Synthesis and Characterization of Carbonaceous Materials for Medical Applications: A Comprehensive Review
Abstract
:1. Introduction
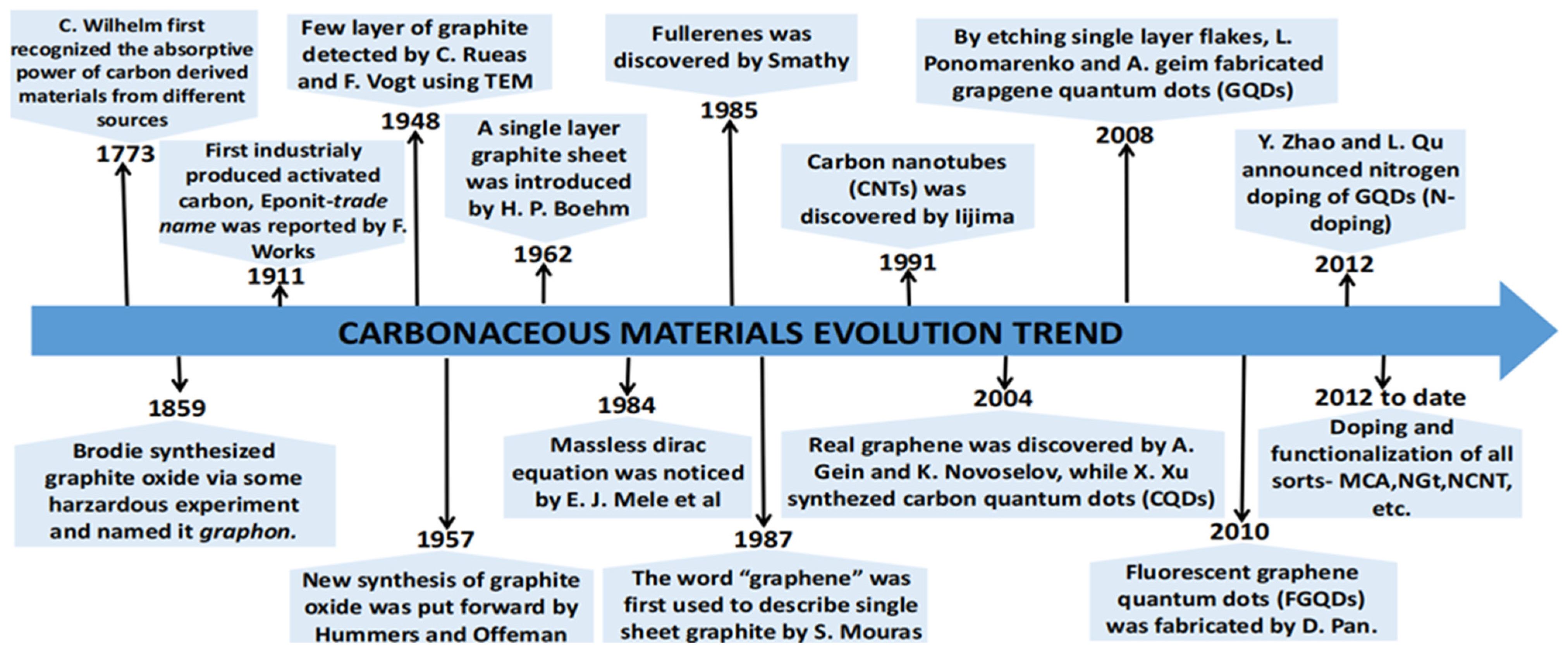
1.1. The History of Carbonaceous Materials (CMs)
Development of Carbonaceous Materials in Medicine
1.2. Synthesis and Characterization of Carbonaceous Materials for Medical Applications
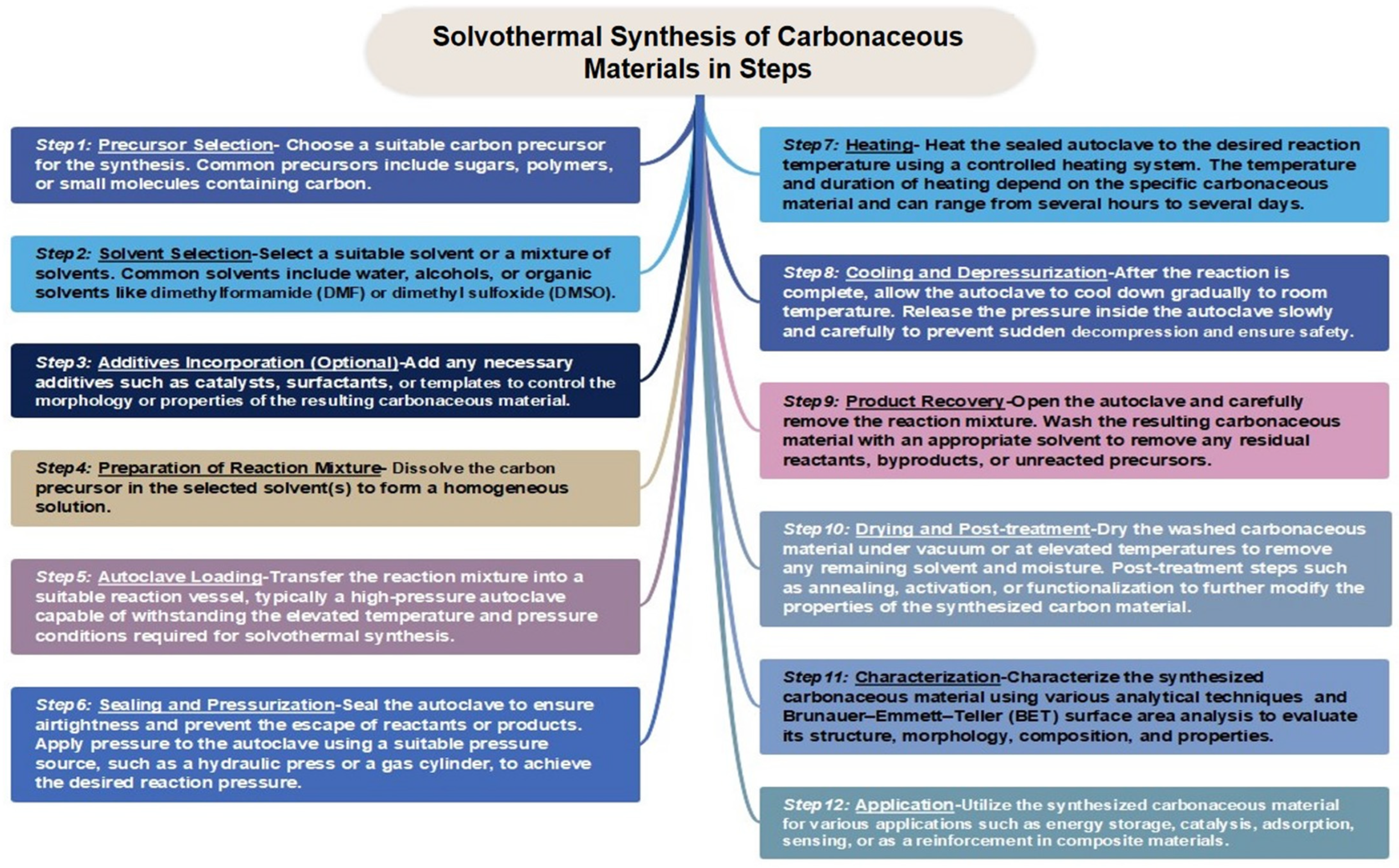
1.3. Various Synthesis Approach of Carbonaceous Materials
1.3.1. Chemical Vapor Deposition (CVD)
1.3.2. Chemical Reduction Methods
1.3.3. Sol–Gel Processes
1.3.4. Arc Discharge
1.3.5. Pyrolysis
1.3.6. Electrochemical Deposition
1.3.7. Template-Assisted Synthesis
1.3.8. Microwave-Assisted Synthesis
1.3.9. Laser Ablation
2. Characterization of Carbonaceous Materials for Medical Applications
2.1. X-Ray Diffraction (XRD) Analysis of Carbonaceous Materials
2.2. Fourier-Transform Infrared (FTIR) Analysis of Carbonaceous Materials
2.3. Raman Spectroscopy of Carbonaceous Materials
2.4. Scanning Electron Microscopy (SEM) of Carbonaceous Materials
2.5. X-Ray Photoelectron Spectroscopy (XPS) of Carbonaceous Materials
2.6. Other Imaging and Related Techniques for Carbonaceous Materials
3. Application of Carbonaceous Materials in Medical Sciences
3.1. Cancer Cell Amelioration (Hyperthermia)
3.2. Magnetic Resonance Imaging (MRI)
3.3. Biosensors in Medicine
3.4. Dental Care Formulation
3.5. Tissue Engineering
3.6. Human System Detoxification
3.7. Wound Healing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rimmer, P.B.; Shorttle, O. Origin of life’s building blocks in carbon-and nitrogen-rich surface hydrothermal vents. Life 2019, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Messel, H. Abridged Science for High School Students: An Integrated Four-Year Course in Physics, Chemistry; Nuclear Research Foundation, University of Sydney: Sydney, NSW, Australia, 1966. [Google Scholar]
- Kim, C.-H.; Lee, S.-Y.; Rhee, K.Y.; Park, S.-J. Carbon-based composites in biomedical applications: A comprehensive review of properties, applications, and future directions. Adv. Compos. Mater. 2024, 7, 55. [Google Scholar] [CrossRef]
- Hu, Z.; Srinivasan, M.P.; Ni, Y. Preparation of mesoporous high-surface-area activated carbon. Adv. Mater. 2000, 12, 62–65. [Google Scholar] [CrossRef]
- Kruss, S.; Hilmer, A.J.; Zhang, J.; Reuel, N.F.; Mu, B.; Strano, M.S. Carbon nanotubes as optical biomedical sensors. Adv. Drug Deliv. Rev. 2013, 65, 1933–1950. [Google Scholar] [CrossRef]
- Chella, S.; Kollu, P.; Komarala, E.V.P.; Doshi, S.; Saranya, M.; Felix, S.; Ramachandran, R.; Saravanan, P.; Koneru, V.L.; Venugopal, V. Solvothermal synthesis of MnFe2O4-graphene composite—Investigation of its adsorption and antimicrobial properties. Appl. Surf. Sci. 2015, 327, 27–36. [Google Scholar] [CrossRef]
- Feng, P.; Cui, K.; Hai, Z.; Wang, J.; Wang, L. Facile synthesis of activated carbon loaded g-C3N4 composite with enhanced photocatalytic performance under visible light. Diam. Relat. Mater. 2023, 136, 109921. [Google Scholar] [CrossRef]
- Reddy, D.H.K.; Lee, S.-M. Magnetic biochar composite: Facile synthesis, characterization, and application for heavy metal removal. Coll. Surf. A Colloid. Surf. A Physicochem. Eng. Asp. 2014, 454, 96–103. [Google Scholar] [CrossRef]
- Aziz, T.; Ullah, A.; Fan, H.; Ullah, R.; Haq, F.; Khan, F.U.; Wei, J. Cellulose nanocrystals applications in health, medicine and catalysis. J. Polym. Environ. 2021, 29, 2062–2071. [Google Scholar] [CrossRef]
- Derbyshire, F.; Jagtoyen, M.; Thwaites, M. Activated carbons-production and applications. In Porosity in Carbons; Halsted Press: Ultimo, NSW, Australia, 1995; Volume 252. [Google Scholar]
- Leimkuehler, E.P. Production, Characterization, and Applications of Activated Carbon; University of Missouri-Columbia: Columbia, MO, USA, 2010. [Google Scholar]
- Ruess, G.; Vogt, F. Höchstlamellarer Kohlenstoff aus Graphitoxyhydroxyd: Über den Ort der aktiven Eigenschaften am Kohlenstoffkristall. Monatshefte Für Chem. Verwandte Teile Anderer Wiss. 1948, 78, 222–242. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Brodie, B.C. XIII. On the atomic weight of graphite. Philos. Trans. R. Soc. Lond. 1859, 149, 249–259. [Google Scholar]
- Boehm, H.-P.; Clauss, A.; Fischer, G.; Hofmann, U. Das adsorptionsverhalten sehr dünner kohlenstoff-folien. Z. Anorg. Und Allg. Chem. 1962, 316, 119–127. [Google Scholar] [CrossRef]
- DiVincenzo, D.; Mele, E. Self-consistent effective-mass theory for intralayer screening in graphite intercalation compounds. Phys. Rev. 1984, 29, 1685. [Google Scholar] [CrossRef]
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Mouras, S.; Hamm, A.; Djurado, D.; Cousseins, J.-C. Synthesis of first stage graphite intercalation compounds with fluorides. Rev. De Chim. Minérale 1987, 24, 572–582. [Google Scholar]
- Lijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Dinadayalane, T.; Lazare, J.; Alzaaqi, N.F.; Herath, D.; Hill, B.; Campbell, A.E. Structures, properties, and applications of nitrogen-doped graphene. In Theoretical and Computational Chemistry; Elsevier: Amsterdam, The Netherlands, 2022; Volume 21, pp. 211–248. [Google Scholar]
- Metwaly, A.M.; Ghoneim, M.M.; Eissa, I.H.; Elsehemy, I.A.; Mostafa, A.E.; Hegazy, M.M.; Afifi, W.M.; Dou, D. Traditional ancient Egyptian medicine: A review. Saudi J. Biol. Sci. 2021, 28, 5823–5832. [Google Scholar] [CrossRef]
- Marketos, S.G.; Androutsos, G. Charcoal: From antiquity to artificial kidney. J. Nephrol. 2004, 17, 453–456. [Google Scholar]
- Al Jumaan, M.A. The Role of Activated Charcoal in Prehospital Care. Med. Arch. 2023, 77, 64. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Eklund, P.C. Science of Fullerenes and Carbon Nanotubes: Their Properties and Applications; Elsevier: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Soonmin, H.; Akram, M.; Rashid, A.; Laila, U.; Zainab, R. Uses of activated carbon in medicine area: Short review. EPRA Int. J. Res. Dev. 2022, 7, 34–39. [Google Scholar]
- Juurlink, D.N. Activated charcoal for acute overdose: A reappraisal. Br. J. Clin. Pharmacol. 2016, 81, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Jacoby, M.; Building, A. Better Gas Mask. C&EN 2014, 92, 34–38. [Google Scholar]
- Editors, H.C. Second Battle of Ypres Begins. Available online: https://www.history.com/this-day-in-history/second-battle-of-ypres-begins (accessed on 5 November 2009).
- Mohamed, E.F.; El-Hashemy, M.A.; Abdel-Latif, N.M.; Shetaya, W.H. Production of sugarcane bagasse-based activated carbon for formaldehyde gas removal from potted plants exposure chamber. J. Air Waste Manag. Assoc. 2015, 65, 1413–1420. [Google Scholar] [CrossRef]
- Navarro, M.; Michiardi, A.; Castano, O.; Planell, J. Biomaterials in orthopaedics. J. R. Soc. Interface. 2008, 5, 1137–1158. [Google Scholar] [CrossRef]
- Alas, M.O.; Alkas, F.B.; Aktas Sukuroglu, A.; Genc Alturk, R.; Battal, D. Fluorescent carbon dots are the new quantum dots: An overview of their potential in emerging technologies and nanosafety. J. Mater. Sci. 2020, 55, 15074–15105. [Google Scholar] [CrossRef]
- El-Shafey, A.M. Carbon dots: Discovery, structure, fluorescent properties, and applications. Green. Process. Synth. 2021, 10, 134–156. [Google Scholar] [CrossRef]
- Dai, B.; Zhou, R.; Ping, J.; Ying, Y.; Xie, L. Recent advances in carbon nanotube-based biosensors for biomolecular detection. TrAC 2022, 154, 116658. [Google Scholar] [CrossRef]
- Mokhtarzadeh, A.; Eivazzadeh-Keihan, R.; Pashazadeh, P.; Hejazi, M.; Gharaatifar, N.; Hasanzadeh, M.; Baradaran, B.; de la Guardia, M. Nanomaterial-based biosensors for detection of pathogenic virus. TrAC 2017, 97, 445–457. [Google Scholar] [CrossRef]
- Kościk, I.; Jankowski, D.; Jagusiak, A. Carbon nanomaterials for theranostic use. C 2021, 8, 3. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Z.; Zhang, Y. The application of carbon nanotubes in target drug delivery systems for cancer therapies. Nanoscale Res. Lett. 2011, 6, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.; Wang, C.-H.K.; Chow, E.K.-H. Nanodiamonds: The intersection of nanotechnology, drug development, and personalized medicine. Sci. Adv. 2015, 1, e1500439. [Google Scholar] [CrossRef]
- Mohan, H.; Fagan, A.; Giordani, S. Carbon Nanomaterials (CNMs) in Cancer Therapy: A Database of CNM-Based Nanocarrier Systems. Pharmaceutics 2023, 15, 1545. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; Nadeem, A.Y.; Qaiser, H.; Kashif, A.S.; Ahmed, A.; Khan, K.; Altaf, A. A review of carbon-based materials and their coating techniques for biomedical implants applications. Carbon. Lett. 2023, 33, 1171–1188. [Google Scholar] [CrossRef]
- Nasir, S.; Hussein, M.Z.; Zainal, Z.; Yusof, N.A. Carbon-based nanomaterials/allotropes: A glimpse of their synthesis, properties, and some applications. Materials 2018, 11, 295. [Google Scholar] [CrossRef]
- Reza, M.S.; Afroze, S.; Kuterbekov, K.; Kabyshev, A.; Bekmyrza, K.Z.; Haque, M.N.; Islam, S.N.; Hossain, M.A.; Hassan, M.; Roy, H. Advanced applications of carbonaceous materials in sustainable water treatment, energy storage, and CO2 capture: A comprehensive review. Sustainability 2023, 15, 8815. [Google Scholar] [CrossRef]
- Kong, J.; Soh, H.T.; Cassell, A.M.; Quate, C.F.; Dai, H. Synthesis of individual single-walled carbon nanotubes on patterned silicon wafers. Nature 1998, 395, 878–881. [Google Scholar] [CrossRef]
- Bhagabati, P.; Rahaman, M.; Bhandari, S.; Roy, I.; Dey, A.; Gupta, P.; Ansari, M.; Dutta, A.; Chattopadhyay, D. Synthesis/preparation of carbon materials. In Carbon-Containing Polymer Composites; Springer: Singapore, 2019; pp. 1–64. [Google Scholar]
- Shah, K.A.; Tali, B.A. Synthesis of carbon nanotubes by catalytic chemical vapour deposition: A review on carbon sources, catalysts and substrates. Mater. Sci. Semicond. Process. 2016, 41, 67–82. [Google Scholar] [CrossRef]
- Magrez, A.; Seo, J.W.; Smajda, R.; Mionić, M.; Forró, L. Catalytic CVD synthesis of carbon nanotubes: Towards high yield and low temperature growth. Materials 2010, 3, 4871–4891. [Google Scholar] [CrossRef]
- Yahya, N.; Koziol, K.; Boskovic, B.O.; Yahya, N. Synthesis of carbon nanostructures by CVD method. In Carbon and Oxide Nanostructures; Springer: Berlin/Heidelberg, 2011; pp. 23–49. [Google Scholar]
- Sahu, S.; Khan, M.S.; Gupta, N.; Chennakesavulu, K.; Sasikumar, C. The hydrogen storage capacity of carbon nano-onions fabricated by thermal chemical vapour deposition. Int. J. Hydrogen Energy 2024, 52, 1371–1383. [Google Scholar] [CrossRef]
- Li, X.; Cai, W.; An, J.; Kim, S.; Nah, J.; Yang, D.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E. Large-area synthesis of high-quality and uniform graphene films on copper foils. Science 2009, 324, 1312–1314. [Google Scholar] [CrossRef] [PubMed]
- Reina, A.; Jia, X.; Ho, J.; Nezich, D.; Son, H.; Bulovic, V.; Dresselhaus, M.S.; Kong, J. Large area, few-layer graphene films on arbitrary substrates by chemical vapor deposition. Nano Lett. 2009, 9, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Ago, H.; Tanaka, I.; Orofeo, C.M.; Tsuji, M.; Ikeda, K.i. Patterned growth of graphene over epitaxial catalyst. Small 2010, 6, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-D.; Vinodgopal, K.; Dai, G.-P. Synthesis of carbon nanotubes by catalytic chemical vapor deposition. In Perspective of Carbon Nanotubes; InTech Open: London, UK, 2019; pp. 1–19. [Google Scholar]
- Gao, L.; Ren, W.; Zhao, J.; Ma, L.-P.; Chen, Z.; Cheng, H.-M. Efficient growth of high-quality graphene films on Cu foils by ambient pressure chemical vapor deposition. Appl. Phys. Lett. 2010, 97, 183109. [Google Scholar] [CrossRef]
- Li, X.; Cai, W.; Colombo, L.; Ruoff, R.S. Evolution of graphene growth on Ni and Cu by carbon isotope labeling. Nano Lett. 2009, 9, 4268–4272. [Google Scholar] [CrossRef]
- Martínez-Castañon, G.-A.; Nino-Martinez, N.; Martinez-Gutierrez, F.; Martínez-Mendoza, J.; Ruiz, F. Synthesis and antibacterial activity of silver nanoparticles with different sizes. J. Nanoparticle Res. 2008, 10, 1343–1348. [Google Scholar] [CrossRef]
- Jana, N.R.; Gearheart, L.; Murphy, C.J. Wet chemical synthesis of high aspect ratio cylindrical gold nanorods. J. Phys. Chem. B 2001, 105, 4065–4067. [Google Scholar] [CrossRef]
- Pillai, Z.S.; Kamat, P.V. What factors control the size and shape of silver nanoparticles in the citrate ion reduction method? J. Phys. Chem. B 2004, 108, 945–951. [Google Scholar] [CrossRef]
- Bressi, V.; Balu, A.M.; Iannazzo, D.; Espro, C. Recent advances in the synthesis of carbon dots from renewable biomass by high-efficient hydrothermal and microwave green approaches. Curr. Opin. Green. Sustain. Chem. 2023, 40, 100742. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, T.; Zhang, G.; Liu, Q.; Kong, G.; Wang, K.; Jiang, Y.; Zhang, X.; Han, L. Sustainable hydrothermal carbon for advanced electrochemical energy storage. J. Mater. Chem. A. 2024, 12, 4996–5039. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Y.-J.; Hao, X.; Peng, P.; Shi, J.-Y.; Peng, F.; Sun, R.-C. Hydrothermal synthesis and applications of advanced carbonaceous materials from biomass: A review. Adv. Compos. Hybrid. Mater. 2020, 3, 267–284. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Nezafat, Z.; Shafiei, N. Lignin chemistry and valorization. In Biopolymer-Based Metal. Nanoparticle Chemistry for Sustainable Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 145–183. [Google Scholar]
- Pathak, M.; Jeong, S.M.; Rout, C.S. Spinel NiCo2O4 based hybrid materials for supercapacitors: Recent developments and future perspectives. J. Energy Storage 2023, 73, 108881. [Google Scholar] [CrossRef]
- Bokov, D.; Turki Jalil, A.; Chupradit, S.; Suksatan, W.; Javed Ansari, M.; Shewael, I.H.; Valiev, G.H.; Kianfar, E. Nanomaterial by sol-gel method: Synthesis and application. Adv. Mater. Sci. Eng. 2021, 2021, 5102014. [Google Scholar] [CrossRef]
- Yilmaz, E.; Soylak, M. Functionalized nanomaterials for sample preparation methods. In Handbook of Nanomaterials in Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2020; pp. 375–413. [Google Scholar]
- Singh, K.; Meena, R.S.; Kumar, S.; Dhyani, S.; Sheoran, S.; Singh, H.M.; Pathak, V.V.; Khalid, Z.; Singh, A.; Chopra, K. India’s renewable energy research and policies to phase down coal: Success after Paris agreement and possibilities post-Glasgow Climate Pact. Biomass Bioenergy 2023, 177, 106944. [Google Scholar] [CrossRef]
- Yang, H.; Zhu, M.; Li, Y. Sol–gel research in China: A brief history and recent research trends in synthesis of sol–gel derived materials and their applications. J. Sol-Gel Sci. Technol. 2023, 106, 406–421. [Google Scholar] [CrossRef]
- Konuk, O.P.; Alsuhile, A.A.; Yousefzadeh, H.; Ulker, Z.; Bozbag, S.E.; García-González, C.A.; Smirnova, I.; Erkey, C. The effect of synthesis conditions and process parameters on aerogel properties. Front. Chem. 2023, 11, 1294520. [Google Scholar]
- Dervin, S.; Pillai, S.C. An introduction to sol-gel processing for aerogels. In Sol-Gel Materials for Energy, Environment and Electronic Applications; Springer: Cham, Switzerland, 2017; pp. 1–22. [Google Scholar]
- Xu, Y.; Liu, Z.; Ren, B.; Wang, S.; Zhang, L. Facile Preparation of Carbon Aerogels with Different Drying Methods. IOP Conf. Ser. Mater. Sci. Eng. 2019, 562, 012101. [Google Scholar] [CrossRef]
- Lin, C.; Ritter, J.A. Carbonization and activation of sol–gel derived carbon xerogels. Carbon 2000, 38, 849–861. [Google Scholar] [CrossRef]
- Théry, A.; Béguin, F.; Kocon, L.; Lillo-Rodenas, M.; Linares-Solano, A.; Rouzaud, J.-N. Influence of carbonisation temperature on the structural and electrochemical properties of carbon aerogels. In Proceedings of the Carbon Conference 2004, Providence, RI, USA, 11–16 July 2004. [Google Scholar]
- Lipeng, Z.; Jianbing, N.; Liming, D.; Zhenhai, X. Effect of Microstructure of Nitrogen-Doped Graphene on Oxygen Reduction Activity in Fuel Cells. Langmuir 2012, 28, 7542–7550. [Google Scholar]
- Chen, F.; Yan, T.-H.; Bashir, S.; Liu, J.L. Synthesis of nanomaterials using top-down methods. In Advanced Nanomaterials and Their Applications in Renewable Energy, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 37–60. [Google Scholar]
- Madhurima, V.; Kumari, K.; Jain, P. Synthesis and study of carbon nanomaterials through arc discharge technique for efficient adsorption of organic dyes. Diam. Relat. Mater. 2024, 141, 110538. [Google Scholar] [CrossRef]
- De Heer, W.A.; Bacsa, W.; Chatelain, A.; Gerfin, T.; Humphrey-Baker, R.; Forro, L.; Ugarte, D. Aligned carbon nanotube films: Production and optical and electronic properties. Science 1995, 268, 845–847. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Wang, D.; Wen, J.; Sennett, M.; Gibson, H.; Ren, Z. Effect of nickel, iron and cobalt on growth of aligned carbon nanotubes. Appl. Phys. A 2002, 74, 387–391. [Google Scholar] [CrossRef]
- Watanabe, T.; Itoh, A.; Watanabe, T.; Kizaki, T.; Inaguma, M.; Hosoi, A.; Kawada, H. Post-synthesis treatment improves the electrical properties of dry-spun carbon nanotube yarns. Carbon 2021, 185, 314–323. [Google Scholar] [CrossRef]
- Arora, N.; Sharma, N.N. Arc discharge synthesis of carbon nanotubes: Comprehensive review. Diam. Relat. Mater. 2014, 50, 135–150. [Google Scholar] [CrossRef]
- Devi, M.; Rawat, S.; Sharma, S. A comprehensive review of the pyrolysis process: From carbon nanomaterial synthesis to waste treatment. Oxf. Open Mater. Sci. 2021, 1, itab014. [Google Scholar] [CrossRef]
- Asif, F.C.; Saha, G.C. Graphene-like carbon structure synthesis from biomass pyrolysis: A critical review on feedstock–process–properties relationship. C 2023, 9, 31. [Google Scholar] [CrossRef]
- Lataf, A.; Jozefczak, M.; Vandecasteele, B.; Viaene, J.; Schreurs, S.; Carleer, R.; Yperman, J.; Marchal, W.; Cuypers, A.; Vandamme, D. The effect of pyrolysis temperature and feedstock on biochar agronomic properties. J. Anal. Appl. Pyrolysis 2022, 168, 105728. [Google Scholar] [CrossRef]
- Predeanu, G.; Slăvescu, V.; Drăgoescu, M.F.; Bălănescu, N.M.; Fiti, A.; Meghea, A.; Samoila, P.; Harabagiu, V.; Ignat, M.; Manea-Saghin, A.-M. Green synthesis of advanced carbon materials used as precursors for adsorbents applied in wastewater treatment. Materials 2023, 16, 1036. [Google Scholar] [CrossRef]
- Ng, C.H.; Mistoh, M.A.; Teo, S.H.; Galassi, A.; Taufiq-Yap, Y.H.; Siambun, N.J.; Foo, J.; Sipaut, C.S.; Seay, J.; Janaun, J. The roles of carbonaceous wastes for catalysis, energy, and environmental remediation. Catal. Commun. 2024, 187, 106845. [Google Scholar] [CrossRef]
- Shang, S.; Zeng, W. Conductive nanofibres and nanocoatings for smart textiles. In Multidisciplinary Know-How for Smart-Textiles Developers; Elsevier: Amsterdam, The Netherlands, 2013; pp. 92–128. [Google Scholar]
- Augello, C.; Liu, H. Surface modification of magnesium by functional polymer coatings for neural applications. In Surface Modification of Magnesium and Its Alloys for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2015; pp. 335–353. [Google Scholar]
- Suzuki, Y.; Takeda, T.; Goto, T. Direct electrochemical formation of carbonaceous material from CO2 in LiCl-KCl melt. Electrochim. Acta 2023, 456, 142464. [Google Scholar] [CrossRef]
- Zhou, D.; Anoshkina, E.V.; Chow, L.; Chai, G. Synthesis of carbon nanotubes by electrochemical deposition at room temperature. Carbon 2006, 44, 1013–1016. [Google Scholar] [CrossRef]
- Arul, P.; Gowthaman, N.; John, S.A.; Tominaga, M. Tunable electrochemical synthesis of 3D nucleated microparticles like Cu-BTC MOF-carbon nanotubes composite: Enzyme free ultrasensitive determination of glucose in a complex biological fluid. Electrochim. Acta 2020, 354, 136673. [Google Scholar] [CrossRef]
- Zhou, D.; Chow, L. Electrochemical Deposition of Carbon Nanoparticles from Organic Solutions. U.S. Patent US7422667B1, 9 September 2008. [Google Scholar]
- Ren, Z.; Meng, N.; Shehzad, K.; Xu, Y.; Qu, S.; Yu, B.; Luo, J. Mechanical properties of nickel-graphene composites synthesized by electrochemical deposition. Nanotechnology 2015, 26, 065706. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Xie, L.; Wang, Y.; He, H.; Yu, H.; Cui, J.; Feng, X.; Lou, Z.; Xiong, Y. Efficient uranium electrochemical deposition with a functional phytic Acid-Doped Polyaniline/Graphite sheet electrode by Adsorption-electrodeposition strategy. Chem. Eng. J. 2023, 457, 141221. [Google Scholar] [CrossRef]
- Wang, Z.; Du, J.; Zhang, M.; Yu, J.; Liu, H.; Chai, X.; Yang, B.; Zhu, C.; Xu, J. Continuous preparation of high performance flexible asymmetric supercapacitor with a very fast, low-cost, simple and scalable electrochemical co-deposition method. J. Power Sources 2019, 437, 226827. [Google Scholar] [CrossRef]
- Pavlenko, V.; Żółtowska, S.; Haruna, A.; Zahid, M.; Mansurov, Z.; Supiyeva, Z.; Galal, A.; Ozoemena, K.; Abbas, Q.; Jesionowski, T. A comprehensive review of template-assisted porous carbons: Modern preparation methods and advanced applications. Mater. Sci. Eng. R Rep. 2022, 149, 100682. [Google Scholar] [CrossRef]
- Xu, X.; He, Z.; Tang, H.; Sun, Y.; Zhang, S.; Shi, D.; Ji, F. Removal of diclofenac and oxytetracycline from synthetic urine by furfuryl alcohol-derived mesoporous carbon. Chemosphere 2022, 288, 132317. [Google Scholar] [CrossRef]
- Xia, W.; Qiu, B.; Xia, D.; Zou, R. Facile preparation of hierarchically porous carbons from metal-organic gels and their application in energy storage. Sci. Rep. 2013, 3, 1935. [Google Scholar] [CrossRef]
- Dong, S.; Song, Y.; Fang, Y.; Zhu, K.; Ye, K.; Gao, Y.; Yan, J.; Wang, G.; Cao, D. Microwave-assisted synthesis of carbon dots modified graphene for full carbon-based potassium ion capacitors. Carbon 2021, 178, 1–9. [Google Scholar] [CrossRef]
- Sathish, S.; Nirmala, R.; Kim, H.Y.; Navamathavan, R. Deriving activated carbon using microwave combustion technique and its energy storage applications: A topical review. Carbon Lett. 2022, 32, 1151–1171. [Google Scholar] [CrossRef]
- Xia, X.; Cheng, C.-F.; Zhu, Y.; Vogt, B.D. Ultrafast microwave-assisted synthesis of highly nitrogen-doped ordered mesoporous carbon. Microporous Mesoporous Mater. 2021, 310, 110639. [Google Scholar] [CrossRef]
- Onwudiwe, D.C. Microwave-assisted synthesis of PbS nanostructures. Heliyon 2019, 5, e01413. [Google Scholar] [CrossRef] [PubMed]
- Brazil, T.R.; Gonçalves, M.; dos Anjos, E.G.R.; de Oliveira Junior, M.S.; Rezende, M.C. Microwave-assisted production of activated carbon in an adapted domestic oven from lignocellulosic waste. Biomass Convers. Biorefin. 2024, 14, 255–268. [Google Scholar] [CrossRef]
- Zhou, Y.; Yan, W.; Yu, X.; Chen, T.; Wang, S.; Zhao, W. Boron and nitrogen co-doped porous carbon for supercapacitors: A comparison between a microwave-assisted and a conventional hydrothermal process. J. Energy Storage 2020, 32, 101706. [Google Scholar] [CrossRef]
- Baghbanzadeh, M.; Carbone, L.; Cozzoli, P.D.; Kappe, C.O. Microwave-assisted synthesis of colloidal inorganic nanocrystals. Angew. Chem. Int. Ed. 2011, 50, 11312–11359. [Google Scholar] [CrossRef]
- Albuquerque, H.M.; Pinto, D.C.; Silva, A.M. Microwave irradiation: Alternative heating process for the synthesis of biologically applicable chromones, quinolones, and their precursors. Molecules 2021, 26, 6293. [Google Scholar] [CrossRef]
- Larosi, M.B.; García, J.d.V.; Rodríguez, A.R. Laser synthesis of nanomaterials. Nanomaterials 2022, 12, 2903. [Google Scholar] [CrossRef]
- Myungjoon, K.; Saho, O.; Taesung, K.; Hidenori, H.; Takafumi, S. Synthesis of nanoparticles by laser ablation: A review. KONA Powder Part. J. 2017, 34, 80–90. [Google Scholar]
- Guo, T.; Nikolaev, P.; Thess, A.; Colbert, D.T.; Smalley, R.E. Catalytic growth of single-walled manotubes by laser vaporization. Chem. Phys. Lett. 1995, 243, 49–54. [Google Scholar] [CrossRef]
- Ganash, E.A.; Al-Jabarti, G.A.; Altuwirqi, R.M. The synthesis of carbon-based nanomaterials by pulsed laser ablation in water. Mater. Res. Express 2019, 7, 015002. [Google Scholar] [CrossRef]
- Yang, G. Laser ablation in liquids: Applications in the synthesis of nanocrystals. Prog. Mater. Sci. 2007, 52, 648–698. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Sun, X.; Ma, Y. Shape-controlled synthesis of nanocarbons through direct conversion of carbon dioxide. Sci. Rep. 2013, 3, 3534. [Google Scholar] [CrossRef] [PubMed]
- Al-Hamaoy, A.; Chikarakara, E.; Jawad, H.; Gupta, K.; Kumar, D.; Rao, M.R.; Krishnamurthy, S.; Morshed, M.; Fox, E.; Brougham, D. Liquid Phase–Pulsed Laser Ablation: A route to fabricate different carbon nanostructures. Appl. Surf. Sci. 2014, 302, 141–144. [Google Scholar] [CrossRef]
- Kazemizadeh, F.; Malekfar, R.; Parvin, P. Pulsed laser ablation synthesis of carbon nanoparticles in vacuum. J. Phys. Chem. Solids 2017, 104, 252–256. [Google Scholar] [CrossRef]
- Khairani, I.Y.; Mínguez-Vega, G.; Doñate-Buendía, C.; Gökce, B. Green nanoparticle synthesis at scale: A perspective on overcoming the limits of pulsed laser ablation in liquids for high-throughput production. Phys. Chem. Chem. Phys. 2023, 25, 19380–19408. [Google Scholar] [CrossRef]
- Neves, V.; Heister, E.; Costa, S.; Tîlmaciu, C.; Flahaut, E.; Soula, B.; Coley, H.M.; Mcfadden, J.; Silva, S.R.P. Design of double-walled carbon nanotubes for biomedical applications. Nanotechnology 2012, 23, 365102. [Google Scholar] [CrossRef]
- Zhang, F.; Xia, Y.; Xu, L.; Gu, N. Surface modification and microstructure of single-walled carbon nanotubes for dental resin-based composites. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 86, 90–97. [Google Scholar] [CrossRef]
- González-García, P.; Centeno, T.; Urones-Garrote, E.; Ávila-Brande, D.; Otero-Díaz, L. Microstructure and surface properties of lignocellulosic-based activated carbons. Appl. Surf. Sci. 2013, 265, 731–737. [Google Scholar] [CrossRef]
- Chukwuike, V.; Sankar, S.S.; Kundu, S.; Barik, R. Capped and uncapped nickel tungstate (NiWO4) nanomaterials: A comparison study for anti-corrosion of copper metal in NaCl solution. Corros. Sci. 2019, 158, 108101. [Google Scholar] [CrossRef]
- Shao, L.; Ren, Z.; Zhang, G.; Chen, L. Facile synthesis, characterization of a MnFe2O4/activated carbon magnetic composite and its effectiveness in tetracycline removal. Mater. Chem. Phys. 2012, 135, 16–24. [Google Scholar] [CrossRef]
- Ai, L.; Huang, H.; Chen, Z.; Wei, X.; Jiang, J. Activated carbon/CoFe2O4 composites: Facile synthesis, magnetic performance and their potential application for the removal of malachite green from water. Chem. Eng. J. 2010, 156, 243–249. [Google Scholar] [CrossRef]
- Argun, M.E.; Dursun, S.; Karatas, M. Removal of Cd (II), Pb (II), Cu (II) and Ni (II) from water using modified pine bark. Desalination 2009, 249, 519–527. [Google Scholar] [CrossRef]
- Muniyalakshmi, M.; Sethuraman, K.; Silambarasan, D. Synthesis and characterization of graphene oxide nanosheets. Mater. Today Proc. 2020, 21, 408–410. [Google Scholar] [CrossRef]
- Zhang, X.; Li, K.; Li, H.; Lu, J.; Fu, Q.; Chu, Y. Graphene nanosheets synthesis via chemical reduction of graphene oxide using sodium acetate trihydrate solution. Synth. Met. 2014, 193, 132–138. [Google Scholar] [CrossRef]
- Rao, A.M.; Richter, E.; Bandow, S.; Chase, B.; Eklund, P.; Williams, K.; Fang, S.; Subbaswamy, K.; Menon, M.; Thess, A. Diameter-selective Raman scattering from vibrational modes in carbon nanotubes. Science 1997, 275, 187–191. [Google Scholar] [CrossRef]
- Rao, C.; Subrahmanyam, K.; Ramakrishna Matte, H.; Maitra, U.; Moses, K.; Govindaraj, A. Graphene: Synthesis, functionalization and properties. Int. J. Mod. Phys. B 2011, 25, 4107–4143. [Google Scholar] [CrossRef]
- Titirici, M.M.; Thomas, A.; Yu, S.-H.; Müller, J.-O.; Antonietti, M. A direct synthesis of mesoporous carbons with bicontinuous pore morphology from crude plant material by hydrothermal carbonization. Chem. Mat. 2007, 19, 4205–4212. [Google Scholar] [CrossRef]
- Servant, A.; Jacobs, I.; Bussy, C.; Fabbro, C.; Da Ros, T.; Pach, E.; Ballesteros, B.; Prato, M.; Nicolay, K.; Kostarelos, K. Gadolinium-functionalised multi-walled carbon nanotubes as a T1 contrast agent for MRI cell labelling and tracking. Carbon 2016, 97, 126–133. [Google Scholar] [CrossRef]
- Liu, F.; Wu, J.; Chen, K.; Xue, D. Morphology study by using scanning electron microscopy. Microsc. Sci.Technol. Appl. Educ. 2010, 3, 1781–1792. [Google Scholar]
- Wang, Z.G.; Liu, K.G.; Jing, M.; Pang, B. SEM analysis of non-conducting materials under low vacuum conditions. Adv. Mater. Res. 2011, 152, 897–901. [Google Scholar] [CrossRef]
- Hanifah, M.F.R.; Jaafar, J.; Othman, M.; Ismail, A.; Rahman, M.A.; Yusof, N.; Salleh, W.; Aziz, F. Facile synthesis of highly favorable graphene oxide: Effect of oxidation degree on the structural, morphological, thermal and electrochemical properties. Materialia 2019, 6, 100344. [Google Scholar] [CrossRef]
- Chambers, S.A. Elastic scattering and interference of backscattered primary, Auger and X-ray photoelectrons at high kinetic energy: Principles and applications. Surf. Sci. Rep. 1992, 16, 261–331. [Google Scholar] [CrossRef]
- Beny-Bassez, C.; Rouzaud, J. Characterization of carbonaceous materials by correlated electron and optical microscopy and Raman microspectroscopy. Scanning Electron Microsc. 1985, 1, 119–132. [Google Scholar]
- Crelling, J.; Bensley, D. Characterization of Carbon Materials Using Quantitative Optical Microscopy. Preprints of Papers, American Chemical Society, Division of Fuel Chemistry; 1995. Available online: https://www.osti.gov/biblio/420577 (accessed on 29 October 2024). [CrossRef]
- Peng, X.; Barber, Z.; Clyne, T. Surface roughness of diamond-like carbon films prepared using various techniques. Surf. Coat. Technol. 2001, 138, 23–32. [Google Scholar] [CrossRef]
- Usachov, D.; Dobrotvorskii, A.; Varykhalov, A.; Rader, O.; Gudat, W.; Shikin, A.; Adamchuk, V. Experimental and theoretical study of the morphology of commensurate and incommensurate graphene layers on Ni single-crystal surfaces. Phys. Rev. B 2008, 78, 085403. [Google Scholar] [CrossRef]
- Robertson, A.W.; Warner, J.H. Atomic resolution imaging of graphene by transmission electron microscopy. Nanoscale 2013, 5, 4079–4093. [Google Scholar] [CrossRef]
- Trudeau, M.; Laul, D.; Veillette, R.; Serventi, A.; Mauger, A.; Julien, C.; Zaghib, K. In situ high-resolution transmission electron microscopy synthesis observation of nanostructured carbon coated LiFePO4. J. Power Sources 2011, 196, 7383–7394. [Google Scholar] [CrossRef]
- Menzel, R.; Bismarck, A.; Shaffer, M.S. Deconvolution of the structural and chemical surface properties of carbon nanotubes by inverse gas chromatography. Carbon 2012, 50, 3416–3421. [Google Scholar] [CrossRef]
- Legras, A.; Kondor, A.; Alcock, M.; Heitzmann, M.; Truss, R. Inverse gas chromatography for natural fibre characterisation: Dispersive and acid-base distribution profiles of the surface energy. Cellulose 2017, 24, 4691–4700. [Google Scholar] [CrossRef]
- Belgacem, M.N.; Gandini, A. Inverse gas chromatography as a tool to characterize dispersive and acid-base properties of the surface of fibers and powders. In Interfacial Phenomena In Chromatography; Surfactant Science Series; CRC Press: New York, NY, USA, 1999; pp. 41–124. [Google Scholar]
- Farivar, F.; Yap, P.L.; Karunagaran, R.U.; Losic, D. Thermogravimetric analysis (TGA) of graphene materials: Effect of particle size of graphene, graphene oxide and graphite on thermal parameters. C 2021, 7, 41. [Google Scholar] [CrossRef]
- WHO. Ambient Air Pollution: A Global Assessment of Exposure and Burden of Disease; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Wassersug, R.J.; Fox, I.N. The molecule that makes prostate cancer easy to find shows why it will be so difficult to cure. J. Men’s Health 2021, 17, 1–3. [Google Scholar]
- Upadhyay, A. Cancer: An unknown territory; rethinking before going ahead. Genes. Dis. 2021, 8, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.S.; Lasley, F.D.; Das, I.J.; Mendonca, M.S.; Dynlacht, J.R.; Chang, D.S.; Lasley, F.D.; Das, I.J.; Mendonca, M.S.; Dynlacht, J.R. Hyperthermia. In Basic Radiotherapy Physics and Biology; Springer: Berlin/Heidelberg, Germany, 2021; pp. 331–336. [Google Scholar]
- Szwed, M.; Marczak, A. Application of Nanoparticles for Magnetic Hyperthermia for Cancer Treatment—The Current State of Knowledge. Cancers 2024, 16, 1156. [Google Scholar] [CrossRef]
- Suleman, M.; Riaz, S.; Jalil, R. A mathematical modeling approach toward magnetic fluid hyperthermia of cancer and unfolding heating mechanism. J. Therm. Anal. Calorim. 2021, 146, 1193–1219. [Google Scholar] [CrossRef]
- Osaci, M.; Cacciola, M. Influence of the magnetic nanoparticle coating on the magnetic relaxation time. Beilstein J. Nanotechnol. 2020, 11, 1207–1216. [Google Scholar] [CrossRef]
- Anand, M. Magnetic relaxation in two-dimensional assembly of dipolar interacting nanoparticles. J. Magn. Magn. Mater. 2022, 552, 169201. [Google Scholar] [CrossRef]
- Barrera, C.A.; Francavilla, M.L.; Serai, S.D.; Edgar, J.C.; Jaimes, C.; Gee, M.S.; Roberts, T.P.; Otero, H.J.; Adzick, N.S.; Victoria, T. Specific absorption rate and specific energy dose: Comparison of 1.5-T versus 3.0-T fetal MRI. Radiology 2020, 295, 664–674. [Google Scholar] [CrossRef]
- Das, P.; Salvioni, L.; Malatesta, M.; Vurro, F.; Mannucci, S.; Gerosa, M.; Rizzuto, M.A.; Tullio, C.; Degrassi, A.; Colombo, M. Colloidal polymer-coated Zn-doped iron oxide nanoparticles with high relaxivity and specific absorption rate for efficient magnetic resonance imaging and magnetic hyperthermia. J. Colloid Interface Sci. 2020, 579, 186–194. [Google Scholar] [CrossRef]
- Huang, P.-C.; Chaney, E.J.; Aksamitiene, E.; Barkalifa, R.; Spillman Jr, D.R.; Bogan, B.J.; Boppart, S.A. Biomechanical sensing of in vivo magnetic nanoparticle hyperthermia-treated melanoma using magnetomotive optical coherence elastography. Theranostics 2021, 11, 5620. [Google Scholar] [CrossRef]
- Liu, F.; Wu, H.; Peng, B.; Zhang, S.; Ma, J.; Deng, G.; Zou, P.; Liu, J.; Chen, A.T.; Li, D. Vessel-targeting nanoclovers enable noninvasive delivery of magnetic hyperthermia–chemotherapy combination for brain cancer treatment. Nano Lett. 2021, 21, 8111–8118. [Google Scholar] [CrossRef] [PubMed]
- Le Guevelou, J.; Chirila, M.E.; Achard, V.; Guillemin, P.C.; Lorton, O.; Uiterwijk, J.W.; Dipasquale, G.; Salomir, R.; Zilli, T. Combined hyperthermia and radiotherapy for prostate cancer: A systematic review. Int. J. Hyperth. 2022, 39, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Hatamie, S.; Balasi, Z.M.; Ahadian, M.M.; Mortezazadeh, T.; Shams, F.; Hosseinzadeh, S. Hyperthermia of breast cancer tumor using graphene oxide-cobalt ferrite magnetic nanoparticles in mice. J. Drug Deliv. Sci. Technol. 2021, 65, 102680. [Google Scholar] [CrossRef]
- Moorthy, M.S.; Oh, Y.; Bharathiraja, S.; Manivasagan, P.; Rajarathinam, T.; Jang, B.; Phan, T.T.V.; Jang, H.; Oh, J. Synthesis of amine-polyglycidol functionalised Fe3O4@SiO2 nanocomposites for magnetic hyperthermia, pH-responsive drug delivery, and boimaging applications. RSC Adv. 2016, 6, 110444–110453. [Google Scholar] [CrossRef]
- Cole, A.J.; David, A.E.; Wang, J.; Galbán, C.J.; Hill, H.L.; Yang, V.C. Polyethylene glycol modified, cross-linked starch-coated iron oxide nanoparticles for enhanced magnetic tumor targeting. Biomaterials 2011, 32, 2183–2193. [Google Scholar] [CrossRef]
- Liu, X.L.; Fan, H.M.; Yi, J.B.; Yang, Y.; Choo, E.S.G.; Xue, J.M.; Di Fan, D.; Ding, J. Optimization of surface coating on Fe3O4 nanoparticles for high performance magnetic hyperthermia agents. J. Mater. Chem. 2012, 22, 8235–8244. [Google Scholar] [CrossRef]
- Khot, V.; Salunkhe, A.; Thorat, N.; Ningthoujam, R.; Pawar, S. Induction heating studies of dextran coated MgFe2O4 nanoparticles for magnetic hyperthermia. Dalton Trans. 2013, 42, 1249–1258. [Google Scholar] [CrossRef]
- Thorat, N.; Patil, R.; Khot, V.; Salunkhe, A.; Prasad, A.; Barick, K.; Ningthoujam, R.; Pawar, S. Highly water-dispersible surface-functionalized LSMO nanoparticles for magnetic fluid hyperthermia application. New J. Chem. 2013, 37, 2733–2742. [Google Scholar] [CrossRef]
- Thomas, L.A.; Dekker, L.; Kallumadil, M.; Southern, P.; Wilson, M.; Nair, S.P.; Pankhurst, Q.A.; Parkin, I.P. Carboxylic acid-stabilised iron oxide nanoparticles for use in magnetic hyperthermia. J. Mater. Chem. 2009, 19, 6529–6535. [Google Scholar] [CrossRef]
- Céspedes, E.; Byrne, J.M.; Farrow, N.; Moise, S.; Coker, V.S.; Bencsik, M.; Lloyd, J.R.; Telling, N.D. Bacterially synthesized ferrite nanoparticles for magnetic hyperthermia applications. Nanoscale 2014, 6, 12958–12970. [Google Scholar] [CrossRef]
- Nayak, K.S.; Lim, Y.; Campbell-Washburn, A.E.; Steeden, J. Real-time magnetic resonance imaging. J. Magn. Reson. Imaging 2022, 55, 81–99. [Google Scholar] [CrossRef] [PubMed]
- Serai, S.D. Basics of magnetic resonance imaging and quantitative parameters T1, T2, T2*, T1rho and diffusion-weighted imaging. Pediatr. Radiol. 2022, 52, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, X.; Zhao, Z.; Tang, S.; Huang, X.; Lin, C.; Cai, C.; Zheng, N. Synthesis of magnetic, fluorescent and mesoporous core-shell-structured nanoparticles for imaging, targeting and photodynamic therapy. J. Mater. Chem. 2011, 21, 11244–11252. [Google Scholar] [CrossRef]
- Fang, Y.; Zhou, L.; Zhao, J.; Zhang, Y.; Yang, M.; Yi, C. Facile synthesis of pH-responsive gadolinium (III)-doped carbon nanodots with red fluorescence and magnetic resonance properties for dual-readout logic gate operations. Carbon 2020, 166, 265–272. [Google Scholar] [CrossRef]
- Song, G.; Kenney, M.; Chen, Y.-S.; Zheng, X.; Deng, Y.; Chen, Z.; Wang, S.X.; Gambhir, S.S.; Dai, H.; Rao, J. Carbon-coated FeCo nanoparticles as sensitive magnetic-particle-imaging tracers with photothermal and magnetothermal properties. Nat. Biomed. Eng. 2020, 4, 325–334. [Google Scholar] [CrossRef]
- Karawdeniya, B.I.; Damry, A.M.; Murugappan, K.; Manjunath, S.; Bandara, Y.N.D.; Jackson, C.J.; Tricoli, A.; Neshev, D. Surface functionalization and texturing of optical metasurfaces for sensing applications. Chem. Rev. 2022, 122, 14990–15030. [Google Scholar] [CrossRef]
- Pan, C.; Wei, H.; Han, Z.; Wu, F.; Mao, L. Enzymatic electrochemical biosensors for in situ neurochemical measurement. Curr. Opin. Electrochem. 2020, 19, 162–167. [Google Scholar] [CrossRef]
- Huang, X.; Zhu, Y.; Kianfar, E. Nano biosensors: Properties, applications and electrochemical techniques. J. Mater. Res. Technol. 2021, 12, 1649–1672. [Google Scholar] [CrossRef]
- Bucur, B.; Purcarea, C.; Andreescu, S.; Vasilescu, A. Addressing the selectivity of enzyme biosensors: Solutions and perspectives. Sensors 2021, 21, 3038. [Google Scholar] [CrossRef]
- Liu, D.; Wang, J.; Wu, L.; Huang, Y.; Zhang, Y.; Zhu, M.; Wang, Y.; Zhu, Z.; Yang, C. Trends in miniaturized biosensors for point-of-care testing. TrAC 2020, 122, 115701. [Google Scholar] [CrossRef]
- Tu, J.; Torrente-Rodríguez, R.M.; Wang, M.; Gao, W. The era of digital health: A review of portable and wearable affinity biosensors. Adv. Funct. Mater. 2020, 30, 1906713. [Google Scholar] [CrossRef]
- Sun, B.; Gou, X.; Bai, R.; Abdelmoaty, A.A.A.; Ma, Y.; Zheng, X.; Hu, F. Direct electrochemistry and electrocatalysis of lobetyolin via magnetic functionalized reduced graphene oxide film fabricated electrochemical sensor. Mater. Sci. Eng. C 2017, 74, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wang, Y.; Peng, C.; Zhang, Z.; Chen, J.; Zhou, X.; Jiang, H. Enantiorecognition of tyrosine based on a novel magnetic electrochemical chiral sensor. Electrochim. Acta 2017, 241, 386–394. [Google Scholar] [CrossRef]
- Bagheri, H.; Khoshsafar, H.; Amidi, S.; Ardakani, Y.H. Fabrication of an electrochemical sensor based on magnetic multi-walled carbon nanotubes for the determination of ciprofloxacin. Anal. Methods 2016, 8, 3383–3390. [Google Scholar] [CrossRef]
- Jeong, S.; Park, J.; Pathania, D.; Castro, C.M.; Weissleder, R.; Lee, H. Integrated magneto–electrochemical sensor for exosome analysis. ACS Nano 2016, 10, 1802–1809. [Google Scholar] [CrossRef]
- Thakur, A.; Ganeshpurkar, A.; Jaiswal, A. Charcoal in dentistry. In Natural Oral Care in Dental Therapy; Scrivener Publishing LLC: Austin, TX, USA, 2020; pp. 197–209. [Google Scholar]
- Aziz, A.; Ayesha, A.; Sevianti, E.; Makalalag, G.V.; Noordiansyah, M.A.; Wicaksono, M.P.; Khairunnisa, S.; Yuniarsih, N. Effectiveness of Toothpaste from Activated Charcoal as Teeth Whitening: A Systematic Literature Review. Eureka Herba Indones. 2023, 4, 263–267. [Google Scholar] [CrossRef]
- Balasubramanian, P.; Selvam, S.M. Valorization of biomass to activated carbon for wound dressing applications: Recent trends and future challenges. Bioresour. Technol. 2023, 23, 101562. [Google Scholar] [CrossRef]
- Roberts, E.; Mason, S. Oral Care–A Mouthful of Chemistry; The Royal Society of Chemistry: London, UK, 2020. [Google Scholar]
- Rajendiran, M.; Trivedi, H.M.; Chen, D.; Gajendrareddy, P.; Chen, L. Recent development of active ingredients in mouthwashes and toothpastes for periodontal diseases. Molecules 2021, 26, 2001. [Google Scholar] [CrossRef]
- Huaman-Sarmiento, E.; Mayta-Tovalino, F.; Munive-Degregori, A.A.; Mendoza, R.; Barja-Ore, J.; Mauricio-Vilchez, C. Uses and applications of activated charcoal in the manufacture of toothpastes and oral rinses: A narrative review. J. Int. Oral Health 2023, 15, 237–241. [Google Scholar]
- Hamza, B.; Tanner, M.L.; Attin, T.; Wegehaupt, F.J. Dentin abrasivity and cleaning efficacy of novel/alternative toothpastes. Oral Health Prev. Dent. 2020, 18, 713–718. [Google Scholar]
- Heydari, Z.; Najimi, M.; Mirzaei, H.; Shpichka, A.; Ruoss, M.; Farzaneh, Z.; Montazeri, L.; Piryaei, A.; Timashev, P.; Gramignoli, R. Tissue engineering in liver regenerative medicine: Insights into novel translational technologies. Cells 2020, 9, 304. [Google Scholar] [CrossRef] [PubMed]
- Qasim, M.; Chae, D.S.; Lee, N.Y. Bioengineering strategies for bone and cartilage tissue regeneration using growth factors and stem cells. J. Biomed. Mater. Res. A 2020, 108, 394–411. [Google Scholar] [CrossRef] [PubMed]
- Zurina, I.M.; Presniakova, V.S.; Butnaru, D.V.; Svistunov, A.A.; Timashev, P.S.; Rochev, Y.A. Tissue engineering using a combined cell sheet technology and scaffolding approach. Acta Biomater. 2020, 113, 63–83. [Google Scholar] [CrossRef] [PubMed]
- Serafin, A.; Murphy, C.; Rubio, M.C.; Collins, M.N. Printable alginate/gelatin hydrogel reinforced with carbon nanofibers as electrically conductive scaffolds for tissue engineering. Mater. Sci. Eng. C 2021, 122, 111927. [Google Scholar] [CrossRef] [PubMed]
- Samadian, H.; Mobasheri, H.; Azami, M.; Faridi-Majidi, R. Osteoconductive and electroactive carbon nanofibers/hydroxyapatite nanocomposite tailored for bone tissue engineering: In vitro and in vivo studies. Sci. Rep. 2020, 10, 14853. [Google Scholar] [CrossRef]
- Ghosh, P.; Peters, J. Impulsive differential equation model in methanol poisoning detoxification: Efficacy of activated charcoal antidote in combating methanol poisoning. J. Math. Chem. 2020, 58, 126–145. [Google Scholar] [CrossRef]
- Al-Jelaify, M.; AlHomidah, S. The individualized management approach for acute poisoning. Adv. Pharmacol. Pharm. Sci. 2021, 2021, 9926682. [Google Scholar] [CrossRef]
- Sharma, G.; Sharma, S.; Kumar, A.; Lai, C.W.; Naushad, M.; Shehnaz; Iqbal, J.; Stadler, F.J. Activated carbon as superadsorbent and sustainable material for diverse applications. Adsorpt. Sci. Technol. 2022, 2022, 4184809. [Google Scholar] [CrossRef]
- Vinarov, Z.; Abdallah, M.; Agundez, J.A.; Allegaert, K.; Basit, A.W.; Braeckmans, M.; Ceulemans, J.; Corsetti, M.; Griffin, B.T.; Grimm, M. Impact of gastrointestinal tract variability on oral drug absorption and pharmacokinetics: An UNGAP review. Eur. J. Pharm. Sci. 2021, 162, 105812. [Google Scholar] [CrossRef]
- Nnadozie, E.C.; Ajibade, P.A. Adsorption, kinetic and mechanistic studies of Pb (II) and Cr (VI) ions using APTES functionalized magnetic biochar. Microporous Mesoporous Mater. 2020, 309, 110573. [Google Scholar] [CrossRef]
- Skov, K.; Graudal, N.A.; Jürgens, G. The effect of activated charcoal on drug exposure following intravenous administration: A meta-analysis. Basic Clin. Pharmacol. Toxicol. 2021, 128, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wei, C.; Hop, C.E.; Wright, M.R.; Hu, M.; Lai, Y.; Khojasteh, S.C.; Humphreys, W.G. Intestinal excretion, intestinal recirculation, and renal tubule reabsorption are underappreciated mechanisms that drive the distribution and pharmacokinetic behavior of small molecule drugs. J. Med. Chem. 2021, 64, 7045–7059. [Google Scholar] [CrossRef] [PubMed]
- Lupaşcu, T.; Petuhov, O.; Ţîmbaliuc, N.; Cibotaru, S.; Rotaru, A. Adsorption capacity of vitamin B12 and creatinine on highly-mesoporous activated carbons obtained from lignocellulosic raw materials. Molecules 2020, 25, 3095. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.-Y.; Azmi, F.; Ng, S.-F. LL37 Microspheres Loaded on Activated Carbon-chitosan Hydrogel: Anti-bacterial and Anti-toxin Wound Dressing for Chronic Wound Infections. AAPS PharmSciTech 2024, 25, 110. [Google Scholar] [CrossRef]
- Brown, G.C.; Heneka, M.T. The endotoxin hypothesis of Alzheimer’s disease. Mol. Neurodegener. 2024, 19, 30. [Google Scholar] [CrossRef]
- Uberoi, A.; McCready-Vangi, A.; Grice, E.A. The wound microbiota: Microbial mechanisms of impaired wound healing and infection. Nat. Rev. Microbiol. 2024, 22, 507–521. [Google Scholar] [CrossRef]
- Rameli, N.; Lim, B.-Y.; Leong, P.-Y.; Lim, C.-C.; Ng, S.-F. Chitosan-reinforced nanocrystalline cellulose hydrogels containing activated carbon as antitoxin wound dressing. Macromol. Res. 2024, 32, 861–872. [Google Scholar]
- Liu, Y.; Xu, B.; Lu, M.; Li, S.; Guo, J.; Chen, F.; Xiong, X.; Yin, Z.; Liu, H.; Zhou, D. Ultrasmall Fe-doped carbon dots nanozymes for photoenhanced antibacterial therapy and wound healing. Bioact. Mater. 2022, 12, 246–256. [Google Scholar] [CrossRef]
- Shakiba, M.; Jahangiri, P.; Rahmani, E.; Hosseini, S.M.; Bigham, A.; Foroozandeh, A.; Tajiki, A.; Pourmadadi, M.; Nasiri, S.; Jouybar, S. Drug-loaded carbon nanotube incorporated in nanofibers: A multifunctional nanocomposite for smart chronic wound healing. ACS Appl. Polym. Mater. 2023, 5, 5662–5675. [Google Scholar] [CrossRef]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nnadozie, E.C.; Ogunwa, K.I.; Chukwuike, V.I.; Nnadozie, O.O.; Ehikhase, C. Synthesis and Characterization of Carbonaceous Materials for Medical Applications: A Comprehensive Review. BioMed 2024, 4, 464-492. https://doi.org/10.3390/biomed4040036
Nnadozie EC, Ogunwa KI, Chukwuike VI, Nnadozie OO, Ehikhase C. Synthesis and Characterization of Carbonaceous Materials for Medical Applications: A Comprehensive Review. BioMed. 2024; 4(4):464-492. https://doi.org/10.3390/biomed4040036
Chicago/Turabian StyleNnadozie, Ebenezer C., Kennedy I. Ogunwa, Vitalis I. Chukwuike, Onyinyechukwu O. Nnadozie, and Charles Ehikhase. 2024. "Synthesis and Characterization of Carbonaceous Materials for Medical Applications: A Comprehensive Review" BioMed 4, no. 4: 464-492. https://doi.org/10.3390/biomed4040036
APA StyleNnadozie, E. C., Ogunwa, K. I., Chukwuike, V. I., Nnadozie, O. O., & Ehikhase, C. (2024). Synthesis and Characterization of Carbonaceous Materials for Medical Applications: A Comprehensive Review. BioMed, 4(4), 464-492. https://doi.org/10.3390/biomed4040036







