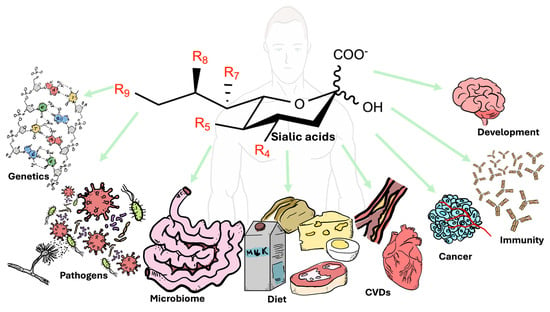
Sialic Acids in Health and Disease
Abstract
1. Introduction
1.1. Analysis of Sialic Acids
1.2. Structures of Sialic Acids
1.3. Synthesis
1.4. Functions
1.5. Genetic Disorders
1.5.1. UDP-GlcNAc 2-Epimerase/ManNAc Kinase (GNE) Myopathy
1.5.2. Free Sialic Acid Storage Disorder (FSASD)
2. Diet
Microbiome and Sialic Acids
3. Interactions with Pathogens
3.1. Sialic Acids and Bacteria
3.1.1. Porphyromonas gingivalis
3.1.2. Streptococcus pneumoniae
3.1.3. Haemophilus influenzae
3.1.4. Pseudomonas aeruginosa
3.1.5. Clostridium perfringens
3.1.6. Neisseria gonorrhoeae
3.2. Sialic Acids and Viral Interactions
3.2.1. Influenza Virus
3.2.2. SARS-CoV-2
3.3. Sialic Acids and Fungi
3.3.1. Cryptococcus neoformans
3.3.2. Histoplasma capsulatum
3.3.3. Candida albicans
3.4. Sialic Acids and Parasites
3.4.1. Trypanosoma cruzi
3.4.2. Plasmodium spp.
4. Inflammatory Processes
4.1. Cardiovascular Disease
4.2. Cancer
| Title | Approach | Year | Findings | Reference | |
|---|---|---|---|---|---|
| Animal | Human | ||||
| Relation of serum sialic acid to blood coagulation activity in type 2 diabetes | x | 2002 | Serum sialic acid level is positive correlated with (a) blood coagulation activity and (b) circulatory fibrinogen levels. | [147] | |
| High fat diet-induced inflammation and oxidative stress are attenuated by N-acetylneuraminic acid in rats | x | 2005 | Neu5Ac could be useful for preventing inflammation and oxidative stress associated with hyperlipidemia. | [395] | |
| Relationship between Sialic acid and metabolic variables in Indian type 2 diabetic patients | x | 2005 | Among Indian patients with type 2 diabetes, elevated levels of serum and urinary sialic acid, as well as microalbumin, are strongly associated with microvascular complications. | [396] | |
| Percentage of body fat and plasma glucose predict plasma sialic acid concentration in type 2 diabetes mellitus | x | 2006 | Percentage of body fat correlates with plasma sialic acid levels and contributes to elevated sialic acid concentrations in patients with type 2 diabetes mellitus. | [146] | |
| Sialic acid and oxidizability of lipid and proteins and antioxidant status in patients with coronary artery disease | x | 2007 | Patients with CAD show significant increases in total sialic acid levels and markers of oxidative stress. Furthermore, higher TSA levels correlate with greater CAD severity. | [370] | |
| N-Acetylneuraminic Acid Supplementation Prevents High Fat Diet-Induced Insulin Resistance in Rats through Transcriptional and Nontranscriptional Mechanisms | x | 2015 | Administering a low dose of sialic acids prevents insulin resistance in rats fed a high-fat diet. | [397] | |
| N-Acetylneuraminic acid attenuates hypercoagulation on high fat diet-induced hyperlipidemic rats | x | 2015 | Data show that Neu5Ac prevents high-fat diet-induced high blood lipid levels and associated increased blood clotting in rats. | [385] | |
| Sialidase downregulation reduces non-HDL cholesterol, inhibits leukocyte transmigration, and attenuates atherosclerosis in ApoE knockout mice | x | 2018 | Decreasing Neu1 expression or function reduces atherosclerosis in mice by substantially impacting lipid metabolism and inflammation. | [398] | |
| Supplementation with the Sialic Acid Precursor N-Acetyl-D-Mannosamine Breaks the Link between Obesity and Hypertension | x | x | 2019 | Interventions targeting hyposialylated IgG and FcγRIIB, such as ManNAc supplementation, could potentially break link between obesity and hypertension and provide novel therapeutic approaches. | [386] |
| Neuraminidases 1 and 3 trigger atherosclerosis by desialylating low-density lipoproteins and increasing their uptake by macrophages | x | x | 2021 | Neuraminidases 1 and 3 initiate atherosclerosis and formation of aortic fatty streaks. | [149] |
| Sialic acid metabolism as a potential therapeutic target of atherosclerosis Sialic acids Neu5Ac and KDN in adipose tissue samples from individuals following habitual vegetarian or non-vegetarian dietary patterns | x | 2023 | Concentrations of Neu5Ac are significantly higher in vegans and lacto-ovo-vegetarians compared to non-vegetarians. Significant inverse association observed between KDN levels and body mass index. | [39] | |
Colorectal Cancer
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADP | adenosine diphosphate |
| ATP | adenosine triphosphate |
| CMAS | CMP-N-acetylneuraminate synthetase |
| Cmah | cytidine monophosphate-N-acetylneuraminic acid hydroxylase; CMP |
| CMP | cytidine monophosphate |
| CTP | cytidine triphosphate |
| GalNAc | N-acetylgalactosamine |
| GlcNAc | N-acetylglucosamine |
| GNE | UDP-GlcNAc 2-epimerase/ManNAc kinase |
| GD2 | disialoganglioside |
| GM2 | ganglioside-mono sialic acid 2 |
| GlmM | phosphoglucosamine mutase |
| GlmS | glucosamine-6-phosphate synthase |
| GlmU | glucosamine-1-phosphate acetyltransferase/N-acetylglucosamine-1-phosphate uridyltransferase |
| HBP | hexosamine biosynthesis pathway |
| ITAM | immunoreceptor tyrosine-based activatory motif |
| ITIM | immunoreceptor tyrosine-based inhibitory motif |
| KDN | 2-keto-3-deoxy-D-glycero-D-galacto-nononic acid |
| LC-MS/MS | liquid chromatography with tandem mass spectrometry |
| Man | mannose |
| ManNAc | N-acetylmannosamine |
| MS | mass spectrometry |
| NANP | N-acetylneuraminic acid phosphatase |
| NANS | N-acetylneuraminic acid 9-phosphate synthase |
| Neu | neuraminic acid |
| Neu5Ac | N-acetylneuraminic acid |
| Neu5Gc | N-glycolylneuraminic acid |
| NeuA | sialic acid synthase A |
| NeuB | sialic acid synthase B |
| NeuC | sialic acid synthase C |
| NulO | nonulosonic acid |
| PEP | phosphoenolpyruvate |
| PGI | glucose-6-phosphate isomerase |
| ROS | reactive oxygen species |
| SLeA | sialyl-Lewis A |
| SLeX | sialyl-Lewis X |
| STn | sialyl-Tn |
| SCFAs | short-chain fatty acids |
| UDP | uridine diphosphate |
| SARS-CoV-2 | severe-acute-respiratory-syndrome-related coronavirus 2. |
References
- Tarbell, J.M.; Cancel, L.M. The glycocalyx and its significance in human medicine. J. Intern. Med. 2016, 280, 97–113. [Google Scholar] [CrossRef]
- Lucas, T.M.; Gupta, C.; Altman, M.O.; Sanchez, E.; Naticchia, M.R.; Gagneux, P.; Singharoy, A.; Godula, K. Mucin-mimetic glycan arrays integrating machine learning for analyzing receptor pattern recognition by influenza A viruses. Chem 2021, 7, 3393–3411. [Google Scholar] [CrossRef]
- Ebong, E.E.; Macaluso, F.P.; Spray, D.C.; Tarbell, J.M. Imaging the Endothelial Glycocalyx In Vitro by Rapid Freezing/Freeze Substitution Transmission Electron Microscopy. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1908–1915. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zhang, Q.; Li, J.; Zheng, Q. LC-MS in combination with DMBA derivatization for sialic acid speciation and distribution analysis in fish tissues. Anal. Methods 2020, 12, 2221–2227. [Google Scholar] [CrossRef]
- Möckl, L.; Pedram, K.; Roy, A.R.; Krishnan, V.; Gustavsson, A.-K.; Dorigo, O.; Bertozzi, C.R.; Moerner, W.E. Quantitative Super-Resolution Microscopy of the Mammalian Glycocalyx. Dev. Cell 2019, 50, 57–72.e6. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.L.; Toukach, P.; Bolton, E.; Chen, X.; Frank, M.; Lütteke, T.; Knirel, Y.; Schoenhofen, I.; Varki, A.; Vinogradov, E.; et al. The SNFG Discussion Group. Cataloging natural sialic acids and other nonulosonic acids (NulOs), and their representation using the Symbol Nomenclature for Glycans. Glycobiology 2023, 33, 99–103. [Google Scholar] [CrossRef]
- Kooner, A.S.; Yu, H.; Chen, X. Synthesis of N-glycolylneuraminic acid (Neu5Gc) and its glycosides. Front. Immunol. 2019, 10, 2004. [Google Scholar] [CrossRef]
- Lewis, A.L.; Chen, X.; Schnaar, R.L.; Varki, A. Sialic acids and other Nonulosonic Acids. In Essential of Glycobiology, 4th ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2022; pp. 316–344. [Google Scholar]
- Chen, X.; Varki, A. Advances in the biology and chemistry of sialic acids. ACS Chem. Biol. 2010, 5, 163–176. [Google Scholar] [CrossRef]
- Traving, C.; Schauer, R. Structure, function and metabolism of sialic acids. Cell. Mol. Life Sci. 1998, 54, 1330–1349. [Google Scholar] [CrossRef]
- Inoue, S.; Lin, S.L.; Chang, T.; Wu, S.H.; Yao, C.W.; Chu, T.Y.; Troy, F.A.; Inoue, Y. Identification of free deaminated sialic acid (2-keto-3-deoxy-D-glycero- D-galacto-nononic acid) in human red blood cells and its elevated expression in fetal cord red blood cells and ovarian cancer cells. J. Biol. Chem. 1998, 273, 27199–27204. [Google Scholar] [CrossRef][Green Version]
- Inoue, S.; Kitajima, K.; Sato, C.; Go, S. Human KDN (Deaminated Neuraminic Acid) and Its Elevated Expression in Cancer Cells: Mechanism and Significance. In The Molecular Immunology of Complex Carbohydrates-3; Wu, A.M., Ed.; Springer: Boston, MA, USA, 2011; Volume 705, pp. 669–678. [Google Scholar]
- Schauer, R. Sialic acids: Fascinating sugars in higher animals and man. Zoology 2004, 107, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Persat, F.; Bouhours, J.-F.; Petavy, A.-F.; Mojon, M. Gangliosides of Echinococcus multilocularis metacestodes. Biochim. Biophys. Acta BBA—Mol. Basis Dis. 1994, 1225, 297–303. [Google Scholar] [CrossRef]
- Yeşilyurt, B.; Şahar, U.; Deveci, R. Determination of the type and quantity of sialic acid in the egg jelly coat of the sea urchin Paracentrotus lividus using capillary LC-ESI-MS/MS. Mol. Reprod. Dev. 2015, 82, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Kitamura, H.; Sugiyama, K. Occurrence of gangliosides in the common squid and pacific octopus among protostomia. Biochim. Biophys. Acta BBA—Biomembr. 2001, 1511, 271–280. [Google Scholar] [CrossRef]
- Elayabharathi, T.; Vinoliya Josephine Mary, J.; Mary Mettilda Bai, S. Characterization of a novel O-acetyl sialic acid specific lectin from the hemolymph of the marine crab, Atergatis integerrimus (Lamarck, 1818). Fish Shellfish Immunol. 2020, 106, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Guerardel, Y.; Chang, L.-Y.; Fujita, A.; Coddeville, B.; Maes, E.; Sato, C.; Harduin-Lepers, A.; Kubokawa, K.; Kitajima, K. Sialome analysis of the cephalochordate Branchiostoma belcheri, a key organism for vertebrate evolution. Glycobiology 2012, 22, 479–491. [Google Scholar] [CrossRef]
- Varki, A. Glycan-based interactions involving vertebrate sialic-acid-recognizing proteins. Nature 2007, 446, 1023–1029. [Google Scholar] [CrossRef]
- Eneva, R.; Engibarov, S.; Abrashev, R.; Krumova, E.; Angelova, M. Sialic acids, sialoconjugates and enzymes of their metabolism in fungi. Biotechnol. Biotechnol. Equip. 2021, 35, 346–357. [Google Scholar] [CrossRef]
- Ghosh, S. Sialic acid and biology of life: An introduction. In Sialic Acids and Sialoglycoconjugates in the Biology of Life, Health and Disease; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–61. [Google Scholar]
- Campetella, O.; Buscaglia, C.A.; Mucci, J.; Leguizamón, M.S. Parasite-host glycan interactions during Trypanosoma cruzi infection: Trans-Sialidase rides the show. Biochim. Biophys. Acta BBA—Mol. Basis Dis. 2020, 1866, 165692. [Google Scholar] [CrossRef]
- Iijima, R.; Takahashi, H.; Namme, R.; Ikegami, S.; Yamazaki, M. Novel biological function of sialic acid (N-acetylneuraminic acid) as a hydrogen peroxide scavenger. FEBS Lett. 2004, 561, 163–166. [Google Scholar] [CrossRef]
- Yang, H.; Lu, L.; Chen, X. An overview and future prospects of sialic acids. Biotechnol. Adv. 2021, 46, 107678. [Google Scholar] [CrossRef]
- Schauer, R. Achievements and challenges of sialic acid research. Glycoconj. J. 2000, 17, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Gopaul, K.P.; Crook, M.A. Sialic acid: A novel marker of cardiovascular disease? Clin. Biochem. 2006, 39, 667–681. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Schweiker, S.; Powell, K.; Levonis, S. An efficient and robust HPLC method to determine the sialylation levels of human epithelial cells. PLoS ONE 2022, 17, e0257178. [Google Scholar] [CrossRef]
- Szabo, Z.; Bones, J.; Guttman, A.; Glick, J.; Karger, B.L. Sialic acid speciation using capillary electrophoresis: Optimization of analyte derivatization and separation. Anal. Chem. 2012, 84, 7638–7642. [Google Scholar] [CrossRef]
- Tang, S.; Li, L.; Wang, R.; Regmi, S.; Zhang, X.; Yang, G.; Ju, J. A Schematic Colorimetric Assay for Sialic Acid Assay Based on PEG-Mediated Interparticle Crosslinking Aggregation of Gold Nanoparticles. Biosensors 2023, 13, 164. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.B.S.; de Paula, N.T.G.; da Silva, P.A.B.; de Souza, G.C.S.; Paim, A.P.S.; Lavorante, A.F. A spectrophotometric procedure for sialic acid determination in milk employing a flow-batch analysis system with direct heating. Microchem. J. 2019, 147, 782–788. [Google Scholar] [CrossRef]
- Wang, M.-H.; Wang, Z.-F.; Yuan, M.; Yang, C.-G.; Wang, D.-L.; Wang, S.-Q. Changes in the Serum and Tissue Levels of Free and Conjugated Sialic Acids, Neu5Ac, Neu5Gc, and KDN in Mice after the Oral Administration of Edible Bird’s Nests: An LC–MS/MS Quantitative Analysis. Separations 2024, 11, 107. [Google Scholar] [CrossRef]
- Ye, L.; Mu, L.; Li, G.; Bao, Y. Assessing sialic acid content in food by hydrophilic chromatography-high performance liquid chromatography. J. Food Compos. Anal. 2020, 87, 103393. [Google Scholar] [CrossRef]
- Wang, L.; Wang, D.; Zhou, X.; Wu, L.; Sun, X.-L. Systematic investigation of quinoxaline derivatization of sialic acids and their quantitation applicability using high performance liquid chromatography. RSC Adv. 2014, 4, 45797–45803. [Google Scholar] [CrossRef]
- Reversed-Phase High-Performance Liquid Chromatography: A Robust Method for Quantitation of Fluorescently Labeled and Derivatized Sialic Acids Isolated from Mouse Liver. Available online: https://app.jove.com/v/21130/reversed-phase-high-performance-liquid-chromatography-robust-method?playlist=2655796&isFrc=true (accessed on 27 January 2025).
- Palmisano, G.; Larsen, M.R.; Packer, N.H.; Thaysen-Andersen, M. Structural analysis of glycoprotein sialylation-part II: LC-MS based detection. RSC Adv. 2013, 3, 22706–22726. [Google Scholar] [CrossRef]
- Wang, F.; Xie, B.; Wang, B.; Troy, F.A. LC-MS/MS glycomic analyses of free and conjugated forms of the sialic acids, Neu5Ac, Neu5Gc and KDN in human throat cancers. Glycobiology 2015, 25, 1362–1374. [Google Scholar] [CrossRef] [PubMed]
- van der Ham, M.; Prinsen, B.H.C.M.T.; Huijmans, J.G.M.; Abeling, N.G.G.M.; Dorland, B.; Berger, R.; de Koning, T.J.; de Sain-van der Velden, M.G.M. Quantification of free and total sialic acid excretion by LC-MS/MS. J. Chromatogr. B Analyt. Technol. Biomed. Life. Sci. 2007, 848, 251–257. [Google Scholar] [CrossRef]
- Zhou, S.; Dong, X.; Veillon, L.; Huang, Y.; Mechref, Y. LC-MS/MS analysis of permethylated N-glycans facilitating isomeric characterization. Anal. Bioanal. Chem. 2017, 409, 453–466. [Google Scholar] [CrossRef]
- Guerrero-Flores, G.N.; Pacheco, F.J.; Boskovic, D.S.; Pacheco, S.O.S.; Zhang, G.; Fraser, G.E.; Miles, F.L. Sialic acids Neu5Ac and KDN in adipose tissue samples from individuals following habitual vegetarian or non-vegetarian dietary patterns. Sci. Rep. 2023, 13, 12593. [Google Scholar] [CrossRef]
- Ho, C.; Lam, C.; Chan, M.; Cheung, R.; Law, L.; Lit, L.; Ng, K.; Suen, M.; Tai, H. Electrospray Ionisation Mass Spectrometry: Principles and Clinical Applications. Clin. Biochem. Rev. 2003, 24, 3–12. [Google Scholar]
- Mellon, F.A. MASS SPECTROMETRY|Principles and Instrumentation. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Oxford, UK, 2003; pp. 3739–3749. ISBN 978-0-12-227055-0. [Google Scholar]
- Kaklamanos, G.; Aprea, E.; Theodoridis, G. 11—Mass spectrometry: Principles and instrumentation. In Chemical Analysis of Food, 2nd ed.; Pico, Y., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 525–552. [Google Scholar]
- Banerjee, S.; Mazumdar, S. Electrospray Ionization Mass Spectrometry: A Technique to Access the Information beyond the Molecular Weight of the Analyte. Int. J. Anal. Chem. 2012, 2012, 282574. [Google Scholar] [CrossRef]
- Thomas, S.N.; French, D.; Jannetto, P.J.; Rappold, B.A.; Clarke, W.A. Liquid chromatography–tandem mass spectrometry for clinical diagnostics. Nat. Rev. Methods Primer 2022, 2, 96. [Google Scholar] [CrossRef]
- Bhardwaj, C.; Hanley, L. Ion sources for mass spectrometric identification and imaging of molecular species. Nat. Prod. Rep. 2014, 31, 756–767. [Google Scholar] [CrossRef]
- Malik, A.K.; Kumar, R. Heena Spectroscopy: Types. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 64–72. ISBN 978-0-12-384953-3. [Google Scholar]
- Ravelli, M.N.; Shriver, T.C.; Schoeller, D.A. Doubly labeled water. In Encyclopedia of Human Nutrition, 4th ed.; Caballero, B., Ed.; Academic Press: Oxford, UK, 2023; pp. 52–61. ISBN 978-0-323-90816-0. [Google Scholar]
- Nishikaze, T. Sialic acid derivatization for glycan analysis by mass spectrometry. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2019, 95, 523–537. [Google Scholar] [CrossRef]
- Priego-Capote, F.; Orozco-Solano, M.I.; Calderón-Santiago, M.; Luque de Castro, M.D. Quantitative determination and confirmatory analysis of N-acetylneuraminic and N-glycolylneuraminic acids in serum and urine by solid-phase extraction on-line coupled to liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2014, 1346, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Padler-Karavani, V. Evolution of sialic acids: Implications in xenotransplant biology. Xenotransplantation 2018, 25, e12424. [Google Scholar] [CrossRef]
- Angata, T.; Varki, A. Chemical diversity in the sialic acids and related α-keto acids: An evolutionary perspective. Chem. Rev. 2002, 102, 439–469. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, J.; Liu, Y.; Xu, D. Sialic acid metabolism as a potential therapeutic target of atherosclerosis. Lipids Health Dis. 2019, 18, 173. [Google Scholar] [CrossRef] [PubMed]
- Soulillou, J.P.; Süsal, C.; Döhler, B.; Opelz, G. No increase in colon cancer risk following induction with neu5Gc-bearing rabbit anti-T cell igg (ATG) in recipients of kidney transplants. Cancers 2018, 10, 324. [Google Scholar] [CrossRef]
- Inoue, S.; Kitajima, K.; Inoue, Y. Identification of 2-keto-3-deoxy-D-glycero-D-galactonononic acid (KDN, deaminoneuraminic acid) residues in mammalian tissues and human lung carcinoma cells. Chemical evidence of the occurrence of KDN glycoconjugates in mammals. J. Biol. Chem. 1996, 271, 24341–24344. [Google Scholar] [CrossRef]
- Inoue, S.; Kitajima, K. KDN (Deaminated neuraminic acid): Dreamful past and exciting future of the newest member of the sialic acid family. Glycoconj. J. 2006, 23, 277–290. [Google Scholar] [CrossRef]
- Chen, Y.; Pan, L.; Liu, N.; Troy, F.A.; Wang, B. LC-MS/MS quantification of N-acetylneuraminic acid, N-glycolylneuraminic acid and ketodeoxynonulosonic acid levels in the urine and potential relationship with dietary sialic acid intake and disease in 3-to 5-year-old children. Br. J. Nutr. 2014, 111, 332–341. [Google Scholar] [CrossRef]
- Li, D.; Lin, Q.; Luo, F.; Wang, H. Insights into the Structure, Metabolism, Biological Functions and Molecular Mechanisms of Sialic Acid: A Review. Foods 2024, 13, 145. [Google Scholar] [CrossRef]
- Guin, S.K.; Velasco-Torrijos, T.; Dempsey, E. Explorations in a galaxy of sialic acids: A review of sensing horizons, motivated by emerging biomedical and nutritional relevance. Sens. Diagn. 2022, 1, 10–70. [Google Scholar] [CrossRef]
- Vos, G.M.; Hooijschuur, K.C.; Li, Z.; Fjeldsted, J.; Klein, C.; de Vries, R.P.; Toraño, J.S.; Boons, G.-J. Sialic acid O-acetylation patterns and glycosidic linkage type determination by ion mobility-mass spectrometry. Nat. Commun. 2023, 14, 6795. [Google Scholar] [CrossRef] [PubMed]
- Chou, W.K.; Hinderlich, S.; Reutter, W.; Tanner, M.E. Sialic Acid Biosynthesis: Stereochemistry and Mechanism of the Reaction Catalyzed by the Mammalian UDP-N-Acetylglucosamine 2-Epimerase. J. Am. Chem. Soc. 2003, 125, 2455–2461. [Google Scholar] [CrossRef] [PubMed]
- Wickramasinghe, S.; Medrano, J.F. Primer on genes encoding enzymes in sialic acid metabolism in mammals. Biochimie 2011, 93, 1641–1646. [Google Scholar] [CrossRef]
- Akella, N.M.; Ciraku, L.; Reginato, M.J. Fueling the fire: Emerging role of the hexosamine biosynthetic pathway in cancer. BMC Biol. 2019, 17, 52. [Google Scholar] [CrossRef]
- Carrillo, N.; Malicdan, M.C.; Leoyklang, P.; Shrader, J.A.; Joe, G.; Slota, C.; Perreault, J.; Heiss, J.D.; Class, B.; Liu, C.-Y.; et al. Safety and efficacy of N-acetylmannosamine (ManNAc) in patients with GNE myopathy: An open-label phase 2 study. Genet. Med. 2021, 23, 2067–2075. [Google Scholar] [CrossRef]
- Hagenhaus, V.; Gorenflos López, J.L.; Rosenstengel, R.; Neu, C.; Hackenberger, C.P.R.; Celik, A.; Weinert, K.; Nguyen, M.-B.; Bork, K.; Horstkorte, R.; et al. Glycation Interferes with the Activity of the Bi-Functional UDP-N-Acetylglucosamine 2-Epimerase/N-Acetyl-mannosamine Kinase (GNE). Biomolecules 2023, 13, 422. [Google Scholar] [CrossRef]
- Glaser, L. On the mechanism of N-acetylmannosamine formation. Biochim. Biophys. Acta 1960, 41, 534–536. [Google Scholar] [CrossRef]
- Comb, D.G.; Roseman, S. Enzymic synthesis of N-acetyl-D-mannosamine. Biochim. Biophys. Acta 1958, 29, 653–654. [Google Scholar] [CrossRef][Green Version]
- Hinderlich, S.; Weidemann, W.; Yardeni, T.; Horstkorte, R.; Huizing, M. UDP-GlcNAc 2-epimerase/ManNAc kinase (GNE), a master regulator of sialic acid synthesis. Top. Curr. Chem. 2015, 366, 97–137. [Google Scholar] [CrossRef]
- Willems, A.P.; Sun, L.; Schulz, M.A.; Tian, W.; Ashikov, A.; van Scherpenzeel, M.; Hermans, E.; Clausen, H.; Yang, Z.; Lefeber, D.J. Activity of N-acylneuraminate-9-phosphatase (NANP) is not essential for de novo sialic acid biosynthesis. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 1471–1479. [Google Scholar] [CrossRef]
- Teoh, S.T.; Ogrodzinski, M.P.; Ross, C.; Hunter, K.W.; Lunt, S.Y. Sialic acid metabolism: A key player in breast cancer metastasis revealed by metabolomics. Front. Oncol. 2018, 8, 174. [Google Scholar] [CrossRef]
- Kozutsumi, Y.; Kawano, T.; Yamakawa, T.; Suzuki, A. Participation of Cytochrome b5 in CMP-N- Acetylneuraminic Acid Hydroxylation in Mouse Liver Cytosol1. J. Biochem. 1990, 108, 704–706. [Google Scholar] [CrossRef] [PubMed]
- Kawano, T.; Kozutsumi, Y.; Takematsu, H.; Kawasaki, T.; Suzuki, A. Regulation of biosynthesis ofN-glycolylneuraminic acid-containing glycoconjugates: Characterization of factors required for NADH-dependent cytidine 5′monophosphate-N-acetylneuraminic acid hydroxylation. Glycoconj. J. 1993, 10, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, X. Sialic acid metabolism and sialyltransferases: Natural functions and applications. Appl. Microbiol. Biotechnol. 2012, 94, 887–905. [Google Scholar] [CrossRef] [PubMed]
- Schauer, R.; Kelm, S.; Reuter, G.; Roggentin, P.; Shaw, L. Biochemistry and Role of Sialic Acids. In Biology of the Sialic Acids; Rosenberg, A., Ed.; Springer: Boston, MA, USA, 1995; pp. 7–67. [Google Scholar]
- Angata, T.; Nakata, D.; Matsuda, T.; Kitajima, K.; Troy, F.A. Biosynthesis of KDN (2-Keto-3-deoxy-d-glycero -d-galacto -nononic acid). J. Biol. Chem. 1999, 274, 22949–22956. [Google Scholar] [CrossRef]
- Crich, D.; Navuluri, C. Practical Synthesis of 2-Keto-3-deoxy-D- glycero-D-galactononulosonic Acid (KDN). Org. Lett. 2011, 13, 6288–6291. [Google Scholar] [CrossRef]
- Kawanishi, K.; Saha, S.; Diaz, S.; Vaill, M.; Sasmal, A.; Siddiqui, S.S.; Choudhury, B.; Sharma, K.; Chen, X.; Schoenhofen, I.C.; et al. Evolutionary conservation of human ketodeoxynonulosonic acid production is independent of sialoglycan biosynthesis. J. Clin. Investig. 2021, 131, e137681. [Google Scholar] [CrossRef]
- Varki, A. Sialic acids in human health and disease. Trends Mol. Med. 2008, 14, 351–360. [Google Scholar] [CrossRef]
- Pawluczyk, I.Z.A.; Najafabadi, M.G.; Brown, J.R.; Bevington, A.; Topham, P.S. Sialic acid supplementation ameliorates puromycin aminonucleoside nephrosis in rats. Lab. Investig. J. Tech. Methods Pathol. 2015, 95, 1019–1028. [Google Scholar] [CrossRef]
- van Karnebeek, C.D.M.; Bonafé, L.; Wen, X.-Y.; Tarailo-Graovac, M.; Balzano, S.; Royer-Bertrand, B.; Ashikov, A.; Garavelli, L.; Mammi, I.; Turolla, L.; et al. NANS-mediated synthesis of sialic acid is required for brain and skeletal development. Nat. Genet. 2016, 48, 777–784. [Google Scholar] [CrossRef]
- Möckl, L. The Emerging Role of the Mammalian Glycocalyx in Functional Membrane Organization and Immune System Regulation. Front. Cell Dev. Biol. 2020, 8, 253. [Google Scholar] [CrossRef]
- Wang, B. Molecular mechanism underlying sialic acid as an essential nutrient for brain development and cognition. Adv. Nutr. 2012, 3, 465–472. [Google Scholar] [CrossRef]
- Bian, D.; Wang, X.; Huang, J.; Chen, X.; Li, H. Maternal Neu5Ac Supplementation During Pregnancy Improves Offspring Learning and Memory Ability in Rats. Front. Nutr. 2021, 8, 641027. [Google Scholar] [CrossRef]
- Cho, A.; Christine, M.; Malicdan, V.; Miyakawa, M.; Nonaka, I.; Nishino, I.; Noguchi, S. Sialic acid deficiency is associated with oxidative stress leading to muscle atrophy and weakness in GNE myopathy. Hum. Mol. Genet. 2017, 26, 3081–3093. [Google Scholar] [CrossRef]
- Pogoryelova, O.; González Coraspe, J.A.; Nikolenko, N.; Lochmüller, H.; Roos, A. GNE myopathy: From clinics and genetics to pathology and research strategies. Orphanet J. Rare Dis. 2018, 13, 70. [Google Scholar] [CrossRef]
- den Hollander, B.; Rasing, A.; Post, M.A.; Klein, W.M.; Oud, M.M.; Brands, M.M.; de Boer, L.; Engelke, U.F.H.; van Essen, P.; Fuchs, S.A.; et al. NANS-CDG: Delineation of the Genetic, Biochemical, and Clinical Spectrum. Front. Neurol. 2021, 12, 668640. [Google Scholar] [CrossRef]
- Betenbaugh, M.J.; Yin, B.; Blake, E.; Kristoffersen, L.; Narang, S.; Viswanathan, K. N-Acetylneuraminic Acid Synthase (NANS). In Handbook of Glycosyltransferases and Related Genes; Taniguchi, N., Honke, K., Fukuda, M., Narimatsu, H., Yamaguchi, Y., Angata, T., Eds.; Springer: Tokyo, Japan, 2014; pp. 1523–1536. [Google Scholar]
- Jin, J.; Fang, F.; Gao, W.; Chen, H.; Wen, J.; Wen, X.; Chen, J. The Structure and Function of the Glycocalyx and Its Connection with Blood-Brain Barrier. Front. Cell. Neurosci. 2021, 15, 739699. [Google Scholar] [CrossRef]
- Houghton, A.N.; Guevara-Patiño, J.A. Immune recognition of self in immunity against cancer. J. Clin. Investig. 2004, 114, 468–471. [Google Scholar] [CrossRef]
- Li, F.; Ding, J. Sialylation is involved in cell fate decision during development, reprogramming and cancer progression. Protein Cell 2019, 10, 550–565. [Google Scholar] [CrossRef] [PubMed]
- Ghasempour, S.; Freeman, S.A. The glycocalyx and immune evasion in cancer. FEBS J. 2023, 290, 55–65. [Google Scholar] [CrossRef]
- Wang, M.; Zhu, J.; Lubman, D.M.; Gao, C. Aberrant glycosylation and cancer biomarker discovery: A promising and thorny journey. Clin. Chem. Lab. Med. CCLM 2019, 57, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Hugonnet, M.; Singh, P.; Haas, Q.; von Gunten, S. The Distinct Roles of Sialyltransferases in Cancer Biology and Onco-Immunology. Front. Immunol. 2021, 12, 799861. [Google Scholar] [CrossRef]
- Le, T.; Ferling, I.; Qiu, L.; Nabaile, C.; Assunção, L.; Roskelley, C.D.; Grinstein, S.; Freeman, S.A. Redistribution of the glycocalyx exposes phagocytic determinants on apoptotic cells. Dev. Cell 2024, 59, 853–868.e7. [Google Scholar] [CrossRef]
- Cho, S.; Zhu, Z.; Li, T.; Baluyot, K.; Howell, B.R.; Hazlett, H.C.; Elison, J.T.; Hauser, J.; Sprenger, N.; Wu, D.; et al. Human milk 3′-Sialyllactose is positively associated with language development during infancy. Am. J. Clin. Nutr. 2021, 114, 588–597. [Google Scholar] [CrossRef]
- Schnaar, R.L.; Gerardy-Schahn, R.; Hildebrandt, H. Sialic Acids in the Brain: Gangliosides and Polysialic Acid in Nervous System Development, Stability, Disease, and Regeneration. Physiol. Rev. 2014, 94, 461–518. [Google Scholar] [CrossRef]
- Liu, F.; Simpson, A.B.; D’Costa, E.; Bunn, F.S.; van Leeuwen, S.S. Sialic acid, the secret gift for the brain. Crit. Rev. Food Sci. Nutr. 2023, 63, 9875–9894. [Google Scholar] [CrossRef]
- Röhrig, C.H.; Choi, S.S.H.; Baldwin, N. The nutritional role of free sialic acid, a human milk monosaccharide, and its application as a functional food ingredient. Crit. Rev. Food Sci. Nutr. 2017, 57, 1017–1038. [Google Scholar] [CrossRef] [PubMed]
- Pang, W.W.; Tan, P.T.; Cai, S.; Fok, D.; Chua, M.C.; Lim, S.B.; Shek, L.P.; Chan, S.-Y.; Tan, K.H.; Yap, F.; et al. Nutrients or nursing? Understanding how breast milk feeding affects child cognition. Eur. J. Nutr. 2020, 59, 609–619. [Google Scholar] [CrossRef]
- Wang, B.; Brand-Miller, J.; McVeagh, P.; Petocz, P. Concentration and distribution of sialic acid in human milk and infant formulas. Am. J. Clin. Nutr. 2001, 74, 510–515. [Google Scholar] [CrossRef]
- Rawal, P.; Zhao, L. Sialometabolism in Brain Health and Alzheimer’s Disease. Front. Neurosci. 2021, 15, 648617. [Google Scholar] [CrossRef]
- Wang, B.; Yu, B.; Karim, M.; Hu, H.; Sun, Y.; McGreevy, P.; Petocz, P.; Held, S.; Brand-Miller, J. Dietary sialic acid supplementation improves learning and memory in piglets2. Matern. Nutr. Optim. Infant Feed. Pract. 2007, 85, 561–569. [Google Scholar] [CrossRef]
- Carrillo, N.; Malicdan, M.C.; Huizing, M. GNE Myopathy: Etiology, Diagnosis, and Therapeutic Challenges. Neurotherapeutics 2018, 15, 900–914. [Google Scholar] [CrossRef]
- Mori-Yoshimura, M.; Oya, Y.; Yajima, H.; Yonemoto, N.; Kobayashi, Y.; Hayashi, Y.K.; Noguchi, S.; Nishino, I.; Murata, M. GNE myopathy: A prospective natural history study of disease progression. Neuromuscul. Disord. 2014, 24, 380–386. [Google Scholar] [CrossRef]
- Xu, X.; Wang, A.Q.; Latham, L.L.; Celeste, F.; Ciccone, C.; Malicdan, M.C.; Goldspiel, B.; Terse, P.; Cradock, J.; Yang, N.; et al. Safety, pharmacokinetics and sialic acid production after oral administration of N -acetylmannosamine (ManNAc) to subjects with GNE myopathy. Mol. Genet. Metab. 2017, 122, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.R.; Gahl, W.A. Lysosomal storage diseases. Transl. Sci. Rare Dis. 2017, 2, 1–71. [Google Scholar] [CrossRef]
- Liu, L.; Lee, W.-S.; Doray, B.; Kornfeld, S. Engineering of GlcNAc-1-Phosphotransferase for Production of Highly Phosphorylated Lysosomal Enzymes for Enzyme Replacement Therapy. Mol. Ther.—Methods Clin. Dev. 2017, 5, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Ito, M. 3.11—Degradation of Glycolipids. In Comprehensive Glycoscience; Kamerling, H., Ed.; Elsevier: Oxford, UK, 2007; pp. 193–208. ISBN 978-0-444-51967-2. [Google Scholar]
- Xiao, C.; Toro, C.; Tifft, C. GM2 Activator Deficiency. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Chapleau, A.; Mirchi, A.; Tran, L.T.; Poulin, C.; Bernard, G. Longitudinal Characterization of the Clinical Course of Intermediate-Severe Salla Disease. Pediatr. Neurol. 2023, 148, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Schauer, R.; Kamerling, J.P. Exploration of the Sialic Acid World. In Advances in Carbohydrate Chemistry and Biochemistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–213. [Google Scholar]
- Tietze, F.; Seppala, R.; Renlund, M.; Hopwood, J.J.; Harper, G.S.; Thomas, G.H.; Gahl, W.A. Defective lysosomal egress of free sialic acid (N-acetylneuraminic acid) in fibroblasts of patients with infantile free sialic acid storage disease. J. Biol. Chem. 1989, 264, 15316–15322. [Google Scholar] [CrossRef]
- Adams, D.; Wasserstein, M. Free Sialic Acid Storage Disorders. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Huizing, M.; Hackbarth, M.E.; Adams, D.R.; Wasserstein, M.; Patterson, M.C.; Walkley, S.U.; Gahl, W.A.; Adams, D.R.; Dobrenis, K.; Foglio, J.; et al. Free sialic acid storage disorder: Progress and promise. Neurosci. Lett. 2021, 755, 135896. [Google Scholar] [CrossRef]
- Sabir, M.S.; Leoyklang, P.; Hackbarth, M.E.; Pak, E.; Dutra, A.; Tait, R.; Pollard, L.; Adams, D.R.; Gahl, W.A.; Huizing, M.; et al. Generation and characterization of two iPSC lines derived from subjects with Free Sialic Acid Storage Disorder (FSASD). Stem Cell Res. 2024, 81, 103600. [Google Scholar] [CrossRef]
- Barmherzig, R.; Bullivant, G.; Cordeiro, D.; Sinasac, D.S.; Blaser, S.; Mercimek-Mahmutoglu, S. A New Patient with Intermediate Severe Salla Disease with Hypomyelination: A Literature Review for Salla Disease. Pediatr. Neurol. 2017, 74, 87–91.e2. [Google Scholar] [CrossRef]
- Tangvoranuntakul, P.; Gagneux, P.; Diaz, S.; Bardor, M.; Varki, N.; Varki, A.; Muchmore, E. Human uptake and incorporation of an immunogenic nonhuman dietary sialic acid. Proc. Natl. Acad. Sci. USA 2003, 100, 12045–12050. [Google Scholar] [CrossRef]
- Nöhle, U.; Schauer, R. Metabolism of Sialic Acids from Exogeneously Administered Sialyllactose and Mucin in Mouse and Rat. Biol. Chem. 1984, 365, 1457–1468. [Google Scholar] [CrossRef]
- Jahan, M.; Thomson, P.C.; Wynn, P.C.; Wang, B. Red Meat Derived Glycan, N-acetylneuraminic Acid (Neu5Ac) Is a Major Sialic Acid in Different Skeletal Muscles and Organs of Nine Animal Species—A Guideline for Human Consumers. Foods 2023, 12, 337. [Google Scholar] [CrossRef] [PubMed]
- Samraj, A.N.; Läubli, H.; Varki, N.; Varki, A. Involvement of a Non-Human Sialic Acid in Human Cancer. Front. Oncol. 2014, 4, 33. [Google Scholar] [CrossRef]
- Yao, H.L.; Conway, L.P.; Wang, M.M.; Huang, K.; Liu, L.; Voglmeir, J. Quantification of sialic acids in red meat by UPLC-FLD using indoxylsialosides as internal standards. Glycoconj. J. 2016, 33, 219–226. [Google Scholar] [CrossRef]
- Bardor, M.; Nguyen, D.H.; Diaz, S.; Varki, A. Mechanism of Uptake and Incorporation of the Non-human Sialic Acid N-Glycolylneuraminic Acid into Human Cells. J. Biol. Chem. 2005, 280, 4228–4237. [Google Scholar] [CrossRef]
- Dhar, C.; Sasmal, A.; Varki, A. From “Serum Sickness” to “Xenosialitis”: Past, Present, and Future Significance of the Non-human Sialic Acid Neu5Gc. Front. Immunol. 2019, 10, 807. [Google Scholar] [CrossRef]
- Sroga, J.M.; Wu, D.H.; Ma, F.; Tecle, E.; Ressler, I.B.; Maxwell, R.; Ferrari, R.; Whigham, L.; Gagneux, P.; Lindheim, S.R. Detection of the dietary xenoglycan N-glycolylneuraminic acid (Neu5Gc) and anti-Neu5Gc antibodies within reproductive tracts of male and female infertility subjects. Clin. Obstet. Gynecol. Reprod. Med. 2015, 1, 72–78. [Google Scholar] [CrossRef]
- Leviatan Ben-Arye, S.; Yu, H.; Chen, X.; Padler-Karavani, V. Profiling Anti-Neu5Gc IgG in Human Sera with a Sialoglycan Microarray Assay. J. Vis. Exp. 2017, 125, e56094. [Google Scholar] [CrossRef]
- Padler-karavani, V.; Yu, H.; Cao, H.; Karp, F.; Varki, N.; Chen, X. Diversity in specificity, abundance, and composition of anti-Neu5Gc antibodies in normal humans: Potential implications for disease. Glycobiology 2008, 18, 818–830. [Google Scholar] [CrossRef]
- Tran, C.; Turolla, L.; Ballhausen, D.; Buros, S.C.; Teav, T.; Gallart-Ayala, H.; Ivanisevic, J.; Faouzi, M.; Lefeber, D.J.; Ivanovski, I.; et al. The fate of orally administered sialic acid: First insights from patients with N-acetylneuraminic acid synthase deficiency and control subjects. Mol. Genet. Metab. Rep. 2021, 28, 100777. [Google Scholar] [CrossRef] [PubMed]
- Soares, C.O.; Grosso, A.S.; Ereño-Orbea, J.; Coelho, H.; Marcelo, F. Molecular Recognition Insights of Sialic Acid Glycans by Distinct Receptors Unveiled by NMR and Molecular Modeling. Front. Mol. Biosci. 2021, 8, 727847. [Google Scholar]
- Wang, B.; Brand-Miller, J. The role and potential of sialic acid in human nutrition. Eur. J. Clin. Nutr. 2003, 57, 1351–1369. [Google Scholar] [CrossRef]
- Zeleny, R.; Kolarich, D.; Strasser, R.; Altmann, F. Sialic acid concentrations in plants are in the range of inadvertent contamination. Planta 2006, 224, 222–227. [Google Scholar] [CrossRef]
- Ylönen, A.; Kalkkinen, N.; Saarinen, J.; Bøgwald, J.; Helin, J. Glycosylation analysis of two cysteine proteinase inhibitors from Atlantic salmon skin: Di-O-acetylated sialic acids are the major sialic acid species on N-glycans. Glycobiology 2001, 11, 523–531. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bashir, S.; Fezeu, L.K.; Leviatan Ben-Arye, S.; Yehuda, S.; Reuven, E.M.; Szabo De Edelenyi, F.; Fellah-Hebia, I.; Le Tourneau, T.; Imbert-Marcille, B.M.; Drouet, E.B.; et al. Association between Neu5Gc carbohydrate and serum antibodies against it provides the molecular link to cancer: French NutriNet-Santé study. BMC Med. 2020, 18, 262. [Google Scholar] [CrossRef]
- Altman, M.O.; Gagneux, P. Absence of Neu5Gc and Presence of Anti-Neu5Gc Antibodies in Humans-An Evolutionary Perspective. Front. Immunol. 2019, 10, 789. [Google Scholar] [CrossRef]
- Schauer, R.; Srinivasan, G.V.; Coddeville, B.; Zanetta, J.-P.; Guérardel, Y. Low incidence of N-glycolylneuraminic acid in birds and reptiles and its absence in the platypus. Carbohydr. Res. 2009, 344, 1494–1500. [Google Scholar] [CrossRef]
- Peri, S.; Kulkarni, A.; Feyertag, F.; Berninsone, P.M.; Alvarez-Ponce, D. Phylogenetic Distribution of CMP-Neu5Ac Hydroxylase (CMAH), the Enzyme Synthetizing the Proinflammatory Human Xenoantigen Neu5Gc. Genome Biol. Evol. 2018, 10, 207–219. [Google Scholar] [CrossRef]
- Koropatkin, N.M.; Cameron, E.A.; Martens, E.C. How glycan metabolism shapes the human gut microbiota. Nat. Rev. Microbiol. 2012, 10, 323–335. [Google Scholar] [CrossRef]
- Tannock Gerald, W. Modulating the Gut Microbiota of Humans by Dietary Intervention with Plant Glycans. Appl. Environ. Microbiol. 2021, 87, e02757-20. [Google Scholar] [CrossRef] [PubMed]
- Adak, A.; Khan, M.R. An insight into gut microbiota and its functionalities. Cell. Mol. Life Sci. 2019, 76, 473–493. [Google Scholar] [CrossRef]
- El-Sayed, A.; Aleya, L.; Kamel, M. Microbiota’s role in health and diseases. Environ. Sci. Pollut. Res. 2021, 28, 36967–36983. [Google Scholar] [CrossRef]
- Ottman, N.; Smidt, H.; de Vos, W.M.; Belzer, C. The function of our microbiota: Who is out there and what do they do? Front. Cell. Infect. Microbiol. 2012, 2, 104. [Google Scholar] [CrossRef]
- Cronin, P.; Joyce, S.A.; O’Toole, P.W.; O’Connor, E.M. Dietary Fibre Modulates the Gut Microbiota. Nutrients 2021, 13, 1655. [Google Scholar] [CrossRef] [PubMed]
- Heimann, E.; Nyman, M.; Degerman, E. Propionic acid and butyric acid inhibit lipolysis and de novo lipogenesis and increase insulin-stimulated glucose uptake in primary rat adipocytes. Adipocyte 2015, 4, 81–88. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, J. Role of intestinal microbiota and metabolites on gut homeostasis and human diseases. BMC Immunol. 2017, 18, 2. [Google Scholar] [CrossRef]
- Coker, J.K.; Moyne, O.; Rodionov, D.A.; Zengler, K. Carbohydrates great and small, from dietary fiber to sialic acids: How glycans influence the gut microbiome and affect human health. Gut Microbes 2021, 13, 1869502. [Google Scholar] [CrossRef]
- Butler, T.L.; Fraser, G.E.; Beeson, W.L.; Knutsen, S.F.; Herring, R.P.; Chan, J.; Sabaté, J.; Montgomery, S.; Haddad, E.; Preston-Martin, S.; et al. Cohort Profile: The Adventist Health Study-2 (AHS-2). Int. J. Epidemiol. 2008, 37, 260–265. [Google Scholar] [CrossRef]
- Davey, G.K.; Spencer, E.A.; Appleby, P.N.; Allen, N.E.; Knox, K.H.; Key, T.J. EPIC–Oxford:lifestyle characteristics and nutrient intakes in a cohort of 33 883 meat-eaters and 31 546 non meat-eaters in the UK. Public Health Nutr. 2003, 6, 259–268. [Google Scholar] [CrossRef]
- Englyst, N.A.; Crook, M.A.; Lumb, P.; Stears, A.J.; Masding, M.G.; Wootton, S.A.; Sandeman, D.D.; Byrne, C.D. Percentage of body fat and plasma glucose predict plasma sialic acid concentration in type 2 diabetes mellitus. Metabolism 2006, 55, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, I.; Masuda, H. Relation of serum sialic acid to blood coagulation activity in type 2 diabetes. Blood Coagul. Fibrinolysis 2002, 13, 691–696. [Google Scholar] [CrossRef]
- Cheeseman, J.; Badia, C.; Elgood-Hunt, G.; Gardner, R.A.; Trinh, D.N.; Monopoli, M.P.; Kuhnle, G.; Spencer, D.I.R.; Osborn, H.M.I. Elevated Concentrations of Neu5Ac and Neu5,9Ac2 in Human Plasma: Potential Biomarkers of Cardiovascular Disease. Glycoconj. J. 2023, 40, 645–654. [Google Scholar] [CrossRef]
- Demina, E.P.; Smutova, V.; Pan, X.; Fougerat, A.; Guo, T.; Zou, C.; Chakraberty, R.; Snarr, B.D.; Shiao, T.C.; Roy, R.; et al. Neuraminidases 1 and 3 Trigger Atherosclerosis by Desialylating Low-Density Lipoproteins and Increasing Their Uptake by Macrophages. J. Am. Heart Assoc. 2021, 10, e018756. [Google Scholar] [CrossRef]
- Samraj, A.N.; Bertrand, K.A.; Luben, R.; Khedri, Z.; Yu, H.; Nguyen, D.; Gregg, C.J.; Diaz, S.L.; Sawyer, S.; Chen, X.; et al. Polyclonal human antibodies against glycans bearing red meat-derived non-human sialic acid N-glycolylneuraminic acid are stable, reproducible, complex and vary between individuals: Total antibody levels are associated with colorectal cancer risk. PLoS ONE 2018, 13, e0197464. [Google Scholar] [CrossRef] [PubMed]
- Yabu, M.; Korekane, H.; Takahashi, H.; Ohigashi, H.; Ishikawa, O.; Miyamoto, Y. Accumulation of free Neu5Ac-containing complex-type N-glycans in human pancreatic cancers. Glycoconj. J. 2013, 30, 247–256. [Google Scholar] [CrossRef]
- Cornelissen, L.A.M.; Blanas, A.; van der Horst, J.C.; Kruijssen, L.; Zaal, A.; O’Toole, T.; Wiercx, L.; van Kooyk, Y.; van Vliet, S.J. Disruption of sialic acid metabolism drives tumor growth by augmenting CD8 + T cell apoptosis. Int. J. Cancer 2019, 144, 2290–2302. [Google Scholar] [CrossRef] [PubMed]
- Zaramela, L.S.; Martino, C.; Alisson-Silva, F.; Rees, S.D.; Diaz, S.L.; Chuzel, L.; Ganatra, M.B.; Taron, C.H.; Secrest, P.; Zuñiga, C.; et al. Gut bacteria responding to dietary change encode sialidases that exhibit preference for red meat-associated carbohydrates. Nat. Microbiol. 2019, 4, 2082–2089. [Google Scholar] [CrossRef]
- McDonald, N.D.; Boyd, E.F. Structural and Biosynthetic Diversity of Nonulosonic Acids (NulOs) That Decorate Surface Structures in Bacteria. Trends Microbiol. 2021, 29, 142–157. [Google Scholar] [CrossRef]
- Bell, A.; Severi, E.; Lee, M.; Monaco, S.; Latousakis, D.; Angulo, J.; Thomas, G.H.; Naismith, J.H.; Juge, N. Uncovering a novel molecular mechanism for scavenging sialic acids in bacteria. J. Biol. Chem. 2020, 295, 13724–13736. [Google Scholar] [CrossRef]
- Aruni, W.; Vanterpool, E.; Osbourne, D.; Roy, F.; Muthiah, A.; Dou, Y.; Fletcher, H.M. Sialidase and Sialoglycoproteases Can Modulate Virulence in Porphyromonas gingivalis. Infect. Immun. 2011, 79, 2779–2791. [Google Scholar] [CrossRef]
- Jennings, M.P.; Day, C.J.; Atack, J.M. How bacteria utilize sialic acid during interactions with the host: Snip, snatch, dispatch, match and attach: This article is part of the Bacterial Cell Envelopes collection. Microbiology 2022, 168, 001157. [Google Scholar] [CrossRef]
- Dedola, S.; Ahmadipour, S.; de Andrade, P.; Baker, A.N.; Boshra, A.N.; Chessa, S.; Gibson, M.I.; Hernando, P.J.; Ivanova, I.M.; Lloyd, J.E.; et al. Sialic acids in infection and their potential use in detection and protection against pathogens. RSC Chem. Biol. 2023, 5, 167. [Google Scholar] [CrossRef]
- Cavalcante, T.; Medeiros, M.M.; Mule, S.N.; Palmisano, G.; Stolf, B.S. The Role of Sialic Acids in the Establishment of Infections by Pathogens, with Special Focus on Leishmania. Front. Cell. Infect. Microbiol. 2021, 11, 671913. [Google Scholar] [CrossRef]
- Matrosovich, M.; Herrler, G.; Klenk, H.D. Sialic Acid Receptors of Viruses. In SialoGlyco Chemistry and Biology II; Springer: Cham, Switzerland, 2013; Volume 367, pp. 1–28. [Google Scholar] [CrossRef]
- Burzyńska, P.; Sobala, Ł.; Mikołajczyk, K.; Jodłowska, M.; Jaśkiewicz, E. Sialic Acids as Receptors for Pathogens. Biomolecules 2021, 11, 831. [Google Scholar] [CrossRef] [PubMed]
- Tolia, N.H.; Enemark, E.J.; Sim, B.K.L.; Joshua-Tor, L. Structural Basis for the EBA-175 Erythrocyte Invasion Pathway of the Malaria Parasite Plasmodium falciparum. Cell 2005, 122, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Morrot, A.; Villar, S.R.; González, F.B.; Pérez, A.R. Evasion and Immuno-Endocrine Regulation in Parasite Infection: Two Sides of the Same Coin in Chagas Disease? Front. Microbiol. 2016, 7, 704. [Google Scholar] [CrossRef]
- Both, P.; Riese, M.; Gray, C.J.; Huang, K.; Pallister, E.G.; Kosov, I.; Conway, L.P.; Voglmeir, J.; Flitsch, S.L. Applications of a highly α2,6-selective pseudosialidase. Glycobiology 2018, 28, 261–268. [Google Scholar] [CrossRef]
- Haines-Menges, B.L.; Whitaker, W.B.; Lubin, J.B.; Boyd, E. Fidelma Host Sialic Acids: A Delicacy for the Pathogen with Discerning Taste. Microbiol. Spectr. 2015, 3, MBP-0005-2014. [Google Scholar] [CrossRef]
- Liu, F.; Dong, Q.; Myers, A.M.; Fromm, H.J. Expression of human brain hexokinase in Escherichiacoli: Purification and characterization of the expressed enzyme. Biochem. Biophys. Res. Commun. 1991, 177, 305–311. [Google Scholar] [CrossRef]
- Bell, A.; Severi, E.; Owen, C.D.; Latousakis, D.; Juge, N. Biochemical and structural basis of sialic acid utilization by gut microbes. J. Biol. Chem. 2023, 299, 102989. [Google Scholar] [CrossRef]
- Zhao, M.; Zhu, Y.; Wang, H.; Zhang, W.; Mu, W. Recent advances on N-acetylneuraminic acid: Physiological roles, applications, and biosynthesis. Synth. Syst. Biotechnol. 2023, 8, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.-X.; Qiao, Y.; Shi, B.; Tao, Y. Polysialic acid biosynthesis and production in Escherichia coli: Current state and perspectives. Appl. Microbiol. Biotechnol. 2016, 100, 1–8. [Google Scholar] [CrossRef]
- Feng, Y.; Cao, M.; Shi, J.; Zhang, H.; Hu, D.; Zhu, J.; Zhang, X.; Geng, M.; Zheng, F.; Pan, X.; et al. Attenuation of Streptococcus suis virulence by the alteration of bacterial surface architecture. Sci. Rep. 2012, 2, 710. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Díaz, J.; Rubio-del-Campo, A.; Yebra, M.J. Regulatory insights into the production of UDP-N-acetylglucosamine by Lactobacillus casei. Bioengineered 2012, 3, 339–342. [Google Scholar] [CrossRef]
- Ko, T.-P.; Lai, S.-J.; Hsieh, T.-J.; Yang, C.-S.; Chen, Y. The tetrameric structure of sialic acid–synthesizing UDP-GlcNAc 2-epimerase from Acinetobacter baumannii: A comparative study with human GNE. J. Biol. Chem. 2018, 293, 10119–10127. [Google Scholar] [CrossRef]
- Zapata, G.; Crowley, J.M.; Vann, W.F. Sequence and expression of the Escherichia coli K1 neuC gene product. J. Bacteriol. 1992, 174, 315–319. [Google Scholar] [CrossRef][Green Version]
- Vimr, E.R.; Aaronson, W.; Silver, R.P. Genetic analysis of chromosomal mutations in the polysialic acid gene cluster of Escherichia coli K1. J. Bacteriol. 1989, 171, 1106–1117. [Google Scholar] [CrossRef]
- Silver, R.P.; Vann, W.F.; Aaronson, W. Genetic and Molecular Analyses of Escherichia coli KI Antigen Genes. J. Bacteriol. 1984, 157, 568–575. [Google Scholar] [CrossRef]
- Vimr, E.R.; Kalivoda, K.A.; Deszo, E.L.; Steenbergen, S.M. Diversity of Microbial Sialic Acid Metabolism. Microbiol. Mol. Biol. Rev. 2004, 68, 132–153. [Google Scholar] [CrossRef]
- Vimr, E.; Lichtensteiger, C.; Steenbergen, S. Sialic acid metabolism’s dual function in Haemophilus influenzae. Mol. Microbiol. 2000, 36, 1113–1123. [Google Scholar] [CrossRef] [PubMed]
- Flack, E.K.; Chidwick, H.S.; Best, M.; Thomas, G.H.; Fascione, M.A. Synthetic Approaches for Accessing Pseudaminic Acid (Pse) Bacterial Glycans. ChemBioChem 2020, 21, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Chidwick, H.S.; Flack, E.K.P.; Keenan, T.; Walton, J.; Thomas, G.H.; Fascione, M.A. Reconstitution and optimisation of the biosynthesis of bacterial sugar pseudaminic acid (Pse5Ac7Ac) enables preparative enzymatic synthesis of CMP-Pse5Ac7Ac. Sci. Rep. 2021, 11, 4756. [Google Scholar] [CrossRef]
- Wei, R.; Liu, H.; Li, X. De novo synthesis of novel bacterial monosaccharide fusaminic acid. J. Antibiot. 2019, 72, 420–431. [Google Scholar] [CrossRef]
- Kenyon, J.J.; Marzaioli, A.M.; De Castro, C.; Hall, R.M. 5,7-di-N-acetyl-acinetaminic acid: A novel non-2-ulosonic acid found in the capsule of an Acinetobacter baumannii isolate. Glycobiology 2015, 25, 644–654. [Google Scholar] [CrossRef]
- Hassan, M.I.; Lundgren, B.R.; Chaumun, M.; Whitfield, D.M.; Clark, B.; Schoenhofen, I.C.; Boddy, C.N. Total Biosynthesis of Legionaminic Acid, a Bacterial Sialic Acid Analogue. Angew. Chem. Int. Ed. Engl. 2016, 55, 12018–12021. [Google Scholar] [CrossRef]
- Tomás-Martínez, S.; Kleikamp, H.B.C.; Neu, T.R.; Pabst, M.; Weissbrodt, D.G.; van Loosdrecht, M.C.M.; Lin, Y. Production of nonulosonic acids in the extracellular polymeric substances of “Candidatus Accumulibacter phosphatis”. Appl. Microbiol. Biotechnol. 2021, 105, 3327–3338. [Google Scholar] [CrossRef]
- Eke, P.I.; Borgnakke, W.S.; Genco, R.J. Recent epidemiologic trends in periodontitis in the USA. Periodontol. 2000 2020, 82, 257–267. [Google Scholar] [CrossRef]
- Yu, S.; Fan, X.; Zheng, S.; Lin, L.; Liu, J.; Pan, Y.; Li, C. The sialidase inhibitor, DANA, reduces Porphyromonas gingivalis pathogenicity and exerts anti-inflammatory effects: An in vitro and in vivo experiment. J. Periodontol. 2021, 92, 286–297. [Google Scholar] [CrossRef]
- Bostanci, N.; Bao, K.; Greenwood, D.; Silbereisen, A.; Belibasakis, G.N. Chapter Six—Periodontal disease: From the lenses of light microscopy to the specs of proteomics and next-generation sequencing. In Advances in Clinical Chemistry; Makowski, G.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 93, pp. 263–290. [Google Scholar] [CrossRef]
- Chen, W.A.; Fletcher, H.M.; Gheorghe, J.D.; Oyoyo, U.; Boskovic, D.S. Platelet plug formation in whole blood is enhanced in the presence of Porphyromonas gingivalis. Mol. Oral Microbiol. 2020, 35, 251–259. [Google Scholar] [CrossRef]
- Chen, W.A.; Fletcher, H.M.; Payne, K.J.; Aka, S.; Thornburg, M.B.; Gheorghe, J.D.; Safi, S.B.; Shavlik, D.; Oyoyo, U.; Boskovic, D.S. Platelet and neutrophil responses to Porphyromonas gingivalis in human whole blood. Mol. Oral Microbiol. 2021, 36, 202–213. [Google Scholar] [CrossRef]
- Frey, A.M.; Satur, M.J.; Phansopa, C.; Honma, K.; Urbanowicz, P.A.; Spencer, D.I.R.; Pratten, J.; Bradshaw, D.; Sharma, A.; Stafford, G. Characterization of Porphyromonas gingivalis sialidase and disruption of its role in host–pathogen interactions. Microbiology 2019, 165, 1181–1197. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.A.; Dou, Y.; Fletcher, H.M.; Boskovic, D.S. Local and Systemic Effects of Porphyromonas gingivalis Infection. Microorganisms 2023, 11, 470. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.J.; Jenkinson, H.F. Life Below the Gum Line: Pathogenic Mechanisms of Porphyromonas gingivalis. Microbiol. Mol. Biol. Rev. 1998, 62, 1244–1263. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, T.K.; Ohara-Nemoto, Y. Exopeptidases and gingipains in Porphyromonas gingivalis as prerequisites for its amino acid metabolism. Jpn. Dent. Sci. Rev. 2016, 52, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Moradali, M.F.; Davey, M.E. Metabolic plasticity enables lifestyle transitions of Porphyromonas gingivalis. Npj Biofilms Microbiomes 2021, 7, 46. [Google Scholar] [CrossRef]
- Nakayama, M.; Ohara, N. Molecular mechanisms of Porphyromonas gingivalis-host cell interaction on periodontal diseases. Jpn. Dent. Sci. Rev. 2017, 53, 134–140. [Google Scholar] [CrossRef]
- Mysak, J.; Podzimek, S.; Sommerova, P.; Lyuya-Mi, Y.; Bartova, J.; Janatova, T.; Prochazkova, J.; Duskova, J. Porphyromonas gingivalis: Major Periodontopathic Pathogen Overview. J. Immunol. Res. 2014, 2014, 476068. [Google Scholar] [CrossRef]
- Cekici, A.; Kantarci, A.; Hasturk, H.; Van Dyke, T.E. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontol. 2000 2014, 64, 57–80. [Google Scholar] [CrossRef]
- Werheim, E.R.; Senior, K.G.; Shaffer, C.A.; Cuadra, G.A. Oral Pathogen Porphyromonas gingivalis Can Escape Phagocytosis of Mammalian Macrophages. Microorganisms 2020, 8, 1432. [Google Scholar] [CrossRef]
- Cueno, M.E.; Kamio, N.; Imai, K.; Ohya, M.; Tamura, M.; Ochiai, K. Structural Significance of the β1K396 Residue Found in the Porphyromonas gingivalis Sialidase β-Propeller Domain: A Computational Study with Implications for Novel Therapeutics Against Periodontal Disease. OMICS J. Integr. Biol. 2014, 18, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Weiser, J.N.; Ferreira, D.M.; Paton, J.C. Streptococcus pneumoniae: Transmission, colonization and invasion. Nat. Rev. Microbiol. 2018, 16, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Loughran, A.J.; Orihuela, C.J.; Tuomanen, E.I. Streptococcus pneumoniae: Invasion and Inflammation. Microbiol. Spectr. 2019, 7, GPP3-0004-2018. [Google Scholar] [CrossRef]
- Xiao, A.; Slack, T.J.; Li, Y.; Shi, D.; Yu, H.; Li, W.; Liu, Y.; Chen, X. Streptococcus pneumoniae Sialidase SpNanB-Catalyzed One-Pot Multienzyme (OPME) Synthesis of 2,7-Anhydro-Sialic acids as Selective Sialidase Inhibitors. J. Org. Chem. 2018, 83, 10798–10804. [Google Scholar] [CrossRef]
- Hentrich, K.; Löfling, J.; Pathak, A.; Nizet, V.; Varki, A.; Henriques-Normark, B. Streptococcus pneumoniae Senses a Human-like Sialic Acid Profile via the Response Regulator CiaR. Cell Host Microbe 2016, 20, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Versluys, K.A.; Eurich, D.T.; Marrie, T.J.; Forgie, S.; Tyrrell, G.J. Invasive pneumococcal disease and long-term outcomes in children: A 20-year population cohort study. Lancet Reg. Health—Am. 2022, 14, 100341. [Google Scholar] [CrossRef] [PubMed]
- Werren, J.P.; Troxler, L.J.; Oyewole, O.R.-A.; Ramette, A.; Brugger, S.D.; Bruggmann, R.; van der Linden, M.; Nahm, M.H.; Gjuroski, I.; Casanova, C.; et al. Carbon Source-Dependent Changes of the Structure of Streptococcus pneumoniae Capsular Polysaccharide with Serotype 6F. Int. J. Mol. Sci. 2021, 22, 4580. [Google Scholar] [CrossRef]
- Mathew, B.J.; Gupta, P.; Naaz, T.; Rai, R.; Gupta, S.; Gupta, S.; Chaurasiya, S.K.; Purwar, S.; Biswas, D.; Vyas, A.K.; et al. Role of Streptococcus pneumoniae extracellular glycosidases in immune evasion. Front. Cell. Infect. Microbiol. 2023, 13, 1109449. [Google Scholar] [CrossRef]
- Trappetti, C.; Kadioglu, A.; Carter, M.; Hayre, J.; Iannelli, F.; Pozzi, G.; Andrew, P.W.; Oggioni, M.R. Sialic acid: A preventable signal for pneumococcal biofilm formation, colonization, and invasion of the host. J. Infect. Dis. 2009, 199, 1497–1505. [Google Scholar] [CrossRef]
- Manco, S.; Hernon, F.; Yesilkaya, H.; Paton, J.C.; Andrew, P.W.; Kadioglu, A. Pneumococcal neuraminidases A and B both have essential roles during infection of the respiratory tract and sepsis. Infect. Immun. 2006, 74, 4014–4020. [Google Scholar] [CrossRef]
- Xu, G.; Kiefel, M.J.; Wilson, J.C.; Andrew, P.W.; Oggioni, M.R.; Taylor, G.L. Three Streptococcus pneumoniae sialidases: Three different products. J. Am. Chem. Soc. 2011, 133, 1718–1721. [Google Scholar] [CrossRef]
- Agarwal, H.S.; Latifi, S.Q. Streptococcus Pneumoniae-Associated Hemolytic Uremic Syndrome in the Era of Pneumococcal Vaccine. Pathogens 2021, 10, 727. [Google Scholar] [CrossRef] [PubMed]
- Parker, D.; Soong, G.; Planet, P.; Brower, J.; Ratner, A.J.; Prince, A. The NanA neuraminidase of Streptococcus pneumoniae is involved in biofilm formation. Infect. Immun. 2009, 77, 3722–3730. [Google Scholar] [CrossRef] [PubMed]
- Ng, P.S.K.; Day, C.J.; Atack, J.M.; Hartley-Tassell, L.E.; Winter, L.E.; Marshanski, T.; Padler-Karavani, V.; Varki, A.; Barenkamp, S.J.; Apicella, M.A.; et al. Nontypeable Haemophilus influenzae Has Evolved Preferential Use of N-Acetylneuraminic Acid as a Host Adaptation. mBio 2019, 10, e00422-19. [Google Scholar] [CrossRef] [PubMed]
- Riesbeck, K. Complement evasion by the human respiratory tract pathogens Haemophilus influenzae and Moraxella catarrhalis. FEBS Lett. 2020, 594, 2586–2597. [Google Scholar] [CrossRef]
- Pittman, M. Variation and Type Specificity in the Bacterial Species Hemophilus influenzae. J. Exp. Med. 1931, 53, 471–492. [Google Scholar] [CrossRef]
- Paulo, A.C.; Lança, J.; Almeida, S.T.; Hilty, M.; Sá-Leão, R. The upper respiratory tract microbiota of healthy adults is affected by Streptococcus pneumoniae carriage, smoking habits, and contact with children. Microbiome 2023, 11, 199. [Google Scholar] [CrossRef]
- Langereis, J.D.; de Jonge, M.I. Invasive Disease Caused by Nontypeable Haemophilus influenzae. Emerg. Infect. Dis. 2015, 21, 1711–1718. [Google Scholar] [CrossRef]
- Landwehr, K.R.; Granland, C.M.; Martinovich, K.M.; Scott, N.M.; Seppanen, E.J.; Berry, L.; Strickland, D.; Fulurija, A.; Richmond, P.C.; Kirkham, L.-A.S. An infant mouse model of influenza-driven nontypeable Haemophilus influenzae colonization and acute otitis media suitable for preclinical testing of novel therapies. Infect. Immun. 2024, 92, e00453-23. [Google Scholar] [CrossRef]
- Oliver, S.E.; Rubis, A.B.; Soeters, H.M.; Reingold, A.; Barnes, M.; Petit, S.; Farley, M.M.; Harrison, L.H.; Como-Sabetti, K.; Khanlian, S.A.; et al. Epidemiology of Invasive Nontypeable Haemophilus influenzae Disease—United States, 2008–2019. Clin. Infect. Dis. 2023, 76, 1889–1895. [Google Scholar] [CrossRef]
- Van Eldere, J.; Slack, M.P.E.; Ladhani, S.; Cripps, A.W. Non-typeable Haemophilus influenzae, an under-recognised pathogen. Lancet Infect. Dis. 2014, 14, 1281–1292. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.; Litt, D.J.; Flynn, S.; Ramsay, M.E.; Slack, M.P.E.; Ladhani, S.N. Neonatal Invasive Haemophilus influenzae Disease in England and Wales: Epidemiology, Clinical Characteristics, and Outcome. Clin. Infect. Dis. 2015, 60, 1786–1792. [Google Scholar] [CrossRef]
- Phillips, Z.N.; Brizuela, C.; Jennison, A.V.; Staples, M.; Grimwood, K.; Seib, K.L.; Jennings, M.P.; Atack, J.M. Analysis of Invasive Nontypeable Haemophilus influenzae Isolates Reveals Selection for the Expression State of Particular Phase-Variable Lipooligosaccharide Biosynthetic Genes. Infect. Immun. 2019, 87, e00093-19. [Google Scholar] [CrossRef]
- Ladhani, S.; Slack, M.P.E.; Heath, P.T.; von Gottberg, A.; Chandra, M.; Ramsay, M.E.; European Union Invasive Bacterial Infection Surveillance Participants. Invasive Haemophilus influenzae Disease, Europe, 1996–2006. Emerg. Infect. Dis. 2010, 16, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.D.; Wong, S.M.; Akerley, B.J. Sialic Acid Protects Nontypeable Haemophilus influenzae from Natural IgM and Promotes Survival in Murine Respiratory Tract. Infect. Immun. 2021, 89, e00676-20. [Google Scholar] [CrossRef] [PubMed]
- Heise, T.; Langereis, J.D.; Rossing, E.; de Jonge, M.I.; Adema, G.J.; Büll, C.; Boltje, T.J. Selective Inhibition of Sialic Acid-Based Molecular Mimicry in Haemophilus influenzae Abrogates Serum Resistance. Cell Chem. Biol. 2018, 25, 1279–1285.e8. [Google Scholar] [CrossRef]
- Jenkins, G.A.; Figueira, M.; Kumar, G.A.; Sweetman, W.A.; Makepeace, K.; Pelton, S.I.; Moxon, R.; Hood, D.W. Sialic acid mediated transcriptional modulation of a highly conserved sialometabolism gene cluster in Haemophilus influenzae and its effect on virulence. BMC Microbiol. 2010, 10, 48. [Google Scholar] [CrossRef]
- Kariminik, A.; Baseri-Salehi, M.; Kheirkhah, B. Pseudomonas aeruginosa quorum sensing modulates immune responses: An updated review article. Immunol. Lett. 2017, 190, 1–6. [Google Scholar] [CrossRef]
- Estahbanati, H.K.; Kashani, P.P.; Ghanaatpisheh, F. Frequency of Pseudomonas aeruginosa serotypes in burn wound infections and their resistance to antibiotics. Burns J. Int. Soc. Burn Inj. 2002, 28, 340–348. [Google Scholar] [CrossRef]
- Mukherjee, K.; Khatua, B.; Mandal, C. Sialic Acid-Siglec-E Interactions During Pseudomonas aeruginosa Infection of Macrophages Interferes with Phagosome Maturation by Altering Intracellular Calcium Concentrations. Front. Immunol. 2020, 11, 332. [Google Scholar] [CrossRef]
- Khatua, B.; Ghoshal, A.; Bhattacharya, K.; Mandal, C.; Saha, B.; Crocker, P.R.; Mandal, C. Sialic acids acquired by Pseudomonas aeruginosa are involved in reduced complement deposition and siglec mediated host-cell recognition. Febs Lett. 2010, 584, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Avril, T.; Wagner, E.R.; Willison, H.J.; Crocker, P.R. Sialic acid-binding immunoglobulin-like lectin 7 mediates selective recognition of sialylated glycans expressed on Campylobacter jejuni lipooligosaccharides. Infect. Immun. 2006, 74, 4133–4141. [Google Scholar] [CrossRef]
- Carlin, A.F.; Lewis, A.L.; Varki, A.; Nizet, V. Group B streptococcal capsular sialic acids interact with siglecs (immunoglobulin-like lectins) on human leukocytes. J. Bacteriol. 2007, 189, 1231–1237. [Google Scholar] [CrossRef]
- Crocker, P.R.; Paulson, J.C.; Varki, A. Siglecs and their roles in the immune system. Nat. Rev. Immunol. 2007, 7, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y. Sialidases from Clostridium perfringens and Their Inhibitors. Front. Cell. Infect. Microbiol. 2020, 9, 462. [Google Scholar] [CrossRef]
- Li, J.; Uzal, F.A.; McClane, B.A. Clostridium perfringens Sialidases: Potential Contributors to Intestinal Pathogenesis and Therapeutic Targets. Toxins 2016, 8, 341. [Google Scholar] [CrossRef]
- Yao, P.Y.; Annamaraju, P. Clostridium perfringens Infection. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Schauer, R.; Sommer, U.; Krüger, D.; van Unen, H.; Traving, C. The Terminal Enzymes of Sialic Acid Metabolism: Acylneuraminate Pyruvate-Lyases. Biosci. Rep. 1999, 19, 373–383. [Google Scholar] [CrossRef]
- Navarro, M.A.; Li, J.; McClane, B.A.; Morrell, E.; Beingesser, J.; Uzal, F.A. NanI Sialidase Is an Important Contributor to Clostridium perfringens Type F Strain F4969 Intestinal Colonization in Mice. Infect. Immun. 2018, 86. [Google Scholar] [CrossRef]
- Camargo, A.; Ramírez, J.D.; Kiu, R.; Hall, L.J.; Muñoz, M. Unveiling the pathogenic mechanisms of Clostridium perfringens toxins and virulence factors. Emerg. Microbes Infect. 2024, 13, 2341968. [Google Scholar] [CrossRef]
- Cioffi, D.L.; Pandey, S.; Alvarez, D.F.; Cioffi, E.A. Terminal sialic acids are an important determinant of pulmonary endothelial barrier integrity. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2012, 302, L1067–L1077. [Google Scholar] [CrossRef]
- Li, X.; Xiong, Y.; Qing, G.; Jiang, G.; Li, X.; Sun, T.; Liang, X. Bioinspired Saccharide-Saccharide Interaction and Smart Polymer for Specific Enrichment of Sialylated Glycopeptides. ACS Appl. Mater. Interfaces 2016, 8, 13294–13302. [Google Scholar] [CrossRef]
- Li, J.; Sayeed, S.; Robertson, S.; Chen, J.; McClane, B.A. Sialidases affect the host cell adherence and epsilon toxin-induced cytotoxicity of Clostridium perfringens type D strain CN3718. PLoS Pathog. 2011, 7, e1002429. [Google Scholar] [CrossRef]
- Li, J.; McClane, B.A. NanI Sialidase Can Support the Growth and Survival of Clostridium perfringens Strain F4969 in the Presence of Sialyated Host Macromolecules (Mucin) or Caco-2 Cells. Infect. Immun. 2018, 86, e00547-17. [Google Scholar] [CrossRef] [PubMed]
- Belcher, T.; Rollier, C.S.; Dold, C.; Ross, J.D.C.; MacLennan, C.A. Immune responses to Neisseria gonorrhoeae and implications for vaccine development. Front. Immunol. 2023, 14, 1248613. [Google Scholar] [CrossRef]
- Cardenas, A.J.; Thomas, K.S.; Broden, M.W.; Ferraro, N.J.; Pires, M.M.; John, C.M.; Jarvis, G.A.; Criss, A.K. Neisseria gonorrhoeae scavenges host sialic acid for Siglec-mediated, complement-independent suppression of neutrophil activation. mBio 2024, 15, e00119-24. [Google Scholar] [CrossRef]
- Kirkcaldy, R.D.; Weston, E.; Segurado, A.C.; Hughes, G. Epidemiology of gonorrhoea: A global perspective. Sex. Health 2019, 16, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Handing, J.W.; Criss, A.K. The lipooligosaccharide-modifying enzyme LptA enhances gonococcal defence against human neutrophils. Cell. Microbiol. 2015, 17, 910–921. [Google Scholar] [CrossRef]
- Cartee, J.C.; Joseph, S.J.; Weston, E.; Pham, C.D.; Thomas, J.C., IV; Schlanger, K.; St Cyr, S.B.; Farley, M.M.; Moore, A.E.; Tunali, A.K.; et al. Phylogenomic Comparison of Neisseria gonorrhoeae Causing Disseminated Gonococcal Infections and Uncomplicated Gonorrhea in Georgia, United States. Open Forum Infect. Dis. 2022, 9, ofac247. [Google Scholar] [CrossRef]
- Gulati, S.; Cox, A.; Lewis, L.A.; St. Michael, F.; Li, J.; Boden, R.; Ram, S.; Rice, P.A. Enhanced Factor H Binding to Sialylated Gonococci Is Restricted to the Sialylated Lacto-N-Neotetraose Lipooligosaccharide Species: Implications for Serum Resistance and Evidence for a Bifunctional Lipooligosaccharide Sialyltransferase in Gonococci. Infect. Immun. 2005, 73, 7390–7397. [Google Scholar] [CrossRef]
- Jen, F.E.-C.; Ketterer, M.R.; Semchenko, E.A.; Day, C.J.; Seib, K.L.; Apicella, M.A.; Jennings, M.P. The Lst Sialyltransferase of Neisseria gonorrhoeae Can Transfer Keto-Deoxyoctanoate as the Terminal Sugar of Lipooligosaccharide: A Glyco-Achilles Heel That Provides a New Strategy for Vaccines to Prevent Gonorrhea. mBio 2021, 12, e03666-20. [Google Scholar] [CrossRef]
- Gil-Farina, I.; Schmidt, M. Interaction of vectors and parental viruses with the host genome. Curr. Opin. Virol. 2016, 21, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Maginnis, M.S. Virus–Receptor Interactions: The Key to Cellular Invasion. J. Mol. Biol. 2018, 430, 2590–2611. [Google Scholar] [CrossRef] [PubMed]
- Wallace, L.E.; Liu, M.; van Kuppeveld, F.J.M.; de Vries, E.; de Haan, C.A.M. Respiratory mucus as a virus-host range determinant. Trends Microbiol. 2021, 29, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Payne, S. 3—Virus interactions with the cell. In Viruses, 2nd ed.; Payne, S., Ed.; Academic Press: Cambridge, MA, USA, 2023; pp. 25–37. [Google Scholar]
- Reeves, J.D.; Doms, R.W. CHAPTER 33—The Role of Chemokine Receptors in HIV Infection of Host Cells. In Handbook of Cell Signaling; Bradshaw, R.A., Dennis, E.A., Eds.; Academic Press: Burlington, VT, USA, 2003; pp. 191–196. [Google Scholar]
- Nicholls, J.M.; Moss, R.B.; Haslam, S.M. The use of sialidase therapy for respiratory viral infections. Antiviral Res. 2013, 98, 401–409. [Google Scholar] [CrossRef]
- Modrow, S.; Falke, D.; Truyen, U.; Schätzl, H. Viruses with Single-Stranded, Segmented, Negative-Sense RNA Genomes. In Molecular Virology; Modrow, S., Falke, D., Truyen, U., Schätzl, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 437–520. [Google Scholar]
- Li, X.; Gu, M.; Zheng, Q.; Gao, R.; Liu, X. Packaging signal of influenza A virus. Virol. J. 2021, 18, 36. [Google Scholar] [CrossRef]
- Hutchinson, E.C. Influenza Virus. Trends Microbiol. 2018, 26, 809–810. [Google Scholar] [CrossRef]
- Kumlin, U.; Olofsson, S.; Dimock, K.; Arnberg, N. Sialic acid tissue distribution and influenza virus tropism. Influenza Other Respir. Viruses 2008, 2, 147–154. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, W.; Wang, Q.; Qin, Y.; Wang, X.; Ma, T.; Zhang, P.; Li, X.; Wang, X.; Ding, L.; et al. Comparative analysis of sialic acid α2–3/6 galactose glycan-binding proteins in human saliva and serum. J. Mol. Struct. 2021, 1230, 129859. [Google Scholar] [CrossRef]
- Liu, M.; Huang, L.Z.X.; Smits, A.A.; Büll, C.; Narimatsu, Y.; van Kuppeveld, F.J.M.; Clausen, H.; de Haan, C.A.M.; de Vries, E. Human-type sialic acid receptors contribute to avian influenza A virus binding and entry by hetero-multivalent interactions. Nat. Commun. 2022, 13, 4054. [Google Scholar] [CrossRef]
- Hatakeyama, S.; Sakai-Tagawa, Y.; Kiso, M.; Goto, H.; Kawakami, C.; Mitamura, K.; Sugaya, N.; Suzuki, Y.; Kawaoka, Y. Enhanced Expression of an α2,6-Linked Sialic Acid on MDCK Cells Improves Isolation of Human Influenza Viruses and Evaluation of Their Sensitivity to a Neuraminidase Inhibitor. J. Clin. Microbiol. 2005, 43, 4139–4146. [Google Scholar] [CrossRef]
- Dawre, S.; Maru, S. Human respiratory viral infections: Current status and future prospects of nanotechnology-based approaches for prophylaxis and treatment. Life Sci. 2021, 278, 119561. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F.; Smith, G.J.D.; Fouchier, R.A.M.; Peiris, M.; Kedzierska, K.; Doherty, P.C.; Palese, P.; Shaw, M.L.; Treanor, J.; Webster, R.G.; et al. Influenza. Nat. Rev. Dis. Primer 2018, 4, 3. [Google Scholar] [CrossRef]
- Javanian, M.; Barary, M.; Ghebrehewet, S.; Koppolu, V.; Vasigala, V.; Ebrahimpour, S. A brief review of influenza virus infection. J. Med. Virol. 2021, 93, 4638–4646. [Google Scholar] [CrossRef] [PubMed]
- Sulpiana; Amalia, R.; Atik, N. The Roles of Endocytosis and Autophagy at the Cellular Level During Influenza Virus Infection: A Mini-Review. Infect. Drug Resist. 2024, 17, 3199–3208. [Google Scholar] [CrossRef]
- McAuley, J.L.; Gilbertson, B.P.; Trifkovic, S.; Brown, L.E.; McKimm-Breschkin, J.L. Influenza Virus Neuraminidase Structure and Functions. Front. Microbiol. 2019, 10, 39. [Google Scholar] [CrossRef]
- Gallagher, J.R.; McCraw, D.M.; Torian, U.; Gulati, N.M.; Myers, M.L.; Conlon, M.T.; Harris, A.K. Characterization of Hemagglutinin Antigens on Influenza Virus and within Vaccines Using Electron Microscopy. Vaccines 2018, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, A. The Influenza Virus Neuraminidase. Nature 1958, 181, 377–378. [Google Scholar] [CrossRef]
- Wu, N.C.; Wilson, I.A. Influenza Hemagglutinin Structures and Antibody Recognition. Cold Spring Harb. Perspect. Med. 2020, 10, a038778. [Google Scholar] [CrossRef]
- Fitz, W.; Rosenthal, P.B.; Wong, C.H. Synthesis and inhibitory properties of a thiomethylmercuric sialic acid with application to the X-ray structure determination of 9-O-acetylsialic acid esterase from influenza C virus. Bioorg. Med. Chem. 1996, 4, 1349–1353. [Google Scholar] [CrossRef]
- Rossman, J.S.; Leser, G.P.; Lamb, R.A. Filamentous Influenza Virus Enters Cells via Macropinocytosis. J. Virol. 2012, 86, 10950–10960. [Google Scholar] [CrossRef]
- de Vries, E.; Tscherne, D.M.; Wienholts, M.J.; Cobos-Jiménez, V.; Scholte, F.; García-Sastre, A.; Rottier, P.J.M.; de Haan, C.A.M. Dissection of the Influenza A Virus Endocytic Routes Reveals Macropinocytosis as an Alternative Entry Pathway. PLOS Pathog. 2011, 7, e1001329. [Google Scholar] [CrossRef]
- Matrosovich, M.N.; Matrosovich, T.Y.; Gray, T.; Roberts, N.A.; Klenk, H.-D. Neuraminidase Is Important for the Initiation of Influenza Virus Infection in Human Airway Epithelium. J. Virol. 2004, 78, 12665–12667. [Google Scholar] [CrossRef]
- Ohuchi, M.; Asaoka, N.; Sakai, T.; Ohuchi, R. Roles of neuraminidase in the initial stage of influenza virus infection. Microbes Infect. 2006, 8, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Giurgea, L.T.; Morens, D.M.; Taubenberger, J.K.; Memoli, M.J. Influenza Neuraminidase: A Neglected Protein and Its Potential for a Better Influenza Vaccine. Vaccines 2020, 8, 409. [Google Scholar] [CrossRef]
- Escuret, V.; Terrier, O. Co-infection of the respiratory epithelium, scene of complex functional interactions between viral, bacterial, and human neuraminidases. Front. Microbiol. 2023, 14, 1137336. [Google Scholar] [CrossRef]
- Peltola, V.T.; Murti, K.G.; McCullers, J.A. Influenza Virus Neuraminidase Contributes to Secondary Bacterial Pneumonia. J. Infect. Dis. 2005, 192, 249–257. [Google Scholar] [CrossRef]
- Creytens, S.; Pascha, M.N.; Ballegeer, M.; Saelens, X.; de Haan, C.A.M. Influenza Neuraminidase Characteristics and Potential as a Vaccine Target. Front. Immunol. 2021, 12, 786617. [Google Scholar] [CrossRef]
- Eurosurveillance Editorial Team. Note from the editors: World Health Organization declares novel coronavirus (2019-nCoV) sixth public health emergency of international concern. Eurosurveillance 2020, 25, 200131e. [Google Scholar] [CrossRef]
- Majumder, J.; Minko, T. Recent Developments on Therapeutic and Diagnostic Approaches for COVID-19. AAPS J. 2021, 23, 14. [Google Scholar] [CrossRef]
- Su, S.; Wong, G.; Shi, W.; Liu, J.; Lai, A.C.K.; Zhou, J.; Liu, W.; Bi, Y.; Gao, G.F. Epidemiology, Genetic Recombination, and Pathogenesis of Coronaviruses. Trends Microbiol. 2016, 24, 490–502. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.-L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef]
- Casalino, L.; Gaieb, Z.; Goldsmith, J.A.; Hjorth, C.K.; Dommer, A.C.; Harbison, A.M.; Fogarty, C.A.; Barros, E.P.; Taylor, B.C.; McLellan, J.S.; et al. Beyond Shielding: The Roles of Glycans in the SARS-CoV-2 Spike Protein. ACS Cent. Sci. 2020, 6, 1722–1734. [Google Scholar] [CrossRef]
- Tortorici, M.A.; Walls, A.C.; Lang, Y.; Wang, C.; Li, Z.; Koerhuis, D.; Boons, G.-J.; Bosch, B.-J.; Rey, F.A.; de Groot, R.J.; et al. Structural basis for human coronavirus attachment to sialic acid receptors. Nat. Struct. Mol. Biol. 2019, 26, 481–489. [Google Scholar] [CrossRef]
- Yan, R.; Zhang, Y.; Li, Y.; Xia, L.; Guo, Y.; Zhou, Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020, 367, 1444–1448. [Google Scholar] [CrossRef]
- Milanetti, E.; Miotto, M.; Di Rienzo, L.; Nagaraj, M.; Monti, M.; Golbek, T.W.; Gosti, G.; Roeters, S.J.; Weidner, T.; Otzen, D.E.; et al. In-Silico Evidence for a Two Receptor Based Strategy of SARS-CoV-2. Front. Mol. Biosci. 2021, 8, 690655. [Google Scholar] [CrossRef]
- Vandelli, A.; Monti, M.; Milanetti, E.; Armaos, A.; Rupert, J.; Zacco, E.; Bechara, E.; Delli Ponti, R.; Tartaglia, G.G. Structural analysis of SARS-CoV-2 genome and predictions of the human interactome. Nucleic Acids Res. 2020, 48, 11270–11283. [Google Scholar] [CrossRef]
- Pronker, M.F.; Creutznacher, R.; Drulyte, I.; Hulswit, R.J.G.; Li, Z.; van Kuppeveld, F.J.M.; Snijder, J.; Lang, Y.; Bosch, B.-J.; Boons, G.-J.; et al. Sialoglycan binding triggers spike opening in a human coronavirus. Nature 2023, 624, 201–206. [Google Scholar] [CrossRef]
- Maity, S.; Acharya, A. Many Roles of Carbohydrates: A Computational Spotlight on the Coronavirus S Protein Binding. ACS Appl. Bio Mater. 2024, 7, 646–656. [Google Scholar] [CrossRef]
- Awasthi, M.; Gulati, S.; Sarkar, D.P.; Tiwari, S.; Kateriya, S.; Ranjan, P.; Verma, S.K. The Sialoside-Binding Pocket of SARS-CoV-2 Spike Glycoprotein Structurally Resembles MERS-CoV. Viruses 2020, 12, 909. [Google Scholar] [CrossRef]
- Baker, A.N.; Richards, S.-J.; Guy, C.S.; Congdon, T.R.; Hasan, M.; Zwetsloot, A.J.; Gallo, A.; Lewandowski, J.R.; Stansfeld, P.J.; Straube, A.; et al. The SARS-COV-2 Spike Protein Binds Sialic Acids and Enables Rapid Detection in a Lateral Flow Point of Care Diagnostic Device. ACS Cent. Sci. 2020, 6, 2046–2052. [Google Scholar] [CrossRef]
- Nguyen, L.; McCord, K.A.; Bui, D.T.; Bouwman, K.M.; Kitova, E.N.; Elaish, M.; Kumawat, D.; Daskhan, G.C.; Tomris, I.; Han, L.; et al. Sialic acid-containing glycolipids mediate binding and viral entry of SARS-CoV-2. Nat. Chem. Biol. 2022, 18, 81–90. [Google Scholar] [CrossRef]
- Cavezzi, A.; Menicagli, R.; Troiani, E.; Corrao, S. COVID-19, Cation Dysmetabolism, Sialic Acid, CD147, ACE2, Viroporins, Hepcidin and Ferroptosis: A Possible Unifying Hypothesis. F1000Research 2022, 11, 102. [Google Scholar] [CrossRef]
- Jiang, X.; Tan, M.; Xia, M.; Huang, P.; Kennedy, M.A. Intra-species sialic acid polymorphism in humans: A common niche for influenza and coronavirus pandemics? Emerg. Microbes Infect. 2021, 10, 1191–1199. [Google Scholar] [CrossRef]
- Dhar, C.; Sasmal, A.; Diaz, S.; Verhagen, A.; Yu, H.; Li, W.; Chen, X.; Varki, A. Are sialic acids involved in COVID-19 pathogenesis? Glycobiology 2021, 31, 1068–1071. [Google Scholar] [CrossRef]
- Unione, L.; Moure, M.J.; Lenza, M.P.; Oyenarte, I.; Ereño-Orbea, J.; Ardá, A.; Jiménez-Barbero, J. The SARS-CoV-2 Spike Glycoprotein Directly Binds Exogeneous Sialic Acids: A NMR View. Angew. Chem. 2022, 134, e202201432. [Google Scholar] [CrossRef]
- Frąc, M.; Hannula, S.E.; Bełka, M.; Jędryczka, M. Fungal Biodiversity and Their Role in Soil Health. Front. Microbiol. 2018, 9, 707. [Google Scholar] [CrossRef]
- Belvoncikova, P.; Splichalova, P.; Videnska, P.; Gardlik, R. The Human Mycobiome: Colonization, Composition and the Role in Health and Disease. J. Fungi 2022, 8, 1046. [Google Scholar] [CrossRef]
- Köhler, J.R.; Casadevall, A.; Perfect, J. The Spectrum of Fungi That Infects Humans. Cold Spring Harb. Perspect. Med. 2015, 5, a019273. [Google Scholar] [CrossRef]
- Rodrigues, M.L.; Dobroff, A.S.; Couceiro, J.N.D.S.; Alviano, C.S.; Schauer, R.; Travassos, L.R. Sialylglycoconjugates and sialyltransferase activity in the fungus Cryptococcus neoformans. Glycoconj. J. 2002, 19, 165–173. [Google Scholar] [CrossRef]
- Bose, I.; Reese, A.J.; Ory, J.J.; Janbon, G.; Doering, T.L. A Yeast under Cover: The Capsule of Cryptococcus neoformans. Eukaryot. Cell 2003, 2, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Warwas, M.L.; Watson, J.N.; Bennet, A.J.; Moore, M.M. Structure and role of sialic acids on the surface of Aspergillus fumigatus conidiospores. Glycobiology 2007, 17, 401–410. [Google Scholar] [CrossRef]
- Alviano, C.S.; Travassos, L.R.; Schauer, R. Sialic acids in fungi. Glycoconj. J. 1999, 16, 545–554. [Google Scholar] [CrossRef]
- Maziarz, E.K.; Perfect, J.R. Cryptococcosis. Infect. Dis. Clin. North Am. 2016, 30, 179–206. [Google Scholar] [CrossRef]
- Horwath, M.C.; Fecher, R.A.; Deepe, G.S. Histoplasma capsulatum, lung infection and immunity. Future Microbiol. 2015, 10, 967–975. [Google Scholar] [CrossRef]
- Sil, A.; Andrianopoulos, A. Thermally Dimorphic Human Fungal Pathogens—Polyphyletic Pathogens with a Convergent Pathogenicity Trait. Cold Spring Harb. Perspect. Med. 2015, 5, a019794. [Google Scholar] [CrossRef] [PubMed]
- Valdez, A.F.; Miranda, D.Z.; Guimarães, A.J.; Nimrichter, L.; Nosanchuk, J.D. Pathogenicity & virulence of Histoplasma capsulatum—A multifaceted organism adapted to intracellular environments. Virulence 2022, 13, 1900. [Google Scholar] [CrossRef]
- Burgess, T.B.; Condliffe, A.M.; Elks, P.M. A Fun-Guide to Innate Immune Responses to Fungal Infections. J. Fungi 2022, 8, 805. [Google Scholar] [CrossRef]
- Erwig, L.P.; Gow, N.A.R. Interactions of fungal pathogens with phagocytes. Nat. Rev. Microbiol. 2016, 14, 163–176. [Google Scholar] [CrossRef]
- Guimaraes, A.J.; de Cerqueira, M.D.; Nosanchuk, J.D. Surface Architecture of Histoplasma Capsulatum. Front. Microbiol. 2011, 2, 225. [Google Scholar] [CrossRef]
- Uribe-Querol, E.; Rosales, C. Control of Phagocytosis by Microbial Pathogens. Front. Immunol. 2017, 8, 1368. [Google Scholar] [CrossRef] [PubMed]
- Mittal, J.; Ponce, M.G.; Gendlina, I.; Nosanchuk, J.D. Histoplasma Capsulatum: Mechanisms for Pathogenesis. Curr. Top. Microbiol. Immunol. 2019, 422, 157–191. [Google Scholar] [CrossRef]
- Huang, S.; Deepe, G.S., Jr. Notch regulates Histoplasma capsulatum clearance in mouse lungs during innate and adaptive immune response phases in primary infection. J. Leukoc. Biol. 2022, 112, 1137–1154. [Google Scholar] [CrossRef] [PubMed]
- Sharon, N. Carbohydrates as recognition determinants in phagocytosis and in lectin-mediated killing of target cells. Biol. Cell 1984, 51, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Ielasi, F.S.; Alioscha-Perez, M.; Donohue, D.; Claes, S.; Sahli, H.; Schols, D.; Willaert, R.G. Lectin-Glycan Interaction Network-Based Identification of Host Receptors of Microbial Pathogenic Adhesins. mBio 2016, 7, e00584-16. [Google Scholar] [CrossRef]
- Singh, R.S.; Bhari, R.; Rana, V.; Tiwary, A.K. Immunomodulatory and therapeutic potential of a mycelial lectin from Aspergillus nidulans. Appl. Biochem. Biotechnol. 2011, 165, 624–638. [Google Scholar] [CrossRef]
- Duarte-Escalante, E.; Zenteno, E.; Taylor, M.L. Interaction of Histoplasma capsulatum Yeasts with Galactosylated Surface Molecules of Murine Macrophages. Arch. Med. Res. 2003, 34, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.L.; Duarte-Escalante, E.; Pérez, A.; Zenteno, E.; Toriello, C. Histoplasma capsulatum yeast cells attach and agglutinate human erythrocytes. Med. Mycol. 2004, 42, 287–292. [Google Scholar] [CrossRef][Green Version]
- Lehmann, F.; Tiralongo, E.; Tiralongo, J. Sialic acid-specific lectins: Occurrence, specificity and function. Cell. Mol. Life Sci. CMLS 2006, 63, 1331–1354. [Google Scholar] [CrossRef]
- Talapko, J.; Juzbašić, M.; Matijević, T.; Pustijanac, E.; Bekić, S.; Kotris, I.; Škrlec, I. Candida albicans—The Virulence Factors and Clinical Manifestations of Infection. J. Fungi 2021, 7, 79. [Google Scholar] [CrossRef]
- Sonesson, A.; Bartosik, J.; Christiansen, J.; Roscher, I.; Nilsson, F.; Schmidtchen, A.; Bäck, O. Sensitization to skin-associated microorganisms in adult patients with atopic dermatitis is of importance for disease severity. Acta Derm. Venereol. 2013, 93, 340–345. [Google Scholar] [CrossRef]
- Kobiela, A.; Frackowiak, J.E.; Biernacka, A.; Hovhannisyan, L.; Bogucka, A.E.; Panek, K.; Paul, A.A.; Lukomska, J.; Wang, X.; Giannoulatou, E.; et al. Exposure of Keratinocytes to Candida Albicans in the Context of Atopic Milieu Induces Changes in the Surface Glycosylation Pattern of Small Extracellular Vesicles to Enhance Their Propensity to Interact with Inhibitory Siglec Receptors. Front. Immunol. 2022, 13, 884530. [Google Scholar] [CrossRef]
- Flevari, A.; Theodorakopoulou, M.; Velegraki, A.; Armaganidis, A.; Dimopoulos, G. Treatment of invasive candidiasis in the elderly: A review. Clin. Interv. Aging 2013, 8, 1199–1208. [Google Scholar] [CrossRef]
- R, A.N.; Rafiq, N.B. Candidiasis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Valentijn-Benz, M.; Nazmi, K.; Brand, H.S.; van’t Hof, W.; Veerman, E.C.I. Growth of Candida albicans in human saliva is supported by low-molecular-mass compounds. FEMS Yeast Res. 2015, 15, fov088. [Google Scholar] [CrossRef][Green Version]
- Lopes, J.P.; Lionakis, M.S. Pathogenesis and virulence of Candida albicans. Virulence 2022, 13, 89–121. [Google Scholar] [CrossRef]
- Masuoka, J. Surface Glycans of Candida albicans and Other Pathogenic Fungi: Physiological Roles, Clinical Uses, and Experimental Challenges. Clin. Microbiol. Rev. 2004, 17, 281–310. [Google Scholar] [CrossRef]
- Soares, R.M.A.; de A. Soares, R.M.; Alviano, D.S.; Angluster, J.; Alviano, C.S.; Travassos, L.R. Identification of sialic acids on the cell surface of Candida albicans. Biochim. Biophys. Acta BBA—Gen. Subj. 2000, 1474, 262–268. [Google Scholar] [CrossRef]
- Roggentin, P.; Krug, G.; Schauer, R.; Brasch, J. Lack of sialidase activity in Candida albicans and Candida glabrata. Mycoses 1999, 42, 33–36. [Google Scholar] [CrossRef]
- Theel, E.S.; Pritt, B.S. Parasites. Microbiol. Spectr. 2016, 4, DMIH2-0013-2015. [Google Scholar] [CrossRef]
- Iwasaki, A.; Medzhitov, R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 2015, 16, 343–353. [Google Scholar] [CrossRef]
- Coakley, G.; Buck, A.H.; Maizels, R.M. Host parasite communications—Messages from helminths for the immune system: Parasite communication and cell-cell interactions. Sens. Signal. Parasit. 2016, 208, 33–40. [Google Scholar] [CrossRef]
- Agusti, R.; Gallo-Rodriguez, C.; de Lederkremer, R.M. Trypanosoma cruzi trans-sialidase. A tool for the synthesis of sialylated oligosaccharides. Carbohydr. Res. 2019, 479, 48–58. [Google Scholar] [CrossRef]
- Schmid-Hempel, P. Immune defence, parasite evasion strategies and their relevance for ‘macroscopic phenomena’ such as virulence. Philos. Trans. R. Soc. B Biol. Sci. 2008, 364, 85–98. [Google Scholar] [CrossRef]
- Useche, Y.; Pérez, A.R.; de Meis, J.; Bonomo, A.; Savino, W. Central nervous system commitment in Chagas disease. Front. Immunol. 2022, 13, 975106. [Google Scholar] [CrossRef]
- da Silva Oliveira Barbosa, E.; Roggero, E.A.; González, F.B.; Fernández, R.D.V.; Carvalho, V.F.; Bottasso, O.A.; Pérez, A.R.; Villar, S.R. Evidence in Favor of an Alternative Glucocorticoid Synthesis Pathway During Acute Experimental Chagas Disease. Front. Endocrinol. 2020, 10, 866. [Google Scholar] [CrossRef]
- González, F.B.; Savino, W.; Pérez, A.R. Brain-Thymus Connections in Chagas Disease. Neuroimmunomodulation 2024, 31, 78–88. [Google Scholar] [CrossRef]
- Cardoso, M.S.; Reis-Cunha, J.L.; Bartholomeu, D.C. Evasion of the Immune Response by Trypanosoma cruzi during Acute Infection. Front. Immunol. 2016, 6, 659. [Google Scholar] [CrossRef]
- Savino, W.; Villa-Verde, D.M.; Mendes-da-Cruz, D.A.; Silva-Monteiro, E.; Perez, A.R.; Aoki Mdel, P.; Bottasso, O.; Guiñazú, N.; Silva-Barbosa, S.D.; Gea, S. Cytokines and cell adhesion receptors in the regulation of immunity to Trypanosoma cruzi. Cytokines Coming Age S. Am. 2007, 18, 107–124. [Google Scholar] [CrossRef]
- Flávia Nardy, A.; Freire-de-Lima, C.G.; Morrot, A. Immune Evasion Strategies of Trypanosoma cruzi. J. Immunol. Res. 2015, 2015, 178947. [Google Scholar] [CrossRef]
- Muiá, R.; Yu, H.; Prescher, J.; Hellman, U.; Bertozzi, C.; Campetella, O. Identification of glycoproteins targeted by Trypanosoma cruzi trans-sialidase, a virulence factor that disturbs lymphocyte glycosylation. Glycobiology 2010, 20, 833–842. [Google Scholar] [CrossRef]
- Piacenza, L.; Peluffo, G.; Alvarez, M.N.; Kelly, J.M.; Wilkinson, S.R.; Radi, R. Peroxiredoxins play a major role in protecting Trypanosoma cruzi against macrophage- and endogenously-derived peroxynitrite. Biochem. J. 2008, 410, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Martín-Escolano, J.; Marín, C.; Rosales, M.J.; Tsaousis, A.D.; Medina-Carmona, E.; Martín-Escolano, R. An Updated View of the Trypanosoma cruzi Life Cycle: Intervention Points for an Effective Treatment. ACS Infect. Dis. 2022, 8, 1107–1115. [Google Scholar] [CrossRef]
- Schacht, P.; Arcieri, G.; Hullmann, R. Safety of oral ciprofloxacin: An update based on clinical trial results. Am. J. Med. 1989, 87, S98–S102. [Google Scholar] [CrossRef]
- Adasme, M.F.; Bolz, S.N.; Adelmann, L.; Salentin, S.; Haupt, V.J.; Moreno-Rodríguez, A.; Nogueda-Torres, B.; Castillo-Campos, V.; Yepez-Mulia, L.; De Fuentes-Vicente, J.A.; et al. Repositioned Drugs for Chagas Disease Unveiled via Structure-Based Drug Repositioning. Int. J. Mol. Sci. 2020, 21, 8809. [Google Scholar] [CrossRef]
- Goerdeler, F.; Seeberger, P.H.; Moscovitz, O. Unveiling the Sugary Secrets of Plasmodium Parasites. Front. Microbiol. 2021, 12, 712538. [Google Scholar] [CrossRef]
- Fact Sheet About Malaria. Available online: https://www.who.int/news-room/fact-sheets/detail/malaria (accessed on 28 January 2025).
- Hanboonkunupakarn, B.; White, N.J. The threat of antimalarial drug resistance. Trop. Dis. Travel Med. Vaccines 2016, 2, 1–5. [Google Scholar] [CrossRef]
- Rasmussen, C.; Alonso, P.; Ringwald, P. Current and emerging strategies to combat antimalarial resistance. Expert Rev. Anti Infect. Ther. 2022, 20, 353–372. [Google Scholar] [CrossRef]
- Chuang, Y.-M.; Agunbiade, T.A.; Tang, X.-D.; Freudzon, M.; Almeras, L.; Fikrig, E. The Effects of A Mosquito Salivary Protein on Sporozoite Traversal of Host Cells. J. Infect. Dis. 2020, 224, 544–553. [Google Scholar] [CrossRef]
- Suwanarusk, R.; Cooke, B.M.; Dondorp, A.M.; Silamut, K.; Sattabongkot, J.; White, N.J.; Udomsangpetch, R. The deformability of red blood cells parasitized by Plasmodium falciparum and P. vivax. J. Infect. Dis. 2004, 189, 190–194. [Google Scholar] [CrossRef]
- Valenciano, A.L.; Gomez-Lorenzo, M.G.; Vega-Rodríguez, J.; Adams, J.H.; Roth, A. In vitro models for human malaria: Targeting the liver stage. Trends Parasitol. 2022, 38, 758–774. [Google Scholar] [CrossRef]
- Deroost, K.; Pham, T.-T.; Opdenakker, G.; Van den Steen, P.E. The immunological balance between host and parasite in malaria. FEMS Microbiol. Rev. 2016, 40, 208–257. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, D.E.; Zimmerberg, J. Hardly Vacuous: The Parasitophorous Vacuolar Membrane of Malaria Parasites. Trends Parasitol. 2020, 36, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Molina-Franky, J.; Patarroyo, M.E.; Kalkum, M.; Patarroyo, M.A. The Cellular and Molecular Interaction Between Erythrocytes and Plasmodium falciparum Merozoites. Front. Cell. Infect. Microbiol. 2022, 12, 816574. [Google Scholar] [CrossRef]
- Cowman, A.F.; Crabb, B.S. Invasion of Red Blood Cells by Malaria Parasites. Cell 2006, 124, 755–766. [Google Scholar] [CrossRef]
- Ezema, C.A.; Okagu, I.U.; Ezeorba, T.P.C. Escaping the enemy’s bullets: An update on how malaria parasites evade host immune response. Parasitol. Res. 2023, 122, 1715–1731. [Google Scholar] [CrossRef]
- Venugopal, K.; Hentzschel, F.; Valkiūnas, G.; Marti, M. Plasmodium asexual growth and sexual development in the haematopoietic niche of the host. Nat. Rev. Microbiol. 2020, 18, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Butcher, G.A.; Mitchell, G.H.; Cohen, S. Mechanism of Host Specificity in Malarial Infection. Nature 1973, 244, 40–42. [Google Scholar] [CrossRef]
- Habtamu, K.; Petros, B.; Yan, G. Plasmodium vivax: The potential obstacles it presents to malaria elimination and eradication. Trop. Dis. Travel Med. Vaccines 2022, 8, 27. [Google Scholar] [CrossRef]
- Jaskiewicz, E.; Jodłowska, M.; Kaczmarek, R.; Zerka, A. Erythrocyte glycophorins as receptors for Plasmodium merozoites. Parasit. Vectors 2019, 12, 317. [Google Scholar] [CrossRef]
- Alves-Rosa, M.F.; Tayler, N.M.; Dorta, D.; Coronado, L.M.; Spadafora, C.P. falciparum Invasion and Erythrocyte Aging. Cells 2024, 13, 334. [Google Scholar] [CrossRef]
- Moskovitz, R.; Pholcharee, T.; DonVito, S.M.; Guloglu, B.; Lowe, E.; Mohring, F.; Moon, R.W.; Higgins, M.K. Structural basis for DARC binding in reticulocyte invasion by Plasmodium vivax. Nat. Commun. 2023, 14, 3637. [Google Scholar] [CrossRef] [PubMed]
- Day, C.J.; Favuzza, P.; Bielfeld, S.; Haselhorst, T.; Seefeldt, L.; Hauser, J.; Shewell, L.K.; Flueck, C.; Poole, J.; Jen, F.E.-C.; et al. The essential malaria protein PfCyRPA targets glycans to invade erythrocytes. Cell Rep. 2024, 43, 114012. [Google Scholar] [CrossRef]
- Yang, P.-K.; Liang, X.-Y.; Lin, M.; Chen, J.-T.; Huang, H.-Y.; Lin, L.-Y.; Ehapo, C.S.; Eyi, U.M.; Zheng, Y.-Z.; Xie, D.-D.; et al. Population genetic analysis of the Plasmodium falciparum erythrocyte binding antigen-175 (EBA-175) gene in Equatorial Guinea. Malar. J. 2021, 20, 374. [Google Scholar] [CrossRef]
- Jiang, L.; Gaur, D.; Mu, J.; Zhou, H.; Long, C.A.; Miller, L.H. Evidence for erythrocyte-binding antigen 175 as a component of a ligand-blocking blood-stage malaria vaccine. Proc. Natl. Acad. Sci. USA 2011, 108, 7553–7558. [Google Scholar] [CrossRef] [PubMed]
- Takashima, E.; Otsuki, H.; Morita, M.; Ito, D.; Nagaoka, H.; Yuguchi, T.; Hassan, I.; Tsuboi, T. The Need for Novel Asexual Blood-Stage Malaria Vaccine Candidates for Plasmodium falciparum. Biomolecules 2024, 14, 100. [Google Scholar] [CrossRef]
- Estrada-Martínez, L.E.; Moreno-Celis, U.; Cervantes-Jiménez, R.; Ferriz-Martínez, R.A.; Blanco-Labra, A.; García-Gasca, T. Plant lectins as medical tools against digestive system cancers. Int. J. Mol. Sci. 2017, 18, 1403. [Google Scholar] [CrossRef] [PubMed]
- Serdar, Z.; Yeşilbursa, D.; Dirican, M.; Sarandöl, E.; Serdar, A. Sialic acid and oxidizability of lipid and proteins and antioxidant status in patients with coronary artery disease. Cell Biochem. Funct. 2007, 25, 655–664. [Google Scholar] [CrossRef]
- Volkhina, I.V.; Butolin, E.G. Clinical and Diagnostic Significance of Sialic Acids Determination in Biological Material. Biochem. Mosc. Suppl. Ser. B Biomed. Chem. 2022, 16, 165–174. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Fuster, V. The Global Burden of Cardiovascular Diseases and Risks: A Compass for Global Action. J. Am. Coll. Cardiol. 2020, 76, 2980–2981. [Google Scholar] [CrossRef]
- Teo, K.K.; Rafiq, T. Cardiovascular Risk Factors and Prevention: A Perspective From Developing Countries. Can. J. Cardiol. 2021, 37, 733–743. [Google Scholar] [CrossRef]
- Cocciolone, A.J.; Hawes, J.Z.; Staiculescu, M.C.; Johnson, E.O.; Murshed, M.; Wagenseil, J.E. Elastin, arterial mechanics, and cardiovascular disease. Am. J. Physiol.-Heart Circ. Physiol. 2018, 315, H189–H205. [Google Scholar] [CrossRef]
- Milusev, A.; Rieben, R.; Sorvillo, N. The Endothelial Glycocalyx: A Possible Therapeutic Target in Cardiovascular Disorders. Front. Cardiovasc. Med. 2022, 9, 897087. [Google Scholar] [CrossRef]
- D’Addio, M.; Frey, J.; Otto, V.I. The manifold roles of sialic acid for the biological functions of endothelial glycoproteins. Glycobiology 2020, 30, 490–499. [Google Scholar] [CrossRef]
- Wei, M.; Wang, P.G. Desialylation in physiological and pathological processes: New target for diagnostic and therapeutic development. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2019; Volume 162, pp. 25–57. [Google Scholar] [CrossRef]
- Gokmen, S.S.; Kilicli, G.; Ozcelik, F.; Ture, M.; Gulen, S. Association between serum total and lipid-bound sialic acid concentration and the severity of coronary atherosclerosis. J. Lab. Clin. Med. 2002, 140, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Mezentsev, A.; Bezsonov, E.; Kashirskikh, D.; Baig, M.S.; Eid, A.H.; Orekhov, A. Proatherogenic Sialidases and Desialylated Lipoproteins: 35 Years of Research and Current State from Bench to Bedside. Biomedicines 2021, 9, 600. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, K.; Coker, J.K.; Grunddal, K.V.; Dhar, C.; Hsiao, J.; Zengler, K.; Varki, N.; Varki, A.; Gordts, P.L.S.M. Dietary Neu5Ac Intervention Protects Against Atherosclerosis Associated with Human-Like Neu5Gc Loss—Brief Report. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2730–2739. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-N.; Qian, S.-H.; Yao, Z.-Y.; Ming, S.-P.; Shi, X.-J.; Kang, P.-F.; Zhang, N.-R.; Wang, X.-J.; Gao, D.-S.; Gao, Q.; et al. Correlation of serum N-Acetylneuraminic acid with the risk and prognosis of acute coronary syndrome: A prospective cohort study. BMC Cardiovasc. Disord. 2020, 20, 404. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, T.-T.; Li, Y.; Li, J.; Fan, Y.; Huang, F.-Q.; Cai, Y.-Y.; Ma, G.; Liu, J.-F.; Chen, Q.-Q.; et al. Functional Metabolomics Characterizes a Key Role for N-Acetylneuraminic Acid in Coronary Artery Diseases. Circulation 2018, 137, 1374–1390. [Google Scholar] [CrossRef]
- Li, C.; Zhao, M.; Xiao, L.; Wei, H.; Wen, Z.; Hu, D.; Yu, B.; Sun, Y.; Gao, J.; Shen, X.; et al. Prognostic Value of Elevated Levels of Plasma N-Acetylneuraminic Acid in Patients with Heart Failure. Circ. Heart Fail. 2021, 14, e008459. [Google Scholar] [CrossRef]
- Pham, T.; Gregg, C.J.; Karp, F.; Chow, R.; Padler-Karavani, V.; Cao, H.; Chen, X.; Witztum, J.L.; Varki, N.M.; Varki, A. Evidence for a novel human-specific xeno-auto-antibody response against vascular endothelium. Blood 2009, 114, 5225–5235. [Google Scholar] [CrossRef]
- Yida, Z.; Imam, M.U.; Ismail, M.; Wong, W.; Abdullah, M.A.; Ideris, A.; Ismail, N. N-Acetylneuraminic acid attenuates hypercoagulation on high fat diet-induced hyperlipidemic rats. Food Nutr. Res. 2015, 59, 29046. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Vongpatanasin, W.; Sacharidou, A.; Kifer, D.; Yuhanna, I.S.; Banerjee, S.; Tanigaki, K.; Polasek, O.; Chu, H.; Sundgren, N.C.; et al. Supplementation with the Sialic Acid Precursor N-Acetyl-D-Mannosamine Breaks the Link Between Obesity and Hypertension. Circulation 2019, 140, 2005–2018. [Google Scholar] [CrossRef]
- Orlich, M.J.; Singh, P.N.; Sabaté, J.; Fan, J.; Sveen, L.; Bennett, H.; Knutsen, S.F.; Beeson, W.L.; Jaceldo-Siegl, K.; Butler, T.L.; et al. Vegetarian Dietary Patterns and the Risk of Colorectal Cancers. JAMA Intern. Med. 2015, 175, 767. [Google Scholar] [CrossRef]
- Liu, L.; Shi, Z.; Ji, X.; Zhang, W.; Luan, J.; Zahr, T.; Qiang, L. Adipokines, adiposity, and atherosclerosis. Cell. Mol. Life Sci. 2022, 79, 272. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, N.S.; Sabaté, J.; Jaceldo-Siegl, K.; Fraser, G.E. Vegetarian Dietary Patterns Are Associated with a Lower Risk of Metabolic Syndrome: The Adventist Health Study 2. Diabetes Care 2011, 34, 1225–1227. [Google Scholar] [CrossRef]
- Tonstad, S.; Stewart, K.; Oda, K.; Batech, M.; Herring, R.P.; Fraser, G.E. Vegetarian diets and incidence of diabetes in the Adventist Health Study-2. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Fraser, G.; Katuli, S.; Anousheh, R.; Knutsen, S.; Herring, P.; Fan, J. Vegetarian diets and cardiovascular risk factors in black members of the Adventist Health Study-2. Public Health Nutr. 2015, 18, 537–545. [Google Scholar] [CrossRef]
- Matar, P.; Alaniz, L.; Rozados, V.; Aquino, J.B.; Malvicini, M.; Atorrasagasti, C.; Gidekel, M.; Silva, M.; Scharovsky, O.G.; Mazzolini, G. Immunotherapy for liver tumors: Present status and future prospects. J. Biomed. Sci. 2009, 16, 30. [Google Scholar] [CrossRef]
- Varki, A. Letter to the Glyco-Forum: Since there are PAMPs and DAMPs, there must be SAMPs? Glycan “self-associated molecular patterns” dampen innate immunity, but pathogens can mimic them. Glycobiology 2011, 21, 1121–1124. [Google Scholar] [CrossRef]
- Lübbers, J.; Rodríguez, E.; van Kooyk, Y. Modulation of Immune Tolerance via Siglec-Sialic Acid Interactions. Front. Immunol. 2018, 9, 2807. [Google Scholar] [CrossRef]
- Pilling, D.; Karhadkar, T.R.; Gomer, R.H. High-Fat Diet–Induced Adipose Tissue and Liver Inflammation and Steatosis in Mice Are Reduced by Inhibiting Sialidases. Am. J. Pathol. 2021, 191, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Nayak, B.S.; Bhaktha, G. Relationship between Sialic acid and metabolic variables in Indian type 2 diabetic patients. Lipids Health Dis. 2005, 4, 15. [Google Scholar] [CrossRef]
- Yida, Z.; Imam, M.U.; Ismail, M.; Ismail, N.; Azmi, N.H.; Wong, W.; Altine Adamu, H.; Md Zamri, N.D.; Ideris, A.; Abdullah, M.A. N-Acetylneuraminic Acid Supplementation Prevents High Fat Diet-Induced Insulin Resistance in Rats through Transcriptional and Nontranscriptional Mechanisms. BioMed Res. Int. 2015, 2015, 602313. [Google Scholar] [CrossRef]
- White, E.J.; Gyulay, G.; Lhoták, Š.; Szewczyk, M.M.; Chong, T.; Fuller, M.T.; Dadoo, O.; Fox-Robichaud, A.E.; Austin, R.C.; Trigatti, B.L.; et al. Sialidase down-regulation reduces non-HDL cholesterol, inhibits leukocyte transmigration, and attenuates atherosclerosis in ApoE knockout mice. J. Biol. Chem. 2018, 293, 14689–14706. [Google Scholar] [CrossRef] [PubMed]
- Munkley, J.; Scott, E. Targeting Aberrant Sialylation to Treat Cancer. Medicines 2019, 6, 102. [Google Scholar] [CrossRef]
- Rabinovich, G.A.; Croci, D.O. Regulatory Circuits Mediated by Lectin-Glycan Interactions in Autoimmunity and Cancer. Immunity 2012, 36, 322–335. [Google Scholar] [CrossRef]
- Rodrigues, E.; Macauley, M.S. Hypersialylation in cancer: Modulation of inflammation and therapeutic opportunities. Cancers 2018, 10, 207. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Croci, D.O.; Zacarías Fluck, M.F.; Rico, M.J.; Matar, P.; Rabinovich, G.A.; Scharovsky, O.G. Dynamic cross-talk between tumor and immune cells in orchestrating the immunosuppressive network at the tumor microenvironment. Cancer Immunol. Immunother. 2007, 56, 1687–1700. [Google Scholar] [CrossRef]
- Cagnoni, A.J.; Pérez Sáez, J.M.; Rabinovich, G.A.; Mariño, K.V. Turning-Off Signaling by Siglecs, Selectins, and Galectins: Chemical Inhibition of Glycan-Dependent Interactions in Cancer. Front. Oncol. 2016, 6, 109. [Google Scholar] [CrossRef]
- van de Wall, S.; Santegoets, K.C.M.; van Houtum, E.J.H.; Büll, C.; Adema, G.J. Sialoglycans and Siglecs Can Shape the Tumor Immune Microenvironment. Trends Immunol. 2020, 41, 274–285. [Google Scholar] [CrossRef]
- Büll, C.; den Brok, M.H.; Adema, G.J. Sweet escape: Sialic acids in tumor immune evasion. Biochim. Biophys. Acta BBA—Rev. Cancer 2014, 1846, 238–246. [Google Scholar] [CrossRef]
- Dall’Olio, F.; Malagolini, N.; Trinchera, M.; Chiricolo, M. Sialosignaling: Sialyltransferases as engines of self-fueling loops in cancer progression. Biochim. Biophys. Acta BBA—Gen. Subj. 2014, 1840, 2752–2764. [Google Scholar] [CrossRef]
- Sun, J.; Lu, Q.; Sanmamed, M.F.; Wang, J. Siglec-15 as an Emerging Target for Next-generation Cancer Immunotherapy. Clin. Cancer Res. 2021, 27, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Altevogt, P.; Sammar, M.; Hüser, L.; Kristiansen, G. Novel insights into the function of CD24: A driving force in cancer. Int. J. Cancer 2021, 148, 546–559. [Google Scholar] [CrossRef] [PubMed]
- Stanczak, M.A.; Rodrigues Mantuano, N.; Kirchhammer, N.; Sanin, D.E.; Jacob, F.; Coelho, R.; Everest-Dass, A.V.; Wang, J.; Trefny, M.P.; Monaco, G.; et al. Targeting cancer glycosylation repolarizes tumor-associated macrophages allowing effective immune checkpoint blockade. Sci. Transl. Med. 2022, 14, eabj1270. [Google Scholar] [CrossRef]
- Diamantopoulou, S.; Stagiannis, K.D.; Vasilopoulos, K.; Barlas, P.; Tsegenidis, T.; Karamanos, N.K. Importance of high-performance liquid chromatographic analysis of serum N-acylneuraminic acids in evaluating surgical treatment in patients with early endometrial cancer. J. Chromatogr. B. Biomed. Sci. App. 1999, 732, 375–381. [Google Scholar] [CrossRef]
- Phitak, T.; Klangjorhor, J.; Pothacharoen, P.; Pruksakorn, D.; Kongtawelert, P. Level and distribution of secreted and cell-associated N-acetyl, N-glycolylneuraminic, and deaminoneuraminic acids in osteosarcoma cells isolated from patients. ScienceAsia 2017, 43, 15–25. [Google Scholar] [CrossRef][Green Version]
- Yabu, M.; Korekane, H.; Hatano, K.; Kaneda, Y.; Nonomura, N.; Sato, C.; Kitajima, K.; Miyamoto, Y. Occurrence of free deaminoneuraminic acid (KDN)-containing complex-type N-glycans in human prostate cancers. Glycobiology 2013, 23, 634–642. [Google Scholar] [CrossRef]
- Tzanakakis, G.N.; Syrokou, A.; Kanakis, I.; Karamanos, N.K. Determination and distribution of N-acetyl- and N-glycolylneuraminic acids in culture media and cell associated glycoconjugates from human malignant mesothelioma and adenocarcinoma cells. Biomed. Chromatogr. 2006, 20, 434–439. [Google Scholar] [CrossRef]
- Tzanakakis, G.N.; Nikitovic, D.; Katonis, P.; Kanakis, I.; Karamanos, N.K. Expression and distribution of N-acetyl and N-glycolylneuraminic acids in secreted and cell-associated glycoconjugates by two human osteosarcoma cell lines. Biomed. Chromatogr. 2007, 21, 406–409. [Google Scholar] [CrossRef] [PubMed]
- Dobie, C.; Skropeta, D. Insights into the role of sialylation in cancer progression and metastasis. Br. J. Cancer 2021, 124, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Ostrand-Rosenberg, S. Immune surveillance: A balance between protumor and antitumor immunity. Curr. Opin. Genet. Dev. 2008, 18, 11–18. [Google Scholar] [CrossRef]
- Smith, B.A.H.; Bertozzi, C.R. The clinical impact of glycobiology: Targeting selectins, Siglecs and mammalian glycans. Nat. Rev. Drug Discov. 2021, 20, 217–243. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Paulson, J.C. Siglecs as Immune Cell Checkpoints in Disease. Annu. Rev. Immunol. 2020, 38, 365–395. [Google Scholar] [CrossRef]
- Läubli, H.; Borsig, L. Altered Cell Adhesion and Glycosylation Promote Cancer Immune Suppression and Metastasis. Front. Immunol. 2019, 10, 2120. [Google Scholar] [CrossRef]
- Anselmino, L.E.; Baglioni, M.V.; Malizia, F.; Laluce, N.C.; Etichetti, C.B.; Marignac, V.L.M.; Rozados, V.; Scharovsky, O.G.; Girardini, J.; Rico, M.J.; et al. Repositioning metformin and propranolol for colorectal and triple negative breast cancers treatment. Sci. Rep. 2021, 11, 8091. [Google Scholar] [CrossRef]
- Veettil, S.K.; Wong, T.Y.; Loo, Y.S.; Playdon, M.C.; Lai, N.M.; Giovannucci, E.L.; Chaiyakunapruk, N. Role of Diet in Colorectal Cancer Incidence: Umbrella Review of Meta-analyses of Prospective Observational Studies. JAMA Netw. Open 2021, 4, e2037341. [Google Scholar] [CrossRef]
- Buja, A.; Pierbon, M.; Lago, L.; Grotto, G.; Baldo, V. Breast Cancer Primary Prevention and Diet: An Umbrella Review. Int. J. Environ. Res. Public. Health 2020, 17, 4731. [Google Scholar] [CrossRef]
- Debras, C.; Chazelas, E.; Srour, B.; Kesse-Guyot, E.; Julia, C.; Zelek, L.; Agaësse, C.; Druesne-Pecollo, N.; Galan, P.; Hercberg, S.; et al. Total and added sugar intakes, sugar types, and cancer risk: Results from the prospective NutriNet-Santé cohort. Am. J. Clin. Nutr. 2020, 112, 1267–1279. [Google Scholar] [CrossRef]
- Gaesser, G.A. Whole Grains, Refined Grains, and Cancer Risk: A Systematic Review of Meta-Analyses of Observational Studies. Nutrients 2020, 12, 3756. [Google Scholar] [CrossRef] [PubMed]
- Orlich, M.J.; Mashchak, A.D.; Jaceldo-Siegl, K.; Utt, J.T.; Knutsen, S.F.; Sveen, L.E.; Fraser, G.E. Dairy foods, calcium intakes, and risk of incident prostate cancer in Adventist Health Study–2. Am. J. Clin. Nutr. 2022, 116, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Penniecook-Sawyers, J.A.; Jaceldo-Siegl, K.; Fan, J.; Beeson, L.; Knutsen, S.; Herring, P.; Fraser, G.E. Vegetarian dietary patterns and the risk of breast cancer in a low-risk population. Br. J. Nutr. 2016, 115, 1790–1797. [Google Scholar] [CrossRef]
- Vargas, A.J.; Thompson, P.A. Diet and Nutrient Factors in Colorectal Cancer Risk. Nutr. Clin. Pract. 2012, 27, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Aykan, N.F. Red meat and colorectal cancer. Oncol. Rev. 2015, 9, 38–44. [Google Scholar] [CrossRef]
- Oostindjer, M.; Alexander, J.; Amdam, G.V.; Andersen, G.; Bryan, N.S.; Chen, D.; Corpet, D.E.; De Smet, S.; Dragsted, L.O.; Haug, A.; et al. The role of red and processed meat in colorectal cancer development: A perspective. Meat Sci. 2014, 97, 583–596. [Google Scholar] [CrossRef]
- Sesink, A.L.A.; Termont, D.S.M.L.; Kleibeuker, J.H.; Meer, R.V.D. Red meat and colon cancer: Dietary haem, but not fat, has cytotoxic and hyperproliferative effects on rat colonic epithelium Aloys. Carcinogenesis 2000, 21, 1909–1915. [Google Scholar]
- Turner, N.D.; Lloyd, S.K. Association between red meat consumption and colon cancer: A systematic review of experimental results. Exp. Biol. Med. 2017, 242, 813–839. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (EFSA CONTAM Panel); Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.-C.; Nebbia, C.S.; et al. Risk assessment of N-nitrosamines in food. EFSA J. 2023, 21, e07884. [Google Scholar] [CrossRef]
- Wu, B.; Yang, D.; Yang, S.; Zhang, G. Dietary Salt Intake and Gastric Cancer Risk: A Systematic Review and Meta-Analysis. Front. Nutr. 2021, 8, 801228. [Google Scholar] [CrossRef]
- Fiorito, V.; Chiabrando, D.; Petrillo, S.; Bertino, F.; Tolosano, E. The Multifaceted Role of Heme in Cancer. Front. Oncol. 2020, 9, 1540. [Google Scholar] [CrossRef]
- Alisson-Silva, F.; Kawanishi, K.; Varki, A. Human risk of diseases associated with red meat intake: Analysis of current theories and proposed role for metabolic incorporation of a non-human sialic acid. Mol. Asp. Med. 2016, 51, 16–30. [Google Scholar] [CrossRef]
- zur Hausen, H. Red meat consumption and cancer: Reasons to suspect involvement of bovine infectious factors in colorectal cancer. Int. J. Cancer 2012, 130, 2475–2483. [Google Scholar] [CrossRef]
- Crisà, A.; Marchitelli, C.; Failla, S.; Contò, M. Determination of N-acetylneuraminic and N-glycolylneuraminic acids in unprocessed milk of four cattle breeds. J. Dairy Res. 2022, 89, 299–301. [Google Scholar] [CrossRef]
- Hinterseher, J.; Günther, J.; Zlatina, K.; Isernhagen, L.; Viergutz, T.; Wirthgen, E.; Hoeflich, A.; Vernunft, A.; Galuska, S.P. Milk Polysialic Acid Levels Rapidly Decrease in Line with the N-Acetylneuraminic Acid Concentrations during Early Lactation in Dairy Cows. Biology 2022, 12, 5. [Google Scholar] [CrossRef]
- Frei, R.; Roduit, C.; Ferstl, R.; Mahony, L.O.; Lauener, R.P. Exposure of children to rural lifestyle factors associated with protection against allergies induces an Anti-Neu5Gc antibody response. Front. Immunol. 2019, 10, 1628. [Google Scholar] [CrossRef]
- Leviatan Ben-Arye, S.; Schneider, C.; Yu, H.; Bashir, S.; Chen, X.; Von Gunten, S.; Padler-Karavani, V. Differential recognition of diet-derived Neu5Gc-Neoantigens on glycan microarrays by carbohydrate-specific pooled human IgG and IgA antibodies. Bioconjug. Chem. 2019, 30, 1565–1574. [Google Scholar] [CrossRef]
- He, E.; Quan, W.; Luo, J.; Liu, C.; Zheng, W.; Shen, Q. Absorption and Transport Mechanism of Red Meat-Derived N-glycolylneuraminic Acid and Its Damage to Intestinal Barrier Function through the NF-κB Signaling Pathway. Toxins 2023, 15, 132. [Google Scholar] [CrossRef]
- Salama, A.; Evanno, G.; Lim, N.; Rousse, J.; Le Berre, L.; Nicot, A.; Brouard, S.; Harris, K.M.M.; Ehlers, M.R.R.; Gitelman, S.E.E.; et al. Anti-Gal and anti-Neu5Gc responses in nonimmunosuppressed patients following treatment with rabbit anti-thymocyte polyclonal IgGs. Transplantation 2017, 101, 2501–2507. [Google Scholar] [CrossRef]

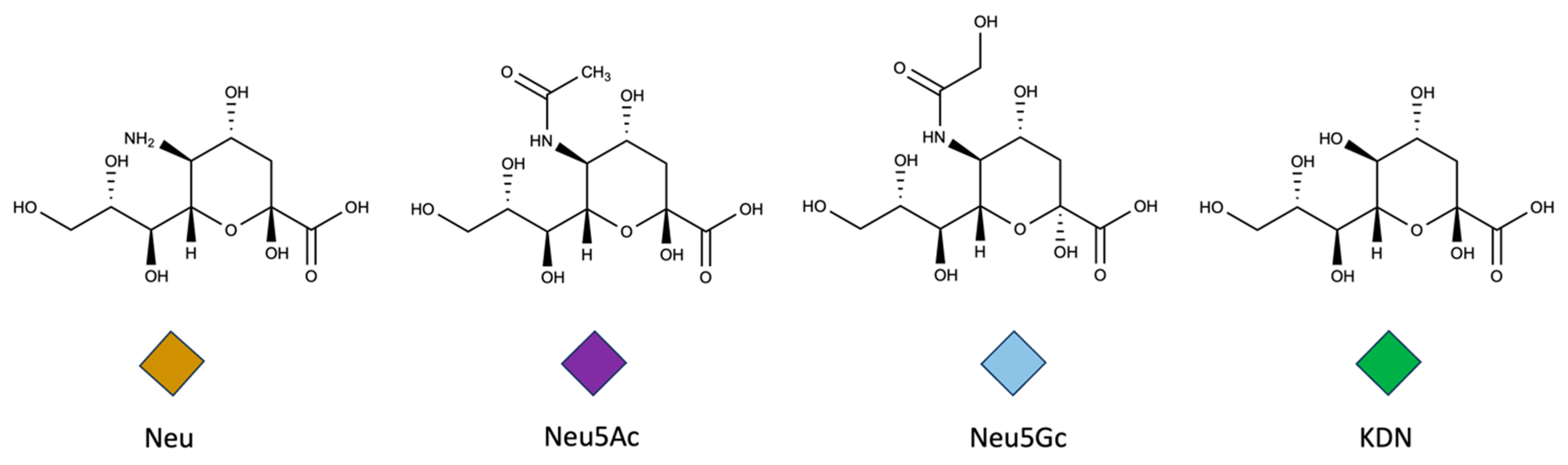
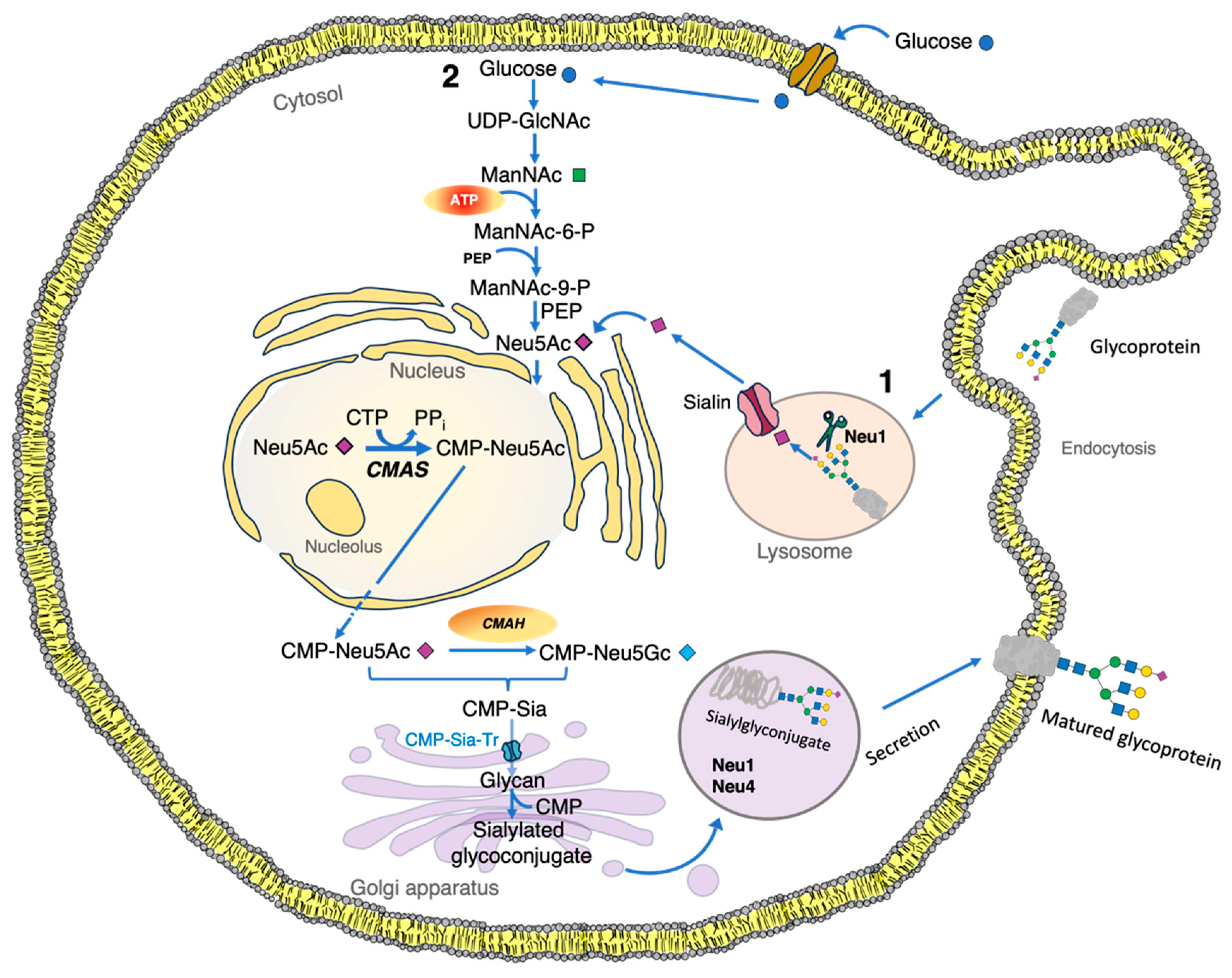
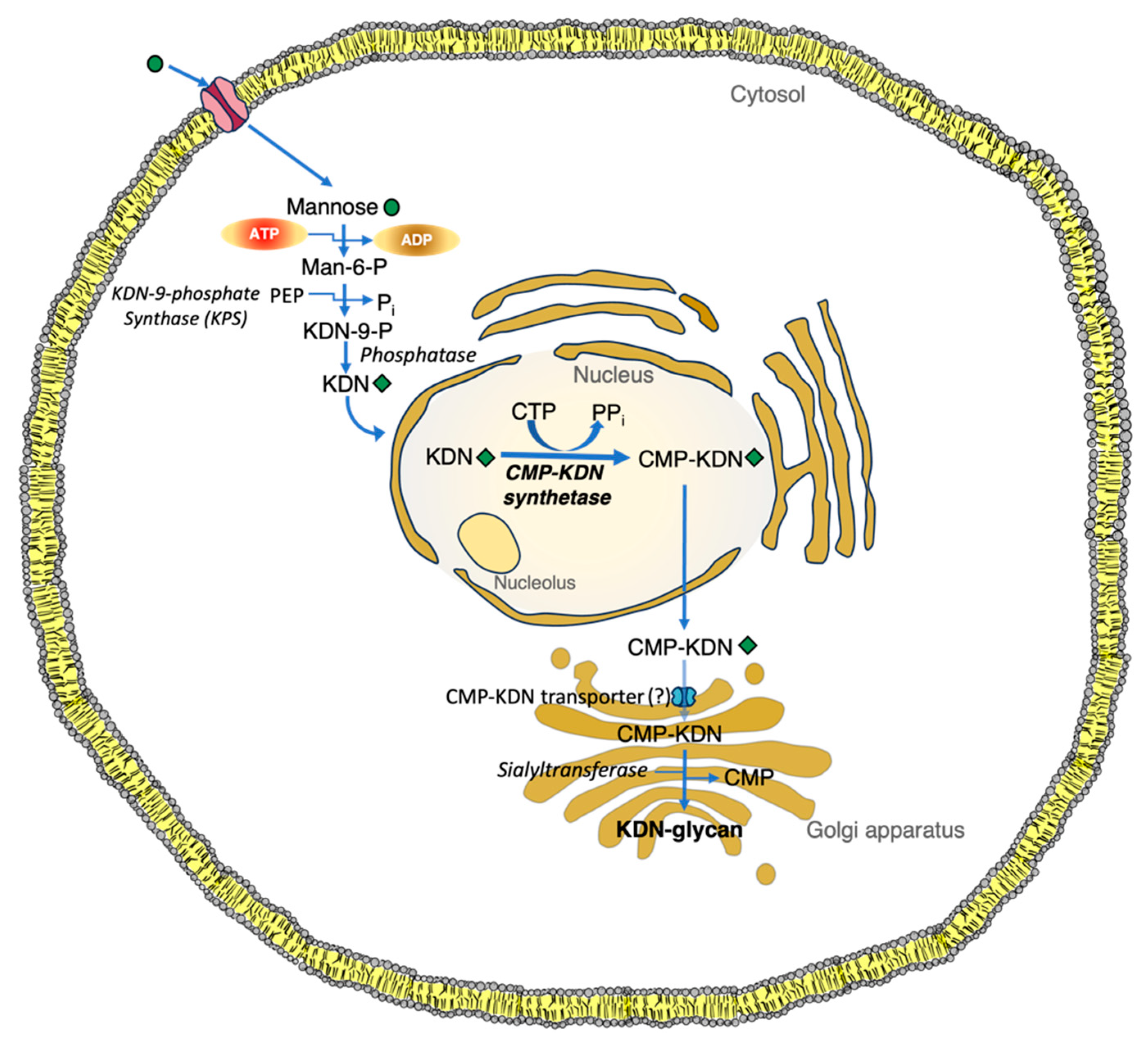
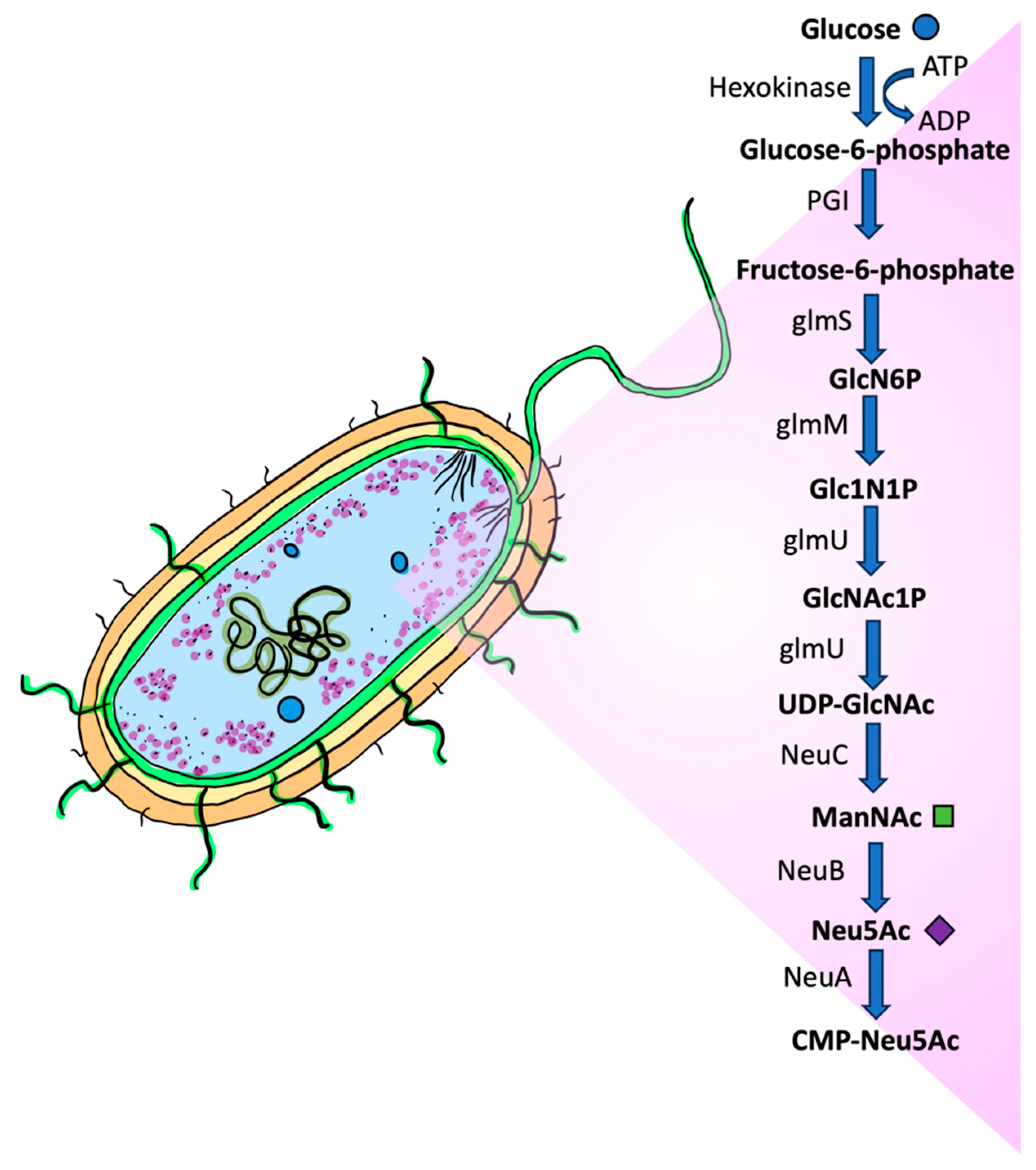

| Cancer | Neu5Ac | Neu5Gc | KDN | Reference | ||
|---|---|---|---|---|---|---|
| Endometrial cancer (µg/g) | 6.99 × 105 ± 3.05 × 102 | 1.3 × 104 ± 5 × 103 | - | [411] | ||
| Osteosarcoma cells (µg/mL) | 53.6 | 17.5 | 0.36 | [412] | ||
| Prostate cancer (µg/100 μg protein) | 3.09 × 10−5–6.04 × 10−2 | - | - | [413] | ||
| Pancreatic cancer (µg/L) | 9.27 × 10−3–3.7 × 10−3 | - | - | [151] | ||
| Throat cancer (µg/g) | 85 | 0.03 | 2 | [36] | ||
| Malignant mesothelioma (µg per 106 cells) | Culture media | Cell membrane extracts | Culture media | Cell membrane extracts | - | [414] |
| STAV-FCS | 5.05 | Traces | 35.77 | 20.37 | ||
| STAV-AB | 10.85 | ND | 13.66 | 19.23 | ||
| Wester | 11.53 | ND | 18.65 | 25.3 | ||
| Adenocarcinoma cells (µg per 106 cells) | ||||||
| Wart | 6 | Traces | 16.51 | 21.12 | ||
| Osteosarcoma cells (µg per 106 cells) | 3.97 | - | 2.75 | 4.48 | - | [415] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerrero-Flores, G.N.; Butler, F.M.; Martinez Marignac, V.L.; Zhang, G.; Pacheco, F.J.; Boskovic, D.S. Sialic Acids in Health and Disease. Biologics 2025, 5, 10. https://doi.org/10.3390/biologics5020010
Guerrero-Flores GN, Butler FM, Martinez Marignac VL, Zhang G, Pacheco FJ, Boskovic DS. Sialic Acids in Health and Disease. Biologics. 2025; 5(2):10. https://doi.org/10.3390/biologics5020010
Chicago/Turabian StyleGuerrero-Flores, Gerardo N., Fayth M. Butler, Veronica L. Martinez Marignac, Guangyu Zhang, Fabio J. Pacheco, and Danilo S. Boskovic. 2025. "Sialic Acids in Health and Disease" Biologics 5, no. 2: 10. https://doi.org/10.3390/biologics5020010
APA StyleGuerrero-Flores, G. N., Butler, F. M., Martinez Marignac, V. L., Zhang, G., Pacheco, F. J., & Boskovic, D. S. (2025). Sialic Acids in Health and Disease. Biologics, 5(2), 10. https://doi.org/10.3390/biologics5020010