Abstract
Cardiovascular disease remains the main cause of mortality in the 21st century. Hypertension, vessel atherosclerosis, and familial hypercholesterolemia (FH) are responsible for increased mortality and morbidity in patients. Therapies for cardiovascular disease are based on drug treatment options, but in the era of precision medicine, personalized treatments are being developed. Studies have shown that these conditions have a strong genetic background, creating an opportunity for the implementation of gene therapy for these diseases. Currently, gene therapy is not widely used in clinical practice. Recent advances in this research field are making gene therapy a very promising preventive and therapeutic tool for cardiovascular disease. Essential hypertension’s (EH) pathophysiology is mostly based on the activation of both the sympathetic nervous system and the renin angiotensin aldosterone system (RAAS), natriuretic peptide production, and endothelial dysfunction. Plasmid DNA and viral vectors can be used, targeting the main mechanisms in the pathogenesis of EH. Many preclinical studies have been developed across the years, presenting a significant decrease in blood pressure. Nevertheless, no clinical studies have been developed studying the implementation of gene therapy in EH. Atherosclerotic damage is caused by monogenic diseases or is deteriorated by the activation of inflammation in the vessel wall. Gene therapy studies have been developed in the pre- and clinical phases targeting the lipoprotein and cholesterol metabolism and the inflammation of the vessels. FH is a common inherited metabolic disease associated with high levels of cholesterol in the blood. Clinical trials of gene therapy have been developed and presented optimistic results. In this review, the challenges of gene therapy for cardiovascular disease are outlined. Nevertheless, more clinical trials are needed to be performed for the development of convenient and safe drug schemes for our patients.
1. Introduction
Cardiovascular disease (CVD) remains the main cause of mortality worldwide [1]. More than one billion adults aged 19 to 79 have been diagnosed with hypertension, and a major number of individuals remain undiagnosed [2]. More than 40% of hypertensives are untreated, and that is associated with young age (age < 45 years), male sex, and obstacles related to access to healthcare [3]. Thus, hypertension could be a target for gene therapy because an increased percentage of hypertensives are non- or under-treated [4]. The progress in the field of gene therapy and the development of up-to-date vector systems are making hypertension a possible therapeutic target of gene therapy [5]. Evidence from clinical and pre-clinical studies has shown that hypertension disease has a strong genetic background [6].
More than 50% of deaths in contemporary Western societies are the result of vessel atherosclerosis [7]. Atherosclerosis is connected to an increased danger of cardiovascular incidents and increased morbidity and mortality [8]. Ischemic heart disease, stroke, peripheral artery disease, and chronic kidney disease (CKD) relate to atherosclerosis [9]. Atherosclerosis has a genetic background, making this disease a target for potential gene therapy [10]. Atherosclerosis is the main pathophysiological finding in familial hypercholesterolemia (FH), a mendelian inherited disease [11]. Gene therapy is already implemented in humans for FH and certain types of atherosclerosis damage, but not for hypertension. Studies in humans and other species have been developed and presented in this review [12].
Proteins, viruses, DNA particles, or RNA particles can be used as vectors for gene administration in gene therapy. Ex vivo gene therapy is implemented with patient’s cell extraction and genetic modification and then returned to the patients. Adeno-associated viruses (AAVs) and liposomes have been extensively utilized for gene administration at in in vivo gene therapy [13]. In the era of precision medicine, gene therapeutics can be crucial for the management of patients with CVD.
2. Materials and Methods
The main objective of this review was to present the current literature on gene therapy for CVD, especially in essential hypertension (EH), vessel atherosclerosis, and FH. A search of the literature in PubMed, Google Scholar, and Scopus was conducted to assemble the current and past studies on the topic. The search process involved utilizing combinations of MeSH terms. The inclusion criteria for the present review were peer-reviewed articles, systematic reviews, randomized controlled trials (RCTs), clinical trials (CTs), and research articles in humans and other species.
3. Gene Therapy for EH
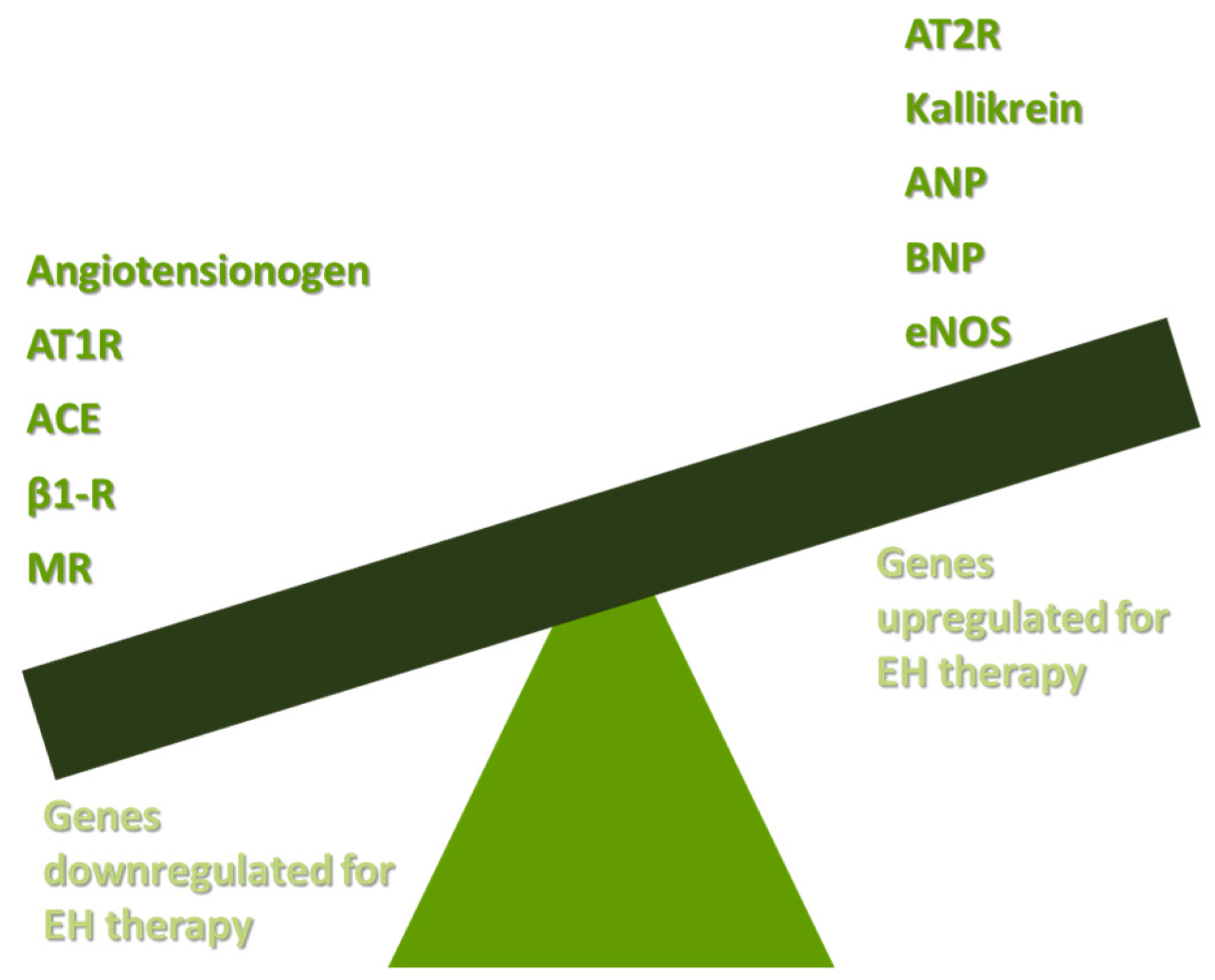
The pathophysiology of EH is based both on increased peripheral resistance of vessels and on increased cardiac output [14]. Studies in humans and other species have shown that functional vasoconstriction and specific defects in vascular smooth muscle walls are responsible for the total increase in peripheral resistance [15]. The increase in total cardiac output is a result of shortages in renal sodium homeostasis [16]. Potential targets for EH gene therapy are the renin angiotensin aldosterone system (RAAS), the B-1-adrenergic receptors (β1-Rs), the endothelial dysfunction mediated by nitric oxide (NO) decrease, and other genes responsible for vasoconstriction or vasodilation. The main axis of gene therapy in EH is presented in Figure 1.
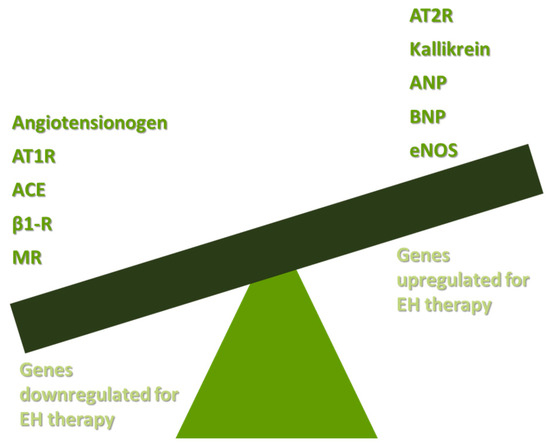
Figure 1.
Basic targets of gene therapy in EH. AT1R: angiotensin II type receptor; ACE: angiotensin-converting enzyme; β1-R: B-1-adrenergic receptor; MR: mineralocorticoid receptor; AT2R: angiotensin II type 2 receptor; ANP: atrial natriuretic peptide; BNP: brain natriuretic peptide; eNOS: endothelial nitric oxide synthase; and EH: essential hypertension.
3.1. Targeting the RAAS
Activation of RAAS is one of the main mechanisms contributing to the pathophysiology of EH [17]. Angiotensinogen is produced from the liver and is converted to angiotensin I (AngI) under the influence of renin, a hormone produced from the kidney’s juxtaglomerular cells (JGCs) [18]. Polymorphisms of angiotensinogen genes in humans or other populations (variation between hypertensive and non-hypertensive individuals) have been detected, showing that these genetic alterations are contributing to the pathogenesis of EH [19,20,21,22]. Thus, angiotensinogen genes can be used as a therapeutic target for EH using gene therapy. The angiotensin-converting enzyme (ACE) of renal and pulmonary endothelium converts AngI to Angiotensin II (AngII). AngII has many cellular actions via two receptors, the angiotensin I receptor (AT1R) and the angiotensin II receptor (AT2R) [23]. The linkage of AngII with AT1R results in constriction, inflammation, and fibrosis of peripheral vessel walls, while the linkage with AT2R vasodilates and promotes anti-fibrotic and anti-inflammatory actions [24]. The genetic pathways for the production of AT1R and AT2R have been studied as targets for genetic therapy for EH [25]. Thus, downregulation of AT1R using different modes of gene therapy can contribute to lower grades of blood pressure (BP) in patients with EH, while activation of AT2R promotes vasodilation and reduces inflammation [26,27]. In the adrenal glands, especially the zona glomerulosa, a high expression of AT1R has been reported. The connection of AngII to AT1R in adrenals results in aldosterone production [28]. The linkage of aldosterone to MR is responsible for aldosterone’s cellular actions [29].
Gyurko et al. were one of the first research groups to use the method of AS-ODNs targeting the AT1R and angiotensinogen messenger ribonucleic acid (mRNA) in spontaneously hypertensive rats (SHRs). The antisense synthetic oligodeoxynucleotides (AS-ODNs) contributed to lower BP levels (p < 0.005) and lower levels of AngII [30]. One year later, Philips et al. presented the use of AS-ODNs targeting 5′ coding areas of angiotensinogen mRNA and of ATR1 in vivo. Their study showed that AS-ODNs contributed to decreased levels of AngII, reduced expression of AT1R, and lower levels of BP (p < 0.01) [31]. Wielbo et al. conducted a study in SHRs that showed that direct brain administration of AS-ODNs is an effective way to decrease BP compared to intravenous administration [32]. Studies have been developed using liposomes for the administration of angiotensinogen mRNA’s AS-ODNS via the portal vein of SHRs, presenting a transient decrease in BP (one to four days) and accordingly lower mRNA levels of angiotensinogen and AngII [33,34]. Retroviruses have been used as vectors for AS-ODN gene therapy. One such study was conducted by Lu et al., showing successful downregulation of AT1-R in astroglia and neuronal cells [35]. Constant suspension of angiotensinogen mRNA synthesis can be promoted by AS-ODNs against the precursor hormone angiotensinogen gene (AOGEN) in SHRs [36]. Other studies have been developed showing that AS-ODNs targeting AT1R can contribute to lower AP in SHR [37,38,39,40]. Makino et al. reported that the administration of AS-ODNs is more effective in the downregulation of RAAS compared to sense-ODNs [41].
For the first time, Lu et al. used adenoviruses as vectors for AS-ODN complementary deoxyribonucleic acids (cDNAs) targeting the downregulation of the AT1R gene, proposing an innovative solution route for EH’s gene therapy [42]. Tang and colleagues administered AS-ODNs by AAVs. Their study showed that the greater the dose of AS-ODNs, the greater the reduction in BP (22.5 +/− 5.2 mmHg) in rats compared with the control (p < 0.01) [43]. Pachori et al., using retrovirus as a vector, developed a prevention method for hypertension in healthy rats using AS-ODNs against AT1R [44]. Kimura et al. developed a study in rats and utilized AAV vectors for AS-ODNs against angiotensinogen synthesis. The application of this technique contributed to lower levels of angiotensinogen (p < 0.01), systolic blood pressure (SBP) (up to 23 mmHg), late onset of hypertension, and decreased left ventricular hypertrophy (LVH) (p = 0.01) compared to controls [45]. A study by Katovich et al. noted that AS-ODNs targeting RAAS are an effective method for the treatment of insulin-resistant hypertension [46]. AT1R downregulation decreases cardiac hypertrophy, as shown by Pachori et al. [47].
Reaves et al. conducted a study causing endothelial injury in rats, contributing to hypertension onset. Developing AS-ODNs against AT1R, this study noted that gene therapy decreases AP despite the endothelial injury [48]. Wang et al., using AAV vectors for shRNA interference, targeted mineralocorticoid receptor (MR) downregulation. The downregulation of the MR contributed to lower levels of BP compared to controls (p < 0.01). Fan mutation is a genetic deficiency in PVN leading to lower levels of AT1R. Artificial microribonucleic acid (amiRNA) induction in AAV vectors has been developed for the administration of the Fan gene in PVN, resulting in upregulation of AT1R and a decrease in BP compared to controls (p < 0.05) [49]. AAVs have been successfully used for siRNA gene therapy for AT1R downregulation in the paraventricular nucleus (PVN) [50]. Repkova et al. presented that a reduction of 20–30 mmHg in SBP can be implemented using titanium dioxide (TiO2)-based nanocomposites with parts of ACE mRNA [51]. Sun et al. observed that CRISPR/Cas9 (a deletion in the angiotensinogen gene) is responsible for lower BP in SHR. With the administration of CRISPR/Cas9 with the AAV vector, BP remained at lower levels compared to controls (p < 0.001) one year after administration [52]. CACNA1D gene polymorphism is associated with hypertension in white adults and increased levels of renin, making it a potential target for gene therapy in humans [53].
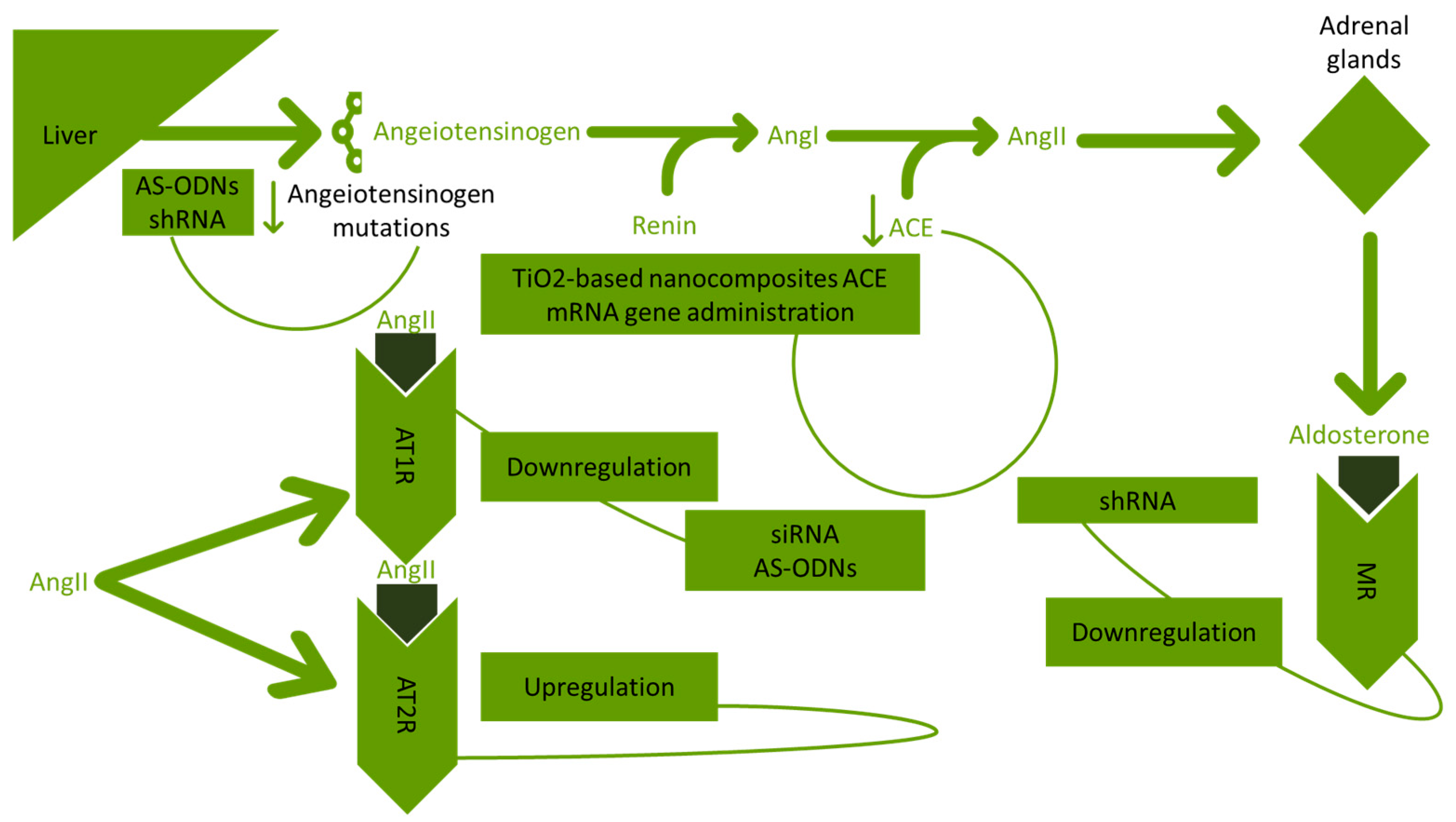
The upregulation of the AT2R gene with AAV vectors is contributing to the reduction of neuronal effects generated from RAAS [54]. In Figure 2, the gene therapy approaches for RAAS in hypertension are presented.
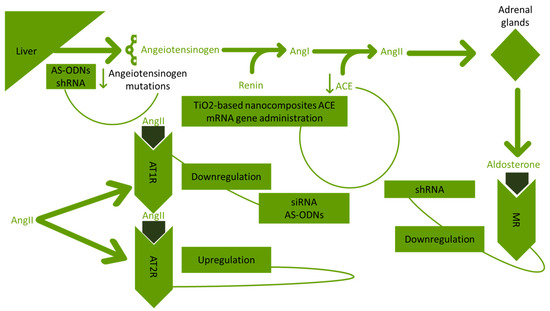
Figure 2.
Approaches developed targeting RAAS in hypertension. RAAS: renin angiotensin aldosterone system; AS-ODNs: antisense synthetic oligodeoxynucleotides; shRNA: shRNA short hairpin ribonucleic acid; AngI: angiotensin I; AngII: angiotensin II; ACE: angiotensin-converting enzyme; TiO2: titanium dioxide; mRNA: messenger ribonucleic acid; AT1R: angiotensin I receptor; AT2R: angiotensin II receptor; siRNA: small interfering ribonucleic acid; and MR: mineralocorticoid receptor.
3.2. Targeting the β1-Rs
β1-Rs are mostly found in the heart, kidney, and fat tissue cells. β1-Rs are connected with G-protein-coupled receptors [55]. Zhang et al. showed that AS-ODNs against β1-R mRNA can be used as an agent for the downregulation of the receptor. The above study reported a reduction in BP (38 ± 5 mmHg) compared to control rats [56]. Liang et al. presented a reduction up to 37 mmHg in hypertensive rats using AS-ODNs against β1-R [57]. Data reported that the downstream of β1-R using AAV vector gene therapy and shRNA is resulting in a reduction of SBP compared to controls in SHRs (p < 0.001) [58].
3.3. Targeting Kallikrein
Plasma and tissue kallikrein represent a potential antihypertensive gene therapy target. Kallikrein is a hormone that is responsible for vasodilation at the cellular level [59]. Wang et al. successfully developed somatic gene therapy using direct DNA administration of the kallikrein gene to control BP in SHR [60]. Yayama et al. used AAV vectors to deliver the human kallikrein gene to hypertensive rats. The kallikrein gene administration resulted in a significant reduction in BP compared to controls (160 ± 5 vs. 186 ± 7, n = 6, p < 0.01) [61]. Intramuscular injection of the kallikrein gene is an effective way of lowering BP in rats [62]. Using AAV as a vector, Dobrynski et al. administered the kallikrein gene to deoxycorticosterone acetate-salt hypertensive rats (DOCA-HRs), resulting in a lower level of BP compared to controls (172 ± 5 vs. 222 ± 13 mm Hg, n = 6, p < 0.01) [63]. The study of Zhao et al. in an insulin-resistant model of hypertension noted that the intravenous administration of the kallikrein gene’s cDNA contributes to lower SBP rates and lower levels of AT1R [64]. Wang et al. conducted a study in SHR, presenting a reduction in BP using human kallikrein cDNA [65]. In SHRs, recombinant AAV vectors expressing human kallikrein can result in a mean decrease of 12.6 mmHg in BP [66]. The safety of AAV vectors in SHR was evaluated by Yan et al. [67].
Kallikrein therapeutics are not only restricted to the field of hypertension. Kallikrein inhibition has been further implemented in the therapy of hereditary angioedema (HAE), a disease caused by genetic alterations in the C1INH [68]. The BMN 311 HAE clinical trial noted that the gene therapy of C1INH administration can prevent the onset of HAE [69]. The IONIS-PKKRx is based on AS-ODNs inhibition of mutations observed in the HAE. The study presented lower levels of kallikrein and a potential therapy for HAE [70]. The NTLA-2002 clinical trial presented the implications of CRISPR/CAS9 KLKB1 gene therapy. A dose-dependent decrease in kallikrein levels was observed in the patients who received gene therapy [71]. Kallikrein administration has been implemented in the therapy of acute ischemic stroke [72,73].
3.4. Targeting Nitric Oxide Synthetase (NOS)
NOS is an enzyme that contributes to NO synthesis in endothelial and smooth muscle cells [74]. NO is responsible for the vasodilation of peripheral vessel walls [75]. The pathophysiology of EH is partially based on a decrease in NO, leading to endothelial dysfunction [76]. Chao et al. showed that direct endothelial nitric oxide synthetase (eNOS) gene administration is an effective way to decrease BP compared to viral vectors in salt-sensitive rats [77]. Miller et al. in SHR using AAV vectors administered the eNOS gene to SHRs. The administration of the eNOS gene resulted in decreased BP compared to controls (p = 0.007) [78]. eNOS AAV vector gene therapy is effective for the prevention of renovascular hypertension (eNOSAAV rats 121 + 3 vs. control 96 + 2 mm Hg, p < 0.01) [79]. Zhao et al. noted that eNOS cDNA gene therapy is effective in reducing BP in fructose-fed rats [80]. The studies on NOS gene therapy are presented in Table 1.

Table 1.
Studies and outcomes of NOS gene targeting in hypertension.
3.5. Targeting Atrial Natriuretic Peptide (ANP)
ANP is a hormone produced from cardiac atria, and its biological actions include the promotion of natriuresis and diuresis, resulting in a reduction in blood volume and BP [81]. Lin et al. showed that the administration of the ANP gene via an AAV vector offers a BP reduction in SHRs [82]. Skin gene therapy with the human ANP gene was developed by Therrien et al. in SHRs. In rats receiving gene therapy, an increase in BP was not observed [83].
3.6. Targeting Brain Natriuretic Peptide (BNP)
BNP is a hormone secreted from the atria and ventricles, and its biological functions include peripheral vasodilation, resulting in a decrease in BP [84]. Catalloti et al. developed AAV vectors for somatic gene therapy with the proBNP gene in rats. Their study showed that BNP gene administration improves LVH and, when used for a long-term period, is an efficient tool for SBP and diastolic blood pressure (DBP) reduction [85]. Tone et al. presented a mean BP reduction of 15 mmHg in SHRs using BNP-AAV-mediated gene therapy in SHRs [86].
3.7. Targeting Adrenomedullin (ADM)
ADM is a hormone that is characterized by anti-hypertensive actions [87]. A long-term reduction in BP was observed in the study of Chao et al., reporting that the use of CMV or RSV as vectors is effective for ADM gene transfer [88]. Zhang et al. showed that ADM cDNA using cytomegalovirus (CMV) as a vector is resulting in BP reduction [89].
3.8. Clinical Trials Developed Evaluating the Pharmacological Correspondence and Clinical Characteristics Based on the Personal Gene Polymorphisms
The NCT02524873 trial examined the genes involved in the pathogenesis of EH in adults. This study showed the interplay between gene polymorphisms and the correspondence to pharmacological therapy [90]. The MDR1 gene polymorphism does not decrease losartan concentration in plasma but offers a greater reduction in BP compared with the presence of the polymorphism [91]. The RCG trial conducted a gene analysis in order to evaluate the efficacy of the precision medicine approach (gene detection for drugs); nevertheless, this trial’s results have not been published yet [92].
4. Gene Therapy for Atherosclerosis
Lipid metabolism includes the intake of dietary lipids by the gastrointestinal tract. The intestine is associated with absorption and chylomicron formation. Chylomicrons are transmitted in chylomicron remnants in portal circulation and transferred to the liver. Apolipoprotein AI (ApoA-I) is a lipoprotein responsible for the cellular intake of cholesterol and is a structural part of high-density lipoprotein (HDL) cholesterol. Apolipoprotein A-I Milano (ApoA-IM) is a genetic variant of ApoA-I found in humans, and it has been associated with potential cardioprotective actions [93]. Very low-density lipoprotein (VLDL) is synthesized by the liver. VLDL particles release triglycerides (TGs) in circulation and then transmit them to VLDL remnants. Low-density lipoprotein (LDL) is the final particle of VLDL metabolism [94].
Atherosclerotic plaques are associated with a smaller artery diameter. Athero arteries are characterized by endothelial damage, smooth muscle cell hyperactivation, differentiation of macrophages, and increased inflammation in smooth muscle vessel cells. Impaired lipid metabolism is associated with increased atherosclerotic lesions in the vessel wall [9]. Distinct mechanisms associated with atherosclerosis can be used as a potential target in gene therapy.
4.1. Targeting Lipoprotein Metabolism
Pastzy et al. conducted a study using apolipoprotein E (ApoE)-deficient mice and showed that ApoA-I gene therapy increases the levels of HDL and ApoA-I, offering a potential cardioprotective action and promoting anti-atherogenic actions [95]. Soma’s et al. study was implemented on rabbits and showed that the administration of the recombinant ApoA-IM gene using liposomes offers an anti-atherogenic action and lowers the levels of inflammation in the smooth muscle cell wall [96]. In the study by Tangirila et al., the ApoA-I gene was successfully administered by AAV vectors compared to controls (only vector) (p < 0.001). This study noted that ApoA-I AAV administration elevates the levels of HDL compared to controls (189 ± 21 mg/dL vs. 123 ± 8 mg/dL, p < 0.02) [97]. The study by Ishiguro et al. in ApoE-deficient mice noted that human ApoA-I gene administration with a retrovirus has effective anti-atherogenic actions (95% less atherosclerotic damage compared to controls) [98]. Kitjama et al. aimed to determine the effect of ApoE gene delivery in ApoE genetically deficient mice using different types of AAV vectors (AAV2, AAV7, and AAV8). The combination of AAV2/AAV7 and AAV2/AAV8 fully prevented atherosclerosis [99].
In the study performed by Lebherz et al., ApoA-I AAV administration was more efficacious versus ApoA-IM AAV administration (32% vs. 24% reduction in atherosclerotic lesions) [100]. Evans and colleagues showed that intramuscular AAV ApoE administration is safe for gene therapy but does not increase apoE plasma levels (remains < 15 ng/dL) [101]. Feng et al. conducted a study using ApoE transgenic mice using ApoA-I and ApoA-IM and showed that ApoA-IM is not superior in atherosclerosis progression [102]. Vaesen et al., using AAV8 vectors, noted that ApoA-I gene therapy is effective and offers an increase in levels of ApoA-I (634 + 69 mg/dL) and HDL in mice [103]. Koorneef and colleagues used shRNA ApoB knockdown and AAV as a vector and showed that this method of gene therapy can result in one single dose of a reduction in LDL cholesterol but stable levels of HDL [104]. Sharma et al. developed a study using mice with a human APOA5 mutation, a genetic alteration connected with higher levels of triglycerides. Researchers used AAV vectors to infuse the APOA-V gene (the normal action of APOA-V is to lower plasma triglycerides). This study noted a significant effect on plasma LDL [105].
Tian and colleagues used the APOA-IM gene and administered it to mice using AAV2 or AAV8 vectors. The rats that received the APOA-IM gene presented a significant reduction in atherosclerotic lesions of the aorta compared to controls (p < 0.01) [106]. Wacker et al. presented that APOI-A gene therapy in rabbits offers anti-atherogenic and anti-inflammatory benefits (reduction in vascular cell adhesion molecule-1 (VCAM-1) by 36%, p = 0.03) [107]. Bi et al. studied vein-graft atherosclerosis in fat-fed rabbits. Using AAV as a vector for APOA-I gene administration, this study showed a reduction in cholesterol compared to controls (p = 0.003) [108].
Gaudet et al., in their study, administered the lipoprotein lipase (LPL) gene using AAV vectors to 14 adults with LPL deficiency and a previous medical history of pancreatitis due to increased triglycerides. The study noted a more than 40% reduction in plasma triglycerides and increased levels of LPL (long-term effect) [109,110]. The NCT00068133 trial evaluated the impact of gene therapy (VLTS-589) in peripheral atherosclerotic disease by administering the gene of angiomatrix protein Del-1 in conjunction with poloxamer 188 with a plasmid vector. This study noted a significant clinical improvement in the patients [111]. Gene therapy for critical lower limb ischemia (caused by atherosclerotic CVD) has been studied in several studies, optimizing the results presented in most of them [112,113,114,115]. The NCT06112327 in up to 15-year follow-up presented a reduction in LDL-C in patients with atherosclerotic CVD [116].
4.2. Targeting Inflammation
Timp et al. conducted a study on ApoE gene-deficient mice and administered the tissue metallopeptidase inhibitor-1 (TIMP-1) gene using AAV vectors. The main outcome was the increase of collagen and elastin in the vessel wall and the decrease of inflammation (a reduction of macrophage cells) [117]. Qian and colleagues showed that gene therapy of neuronal nitric oxide synthase (nNos) with an AAV vector successfully lowers the monocytes on the vessel wall [118]. Thusen et al. proved that systematic administration of Intereukin-10 (IL-10) with AAV vectors is effective in atherosclerosis prevention [119]. Yoshioka reported that gene therapy of IL-10 successfully contributes to circulatory MCP-1 decrease and the suspension of Β-Hydroxy Β-Methylglutaryl-CoA (HMG-CoA) reductase, a molecule responsible for cholesterol synthesis [120]. Liu et al. and Namiki et al. reported suspension of atherogenesis using IL-10 gene therapy [121,122].
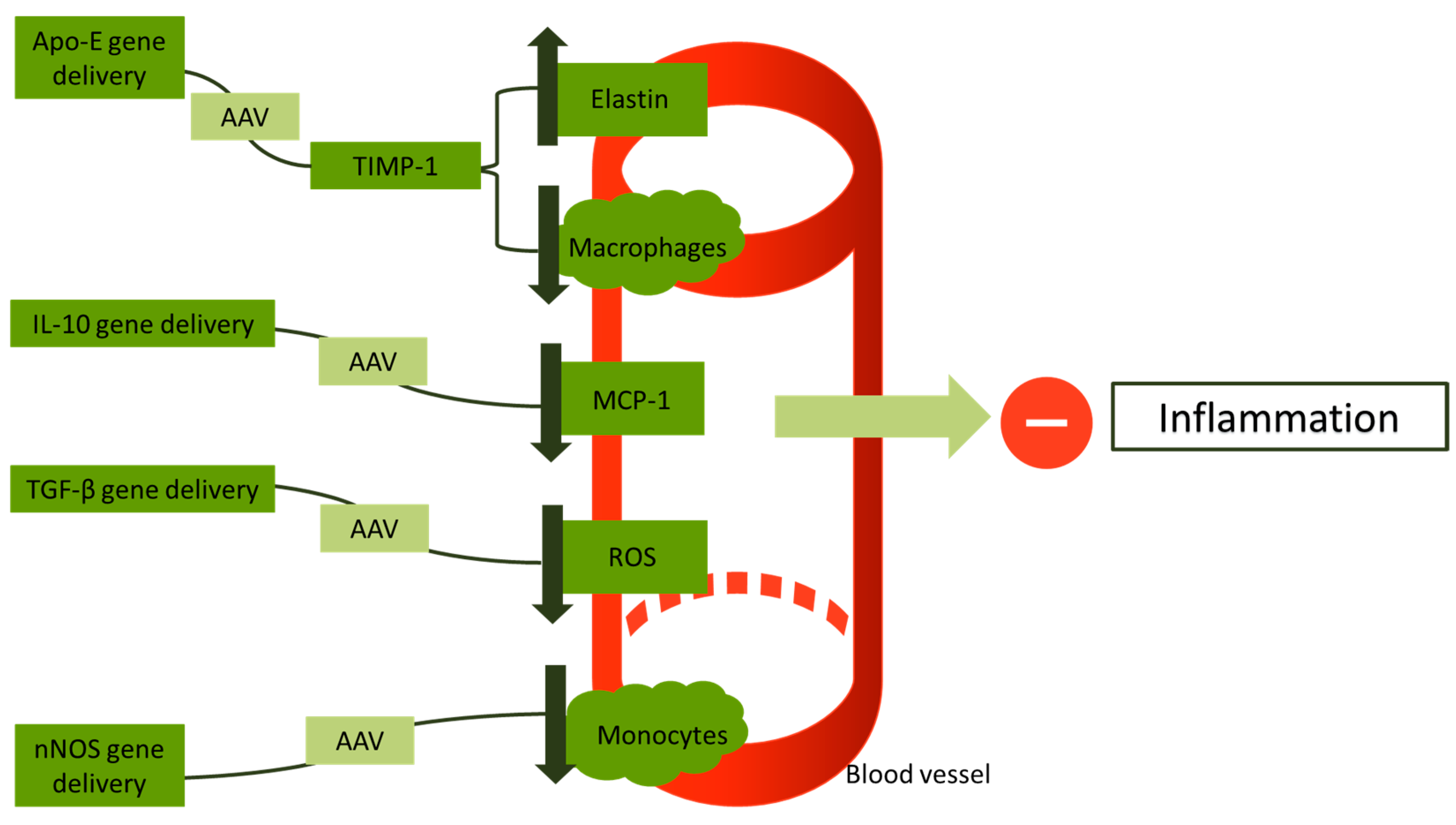
Li et al., for the first time in their study, used the transforming growth factor beta (TGF-β) gene using AAV vectors in low-density lipoprotein receptor (LDLR)-deficient mice. This study reports a reduction of reactive oxygen species (ROS) in vessel walls and a retraction of inflammation compared to controls (p < 0.05) [123]. Khan et al. conducted a study in which they used rats with a deficiency of LDLR and administered the human STAT3 gene via an AAV8 vector. This study reports a suspension of atherogenesis in rats who received gene therapy, showing that STAT3 has a potential cardioprotective action [124]. Cao and his colleagues implemented a combining gene therapy of IL-10/STAT-3, but their study did not offer a superior cardioprotective outcome compared to STAT-3 or IL-10 administration [125]. Vi et al. successfully showed that AAV vectors are an efficient vector for use in gene therapy for vein graft atherosclerosis [126]. Figure 3 gives a short illustration on the approaches that have been proposed as gene therapy for inflammation in atherosclerosis.
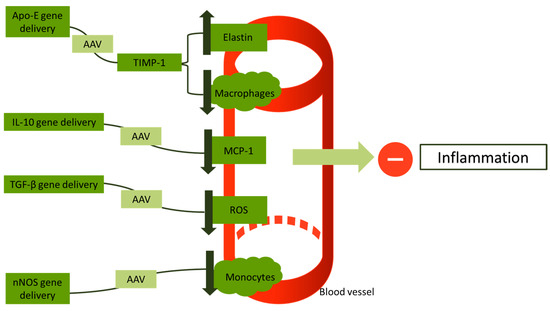
Figure 3.
An overview of gene therapies approaches targeting inflammation in atherosclerotic vessels. Apo-E: apolipoprotein E; AAV: adeno-associated virus; TIMP-1: tissue metallopeptidase inhibitor-1; IL-10: interleukin-10; TGF-β: transforming growth factor beta; ROS: reactive oxygen species; and nNOS: neuronal nitric oxide synthase.
5. Gene Therapy for FH
Heterozygous (He) FH is presented in one of 250 births with levels of LDL cholesterol above 190 mg/dL and onset between 30 and 60 years [127]. Homozygous (HoFH) is characterized by the presence of two mutated genes, onset in childhood, and levels of LDL cholesterol above 400 mg/dL. Mutations have been detected in LDLR (the most common) and in proprotein convertase subtilisin/kexin type 9 (PCSK9) and are inherited with an autosomal dominant type of inheritance [128]. A small percentage of FH clinical cases are inherited with an autosomal recessive type of inheritance.
5.1. Animal Models for FH Gene Therapy
Chowdbury et al. developed an animal rabbit model and used AAV vectors to transfer the LDLR gene. Animals receiving the therapy had a total reduction of 30–50% of plasma cholesterol [129]. Chen and colleagues using mice with Ho FH used AAV to administer the very low-density lipoprotein (VLDLR) gene. Their study noted a 40% reduction in plasma cholesterol and triglycerides [130]. Kankkoken et al., in their study of 24 rabbits with FH, noted that LDLR gene therapy is effective in LDL reduction (in 2 years, 34 ± 10%) [131]. Kasim et al., using AAV8 vector gene therapy, administered the LDLR gene to rats with Ho FH. Their study showed an important decrease in plasma cholesterol [132]. The study by Hibbit et al. shows that LDLR gene transfer is effective and increases the level of LDLR in vivo [133]. Kasim et al., in their studies, evaluated that AAV8 LDLR administration is very effective in LDL cholesterol reduction [134].
Somanthan and colleagues conducted a study using three different types of genes. The study notes an increase in LDLR, lower levels of LDL cholesterol, and improved resistance to PCKS9 [135]. Wang et al. used a new type of AAV vector in LDLR deficient mice. Their study noted a 98% reduction in LDL cholesterol in male mice, and the effects lasted more than 120 days [136]. Li et al. used exosomes as vectors for LDLR mRNA transfer in mice with Ho FH. A significant reduction in LDL cholesterol was observed compared to controls (p < 0.05) [137].
5.2. FH Gene Therapy Studies in Humans
Grossman and colleagues reported the first case of ex vivo gene therapy for Ho FH in a woman patient using a retrovirus for the administration of the LDLR gene [138]. A mean reduction of 150 mg/dL was implemented in the first study for FH gene therapy in 5 patients by Grossman et al. one year later from their first case report published [139]. Gaudet et al. successfully used AAV to administer the LPL gene to patients with LPL deficiency, a subtype of FH [110]. The NCT02651675 trial conducted both in phase one and phase two used AAV8 to administer the LDLR gene in nine patients with Ho FH, but the study did not have a clinically important effect on total lipids [140].
Exosome administration has been developed in the NCT05043181 trial. This study used exosomes for the administration of LDLR mRNA in 30 patients, resulting in lower levels of LDL cholesterol and triglycerides [141]. The NCT03400800 trial, with 1617 participants, presents siRNA as a potential mechanism for PCSK9 mutations in FH, offering a mean 50% reduction in LDL cholesterol [142]. The NCT03747224 trial used exosomes to administer ribonucleic acid interference (RNAi0) for PCSK9 genetic alterations, offering a 42% reduction in LDL cholesterol [143]. Three NCTs used the siRNA technique targeting Lp(a), offering reductions in Lp(a) and cardioprotective outcomes [144,145,146].
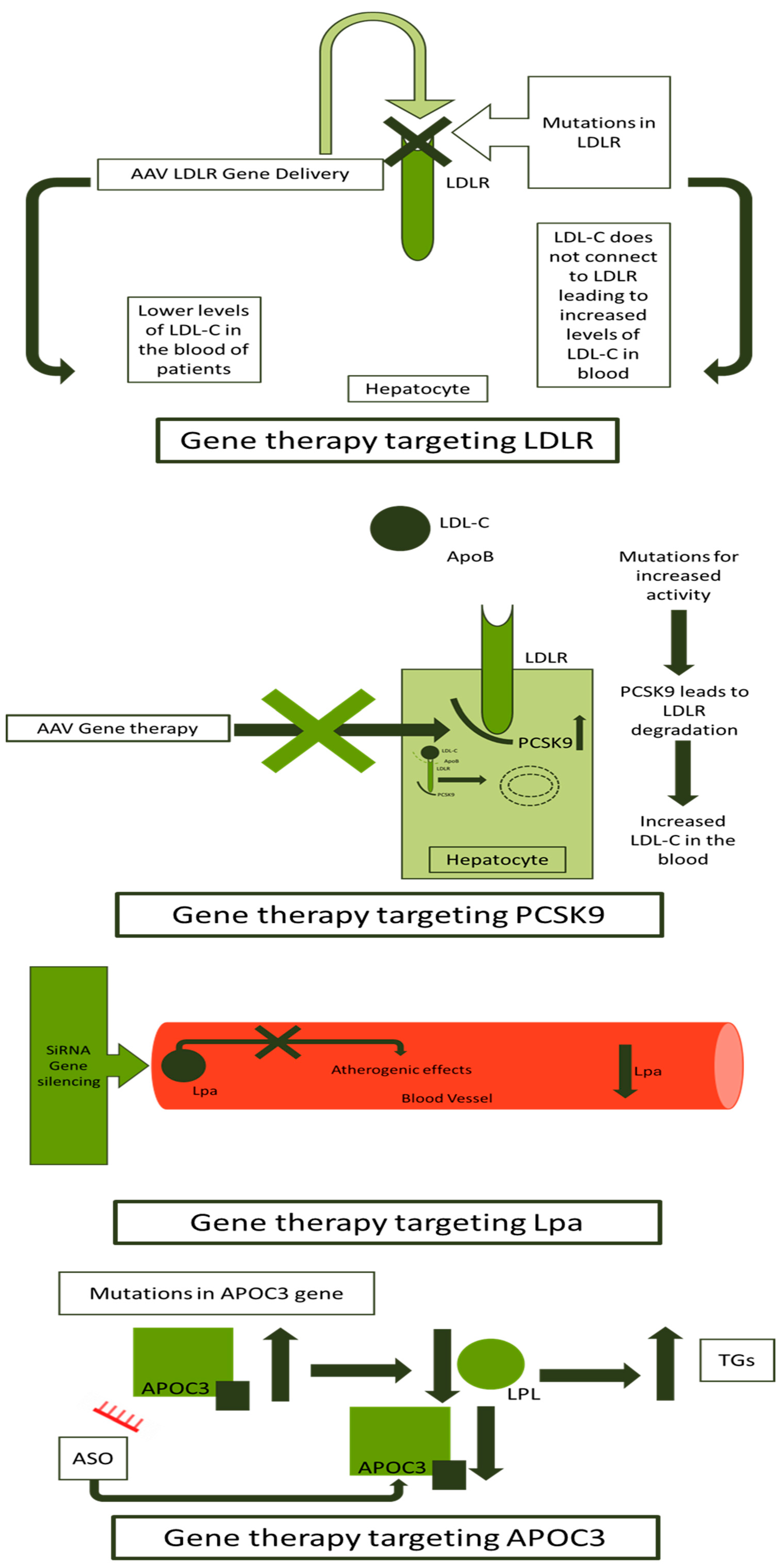
AS-ODNs have been used for FH gene therapy in different clinical trials. The NCT02900027 uses AS-ODNs to downregulate the APOC3 gene, offering a 73% mean reduction in APOC3 and 77% of triglycerides [147]. Two other gene therapies using AS-ODNs against the APOC3 gene presented a significant reduction in APOC3 and triglycerides [148,149]. A short summary of clinical trials developed for FH gene therapy is presented in Table 2. An overview of methods and targets for gene therapy in FH is presented in Figure 4.

Table 2.
Clinical trials of gene therapy in FH.
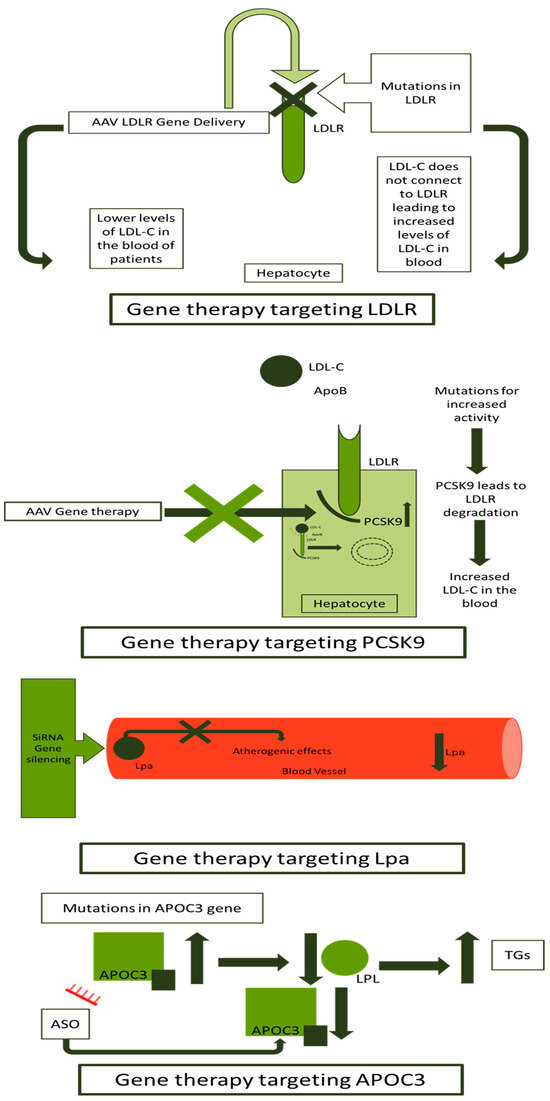
Figure 4.
Gene therapy approaches in FH. FH: familial hypercholesterolemia; LDLR: low-density lipoprotein receptor; LDL-C: low-density lipoprotein-cholesterol; ApoB: apolipoprotein B; AAV: adeno-associated virus; PCKS9: proprotein convertase subtilisin/kexin type 9; siRNA: small interfering ribonucleic acid; Lp(a): lipoprotein a; LPL: lipoprotein lipase; TGs: triglycerides; ASO: antisense synthetic oligodeoxynucleotide; and APOC3: apolipoprotein C3.
6. Limitations of the Clinical Implications of Gene Therapy
Since the first implication of gene therapy in 1990, many breakthrough advances have been made [151]. Several pharmaceutical products have already contributed to the therapy of multiple genetic conditions (e.g., cystic fibrosis, alpha-1 antitrypsin deficiency, hemophilia, beta thalassemia, and sickle cell disease) [152]. In the field of CVD research, many pre-clinical studies have been conducted, as presented in the current review. Nevertheless, a significant lack of clinical trials is observed, especially in the field of hypertension.
The viral vectors have been developed for gene administration, but toxicities have been referred to in the literature. Immune-mediated reactions and the development of neutralizing antibodies against AAVs are the two main implications of gene therapy administered with viral vectors [153]. A limitation in the use of AAVs is their small packaging capacity [154,155]. Medical research has come up with non-viral vectors, decreasing the danger of immune-mediated or inflammatory reactions [156]. Moreover, the increased prevalence of CVD and the significant cost of gene therapy are barriers to the massive use of gene therapy in the field of CVD. The complex pathophysiology of CVD and the non-fully known mechanisms leading to the disease onset remain obstacles to the wide use of gene therapy in clinical practice [12]. The CRISPR delivery system has been associated with numerous adverse events, such as off-target events (in more than 50% of cases referred to in some studies), DNA damage, and immunotoxicity [157].
7. Conclusions
EH can potentially be treated using gene therapy, but organized RCTs are needed in order to evaluate its safety and efficacy in human patients. The increased cost of gene therapy and the significant number of hypertensive individuals make current gene therapy techniques improbable for implementation. Models for atherosclerosis gene therapy have been developed, and RCTs have already been implemented, leading CVD therapies into a new era. Many clinical trials have been implemented in the field of FH, and promising data have been published. In this review, the challenges of gene therapy for cardiovascular disease are outlined.
Author Contributions
N.E. and P.E. contributed to writing and designing the manuscript, collecting or analyzing the data, and drafting and critically revising the paper regarding its important intellectual content. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| AAV | Adeno-associated virus |
| ACE | Angiotensin-converting enzyme |
| AdeNOS | Ad-encoding endothelial nitric oxide synthase |
| ADM | Adrenomedullin |
| AmiRNA | Artificial microribonucleic acid |
| AngI | Angiotensin I |
| AngII | Angiotensin II |
| ANP | Atrial natriuretic peptide |
| AOGEN | Precursor hormone angiotensinogen gene |
| AP | Arterial pressure |
| ApoA-I | Apolipoprotein AI |
| ApoA-IM | Apolipoprotein A-I Milano |
| APOA-V | Apolipoprotein A-V |
| ApoB | Apolipoprotein B |
| APOC3 | Apolipoprotein C3 |
| ApoE | Apolipoprotein E |
| AS-ODNs | Antisense synthetic oligodeoxynucleotides |
| AT1R | Angiotensin II type 1 receptor |
| AT2R | Angiotensin II type 2 receptor |
| BNP | Brain natriuretic peptide |
| Cdna | Complementary deoxyribonucleic acid |
| CMV | Cytomegalovirus |
| CVD | Cardiovascular disease |
| DBP | Diastolic blood pressure |
| DOCA-HR | Deoxycorticosterone acetate-salt hypertensive rats |
| EH | Essential hypertension |
| eNOS | Endothelial nitric oxide synthase |
| FH | Familial hypercholesterolemia |
| HDL | High-density lipoprotein |
| He | Heterozygous |
| HMG-CoA | Β-Hydroxy Β-Methylglutaryl-CoA |
| Ho | Homozygous |
| IL-10 | Intereukin-10 |
| JGCs | Juxtaglomerular cells |
| LDL | Low-Density lipoprotein |
| LDLR | Low-Density lipoprotein receptor |
| Lp(a) | Lipoprotein a |
| LPL | Lipoprotein lipase |
| LVH | Left ventricular hypertrophy |
| MESH | Medical subject headings |
| MR | Mineralocorticoid receptor |
| mRNA | Messenger ribonucleic acid |
| nNOS | Neuronal nitric oxide synthase |
| NO | Nitric oxide |
| NOS | Nitric oxide synthetase |
| PCKS9 | Proprotein convertase subtilisin/kexin type 9 |
| PVN | Paraventricular nucleus |
| RAAS | Renin–angiotensin–aldosterone system |
| RNAi | Ribonucleic acid interference |
| ROS | Reactive oxygen species |
| RSV | Rous sarcoma virus |
| SBP | Systolic blood pressure |
| SHR | Spontaneously hypertensive rats |
| shRNA | Short hairpin ribonucleic acid |
| siRNA | Small interfering ribonucleic acid |
| STAT3 | Signal transducer and activator of transcription 3 |
| TGF-β | Transforming growth factor beta |
| TGs | Triglycerides |
| TIMP-1 | Tissue metallopeptidase inhibitor- 1 |
| TiO2 | Titanium dioxide |
| VCAM-1 | Vascular cell adhesion molecule-1 |
| VLDL | Very low-density lipoprotein |
| β1-Rs | B-1-adrenergic receptors |
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Mills, K.T.; Stefanescu, A.; He, J. The Global Epidemiology of Hypertension. Nat. Rev. Nephrol. 2020, 16, 223–237. [Google Scholar] [CrossRef]
- Appleton, S.L.; Neo, C.; Hill, C.L.; Douglas, K.A.; Adams, R.J. Untreated Hypertension: Prevalence and Patient Factors and Beliefs Associated with under-Treatment in a Population Sample. J. Hum. Hypertens. 2013, 27, 453–462. [Google Scholar] [CrossRef]
- Paulis, L.; Franke, H.; Simko, F. Gene Therapy for Hypertension. Expert. Opin. Biol. Ther. 2017, 17, 1345–1361. [Google Scholar] [CrossRef]
- Phillips, M.I. Is Gene Therapy for Hypertension Possible? Hypertension 1999, 33, 8–13. [Google Scholar] [CrossRef][Green Version]
- Gluba, A.; Banach, M.; Mikhailidis, D.P.; Rysz, J. Genetic Determinants of Cardiovascular Disease: The Renin-Angiotensin-Aldosterone System, Paraoxonases, Endothelin-1, Nitric Oxide Synthase and Adrenergic Receptors. In Vivo 2009, 23, 797–812. [Google Scholar]
- Dichgans, M.; Pulit, S.L.; Rosand, J. Stroke Genetics: Discovery, Biology, and Clinical Applications. Lancet Neurol. 2019, 18, 587–599. [Google Scholar] [CrossRef]
- Pahwa, R.; Jialal, I. Atherosclerosis; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Jebari-Benslaiman, S.; Galicia-García, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martín, C. Pathophysiology of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef]
- Kovacic, S.; Bakran, M. Genetic Susceptibility to Atherosclerosis. Stroke Res. Treat. 2012, 2012, 362941. [Google Scholar] [CrossRef] [PubMed]
- Ison, H.E.; Clarke, S.L.; Knowles, J.W. Familial Hypercholesterolemia; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Cao, G.; Xuan, X.; Zhang, R.; Hu, J.; Dong, H. Gene Therapy for Cardiovascular Disease: Basic Research and Clinical Prospects. Front. Cardiovasc. Med. 2021, 8, 760140. [Google Scholar] [CrossRef] [PubMed]
- Scheller, E.L.; Krebsbach, P.H. Gene Therapy: Design and Prospects for Craniofacial Regeneration. J. Dent. Res. 2009, 88, 585–596. [Google Scholar] [CrossRef]
- Saxena, T.; Ali, A.O.; Saxena, M. Pathophysiology of Essential Hypertension: An Update. Expert. Rev. Cardiovasc. Ther. 2018, 16, 879–887. [Google Scholar] [CrossRef]
- Beevers, G.; Lip, G.Y.; O’Brien, E. ABC of Hypertension: The Pathophysiology of Hypertension. BMJ 2001, 322, 912–916. [Google Scholar] [CrossRef]
- Shin, J.; Lee, C.H. The Roles of Sodium and Volume Overload on Hypertension in Chronic Kidney Disease. Kidney Res. Clin. Pract. 2021, 40, 542–554. [Google Scholar] [CrossRef]
- Wu, C.-H.; Mohammadmoradi, S.; Chen, J.Z.; Sawada, H.; Daugherty, A.; Lu, H.S. Renin-Angiotensin System and Cardiovascular Functions. Arterioscler. Thromb. Vasc. Biol. 2018, 38, e108–e116. [Google Scholar] [CrossRef] [PubMed]
- Sequeira-Lopez, M.L.S.; Gomez, R.A. Renin Cells, the Kidney, and Hypertension. Circ. Res. 2021, 128, 887–907. [Google Scholar] [CrossRef] [PubMed]
- Benetos, A.; Gautier, S.; Ricard, S.; Topouchian, J.; Asmar, R.; Poirier, O.; Larosa, E.; Guize, L.; Safar, M.; Soubrier, F.; et al. Influence of Angiotensin-Converting Enzyme and Angiotensin II Type 1 Receptor Gene Polymorphisms on Aortic Stiffness in Normotensive and Hypertensive Patients. Circulation 1996, 94, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Caulfield, M.; Lavender, P.; Farrall, M.; Munroe, P.; Lawson, M.; Turner, P.; Clark, A.J. Linkage of the Angiotensinogen Gene to Essential Hypertension. N. Engl. J. Med. 1994, 330, 1629–1633. [Google Scholar] [CrossRef]
- Bloem, L.J.; Foroud, T.M.; Ambrosius, W.T.; Hanna, M.P.; Tewksbury, D.A.; Pratt, J.H. Association of the Angiotensinogen Gene to Serum Angiotensinogen in Blacks and Whites. Hypertension 1997, 29, 1078–1082. [Google Scholar] [CrossRef]
- Davisson, R.L.; Ding, Y.; Stec, D.E.; Catterall, J.F.; Sigmund, C.D. Novel Mechanism of Hypertension Revealed by Cell-Specific Targeting of Human Angiotensinogen in Transgenic Mice. Physiol. Genom. 1999, 1, 3–9. [Google Scholar] [CrossRef]
- Danigo, A.; Rovini, A.; Bessaguet, F.; Bouchenaki, H.; Bernard, A.; Sturtz, F.; Bourthoumieu, S.; Desmoulière, A.; Magy, L.; Demiot, C. The Angiotensin II Type 2 Receptor, a Target for Protection and Regeneration of the Peripheral Nervous System? Pharmaceuticals 2021, 14, 175. [Google Scholar] [CrossRef] [PubMed]
- Janc, J.; Suchański, M.; Mierzchała-Pasierb, M.; Woźnica-Niesobska, E.; Łysenko, L.; Leśnik, P. Does the Serum Concentration of Angiotensin II Type 1 Receptor Have an Effect on the Severity of COVID-19? A Prospective Preliminary Observational Study among Healthcare Professionals. J. Clin. Med. 2022, 11, 1769. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.I.; Kimura, B. Gene Therapy for Hypertension: Antisense Inhibition of the Renin-Angiotensin System. Methods Mol. Med. 2005, 108, 363–379. [Google Scholar] [CrossRef] [PubMed]
- St Paul, A.; Corbett, C.B.; Okune, R.; Autieri, M. V Angiotensin II, Hypercholesterolemia, and Vascular Smooth Muscle Cells: A Perfect Trio for Vascular Pathology. Int. J. Mol. Sci. 2020, 21, 4525. [Google Scholar] [CrossRef] [PubMed]
- Gaspari, T.A.; Vinh, A.; Jones, E.S.; Widdop, R.E. Ganging up on Angiotensin II Type 1 Receptors in Vascular Remodeling. Hypertension 2012, 60, 17–19. [Google Scholar] [CrossRef][Green Version]
- Morimoto, S.; Ichihara, A. Management of Primary Aldosteronism and Mineralocorticoid Receptor-Associated Hypertension. Hypertens. Res. 2020, 43, 744–753. [Google Scholar] [CrossRef]
- Fujii, W.; Shibata, S. Mineralocorticoid Receptor Antagonists for Preventing Chronic Kidney Disease Progression: Current Evidence and Future Challenges. Int. J. Mol. Sci. 2023, 24, 7719. [Google Scholar] [CrossRef] [PubMed]
- Gyurko, R.; Wielbo, D.; Phillips, M.I. Antisense Inhibition of AT1 Receptor MRNA and Angiotensinogen MRNA in the Brain of Spontaneously Hypertensive Rats Reduces Hypertension of Neurogenic Origin. Regul. Pept. 1993, 49, 167–174. [Google Scholar] [CrossRef]
- Phillips, M.I.; Wielbo, D.; Gyurko, R. Antisense Inhibition of Hypertension: A New Strategy for Renin-Angiotensin Candidate Genes. Kidney Int. 1994, 46, 1554–1556. [Google Scholar] [CrossRef]
- Wielbo, D.; Sernia, C.; Gyurko, R.; Phillips, M.I. Antisense Inhibition of Hypertension in the Spontaneously Hypertensive Rat. Hypertension 1995, 25, 314–319. [Google Scholar] [CrossRef]
- Wielbo, D.; Simon, A.; Phillips, M.I.; Toffolo, S. Inhibition of Hypertension by Peripheral Administration of Antisense Oligodeoxynucleotides. Hypertension 1996, 28, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Tomita, N.; Morishita, R.; Higaki, J.; Aoki, M.; Nakamura, Y.; Mikami, H.; Fukamizu, A.; Murakami, K.; Kaneda, Y.; Ogihara, T. Transient Decrease in High Blood Pressure by In Vivo Transfer of Antisense Oligodeoxynucleotides against Rat Angiotensinogen. Hypertension 1995, 26, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Yu, K.; Raizada, M.K. Retrovirus-Mediated Transfer of an Angiotensin Type I Receptor (AT1-R) Antisense Sequence Decreases AT1-Rs and Angiotensin II Action in Astroglial and Neuronal Cells in Primary Cultures from the Brain. Proc. Natl. Acad. Sci. USA 1995, 92, 1162–1166. [Google Scholar] [CrossRef] [PubMed]
- Schinke, M.; Böhm, M.; Bricca, G.; Ganten, D.; Bader, M. Permanent Inhibition of Angiotensinogen Synthesis by Antisense RNA Expression. Hypertension 1996, 27, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.I.; Mohuczy-Dominiak, D.; Coffey, M.; Galli, S.M.; Kimura, B.; Wu, P.; Zelles, T. Prolonged Reduction of High Blood Pressure with an In Vivo, Nonpathogenic, Adeno-Associated Viral Vector Delivery of AT1-R MRNA Antisense. Hypertension 1997, 29, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.I. Antisense Inhibition and Adeno-Associated Viral Vector Delivery for Reducing Hypertension. Hypertension 1997, 29, 177–187. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gyurko, R.; Tran, D.; Phillips, M.I. Time Course of Inhibition of Hypertension by Antisense Oligonucleotides Targeted to AT1 Angiotensin Receptor MRNA in Spontaneously Hypertensive Rats. Am. J. Hypertens. 1997, 10, 56S–62S. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.N.; Lu, D.; Katovich, M.J.; Raizada, M.K. Chronic Control of High Blood Pressure in the Spontaneously Hypertensive Rat by Delivery of Angiotensin Type 1 Receptor Antisense. Proc. Natl. Acad. Sci. USA 1996, 93, 9960–9965. [Google Scholar] [CrossRef] [PubMed]
- Makino, N.; Sugano, M.; Ohtsuka, S.; Sawada, S. Intravenous Injection with Antisense Oligodeoxynucleotides against Angiotensinogen Decreases Blood Pressure in Spontaneously Hypertensive Rats. Hypertension 1998, 31, 1166–1170. [Google Scholar] [CrossRef][Green Version]
- Lu, D.; Yang, H.; Raizada, M.K. Attenuation of ANG II Actions by Adenovirus Delivery of AT1 Receptor Antisense in Neurons and SMC. Am. J. Physiol. 1998, 274, H719–H727. [Google Scholar] [CrossRef]
- Tang, X.; Mohuczy, D.; Zhang, Y.C.; Kimura, B.; Galli, S.M.; Phillips, M.I. Intravenous Angiotensinogen Antisense in AAV-Based Vector Decreases Hypertension. Am. J. Physiol. 1999, 277, H2392–H2399. [Google Scholar] [CrossRef]
- Pachori, A.S.; Wang, H.; Gelband, C.H.; Ferrario, C.M.; Katovich, M.J.; Raizada, M.K. Inability to Induce Hypertension in Normotensive Rat Expressing AT(1) Receptor Antisense. Circ. Res. 2000, 86, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Kimura, B.; Mohuczy, D.; Tang, X.; Phillips, M.I. Attenuation of Hypertension and Heart Hypertrophy by Adeno-Associated Virus Delivering Angiotensinogen Antisense. Hypertension 2001, 37, 376–380. [Google Scholar] [CrossRef][Green Version]
- Katovich, M.J.; Reaves, P.Y.; Francis, S.C.; Pachori, A.S.; Wang, H.W.; Raizada, M.K. Gene Therapy Attenuates the Elevated Blood Pressure and Glucose Intolerance in an Insulin-Resistant Model of Hypertension. J. Hypertens. 2001, 19, 1553–1558. [Google Scholar] [CrossRef] [PubMed]
- Pachori, A.S.; Numan, M.T.; Ferrario, C.M.; Diz, D.M.; Raizada, M.K.; Katovich, M.J. Blood Pressure-Independent Attenuation of Cardiac Hypertrophy by AT(1)R-AS Gene Therapy. Hypertension 2002, 39, 969–975. [Google Scholar] [CrossRef]
- Reaves, P.Y.; Beck, C.R.; Wang, H.-W.; Raizada, M.K.; Katovich, M.J. Endothelial-Independent Prevention of High Blood Pressure in L-NAME-Treated Rats by Angiotensin II Type I Receptor Antisense Gene Therapy. Exp. Physiol. 2003, 88, 467–473. [Google Scholar] [CrossRef]
- Fan, Z.-D.; Zhang, L.; Shi, Z.; Gan, X.-B.; Gao, X.-Y.; Zhu, G.-Q. Artificial MicroRNA Interference Targeting AT(1a) Receptors in Paraventricular Nucleus Attenuates Hypertension in Rats. Gene Ther. 2012, 19, 810–817. [Google Scholar] [CrossRef]
- Chen, A.; Huang, B.S.; Wang, H.-W.; Ahmad, M.; Leenen, F.H.H. Knockdown of Mineralocorticoid or Angiotensin II Type 1 Receptor Gene Expression in the Paraventricular Nucleus Prevents Angiotensin II Hypertension in Rats. J. Physiol. 2014, 592, 3523–3536. [Google Scholar] [CrossRef] [PubMed]
- Repkova, M.N.; Levina, A.S.; Seryapina, A.A.; Shikina, N.V.; Bessudnova, E.V.; Zarytova, V.F.; Markel, A.L. Toward Gene Therapy of Hypertension: Experimental Study on Hypertensive ISIAH Rats. Biochemistry 2017, 82, 454–457. [Google Scholar] [CrossRef]
- Sun, H.; Hodgkinson, C.P.; Pratt, R.E.; Dzau, V.J. CRISPR/Cas9 Mediated Deletion of the Angiotensinogen Gene Reduces Hypertension: A Potential for Cure? Hypertension 2021, 77, 1990–2000. [Google Scholar] [CrossRef]
- Stanton, A.M.; Heydarpour, M.; Williams, J.S.; Williams, G.H.; Adler, G.K. CACNA1D Gene Polymorphisms Associate with Increased Blood Pressure and Salt Sensitivity of Blood Pressure in White Individuals. Hypertension 2023, 80, 2665–2673. [Google Scholar] [CrossRef]
- Ruchaya, P.J.; Speretta, G.F.; Blanch, G.T.; Li, H.; Sumners, C.; Menani, J.V.; Colombari, E.; Colombari, D.S.A. Overexpression of AT2R in the Solitary-Vagal Complex Improves Baroreflex in the Spontaneously Hypertensive Rat. Neuropeptides 2016, 60, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Alhayek, S.; Preuss, C.V. Beta 1 Receptors; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Clare Zhang, Y.; Kimura, B.; Shen, L.; Phillips, M.I. New Beta-Blocker: Prolonged Reduction in High Blood Pressure with Beta(1) Antisense Oligodeoxynucleotides. Hypertension 2000, 35, 219–224. [Google Scholar] [CrossRef]
- Liang, Y.; Lin, S.; Zhou, Y.; Wang, J.; Yu, X. Beta-1 Adrenergic Receptor Antisense-Oligodeoxynucleotides Ameliorates Left Ventricular Remodeling in 2-Kidney, 1-Clip Rats. J. Biomed. Sci. 2007, 14, 155–164. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, X.-L.; Wen, J.; Huang, L.-H.; Lu, Y.; Miao, R.-J.; Liu, X.; Li, Y.; Xing, X.-W.; Yuan, H. Downregulation of the Β1 Adrenergic Receptor in the Myocardium Results in Insensitivity to Metoprolol and Reduces Blood Pressure in Spontaneously Hypertensive Rats. Mol. Med. Rep. 2017, 15, 703–711. [Google Scholar] [CrossRef]
- Dagnino, A.P.A.; Campos, M.M.; Silva, R.B.M. Kinins and Their Receptors in Infectious Diseases. Pharmaceuticals 2020, 13, 215. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chao, L.; Chao, J. Direct Gene Delivery of Human Tissue Kallikrein Reduces Blood Pressure in Spontaneously Hypertensive Rats. J. Clin. Investig. 1995, 95, 1710–1716. [Google Scholar] [CrossRef] [PubMed]
- Yayama, K.; Wang, C.; Chao, L.; Chao, J. Kallikrein Gene Delivery Attenuates Hypertension and Cardiac Hypertrophy and Enhances Renal Function in Goldblatt Hypertensive Rats. Hypertension 1998, 31, 1104–1110. [Google Scholar] [CrossRef]
- Zhang, J.J.; Wang, C.; Lin, K.F.; Chao, L.; Chao, J. Human Tissue Kallikrein Attenuates Hypertension and Secretes into Circulation and Urine after Intramuscular Gene Delivery in Hypertensive Rats. Clin. Exp. Hypertens. 1999, 21, 1145–1160. [Google Scholar] [CrossRef]
- Dobrzynski, E.; Yoshida, H.; Chao, J.; Chao, L. Adenovirus-Mediated Kallikrein Gene Delivery Attenuates Hypertension and Protects against Renal Injury in Deoxycorticosterone-Salt Rats. Immunopharmacology 1999, 44, 57–65. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, P.; Xiao, X.; Chao, J.; Chao, L.; Wang, D.W.; Zeldin, D.C. Gene Therapy with Human Tissue Kallikrein Reduces Hypertension and Hyperinsulinemia in Fructose-Induced Hypertensive Rats. Hypertension 2003, 42, 1026–1033. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, T.; Li, H.; Zhao, C.; Chen, C.; Li, J.; Chao, J.; Chao, L.; Xiao, X.; Wang, D.W. Recombinant Adeno-Associated Virus-Mediated Kallikrein Gene Therapy Reduces Hypertension and Attenuates Its Cardiovascular Injuries. Gene Ther. 2004, 11, 1342–1350. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Hou, L.; Liu, Z.; Wang, Y.; Chen, C.; Xiao, X.; Wang, D. Intramuscular Delivery of RAAV-Mediated Kallikrein Gene Reduces Hypertension and Prevents Cardiovascular Injuries in Model Rats. Acta Pharmacol. Sin. 2007, 28, 1898–1906. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yan, J.; Wang, T.; Li, J.; Xiao, X.; Wang, D. Recombinant Adeno-Associated Virus-Mediated Human Kallikrein Gene Therapy Prevents High-Salt Diet-Induced Hypertension without Effect on Basal Blood Pressure. Acta Pharmacol. Sin. 2008, 29, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Valerieva, A.; Longhurst, H.J. Treatment of Hereditary Angioedema-Single or Multiple Pathways to the Rescue. Front. Allergy 2022, 3, 952233. [Google Scholar] [CrossRef] [PubMed]
- Marceau, F. Correction: Marceau, F. Drugs of the Kallikrein–Kinin System: An Overview. Drugs Drug Candidates 2023, 2, 538–553. Drugs Drug Candidates 2024, 3, 208. [Google Scholar] [CrossRef]
- Ferrone, J.D.; Bhattacharjee, G.; Revenko, A.S.; Zanardi, T.A.; Warren, M.S.; Derosier, F.J.; Viney, N.J.; Pham, N.C.; Kaeser, G.E.; Baker, B.F.; et al. IONIS-PKKRx a Novel Antisense Inhibitor of Prekallikrein and Bradykinin Production. Nucleic Acid. Ther. 2019, 29, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.intelliatx.com/Our-Science/Publications-and-Presentations/ (accessed on 26 February 2024).
- Shehjar, F.; Maktabi, B.; Rahman, Z.A.; Bahader, G.A.; James, A.W.; Naqvi, A.; Mahajan, R.; Shah, Z.A. Stroke: Molecular Mechanisms and Therapies: Update on Recent Developments. Neurochem. Int. 2023, 162, 105458. [Google Scholar] [CrossRef] [PubMed]
- Alexander-Curtis, M.; Pauls, R.; Chao, J.; Volpi, J.J.; Bath, P.M.; Verdoorn, T.A. Human Tissue Kallikrein in the Treatment of Acute Ischemic Stroke. Ther. Adv. Neurol. Disord. 2019, 12, 1756286418821918. [Google Scholar] [CrossRef]
- Tulloh, R. Etiology, Diagnosis, and Pharmacologic Treatment of Pediatric Pulmonary Hypertension. Paediatr. Drugs 2009, 11, 115–128. [Google Scholar] [CrossRef]
- Da Silva, G.; da Silva, M.; Nascimento, D.; Lima Silva, E.; Gouvêa, F.; de França Lopes, L.; Araújo, A.; Ferraz Pereira, K.; de Queiroz, T. Nitric Oxide as a Central Molecule in Hypertension: Focus on the Vasorelaxant Activity of New Nitric Oxide Donors. Biology 2021, 10, 1041. [Google Scholar] [CrossRef]
- Bryan, N.S. Nitric Oxide Deficiency Is a Primary Driver of Hypertension. Biochem. Pharmacol. 2022, 206, 115325. [Google Scholar] [CrossRef] [PubMed]
- Chao, J.; Zhang, J.J.; Lin, K.F.; Chao, L. Human Kallikrein Gene Delivery Attenuates Hypertension, Cardiac Hypertrophy, and Renal Injury in Dahl Salt-Sensitive Rats. Hum. Gene Ther. 1998, 9, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.H.; Brosnan, M.J.; Graham, D.; Nicol, C.G.; Morecroft, I.; Channon, K.M.; Danilov, S.M.; Reynolds, P.N.; Baker, A.H.; Dominiczak, A.F. Targeting Endothelial Cells with Adenovirus Expressing Nitric Oxide Synthase Prevents Elevation of Blood Pressure in Stroke-Prone Spontaneously Hypertensive Rats. Mol. Ther. 2005, 12, 321–327. [Google Scholar] [CrossRef]
- Gava, A.L.; Peotta, V.A.; Cabral, A.M.; Vasquez, E.C.; Meyrelles, S.S. Overexpression of ENOS Prevents the Development of Renovascular Hypertension in Mice. Can. J. Physiol. Pharmacol. 2008, 86, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.X.; Xu, X.; Cui, Y.; Wang, P.; Wei, X.; Yang, S.; Edin, M.L.; Zeldin, D.C.; Wang, D.W. Increased Endothelial Nitric-Oxide Synthase Expression Reduces Hypertension and Hyperinsulinemia in Fructose-Treated Rats. J. Pharmacol. Exp. Ther. 2009, 328, 610–620. [Google Scholar] [CrossRef]
- Cannone, V.; Cabassi, A.; Volpi, R.; Burnett, J.C., Jr. Atrial Natriuretic Peptide: A Molecular Target of Novel Therapeutic Approaches to Cardio-Metabolic Disease. Int. J. Mol. Sci. 2019, 20, 3265. [Google Scholar] [CrossRef]
- Lin, K.F.; Chao, J.; Chao, L. Atrial Natriuretic Peptide Gene Delivery Attenuates Hypertension, Cardiac Hypertrophy, and Renal Injury in Salt-Sensitive Rats. Hum. Gene Ther. 1998, 9, 1429–1438. [Google Scholar] [CrossRef]
- Therrien, J.-P.; Kim, S.M.; Terunuma, A.; Qin, Y.; Tock, C.L.; Pfützner, W.; Ohyama, M.; Schnermann, J.; Vogel, J.C. A Gene Therapy Approach for Long-Term Normalization of Blood Pressure in Hypertensive Mice by ANP-Secreting Human Skin Grafts. Proc. Natl. Acad. Sci. USA 2010, 107, 1178–1183. [Google Scholar] [CrossRef]
- Calzetta, L.; Orlandi, A.; Page, C.; Rogliani, P.; Rinaldi, B.; Rosano, G.; Cazzola, M.; Matera, M.G. Brain Natriuretic Peptide: Much More than a Biomarker. Int. J. Cardiol. 2016, 221, 1031–1038. [Google Scholar] [CrossRef]
- Cataliotti, A.; Tonne, J.M.; Bellavia, D.; Martin, F.L.; Oehler, E.A.; Harders, G.E.; Campbell, J.M.; Peng, K.-W.; Russell, S.J.; Malatino, L.S.; et al. Long-Term Cardiac pro-B-Type Natriuretic Peptide Gene Delivery Prevents the Development of Hypertensive Heart Disease in Spontaneously Hypertensive Rats. Circulation 2011, 123, 1297–1305. [Google Scholar] [CrossRef]
- Tonne, J.M.; Holditch, S.J.; Oehler, E.A.; Schreiber, C.A.; Ikeda, Y.; Cataliotti, A. Cardiac BNP Gene Delivery Prolongs Survival in Aged Spontaneously Hypertensive Rats with Overt Hypertensive Heart Disease. Aging 2014, 6, 311–319. [Google Scholar] [CrossRef]
- Qian, P.; Wang, Q.; Wang, F.-Z.; Dai, H.-B.; Wang, H.-Y.; Gao, Q.; Zhou, H.; Zhou, Y.-B. Adrenomedullin Improves Cardiac Remodeling and Function in Obese Rats with Hypertension. Pharmaceuticals 2022, 15, 719. [Google Scholar] [CrossRef]
- Chao, J.; Jin, L.; Lin, K.F.; Chao, L. Adrenomedullin Gene Delivery Reduces Blood Pressure in Spontaneously Hypertensive Rats. Hypertens. Res. 1997, 20, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Yoshida, H.; Chao, L.; Chao, J. Human Adrenomedullin Gene Delivery Protects against Cardiac Hypertrophy, Fibrosis, and Renal Damage in Hypertensive Dahl Salt-Sensitive Rats. Hum. Gene Ther. 2000, 11, 1817–1827. [Google Scholar] [CrossRef] [PubMed]
- Phelps, P.K.; Kelley, E.F.; Walla, D.M.; Ross, J.K.; Simmons, J.J.; Bulock, E.K.; Ayres, A.; Akre, M.K.; Sprissler, R.; Olson, T.P.; et al. Relationship between a Weighted Multi-Gene Algorithm and Blood Pressure Control in Hypertension. J. Clin. Med. 2019, 8, 289. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05349825 (accessed on 10 April 2024).
- Available online: https://clinicaltrials.gov/study/NCT04660630?Cond=Hypertension&intr=therapy%20gene&rank=1 (accessed on 10 April 2024).
- Chiesa, G.; Sirtori, C.R. Apolipoprotein A-I(Milano): Current Perspectives. Curr. Opin. Lipidol. 2003, 14, 159–163. [Google Scholar] [CrossRef]
- Sanllorente, A.; Lassale, C.; Soria-Florido, M.T.; Castañer, O.; Fitó, M.; Hernáez, Á. Modification of High-Density Lipoprotein Functions by Diet and Other Lifestyle Changes: A Systematic Review of Randomized Controlled Trials. J. Clin. Med. 2021, 10, 5897. [Google Scholar] [CrossRef]
- Pászty, C.; Maeda, N.; Verstuyft, J.; Rubin, E.M. Apolipoprotein AI Transgene Corrects Apolipoprotein E Deficiency-Induced Atherosclerosis in Mice. J. Clin. Investig. 1994, 94, 899–903. [Google Scholar] [CrossRef]
- Soma, M.R.; Donetti, E.; Parolini, C.; Sirtori, C.R.; Fumagalli, R.; Franceschini, G. Recombinant Apolipoprotein A-I Milano Dimer Inhibits Carotid Intimal Thickening Induced by Perivascular Manipulation in Rabbits. Circ. Res. 1995, 76, 405–411. [Google Scholar] [CrossRef]
- Tangirala, R.K.; Tsukamoto, K.; Chun, S.H.; Usher, D.; Puré, E.; Rader, D.J. Regression of Atherosclerosis Induced by Liver-Directed Gene Transfer of Apolipoprotein A-I in Mice. Circulation 1999, 100, 1816–1822. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, H.; Yoshida, H.; Major, A.S.; Zhu, T.; Babaev, V.R.; Linton, M.F.; Fazio, S. Retrovirus-Mediated Expression of Apolipoprotein A-I in the Macrophage Protects against Atherosclerosis In Vivo. J. Biol. Chem. 2001, 276, 36742–36748. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, K.; Marchadier, D.H.L.; Miller, G.C.; Gao, G.; Wilson, J.M.; Rader, D.J. Complete Prevention of Atherosclerosis in ApoE-Deficient Mice by Hepatic Human ApoE Gene Transfer with Adeno-Associated Virus Serotypes 7 and 8. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1852–1857. [Google Scholar] [CrossRef] [PubMed]
- Lebherz, C.; Sanmiguel, J.; Wilson, J.M.; Rader, D.J. Gene Transfer of Wild-Type ApoA-I and ApoA-I Milano Reduce Atherosclerosis to a Similar Extent. Cardiovasc. Diabetol. 2007, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Evans, V.; Foster, H.; Graham, I.R.; Foster, K.; Athanasopoulos, T.; Simons, J.P.; Dickson, G.; Owen, J.S. Human Apolipoprotein E Expression from Mouse Skeletal Muscle by Electrotransfer of Nonviral DNA (Plasmid) and Pseudotyped Recombinant Adeno-Associated Virus (AAV2/7). Hum. Gene Ther. 2008, 19, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Van Craeyveld, E.; Jacobs, F.; Lievens, J.; Snoeys, J.; De Geest, B. Wild-Type Apo A-I and Apo A-I (Milano) Gene Transfer Reduce Native and Transplant Arteriosclerosis to a Similar Extent. J. Mol. Med. 2009, 87, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Vaessen, S.F.C.; Veldman, R.J.; Comijn, E.M.; Snapper, J.; Sierts, J.A.; van den Oever, K.; Beattie, S.G.; Twisk, J.; Kuivenhoven, J.A. AAV Gene Therapy as a Means to Increase Apolipoprotein (Apo) A-I and High-Density Lipoprotein-Cholesterol Levels: Correction of Murine ApoA-I Deficiency. J. Gene Med. 2009, 11, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Koornneef, A.; Maczuga, P.; van Logtenstein, R.; Borel, F.; Blits, B.; Ritsema, T.; van Deventer, S.; Petry, H.; Konstantinova, P. Apolipoprotein B Knockdown by AAV-Delivered ShRNA Lowers Plasma Cholesterol in Mice. Mol. Ther. 2011, 19, 731–740. [Google Scholar] [CrossRef]
- Sharma, V.; Beckstead, J.A.; Simonsen, J.B.; Nelbach, L.; Watson, G.; Forte, T.M.; Ryan, R.O. Gene Transfer of Apolipoprotein A-V Improves the Hypertriglyceridemic Phenotype of Apoa5 (−/−) Mice. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 474–480. [Google Scholar] [CrossRef]
- Tian, F.; Wang, L.; Arias, A.; Yang, M.; Sharifi, B.G.; Shah, P.K. Comparative Antiatherogenic Effects of Intravenous AAV8- and AAV2-Mediated ApoA-IMilano Gene Transfer in Hypercholesterolemic Mice. J. Cardiovasc. Pharmacol. Ther. 2015, 20, 66–75. [Google Scholar] [CrossRef]
- Wacker, B.K.; Dronadula, N.; Bi, L.; Stamatikos, A.; Dichek, D.A. Apo A-I (Apolipoprotein A-I) Vascular Gene Therapy Provides Durable Protection Against Atherosclerosis in Hyperlipidemic Rabbits. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Bi, L.; Wacker, B.K.; Komandur, K.; Sanford, N.; Dichek, D.A. Apolipoprotein A-I Vascular Gene Therapy Reduces Vein-Graft Atherosclerosis. Mol. Ther. Methods Clin. Dev. 2023, 30, 558–572. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.; Twisk, J.; Kwikkers, K.; Aronica, E.; Brisson, D.; Methot, J.; Petry, H.; Gaudet, D. Immune Responses to Intramuscular Administration of Alipogene Tiparvovec (AAV1-LPL(S447X)) in a Phase II Clinical Trial of Lipoprotein Lipase Deficiency Gene Therapy. Hum. Gene Ther. 2014, 25, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, D.; Méthot, J.; Déry, S.; Brisson, D.; Essiembre, C.; Tremblay, G.; Tremblay, K.; de Wal, J.; Twisk, J.; van den Bulk, N.; et al. Efficacy and Long-Term Safety of Alipogene Tiparvovec (AAV1-LPLS447X) Gene Therapy for Lipoprotein Lipase Deficiency: An Open-Label Trial. Gene Ther. 2013, 20, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Grossman, P.M.; Mendelsohn, F.; Henry, T.D.; Hermiller, J.B.; Litt, M.; Saucedo, J.F.; Weiss, R.J.; Kandzari, D.E.; Kleiman, N.; Anderson, R.D.; et al. Results from a Phase II Multicenter, Double-Blind Placebo-Controlled Study of Del-1 (VLTS-589) for Intermittent Claudication in Subjects with Peripheral Arterial Disease. Am. Heart J. 2007, 153, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://clinicaltrials.gov/study/NCT00696124?Cond=Atherosclerosis&intr=gene%20therapy&page=2&rank=13 (accessed on 10 April 2024).
- Available online: https://clinicaltrials.gov/study/NCT02144610?Cond=Atherosclerosis&intr=gene%20therapy&page=2&rank=12 (accessed on 10 April 2024).
- Available online: https://clinicaltrials.gov/study/NCT01064440?Cond=Atherosclerosis&intr=gene%20therapy&page=2&rank=11 (accessed on 10 April 2024).
- Available online: https://clinicaltrials.gov/study/NCT02016755?Cond=Atherosclerosis&intr=gene%20therapy&page=3&rank=21 (accessed on 10 April 2024).
- Available online: https://classic.clinicaltrials.gov/ct2/show/NCT06112327 (accessed on 10 April 2024).
- Rouis, M.; Adamy, C.; Duverger, N.; Lesnik, P.; Horellou, P.; Moreau, M.; Emmanuel, F.; Caillaud, J.M.; Laplaud, P.M.; Dachet, C.; et al. Adenovirus-Mediated Overexpression of Tissue Inhibitor of Metalloproteinase-1 Reduces Atherosclerotic Lesions in Apolipoprotein E-Deficient Mice. Circulation 1999, 100, 533–540. [Google Scholar] [CrossRef]
- Qian, H.; Neplioueva, V.; Shetty, G.A.; Channon, K.M.; George, S.E. Nitric Oxide Synthase Gene Therapy Rapidly Reduces Adhesion Molecule Expression and Inflammatory Cell Infiltration in Carotid Arteries of Cholesterol-Fed Rabbits. Circulation 1999, 99, 2979–2982. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Von Der Thüsen, J.H.; Kuiper, J.; Fekkes, M.L.; De Vos, P.; Van Berkel, T.J.; Biessen, E.A. Attenuation of Atherogenesis by Systemic and Local Adenovirus-Mediated Gene Transfer of Interleukin-10 in LDLr−/− Mice. FASEB J. 2001, 15, 2730–2732. [Google Scholar] [CrossRef]
- Yoshioka, T.; Okada, T.; Maeda, Y.; Ikeda, U.; Shimpo, M.; Nomoto, T.; Takeuchi, K.; Nonaka-Sarukawa, M.; Ito, T.; Takahashi, M.; et al. Adeno-Associated Virus Vector-Mediated Interleukin-10 Gene Transfer Inhibits Atherosclerosis in Apolipoprotein E-Deficient Mice. Gene Ther. 2004, 11, 1772–1779. [Google Scholar] [CrossRef]
- Liu, Y.; Li, D.; Chen, J.; Xie, J.; Bandyopadhyay, S.; Zhang, D.; Nemarkommula, A.R.; Liu, H.; Mehta, J.L.; Hermonat, P.L. Inhibition of Atherogenesis in LDLR Knockout Mice by Systemic Delivery of Adeno-Associated Virus Type 2-HIL-10. Atherosclerosis 2006, 188, 19–27. [Google Scholar] [CrossRef]
- Namiki, M.; Kawashima, S.; Yamashita, T.; Ozaki, M.; Sakoda, T.; Inoue, N.; Hirata, K.-I.; Morishita, R.; Kaneda, Y.; Yokoyama, M. Intramuscular Gene Transfer of Interleukin-10 CDNA Reduces Atherosclerosis in Apolipoprotein E-Knockout Mice. Atherosclerosis 2004, 172, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, Y.; Chen, J.; Velchala, N.; Amani, F.; Nemarkommula, A.; Chen, K.; Rayaz, H.; Zhang, D.; Liu, H.; et al. Suppression of Atherogenesis by Delivery of TGFbeta1ACT Using Adeno-Associated Virus Type 2 in LDLR Knockout Mice. Biochem. Biophys. Res. Commun. 2006, 344, 701–707. [Google Scholar] [CrossRef]
- Khan, J.A.; Cao, M.; Kang, B.-Y.; Liu, Y.; Mehta, J.L.; Hermonat, P.L. AAV/HSTAT3-Gene Delivery Lowers Aortic Inflammatory Cell Infiltration in LDLR KO Mice on High Cholesterol. Atherosclerosis 2010, 213, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Khan, J.A.; Kang, B.-Y.; Mehta, J.L.; Hermonat, P.L. Dual AAV/IL-10 Plus STAT3 Anti-Inflammatory Gene Delivery Lowers Atherosclerosis in LDLR KO Mice, but without Increased Benefit. Int. J. Vasc. Med. 2012, 2012, 524235. [Google Scholar] [CrossRef]
- Bi, L.; Wacker, B.K.; Bueren, E.; Ham, E.; Dronadula, N.; Dichek, D.A. A Rabbit Model for Testing Helper-Dependent Adenovirus-Mediated Gene Therapy for Vein Graft Atherosclerosis. Mol. Ther. Methods Clin. Dev. 2017, 7, 96–111. [Google Scholar] [CrossRef]
- Henderson, R.; O’Kane, M.; McGilligan, V.; Watterson, S. The Genetics and Screening of Familial Hypercholesterolaemia. J. Biomed. Sci. 2016, 23, 39. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.P.; Ahmed, H.M.; Wilson Tang, W.H. Familial Hypercholesterolemia: Detect, Treat, and Ask about Family. Cleve Clin. J. Med. 2020, 87, 109–120. [Google Scholar] [CrossRef]
- Chowdhury, J.R.; Grossman, M.; Gupta, S.; Chowdhury, N.R.; Baker, J.R.; Wilson, J.M. Long-Term Improvement of Hypercholesterolemia after Ex Vivo Gene Therapy in LDLR-Deficient Rabbits. Science 1991, 254, 1802–1805. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.J.; Rader, D.J.; Tazelaar, J.; Kawashiri, M.; Gao, G.; Wilson, J.M. Prolonged Correction of Hyperlipidemia in Mice with Familial Hypercholesterolemia Using an Adeno-Associated Viral Vector Expressing Very-Low-Density Lipoprotein Receptor. Mol. Ther. 2000, 2, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Kankkonen, H.M.; Vähäkangas, E.; Marr, R.A.; Pakkanen, T.; Laurema, A.; Leppänen, P.; Jalkanen, J.; Verma, I.M.; Ylä-Herttuala, S. Long-Term Lowering of Plasma Cholesterol Levels in LDL-Receptor-Deficient WHHL Rabbits by Gene Therapy. Mol. Ther. 2004, 9, 548–556. [Google Scholar] [CrossRef]
- Kassim, S.H.; Li, H.; Vandenberghe, L.H.; Hinderer, C.; Bell, P.; Marchadier, D.; Wilson, A.; Cromley, D.; Redon, V.; Yu, H.; et al. Gene Therapy in a Humanized Mouse Model of Familial Hypercholesterolemia Leads to Marked Regression of Atherosclerosis. PLoS ONE 2010, 5, e13424. [Google Scholar] [CrossRef]
- Hibbitt, O.; Agkatsev, S.; Owen, C.; Cioroch, M.; Seymour, L.; Channon, K.; Wade-Martins, R. RNAi-Mediated Knockdown of HMG CoA Reductase Enhances Gene Expression from Physiologically Regulated Low-Density Lipoprotein Receptor Therapeutic Vectors In Vivo. Gene Ther. 2012, 19, 463–467. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kassim, S.H.; Li, H.; Bell, P.; Somanathan, S.; Lagor, W.; Jacobs, F.; Billheimer, J.; Wilson, J.M.; Rader, D.J. Adeno-Associated Virus Serotype 8 Gene Therapy Leads to Significant Lowering of Plasma Cholesterol Levels in Humanized Mouse Models of Homozygous and Heterozygous Familial Hypercholesterolemia. Hum. Gene Ther. 2013, 24, 19–26. [Google Scholar] [CrossRef]
- Somanathan, S.; Jacobs, F.; Wang, Q.; Hanlon, A.L.; Wilson, J.M.; Rader, D.J. AAV Vectors Expressing LDLR Gain-of-Function Variants Demonstrate Increased Efficacy in Mouse Models of Familial Hypercholesterolemia. Circ. Res. 2014, 115, 591–599. [Google Scholar] [CrossRef]
- Wang, L.; Muthuramu, I.; Somanathan, S.; Zhang, H.; Bell, P.; He, Z.; Yu, H.; Zhu, Y.; Tretiakova, A.P.; Wilson, J.M. Developing a Second-Generation Clinical Candidate AAV Vector for Gene Therapy of Familial Hypercholesterolemia. Mol. Ther. Methods Clin. Dev. 2021, 22, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhao, P.; Zhang, Y.; Wang, J.; Wang, C.; Liu, Y.; Yang, G.; Yuan, L. Exosome-Based Ldlr Gene Therapy for Familial Hypercholesterolemia in a Mouse Model. Theranostics 2021, 11, 2953–2965. [Google Scholar] [CrossRef]
- Grossman, M.; Raper, S.E.; Kozarsky, K.; Stein, E.A.; Engelhardt, J.F.; Muller, D.; Lupien, P.J.; Wilson, J.M. Successful Ex Vivo Gene Therapy Directed to Liver in a Patient with Familial Hypercholesterolaemia. Nat. Genet. 1994, 6, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Grossman, M.; Rader, D.J.; Muller, D.W.; Kolansky, D.M.; Kozarsky, K.; Clark, B.J.; Stein, E.A.; Lupien, P.J.; Brewer, H.B.; Raper, S.E. A Pilot Study of Ex Vivo Gene Therapy for Homozygous Familial Hypercholesterolaemia. Nat. Med. 1995, 1, 1148–1154. [Google Scholar] [CrossRef]
- Available online: https://clinicaltrials.gov/Study/NCT02651675 (accessed on 27 February 2024).
- Available online: https://clinicaltrials.gov/study/NCT05043181 (accessed on 27 February 2024).
- Available online: https://clinicaltrials.gov/study/NCT03400800 (accessed on 27 February 2024).
- Available online: https://www.clinicaltrials.gov/study/NCT03747224 (accessed on 27 February 2024).
- Available online: https://clinicaltrials.gov/study/NCT04606602 (accessed on 27 February 2024).
- Available online: https://clinicaltrials.gov/study/NCT04270760 (accessed on 27 February 2024).
- Available online: https://clinicaltrials.gov/study/NCT03626662 (accessed on 27 February 2024).
- Available online: https://classic.clinicaltrials.gov/Ct2/Show/NCT02900027 (accessed on 27 February 2024).
- Available online: https://clinicaltrials.gov/study/NCT03385239 (accessed on 27 February 2024).
- Available online: https://www.clinicaltrials.gov/study/NCT02211209 (accessed on 27 February 2024).
- O’Donoghue, M.L.; Rosenson, R.S.; Gencer, B.; López, J.A.G.; Lepor, N.E.; Baum, S.J.; Stout, E.; Gaudet, D.; Knusel, B.; Kuder, J.F.; et al. Small Interfering RNA to Reduce Lipoprotein(a) in Cardiovascular Disease. N. Engl. J. Med. 2022, 387, 1855–1864. [Google Scholar] [CrossRef]
- Anderson, W.F. September 14, 1990: The Beginning. Hum. Gene Ther. 1990, 1, 371–372. [Google Scholar] [CrossRef]
- Arabi, F.; Mansouri, V.; Ahmadbeigi, N. Gene Therapy Clinical Trials, Where Do We Go? An Overview. Biomed. Pharmacother. 2022, 153, 113324. [Google Scholar] [CrossRef] [PubMed]
- Gardin, A.; Ronzitti, G. Current Limitations of Gene Therapy for Rare Pediatric Diseases: Lessons Learned from Clinical Experience with AAV Vectors. Arch. Pédiatrie 2023, 30, 8S46–8S52. [Google Scholar] [CrossRef] [PubMed]
- Grieger, J.C.; Samulski, R.J. Packaging Capacity of Adeno-Associated Virus Serotypes: Impact of Larger Genomes on Infectivity and Postentry Steps. J. Virol. 2005, 79, 9933–9944. [Google Scholar] [CrossRef] [PubMed]
- Athanasopoulos, T.; Munye, M.M.; Yáñez-Muñoz, R.J. Nonintegrating Gene Therapy Vectors. Hematol. Oncol. Clin. N. Am. 2017, 31, 753–770. [Google Scholar] [CrossRef]
- Butt, M.; Zaman, M.; Ahmad, A.; Khan, R.; Mallhi, T.; Hasan, M.; Khan, Y.; Hafeez, S.; Massoud, E.; Rahman, M.; et al. Appraisal for the Potential of Viral and Nonviral Vectors in Gene Therapy: A Review. Genes 2022, 13, 1370. [Google Scholar] [CrossRef]
- Uddin, F.; Rudin, C.M.; Sen, T. CRISPR Gene Therapy: Applications, Limitations, and Implications for the Future. Front. Oncol. 2020, 10, 1387. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).