Sherpabodies—A Highly Versatile and Modular Scaffold for Biomedical Targeting
Abstract
1. Non-Ig Scaffolds
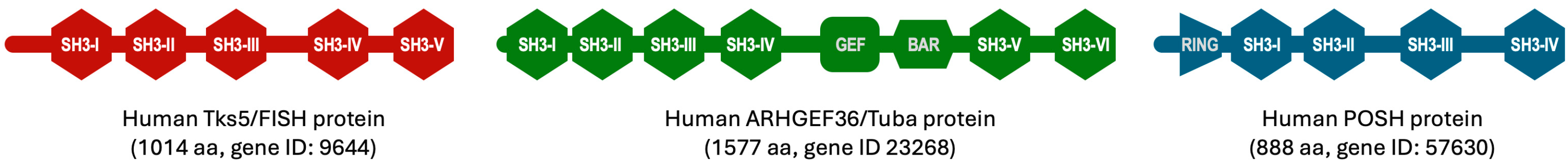
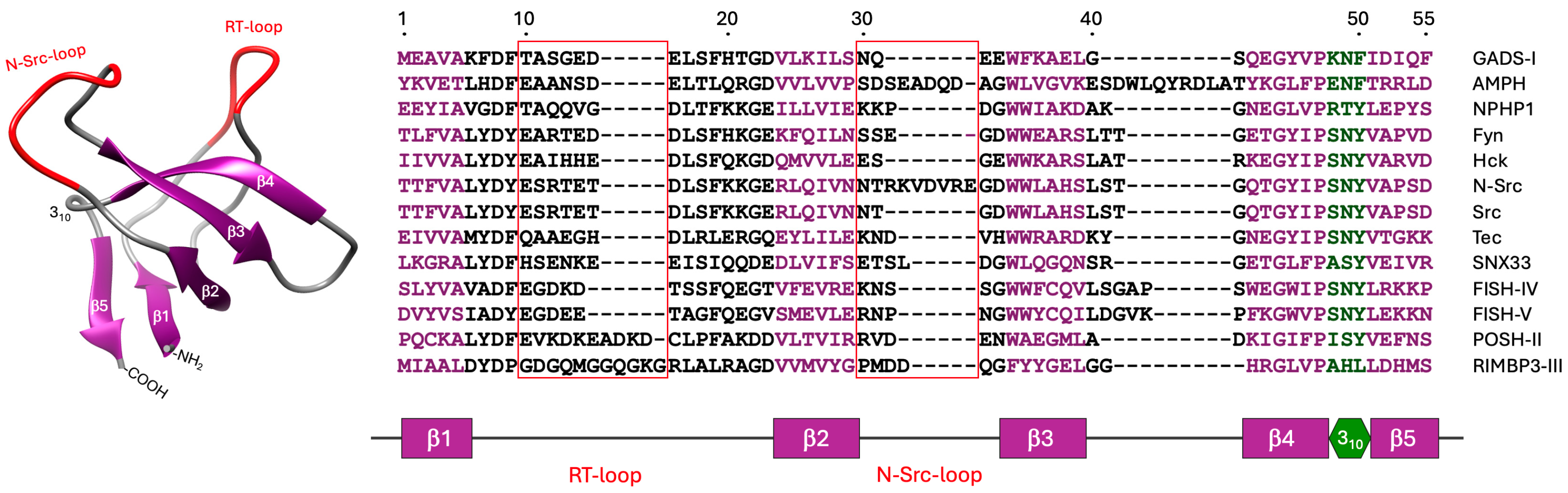
2. What Are SH3 Domains?
3. History of SH3 Scaffold Engineering
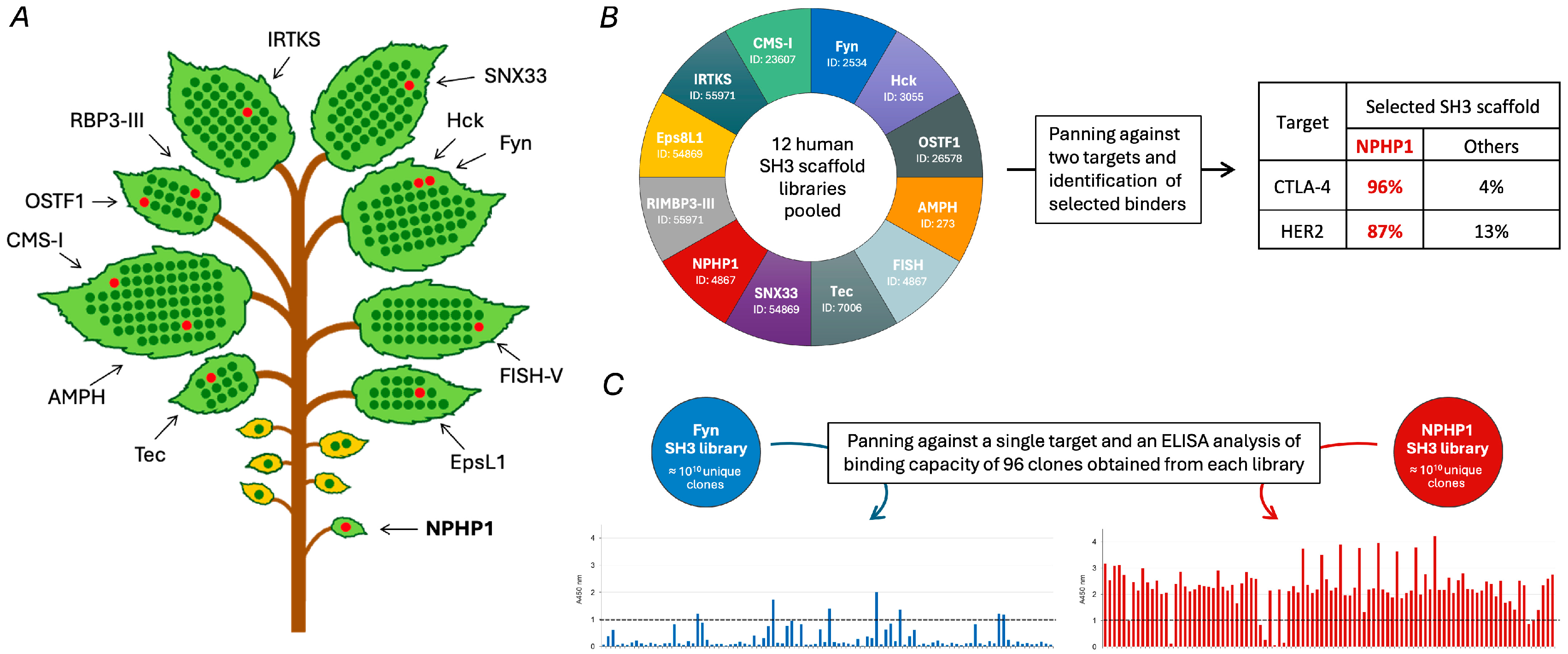
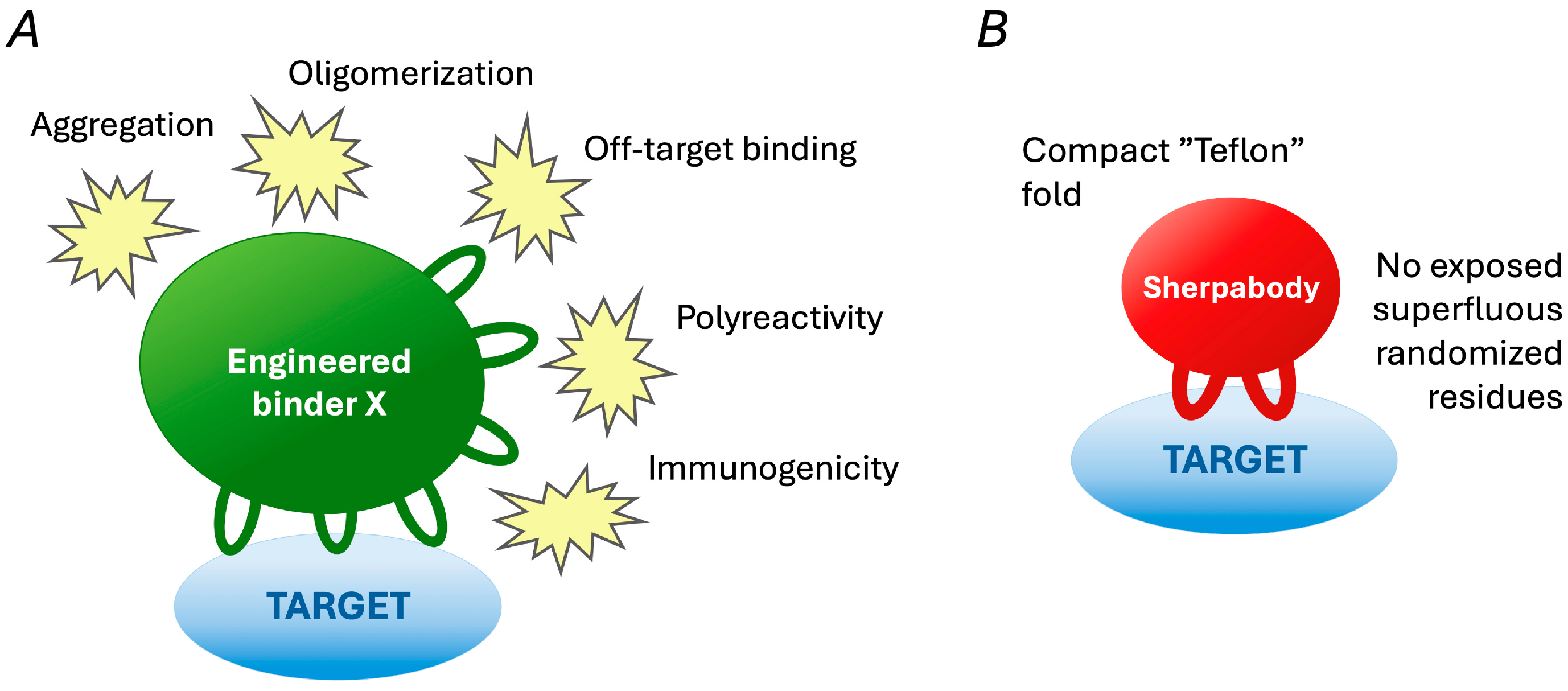
4. Discovery of Sherpabodies
5. Biomedical Applications of Sherpabodies
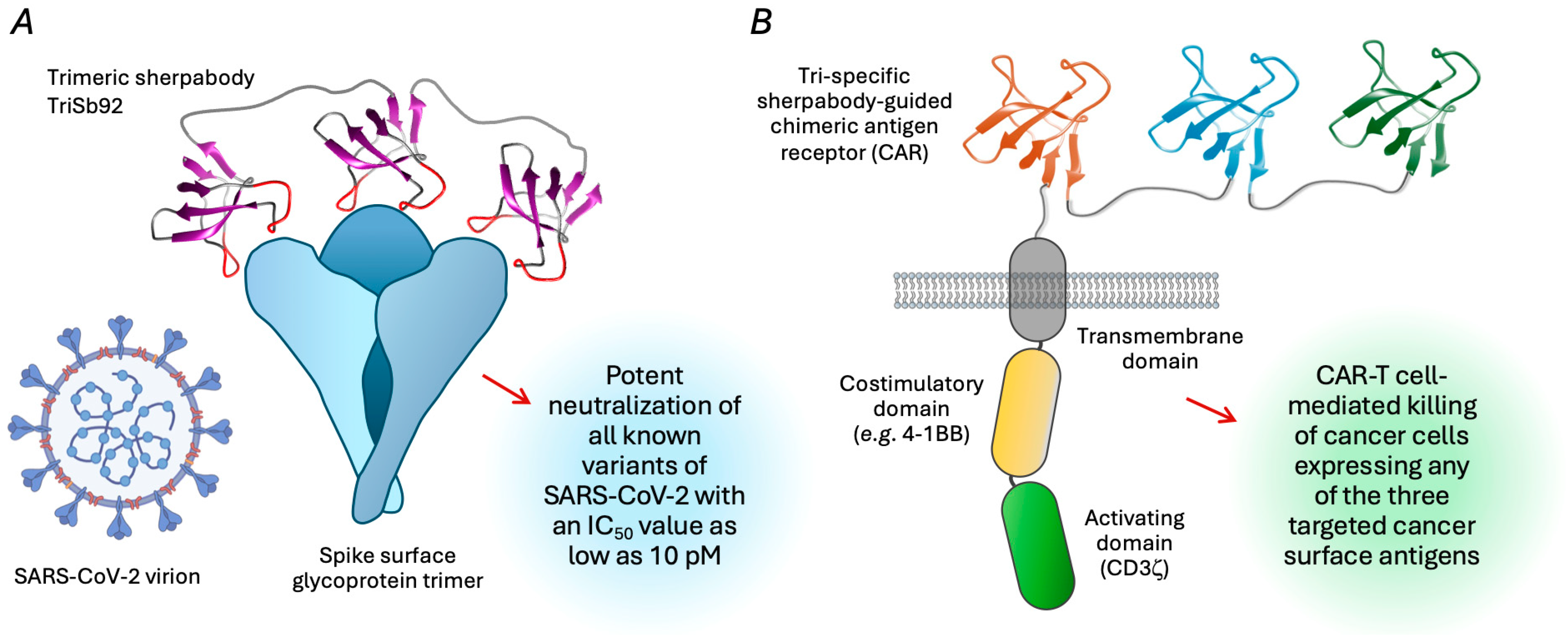
5.1. Multimeric Sherpabodies as Entry-Blocking Antiviral Proteins
5.2. Precision Targeting of Cancer for Killing by CAR-T Cells
6. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sidhu, S.S.; Koide, S. Phage display for engineering and analyzing protein interaction interfaces. Curr. Opin. Struct. Biol. 2007, 17, 481–487. [Google Scholar] [CrossRef]
- Binz, H.K.; Amstutz, P.; Plückthun, A. Engineering novel binding proteins from nonimmunoglobulin domains. Nat. Biotechnol. 2005, 23, 1257–1268. [Google Scholar] [CrossRef]
- Packer, M.S.; Liu, D.R. Methods for the directed evolution of proteins. Nat. Rev. Genet. 2015, 16, 379–394. [Google Scholar] [CrossRef]
- Levin, A.M.; Weiss, G.A. Optimizing the affinity and specificity of proteins with molecular display. Mol. Biosyst. 2006, 2, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Clackson, T.; Wells, J.A. In vitro selection from protein and peptide libraries. Trends Biotech. 1994, 12, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Markland, W.; Ley, A.C.; Lee, S.W.; Ladner, R.C. Iterative optimization of high-affinity proteases inhibitors using phage display. 1. Plasmin. Biochemistry 1996, 35, 8045–8057. [Google Scholar] [CrossRef] [PubMed]
- Roberts, B.L.; Markland, W.; Siranosian, K.; Saxena, M.J.; Guterman, S.K.; Ladner, R.C. Protease inhibitor display M13 phage: Selection of high-affinity neutrophil elastase inhibitors. Gene 1992, 121, 9–15. [Google Scholar] [CrossRef]
- Röttgen, P.; Collins, J. A human pancreatic secretory trypsin inhibitor presenting a hypervariable highly constrained epitope via monovalent phagemid display. Gene 1995, 164, 243–250. [Google Scholar] [CrossRef]
- Abedi, M.R.; Caponigro, G.; Kamb, A. Green fluorescent protein as a scaffold for intracellular presentation of peptides. Nucleic Acids Res. 1998, 26, 623–630. [Google Scholar] [CrossRef]
- Norman, T.C.; Smith, D.L.; Sorger, P.K.; Drees, B.L.; O’Rourke, S.M.; Hughes, T.R.; Roberts, C.J.; Friend, S.H.; Fields, S.; Murray, A.W. Genetic selection of peptide inhibitors of biological pathways. Science 1999, 285, 591–595. [Google Scholar] [CrossRef]
- Lu, Z.; Murray, K.S.; Van Cleave, V.; LaVallie, E.R.; Stahl, M.L.; McCoy, J.M. Expression of thioredoxin random peptide libraries on the Escherichia coli cell surface as functional fusions to flagellin: A system designed for exploring protein-protein interactions. Biotechnology 1995, 13, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Smith, G. Patch engineering: A general approach for creating proteins that have new binding activities. Trends Biochem. Sci. 1998, 23, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Colas, P.; Cohen, B.; Jessen, T.; Grishina, I.; McCoy, J.; Brent, R. Genetic selection of peptide aptamers that recognize and inhibit cyclin-dependent kinase 2. Nature 1996, 380, 548–550. [Google Scholar] [CrossRef]
- Dennis, M.S.; Herzka, A.; Lazarus, R.A. Potent and selective Kunitz domain inhibitors of plasma kallikrein designed by phage display. J. Biol. Chem. 1995, 270, 25411–25417. [Google Scholar] [CrossRef]
- Szakács, D.; Kocsis, A.; Szász, R.; Gál, P.; Pál, G. Novel MASP-2 inhibitors developed via directed evolution of human TFPI1 are potent lectin pathway inhibitors. J. Biol. Chem. 2019, 294, 8227–8237. [Google Scholar] [CrossRef]
- Ku, J.; Schultz, P.G. Alternate protein frameworks for molecular recognition. Proc. Natl. Acad. Sci. USA 1995, 92, 6552–6556. [Google Scholar] [CrossRef]
- McConnell, S.J.; Hoess, R.H. Tendamistat as a scaffold for conformationally constrained phage peptide libraries. J. Mol. Biol. 1995, 250, 460–470. [Google Scholar] [CrossRef]
- Li, R.; Hoess, R.H.; Bennett, J.S.; DeGrado, W.F. Use of phage display to probe the evolution of binding specificity and affinity in integrins. Protein Eng. 2003, 16, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Nord, K.; Gunneriusson, E.; Ringdahl, J.; Ståhl, S.; Uhlén, M.; Nygren, P.A. Binding proteins selected from combinatorial libraries of an alpha-helical bacterial receptor domain. Nat. Biotechnol. 1997, 15, 772–777. [Google Scholar] [CrossRef]
- Ståhl, S.; Gräslund, T.; Eriksson Karlström, A.; Frejd, F.Y.; Nygren, P.; Löfblom, J. Affibody molecules in biotechnological and medical applications. Trends Biotech. 2017, 35, 691–712. [Google Scholar] [CrossRef]
- Koide, A.; Bailey, C.W.; Huang, X.; Koide, S. The fibronectin type III domain as a scaffold for novel binding proteins. J. Mol. Biol. 1998, 284, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Koide, A.; Koide, S. Monobodies: Antibody mimics based on the scaffold of the fibronectin type III domain. Methods Mol. Biol. 2007, 352, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Hiipakka, M.; Poikonen, K.; Saksela, K. SH3 domains with high affinity and engineered ligand specificities targeted to HIV-1 Nef. J. Mol. Biol. 1999, 293, 1097–1106. [Google Scholar] [CrossRef]
- Hiipakka, M.; Saksela, K. Versatile retargeting of SH3 domain binding by modification of non-conserved loop residues. FEBS Lett. 2007, 581, 1735–1741. [Google Scholar] [CrossRef]
- Beste, G.; Schmidt, F.S.; Stibora, T.; Skerra, A. Small antibody-like proteins with prescribed ligand specificities derived from the lipocalin fold. Proc. Natl. Acad. Sci. USA 1999, 96, 1898–1903. [Google Scholar] [CrossRef]
- Skerra, A. Alternative binding proteins: Anticalins-harnessing the structural plasticity of the lipocalin ligand pocket to engineer novel binding activities. FEBS J. 2008, 275, 2677–2683. [Google Scholar] [CrossRef]
- Christmann, A.; Walter, K.; Wentzel, A.; Krätzner, R.; Kolmar, H. The cystine knot of a squash-type protease inhibitor as a structural scaffold for Escherichia coli cell surface display of conformationally constrained peptides. Protein Eng. 1999, 12, 797–806. [Google Scholar] [CrossRef]
- Moore, S.J.; Cochran, J.R. Engineering knottins as novel binding agents. Methods Enzymol. 2012, 503, 223–251. [Google Scholar] [CrossRef]
- Binz, H.K.; Stumpp, M.T.; Forrer, P.; Amstutz, P.; Plückthun, A. Designing repeat proteins: Well-expressed, soluble and stable proteins from combinatorial libraries of consensus ankyrin repeat proteins. J. Mol. Biol. 2003, 332, 489–503. [Google Scholar] [CrossRef]
- Stumpp, M.T.; Binz, H.K.; Amstutz, P. DARPins: A new generation of protein therapeutics. Drug Discov. Today 2008, 13, 695–701. [Google Scholar] [CrossRef]
- Silverman, J.; Liu, Q.; Bakker, A.; To, W.; Duguay, A.; Alba, B.M.; Smith, R.; Rivas, A.; Li, P.; Le, H.; et al. Multivalent avimer proteins evolved by exon shuffling of a family of human receptor domains. Nat. Biotechnol. 2005, 23, 1556–1561. [Google Scholar] [CrossRef]
- Hulme, J.T.; D’Souza, W.N.; McBride, H.J.; Yoon, B.P.; Willee, A.M.; Duguay, A.; Thomas, M.; Fan, B.; Dayao, M.R.; Rottman, J.B.; et al. Novel protein therapeutic joint retention strategy based on collagen-binding Avimers. J. Orthop. Res. 2018, 36, 1238–1247. [Google Scholar] [CrossRef]
- Woodman, R.; Yeh, J.T.; Laurenson, S.; Ko Ferrigno, P. Design and validation of a neutral protein scaffold for the presentation of peptide aptamers. J. Mol. Biol. 2005, 352, 1118–1133. [Google Scholar] [CrossRef] [PubMed]
- Stadler, L.K.; Hoffmann, T.; Tomlinson, D.C.; Song, Q.; Lee, T.; Busby, M.; Nyathi, Y.; Gendra, E.; Tiede, C.; Flanagan, K.; et al. Structure-function studies of an engineered scaffold protein derived from Stefin A. II: Development and applications of the SQT variant. Protein Eng. Des. Sel. 2011, 24, 751–763. [Google Scholar] [CrossRef] [PubMed]
- Tiede, C.; Bedford, R.; Heseltine, S.J.; Smith, G.; Wijetunga, I.; Ross, R.; AlQallaf, D.; Roberts, A.P.; Balls, A.; Curd, A.; et al. Affimer proteins are versatile and renewable affinity reagents. Elife 2017, 6, e24903. [Google Scholar] [CrossRef]
- Ebersbach, H.; Fiedler, E.; Scheuermann, T.; Fiedler, M.; Stubbs, M.T.; Reimann, C.; Proetzel, G.; Rudolph, R.; Fiedler, U. Affilin-novel binding molecules based on human gamma-B-crystallin, an all beta-sheet protein. J. Mol. Biol. 2007, 372, 172–185. [Google Scholar] [CrossRef]
- Mirecka, E.A.; Hey, T.; Fiedler, U.; Rudolph, R.; Hatzfeld, M. Affilin molecules selected against the human papillomavirus E7 protein inhibit the proliferation of target cells. J. Mol. Biol. 2009, 390, 710–721. [Google Scholar] [CrossRef]
- Mouratou, B.; Schaeffer, F.; Guilvout, I.; Tello-Manigne, D.; Pugsley, A.P.; Alzari, P.M.; Pecorari, F. Remodeling a DNA-binding protein as a specific in vivo inhibitor of bacterial secretin PulD. Proc. Natl. Acad. Sci. USA 2007, 104, 17983–17988. [Google Scholar] [CrossRef]
- Mouratou, B.; Béhar, G.; Paillard-Laurance, L.; Colinet, S.; Pecorari, F. Ribosome display for the selection of Sac7d scaffolds. Methods Mol. Biol. 2012, 805, 315–331. [Google Scholar] [CrossRef]
- Grabulovski, D.; Kaspar, M.; Neri, D. A novel, non-immunogenic Fyn SH3-derived binding protein with tumor vascular targeting properties. J. Biol. Chem. 2007, 282, 3196–3204. [Google Scholar] [CrossRef]
- Silacci, M.; Baenziger-Tobler, N.; Lembke, W.; Zha, W.; Batey, S.; Bertschinger, J.; Grabulovski, D. Linker length matters, fynomer-Fc fusion with an optimized linker displaying picomolar IL-17A inhibition potency. J. Biol. Chem. 2014, 289, 14392–14398. [Google Scholar] [CrossRef]
- Byla, P.; Andersen, M.H.; Holtet, T.L.; Jacobsen, H.; Munch, M.; Gad, H.H.; Thøgersen, H.C.; Hartmann, R. Selection of a novel and highly specific tumor necrosis factor alpha (TNFalpha) antagonist: Insight from the crystal structure of the antagonist-TNFalpha complex. J. Biol. Chem. 2010, 285, 12096–12100. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.E.; Ferrini, R.; Dicker, D.T.; Batzer, G.; Chen, E.; Oltean, D.I.; Lin, B.; Renshaw, M.W.; Kretz-Rommel, A.; El-Deiry, W.S. Targeting TRAIL death receptor 4 with trivalent DR4 Atrimer complexes. Mol. Cancer Ther. 2012, 11, 2087–2095. [Google Scholar] [CrossRef] [PubMed]
- Nilvebrant, J.; Alm, T.; Hober, S.; Löfblom, J. Engineering bispecificity into a single albumin-binding domain. PLoS ONE 2011, 6, e25791. [Google Scholar] [CrossRef]
- Garousi, J.; Lindbo, S.; Nilvebrant, J.; Åstrand, M.; Buijs, J.; Sandström, M.; Honarvar, H.; Orlova, A.; Tolmachev, V.; Hober, S. ADAPT, a novel scaffold protein-based probe for radionuclide imaging of molecular targets that are expressed in disseminated cancers. Cancer Res. 2015, 75, 4364–4371. [Google Scholar] [CrossRef]
- Varadamsetty, G.; Tremmel, D.; Hansen, S.; Parmeggiani, F.; Plückthun, A. Designed Armadillo repeat proteins: Library generation, characterization and selection of peptide binders with high specificity. J. Mol. Biol. 2012, 424, 68–87. [Google Scholar] [CrossRef]
- Reichen, C.; Hansen, S.; Plückthun, A. Modular peptide binding: From a comparison of natural binders to designed armadillo repeat proteins. J. Struct. Biol. 2014, 185, 147–162. [Google Scholar] [CrossRef]
- Lee, S.C.; Park, K.; Han, J.; Lee, J.J.; Kim, H.J.; Hong, S.; Heu, W.; Kim, Y.J.; Ha, J.S.; Lee, S.G.; et al. Design of a binding scaffold based on variable lymphocyte receptors of jawless vertebrates by module engineering. Proc. Natl. Acad. Sci. USA 2012, 109, 3299–3304. [Google Scholar] [CrossRef]
- Jung, Y.H.; Choi, Y.; Seo, H.D.; Seo, M.H.; Kim, H.S. A conformation-selective protein binder for a KRAS mutant inhibits the interaction between RAS and RAF. Biochem. Biophys. Res. Commun. 2023, 645, 110–117. [Google Scholar] [CrossRef]
- Jacobs, S.A.; Diem, M.D.; Luo, J.; Teplyakov, A.; Obmolova, G.; Malia, T.; Gilliland, G.L.; O’Neil, K.T. Design of novel FN3 domains with high stability by a consensus sequence approach. Protein Eng. Des. Sel. 2012, 25, 107–117. [Google Scholar] [CrossRef]
- Diem, M.D.; Hyun, L.; Yi, F.; Hippensteel, R.; Kuhar, E.; Lowenstein, C.; Swift, E.J.; O’Neil, K.T.; Jacobs, S.A. Selection of high-affinity Centyrin FN3 domains from a simple library diversified at a combination of strand and loop positions. Protein Eng. Des. Sel. 2014, 27, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Lorey, S.; Fiedler, E.; Kunert, A.; Nerkamp, J.; Lange, C.; Fiedler, M.; Bosse-Doenecke, E.; Meysing, M.; Gloser, M.; Rundfeldt, C.; et al. Novel ubiquitin-derived high affinity binding proteins with tumor targeting properties. J. Biol. Chem. 2014, 289, 8493–8507. [Google Scholar] [CrossRef] [PubMed]
- Job, F.; Settele, F.; Lorey, S.; Rundfeldt, C.; Baumann, L.; Beck-Sickinger, A.G.; Haupts, U.; Lilie, H.; Bosse-Doenecke, E. Ubiquitin is a versatile scaffold protein for the generation of molecules with de novo binding and advantageous drug-like properties. FEBS Open Bio 2015, 5, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Tiede, C.; Tang, A.A.; Deacon, S.E.; Mandal, U.; Nettleship, J.E.; Owen, R.L.; George, S.E.; Harrison, D.J.; Owens, R.J.; Tomlinson, D.C.; et al. Adhiron: A stable and versatile peptide display scaffold for molecular recognition applications. Protein Eng. Des. Sel. 2014, 27, 145–155. [Google Scholar] [CrossRef]
- Desmet, J.; Verstraete, K.; Bloch, Y.; Lorent, E.; Wen, Y.; Devreese, B.; Vandenbroucke, K.; Loverix, S.; Hettmann, T.; Deroo, S.; et al. Structural basis of IL-23 antagonism by an Alphabody protein scaffold. Nat. Commun. 2014, 5, 5237. [Google Scholar] [CrossRef]
- Pannecoucke, E.; Van Trimpont, M.; Desmet, J.; Pieters, T.; Reunes, L.; Demoen, L.; Vuylsteke, M.; Loverix, S.; Vandenbroucke, K.; Alard, P.; et al. Cell-penetrating Alphabody protein scaffolds for intracellular drug targeting. Sci. Adv. 2021, 7, eabe1682. [Google Scholar] [CrossRef]
- Steemson, J.D.; Baake, M.; Rakonjac, J.; Arcus, V.L.; Liddament, M.T. Tracking molecular recognition at the atomic level with a new protein scaffold based on the OB-fold. PLoS ONE 2014, 9, e86050. [Google Scholar] [CrossRef]
- Suderman, R.J.; Rice, D.A.; Gibson, S.D.; Strick, E.J.; Chao, D.M. Development of polyol-responsive antibody mimetics for single-step protein purification. Protein Expr. Purif. 2017, 134, 114–124. [Google Scholar] [CrossRef]
- Suderman, R.J.; Gibson, S.D.; Strecker, M.; Bonner, A.M.; Chao, D.M. Protein engineering of a nanoCLAMP antibody mimetic scaffold as a platform for producing bioprocess-compatible affinity capture ligands. J. Biol. Chem. 2023, 299, 104910. [Google Scholar] [CrossRef]
- Wicke, N.; Bedford, M.R.; Howarth, M. Gastrobodies are engineered antibody mimetics resilient to pepsin and hydrochloric acid. Commun. Biol. 2021, 4, 960. [Google Scholar] [CrossRef]
- Hokanson, C.A.; Zacco, E.; Cappuccilli, G.; Odineca, T.; Crea, R. AXL-receptor targeted 14FN3 based single domain proteins (PronectinsTM) from 3 synthetic human libraries as components for exploring novel bispecific constructs against solid tumors. Biomedicines 2022, 10, 3184. [Google Scholar] [CrossRef]
- Riillo, C.; Polerà, N.; Di Martino, M.T.; Juli, G.; Hokanson, C.A.; Odineca, T.; Signorelli, S.; Grillone, K.; Ascrizzi, S.; Mancuso, A.; et al. A PronectinTM AXL-targeted first-in-class bispecific T cell engager (pAXLxCD3ε) for ovarian cancer. J. Transl. Med. 2023, 21, 301. [Google Scholar] [CrossRef] [PubMed]
- Mäkelä, A.R.; Ugurlu, H.; Hannula, L.; Kant, R.; Salminen, P.; Fagerlund, R.; Mäki, S.; Haveri, A.; Strandin, T.; Kareinen, L.; et al. Intranasal trimeric sherpabody inhibits SARS-CoV-2 including recent immunoevasive Omicron subvariants. Nat. Commun. 2023, 14, 1637. [Google Scholar] [CrossRef]
- Hernández-López, R.A.; Kesti, T.; Mäkelä, A.R.; Zhao, Z.; Yu, W.; Tonai, Y.; Monzo, H.J.; Kalander, K.; Leppä, S.; Ojala, P.M.; et al. Engineered SH3-derived sherpabodies function as a modular platform for targeted T cell immunotherapy. Cancer Res. 2025, in press. [CrossRef] [PubMed]
- Kaneko, T.; Li, L.; Li, S.S. The SH3 domain—A family of versatile peptide- and protein-recognition module. Front. Biosci. 2008, 13, 4938–4952. [Google Scholar] [CrossRef] [PubMed]
- Mayer, B.J. SH3 domains: Complexity in moderation. J. Cell Sci. 2001, 114, 1253–1263. [Google Scholar] [CrossRef]
- Plaxco, K.W.; Guijarro, J.I.; Morton, C.J.; Pitkeathly, M.; Campbell, I.D.; Dobson, C.M. The folding kinetics and thermodynamics of the Fyn-SH3 domain. Biochemistry 1998, 37, 2529–2537. [Google Scholar] [CrossRef]
- Lim, W.A.; Fox, R.O.; Richards, F.M. Stability and peptide binding affinity of an SH3 domain from the Caenorhabditis elegans signaling protein Sem-5. Protein Sci. 1994, 3, 1261–1266. [Google Scholar] [CrossRef]
- Saksela, K.; Permi, P. SH3 domain ligand binding: What’s the consensus and where’s the specificity? FEBS Lett. 2012, 586, 2609–2614. [Google Scholar] [CrossRef]
- Lee, C.H.; Leung, B.; Lemmon, M.A.; Zheng, J.; Cowburn, D.; Kuriyan, J.; Saksela, K. A single amino acid in the SH3 domain of Hck determines its high affinity and specificity in binding to HIV-1 Nef protein. EMBO J. 1995, 14, 5006–5015. [Google Scholar] [CrossRef]
- Lee, C.H.; Saksela, K.; Mirza, U.A.; Chait, B.T.; Kuriyan, J. Crystal structure of the conserved core of HIV-1 Nef complexed with a Src family SH3 domain. Cell 1996, 85, 931–942. [Google Scholar] [CrossRef]
- Schlatter, D.; Brack, S.; Banner, D.W.; Batey, S.; Benz, J.; Bertschinger, J.; Huber, W.; Joseph, C.; Rufer, A.; van der Klooster, A.; et al. Generation, characterization and structural data of chymase binding proteins based on the human Fyn kinase SH3 domain. mAbs 2012, 4, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Brack, S.; Attinger-Toller, I.; Schade, B.; Mourlane, F.; Klupsch, K.; Woods, R.; Hachemi, H.; von der Bey, U.; Koenig-Friedrich, S.; Bertschinger, J.; et al. A bispecific HER2-targeting FynomAb with superior antitumor activity and novel mode of action. Mol. Cancer Ther. 2014, 13, 2030–2039. [Google Scholar] [CrossRef]
- Silacci, M.; Lembke, W.; Woods, R.; Attinger-Toller, I.; Baenziger-Tobler, N.; Batey, S.; Santimaria, R.; von der Bey, U.; Koenig-Friedrich, S.; Zha, W.; et al. Discovery and characterization of COVA322, a clinical-stage bispecific TNF/IL-17A inhibitor for the treatment of inflammatory diseases. mAbs 2016, 8, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000144061-NPHP1 (accessed on 3 March 2025).
- Otto, E.; Kispert, A.; Schätzle, S.; Lescher, B.; Rensing, C.; Hildebrandt, F. Nephrocystin: Gene expression and sequence conservation between human, mouse, and Caenorhabditis elegans. J. Am. Soc. Nephrol. 2000, 11, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Saksela, K. SH3 Domain Derivatives. U.S. Patent US110,060,084 B2, 13 July 2021. [Google Scholar]
- Kazlauskas, A.; Schmotz, C.; Kesti, T.; Hepojoki, J.; Kleino, I.; Kaneko, T.; Li, S.S.; Saksela, K. Large-scale screening of preferred interactions of human Src homology-3 (SH3) domains using native target proteins as affinity ligands. Mol. Cell Proteom. 2016, 15, 3270–3281. [Google Scholar] [CrossRef]
- le Maire, A.; Weber, T.; Saunier, S.; Broutin, I.; Antignac, C.; Ducruix, A.; Dardel, F. Solution NMR structure of the SH3 domain of human nephrocystin and analysis of a mutation-causing juvenile nephronophthisis. Proteins 2005, 59, 347–355. [Google Scholar] [CrossRef][Green Version]
- Wodarczyk, C.; Distefano, G.; Rowe, I.; Gaetani, M.; Bricoli, B.; Muorah, M.; Spitaleri, A.; Mannella, V.; Ricchiuto, P.; Pema, M.; et al. Nephrocystin-1 forms a complex with polycystin-1 via a polyproline motif/SH3 domain interaction and regulates the apoptotic response in mammals. PLoS ONE 2010, 5, e12719. [Google Scholar] [CrossRef]
- White, J.M.; Ward, A.E.; Odongo, L.; Tamm, L.K. Viral membrane fusion: A dance between proteins and lipids. Annu. Rev. Virol. 2023, 10, 139–161. [Google Scholar] [CrossRef]
- Beukenhorst, A.L.; Rice, K.L.; Frallicciardi, J.; Koldijk, M.H.; Boudreau, C.M.; Crawford, J.; Cornelissen, L.; da Costa, K.A.S.; de Jong, B.A.; Fischinger, S.; et al. Intranasal administration of a panreactive influenza antibody reveals Fc-independent mode of protection. Sci. Rep. 2025, 15, 10309. [Google Scholar] [CrossRef]
- Sparrow, E.; Torvaldsen, S.; Newall, A.T.; Wood, J.G.; Sheikh, M.; Kieny, M.P.; Abela-Ridder, B. Recent advances in the development of monoclonal antibodies for rabies post exposure prophylaxis: A review of the current status of the clinical development pipeline. Vaccine 2019, 37 (Suppl. 1), 132–139. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, L.; Li, J.; Zhu, F. Advances in the progress of monoclonal antibodies for rabies. Hum. Vaccin. Immunother. 2022, 18, 2026713. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.A.; June, C.H. The principles of engineering immune cells to treat cancer. Cell 2017, 168, 724–740. [Google Scholar] [CrossRef]
- Guzman, G.; Reed, M.R.; Bielamowicz, K.; Koss, B.; Rodriguez, A. CAR-T therapies in solid tumors: Opportunities and challenges. Curr. Oncol. Rep. 2023, 25, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Kailayangiri, S.; Altvater, B.; Wiebel, M.; Jamitzky, S.; Rossig, C. Overcoming heterogeneity of antigen expression for effective CAR T cell targeting of cancers. Cancers 2020, 12, 1075. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Li, Q.; Zhu, X. Mechanisms of CAR T cell exhaustion and current counteraction strategies. Front. Cell Dev. Biol. 2022, 10, 1034257. [Google Scholar] [CrossRef]
- Zajc, C.U.; Salzer, B.; Taft, J.M.; Reddy, S.T.; Lehner, M.; Traxlmayr, M.W. Driving CARs with alternative navigation tools—The potential of engineered binding scaffolds. FEBS J. 2021, 288, 2103–2118. [Google Scholar] [CrossRef]
- Mouquet, H.; Scheid, J.F.; Zoller, M.J.; Krogsgaard, M.; Ott, R.G.; Shukair, S.; Artyomov, M.N.; Pietzsch, J.; Connors, M.; Pereyra, F.; et al. Polyreactivity increases the apparent affinity of anti-HIV antibodies by heteroligation. Nature 2010, 467, 591–595. [Google Scholar] [CrossRef]
- Jain, T.; Sun, T.; Durand, S.; Hall, A.; Houston, N.R.; Nett, J.H.; Sharkey, B.; Bobrowicz, B.; Caffry, I.; Yu, Y.; et al. Biophysical properties of the clinical-stage antibody landscape. Proc. Natl. Acad. Sci. USA 2017, 114, 944–949. [Google Scholar] [CrossRef]
- Vander Mause, E.R.; Atanackovic, D.; Lim, C.S.; Luetkens, T. Roadmap to affinity-tuned antibodies for enhanced chimeric antigen receptor T cell function and selectivity. Trends Biotech. 2022, 40, 875–890. [Google Scholar] [CrossRef]
- Mao, R.; Kong, W.; He, Y. The affinity of antigen-binding domain on the antitumor efficacy of CAR T cells: Moderate is better. Front. Immunol. 2022, 13, 1032403. [Google Scholar] [CrossRef] [PubMed]
- Buller, F.; Wüllner, U.; Zbinden, I.; Attinger-Toller, I.; von der Bey, U.; König-Friedrich, S.; Bertschinger, J.; Grabulovski, D.; Henne, P. Human Serum Albumin Binding Compounds and Fusion Proteins Thereof. U.S. Patent US10,364,419 B2, 30 July 2019. [Google Scholar]
- Zaman, R.; Islam, R.A.; Ibnat, N.; Othman, I.; Zaini, A.; Lee, C.Y.; Chowdhury, E.H. Current strategies in extending half-lives of therapeutic proteins. J. Control Release 2019, 301, 176–189. [Google Scholar] [CrossRef] [PubMed]
| Scaffold | Protein of Origin | Randomized Surface | Species of Origin | Molar Mass | First Publication | References |
|---|---|---|---|---|---|---|
| Kunitz domains | BPTI or other protease inhibitors | Exposed loops | Cow and others | 6 kDa | 1992 | [7,14,15] |
| Cytochrome b562 | Bacterial c-type cytochrome | Exposed loops | Escherichia coli (bacterium) | 12 kDa | 1995 | [16] |
| Tendamistat | Alpha-amylase inhibitor HOE 467 | Exposed loops | Streptomyces tendae (bacterium) | 8 kDa | 1995 | [17,18] |
| Affibody | Protein A (Z domain) | α-helices | Staphylococcus aureus (bacterium) | 6 kDa | 1997 | [19,20] |
| Monobodies/Adnectins | Fibronectin (10th type III domain) | Exposed loops | Human | 10 kDa | 1998 | [21,22] |
| Hck-SH3 | Hck kinase (SH3 domain) | Exposed loops | Human | 6 kDa | 1999 | [23,24] |
| Anticalin | Lipocalins | Exposed loops | Pieris brassicae (butterfly); later also human lipocalins | 20 kDa | 1999 | [25,26] |
| Knottins | Knottin folds of various proteins | Exposed loops | Squirting cucumber (plant) and other species | 3 kDa | 1999 | [27,28] |
| DARPin | Human ankyrin repeat proteins | β-turn & α-helix | Non-natural protein | 10–19 kDa | 2003 | [29,30] |
| Avimer | Human LDL receptor-like proteins | Exposed loops | Designed consensus of 197 A-domains | 9–18 kDa | 2005 | [31,32] |
| Affimer | Cystatin A (Stefin A) | Exposed loops | Human | 12–14 kDa | 2005 | [33,34,35] |
| Affilin (γB) | Gamma-B crystallin | β-strands | Human | 20 kDa | 2007 | [36,37] |
| Affitin/Nanofitin | Sac7d DNA binding protein | β-strands | Sulfolobus acidocaldarius (archaeon) | 7 kDa | 2007 | [38,39] |
| Fynomer | Fyn kinase (SH3 domain) | Exposed loops | Human | 6 kDa | 2007 | [40,41] |
| Atrimer | Tetranectin (C-type lectin domain) | Exposed loops | Human | 60–70 kDa | 2010 | [42,43] |
| ADAPT | Protein G (3rd albumin binding domain) | α-helices | Streptococcus strain G148 (bacterium) | 5 kDa | 2011 | [44,45] |
| ArmRP | Armadillo repeat proteins | α-helices | Non-natural protein | 30kDa | 2012 | [46,47] |
| Repebody | VLR proteins of jawless vertebrates | β-strands and loops | Designed based on consensus leucine-rich repeats | 30 kDa | 2012 | [48,49] |
| Centyrin | Tenascin and fibronectin | Exposed loops | Designed consensus of different III domains | 10 kDa | 2012 | [50,51] |
| Affilin | Two head-to-tail linked ubiquitins | β-strands | Human | 17 kDa | 2014 | [52,53] |
| Adhiron | Cystatins | Exposed loops | Designed consensus of plant cystatins | 12–14 kDa | 2014 | [35,54] |
| Alphabody | Engineered triple helix coiled coil | α-helices | Non-natural protein | 10 kDa | 2014 | [55,56] |
| Obody | Aspartyl tRNA synthetase (OB-fold) | β-strands and loops | Pyrobaculum aerophilum (archaeon) | 11 kDa | 2014 | [57] |
| nanoCLAMP | NagH (carbohydrate binding module 32-2) | Exposed loops | Clostridium perfringens (bacterium) | 16 kDa | 2017 | [58,59] |
| Gastrobody | Soybean trypsin inhibitor | Exposed loops | Soybean (plant) | 21 kDa | 2021 | [60] |
| Pronectin | Fibronectin (14th type III domain) | Exposed loops | Human | 10 kDa | 2022 | [61,62] |
| Sherpabody | Nephrocystin (SH3 domain) | Exposed loops | Human | 6 kDa | 2023 | [63,64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mäkelä, A.R.; Saksela, K. Sherpabodies—A Highly Versatile and Modular Scaffold for Biomedical Targeting. Biologics 2025, 5, 13. https://doi.org/10.3390/biologics5020013
Mäkelä AR, Saksela K. Sherpabodies—A Highly Versatile and Modular Scaffold for Biomedical Targeting. Biologics. 2025; 5(2):13. https://doi.org/10.3390/biologics5020013
Chicago/Turabian StyleMäkelä, Anna R., and Kalle Saksela. 2025. "Sherpabodies—A Highly Versatile and Modular Scaffold for Biomedical Targeting" Biologics 5, no. 2: 13. https://doi.org/10.3390/biologics5020013
APA StyleMäkelä, A. R., & Saksela, K. (2025). Sherpabodies—A Highly Versatile and Modular Scaffold for Biomedical Targeting. Biologics, 5(2), 13. https://doi.org/10.3390/biologics5020013





