Clinical Characteristics, Outcomes, and Risk Factors of Patients Hospitalized for COVID-19 across the Latest Pandemic Waves: Has Something Changed?
Abstract
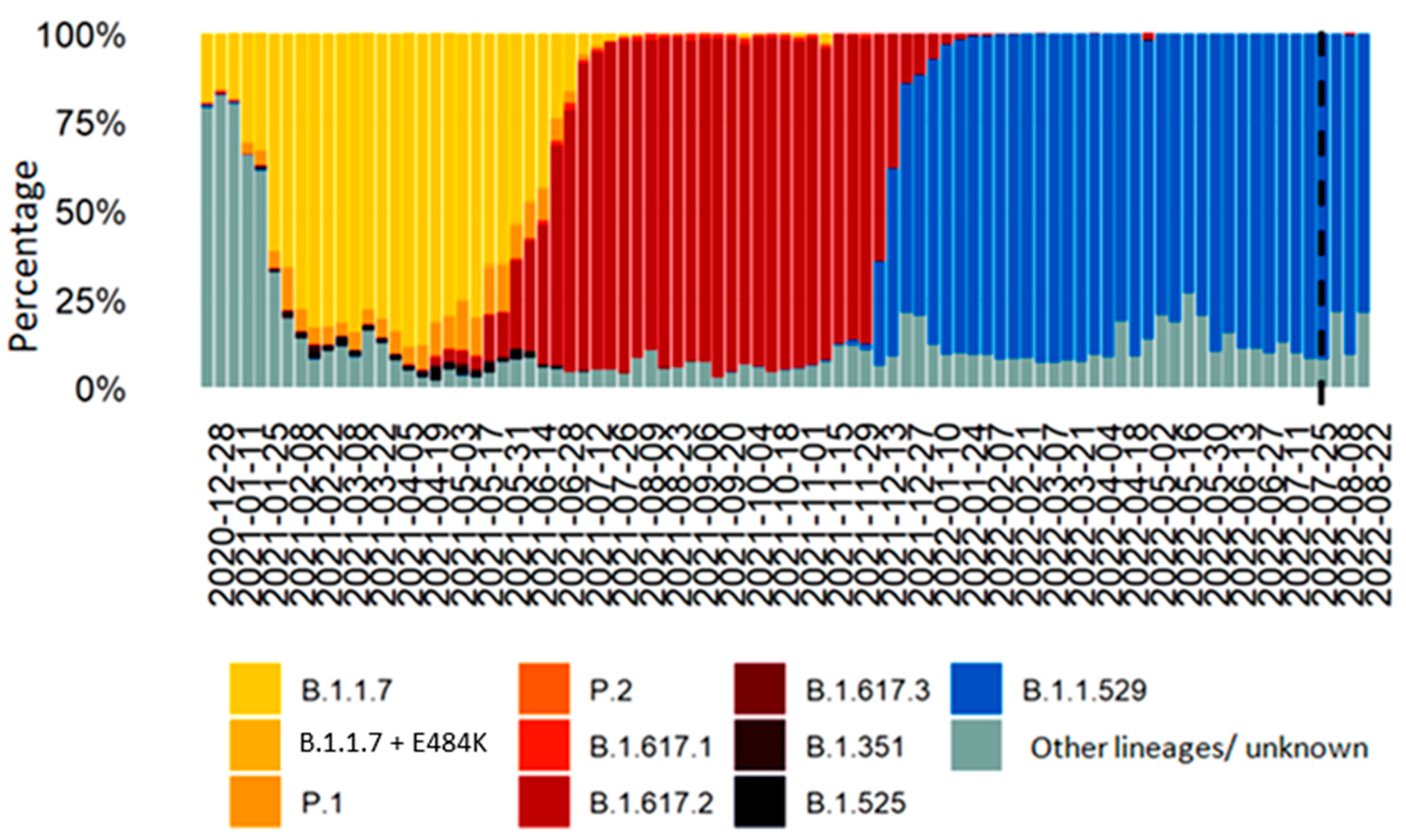
:1. Introduction
2. Materials and Methods
2.1. Study Design and Data Collection
- −
- Group A: included patients hospitalized from 1 August to 9 December 2021;
- −
- Group B: included patients hospitalized from 3 January to 30 June 2022.
2.2. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, B.; Guo, H. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; McGoogan, J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Aleem, A.; Akbar Samad, A.B. Emerging Variants of SARS-CoV-2 And Novel Therapeutics Against Coronavirus (COVID-19). In StatPearls; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Akkız, H. The Biological Functions and Clinical Significance of SARS-CoV-2 Variants of Concern. Front. Med. 2022, 9, 849217. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, T.; Ferguson, N.M. Comparative analysis of the risks of hospitalization and death associated with SARS-CoV-2 Omicron (B.1.1.529) and Delta (B.1.617.2) variants in England: A cohort study. Lancet 2022, 399, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- WHO Coronavirus (COVID-19) Dashboard|WHO Coronavirus (COVID-19) Dashboard with Vaccination Data. Available online: https://covid19.who.int/data/ (accessed on 1 May 2023).
- Iuliano, A.D.; Brunkard, J.M. Trends in Disease Severity and Health Care Utilization during the Early Omicron Variant Period Compared with Previous SARS-CoV-2 High Transmission Periods—United States, December 2020–January 2022. MMWR 2022, 71, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Aggiornamento Casi COVID-19—Dati AggregatiQuotidianiRegioni/PPAA—Ministero Della Salute—IstitutoSuperiore di Sanità. Available online: https://www.salute.gov.it/portale/nuovocoronavirus/dettaglioContenutiNuovoCoronavirus.jsp?id=5351&area=nuovoCoronavirus&menu=vuoto (accessed on 1 May 2023).
- De Vito, A.; Colpani, A. Is the 4C Score Still a Valid Item to Predict In-Hospital Mortality in People with SARS-CoV-2 Infections in the Omicron Variant Era? Life 2023, 13, 183. [Google Scholar] [CrossRef] [PubMed]
- Rovida, F.; Esposito, G.L. Characteristics and outcomes of vaccinated and nonvaccinated patients hospitalized in a single Italian hub for COVID-19 during the Delta and Omicron waves in Northern Italy. IJID 2022, 122, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classification of prognostic co-morbidity for longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- NIH COVID-19 Treatment Guidelines. Clinical Spectrum of SARS-CoV-2 Infection. (Last Updated April 2w0, 2023). Available online: https://www.covid19treatmentguidelines.nih.gov/ (accessed on 1 May 2023).
- Hammond, J.; Leister-Tebbe, H. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with COVID-19. NEJM 2022, 386, 1397–1408. [Google Scholar] [CrossRef] [PubMed]
- Jayk Bernal, A.; Gomes da Silva, M.M. Molnupiravir for Oral Treatment of COVID-19 in Nonhospitalized Patients. NEJM 2022, 386, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, D. Remdesivir in adults with severe COVID-19: A randomized, double-blind, placebo-controlled, multicentre trial. Lancet 2020, 395, 1569–1578. [Google Scholar] [CrossRef] [PubMed]
- Prevalenza e Distribuzione Delle Varianti del Virus SARS-CoV-2 di Interesse per la SanitàPubblica in Italia. Available online: https://www.iss.it/documents/20126/0/Bollettino+varianti+n.15.pdf/be68a65c-b52c-2179-ac39-14bbc3292be2?t=1639148646776 (accessed on 1 May 2023).
- Altman, D.G.; Machin, D. Statistics with Confidence: Confidence Intervals and Statistical Guidelines, 2nd ed.; BMJ BooksTwo Hudson Place: Hoboken, NJ, USA, 2000. [Google Scholar]
- Cox, D.R. Statistical significance tests. Br. J. Clin. Pharm. 1982, 14, 325. [Google Scholar] [CrossRef]
- The Jamovi Project. jamovi, (Version 2.3) [Computer Software]; 2022. Available online: https://www.jamovi.org (accessed on 1 May 2023).
- Ministero Delle Saulte. Available online: https://www.salute.gov.it/portale/nuovocoronavirus/dettagliocomunicatinuovocoronavirus.jsp?Lingua=italiano&menu=salastampa&p=comunicatistampa&id=5835 (accessed on 1 May 2023).
- Country Overview Report: Week 6, 2023. Available online: https://covid19-country-overviews.ecdc.europa.eu/notes.html (accessed on 1 May 2023).
- Lab24: Vaccini in Tempo Reale. Available online: https://lab24.ilsole24ore.com/numeri-vaccini-italia-mondo (accessed on 1 May 2023).
- Post, L.A.; Lorenzo-Redondo, R. Omicron: Fewer adverse outcomes come with new dangers. Lancet 2022, 399, 1280–1281. [Google Scholar] [CrossRef] [PubMed]
- Chenchula, S.; Karunakaran, P. Current evidence on the efficacy of COVID-19 booster dose vaccination against the Omicron variant: A systematic review. J. Med. Virol. 2022, 94, 2969–2976. [Google Scholar] [CrossRef] [PubMed]
- Araf, Y.; Akter, F. Omicron variant of SARS-CoV-2: Genomics, transmissibility, and responses to current COVID-19 vaccines. J. Med. Virol. 2020, 94, 1825–1832. [Google Scholar] [CrossRef] [PubMed]
- Wirth, R.; Becker, C. COVID-19 im Alter—Die geriatric Perspektive [COVID-19 in old age-The geriatric perspective]. Z. Gerontol. Geriatr. 2021, 54, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.D.; Ding, M. Risk factors for severe and critically ill COVID-19 patients: A review. Allergy 2021, 76, 428–455. [Google Scholar] [CrossRef] [PubMed]
- Dhama, K.; Patel, S.K. Geriatric Population during the COVID-19 Pandemic: Problems, Considerations, Exigencies, and Beyond. Front. Public Health 2020, 8, 574198. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, O.P.; Singh, I. Making sound public health policy decisions for COVID-19 vaccination: Vaccine effectiveness, safety, affordability, programmatic logistics and roll-out globally. J. Travel Med. 2021, 28, taab031. [Google Scholar] [CrossRef]
- Tomas-Grau, R.H.; Maldonado-Galdeano, C. Humoral immune response elicited against an adenoviral-based SARS-CoV-2 coronavirus vaccine in elderly patients. Aging 2022, 14, 7193–7205. [Google Scholar] [CrossRef] [PubMed]

| Variables | Overall (N = 310) | Group A § (N = 100) | Group B (N = 210) | p-Value |
|---|---|---|---|---|
| Median (IQR) age, years | 75 (61–83) | 67 (56–80) | 78 (66–84) | <0.001 |
| Male gender, n (%) | 199 (64) | 59 (59) | 140 (67) | 0.19 |
| Co-existing conditions, n (%) | ||||
| Hypertension | 119 (38) | 7 (7) | 112 (53) | <0.001 |
| Heart rhythm abnormalities | 55 (17) | 8 (8) | 47 (22) | 0.002 |
| Ischemic heart disease | 38 (13) | 8 (8) | 30 (15) | 0.08 |
| Chronic heart failure | 28 (9) | 4 (4) | 24 (11) | 0.03 |
| Type II Diabetes | 43 (14) | 6 (6) | 37 (18) | 0.005 |
| Chronic Kidney disease | 35 (11) | 3 (3) | 33 (16) | <0.001 |
| Chronic Liver disease | 8 (3) | 1 (1) | 7 (3) | 0.23 |
| Peripheral vasculopathy | 19 (6) | 2 (2) | 17 (8) | 0.03 |
| Ischemic stroke | 18 (6) | 3 (3) | 15 (7) | 0.12 |
| Dementia | 41 (13) | 4 (4) | 37 (18) | <0.001 |
| Chronic Obstructive Pulmonary Disease | 53 (17) | 8 (8) | 45 (21) | 0.003 |
| Autoimmune Diseases | 17 (5) | 3 (3) | 14 (7) | 0.18 |
| Peptic Ulcer | 16 (5) | 2 (2) | 14 (7) | 0.08 |
| Solid Tumors | 42 (13) | 7 (7) | 35 (17) | 0.02 |
| Hematologic malignancies | 15 (5) | 2 (2) | 13 (6) | 0.09 |
| Bone fractures | 16 (5) | 3 (3) | 13 (6) | 0.23 |
| BMI >= 30 | 27 (9) | 12 (12) | 15 (7) | 0.15 |
| Charlson Co-morbidity Index, median (IQR) | 4 (2–6) | 3 (2–4) | 5 (3–6) | <0.001 |
| Vaccine status | <0.001 | |||
| 0–1 dose | 82 (26) | 44 (44) | 37 (18) | |
| 2 doses | 98 (32) | 56 (56) | 42 (20) | |
| 2 doses + booster dose | 131 (42) | 0 (0) | 131 (62) | |
| Oxygen * required >=24 after admission, n (%) | 206 (66) | 69 (69) | 137 (65) | 0.51 |
| NIV required >=24 h after admission, n (%) | 93 (30) | 33 (33) | 60 (29) | 0.43 |
| Median (IQR) time from symptom onset to hospitalization, days | 4 (2–8) | 5 (3–9) | 4 (1–7) | 0.56 |
| Median (IQR) duration of hospital stay, days | 14 (8–20) | 14 (9–21) | 14 (8–19) | 0.05 |
| Outcome, n (%) | 0.23 | |||
| Discharged with clinical stability | 220 (71) | 77 (77) | 143 (69) | |
| Admitted in ICU | 31 (10) | 9 (9) | 22 (10) | |
| Dead | 59(19) | 14 (14) | 45 (21) |
| Variables | H.R. (95% C.I., p-Value) | aHR (95% C.I., p-Value) |
|---|---|---|
| Male sex | 1.58 (1.00–2.49, p = 0.05) | 1.27 (0.79–2.03, p = 0.32) |
| Age >= 65 years | 2.15 (1.17–3.96, p = 0.01) | 1.70 (0.86–3.34, p = 0.12) |
| Hospitalization during Omicron VOC pandemic wave | 1.60 (1.00–2.58, p = 0.05) | 1.95 (1.09–3.5, p = 0.02) |
| BMI >= 30 | 0.67 (0.27–1.64, p= 0.38) | 0.89 (0.34–2.31, p = 0.81) |
| CCI >= 6 | 1.42 (0.93–2.18, p= 0.11) | 1.03 (0.64–1.65, p = 0.91) |
| >=7 days before hospitalization | 1.12 (0.71–1.76, p= 0.62) | 0.93 (0.57–1.50, p = 0.76) |
| Vaccinate | 1.03 (0.62–1.72, p= 0.89) | 1.14 (0.62–2.09, p = 0.69) |
| Vaccinate with a booster dose | 0.97 (0.63–1.49, p= 0.90) | 0.69 (0.39–1.20, p = 0.19) |
| NIV < 24 h from admission | 3.83 (2.48–5.91, p < 0.001) | 3.88 (2.49–6.06, p < 0.001) |
| Variables | H.R. (95% C.I., p-Value) | aHR (95% C.I., p-Value) |
|---|---|---|
| Male sex | 1.35 (0.58–3.16, p = 0.48) | 0.65 (0.26–1.61, p = 0.35) |
| Age >= 65 years | 3.16 (1.06–9.40, p = 0.04) | 6.80 (1.71–27.04, p = 0.006) |
| CCI >= 6 | 1.24 (0.29–5.35, p = 0.77) | 0.58 (0.13–2.57, p = 0.47) |
| BMI >= 30 | 0.97 (0.29–3.27, p = 0.96) | 2.30 (0.55–9.63, p = 0.25) |
| >=7 days until hospitalization | 0.78 (0.32–1.93, p = 0.59) | 0.81 (0.32–2.06, p = 0.65) |
| Vaccinate * | 1.00 (0.42–2.38, p = 0.99) | 0.35 (0.12–0.98, p = 0.04) |
| NIV < 24 h from admission | 14.14 (4.18–47.78, p < 0.001) | 21.03 (5.34–82.80, p < 0.001) |
| Variables | H.R. (95% C.I., p-Value) | aHR (95% C.I., p-Value) |
|---|---|---|
| Male sex | 1.62 (0.93–2.81, p = 0.09) | 1.45 (0.81–2.59, p = 0.21) |
| Age >= 65 years | 1.73 (0.82–3.65, p = 0.19) | 1.43 (0.62–3.31, p = 0.40) |
| CCI >= 6 | 1.20 (0.74–1.94, p = 0.46) | 1.13 (0.66–1.91, p = 0.66) |
| BMI >= 30 | 0.64 (0.15–2.62, p = 0.53) | 0.67 (0.16–2.85, p = 0.59) |
| >=7 days until hospitalization | 1.25 (0.74–2.13, p = 0.40) | 0.96 (0.55–1.70, p = 0.90) |
| Vaccinate * | 1.05 (0.55–2.03, p = 0.89) | 1.81 (0.83–3.92, p = 0.13) |
| Vaccinate with a booster dose | 0.69 (0.43–1.13, p = 0.14) | 0.58 (0.33–1.02, p = 0.06) |
| NIV < 24 h from admission | 3.19 (1.94–5.26, p < 0.001) | 3.36 (1.99–5.66, p < 0.001) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poliseno, M.; Drago, E.P.; Poli, M.A.; Altamura, M.; Bruno, S.R.; Calamo, A.; Giannelli, A.; Infante, G.; Mazzola, M.; Moschetta, D.; et al. Clinical Characteristics, Outcomes, and Risk Factors of Patients Hospitalized for COVID-19 across the Latest Pandemic Waves: Has Something Changed? BioMed 2023, 3, 272-281. https://doi.org/10.3390/biomed3020024
Poliseno M, Drago EP, Poli MA, Altamura M, Bruno SR, Calamo A, Giannelli A, Infante G, Mazzola M, Moschetta D, et al. Clinical Characteristics, Outcomes, and Risk Factors of Patients Hospitalized for COVID-19 across the Latest Pandemic Waves: Has Something Changed? BioMed. 2023; 3(2):272-281. https://doi.org/10.3390/biomed3020024
Chicago/Turabian StylePoliseno, Mariacristina, Edoardo Paolo Drago, Melita Anna Poli, Maurantonio Altamura, Serena Rita Bruno, Angela Calamo, Anna Giannelli, Giovanni Infante, Michele Mazzola, Damiana Moschetta, and et al. 2023. "Clinical Characteristics, Outcomes, and Risk Factors of Patients Hospitalized for COVID-19 across the Latest Pandemic Waves: Has Something Changed?" BioMed 3, no. 2: 272-281. https://doi.org/10.3390/biomed3020024
APA StylePoliseno, M., Drago, E. P., Poli, M. A., Altamura, M., Bruno, S. R., Calamo, A., Giannelli, A., Infante, G., Mazzola, M., Moschetta, D., Lo Caputo, S., Santantonio, T. A., & Carbonara, S. (2023). Clinical Characteristics, Outcomes, and Risk Factors of Patients Hospitalized for COVID-19 across the Latest Pandemic Waves: Has Something Changed? BioMed, 3(2), 272-281. https://doi.org/10.3390/biomed3020024







