Short-Term Clinical Results of Single-Injection Autologous Bone Marrow Aspirate Concentrate (BMAC) as a Therapeutic Option/Tool in Knee Osteoarthritis
Abstract
1. Introduction
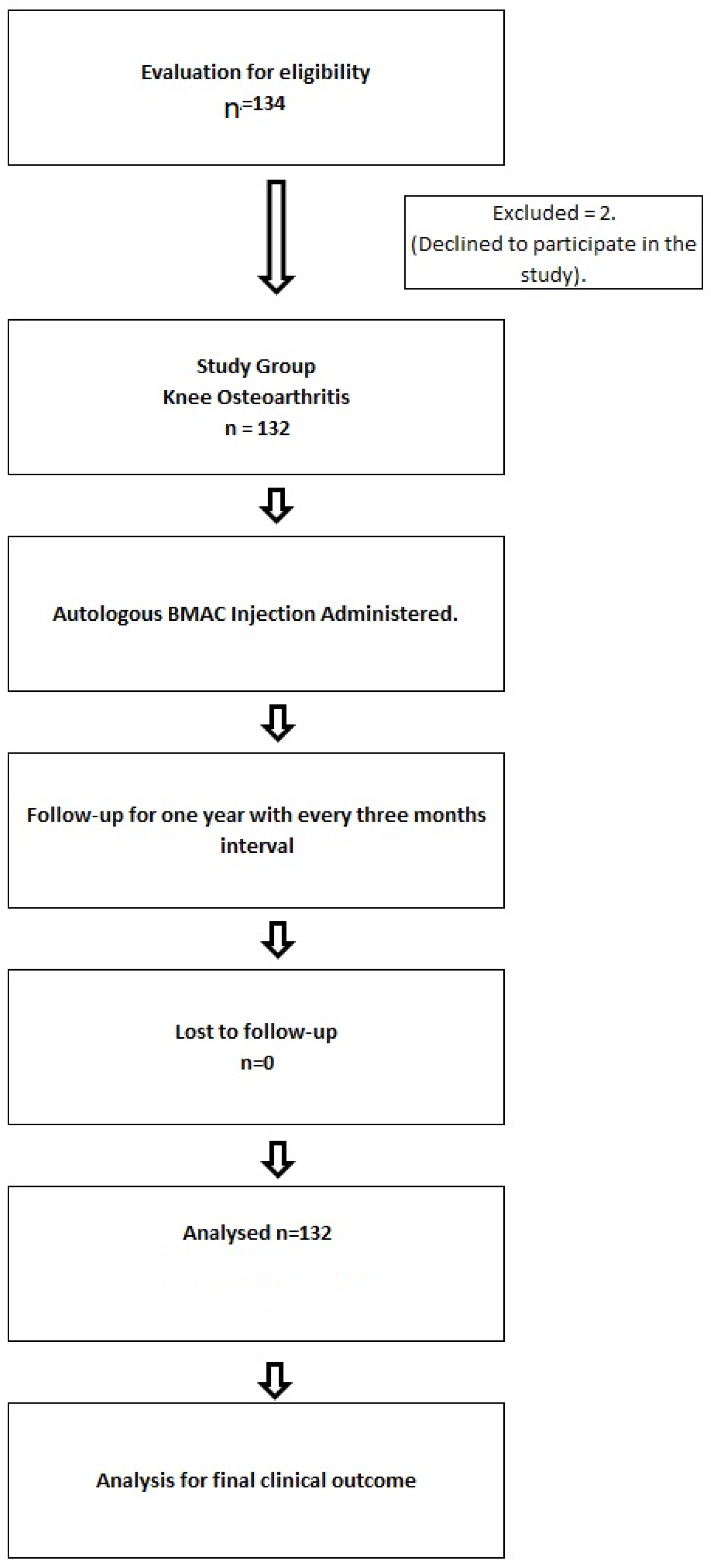
2. Materials and Methods
2.1. Patient Recruitment
2.1.1. Inclusion Criteria
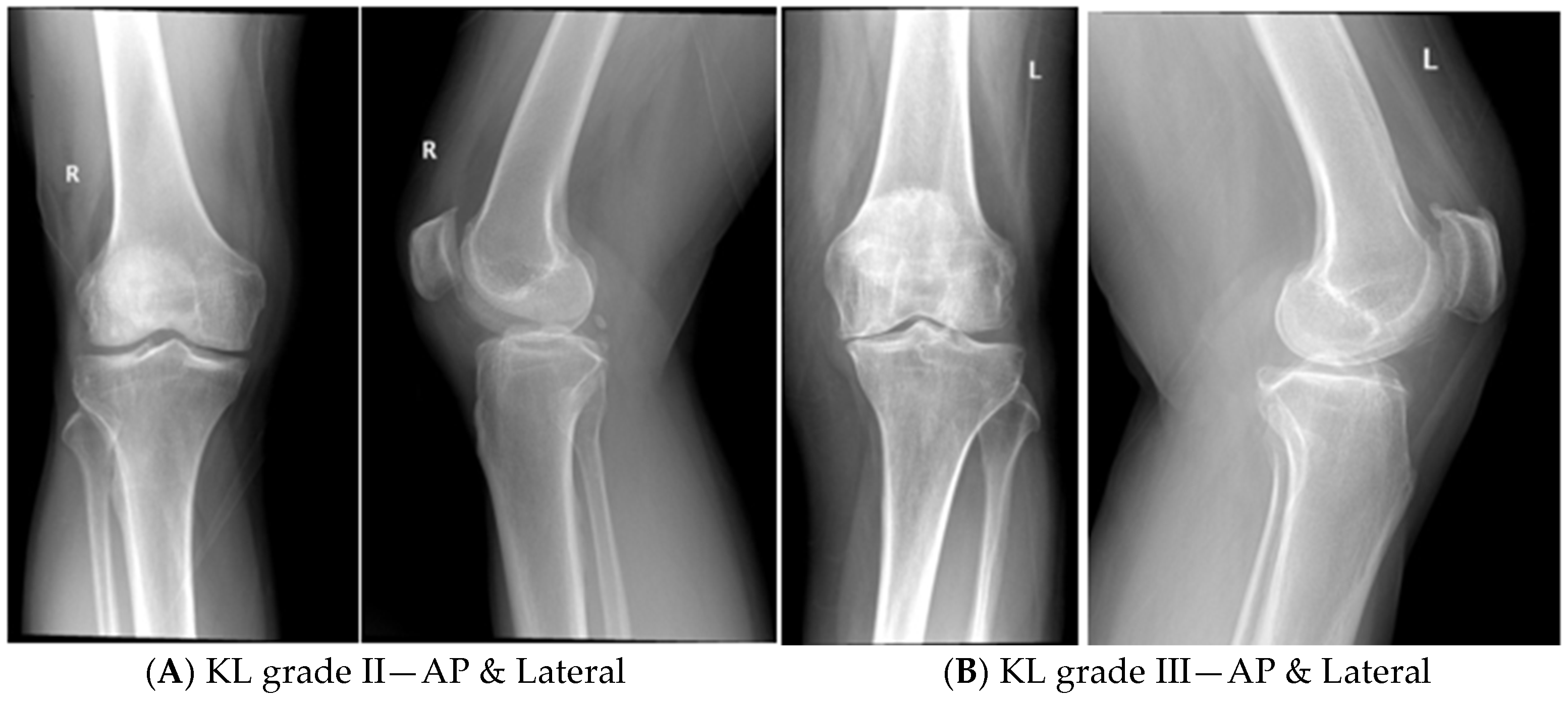
- Patients of KOA with radiological grading of KL grade II and III.
- Age group of the patients between 25 and 60 years of either gender.
- Only patients with primary KOA were included in the study.
2.1.2. Exclusion Criteria
- KOA patients with KL grade IV were excluded, as it has global cartilage loss with subchondral erosions and deformities.
- KOA patients with deformities like varus/valgus/genu recurvatum ligament imbalance and any gross anatomical malalignment of the joints were also excluded.
- Patients with secondary KOA and rheumatoid arthritis were also excluded.
- Patients with metabolic disorders like hyperuricemia were excluded.
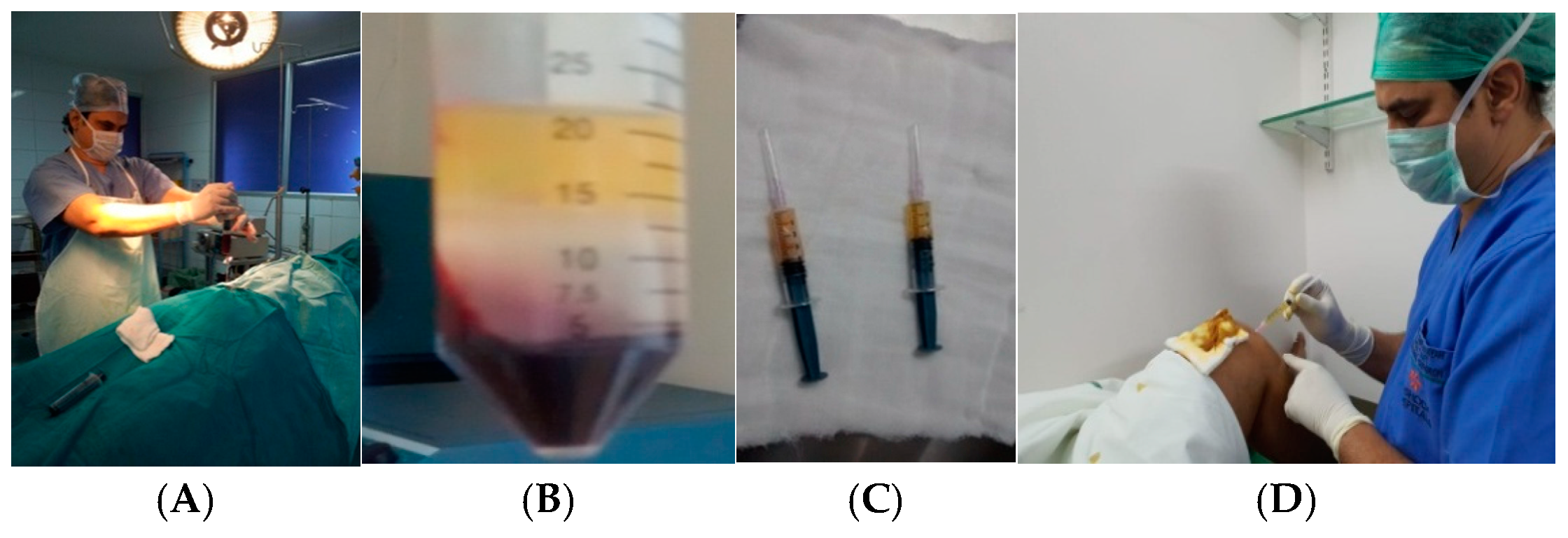
2.2. Procedure for Bone Marrow Aspiration
Density Gradient Centrifugation Method for the Separation of Mononuclear Cells Enriched with Stem Cells
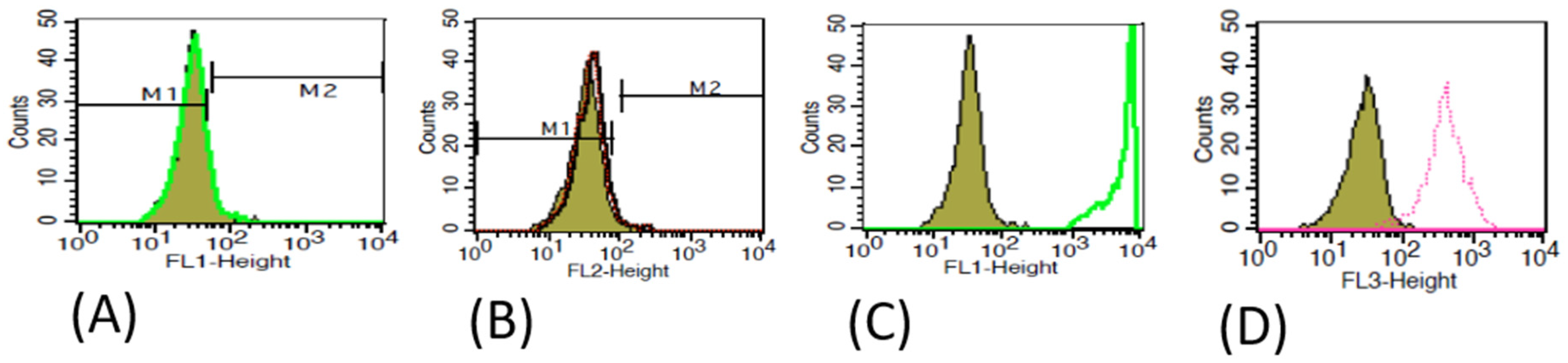
2.3. Characterization of MSCs
2.4. Primary Clinical Outcome Measurements
2.4.1. Subjective Assessment: It Is Assessed by VAS and WOMAC Index Score
2.4.2. Objective Assessment
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Patients
3.2. Primary Clinical Outcome
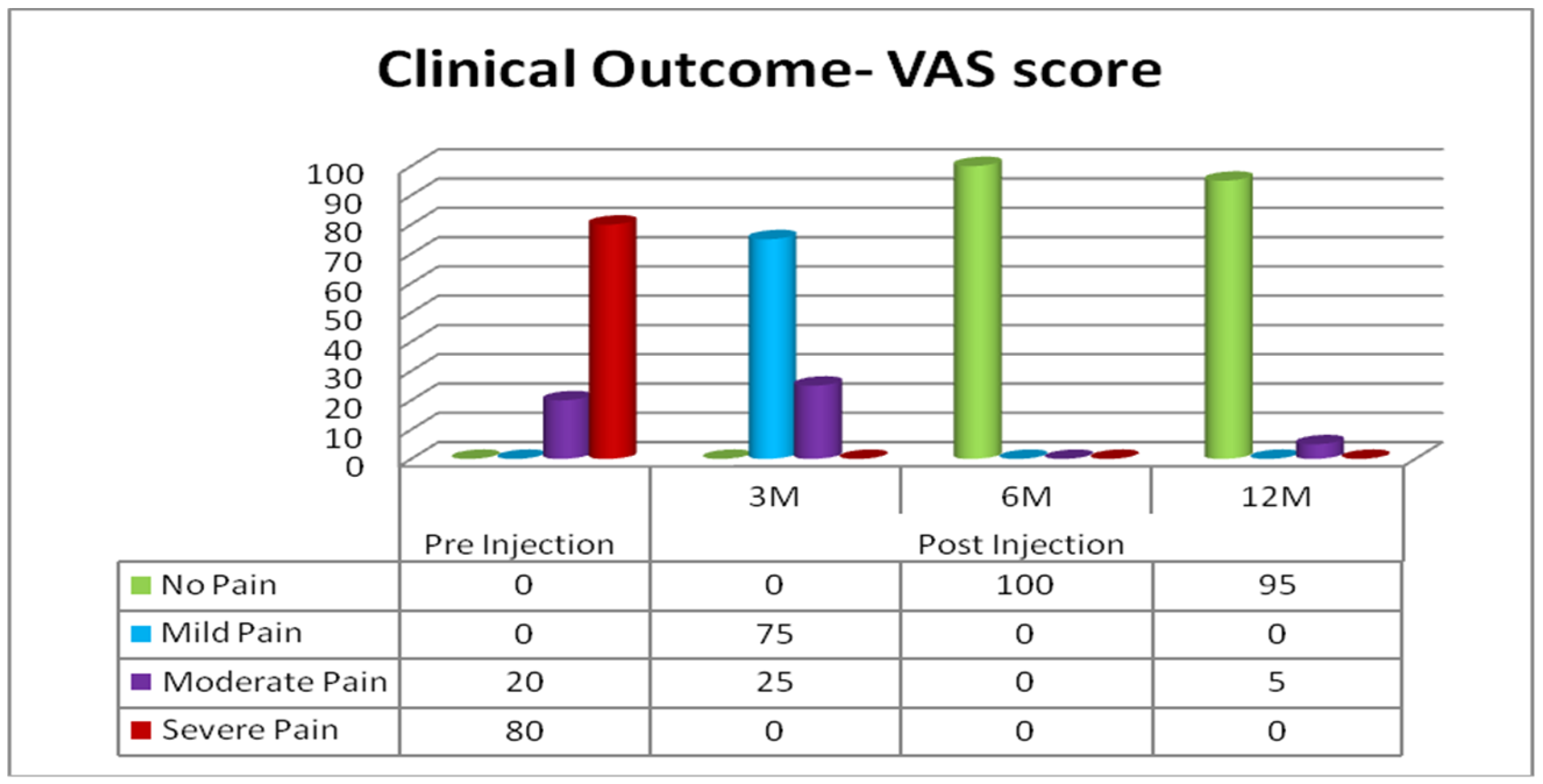
3.2.1. Subjective Assessment: Clinical Outcomes Based on VAS
3.2.2. Evaluation of Statistical Significance Using the VAS score
3.3. Subjective Assessment: Clinical Outcomes with WOMAC
3.3.1. Evaluation of Statistical Significance Using WOMAC
KL Grade II KOA Patients
KL Grade III KOA Patients
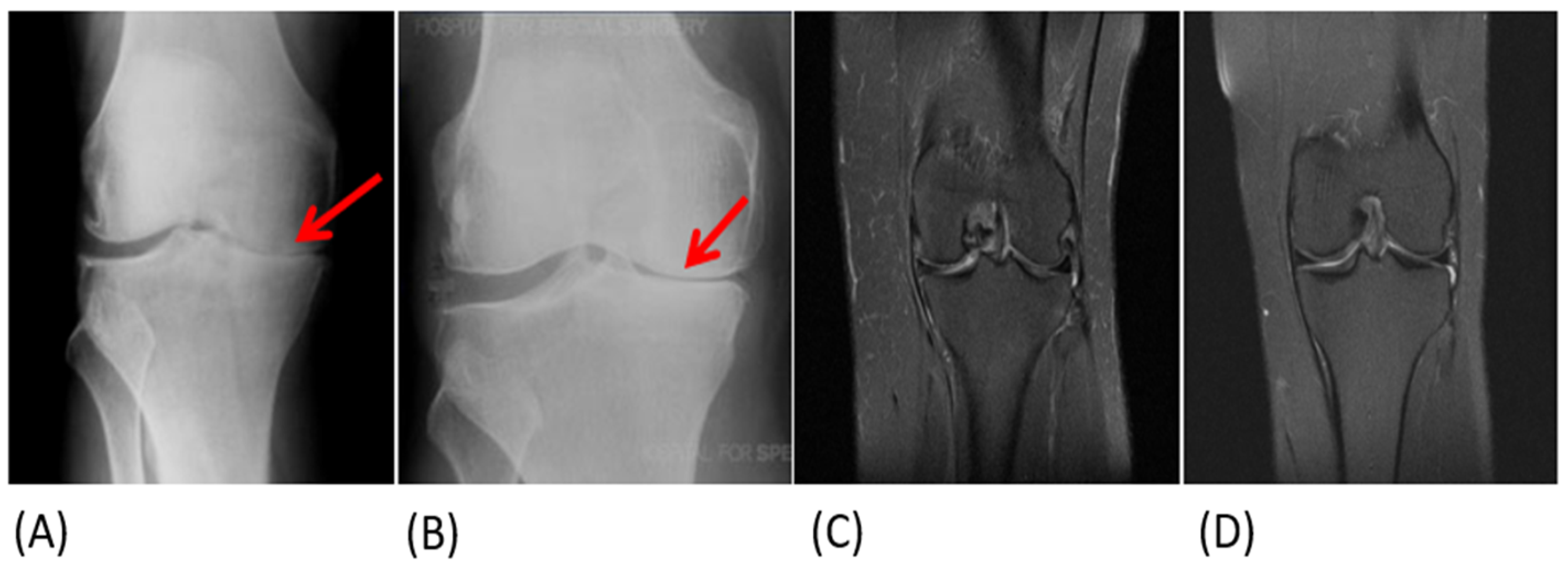
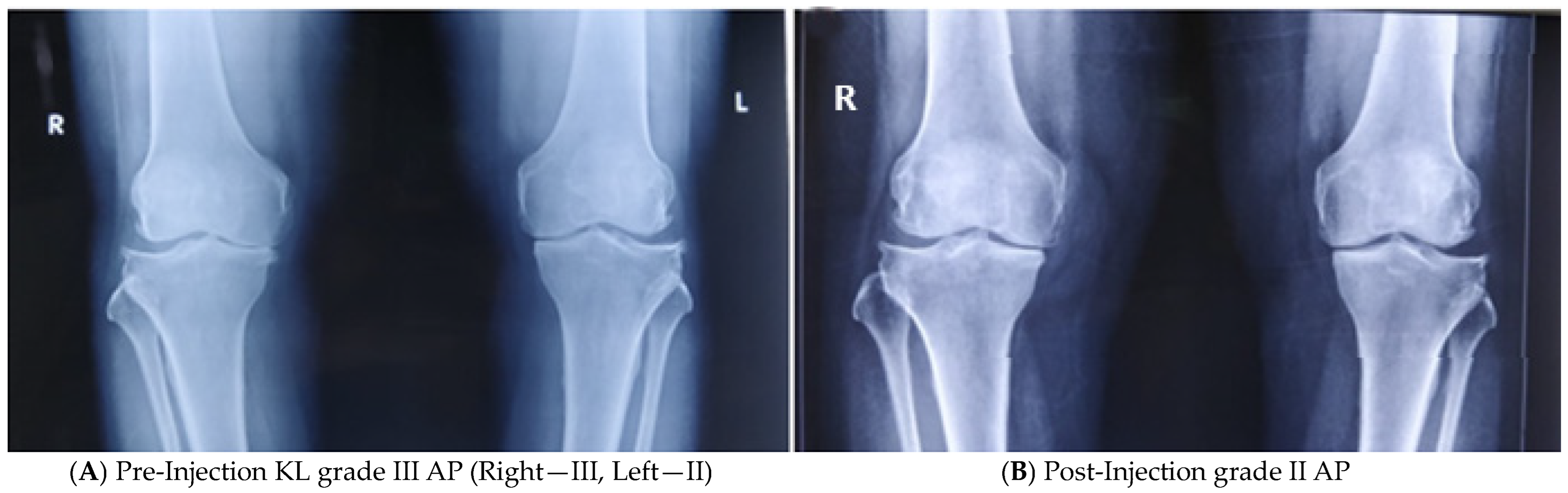
3.4. Objective Assessment
4. Discussion
- The unique features/highlights of the current study
- Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, L. Osteoarthritis of the Knee. N. Engl. J. Med. 2021, 384, 51–59. [Google Scholar] [CrossRef]
- Azad, C.S.; Singh, A.; Pandey, P.; Gambhir, I.S. Osteoarthritis in India: An epidemiologic aspect. Int. J. Recent Sci. Res. 2011, 8, 20918–20922. [Google Scholar]
- Mora, J.C.; Przkora, R.; Cruz-Almeida, Y. Knee osteoarthritis: Pathophysiology and current treatment modalities. J. Pain Res. 2018, 11, 2189. [Google Scholar] [CrossRef]
- Lim, W.B.; Al-Dadah, O. Conservative treatment of knee osteoarthritis: A review of the literature. World J. Orthop. 2022, 13, 212. [Google Scholar] [CrossRef]
- Primorac, D.; Molnar, V.; Matišić, V.; Hudetz, D.; Jeleč, Ž.; Rod, E.; Čukelj, F.; Vidović, D.; Vrdoljak, T.; Dobričić, B.; et al. Comprehensive review of knee osteoarthritis pharmacological treatment and the latest professional societies’ guidelines. Pharmaceuticals 2021, 14, 205. [Google Scholar] [CrossRef]
- Belk, J.W.; Lim, J.J.; Keeter, C.; McCulloch, P.C.; Houck, D.A.; McCarty, E.C.; Frank, R.M.; Kraeutler, M.J. Patients with knee osteoarthritis who receive platelet-rich plasma or bone-marrow aspirate concentrate injections have better outcomes than patients who receive hyaluronic acid: Systematic review and meta-analysis. Arthrosc. J. Arthrosc. Relat. Surg. 2023, 39, 1714–1734. [Google Scholar] [CrossRef]
- Dulic, O.; Rasovic, P.; Lalic, I.; Kecojevic, V.; Gavrilovic, G.; Abazovic, D.; Maric, D.; Miskulin, M.; Bumbasirevic, M. Bone marrow aspirate concentrate versus platelet rich plasma or hyaluronic acid for the treatment of knee osteoarthritis. Medicina 2021, 57, 1193. [Google Scholar] [CrossRef]
- Keeling, L.E.; Belk, J.W.; Kraeutler, M.J.; Kallner, A.C.; Lindsay, A.; McCarty, E.C.; Postma, W.F. Bone marrow aspirate concentrate for the treatment of knee osteoarthritis: A systematic review. Am. J. Sports Med. 2022, 50, 2315–2323. [Google Scholar] [CrossRef]
- Bolia, I.K.; Bougioukli, S.; Hill, W.J.; Trasolini, N.A.; Petrigliano, F.A.; Lieberman, J.R.; Weber, A.E. Clinical efficacy of bone marrow aspirate concentrate versus stromal vascular fraction injection in patients with knee osteoarthritis: A systematic review and meta-analysis. Am. J. Sports Med. 2022, 50, 1451–1461. [Google Scholar] [CrossRef]
- Al-Najar, M.; Khalil, H.; Al-Ajlouni, J.; Al-Antary, E.; Hamdan, M.; Rahmeh, R.; Alhattab, D.; Samara, O.; Yasin, M.; Abdullah, A.A.; et al. Intra-articular injection of expanded autologous bone marrow mesenchymal cells in moderate and severe knee osteoarthritis is safe: A phase I/II study. J. Orthop. Surg. Res. 2017, 12, 190. [Google Scholar] [CrossRef]
- Kon, E.; Boffa, A.; Andriolo, L.; Di Martino, A.; Di Matteo, B.; Magarelli, N.; Marcacci, M.; Onorato, F.; Trenti, N.; Zaffagnini, S.; et al. Subchondral and intra-articular injections of bone marrow concentrate are a safe and effective treatment for knee osteoarthritis: A prospective, multi-center pilot study. Knee Surg. Sports Traumatol. Arthrosc. 2021, 29, 4232–4240. [Google Scholar] [CrossRef]
- Mautner, K.; Bowers, R.; Easley, K.; Fausel, Z.; Robinson, R. Functional outcomes following microfragmented adipose tissue versus bone marrow aspirate concentrate injections for symptomatic knee osteoarthritis. Stem Cells Transl. Med. 2019, 8, 1149–1156. [Google Scholar] [CrossRef]
- Wells, K.; Klein, M.; Hurwitz, N.; Santiago, K.; Cheng, J.; Abutalib, Z.; Beatty, N.; Lutz, G. Cellular and clinical analyses of autologous bone marrow aspirate injectate for knee osteoarthritis: A pilot study. PM&R 2021, 13, 387–396. [Google Scholar]
- Betzler, B.K.; Bin Muhammad Ridzwan Chew, A.H.; Bin Abd Razak, H.R. Intra-articular injection of orthobiologics in patients undergoing high tibial osteotomy for knee osteoarthritis is safe and effective—A systematic review. J. Exp. Orthop. 2021, 8, 83. [Google Scholar] [CrossRef]
- Cavallo, C.; Boffa, A.; Andriolo, L.; Silva, S.; Grigolo, B.; Zaffagnini, S.; Filardo, G. Bone marrow concentrate injections for the treatment of osteoarthritis: Evidence from preclinical findings to the clinical application. Int. Orthop. 2021, 45, 525–538. [Google Scholar] [CrossRef]
- Ding, W.; Xu, Y.Q.; Zhang, Y.; Li, A.X.; Qiu, X.; Wen, H.J.; Tan, H.B. Efficacy and safety of intra-articular cell-based therapy for osteoarthritis: Systematic review and network meta-analysis. Cartilage 2021, 13, 104S–115S. [Google Scholar] [CrossRef]
- Silva, S.; Andriolo, L.; Boffa, A.; Di Martino, A.; Reale, D.; Vara, G.; Miceli, M.; Cavallo, C.; Grigolo, B.; Zaffagnini, S.; et al. Prospective double-blind randomised controlled trial protocol comparing bone marrow aspirate concentrate intra-articular injection combined with subchondral injection versus intra-articular injection alone for the treatment of symptomatic knee osteoarthritis. BMJ Open 2022, 12, e062632. [Google Scholar]
- Rodríguez-Merchán, E.C. Intraarticular injections of mesenchymal stem cells in knee osteoarthritis: A review of their current molecular mechanisms of action and their efficacy. Int. J. Mol. Sci. 2022, 23, 14953. [Google Scholar] [CrossRef]
- Im, G.I. Clinical use of stem cells in orthopaedics. Eur. Cell Mater. 2017, 33, 183–196. [Google Scholar] [CrossRef]
- Gursoy, S.; Akkaya, M.; Simsek, M.E.; Bozkurt, M. Functional outcomes of bone marrow aspirate concentrate application in osteoarthritis of the knee. Int. J. Res. Med. Sci. 2019, 7, 587–591. [Google Scholar] [CrossRef]
- Kim, G.B.; Seo, M.S.; Park, W.T.; Lee, G.W. Bone marrow aspirate concentrate: Its uses in osteoarthritis. Int. J. Mol. Sci. 2020, 21, 3224. [Google Scholar] [CrossRef]
- Themistocleous, G.S.; Chloros, G.D.; Kyrantzoulis, I.M.; Georgokostas, I.A.; Themistocleous, M.S.; Papagelopoulos, P.J.; Savvidou, O.D. Effectiveness of a single intra-articular bone marrow aspirate concentrate (BMAC) injection in patients with grade 3 and 4 knee osteoarthritis. Heliyon 2018, 4, e00871. [Google Scholar] [CrossRef]
- Sundberg, M.; Jansson, L.; Ketolainen, J.; Pihlajamäki, H.; Suuronen, R.; Skottman, H.; Inzunza, J.; Hovatta, O.; Narkilahti, S. CD marker expression profiles of human embryonic stem cells and their neural derivatives, determined using flow-cytometric analysis, reveal a novel CD marker for exclusion of pluripotent stem cells. Stem Cell Res. 2009, 2, 113–124. [Google Scholar] [CrossRef]
- Granero-Molto, F.; Weis, J.A.; Longobardi, L.; Spagnoli, A. Role of mesenchymal stem cells in regenerative medicine: Application to bone and cartilage repair. Expert Opin. Biol. Ther. 2008, 8, 255–268. [Google Scholar] [CrossRef]
- Davatchi, F.; Abdollahi, B.S.; Mohyeddin, M.; Shahram, F.; Nikbin, B. Mesenchymal stem cell therapy for knee osteoarthritis. Preliminary report of four patients. Int. J. Rheum. Dis. 2011, 14, 211–215. [Google Scholar] [CrossRef]
- Boffa, A.; Di Martino, A.; Andriolo, L.; De Filippis, R.; Poggi, A.; Kon, E.; Zaffagnini, S.; Filardo, G. Bone marrow aspirate concentrate injections provide similar results versus viscosupplementation up to 24 months of follow-up in patients with symptomatic knee osteoarthritis. A randomized controlled trial. Knee Surg. Sports Traumatol. Arthrosc. 2022, 30, 3958–3967. [Google Scholar] [CrossRef]
- McCarrel, T.; Fortier, L. Temporal growth factor release from platelet-rich plasma, trehalose lyophilized platelets, and bone marrow aspirate and their effect on tendon and ligament gene expression. J. Orthop. Res. 2009, 27, 1033–1042. [Google Scholar] [CrossRef]
- Sampson, S.; Botto-van Bemden, A.; Aufiero, D. Stem cell therapies for treatment of cartilage and bone disorders: Osteoarthritis, avascular necrosis, and non-union fractures. PM&R 2015, 7, S26–S32. [Google Scholar]
- Pintore, A.; Notarfrancesco, D.; Zara, A.; Oliviero, A.; Migliorini, F.; Oliva, F.; Maffulli, N. Intra-articular injection of bone marrow aspirate concentrate (BMAC) or adipose-derived stem cells (ADSCs) for knee osteoarthritis: A prospective comparative clinical trial. J. Orthop. Surg. Res. 2023, 18, 350. [Google Scholar] [CrossRef]
- Im, G.I. Regeneration of articular cartilage using adipose stem cells. J. Biomed. Mater. Res. Part A 2016, 104, 1830–1844. [Google Scholar] [CrossRef]
- Vinatier, C.; Bouffi, C.; Merceron, C.; Gordeladze, J.; Brondello, J.M.; Jorgensen, C.; Weiss, P.; Guicheux, J.; Noël, D. Cartilage tissue engineering: Towards a biomaterial-assisted mesenchymal stem cell therapy. Curr. Stem Cell Res. Ther. 2009, 4, 318–329. [Google Scholar] [CrossRef]
- Woodell-May, J.; Steckbeck, K.; King, W. Potential Mechanism of Action of Current Point-of-Care Autologous Therapy Treatments for Osteoarthritis of the Knee—A Narrative Review. Int. J. Mol. Sci. 2021, 22, 2726. [Google Scholar] [CrossRef]
- Hernigou, J.; Vertongen, P.; Rasschaert, J.; Hernigou, P. Role of scaffolds, subchondral, intra-articular injections of fresh autologous bone marrow concentrate regenerative cells in treating human knee cartilage lesions: Different approaches and different results. Int. J. Mol. Sci. 2021, 22, 3844. [Google Scholar] [CrossRef]
- Chahla, J.; Mannava, S.; Cinque, M.E.; Geeslin, A.G.; Codina, D.; LaPrade, R.F. Bone marrow aspirate concentrate harvesting and processing technique. Arthrosc. Tech. 2017, 6, e441-5. [Google Scholar] [CrossRef]
- Jeyaraman, M.; Bingi, S.K.; Muthu, S.; Jeyaraman, N.; Packkyarathinam, R.P.; Ranjan, R.; Sharma, S.; Jha, S.K.; Khanna, M.; Rajendran, S.N.; et al. Impact of the process variables on the yield of mesenchymal stromal cells from bone marrow aspirate concentrate. Bioengineering 2022, 9, 57. [Google Scholar] [CrossRef]
- El-Kadiry, A.E.; Lumbao, C.; Salame, N.; Rafei, M.; Shammaa, R. Bone marrow aspirate concentrate versus platelet-rich plasma for treating knee osteoarthritis: A one-year non-randomized retrospective comparative study. BMC Musculoskelet. Disord. 2022, 23, 23. [Google Scholar] [CrossRef]
- Anzillotti, G.; Conte, P.; Di Matteo, B.; Bertolino, E.M.; Marcacci, M.; Kon, E. Injection of biologic agents for treating severe knee osteoarthritis: Is there a chance for a good outcome? A systematic review of clinical evidence. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 5447–5459. [Google Scholar]
- Baria, M.; Pedroza, A.; Kaeding, C.; Durgam, S.; Duerr, R.; Flanigan, D.; Borchers, J.; Magnussen, R. Platelet-rich plasma versus microfragmented adipose tissue for knee osteoarthritis: A randomized controlled trial. Orthop. J. Sports Med. 2022, 10, 23259671221120678. [Google Scholar] [CrossRef]
- Weber, A.E.; Bolia, I.K.; Trasolini, N.A. Biological strategies for osteoarthritis: From early diagnosis to treatment. Int. Orthop. 2021, 45, 335–344. [Google Scholar] [CrossRef]
- Chahla, J.; Dean, C.S.; Moatshe, G.; Pascual-Garrido, C.; Serra Cruz, R.; LaPrade, R.F. Concentrated Bone Marrow Aspirate for the Treatment of Chondral Injuries and Osteoarthritis of the Knee: A Systematic Review of Outcomes. Orthop. J. Sports Med. 2016, 4, 2325967115625481. [Google Scholar] [CrossRef]
- Lopa, S.; Colombini, A.; Moretti, M.; de Girolamo, L. Injective mesenchymal stem cell-based treatments for knee osteoarthritis: From mechanisms of action to current clinical evidences. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 2003–2020. [Google Scholar] [CrossRef]
- Wakitani, S.; Nawata, M.; Tensho, K.; Okabe, T.; Machida, H.; Ohgushi, H. Repair of articular cartilage defects in the patello-femoral joint with autologous bone marrow mesenchymal cell transplantation: Three case reports involving nine defects in five knees. J. Tissue Eng. Regen. Med. 2007, 1, 74–79. [Google Scholar] [CrossRef]
- Haleem, A.M.; Singergy, A.A.E.; Sabry, D.; Atta, H.M.; Rashed, L.A.; Chu, C.R.; El Shewy, M.T.; Azzam, A.; Aziz, M.T.A. The clinical use of human culture–expanded autologous bone marrow mesenchymal stem cells transplanted on platelet-rich fibrin glue in the treatment of articular cartilage defects: A pilot study and preliminary results. Cartilage 2010, 1, 253–261. [Google Scholar] [CrossRef]
- Kasemkijwattana, C.; Hongeng, S.; Kesprayura, S.; Rungsinaporn, V.; Chaipinyo, K.; Chansiri, K. Autologous bone marrow mesenchymal stem cells implantation for cartilage defects: Two cases report. J. Med. Assoc. Thail. 2011, 94, 395. [Google Scholar]
- Giannini, S.; Buda, R.; Vannini, F.; Cavallo, M.; Grigolo, B. One-step bone marrow-derived cell transplantation in talar osteochondral lesions. Clin. Orthop. Relat. Res. 2009, 467, 3307–3320. [Google Scholar] [CrossRef]
- Giannini, S.; Buda, R.; Cavallo, M.; Ruffilli, A.; Cenacchi, A.; Cavallo, C.; Vannini, F. Cartilage repair evolution in post-traumatic osteochondral lesions of the talus: From open field autologous chondrocyte to bone-marrow-derived cells transplantation. Injury 2010, 41, 1196–1203. [Google Scholar] [CrossRef]
- Gobbi, A.; Karnatzikos, G.; Scotti, C.; Mahajan, V.; Mazzucco, L.; Grigolo, B. One-step cartilage repair with bone marrow aspirate concentrated cells and collagen matrix in full-thickness knee cartilage lesions: Results at 2-year follow-up. Cartilage 2011, 2, 286–299. [Google Scholar] [CrossRef]
- Pabinger, C.; Lothaller, H.; Kobinia, G.S. Intra-articular injection of bone marrow aspirate concentrate (mesenchymal stem cells) in KL grade III and IV knee osteoarthritis: 4 year results of 37 knees. Sci. Rep. 2024, 14, 2665. [Google Scholar] [CrossRef]
- Shapiro, S.A.; Kazmerchak, S.E.; Heckman, M.G.; Zubair, A.C.; O’Connor, M.I. A prospective, single-blind, placebo-controlled trial of bone marrow aspirate concentrate for knee osteoarthritis. Am. J. Sports Med. 2017, 45, 82–90. [Google Scholar] [CrossRef]
- Garay-Mendoza, D.; Villarreal-Martínez, L.; Garza-Bedolla, A.; Pérez-Garza, D.M.; Acosta-Olivo, C.; Vilchez-Cavazos, F.; Diaz-Hutchinson, C.; Gómez-Almaguer, D.; Jaime-Pérez, J.C.; Mancías-Guerra, C. The effect of intra-articular injection of autologous bone marrow stem cells on pain and knee function in patients with osteoarthritis. Int. J. Rheum. Dis. 2018, 21, 140–147. [Google Scholar] [CrossRef]
- Anz, A.W.; Hubbard, R.; Rendos, N.K.; Everts, P.A.; Andrews, J.R.; Hackel, J.G. Bone marrow aspirate concentrate is equivalent to platelet-rich plasma for the treatment of knee osteoarthritis at 1 year: A prospective, randomized trial. Orthop. J. Sports Med. 2020, 8, 2325967119900958. [Google Scholar] [CrossRef]
| Characters | Cases (n = 132) |
|---|---|
| Age (Years) | 45 ± 8.8 |
| Sex: (M:F) | 92:40 |
| Height (cm) | 155.7 ± 3.1 |
| Weight (kg) | 74.7 ± 8.7 |
| BMI (kg/m2) | 30.8 ± 3.4 |
| Age of Onset | 42 ± 8 |
| Patients with Unilateral KOA | 80% |
| Patients with Bilateral KOA | 20% |
| Patients with KL grade II | 50% |
| Patients with KL grade III | 50% |
| Pre-Injection | 3 Months | 6 Months | 12 Months | |
|---|---|---|---|---|
| Mean | 8.3 | 3.73 | 1.00 | 1.25 |
| SD | 1.49 | 1.49 | 0 | 1.10 |
| SEM | 0.12 | 0.12 | 0 | 0.09 |
| KL Grade II (n = 66) | KL Grade III (n = 66) | |||||||
|---|---|---|---|---|---|---|---|---|
| WOMAC Index Score (0–96) | Pre-Injection | Post-Injection | Pre-Injection | Post-Injection | ||||
| 3 Months | 6 Months | 12 Months | 3 Months | 6 Months | 12 Months | |||
| Mild (<35) | 0 | 100 | 100 | 100 | 0 | 60 | 100 | 90 |
| Moderate (36–75) | 100 | 0 | 0 | 0 | 25 | 40 | 0 | 10 |
| Severe (76–96) | 0 | 0 | 0 | 0 | 75 | 0 | 0 | 0 |
| Pre-Injection | 3 Months | 6 Months | 12 Months | |
|---|---|---|---|---|
| Mean | 55.59 | 22.34 | 20.83 | 21.60 |
| SD | 10.44 | 8.24 | 9.39 | 8.55 |
| SEM | 1.28 | 1.01 | 1.15 | 1.05 |
| Pre-Injection | 3 Months | 6 Months | 12 Months | |
|---|---|---|---|---|
| Mean | 76.84 | 33.36 | 21.83 | 25.31 |
| SD | 14.33 | 17.15 | 8.26 | 13.15 |
| SEM | 1.76 | 2.11 | 1.01 | 1.61 |
| Authors | Cell Type | Dosage | Study Duration (Months) | No. of Joints | Pre-Injection (WOMAC) | 3 Months (WOMAC) | 6 Months (WOMAC) | 12 Months (WOMAC) | Pre-Injection (VAS) | 3 Months (VAS) | 6 Months (VAS) | 12 Months (VAS) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Shapiro 2017 [49] | BMAC | 6mL | 6 | 25 | NA | NA | NA | NA | 31.0 | 15.0 | 11.0 | NA |
| Garay Mendoza 2018 [50] | BMAC | 10 mL | 6 | 26 | 35.9 | 7.9 | 28.0 | 7.9 | 52.7 | 9.2 | 42.8 | NA |
| Mautner 2019 [12] | BMAC | 8 mL | 6 | 58 | NA | NA | NA | NA | 39 | 25 | 14 | NA |
| Anz 2020 [51] | BMAC | 7 mL | 12 | 45 | 35.3 | 19.4 | 15.9 | 19.4 | NA | NA | NA | NA |
| Current Study | BMAC | 7 ml | 12 | 158 | 53.2 | 21.5 | 17.2 | 18 | 6.82 | 3.8 | 2.6 | 3.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subramanyam, K.; Poornima, S.; Kumar, S.; Hasan, Q. Short-Term Clinical Results of Single-Injection Autologous Bone Marrow Aspirate Concentrate (BMAC) as a Therapeutic Option/Tool in Knee Osteoarthritis. Biologics 2024, 4, 218-231. https://doi.org/10.3390/biologics4020015
Subramanyam K, Poornima S, Kumar S, Hasan Q. Short-Term Clinical Results of Single-Injection Autologous Bone Marrow Aspirate Concentrate (BMAC) as a Therapeutic Option/Tool in Knee Osteoarthritis. Biologics. 2024; 4(2):218-231. https://doi.org/10.3390/biologics4020015
Chicago/Turabian StyleSubramanyam, Krishna, Subhadra Poornima, Satish Kumar, and Qurratulain Hasan. 2024. "Short-Term Clinical Results of Single-Injection Autologous Bone Marrow Aspirate Concentrate (BMAC) as a Therapeutic Option/Tool in Knee Osteoarthritis" Biologics 4, no. 2: 218-231. https://doi.org/10.3390/biologics4020015
APA StyleSubramanyam, K., Poornima, S., Kumar, S., & Hasan, Q. (2024). Short-Term Clinical Results of Single-Injection Autologous Bone Marrow Aspirate Concentrate (BMAC) as a Therapeutic Option/Tool in Knee Osteoarthritis. Biologics, 4(2), 218-231. https://doi.org/10.3390/biologics4020015





