadar, S.; Mumtaz, S.U.; Heinrich, M.; Pascual-Figal, D.A.; Livingstone, S.; Abaidullah, S. Oral Co-Supplementation of Curcumin, Quercetin, and Vitamin D3 as an Adjuvant Therapy for Mild to Moderate Symptoms of COVID-19-Results from a Pilot Open-Label, Randomized Controlled Trial. Front. Pharmacol. 2022, 13, 898062. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Perna, S.; Gasparri, C.; Petrangolini, G.; Allegrini, P.; Cavioni, A.; Faliva, M.A.; Mansueto, F.; Patelli, Z.; Peroni, G.; et al. Promising Effects of 3-Month Period of Quercetin Phytosome® Supplementation in the Prevention of Symptomatic COVID-19 Disease in Healthcare Workers: A Pilot Study. Life 2022, 12, 66. [Google Scholar] [CrossRef] [PubMed]
- Silvagno, F.; Vernone, A.; Pescarmona, G.P. The Role of Glutathione in Protecting against the Severe Inflammatory Response Triggered by COVID-19. Antioxidants 2020, 9, 624. [Google Scholar] [CrossRef] [PubMed]
- Suhail, S.; Zajac, J.; Fossum, C.; Lowater, H.; McCracken, C.; Severson, N.; Laatsch, B.; Narkiewicz-Jodko, A.; Johnson, B.; Liebau, J.; et al. Role of Oxidative Stress on SARS-CoV (SARS) and SARS-CoV-2 (COVID-19) Infection: A Review. Protein J. 2020, 39, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.S.; Czajkowsky, D.M. SARS-CoV-2 infection and oxidative stress: Pathophysiological insight into thrombosis and therapeutic opportunities. Cytokine Growth Factor Rev. 2022, 63, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Erlich, J.R.; To, E.E.; Liong, S.; Brooks, R.; Vlahos, R.; O’Leary, J.J.; Brooks, D.A.; Selemidis, S. Targeting Evolutionary Conserved Oxidative Stress and Immunometabolic Pathways for the Treatment of Respiratory Infectious Diseases. Antioxid. Redox Signal. 2020, 32, 993–1013. [Google Scholar] [CrossRef]
- Potus, F.; Mai, V.; Lebret, M.; Malenfant, S.; Breton-Gagnon, E.; Lajoie, A.C.; Boucherat, O.; Bonnet, S.; Provencher, S. Novel insights on the pulmonary vascular consequences of COVID-19. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 319, L277–L288. [Google Scholar] [CrossRef]
- Fratta Pasini, A.M.; Stranieri, C.; Cominacini, L.; Mozzini, C. Potential Role of Antioxidant and Anti-Inflammatory Therapies to Prevent Severe SARS-CoV-2 Complications. Antioxidants 2021, 10, 272. [Google Scholar] [CrossRef]
- Checconi, P.; De, A.M.; Marcocci, M.E.; Fraternale, A.; Magnani, M.; Palamara, A.T.; Nencioni, L. Redox-Modulating Agents in the Treatment of Viral Infections. Int. J. Mol. Sci. 2020, 21, 4084. [Google Scholar] [CrossRef] [PubMed]
- Bellavite, P.; Donzelli, A. Hesperidin and SARS-CoV-2: New Light on the Healthy Function of Citrus Fruits. Antioxidants 2020, 9, 742. [Google Scholar] [CrossRef] [PubMed]
- Iddir, M.; Brito, A.; Dingeo, G.; Fernandez Del Campo, S.S.; Samouda, H.; La Frano, M.R.; Bohn, T. Strengthening the Immune System and Reducing Inflammation and Oxidative Stress through Diet and Nutrition: Considerations during the COVID-19 Crisis. Nutrients 2020, 12, 1562. [Google Scholar] [CrossRef]
- Filardo, S.; Di, P.M.; Mastromarino, P.; Sessa, R. Therapeutic potential of resveratrol against emerging respiratory viral infections. Pharmacol. Ther. 2020, 214, 107613. [Google Scholar] [CrossRef]
- Marinella, M.A. Indomethacin and resveratrol as potential treatment adjuncts for SARS-CoV-2/COVID-19. Int. J. Clin. Pract. 2020, 74, e13535. [Google Scholar] [CrossRef]
- Mrityunjaya, M.; Pavithra, V.; Neelam, R.; Janhavi, P.; Halami, P.M.; Ravindra, P.V. Immune-Boosting, Antioxidant and Anti-inflammatory Food Supplements Targeting Pathogenesis of COVID-19. Front. Immunol. 2020, 11, 570122. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Liu, Y.; Yang, Y.; Zhang, P.; Zhong, W.; Wang, Y.; Wang, Q.; Xu, Y.; Li, M.; Li, X.; et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B 2020, 10, 766–788. [Google Scholar] [CrossRef]
- Chen, Y.W.; Yiu, C.-P.B.; Wong, K.Y. Prediction of the SARS-CoV-2 (2019-nCoV) 3C-like protease (3CLpro) structure: Virtual screening reveals velpatasvir, ledipasvir, and other drug repurposing candidates. F1000Research 2020, 9, 129. [Google Scholar] [CrossRef] [Green Version]
- Adem, S.; Eyupoglu, V.; Sarfraz, I.; Rasul, A.; Ali, M. Identification of Potent COVID-19 Main Protease (Mpro) Inhibitors from Natural Polyphenols: An In Silico Strategy Unveils a Hope against CORONA. Preprints 2020, 2020030333. [Google Scholar] [CrossRef] [Green Version]
- Utomo, R.Y.; Ikawati, M.; Meiyanto, E. Revealing the potency of citrus and galangal constituents to halt SARS-CoV-2 infection. Preprints 2020, 202003021. [Google Scholar] [CrossRef] [Green Version]
- Das, S.; Sarmah, S.; Lyndem, S.; Roy, A.S. An investigation into the identification of potential inhibitors of SARS-CoV-2 main protease using molecular docking study. J. Biomol. Struct. Dyn. 2020, 39, 3347–3357. [Google Scholar] [CrossRef]
- Joshi, R.S.; Jagdale, S.S.; Bansode, S.B.; Shankar, S.S.; Tellis, M.B.; Pandya, V.K.; Chugh, A.; Giri, A.P.; Kulkarni, M.J. Discovery of potential multi-target-directed ligands by targeting host-specific SARS-CoV-2 structurally conserved main protease. J. Biomol. Struct. Dyn. 2020, 39, 3099–3114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basu, A.; Sarkar, A.; Maulik, U. Molecular docking study of potential phytochemicals and their effects on the complex of SARS-CoV2 spike protein and human ACE2. Sci. Rep. 2020, 10, 17699. [Google Scholar] [CrossRef] [PubMed]
- Bellavite, P. Reappraisal of Dietary Phytochemicals for Coronavirus Infection: Focus on Hesperidin and Quercetin. In Antioxidants: Benefits, Sources, Mechanisms of Action; Waisundara, V.Y., Ed.; IntechOpen: London, UK, 2021; pp. 473–487. [Google Scholar] [CrossRef]
- Zannella, C.; Giugliano, R.; Chianese, A.; Buonocore, C.; Vitale, G.; Sanna, G.; Sarno, F.; Manzin, A.; Nebbioso, A.; Termolino, P.; et al. Antiviral Activity of Vitis vinifera Leaf Extract against SARS-CoV-2 and HSV-1. Viruses 2021, 13, 1263. [Google Scholar] [CrossRef] [PubMed]
- Messina, G.; Polito, R.; Monda, V.; Cipolloni, L.; Di, N.N.; Di, M.G.; Murabito, P.; Carotenuto, M.; Messina, A.; Pisanelli, D.; et al. Functional Role of Dietary Intervention to Improve the Outcome of COVID-19: A Hypothesis of Work. Int. J. Mol. Sci. 2020, 21, 3104. [Google Scholar] [CrossRef]
- Meneguzzo, F.; Ciriminna, R.; Zabini, F.; Pagliaro, M. Review of Evidence Available on Hesperidin-Rich Products as Potential Tools against COVID-19 and Hydrodynamic Cavitation-Based Extraction as a Method of Increasing Their Production. Processes 2020, 8, 549. [Google Scholar] [CrossRef]
- Bhowmik, D.; Nandi, R.; Prakash, A.; Kumar, D. Evaluation of flavonoids as 2019-nCoV cell entry inhibitor through molecular docking and pharmacological analysis. Heliyon 2021, 7, e06515. [Google Scholar] [CrossRef]
- Junior, A.G.; Tolouei, S.E.L.; Dos Reis Lívero, F.A.; Gasparotto, F.; Boeing, T.; de Souza, P. Natural agents modulating ACE-2: A review of compounds with potential against SARS-CoV-2 infections. Curr. Pharm. Des. 2021, 27, 1588–1596. [Google Scholar] [CrossRef]
- Alesci, A.; Aragona, M.; Cicero, N.; Lauriano, E.R. Can nutraceuticals assist treatment and improve COVID-19 symptoms? Nat. Prod. Res. 2021, 36, 2672–2691. [Google Scholar] [CrossRef]
- Harwansh, R.K.; Bahadur, S. Herbal Medicine in Fighting Against COVID-19: New Battle with an Old Weapon. Curr. Pharm. Biotechnol. 2021, 23, 235–260. [Google Scholar] [CrossRef]
- Gour, A.; Manhas, D.; Bag, S.; Gorain, B.; Nandi, U. Flavonoids as potential phytotherapeutics to combat cytokine storm in SARS-CoV-2. Phytother. Res. 2021, 35, 4258–4283. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Rane, J.S.; Chatterjee, A.; Kumar, A.; Khan, R.; Prakash, A.; Ray, S. Targeting SARS-CoV-2 spike protein of COVID-19 with naturally occurring phytochemicals: An in silico study for drug development. J. Biomol. Struct. Dyn. 2020, 39, 1796811. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, B.G.; Ramesh, D.; Joji, A.; Jayachandra, P.J.; Kannan, T. In silico pharmacokinetic and molecular docking studies of natural flavonoids and synthetic indole chalcones against essential proteins of SARS-CoV-2. Eur. J Pharmacol. 2020, 886, 173448. [Google Scholar] [CrossRef]
- Tallei, T.E.; Tumilaar, S.G.; Niode, N.J.; Kepel, B.; Idroes, R.; Effendi, Y.; Sakib, S.A.; Emran, T.B. Potential of Plant Bioactive Compounds as SARS-CoV-2 Main Protease (Mpro) and Spike (S) Glycoprotein Inhibitors: A Molecular Docking Study. Scientifica 2020, 2020, 6307457. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.H.; Woo, H.J.; Kang, H.K.; Nguyen, V.D.; Kim, Y.M.; Kim, D.W.; Ahn, S.A.; Xia, Y.; Kim, D. Flavonoid-mediated inhibition of SARS coronavirus 3C-like protease expressed in Pichia pastoris. Biotechnol. Lett. 2012, 34, 831–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.Y.; Yuk, H.J.; Ryu, H.W.; Lim, S.H.; Kim, K.S.; Park, K.H.; Ryu, Y.B.; Lee, W.S. Evaluation of polyphenols from Broussonetia papyrifera as coronavirus protease inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 504–515. [Google Scholar] [CrossRef] [Green Version]
- Khaerunnisa, S.; Kurniawan, H.; Awaluddin, R.; Suhartati, S.; Soetjipto, S. Potential Inhibitor of COVID-19 Main Protease (Mpro) From Several Medicinal Plant Compounds by Molecular Docking Study. Preprints 2020, 2020030226. [Google Scholar] [CrossRef] [Green Version]
- Abian, O.; Ortega-Alarcon, D.; Jimenez-Alesanco, A.; Ceballos-Laita, L.; Vega, S.; Reyburn, H.T.; Rizzuti, B.; Velazquez-Campoy, A. Structural stability of SARS-CoV-2 3CLpro and identification of quercetin as an inhibitor by experimental screening. Int. J. Biol. Macromol. 2020, 164, 1693–1703. [Google Scholar] [CrossRef]
- Gogoi, N.; Chowdhury, P.; Goswami, A.K.; Das, A.; Chetia, D.; Gogoi, B. Computational guided identification of a citrus flavonoid as potential inhibitor of SARS-CoV-2 main protease. Mol. Divers. 2020, 25, 1745–1759. [Google Scholar] [CrossRef]
- Shaik, Y.B.; Castellani, M.L.; Perrella, A.; Conti, F.; Salini, V.; Tete, S.; Madhappan, B.; Vecchiet, J.; De Lutiis, M.A.; Caraffa, A.; et al. Role of quercetin (a natural herbal compound) in allergy and inflammation. J. Biol. Regul. Homeost. Agents 2006, 20, 47–52. [Google Scholar]
- Saeedi-Boroujeni, A.; Mahmoudian-Sani, M.R. Anti-inflammatory potential of Quercetin in COVID-19 treatment. J. Inflamm. 2021, 18, 3. [Google Scholar] [CrossRef] [PubMed]
- Kimata, M.; Shichijo, M.; Miura, T.; Serizawa, I.; Inagaki, N.; Nagai, H. Effects of luteolin, quercetin and baicalein on immunoglobulin E-mediated mediator release from human cultured mast cells. Clin. Exp. Allergy 2000, 30, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.D.; Choi, C.H.; Bark, H.; Son, H.Y.; Park, H.H.; Lee, S.; Park, J.W.; Park, E.K.; Shin, H.I.; Kim, S.H. Quercetin inhibits expression of inflammatory cytokines through attenuation of NF-kappaB and p38 MAPK in HMC-1 human mast cell line. Inflamm. Res. 2007, 56, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Kalogeromitros, D.; Makris, M.; Chliva, C.; Aggelides, X.; Kempuraj, D.; Theoharides, T.C. A quercetin containing supplement reduces niacin-induced flush in humans. Int. J. Immunopathol. Pharmacol. 2008, 21, 509–514. [Google Scholar] [CrossRef]
- Park, S.J.; Chung, H.Y.; Lee, J.H. Rapid in vivo screening system for anti-oxidant activity using bacterial redox sensor strains. J. Appl. Microbiol. 2009, 108, 1217–1225. [Google Scholar] [CrossRef]
- Lee, E.J.; Ji, G.E.; Sung, M.K. Quercetin and kaempferol suppress immunoglobulin E-mediated allergic inflammation in RBL-2H3 and Caco-2 cells. Inflamm. Res. 2010, 59, 847–854. [Google Scholar] [CrossRef]
- Chirumbolo, S.; Conforti, A.; Ortolani, R.; Vella, A.; Marzotto, M.; Bellavite, P. Stimulus-specific regulation of CD63 and CD203c membrane expression in human basophils by the flavonoid quercetin. Int. Immunopharmacol. 2010, 10, 183–192. [Google Scholar] [CrossRef]
- Chirumbolo, S.; Marzotto, M.; Conforti, A.; Vella, A.; Ortolani, R.; Bellavite, P. Bimodal action of the flavonoid quercetin on basophil function: An investigation of the putative biochemical targets. Clin. Mol. Allergy 2010, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Theoharides, T.C. COVID-19, pulmonary mast cells, cytokine storms, and beneficial actions of luteolin. Biofactors 2020, 46, 306–308. [Google Scholar] [CrossRef]
- Wu, M.L.; Liu, F.L.; Sun, J.; Li, X.; He, X.Y.; Zheng, H.Y.; Zhou, Y.H.; Yan, Q.; Chen, L.; Yu, G.Y.; et al. SARS-CoV-2-triggered mast cell rapid degranulation induces alveolar epithelial inflammation and lung injury. Signal Transduct. Target. Ther. 2021, 6, 428. [Google Scholar] [CrossRef]
- Conti, P.; Caraffa, A.; Tete, G.; Gallenga, C.E.; Ross, R.; Kritas, S.K.; Frydas, I.; Younes, A.; Di Emidio, P.; Ronconi, G. Mast cells activated by SARS-CoV-2 release histamine which increases IL-1 levels causing cytokine storm and inflammatory reaction in COVID-19. J. Biol. Regul. Homeost. Agents 2020, 34, 1629–1632. [Google Scholar] [CrossRef] [PubMed]
- Murota, K.; Nakamura, Y.; Uehara, M. Flavonoid metabolism: The interaction of metabolites and gut microbiota. Biosci. Biotechnol. Biochem. 2018, 82, 600–610. [Google Scholar] [CrossRef] [Green Version]
- Xiang, Z.; Koo, H.; Chen, Q.; Zhou, X.; Liu, Y.; Simon-Soro, A. Potential implications of SARS-CoV-2 oral infection in the host microbiota. J. Oral Microbiol. 2020, 13, 1853451. [Google Scholar] [CrossRef] [PubMed]
- Lamers, M.M.; Beumer, J.; van der Vaart, J.; Knoops, K.; Puschhof, J.; Breugem, T.I.; Ravelli, R.B.G.; van Schayck, J.P.; Mykytyn, A.Z.; Duimel, H.Q.; et al. SARS-CoV-2 productively infects human gut enterocytes. Science 2020, 369, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Burgueño, J.F.; Reich, A.; Hazime, H.; Quintero, M.A.; Fernandez, I.; Fritsch, J.; Santander, A.M.; Brito, N.; Damas, O.M.; Deshpande, A.; et al. Expression of SARS-CoV-2 Entry Molecules ACE2 and TMPRSS2 in the Gut of Patients With IBD. Inflamm. Bowel Dis. 2020, 26, 797–808. [Google Scholar] [CrossRef] [Green Version]
- Zuo, T.; Liu, Q.; Zhang, F.; Lui, G.C.; Tso, E.Y.; Yeoh, Y.K.; Chen, Z.; Boon, S.S.; Chan, F.K.; Chan, P.K.; et al. Depicting SARS-CoV-2 faecal viral activity in association with gut microbiota composition in patients with COVID-19. Gut 2021, 70, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Yeoh, Y.K.; Zuo, T.; Lui, G.C.; Zhang, F.; Liu, Q.; Li, A.Y.; Chung, A.C.; Cheung, C.P.; Tso, E.Y.; Fung, K.S.; et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut 2021, 70, 698–706. [Google Scholar] [CrossRef]
- Augusti, P.R.; Conterato, G.M.M.; Denardin, C.C.; Prazeres, I.D.; Serra, A.T.; Bronze, M.R.; Emanuelli, T. Bioactivity, bioavailability, and gut microbiota transformations of dietary phenolic compounds: Implications for COVID-19. J. Nutr. Biochem. 2021, 97, 108787. [Google Scholar] [CrossRef]
- Licciardello, F.; Arena, E.; Rizzo, V.; Fallico, B. Contribution of Blood Orange-Based Beverages to Bioactive Compounds Intake. Front. Chem. 2018, 6, 374. [Google Scholar] [CrossRef]
- Grosso, G.; Galvano, F.; Mistretta, A.; Marventano, S.; Nolfo, F.; Calabrese, G.; Buscemi, S.; Drago, F.; Veronesi, U.; Scuderi, A. Red orange: Experimental models and epidemiological evidence of its benefits on human health. Oxid. Med. Cell. Longev. 2013, 2013, 157240. [Google Scholar] [CrossRef] [Green Version]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.; Kim, H.; Bae, S.; Choi, J.; Lim, S.Y.; Lee, N.; Kong, J.M.; Hwang, Y.I.; Kang, J.S.; Lee, W.J. Vitamin C Is an Essential Factor on the Anti-viral Immune Responses through the Production of Interferon-alpha/beta at the Initial Stage of Influenza A Virus (H3N2) Infection. Immune Netw. 2013, 13, 70–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.; Jang, M.; Kim, Y.; Choi, J.; Jeon, J.; Kim, J.; Hwang, Y.I.; Kang, J.S.; Lee, W.J. Red ginseng and vitamin C increase immune cell activity and decrease lung inflammation induced by influenza A virus/H1N1 infection. J. Pharm. Pharmacol. 2016, 68, 406–420. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Ward, S.A.; Kalantar-Zadeh, K.; El-Omar, E. Considering the Effects of Microbiome and Diet on SARS-CoV-2 Infection: Nanotechnology Roles. ACS Nano 2020, 14, 5179–5182. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Moore, L.W. Impact of Nutrition and Diet on COVID-19 Infection and Implications for Kidney Health and Kidney Disease Management. J. Ren. Nutr. 2020, 30, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Lehene, M.; Fischer-Fodor, E.; Scurtu, F.; Hădade, N.D.; Gal, E.; Mot, A.C.; Matei, A.; Silaghi-Dumitrescu, R. Excess Ascorbate is a Chemical Stress Agent against Proteins and Cells. Pharmaceuticals 2020, 13, 107. [Google Scholar] [CrossRef]
- Colunga Biancatelli, R.M.L.; Berrill, M.; Catravas, J.D.; Marik, P.E. Quercetin and Vitamin C: An Experimental, Synergistic Therapy for the Prevention and Treatment of SARS-CoV-2 Related Disease (COVID-19). Front. Immunol. 2020, 11, 1451. [Google Scholar] [CrossRef]
- Li, G.; Wei, W.; Suo, L.; Zhang, C.; Yu, H.; Liu, H.; Guo, Q.; Zhen, X.; Yu, Y. Low-Dose Aspirin Prevents Kidney Damage in LPS-Induced Preeclampsia by Inhibiting the WNT5A and NF-kappaB Signaling Pathways. Front. Endocrinol. 2021, 12, 639592. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, N.; Li, A.; Zhou, Y.; Liang, L.; Song, X.; Yang, Z.; Zhou, X. Effect of low-dose aspirin on mortality and viral duration of the hospitalized adults with COVID-19. Medicine 2021, 100, e24544. [Google Scholar] [CrossRef]
- Ma, S.; Su, W.; Sun, C.; Lowe, S.; Zhou, Z.; Liu, H.; Qu, G.; Xia, W.; Xie, P.; Wu, B.; et al. Does aspirin have an effect on risk of death in patients with COVID-19? A meta-analysis. Eur. J. Clin. Pharmacol. 2022, 78, 1403–1420. [Google Scholar] [CrossRef]
- Santoro, F.; Núñez-Gil, I.J.; Vitale, E.; Viana-Llamas, M.C.; Romero, R.; Maroun Eid, C.; Feltes Guzman, G.; Becerra-Muñoz, V.M.; Fernandez Rozas, I.; Uribarri, A.; et al. Aspirin Therapy on Prophylactic Anticoagulation for Patients Hospitalized With COVID-19: A Propensity Score-Matched Cohort Analysis of the HOPE-COVID-19 Registry. J. Am. Heart Assoc. 2022, 11, e024530. [Google Scholar] [CrossRef] [PubMed]
- Massimo Claar, G.; Monaco, S.; Del Veccho Blanco, C.; Capurso, L.; Fusillo, M.; Annibale, B. Omeprazole 20 or 40 mg daily for healing gastroduodenal ulcers in patients receiving non-steroidal anti-inflammatory drugs. Aliment. Pharmacol. Ther. 1998, 12, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Bianchi Porro, G.; Lazzaroni, M.; Petrillo, M.; Manzionna, G.; Montrone, F.; Caruso, I. Prevention of gastroduodenal damage with omeprazole in patients receiving continuous NSAIDs treatment. A double blind placebo controlled study. Ital. J. Gastroenterol. Hepatol. 1998, 30, 43–47. [Google Scholar]
- Gao, J.; Zhang, L.; Liu, X.; Li, F.; Ma, R.; Zhu, Z.; Zhang, J.; Wu, J.; Shi, Y.; Pan, Y.; et al. Repurposing Low-Molecular-Weight Drugs against the Main Protease of Severe Acute Respiratory Syndrome Coronavirus 2. J. Phys. Chem. Lett. 2020, 11, 7267–7272. [Google Scholar] [CrossRef] [PubMed]
- Tanimoto, K.; Hirota, K.; Fukazawa, T.; Matsuo, Y.; Nomura, T.; Tanuza, N.; Hirohashi, N.; Bono, H.; Sakaguchi, T. Inhibiting SARS-CoV-2 infection in vitro by suppressing its receptor, angiotensin-converting enzyme 2, via aryl-hydrocarbon receptor signal. Sci. Rep. 2021, 11, 16629. [Google Scholar] [CrossRef] [PubMed]
- El-Aarag, S.A.; Mahmoud, A.; ElHefnawi, M. Identifying potential novel insights for COVID-19 pathogenesis and therapeutics using an integrated bioinformatics analysis of host transcriptome. Int. J. Biol. Macromol. 2022, 194, 770–780. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.S.; Lee, S.E.; Hong, S.T.; Kim, H.S.; Choi, B.C.; Sim, S.S.; Whang, W.K.; Sohn, U.D. The Inhibitory Effect of Quercetin-3-O-beta-D-Glucuronopyranoside on Gastritis and Reflux Esophagitis in Rats. Korean J. Physiol. Pharmacol. 2009, 13, 295–300. [Google Scholar] [CrossRef] [Green Version]
- Fuentes, J.; de Camargo, A.C.; Atala, E.; Gotteland, M.; Olea-Azar, C.; Speisky, H. Quercetin Oxidation Metabolite Present in Onion Peel Protects Caco-2 Cells against the Oxidative Stress, NF-kB Activation, and Loss of Epithelial Barrier Function Induced by NSAIDs. J. Agric. Food Chem. 2021, 69, 2157–2167. [Google Scholar] [CrossRef]
- Fan, J.; Li, B.R.; Zhang, Q.; Zhao, X.H.; Wang, L. Pretreatment of IEC-6 cells with quercetin and myricetin resists the indomethacin-induced barrier dysfunction via attenuating the calcium-mediated JNK/Src activation. Food Chem. Toxicol. 2021, 147, 111896. [Google Scholar] [CrossRef]
- Rezza, G.; Urbani, A. Circolare Recante “Gestione Domiciliare Dei Pazienti Con Infezione Da SARS-CoV-2 Aggiornata al 26 Aprile 2021”; Ditrezioni Generali Programmazione Sanitaria e Prevenzione Sanitaria; Ministero della Salute: Roma, Italy, 2021. [Google Scholar]
- Frieden, T.R. Evidence for Health Decision Making—Beyond Randomized, Controlled Trials. N. Engl. J. Med. 2017, 377, 465–475. [Google Scholar] [CrossRef]
- Andreoni, M.; Bartoletti, P.L. CORONAVIRUS: Dalla FIMMG Roma una Flow Chart per il Trattamento Farmacologico Dell’infezione da SARS-CoV-2 in (FIMMG Sezione Provinciale di Roma, iccPTV, ed.); FIMMG, Sezione di Roma: Roma, Italy, 2023. [Google Scholar]
| Drug | Chemical Formula | Structure | Molecular Mass (g/mol) | Properties | Dose |
|---|---|---|---|---|---|
| Indomethacin | C19H16ClNO4 |  | 357.8 | Antiviral Anti-inflammatory | 75 mg/day 1 |
| Omeprazole | C17H19N3O3S |  | 345.4 | Gastric protection | 20 mg/day |
| Aspirin | C9H8O4 |  | 180.2 | Anti-thrombotic | 100 mg/day |
| Hesperidin | C28H34O15 |  | 610.6 | Antioxidant Antiviral Anti-inflammatory | 200 mg/day 2 |
| Quercetin | C15H10O7 |  | 302.2 | Antioxidant Antiviral Anti-inflammatory | 200 mg/day 2 |
| Vitamin C | C6H8O6 |  | 176.1 | Antioxidant | 100 mg/day 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fazio, S.; Bellavite, P. Early Multi-Target Treatment of Mild-to-Moderate COVID-19, Particularly in Terms of Non-Steroidal Anti-Inflammatory Drugs and Indomethacin. BioMed 2023, 3, 177-194. https://doi.org/10.3390/biomed3010015
Fazio S, Bellavite P. Early Multi-Target Treatment of Mild-to-Moderate COVID-19, Particularly in Terms of Non-Steroidal Anti-Inflammatory Drugs and Indomethacin. BioMed. 2023; 3(1):177-194. https://doi.org/10.3390/biomed3010015
Chicago/Turabian StyleFazio, Serafino, and Paolo Bellavite. 2023. "Early Multi-Target Treatment of Mild-to-Moderate COVID-19, Particularly in Terms of Non-Steroidal Anti-Inflammatory Drugs and Indomethacin" BioMed 3, no. 1: 177-194. https://doi.org/10.3390/biomed3010015
APA StyleFazio, S., & Bellavite, P. (2023). Early Multi-Target Treatment of Mild-to-Moderate COVID-19, Particularly in Terms of Non-Steroidal Anti-Inflammatory Drugs and Indomethacin. BioMed, 3(1), 177-194. https://doi.org/10.3390/biomed3010015