Microalgae Isolated from Singapore Mangrove Habitat as Promising Microorganisms for the Sustainable Production of Omega-3 Docosahexaenoic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Strain Isolation
2.2. Strain Cultivation
2.3. Strain Identification by 18S rRNA Sequences
2.4. Morphological Analysis
2.5. Measurement of Dry Cell Weight (DCW)
2.6. Measurement of Total Lipids
2.7. Fatty Acid Composition Analysis
2.8. Optimization of Carbon and Nitrogen Content
2.9. Fed-Batch Fermentation
3. Results and Discussion
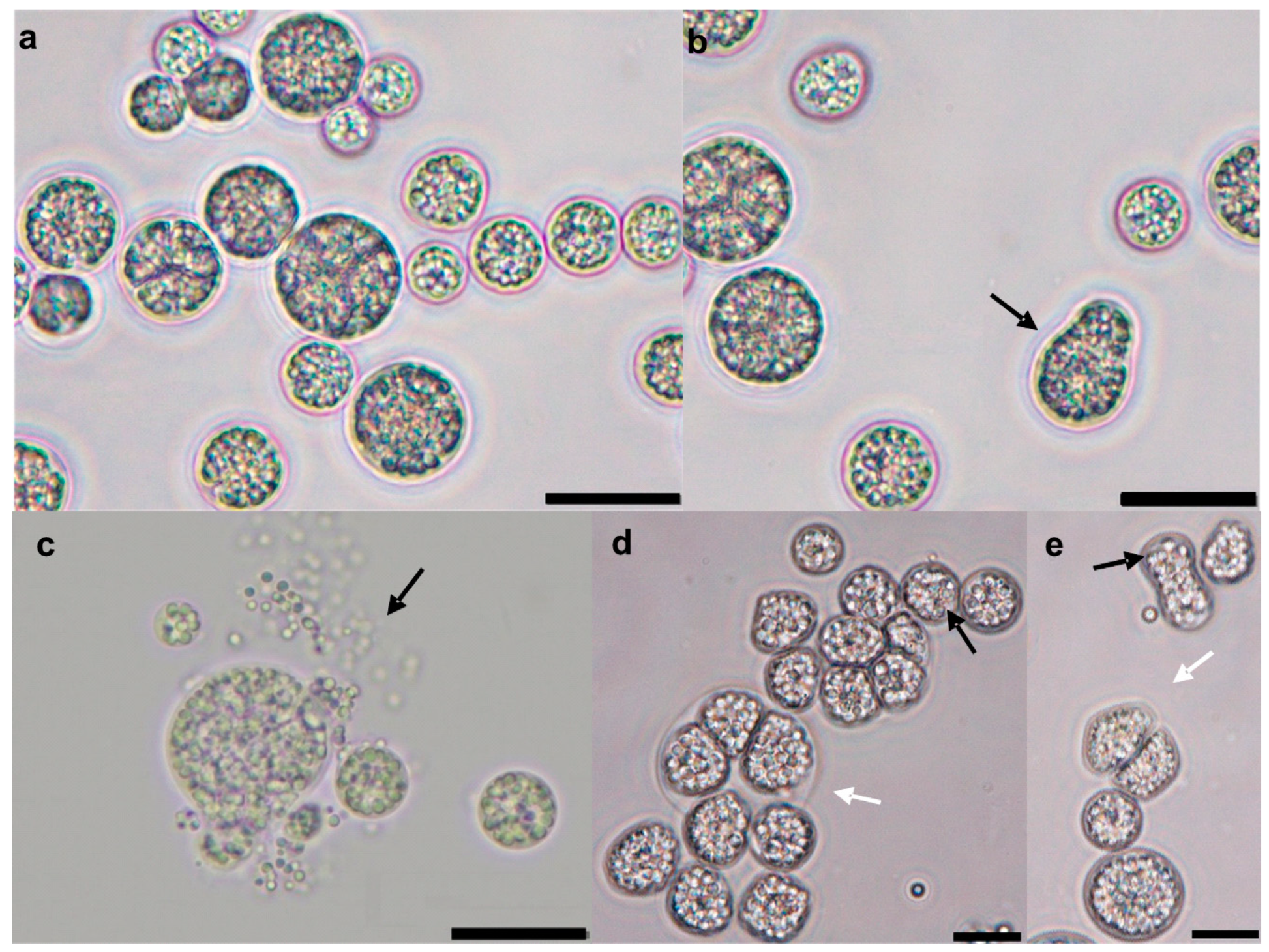
3.1. Isolation and Identification of Microalgae Strains
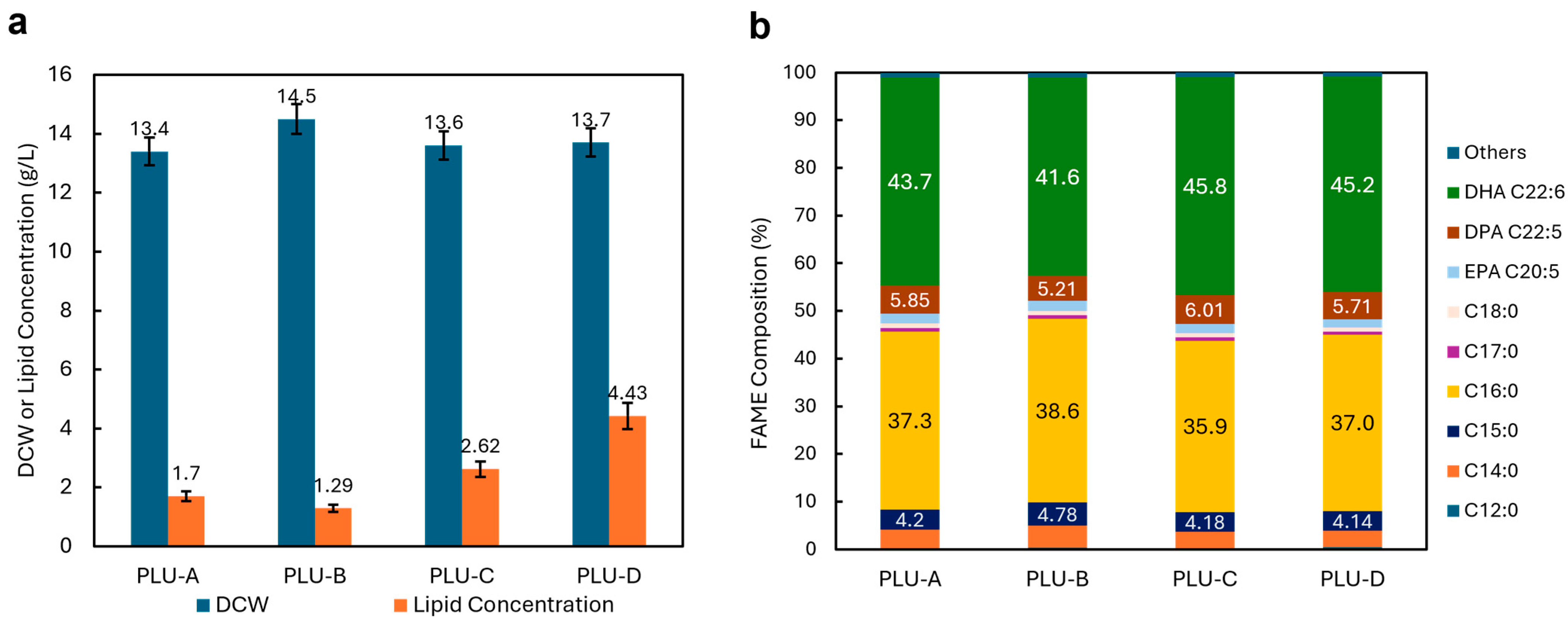
3.2. Characterization of Biomass, Lipid Concentration and DHA Content of Schizochytrium sp. Isolates
3.3. Morphology of Schizochytrium sp. PLU-D
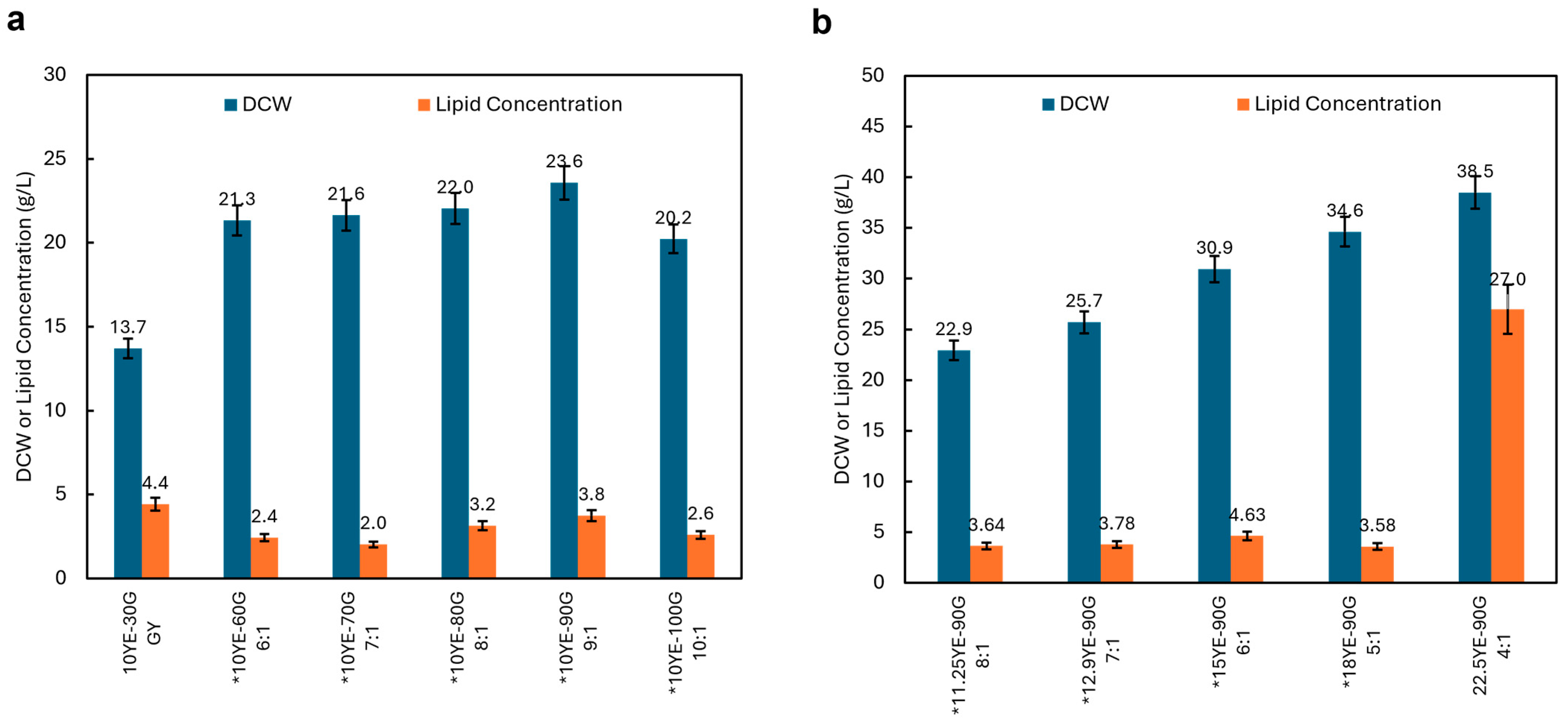
3.4. Optimization of Carbon-to-Nitrogen Ratio
3.5. DHA Production by Schizochytrium sp. PLU-D in Fed-Batch Fermentation
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, H.; Li, X.; Su, X.; Ang, E.L.; Zhang, Y.; Zhao, H. Production of Adipic Acid from Sugar Beet Residue by Combined Biological and Chemical Catalysis. ChemCatChem 2016, 8, 1500–1506. [Google Scholar] [CrossRef]
- Ng, A.M.J.; Yang, R.; Zhang, H.; Xue, B.; Yew, W.S.; Nguyen, G.K.T. A Novel Lipase from Lasiodiplodia theobromae Efficiently Hydrolyses C8-C10 Methyl Esters for the Preparation of Medium-Chain Triglycerides’ Precursors. Int. J. Mol. Sci. 2021, 22, 10339. [Google Scholar] [CrossRef]
- Chow, J.Y.; Nguyen, G.K.T. Rational Design of Lipase ROL to Increase Its Thermostability for Production of Structured Tags. Int. J. Mol. Sci. 2022, 23, 9515. [Google Scholar] [CrossRef] [PubMed]
- Kua, G.K.B.; Nguyen, G.K.T.; Li, Z. Enzyme Engineering for High-Yielding Amide Formation: Lipase-Catalyzed Synthesis of N-Acyl Glycines in Aqueous Media. Angew. Chemie Int. Ed. 2023, 62, e202217878. [Google Scholar] [CrossRef] [PubMed]
- Kua, G.K.B.; Nguyen, G.K.T.; Li, Z. Enzymatic Strategies for the Biosynthesis of N-Acyl Amino Acid Amides. ChemBioChem 2024, 25, e202300672. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, L.; Brambilla, P.; Mazzocchi, A.; Harsløf, L.B.S.; Ciappolino, V.; Agostoni, C. DHA Effects in Brain Development and Function. Nutrients 2016, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.; Lone, A.N.; Khan, M.S.; Virani, S.S.; Blumenthal, R.S.; Nasir, K.; Miller, M.; Michos, E.D.; Ballantyne, C.M.; Boden, W.E.; et al. Effect of omega-3 fatty acids on cardiovascular outcomes: A systematic review and meta-analysis. eClinicalMedicine 2021, 38, 100997. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.S.; Hung, C.F.; Ponnusamy, V.K.; Chen, K.C.; Chen, N.C. Higher Serum DHA and Slower Cognitive Decline in Patients with Alzheimer’s Disease: Two-Year Follow-Up. Nutrients 2022, 14, 1159. [Google Scholar] [CrossRef] [PubMed]
- Aslan, C.; Maralbashi, S.; Kahroba, H.; Asadi, M.; Soltani-Zangbar, M.S.; Javadian, M.; Shanehbandi, D.; Baradaran, B.; Darabi, M.; Kazemi, T. Docosahexaenoic acid (DHA) inhibits pro-angiogenic effects of breast cancer cells via down-regulating cellular and exosomal expression of angiogenic genes and microRNAs. Life Sci. 2020, 258, 118094. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tian, Y.; Cai, W.; Guo, Y.; Xue, C.; Wang, J. DHA/EPA-Enriched Phosphatidylcholine Suppresses Tumor Growth and Metastasis via Activating Peroxisome Proliferator-Activated Receptor γ in Lewis Lung Cancer Mice. J. Agric. Food Chem. 2021, 69, 676–685. [Google Scholar] [CrossRef]
- Oliver, L.; Dietrich, T.; Marañón, I.; Villarán, M.C.; Barrio, R.J. Producing Omega-3 Polyunsaturated Fatty Acids: A Review of Sustainable Sources and Future Trends for the EPA and DHA Market. Resources 2020, 9, 148. [Google Scholar] [CrossRef]
- Wall, R.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Fatty acids from fish: The anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr. Rev. 2010, 68, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Ganuza, E.; Anderson, A.J.; Ratledge, C. High-cell-density cultivation of Schizochytrium sp. in an ammonium/pH-auxostat fed-batch system. Biotechnol. Lett. 2008, 30, 1559–1564. [Google Scholar] [CrossRef] [PubMed]
- Burja, A.M.; Radianingtyas, H.; Windust, A.; Barrow, C.J. Isolation and characterization of polyunsaturated fatty acid producing Thraustochytrium species: Screening of strains and optimization of omega-3 production. Appl. Microbiol. Biotechnol. 2006, 72, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Sharpe, P.L.; Hong, S.-P.; Yadav, N.S.; Xie, D.; Short, D.R.; Damude, H.G.; Rupert, R.A.; Seip, J.E.; Wang, J.; et al. Production of omega-3 eicosapentaenoic acid by metabolic engineering of Yarrowia lipolytica. Nat. Biotechnol. 2013, 31, 734–740. [Google Scholar] [CrossRef]
- Pino-Maureira, N.L.; González-Saldía, R.R.; Capdeville, A.; Srain, B. Rhodotorula Strains Isolated from Seawater That Can Biotransform Raw Glycerol into Docosahexaenoic Acid (DHA) and Carotenoids for Animal Nutrition. Appl. Sci. 2021, 11, 2824. [Google Scholar] [CrossRef]
- Mendes, A.; Reis, A.; Vasconcelos, R.; Guerra, P.; Lopes da Silva, T. Crypthecodinium cohnii with emphasis on DHA production: A review. J. Appl. Phycol. 2009, 21, 199–214. [Google Scholar] [CrossRef]
- Sahin, D.; Altindag, U.H.; Tas, E. Enhancement of docosahexaenoic acid (DHA) and beta-carotene production in Schizochytrium sp. using symbiotic relationship with Rhodotorula glutinis. Process Biochem. 2018, 75, 10–15. [Google Scholar] [CrossRef]
- Morabito, C.; Bournaud, C.; Maës, C.; Schuler, M.; Aiese Cigliano, R.; Dellero, Y.; Maréchal, E.; Amato, A.; Rébeillé, F. The lipid metabolism in thraustochytrids. Prog. Lipid Res. 2019, 76, 101007. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Goda, H.; Hamaguchi, R.; Sakaguchi, K.; Sekiguchi, T.; Ishiwata, Y.; Okita, Y.; Mochinaga, S.; Ikeuchi, S.; Mizobuchi, T.; et al. PUFA synthase-independent DHA synthesis pathway in Parietichytrium sp. and its modification to produce EPA and n-3DPA. Commun. Biol. 2021, 4, 1–11. [Google Scholar] [CrossRef]
- Nagano, N.; Sakaguchi, K.; Taoka, Y.; Okita, Y.; Honda, D.; Ito, M.; Hayashi, M. Detection of Genes Involved in Fatty Acid Elongation and Desaturation in Thraustochytrid Marine Eukaryotes. J. Oleo Sci. 2011, 60, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Meesapyodsuk, D.; Qiu, X. Biosynthetic mechanism of very long chain polyunsaturated fatty acids in Thraustochytrium sp. 26185. J. Lipid Res. 2016, 57, 1854–1864. [Google Scholar] [CrossRef]
- Ju, J.-H.; Ko, D.-J.; Heo, S.-Y.; Lee, J.-J.; Kim, Y.-M.; Lee, B.-S.; Kim, M.-S.; Kim, C.-H.; Seo, J.-W.; Oh, B.-R. Regulation of lipid accumulation using nitrogen for microalgae lipid production in Schizochytrium sp. ABC101. Renew. Energy 2020, 153, 580–587. [Google Scholar] [CrossRef]
- Ren, L.-J.; Huang, H.; Xiao, A.-H.; Lian, M.; Jin, L.-J.; Ji, X.-J. Enhanced docosahexaenoic acid production by reinforcing acetyl-CoA and NADPH supply in Schizochytrium sp. HX-308. Bioprocess Biosyst. Eng. 2009, 32, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.-J.; Ji, X.-J.; Huang, H.; Qu, L.; Feng, Y.; Tong, Q.-Q.; Ouyang, P.-K. Development of a stepwise aeration control strategy for efficient docosahexaenoic acid production by Schizochytrium sp. Appl. Microbiol. Biotechnol. 2010, 87, 1649–1656. [Google Scholar] [CrossRef]
- Ren, L.; Sun, X.; Zhang, L.; Huang, H.; Zhao, Q. Exergy analysis for docosahexaenoic acid production by fermentation and strain improvement by adaptive laboratory evolution for Schizochytrium sp. Bioresour. Technol. 2020, 298, 122562. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-M.; Ren, L.-J.; Bi, Z.-Q.; Ji, X.-J.; Zhao, Q.-Y.; Jiang, L.; Huang, H. Development of a cooperative two-factor adaptive-evolution method to enhance lipid production and prevent lipid peroxidation in Schizochytrium sp. Biotechnol. Biofuels 2018, 11, 65. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Singh, P.; Sun, Y.; Luan, S.; Wang, G. Culturable diversity and biochemical features of thraustochytrids from coastal waters of Southern China. Appl. Microbiol. Biotechnol. 2014, 98, 3241–3255. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Bai, M.; Zhang, S.; Li, J.; Liu, X.; Sen, B.; Wang, G. ARTP Mutagenesis of Schizochytrium sp. PKU#Mn4 and Clethodim-Based Mutant Screening for Enhanced Docosahexaenoic Acid Accumulation. Mar. Drugs 2021, 19, 564. [Google Scholar] [CrossRef]
- Johnson, M.; Zaretskaya, I.; Raytselis, Y.; Merezhuk, Y.; McGinnis, S.; Madden, T.L. NCBI BLAST: A better web interface. Nucleic Acids Res. 2008, 36, W5–W9. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Venn-Watson, S.; Lumpkin, R.; Dennis, E.A. Efficacy of dietary odd-chain saturated fatty acid pentadecanoic acid parallels broad associated health benefits in humans: Could it be essential? Sci. Rep. 2020, 10, 1–14. [Google Scholar]
- Unagul, P.; Suetrong, S.; Preedanon, S.; Klaysuban, A.; Gundool, W.; Suriyachadkun, C.; Sakayaroj, J. Isolation, fatty acid profiles and cryopreservation of marine thraustochytrids from mangrove habitats in Thailand. Bot. Mar. 2017, 60, 363–379. [Google Scholar] [CrossRef]
- Ou, M.-C.; Yeong, H.-Y.; Pang, K.-L.; Phang, S.-M. Fatty acid production of tropical thraustochytrids from Malaysian mangroves. Bot. Mar. 2016, 59, 321–338. [Google Scholar] [CrossRef]
- Manikan, V.; Nazir, M.Y.M.; Kalil, M.S.; Isa, M.H.M.; Kader, A.J.A.; Yusoff, W.M.W.; Hamid, A.A. A new strain of docosahexaenoic acid producing microalga from Malaysian coastal waters. Algal Res. 2015, 9, 40–47. [Google Scholar] [CrossRef]
- Kalidasan, K.; Vinithkumar, N.V.; Peter, D.M.; Dharani, G.; Dufossé, L. Thraustochytrids of mangrove habitats from andaman islands: Species diversity, PUFA profiles and biotechnological potential. Mar. Drugs 2021, 19, 571. [Google Scholar] [CrossRef]
- Ganuza, E.; Izquierdo, M.S. Lipid accumulation in Schizochytrium G13/2S produced in continuous culture. Appl. Microbiol. Biotechnol. 2007, 76, 985–990. [Google Scholar] [CrossRef]
- Ling, X.; Guo, J.; Liu, X.; Zhang, X.; Wang, N.; Lu, Y.; Ng, I.S. Impact of carbon and nitrogen feeding strategy on high production of biomass and docosahexaenoic acid (DHA) by Schizochytrium sp. LU310. Bioresour. Technol. 2015, 184, 139–147. [Google Scholar] [CrossRef]
- Patil, K.P.; Gogate, P.R. Improved synthesis of docosahexaenoic acid (DHA) using Schizochytrium limacinum SR21 and sustainable media. Chem. Eng. J. 2015, 268, 187–196. [Google Scholar] [CrossRef]
- Ratledge, C. Regulation of lipid accumulation in oleaginous micro-organisms. Biochem. Soc. Trans. 2002, 30, 1047–1050. [Google Scholar] [CrossRef]
- Ratledge, C. The role of malic enzyme as the provider of NADPH in oleaginous microorganisms: A reappraisal and unsolved problems. Biotechnol. Lett. 2014, 36, 1557–1568. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Bi, Y.; Guo, P.; Bi, Y.; Wang, T.; Dong, L.; Wang, F.; Chen, L.; Zhang, W. Metabolic Analysis of Schizochytrium Mutants with High DHA Content Achieved With ARTP Mutagenesis Combined with Iodoacetic Acid and Dehydroepiandrosterone Screening. Front. Bioeng. Biotechnol. 2021, 9, 738052. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, A.N.; Aasen, I.M.; Josefsen, K.D.; Strøm, A.R. Accumulation of docosahexaenoic acid-rich lipid in thraustochytrid Aurantiochytrium sp. strain T66: Effects of N and P starvation and O2 limitation. Appl. Microbiol. Biotechnol. 2008, 80, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.Y.; Lu, W.C.; Chu, I.M. A fermentation strategy for producing docosahexaenoic acid in Aurantiochytrium limacinum SR21 and increasing C22:6 proportions in total fatty acid. Bioresour. Technol. 2012, 123, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Hoang, L.A.T.; Nguyen, H.C.; Le, T.T.; Hoang, T.H.Q.; Pham, V.N.; Hoang, M.H.T.; Ngo, H.T.T.; Hong, D.D. Different fermentation strategies by Schizochytrium mangrovei strain pq6 to produce feedstock for exploitation of squalene and omega-3 fatty acids. J. Phycol. 2018, 54, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.W.; Zhang, Y.T.; Jiang, J.Y.; Guo, D.S.; Gao, S.; Gao, Z. Efficient docosahexaenoic acid production by Schizochytrium sp. via a two-phase pH control strategy using ammonia and citric acid as pH regulators. Process Biochem. 2019, 77, 1–7. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, X.; Ye, H.; Wen, Y.; Sen, B.; Wang, G. Low dissolved oxygen supply functions as a global regulator of the growth and metabolism of Aurantiochytrium sp. PKU#Mn16 in the early stages of docosahexaenoic acid fermentation. Microb. Cell Fact. 2023, 22, 1–13. [Google Scholar]
- Chang, G.; Gao, N.; Tian, G.; Wu, Q.; Chang, M.; Wang, X. Improvement of docosahexaenoic acid production on glycerol by Schizochytrium sp. S31 with constantly high oxygen transfer coefficient. Bioresour. Technol. 2013, 142, 400–406. [Google Scholar] [CrossRef]


| Entry | Thraustochytrid Strains | Scale | Fermentation Time (h) | Biomass (g/L DCW) | Lipid Concentration (g/L) | Lipid Content (%) | DHA Titer (g/L) | DHA Content (%) | References |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Aurantiochytrium sp. ATCC PRA-276 (T66) | 5 L fermenter | 180 | 100.0 | 58.0 | 58.0 | 17.4 | 30.0 | [43] |
| 2 | Schizochytrium sp. G13/2S | 5 L fermenter | 48 | 63.3 | 15.8 | 25.0 | 5.47 | 34.6 | [13] |
| 3 | Aurantiochytrium limacinum ATCCMYA-1381(SR21) | 5 L fermenter | 150 | 61.8 | 40.3 | 65.2 | 20.3 | 50.4 | [44] |
| 4 | Schizochytrium mangrovei (PQ6) | 30 L fermenter | 96 | 105.3 | 45.7 | 43.4 | 5.9 | 12.9 | [45] |
| 5 | Schizochytrium sp. CCTCC M209059 (HX-308) | 1500 L fermenter | 132 | 71.0 | 35.8 | 50.4 | 17.5 | 48.9 | [25] |
| 6 | Schizochytrium sp. CCTCC M209059 (HX-308) | 5 L fermenter | 120 | 120.6 | 62.6 | 51.9 | 32.8 | 52.4 | [46] |
| 7 | Schizochytrium sp. CCTCC M209059 (ALE-TF30) | 5 L fermenter | 120 | 126.4 | 71.6 | 56.6 | 38.1 | 53.2 | [27] |
| 8 | Schizochytrium sp. ABC101 | 5 L fermenter | 84 | 86.0 | 37.2 | 43.3 | 16.7 | 44.9 | [23] |
| 9 | Aurantiochytrium sp. PKU#Mn16 | 5 L fermenter | 144 | 56.7 | 19.0 | 33.5 | 6.0 | 31.6 | [47] |
| 10 | Schizochytrium sp. LU310 | 1 L baffled flask | 144 | 88.6 | 52.0 | 58.7 | 24.7 | 47.5 | [38] |
| 11 | Schizochytrium sp. ATCC 20,888 (S31) | 7.5 L fermenter | 96 | 151.4 | 79.7 | 52.6 | 28.9 | 36.3 | [48] |
| 12 | Schizochytrium sp. ATCC20888 (M-6-23) | 5 L fermenter | 96 | 154.8 | 88.2 | 57.0 | 41.4 | 46.9 | [42] |
| 13 | Schizochytrium sp. PLU-D | 2 L fermenter | 84 | 175.3 | 93.6 | 53.4 | 36.2 | 38.7 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kua, G.K.B.; Kong, S.N.; Zhang, H.; Nguyen, G.K.T. Microalgae Isolated from Singapore Mangrove Habitat as Promising Microorganisms for the Sustainable Production of Omega-3 Docosahexaenoic Acid. Biomass 2024, 4, 751-764. https://doi.org/10.3390/biomass4030042
Kua GKB, Kong SN, Zhang H, Nguyen GKT. Microalgae Isolated from Singapore Mangrove Habitat as Promising Microorganisms for the Sustainable Production of Omega-3 Docosahexaenoic Acid. Biomass. 2024; 4(3):751-764. https://doi.org/10.3390/biomass4030042
Chicago/Turabian StyleKua, Glen Kai Bin, Shik Nie Kong, Hongfang Zhang, and Giang Kien Truc Nguyen. 2024. "Microalgae Isolated from Singapore Mangrove Habitat as Promising Microorganisms for the Sustainable Production of Omega-3 Docosahexaenoic Acid" Biomass 4, no. 3: 751-764. https://doi.org/10.3390/biomass4030042
APA StyleKua, G. K. B., Kong, S. N., Zhang, H., & Nguyen, G. K. T. (2024). Microalgae Isolated from Singapore Mangrove Habitat as Promising Microorganisms for the Sustainable Production of Omega-3 Docosahexaenoic Acid. Biomass, 4(3), 751-764. https://doi.org/10.3390/biomass4030042





