Deep Eutectic Solvents for the Valorisation of Lignocellulosic Biomasses towards Fine Chemicals
Abstract
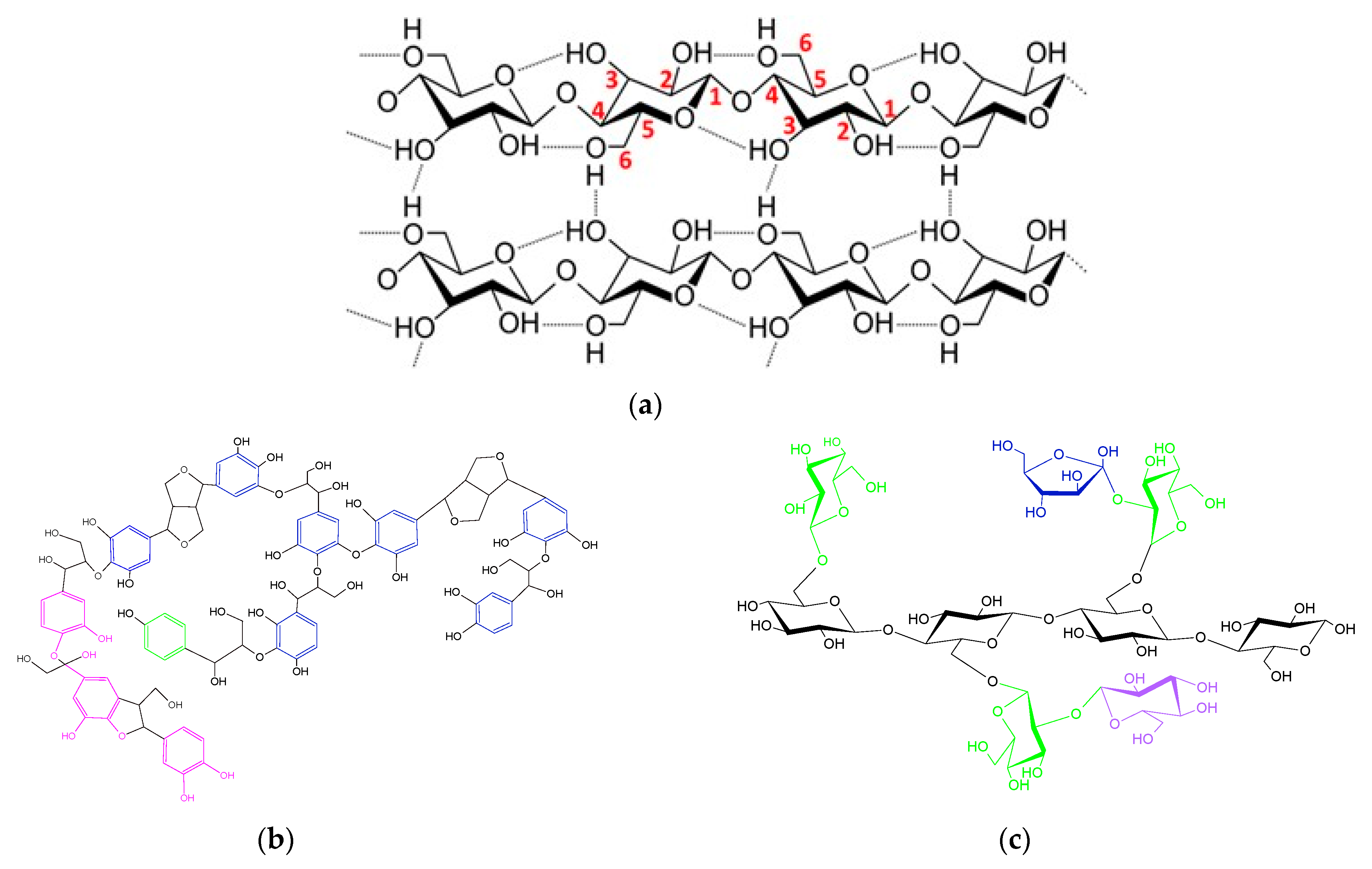
:1. Lignocellulosic Biomass: Main Components and Structures
2. Deep Eutectic Solvents (DES)
2.1. Definition of DES
2.2. DES Preparation and Physicochemical Properties
- -
- Heating up, under constant agitation, the DES components to obtain a clear liquid solution;
- -
- Removal of the solvent (typically water) through evaporation up to the remainder of the DES components initially dissolved therein;
- -
- Removal of the solvent through freeze-drying from HBA and HBD solutions, up to the remainder of the individual components of the DES;
- -
- Continuous feeding of the DES components into a heated extruder until a solution is obtained.
3. Use of DES for the Treatment of Lignocellulosic Biomass
3.1. New DES-Based Combined Pretreatment Strategies
3.1.1. Ternary DES
3.1.2. Alcohols and Organic Solvents
3.1.3. Lewis Acids
3.1.4. DES Pretreatment Coupled with Ultrasonic Irradiation and MW
4. Lignin Valorisation and Novel Hardwood Lignin-Based DES
5. Use of DES in the Valorisation of Cellulose
5.1. DES as a Non-Derivatising Solvent for Cellulose
5.2. Derivatization of Cellulose for the Production of Added Value Polymers
5.3. Direct and Indirect Conversion of Cellulose into Platform Molecules
6. Conclusions: Perspectives and Criticisms on the Use of DES
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nagarajan, D.; Chang, J.S.; Lee, D.J. Pretreatment of microalgal biomass for efficient biohydrogen production—Recent insights and future perspectives. Bioresour. Technol. 2020, 302, 122871. [Google Scholar] [CrossRef]
- Kumar, B.; Bhardwaj, N.; Agrawal, K.; Chaturvedi, V.; Verma, P. Current perspective on pretreatment technologies using lignocellulosic biomass: An emerging biorefinery concept. Fuel Process. Technol. 2020, 199, 106244. [Google Scholar] [CrossRef]
- Haldar, D.; Purkait, M.K. Lignocellulosic conversion into value-added products: A review. Process Biochem. 2020, 89, 110–133. [Google Scholar] [CrossRef]
- Roy, R.; Rahman, M.S.; Raynie, D.E. Recent advances of greener pretreatment technologies of lignocellulose. Curr. Res. Green Sustain. Chem. 2020, 3, 100035. [Google Scholar] [CrossRef]
- Liu, X.; Bouxin, F.P.; Fan, J.; Budarin, V.L.; Hu, C.; Clark, J.H. Recent Advances in the Catalytic Depolymerization of Lignin towards Phenolic Chemicals: A Review. ChemSusChem 2020, 13, 4296–4317. [Google Scholar] [CrossRef]
- Zhuang, X.; Wang, W.; Yu, Q.; Qi, W.; Wang, Q.; Tan, X.; Zhou, G.; Yuan, Z. Liquid hot water pretreatment of lignocellulosic biomass for bioethanol production accompanying with high valuable products. Bioresour. Technol. 2016, 199, 68–75. [Google Scholar] [CrossRef]
- Ciesielski, P.N.; Pecha, M.B.; Lattanzi, A.M.; Bharadwaj, V.S.; Crowley, M.F.M.F.; Bu, L.; Vermaas, J.V.; Steirer, K.X.; Crowley, M.F.M.F. Advances in Multiscale Modeling of Lignocellulosic Biomass. ACS Sustain. Chem. Eng. 2020, 8, 3512–3531. [Google Scholar] [CrossRef]
- Guerriero, G.; Hausman, J.-F.; Strauss, J.; Ertan, H.; Siddiqui, K.S. Lignocellulosic biomass: Biosynthesis, degradation, and industrial utilization. Eng. Life Sci. 2016, 16, 1–16. [Google Scholar] [CrossRef]
- Nishimura, H.; Kamiya, A.; Nagata, T.; Katahira, M.; Watanabe, T. Direct evidence for α ether linkage between lignin and carbohydrates in wood cell walls. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Haldar, D.; Purkait, M.K. A review on the environment-friendly emerging techniques for pretreatment of lignocellulosic biomass: Mechanistic insight and advancements. Chemosphere 2021, 264, 128523. [Google Scholar] [CrossRef]
- Fearon, O.; Kuitunen, S.; Ruuttunen, K.; Alopaeus, V.; Vuorinen, T. Detailed Modeling of Kraft Pulping Chemistry. Delignification. Ind. Eng. Chem. Res. 2020, 59, 12977–12985. [Google Scholar] [CrossRef]
- Mohammad, J.T.; Keikhosro, K. Pretreatment of Lignocellulosic Wastes to Improve Ethanol and Biogas Production: A Review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar]
- Brodeur, G.; Yau, E.; Badal, K.; Collier, J.; Ramachandran, K.B.; Ramakrishnan, S. Chemical and Physicochemical Pretreatment of Lignocellulosic Biomass: A Review. Res. Enzym. Res. 2011, 2011, 17. [Google Scholar]
- Sihvonen, M.; Järvenpää, E.; Hietaniemi, V.; Huopalahti, R. Advances in supercritical carbon dioxide technologies. Trends Food Sci. Technol. 1999. [Google Scholar] [CrossRef]
- Stolarski, M.J.; Warmiński, K.; Krzyżaniak, M.; Tyśkiewicz, K.; Olba-Zięty, E.; Graban, Ł.; Lajszner, W.; Załuski, D.; Wiejak, R.; Kamiński, P.; et al. How does extraction of biologically active substances with supercritical carbon dioxide affect lignocellulosic biomass properties? Wood Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Yoo, C.G.; Pu, Y.; Ragauskas, A.J. Ionic liquids: Promising green solvents for lignocellulosic biomass utilization. Curr. Opin. Green Sustain. Chem. 2017, 5, 5–11. [Google Scholar] [CrossRef]
- Xia, Z.; Li, J.; Zhang, J.; Zhang, X.; Zheng, X.; Zhang, J. Processing and valorization of cellulose, lignin and lignocellulose using ionic liquids. J. Bioresour. Bioprod. 2020, 5, 79–95. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, Y.; Yang, X.; McQueen-Mason, S.J.; Gomez, L.D.; Macquarrie, D.J. Comparative evaluation of microwave-assisted acid, alkaline, and inorganic salt pretreatments of sugarcane bagasse for sugar recovery. Biomass Convers. Biorefin. 2020. [Google Scholar] [CrossRef]
- Mikulski, D.; Kłosowski, G. Microwave-assisted dilute acid pretreatment in bioethanol production from wheat and rye stillages. Biomass Bioenergy 2020, 136, 105528. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, J.; Wang, Y.; Sun, J.; Huang, P.; Chang, K. Effect of ultrasound on ionic liquid-hydrochloric acid pretreatment with rice straw. Biomass Convers. Biorefin. 2020. [Google Scholar] [CrossRef]
- Qing, Q.; Hu, R.; He, Y.; Zhang, Y.; Wang, L. Investigation of a novel acid-catalyzed ionic liquid pretreatment method to improve biomass enzymatic hydrolysis conversion. Appl. Microbiol. Biotechnol. 2014, 98, 5275–5286. [Google Scholar] [CrossRef]
- Mora-Pale, M.; Meli, L.; Doherty, T.V.; Linhardt, R.J.; Dordick, J.S. Room temperature ionic liquids as emerging solvents for the pretreatment of lignocellulosic biomass. Biotechnol. Bioeng. 2011, 108, 1229–1245. [Google Scholar] [CrossRef]
- Morais, E.S.; Lopes, A.M.d.C.; Freire, M.G.; Freire, C.S.R.; Coutinho, J.A.P.; Silvestre, A.J.D. Use of Ionic Liquids and Deep Eutectic Solvents in Polysaccharides Dissolution and Extraction Processes towards Sustainable Biomass Valorization. Molecules 2020, 25, 3652. [Google Scholar] [CrossRef]
- Wahlström, R.; Hiltunen, J.; Pitaluga De Souza Nascente Sirkka, M.; Vuoti, S.; Kruus, K. Comparison of three deep eutectic solvents and 1-ethyl-3-methylimidazolium acetate in the pretreatment of lignocellulose: Effect on enzyme stability, lignocellulose digestibility and one-pot hydrolysis. RSC Adv. 2016, 6, 68100–68110. [Google Scholar] [CrossRef]
- Wahlström, R.; Hiltunen, J.; de Souza Nascente Sirkka, P.M.; Kruus, K.; Vuoti, S. Deep eutectic solvents and ionic liquids in enzymatic lignocellulose hydrolysis. In Proceedings of the NWBC 2015—6th Nordic Wood Biorefinery Conference, Helsinki, Finland, 20–22 October 2015; pp. 326–331. [Google Scholar]
- Xia, S.; Baker, G.A.; Li, H.; Ravula, S.; Zhao, H. Aqueous ionic liquids and deep eutectic solvents for cellulosic biomass pretreatment and saccharification. RSC Adv. 2014, 4, 10586–10596. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 70–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.; Baker, G.A. Ionic liquids and deep eutectic solvents for biodiesel synthesis: A review. J. Chem. Technol. Biotechnol. 2013, 88, 3–12. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hossain, M.A.; Rahaman, M.S.; Yelle, D.; Shang, H.; Sun, Z.; Renneckar, S.; Dong, J.; Tulaphol, S.; Sathitsuksanoh, N. Effects of polyol-based deep eutectic solvents on the efficiency of rice straw enzymatic hydrolysis. Ind. Crops Prod. 2021, 167, 113480. [Google Scholar] [CrossRef]
- Wang, R.; Wang, K.; Zhou, M.; Xu, J.; Jiang, J. Efficient fractionation of moso bamboo by synergistic hydrothermal-deep eutectic solvents pretreatment. Bioresour. Technol. 2021, 328, 124873. [Google Scholar] [CrossRef]
- Li, C.; Huang, C.; Zhao, Y.; Zheng, C.; Su, H.; Zhang, L.; Luo, W.; Zhao, H.; Wang, S.; Huang, L.J. Effect of choline-based deep eutectic solvent pretreatment on the structure of cellulose and lignin in Bagasse. Processes 2021, 9, 384. [Google Scholar] [CrossRef]
- Li, G.; Jiang, Y.; Liu, X.; Deng, D. New levulinic acid-based deep eutectic solvents: Synthesis and physicochemical property determination. J. Mol. Liq. 2016, 222, 201–207. [Google Scholar] [CrossRef]
- De, D.; Naga Sai, M.S.; Aniya, V.; Satyavathi, B. Strategic biorefinery platform for green valorization of agro-industrial residues: A sustainable approach towards biodegradable plastics. J. Clean. Prod. 2021, 290, 125184. [Google Scholar] [CrossRef]
- Ji, Q.; Yu, X.; Yagoub, A.E.G.A.; Chen, L.; Zhou, C. Efficient removal of lignin from vegetable wastes by ultrasonic and microwave-assisted treatment with ternary deep eutectic solvent. Ind. Crops Prod. 2020, 149. [Google Scholar] [CrossRef]
- Bahadori, L.; Chakrabarti, M.H.; Mjalli, F.S.; Alnashef, I.M.; Manan, N.S.A.; Hashim, M.A. Physicochemical properties of ammonium-based deep eutectic solvents and their electrochemical evaluation using organometallic reference redox systems. Electrochim. Acta 2013, 113, 205–211. [Google Scholar] [CrossRef]
- Hayyan, A.; Mjalli, F.S.; Alnashef, I.M.; Al-Wahaibi, Y.M.; Al-Wahaibi, T.; Hashim, M.A.; Hashim, A. Glucose-based deep eutectic solvents: Physical properties. J. Mol. Liq. 2013. [Google Scholar] [CrossRef]
- Craveiro, R.; Aroso, I.; Flammia, V.; Carvalho, T.; Viciosa, M.T.; Dionísio, M.; Barreiros, S.; Reis, R.L.; Duarte, A.R.C.; Paiva, A. Properties and thermal behavior of natural deep eutectic solvents. J. Mol. Liq. 2016, 215, 534–540. [Google Scholar] [CrossRef]
- Gautam, R.; Kumar, N.; Lynam, J.G. Theoretical and experimental study of choline chloride-carboxylic acid deep eutectic solvents and their hydrogen bonds. J. Mol. Struct. 2020, 1222. [Google Scholar] [CrossRef]
- Cui, Y.; Li, C.; Yin, J.; Li, S.; Jia, Y.; Bao, M. Design, synthesis and properties of acidic deep eutectic solvents based on choline chloride. J. Mol. Liq. 2017, 236, 338–343. [Google Scholar] [CrossRef]
- Wang, Y.; Meng, X.; Jeong, K.; Li, S.; Leem, G.; Kim, K.H.; Pu, Y.; Ragauskas, A.J.; Yoo, C.G. Investigation of a lignin-based deep eutectic solvent using p-hydroxybenzoic acid for efficient woody biomass conversion. ACS Sustain. Chem. Eng. 2020, 8, 12542–12553. [Google Scholar] [CrossRef]
- García, G.; Aparicio, S.; Ullah, R.; Atilhan, M. Deep eutectic solvents: Physicochemical properties and gas separation applications. Energy Fuels 2015. [Google Scholar] [CrossRef]
- Zhang, Q.; De Oliveira Vigier, K.; Royer, S.; Jérôme, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Bai, X.; Xue, Z.; Mou, H.; Chen, J.; Liu, Z.; Mu, T. The formation and physicochemical properties of PEGylated deep eutectic solvents. New J. Chem. 2019, 43, 8804–8810. [Google Scholar] [CrossRef]
- Naser, J.; Mjalli, F.; Jibril, B.; Al-Hatmi, S.; Gano, Z. Potassium Carbonate as a Salt for Deep Eutectic Solvents. Int. J. Chem. Eng. Appl. 2013, 114–118. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y.T.; Ngoh, G.C.; Chua, A.S.M. Evaluation of fractionation and delignification efficiencies of deep eutectic solvents on oil palm empty fruit bunch. Ind. Crops Prod. 2018, 123, 271–277. [Google Scholar] [CrossRef]
- Adeyemi, I.; Abu-Zahra, M.R.M.; AlNashef, I.M. Physicochemical properties of alkanolamine-choline chloride deep eutectic solvents: Measurements, group contribution and artificial intelligence prediction techniques. J. Mol. Liq. 2018, 256, 581–590. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, X.; Ali, M.F.; Abdeltawab, A.A.; Yakout, S.M.; Yu, G. Pretreatment of wheat straw using basic ethanolamine-based deep eutectic solvents for improving enzymatic hydrolysis. Bioresour. Technol. 2018, 263, 325–333. [Google Scholar] [CrossRef]
- Satlewal, A.; Agrawal, R.; Bhagia, S.; Sangoro, J.; Ragauskas, A.J. Natural deep eutectic solvents for lignocellulosic biomass pretreatment: Recent developments, challenges and novel opportunities. Biotechnol. Adv. 2018, 36, 2032–2050. [Google Scholar] [CrossRef] [PubMed]
- Kalhor, P.; Ghandi, K. Deep eutectic solvents for pretreatment, extraction, and catalysis of biomass and food waste. Molecules 2019, 24, 4012. [Google Scholar] [CrossRef] [Green Version]
- Sosa, F.H.B.; Abranches, D.O.; Da Costa Lopes, A.M.; Coutinho, J.A.P.; Da Costa, M.C. Kraft Lignin Solubility and Its Chemical Modification in Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2020, 8, 18577–18589. [Google Scholar] [CrossRef]
- Hayyan, M.; Hashim, M.A.; Hayyan, A.; Al-Saadi, M.A.; AlNashef, I.M.; Mirghani, M.E.S.; Saheed, O.K. Are deep eutectic solvents benign or toxic? Chemosphere 2013. [Google Scholar] [CrossRef]
- Choi, Y.H.; van Spronsen, J.; Dai, Y.; Verberne, M.; Hollmann, F.; Arends, I.W.C.E.; Witkamp, G.J.; Verpoorte, R. Are natural deep eutectic solvents the missing link in understanding cellular metabolism and physiology? Plant. Physiol. 2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zulkefli, S.; Abdulmalek, E.; Abdul Rahman, M.B. Pretreatment of oil palm trunk in deep eutectic solvent and optimization of enzymatic hydrolysis of pretreated oil palm trunk. Renew. Energy 2017. [Google Scholar] [CrossRef]
- Francisco, M.; Van Den Bruinhorst, A.; Kroon, M.C. Low-transition-temperature mixtures (LTTMs): A new generation of designer solvents. Angew. Chem. Int. Ed. 2013, 52, 3074–3085. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Chen, W.; Xia, Q.; Guo, B.; Wang, Q.; Liu, S.; Liu, Y.Y.; Li, J.; Yu, H. Efficient Cleavage of Lignin–Carbohydrate Complexes and Ultrafast Extraction of Lignin Oligomers from Wood Biomass by Microwave-Assisted Treatment with Deep Eutectic Solvent. ChemSusChem 2017, 10, 1692–1700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Mu, T. Application of deep eutectic solvents in biomass pretreatment and conversion. Green Energy Environ. 2019, 4, 95–115. [Google Scholar] [CrossRef]
- Kim, K.H.; Eudes, A.; Jeong, K.; Yoo, C.G.; Kim, C.S.; Ragauskas, A. Integration of renewable deep eutectic solvents with engineered biomass to achieve a closed-loop biorefinery. Proc. Natl. Acad. Sci. USA 2019, 116, 13816–13824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Q.; Song, Z.; Zhuang, X.; Liu, L.; Qiu, W.; Shi, J.; Wang, W.; Li, Y.; Wang, Z.; Yuan, Z. Catalytic conversion of herbal residue carbohydrates to furanic derivatives in a deep eutectic solvent accompanied by dissolution and recrystallisation of choline chloride. Cellulose 2019, 26. [Google Scholar] [CrossRef]
- Ling, Z.; Tang, W.; Su, Y.; Shao, L.; Wang, P.; Ren, Y.; Huang, C.; Lai, C.; Yong, Q. Promoting enzymatic hydrolysis of aggregated bamboo crystalline cellulose by fast microwave-assisted dicarboxylic acid deep eutectic solvents pretreatments. Bioresour. Technol. 2021, 333, 125122. [Google Scholar] [CrossRef]
- Lin, X.; Liu, Y.; Zheng, X.; Qureshi, N. High-efficient cellulosic butanol production from deep eutectic solvent pretreated corn stover without detoxification. Ind. Crops Prod. 2021, 162. [Google Scholar] [CrossRef]
- Oh, Y.; Park, S.; Jung, D.; Oh, K.K.; Lee, S.H. Effect of hydrogen bond donor on the choline chloride-based deep eutectic solvent-mediated extraction of lignin from pine wood. Int. J. Biol. Macromol. 2020, 165, 187–197. [Google Scholar] [CrossRef]
- Hu, S.; Meng, F.; Huang, D.; Huang, J.; Lou, W. Hydrolysis of corn stover pretreated by DESs with carbon-based solid acid catalyst. SN Appl. Sci. 2020, 2. [Google Scholar] [CrossRef]
- Ling, Z.; Guo, Z.; Huang, C.; Yao, L.; Xu, F. Deconstruction of oriented crystalline cellulose by novel levulinic acid based deep eutectic solvents pretreatment for improved enzymatic accessibility. Bioresour. Technol. 2020, 305, 123025. [Google Scholar] [CrossRef]
- Shen, X.J.; Wen, J.L.; Mei, Q.Q.; Chen, X.; Sun, D.; Yuan, T.Q.; Sun, R.C. Facile fractionation of lignocelluloses by biomass-derived deep eutectic solvent (DES) pretreatment for cellulose enzymatic hydrolysis and lignin valorization. Green Chem. 2019, 21, 275–283. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.; Yu, J.; Lu, Y.; Jiang, B.; Fan, Y.; Wang, Z. High-purity lignin isolated from poplar wood meal through dissolving treatment with deep eutectic solvents. R. Soc. Open Sci. 2019, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satlewal, A.; Agrawal, R.; Das, P.; Bhagia, S.; Pu, Y.; Puri, S.K.; Ramakumar, S.S.V.; Ragauskas, A.J. Assessing the Facile Pretreatments of Bagasse for Efficient Enzymatic Conversion and Their Impacts on Structural and Chemical Properties. ACS Sustain. Chem. Eng. 2019, 7, 1095–1104. [Google Scholar] [CrossRef]
- Wang, Z.-K.; Hong, S.S.; Wen, J.-L.; Ma, C.-Y.; Tang, L.; Jiang, H.; Chen, J.-J.; Li, S.; Shen, X.-J.; Yuan, T.-Q. Lewis Acid-Facilitated Deep Eutectic Solvent (DES) Pretreatment for Producing High-Purity and Antioxidative Lignin. ACS Sustain. Chem. Eng. 2020, 8, 1050–1057. [Google Scholar] [CrossRef]
- Tian, D.; Guo, Y.; Hu, J.; Yang, G.; Zhang, J.; Luo, L.; Xiao, Y.; Deng, S.; Deng, O.; Zhou, W.; et al. Acidic deep eutectic solvents pretreatment for selective lignocellulosic biomass fractionation with enhanced cellulose reactivity. Int. J. Biol. Macromol. 2020, 142, 288–297. [Google Scholar] [CrossRef]
- Liang, X.; Fu, Y.; Chang, J. Effective separation, recovery and recycling of deep eutectic solvent after biomass fractionation with membrane-based methodology. Sep. Purif. Technol. 2019, 210, 409–416. [Google Scholar] [CrossRef]
- Tan, Y.T.; Ngoh, G.C.; Chua, A.S.M. Effect of functional groups in acid constituent of deep eutectic solvent for extraction of reactive lignin. Bioresour. Technol. 2019, 281, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.D.; Li, A.L.; Lin, K.P.; Wang, Y.Y.; Kuang, Z.Y.; Cao, S.L. Insight into the structure-function relationships of deep eutectic solvents during rice straw pretreatment. Bioresour. Technol. 2018, 249, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.J.; Chen, T.; Wang, H.M.; Mei, Q.; Yue, F.; Sun, S.; Wen, J.L.; Yuan, T.Q.; Sun, R.C. Structural and Morphological Transformations of Lignin Macromolecules during Bio-Based Deep Eutectic Solvent (DES) Pretreatment. ACS Sustain. Chem. Eng. 2020, 8, 2130–2137. [Google Scholar] [CrossRef]
- Fernandes, C.; Melro, E.; Magalhães, S.; Alves, L.; Craveiro, R.; Filipe, A.; Valente, A.J.M.; Martins, G.; Antunes, F.E.; Romano, A.; et al. New deep eutectic solvent assisted extraction of highly pure lignin from maritime pine sawdust (Pinus pinaster Ait.). Int. J. Biol. Macromol. 2021, 177, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Kandanelli, R.; Thulluri, C.; Mangala, R.; Rao, P.V.C.; Gandham, S.; Velankar, H.R. A novel ternary combination of deep eutectic solvent-alcohol (DES-OL) system for synergistic and efficient delignification of biomass. Bioresour. Technol. 2018, 265, 573–576. [Google Scholar] [CrossRef]
- Wang, Z.K.; Shen, X.J.; Chen, J.J.; Jiang, Y.Q.; Hu, Z.Y.; Wang, X.; Liu, L. Lignocellulose fractionation into furfural and glucose by AlCl3-catalyzed DES/MIBK biphasic pretreatment. Int. J. Biol. Macromol. 2018, 117, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, Q.; Zhang, X.X.; Zhang, X.X.; Pan, X.; Xu, F. Subcellular dissolution of xylan and lignin for enhancing enzymatic hydrolysis of microwave assisted deep eutectic solvent pretreated Pinus bungeana Zucc. Bioresour. Technol. 2019, 288. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Yu, X.; Yagoub, A.E.G.A.; Chen, L.; Mustapha, A.T.; Zhou, C. Enhancement of lignin removal and enzymolysis of sugarcane bagasse by ultrasound-assisted ethanol synergized deep eutectic solvent pretreatment. Renew. Energy 2021, 172, 304–316. [Google Scholar] [CrossRef]
- Alvarez-Vasco, C.; Ma, R.; Quintero, M.; Guo, M.; Geleynse, S.; Ramasamy, K.K.; Wolcott, M.; Zhang, X. Unique low-molecular-weight lignin with high purity extracted from wood by deep eutectic solvents (DES): A source of lignin for valorization. Green Chem. 2016, 18, 5133–5141. [Google Scholar] [CrossRef]
- Pan, M.; Zhao, G.; Ding, C.; Wu, B.; Lian, Z.; Lian, H. Physicochemical transformation of rice straw after pretreatment with a deep eutectic solvent of choline chloride/urea. Carbohydr. Polym. 2017, 176. [Google Scholar] [CrossRef]
- Yu, Q.; Zhang, A.; Wang, W.; Chen, L.; Bai, R.; Zhuang, X.; Wang, Q.; Wang, Z.; Yuan, Z. Deep eutectic solvents from hemicellulose-derived acids for the cellulosic ethanol refining of Akebia’ herbal residues. Bioresour. Technol. 2018. [Google Scholar] [CrossRef]
- Xu, H.; Kong, Y.Y.; Peng, J.; Song, X.; Liu, Y.; Su, Z.; Li, B.; Gao, C.; Tian, W. Comprehensive analysis of important parameters of choline chloride-based deep eutectic solvent pretreatment of lignocellulosic biomass. Bioresour. Technol. 2021, 319, 124209. [Google Scholar] [CrossRef]
- Chen, Z.; Bai, X.; Lusi, A.; Zhang, H.; Wan, C. Insights into Structural Changes of Lignin toward Tailored Properties during Deep Eutectic Solvent Pretreatment. ACS Sustain. Chem. Eng. 2020, 8, 9783–9793. [Google Scholar] [CrossRef]
- Hong, S.; Shen, X.J.; Pang, B.; Xue, Z.; Cao, X.F.; Wen, J.L.; Sun, Z.H.; Lam, S.S.; Yuan, T.Q.; Sun, R.C. In-depth interpretation of the structural changes of lignin and formation of diketones during acidic deep eutectic solvent pretreatment. Green Chem. 2020, 22, 1851–1858. [Google Scholar] [CrossRef]
- Lin, W.; Xing, S.; Jin, Y.; Lu, X.; Huang, C.; Yong, Q. Insight into understanding the performance of deep eutectic solvent pretreatment on improving enzymatic digestibility of bamboo residues. Bioresour. Technol. 2020, 306, 123163. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.C.; Ding, J.C.; Han, R.Z.; Dong, J.J.; Ni, Y. Enhancing cellulose accessibility of corn stover by deep eutectic solvent pretreatment for butanol fermentation. Bioresour. Technol. 2016, 203, 364–369. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, T.; Liu, J.; Gao, H.; Bian, H.; Wang, R.; Huang, C.; Sha, J.; Dai, H. Recyclable deep eutectic solvent coupling sodium hydroxide post-treatment for boosting woody/herbaceous biomass conversion at mild condition. Bioresour. Technol. 2021, 320, 124327. [Google Scholar] [CrossRef]
- Su, Y.; Huang, C.; Lai, C.; Yong, Q. Green solvent pretreatment for enhanced production of sugars and antioxidative lignin from poplar. Bioresour. Technol. 2021, 321, 124471. [Google Scholar] [CrossRef]
- Ci, Y.H.; Yu, F.; Zhou, C.X.; Mo, H.E.; Li, Z.Y.; Ma, Y.Q.; Zang, L.H. New ternary deep eutectic solvents for effective wheat straw deconstruction into its high-value utilization under near-neutral conditions. Green Chem. 2020, 22, 8713–8720. [Google Scholar] [CrossRef]
- Shen, X.J.; Wang, B.; Huang, P.L.; Wen, J.L.; Sun, R.C. Effects of aluminum chloride-catalyzed hydrothermal pretreatment on the structural characteristics of lignin and enzymatic hydrolysis. Bioresour. Technol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sharma, S.; Arumugam, S.M.; Miglani, C.; Elumalai, S. Biphasic Separation Approach in the des Biomass Fractionation Facilitates Lignin Recovery for Subsequent Valorization to Phenolics. ACS Sustain. Chem. Eng. 2020, 8, 19140–19154. [Google Scholar] [CrossRef]
- Thulluri, C.; Balasubramaniam, R.; Velankar, H.R. Generation of highly amenable cellulose-Iβ via selective delignification of rice straw using a reusable cyclic ether-assisted deep eutectic solvent system. Sci. Rep. 2021, 11, 1–14. [Google Scholar] [CrossRef]
- Loow, Y.L.; Wu, T.Y.; Yang, G.H.; Ang, L.Y.; New, E.K.; Siow, L.F.; Md. Jahim, J.; Mohammad, A.W.; Teoh, W.H. Deep eutectic solvent and inorganic salt pretreatment of lignocellulosic biomass for improving xylose recovery. Bioresour. Technol. 2018. [Google Scholar] [CrossRef]
- Fang, C.; Thomsen, M.H.; Frankær, C.G.; Brudecki, G.P.; Schmidt, J.E.; Alnashef, I.M. Reviving Pretreatment Effectiveness of Deep Eutectic Solvents on Lignocellulosic Date Palm Residues by Prior Recalcitrance Reduction. Ind. Eng. Chem. Res. 2017. [Google Scholar] [CrossRef]
- Procentese, A.; Johnson, E.; Orr, V.; Garruto Campanile, A.; Wood, J.A.; Marzocchella, A.; Rehmann, L. Deep eutectic solvent pretreatment and subsequent saccharification of corncob. Bioresour. Technol. 2015, 192, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Sun, J.; Cai, C.; Wang, S.; Pei, H.; Zhang, J. Corn stover pretreatment by inorganic salts and its effects on hemicellulose and cellulose degradation. Bioresour. Technol. 2009. [Google Scholar] [CrossRef]
- Ma, Y.; Ji, W.; Zhu, X.; Tian, L.; Wan, X. Effect of extremely low AlCl 3 on hydrolysis of cellulose in high temperature liquid water. Biomass Bioenergy 2012. [Google Scholar] [CrossRef]
- Di Bitonto, L.; Menegatti, S.; Pastore, C. Process intensification for the production of the ethyl esters of volatile fatty acids using aluminium chloride hexahydrate as a catalyst. J. Clean. Prod. 2019, 239. [Google Scholar] [CrossRef] [Green Version]
- Pastore, C.; Barca, E.; Del Moro, G.; Lopez, A.; Mininni, G.; Mascolo, G. Recoverable and reusable aluminium solvated species used as a homogeneous catalyst for biodiesel production from brown grease. Appl. Catal. A Gen. 2016, 501. [Google Scholar] [CrossRef]
- Di Bitonto, L.; Pastore, C. Metal hydrated-salts as efficient and reusable catalysts for pre-treating waste cooking oils and animal fats for an effective production of biodiesel. Renew. Energy 2019, 143, 1193–1200. [Google Scholar] [CrossRef]
- Pastore, C.; Lopez, A.; Mascolo, G. Efficient conversion of brown grease produced by municipal wastewater treatment plant into biofuel using aluminium chloride hexahydrate under very mild conditions. Bioresour. Technol. 2014, 155. [Google Scholar] [CrossRef]
- Wang, Z.K.; Li, H.; Lin, X.C.; Tang, L.; Chen, J.J.; Mo, J.W.; Yu, R.S.; Shen, X.J. Novel recyclable deep eutectic solvent boost biomass pretreatment for enzymatic hydrolysis. Bioresour. Technol. 2020, 307, 123237. [Google Scholar] [CrossRef]
- Lee, K.M.; Hong, J.Y.; Tey, W.Y. Combination of ultrasonication and deep eutectic solvent in pretreatment of lignocellulosic biomass for enhanced enzymatic saccharification. Cellulose 2021. [Google Scholar] [CrossRef]
- Schutyser, W.; Renders, T.; Van Den Bosch, S.; Koelewijn, S.F.; Beckham, G.T.; Sels, B.F. Chemicals from lignin: An interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Gu, F.; Zhu, J.Y.; Sun, K.; Cai, Z.; Yao, S.; Wu, W.; Jin, Y. Fractionation of herbaceous biomass using a recyclable hydrotropic p-toluenesulfonic acid (p-TsOH)/choline chloride (ChCl) solvent system at low temperatures. Ind. Crops Prod. 2020, 150, 112423. [Google Scholar] [CrossRef]
- Gross, A.S.; Bell, A.T.; Chu, J.W. Preferential interactions between lithium chloride and glucan chains in N, N -dimethylacetamide drive cellulose dissolution. J. Phys. Chem. B 2013. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, H.; Kristensen, J.B.; Felby, C. Enzymatic conversion of lignocellulose into fermentable sugars: Challenges and opportunities. Biofuels Bioprod. Biorefin. 2007, 1, 119–134. [Google Scholar] [CrossRef]
- Trygg, J.; Fardim, P. Enhancement of cellulose dissolution in water-based solvent via ethanol-hydrochloric acid pretreatment. Cellulose 2011. [Google Scholar] [CrossRef]
- Sen, S.; Martin, J.D.; Argyropoulos, D.S. Review of cellulose non-derivatizing solvent interactions with emphasis on activity in inorganic molten salt hydrates. ACS Sustain. Chem. Eng. 2013. [Google Scholar] [CrossRef]
- Lindman, B.; Karlström, G.; Stigsson, L. On the mechanism of dissolution of cellulose. J. Mol. Liq. 2010. [Google Scholar] [CrossRef]
- Heinze, T.; Koschella, A. Solvents applied in the field of cellulose chemistry: A mini review. Polímeros 2005. [Google Scholar] [CrossRef]
- Heinze, T.; Liebert, T. Unconventional methods in cellulose functionalization. Prog. Polym. Sci. 2001, 26, 1689–1762. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, L. Rapid dissolution of cellulose in LiOH/urea and NaOH/urea aqueous solutions. Macromol. Biosci. 2005. [Google Scholar] [CrossRef] [PubMed]
- Brandt, A.; Gräsvik, J.; Hallett, J.P.; Welton, T. Deconstruction of lignocellulosic biomass with ionic liquids. Green Chem. 2013, 15, 550–583. [Google Scholar] [CrossRef] [Green Version]
- Zavrel, M.; Bross, D.; Funke, M.; Büchs, J.; Spiess, A.C. High-throughput screening for ionic liquids dissolving (ligno-)cellulose. Bioresour. Technol. 2009. [Google Scholar] [CrossRef]
- Francisco, M.; Van Den Bruinhorst, A.; Kroon, M.C. New natural and renewable low transition temperature mixtures (LTTMs): Screening as solvents for lignocellulosic biomass processing. Green Chem. 2012. [Google Scholar] [CrossRef]
- Zhang, Q.; Benoit, M.; Dea Oliveiraa Vigier, K.; Barrault, J.; Jérǒme, F. Green and inexpensive choline-derived solvents for cellulose decrystallization. Chem. A Eur. J. 2012, 18, 1043–1046. [Google Scholar] [CrossRef]
- Chen, Y.L.; Zhang, X.; You, T.T.; Xu, F. Deep eutectic solvents (DESs) for cellulose dissolution: A mini-review. Cellulose 2019, 26, 205–213. [Google Scholar] [CrossRef]
- Lan, W.; Liu, C.F.; Yue, F.X.; Sun, R.C.; Kennedy, J.F. Ultrasound-assisted dissolution of cellulose in ionic liquid. Carbohydr. Polym. 2011. [Google Scholar] [CrossRef]
- Ren, H.; Chen, C.; Guo, S.; Zhao, D.; Wang, Q. Synthesis of a Novel Allyl-Functionalized Deep Eutectic Solvent to Promote Dissolution of Cellulose. BioResources 2016, 11, 8535–8547. [Google Scholar] [CrossRef] [Green Version]
- Malaeke, H.; Housaindokht, M.R.; Monhemi, H.; Izadyar, M. Deep eutectic solvent as an efficient molecular liquid for lignin solubilization and wood delignification. J. Mol. Liq. 2018. [Google Scholar] [CrossRef]
- Chhotaray, P.K.; Biswal, S.K.; Pandey, S. Development of novel hybrid ionic fluids for efficient CO2 capture and cellulose dissolution. J. Mol. Liq. 2020, 312. [Google Scholar] [CrossRef]
- Ren, H.; Chen, C.; Wang, Q.; Zhao, D.; Guo, S. The properties of choline chloride-based deep eutectic solvents and their performance in the dissolution of cellulose. BioResources 2016. [Google Scholar] [CrossRef]
- Zhou, Y.; Fuentes-Hernandez, C.; Khan, T.M.; Liu, J.C.; Hsu, J.; Shim, J.W.; Dindar, A.; Youngblood, J.P.; Moon, R.J.; Kippelen, B. Recyclable organic solar cells on cellulose nanocrystal substrates. Sci. Rep. 2013. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Khan, T.M.; Liu, J.C.; Fuentes-Hernandez, C.; Shim, J.W.; Najafabadi, E.; Youngblood, J.P.; Moon, R.J.; Kippelen, B. Efficient recyclable organic solar cells on cellulose nanocrystal substrates with a conducting polymer top electrode deposited by film-transfer lamination. Org. Electron. 2014, 15, 661–666. [Google Scholar] [CrossRef]
- Clarke, C.J.; Tu, W.C.; Levers, O.; Bröhl, A.; Hallett, J.P. Green and Sustainable Solvents in Chemical Processes. Chem. Rev. 2018, 118, 747–800. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Oh, K.W.; Choi, H.M. Preparation and characterization of cotton fabrics with antibacterial properties treated by crosslinkable benzophenone derivative in choline chloride-based deep eutectic solvents. Cellulose 2013, 20, 2101–2114. [Google Scholar] [CrossRef]
- Abbott, A.P.; Bell, T.J.; Handa, S.; Stoddart, B. Cationic functionalisation of cellulose using a choline based ionic liquid analogue. Green Chem. 2006, 8, 784–786. [Google Scholar] [CrossRef] [Green Version]
- Willberg-Keyriläinen, P.; Hiltunen, J.; Ropponen, J. Production of cellulose carbamate using urea-based deep eutectic solvents. Cellulose 2018, 25, 195–204. [Google Scholar] [CrossRef]
- Sirviö, J.A.; Visanko, M.; Liimatainen, H. Deep eutectic solvent system based on choline chloride-urea as a pre-treatment for nanofibrillation of wood cellulose. Green Chem. 2015, 17, 3401–3406. [Google Scholar] [CrossRef]
- Selkälä, T.; Sirviö, J.A.; Lorite, G.S.; Liimatainen, H. Anionically Stabilized Cellulose Nanofibrils through Succinylation Pretreatment in Urea–Lithium Chloride Deep Eutectic Solvent. ChemSusChem 2016, 9, 3074–3083. [Google Scholar] [CrossRef]
- Hosseinmardi, A.; Annamalai, P.K.; Wang, L.; Martin, D.; Amiralian, N. Reinforcement of natural rubber latex using lignocellulosic nanofibers isolated from spinifex grass. Nanoscale 2017, 9, 9510–9519. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, B.; Xia, Q.; Meng, J.; Chen, W.; Liu, S.; Wang, Q.; Liu, Y.; Li, J.; Yu, H. Efficient Cleavage of Strong Hydrogen Bonds in Cotton by Deep Eutectic Solvents and Facile Fabrication of Cellulose Nanocrystals in High Yields. ACS Sustain. Chem. Eng. 2017, 5, 7623–7631. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, C.; Zhang, Y.; Wu, H. Synthesis and characterization of cellulose carbamate from wood pulp, Assisted by supercritical carbon dioxide. BioResources 2013, 8, 1398–1408. [Google Scholar] [CrossRef]
- Mormann, W.; Michel, U. Improved synthesis of cellulose carbamates without by-products. Carbohydr. Polym. 2002, 50, 201–208. [Google Scholar] [CrossRef]
- Fu, F.; Xu, M.; Wang, H.; Wang, Y.; Ge, H.; Zhou, J. Improved Synthesis of Cellulose Carbamates with Minimum Urea Based on an Easy Scale-up Method. ACS Sustain. Chem. Eng. 2015, 3, 1510–1517. [Google Scholar] [CrossRef]
- Sirviö, J.A.; Heiskanen, J.P. Synthesis of Alkaline-Soluble Cellulose Methyl Carbamate Using a Reactive Deep Eutectic Solvent. ChemSusChem 2017, 10, 455–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sirviö, J.A.; Ukkola, J.; Liimatainen, H. Direct sulfation of cellulose fibers using a reactive deep eutectic solvent to produce highly charged cellulose nanofibers. Cellulose 2019, 26, 2303–2316. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.; Zhang, Z.; Zhou, Y.; Han, B.; Fan, H.; Li, W.; Song, J.; Xie, Y. Conversion of fructose to 5-hydroxymethylfurfural using ionic liquids prepared from renewable materials. Green Chem. 2008, 10, 1280–1283. [Google Scholar] [CrossRef]
- Assanosi, A.A.; Farah, M.M.; Wood, J.; Al-Duri, B. A facile acidic choline chloride-p-TSA DES-catalysed dehydration of fructose to 5-hydroxymethylfurfural. RSC Adv. 2014, 4, 39359–39364. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, Y.; Zhong, Y.; Du, N.; Huang, X. Alcohol effect and the related mechanism on fructose dehydration into 5-hydroxymethylfurfural in the deep eutectic solvent of [Emim]Cl/alcohol. ACS Sustain. Chem. Eng. 2016, 4, 3995–4002. [Google Scholar] [CrossRef]
- Di Bitonto, L.; Antonopoulou, G.; Braguglia, C.; Campanale, C.; Gallipoli, A.; Lyberatos, G.; Ntaikou, I.; Pastore, C. Lewis-Brønsted acid catalysed ethanolysis of the organic fraction of municipal solid waste for efficient production of biofuels. Bioresour. Technol. 2018, 266. [Google Scholar] [CrossRef]
- Di Bitonto, L.; Locaputo, V.; D’Ambrosio, V.; Pastore, C. Direct Lewis-Brønsted acid ethanolysis of sewage sludge for production of liquid fuels. Appl. Energy 2020, 259, 114163. [Google Scholar] [CrossRef]
- Arslanoğlu, A.; Sert, M. Direct conversion of biomass to platform chemicals, catalyzed using a deep eutectic solvent of N,N diethyl ethanol ammonium chloride-oxalic acid in a microwave reactor. Fuel 2019, 258, 116142. [Google Scholar] [CrossRef]
- Arora, S.; Gupta, N.; Singh, V. Choline Based Basic Ionic Liquid (BIL)/Acidic DES Mediated Cellulose Rich Fractionation of Agricultural Waste Biomass and Valorization to 5-HMF. Waste Biomass Valorization 2020, 11, 3345–3354. [Google Scholar] [CrossRef]
- Lang, J.; Lu, J.; Lan, P.; Wang, N.; Yang, H.; Zhang, H. Preparation of 5-HMF in a des/ethyl n-butyrate two-phase system. Catalysts 2020, 10, 636. [Google Scholar] [CrossRef]
- Qin, Y.Z.; Li, Y.M.; Zong, M.H.; Wu, H.; Li, N. Enzyme-catalyzed selective oxidation of 5-hydroxymethylfurfural (HMF) and separation of HMF and 2,5-diformylfuran using deep eutectic solvents. Green Chem. 2015, 17, 3718–3722. [Google Scholar] [CrossRef]
- Chen, B.; Li, Z.; Feng, Y.; Hao, W.; Sun, Y.; Tang, X.; Zeng, X.; Lin, L. Green Process for 5-(Chloromethyl)furfural Production from Biomass in Three-Constituent Deep Eutectic Solvent. ChemSusChem 2021, 14, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yu, Z.; Gu, T.; Xiang, L.; Shang, M.; Shen, C.; Su, Y. Continuous Synthesis of 5-hydroxymethylfurfural Using Deep Eutectic Solvents and Its Kinetic Study in Microreactors; Elsevier: Amsterdam, The Netherlands, 2020; Volume 391, ISBN 2154738710. [Google Scholar]
- Zuo, M.; Jia, W.; Feng, Y.; Zeng, X.; Tang, X.; Sun, Y.; Lin, L. Effective selectivity conversion of glucose to furan chemicals in the aqueous deep eutectic solvent. Renew. Energy 2021, 164, 23–33. [Google Scholar] [CrossRef]
- Ruan, C.; Mo, F.; Qin, H.; Cheng, H.; Chen, L.; Qi, Z. Bifunctional Imidazole-Benzenesulfonic Acid Deep Eutectic Solvent for Fructose Dehydration to 5-Hydroxymethylfurfural. Catal. Lett. 2021, 151, 445–453. [Google Scholar] [CrossRef]
- Zuo, M.; Li, Z.; Jiang, Y.; Tang, X.; Zeng, X.; Sun, Y.; Lin, L. Green catalytic conversion of bio-based sugars to 5-chloromethyl furfural in deep eutectic solvent, catalyzed by metal chlorides. RSC Adv. 2016, 6, 27004–27007. [Google Scholar] [CrossRef]
- Zuo, M.; Le, K.; Li, Z.; Jiang, Y.; Zeng, X.; Tang, X.; Sun, Y.; Lin, L. Green process for production of 5-hydroxymethylfurfural from carbohydrates with high purity in deep eutectic solvents. Ind. Crops Prod. 2017, 99, 1–6. [Google Scholar] [CrossRef]
- Yang, H.; Lang, J.; Lu, J.; Lan, P.; Zhang, H. Study on catalytic conversion of cellulose to 5-hydroxymethyl furfural by directional degradation in deep eutectic solvents. BioResources 2020, 15, 3344–3355. [Google Scholar] [CrossRef]
- Sert, M.; Arslanoğlu, A.; Ballice, L. Conversion of sunflower stalk based cellulose to the valuable products using choline chloride based deep eutectic solvents. Renew. Energy 2018, 118, 993–1000. [Google Scholar] [CrossRef]
- Shavandi, A.; Jafari, H.; Zago, E.; Hobbi, P.; Nie, L.; De Laet, N. A sustainable solvent based on lactic acid andl-cysteine for the regeneration of keratin from waste wool. Green Chem. 2021, 23, 1171–1174. [Google Scholar] [CrossRef]
- Morais, E.S.; Da Costa Lopes, A.M.; Freire, M.G.; Freire, C.S.R.; Silvestre, A.J.D. Unveiling Modifications of Biomass Polysaccharides during Thermal Treatment in Cholinium Chloride: Lactic Acid Deep Eutectic Solvent. ChemSusChem 2021, 14, 686–698. [Google Scholar] [CrossRef] [PubMed]
- Ai, B.; Li, W.; Woomer, J.; Li, M.; Pu, Y.; Sheng, Z.; Zheng, L.; Adedeji, A.; Ragauskas, A.J.; Shi, J. Natural deep eutectic solvent mediated extrusion for continuous high-solid pretreatment of lignocellulosic biomass. Green Chem. 2020, 22, 6372–6383. [Google Scholar] [CrossRef]
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.N.; Pauli, G.F. Natural Deep Eutectic Solvents: Properties, Applications, and Perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef]
- Hong, S.; Shen, X.J.; Xue, Z.; Sun, Z.; Yuan, T.Q. Structure-function relationships of deep eutectic solvents for lignin extraction and chemical transformation. Green Chem. 2020, 22, 7219–7232. [Google Scholar] [CrossRef]










| DES/NADES | Molar Ratio | d [g/cm3] | Tm [°C] | Tf [°C] | Viscosity [mPa s] | γ [mN m−1] | nD | Conductivity [mS cm−1] | Ref | |
|---|---|---|---|---|---|---|---|---|---|---|
| HBA | HBD | |||||||||
| Acidic DES | ||||||||||
| ChCl | Lactic acid | 1:2 | 1.13 | 212 | 134 | 1.46 | 0.81 | [30,31,32,33] | ||
| ChCl | Oxalic acid | 1:1 | 1.24 | 34 | 1881 | 1.48 | 0.38 | [34,35,36] | ||
| ChCl | Formic Acid | 1:1 | 1.16 | 31 | 234 | 73.1 | >1.50 | [37] | ||
| ChCl | Citric acid | 1:1 | 1.30 | 76 | 9.126 | [38] | ||||
| ChCl | Acetic acid | 1:2 | 1.12 | >150 | 39 | 5.83 | [39] | |||
| ChCl | p-CH3PhSO3H | 1:2 | 1.24281 | 697.9 | 1.5167 | 0.691 | [40] | |||
| ChCl | p-OHPhCOOH | 1:1 | 58 | [41] | ||||||
| ChCl | p-coumaric acid | 1:1 | 78 | [41] | ||||||
| Neutral DES | ||||||||||
| ChCl | Ethylene Glycol | 1:2 | 1.12 | −66 | 35 (30 °C) | 48 | 1.46823 | 7.61 (20 °C) | [30,42] | |
| ChCl | Glycerol | 1:2 | 1.1548 | −40 | −33.5 | 246 | 58 | 1.48675 | 1.7 | |
| ChCl | Sorbitol | 1:1 | 1.12–1.28 | Liq. at 0 | 12,730 | [30,43] | ||||
| ChCl | Xylitol | 1:1 | 1.12–1.28 | Liq. at 0 | 5230 (30 °C) | <2 | [30,43] | |||
| ChCl | PEG | 1:4 | 1.07–1.08 | 150 | <100 | 0.745 | [44] | |||
| ChCl | D(+)-glucose | 1:1 | 1.2978 | 15 | 31 | 0.45663 | 73.1 | >1.50 | [37] | |
| Basic DES | ||||||||||
| ChCl | Urea | 1:2 | 1.24 | 12 | 12 | 449 (30 °C) | 52 | 1.5044 | 2.31 (30 °C) | [42] |
| K2CO3 | Glycerol | 1:6 | 1.44–1.46 | −60 | ≈18,000 (20 °C) | 64 | 1.491 | 7.89 10−3 (20 °C) | [45,46] | |
| ChCl | MEA | 1:5 | 1.0767 | −4 | 51.7 | 48.21 | 1.4830 | 2.62 | [47,48] | |
| DES | Biomass/Conditions | Results | Ref. |
|---|---|---|---|
| ChCl:ethylene glycol 2:1 | Rice straw/80–150 °C, 3–24 h. biomass/DES ratio 5%wt | ChCl-Gly removed 74 wt. % lignin with 91% glucan retention. Residual ChCl-Glycerol limited cellulase enzyme activity to 68%. Sodium carbonate wash removed the residual DES, increasing the glucan digestibility to 87%. | [30] |
| ChCl:glycerol 1:1 | |||
| ChCl:xylitol 1:1 | |||
| ChCl:sorbitol 1:2 | |||
| ChCl: Oxalic/Malonic/Succinic/Malic/L-Tartaric Acid 2:1 | Moso Bamboo/160 °C, 10 min, MW power 600 W | MW-DESs pretreatments reduced xylans content. Lignin still remains with 40% of removal. Enzymatic hydrolysis of pretreated samples produced 60% of glucans. | [60] |
| Betaine:Lactic acid 2:1 ChCl—Lactic acid 1:10 | Moso Bamboo/200 °C,10 min 100–140 °C, 6 h | Total of 98.2 wt. % hemicelluloses degraded and mainly converted into pentose. Delignification reached 80.1% at 140 °C; highly purified lignin (99.49%) was achieved after ChCl/lactic acid system treatment at 140 °C. | [31] |
| Tri-ethylbenzyl ammonium chloride (TEBAC)—LacticaAcid 1:7 | Corn Stover/80–140 °C for 90 min | Glucan contents in pretreated CS were significantly increased from 30.99 to 67.07% with increasing temperature from 80 to 140 °C. Xylan contents decreased from 18.86 to 4.66%. Lignin contents decreased from 22.97 to 9.47%. At 120 °C, the glucan recovery reached 99.23%, the lignin removal was 61.56. The glucose and xylose yields reached 76.41% and 77.43%, respectively. | [61] |
| ChCl—oxalic acid dihydrate 1:1 | Tender coconut husks and tamarind seeds/90 °C for 4 h; 1:15 solid to liquid ratio | Cellulose recovery of 87.5% (92.3% pure). DES was successfully reused with negligible effect on the delignification efficiency. | [34] |
| ChCl—urea/ethylene glycol/glycerol/lactic acid 1:2 ChCl—oxalic acid 1:1 | Bagasse/100 °C, 4 h | Up to 47.85% lignin was solubilized (oxalic acid). Minimum solubility of 8.60% is exhibited by the ethylene glycol DES. Acidic DES improved the crystallinity of bagasse fibre, whereas basic DES had little effect on the crystallinity of cellulose. | [32] |
| ChCl:formic acid 1:2 | Pine wood/130 °C for 6 h | Acid DESs were the most efficient to solubilize hemicellulose and lignin, resulting in a glucan content increased. The lignin could be successfully isolated with high yield and high purity. DES could also be successfully recovered and reused without loss of performance. | [62] |
| ChCl:acetic acid 1:2 | |||
| ChCl:propionic acid 1:2 | |||
| ChCl:lactic acid 1:2 | |||
| ChCl:citric acid 1:1 | |||
| ChCl:malic acid 1:1 | |||
| ChCl:succinic acid 1:1 | |||
| ChCl:glycerol 1:2 | |||
| ChCl:ethylene glycol 1:2 | |||
| ChCl:diethylene glycol 1:2 | |||
| ChCl:triethylene glycol 1:2 | |||
| ChCl:xylitol 1:1 | |||
| ChCl:urea 1:2 | |||
| ChCl:formamide 1:2 | |||
| ChCl:acetamide 1:2 | |||
| ChCl:lactic acid:formic acid 1:1:1 | |||
| ChCl:lactic acid:formamide 1:1:1 | |||
| ChCl:formic acid:ethylene glycol 1:0.4:1.6 | |||
| ChCl:acetic acid:ethylene glycol 1:0.4:1.6 | |||
| ChCl:propionic acid:ethyleneglycol 1:0.4:1.6 | |||
| ChCl:lactic acid:ethylene glycol 1:0.4:1.6 | |||
| ChCl:formic acid:ethylene glycol 1:1.2:0.8 | |||
| ChCl:acetic acid:ethylene glycol 1:1.2:0.8 | |||
| ChCl:propionic acid:ethyleneglycol 1:1.2:0.8 | |||
| ChCl:lactic acid:ethylene glycol 1:1.2:0.8 | |||
| ChCl:glycerol 1:1; 1:2; 2:1 | Garlic skin Green onion root/Biomass-DES 1:10 w/w; 110 °C, 4 h (oil bath) 80 °C, 20 min (MW) ultrasound pretreatment at room temperature | The study demonstrated the effects of ultrasound frequency, the molar ratio, metal chloride, composition of DES at different heating methods on the components of biomass. | [35] |
| ChCl:oxalic acid 1:1; 1:2; 2:1 | |||
| ChCl:urea 1:1; 1:2; 2:1 | |||
| ChCl:glycerol:AlCl3·6H2O 1:2:0.2 | |||
| ChCl:glycerol:FeCl3·6H2O 1:2:0.2 | |||
| ChCl:glycerol:CrCl3·6H2O 1:2:0.2 | |||
| ChCl:Urea 1:2 | Corn stover/80–120 °C, 24 h followed by hydrolysis promoted by the carbon-based solid acid catalyst. | Total of 33.9% of xylose and 6.9% of glucose was recovered from ChCl:formic acid. The comparison between hydrolysis conducted in two steps, after pretreatment with DES (or ILs), and the one-pot method showed that the glucose yield obtained by one-pot hydrolysis was much lower than the other. | [63] |
| ChCl:Glycerol 1:2 | |||
| ChCl:Malonic acid 1:1 | |||
| ChCl:Formic acid 1:2 | |||
| ChCl:Ethylene glycol 1:2 | |||
| ChCl:1,4-butanediol 1:4 | |||
| ChCl:Citric acid 1:1 | |||
| ChCl:Oxalic acid 1:1 | |||
| Acetamide:Levulinic acid 1:2 Betaine:Levulinic acid 1:2 ChCl:Levulinic acid 1:2 | Moso Bamboo/120 °C, 2 h | ChCl:Levulinic acid presented 79.07% glucose conversion yield, Acetamide:Levulinic acid 56.44% and Betaine:Levulinic acid 48.90%. | [64] |
| ChCl:lactic acid 1:10 | Eucalyptus Camaldulensis/90–130 °C, 6 h | Up to 80% lignin was removed and more than 44% could be collected. The enzymatic saccharification yields of cellulose was higher than 90%. | [65] |
| ChCl:lactic acid 1:9 | Poplar wood meal/120 °C, 6 h | Lignins were efficiently removed (95%). The purity of regenerated lignin is very high (up to 98.1%). | [66] |
| ChCl:lactic acid 1:5 | Sugarcane bagasse/80 °C, 12 h | Delignification 50.6% Enzymatic conversion 90.4% | [67] |
| ChCl:Glycerol:Lewis acid 62:124:1 (Ternary DES) Lewis acid: AlCl3·6H2O, FeCl3·6H2O, FeCl2·4H2O, ZnCl2, CuCl2 | Pennisetum/Biomass-DES 1:10 w/w. 60–140 °C, 1–9 h | The use of Lewis acids significantly improved the delignification of Pennisetum, reaching 85% efficiency. | [68] |
| ChCl:Formic acid/Acetic acid/lactic acid 1:2 | Hardwood poplar/Two-step process: Liquid Hot Water Extraction (170 °C for 40 min)—DES treatment (130 °C for 3 h; biomass-DES 1:20 w/w) | After the physical pretreatment, a solid yield of 79.8% and hemicellulose removal of 54.4% were observed. Cellulose content was increased from 61.1 to 79.8–85.4%, corresponding to a relatively high extent of delignification of 73.0–76.5% after acidic DESs pretreatment. | [69] |
| ChCl:Ethylene Glycol 2:1 | Eucalyptus globulus wood/Two-step pretreatment: hydrothermal treatment 170 °C, 4 h +90 °C, 24 h (DES) | The removal of lignin and hemicellulose was 90.2% and 97.4%. Cellulose was effectively retained (94.5% of the original cellulose content). | [70] |
| ChCl:lactic/malic/citric/formic/acetic/propionic/butyric/succinic/maleic acid Various molar ratio combinations at 2:1, 1:1, 1:2, 1:5, 1:10 and 1:15 | Oil palm empty fruit bunch/Biomass/DES ratio 1:10 wt. 120 °C, 8 h | Formic acid (61.9% lignin yield) and lactic acid (33.5% lignin yield)-based DESs emerged for the highest lignin extraction yield. | [71] |
| ChCl:lactic acid 1:5 D(+)-glucose:lactic acid 1:5 ChCl:D(+)-glucose 1:1 ChCl:glycerol 1:2 ChCl:urea 1:2 K2CO3:glycerol 1:6 | Oil palm empty fruit bunch/Biomass/DES ratio 1:10 wt. 120 °C, 8 h. | Delignification: ChCl:lactic acid 88% D(+)-glucose:lactic acid 55% ChCl:D(+)-glucose 17% ChCl:glycerol 22% ChCl:urea 34% K2CO3:glycerol 51% The acidic DES pretreatment allows the destruction of the hemicellulose: after the lactic acid based-DES treatment, no residual hemicellulose was detected in the solid. | [46] |
| ChCl:Monoethanol amine/N-methyldiethanolamine/Urea/Acetamide/Diethanolamine/Glycerol Molar ratios 1:6, 1:8, 1:10, 1:2, 1:2 and 1:2 | Wheat straw/Biomass/DES ratio 1:20 wt. 50–130 °C, 1–24 h | ChCl-MonoethanolAmine is capable of removing 71.4% lignin while reserving 93.7% cellulose; 89.8% and 62.0% of cellulose and xylan conversions were eventually achieved through enzymatic hydrolysis of residue. | [48] |
| Guanidine·HCl:Lactic acid | Rice straw/Biomass/DES 5 wt. %. 80 °C, 6 h or 120 °C, 3–6 h | The pretreatment efficiency of the Lactic acid amide DES varied as the HBD structures changed and the pretreatment efficiency of these DESs displayed a similar trend as the polyol-based DESs. | [72] |
| ChCl-Ethylene glycol | |||
| ChCl-Glycerol | |||
| ChCl-Xylitol | |||
| ChCl- Formamide | |||
| ChCl-Urea | |||
| ChCl-Guanidine·HCl | |||
| ChCl-1,2-Propanediol | |||
| ChCl-1,3-Propanediol | |||
| ChCl-Glycolic acid | |||
| ChCl-Lactic acid | |||
| ChCl-2-Chloropropionic acid | |||
| ChCl-Oxalic acid | |||
| ChCl-Malonic acid | |||
| Molar ratio 1:1 | |||
| ChCl:Lactic acid 1:10 | Eucalyptus Camaldulensis/60–140 °C, 6 h | The DES pretreatment temperatures influence the cleavage of C−O and C−C bonds in the lignin dehydration, and acylation of OH groups and partial recondensation were observed. | [73] |
| Lactic acid/tartaric acid/ChCl 4:1:1 | Pinus pinaster/175 °C, 1 h | Lactic acid:ChCl extracted the highest lignin amount, while the purest lignin was achieved with Tartaric Acid:ChCl. With the ternary DES, it registered a recovery of ca. 95% of lignin present in the biomass with a purity of ca. 89%. | [74] |
| ChCl:oxalic acid 1:1 Cosolvents: butanol, n-propanol and ethyl acetate [DES/cosolvent ratio 1:2, 1:1, 2:1] | Rice husk, rice straw/50–80–120 °C 1 h and wheat straw | Delignification using the ternary combination system (DES-n-butanol) was at least 50% higher than that of pure DES treatment (23–31% delignification). | [75] |
| ChCl:oxalic acid 2:1, 1:1 and 1:10 Cosolvent: MIBK Cat. AlCl3·6H2O | Eucalyptus urophydis/Biomass/DES ratio 1:10 wt. MIBK/DES ratio 1.6 w/w. 100–160 °C, 30–120 min. | At 140 °C for 90 min, the maximum yield of Furfural was 70.3%, and the maximum saccharification was 80.8%. | [76] |
| ChCl:Lactic acid 1:10 | Pinus bungeana Zucc./Biomass/DES ratio 6.25%wt. 120 °C, 4 h (oil bath) 120 °C, 8 min (MW, 800 W). | The cellulose conversion of biomass pretreated with MW-DES achieved 81.9%. DES pretreated samples (41.6%). | [77] |
| ChCl:Glycerol:Lewis acid (AlCl3·6H2O, FeCl3·6H2O, CrCl3·6H2O, MgCl2·6H2O) molar ratio = 1:1:0.3 | Sugarcane Bagasse/Pretreatment with ethanol ultrasound at various ultrasonic frequency. Biomass/DES ratio 1:10 wt. 100–140 °C, 1–4 h | The sequential multifrequency ultrasonic and DES (with Lewis acid) pretreatment enhanced the delignification of lignin and cellulose recovery from biomass. | [78] |
| ChCl:acetic acid 1:2 | Poplar wood Douglas fir/90–180 °C 1–9 h | Delignification: 78% from poplar and 58% from D. fir. Highly pure lignin (95%) with unique structural properties was recovered. | [79] |
| ChCl:lactic acid 1:2 | |||
| ChCl:levulinic acid 1:2 | |||
| ChCl:glycerol 1:2 | |||
| ChCl:p-hydroxybenzoic acid 3:2 | Poplar wood/Biomass/DES ratio 1:10 wt. 100–160 °C for 1–9 h | ChCl- p-hydroxybenzoic acid showed the best performance in terms of delignification (69%) and digestibility of glucan and xylan (90.8% and 88.9%). | [41] |
| ChCl:p-coumaric acid 1:1 | |||
| ChCl:4-hydroxybenzylaldehyde 1:2 |
| Feedstock | DESs | Operative Conditions | S (wt. %) | Ref. |
|---|---|---|---|---|
| MCC | ChCl/urea | T = 110 °C, 12 h | <0.2 | [117] |
| MCC | ChCl/ZnCl2 | T = 110 °C, 12 h | <0.2 | [117] |
| MCC | [Ch]OAc | T = 110 °C, 12 h | 0.5 | [117] |
| MCC | [Ch]OAc/[TBMA]Cl (15 wt. %) | T = 110 °C, 10 min | 6 | [117] |
| Cotton linter pulp (DP = 575.6) | ChCl/urea | ultrasound activation; T = 120 °C | 1.43 | [118] |
| Cotton linter pulp (DP = 575.6) | ChCl/imidazole | ultrasound activation; T = 120 °C | 2.48 | [118] |
| Cotton linter pulp (DP = 575.6) | ChCl/imidazole | ultrasound activation; T = 120 °C cosolvent = 5% PEG | 4.57 | [118] |
| Commercial cellulose (Merck) | ChCl/maleic acid | ultrasound activation; T = 90 °C, 20 min | 2.57 | [119] |
| Commercial cellulose (Merck) | ChCl/a-naphthol | ultrasound activation; T = 90 °C, 20 min | 3.39 | [119] |
| Commercial cellulose (Merck) | ChCl/phenol | ultrasound activation; T = 90 °C, 20 min | 4.70 | [119] |
| Commercial cellulose (Merck) | ChCl/resorcinol | ultrasound activation; T = 90 °C, 20 min | 6.10 | [119] |
| MCC | Glyn-EmimCl | T = 100 °C, 3 h | 5 | [120] |
| MCC | Glyn-EmimCl | MW, 6 s | 5 | [120] |
| Cotton linter pulp (DP = 575.6) | Triethyl-allyl ammonia chloride/oxalic acid | T = 110 °C, 2 h | 6.48 | [121] |
| Feedstock | DES | Operative Conditions | Results | Ref. |
|---|---|---|---|---|
| Cellulose | 3DEACl 3:2oxalic acid | at 170 °C in 5 min, MW reactor | 38.4% total conversion (levulinic acid, HMF, furfural and formic acid) | [144] |
| ChCl/pTSA | 80 °C, 30 min | 93% yield in HMF | [145] | |
| Oxalic acid/SnCl4 Oxalic acid/CrCl3 | 160 °C, 90 min | 11% HMF 22.7% glucose | [145] | |
| ChCl/oxalic acid + SnCl4 | 140 °C, 2 h | 23.5% HMF yield | [146] | |
| ChCl/oxalic acid | 180 °C, 1 min, MW reactor | 76.2% of levulinic acid, 4.07% of HMF, 5.57% of furfural | [147] | |
| ChCl, AlCl3·6H2O, oxalic acid | at 120 °C within 30 min | CMF yield of 30% | [148] | |
| Glucose | ChCl/ethylene glycol + CrCl3 | t 150 °C, 3.64 min | 42% yield of HMF | [149] |
| ChCl, AlCl3·6H2O, oxalic acid | 120 °C, 30 min | CMF yield of 70% | [148] | |
| ChCl/AlCl3 | 120 °C for 2 h and 14 h cosolvent = EtAc | 73.2% furan products, including 52.9% of HMF and 17.5% of AMF | [150] | |
| Fructose | Im/1.5BSA | 100 °C, 3 min | 90.1% HMF | [151] |
| Ch/pTSA | 80 °C, 1 h | 90.7% HMF | [140] | |
| [Emim]Cl/isopropanol | 25 °C, 3 h Cat = [HNMP]Cl | 89% HMF | [141] | |
| ChCl/AlCl3∙6H2O | 120 °C, 5 h | 50.3% of CMF 8.1% of HMF | [152] | |
| DES/acetonitrile (MeCN) biphasic reaction | 100 °C, 4 h | 90.3% HMF | [153] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scelsi, E.; Angelini, A.; Pastore, C. Deep Eutectic Solvents for the Valorisation of Lignocellulosic Biomasses towards Fine Chemicals. Biomass 2021, 1, 29-59. https://doi.org/10.3390/biomass1010003
Scelsi E, Angelini A, Pastore C. Deep Eutectic Solvents for the Valorisation of Lignocellulosic Biomasses towards Fine Chemicals. Biomass. 2021; 1(1):29-59. https://doi.org/10.3390/biomass1010003
Chicago/Turabian StyleScelsi, Enrico, Antonella Angelini, and Carlo Pastore. 2021. "Deep Eutectic Solvents for the Valorisation of Lignocellulosic Biomasses towards Fine Chemicals" Biomass 1, no. 1: 29-59. https://doi.org/10.3390/biomass1010003
APA StyleScelsi, E., Angelini, A., & Pastore, C. (2021). Deep Eutectic Solvents for the Valorisation of Lignocellulosic Biomasses towards Fine Chemicals. Biomass, 1(1), 29-59. https://doi.org/10.3390/biomass1010003








